Introduction
Cervical cancer is a primary malignant tumor of the
female reproductive system and is associated with high mortality.
Its development involves multiple steps of genetic aberrations and
complex biological processes, and is closely linked to persistent
infection with high-risk human papilloma virus (HPV) (1). The overall malignant transformation
process includes a number of genetic and epigenetic alterations and
the mechanisms of carcinogenesis remain to be fully elucidated.
MicroRNAs (miRNAs) are small non-coding RNAs of
19–25 nucleotides in length, which modulate gene expression either
by catalyzing mRNA cleavage or by inhibiting mRNA translation. They
may act as tumor suppressors or oncogenes by regulating the
expression of various target genes (2,3). MiR-21
has been reported to be associated with a wide variety of human
cancers, including cervical cancer (4,5). The
upregulation of miR-21 has been observed in cervical cancer cell
lines and clinical specimens (6,7). MiR-21
acts as an oncogene by promoting the proliferation and migration of
cervical cancer cells, while the underlying molecular mechanisms
have remained to be fully elucidated (8,9).
Epithelial mesenchymal transition (EMT) is a
biological process that involves the polarization of epithelial
cells, which refers to the transformation of epithelial cells to
stroma cells accompanied by loss of cell polarity and gain of
stroma cell features (10). Previous
studies have indicated that EMT plays an important role in cervical
cancer progression and metastasis (10). Of note, miR-21 has been confirmed to
act synergistically with transforming growth factor β (TGF-β) to
accelerate the EMT process in colon carcinoma, involving the direct
targeting of Rac GTPase to enhance cell migration and invasion
(11). MiR-21 has also been reported
to promote the invasion and migration of cholangiocarcinoma cells
by inducing EMT (12). However,
whether EMT is involved in promoting the invasion and metastasis of
cervical cancer cells driven by miR-21 has remained elusive.
This study aims to investigate the role of EMT in
the malignant progression of cervical cancer that is driven by the
increased expression of miR-21, to provide theoretical basis for
individualized treatment and gene targeting interventions in
cervical cancer patients.
Materials and methods
Patients and samples
A total of 45 cervical cancer tissue samples, the
corresponding adjacent non-neoplastic tissues and lymph nodes with
suspected metastasis were collected from patients who underwent
cervical surgical resection without any pre-operative systemic
therapy. These 45 patients were diagnosed with cervical cancer at
the IB1-IIB stage, according to the 2009 International Federation
of Gynecology and Obstetrics (FIGO) Cervical Cancer Staging
Guidelines (13). All tissues
included in the present study were confirmed by pathological
examination. Furthermore, 15 cases of cervical cancer with lymph
node metastasis were included. After surgical removal, the tissues
were immediately frozen in liquid nitrogen and stored at −80°C.
Quantitative real-time PCR
MicroRNA was extracted using the miRNeasy Mini Kit
(QIAGEN) and reverse transcription was performed with a miRNA
Reverse Transcription Kit (QIAGEN). The relative expression levels
of miR-21 in tissues from patients and cell lines were detected
using the miScript SYBR Green PCR Kit (QIAGEN), with U6 as the
internal control (12). The primers
used in the present study were listed in Table SI. The PCR program consisted of
three steps: denaturation at 94°C for 15 sec, annealing at 55°C for
30 sec, and extension at 72°C for 30 sec, with 40 cycles in total.
For the detection of other genes, total RNA was isolated with
TRIzol® (Invitrogen) and reverse transcription was
performed with the PrimeScrip RT reagent Kit with gDNA Eraser
(TaKaRa Bio). Real-time qPCR was performed using PrimeScript RT
Master Mix, with GAPDH as the internal control. The cycling program
consisted of one cycle at 95°C for 1 min, and 40 cycles of 15 sec
at 95°C, 20 sec at 55°C, plus 60 sec at 72°C. Each sample was
evaluated in triplicate. Default threshold settings were used as
threshold cycle (Ct) data. Relative quantification of gene
expression was performed using 2−ΔΔCt method, which
indicates relative fold changes.
Western blot analysis
Total protein was lysed with radio
immunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology) and the protein concentration of each sample was
determined using a bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology). Equal amounts of protein were
separated by SDS-PAGE (10% acrylamide gel) and transferred to
polyvinylidene fluoride membrane. After blocked with 1% BSA,
membranes were incubated with the primary mouse monoclonal
antibodies (Santa Cruz Biotechnology) (dilution, 1:1,000) for
E-cadherin, Vimentin and N-cadherin at 4°C overnight, and then with
the corresponding secondary anti-mouse antibodies (TaKaRa Bio)
(dilution, 1:5,000) at 37°C for 1h. Digital images were acquired
using an enhanced chemiluminescence reagent from EMD Millipore.
Immunohistochemistry
The tissue sections were de-paraffinized and
rehydrated through a series of graded alcohols. Endogenous
peroxidase activity was blocked with H2O2.
Primary antibodies were titrated to determine the optimal antibody
concentration for staining with no background. A negative control
was run on each tissue, where the primary antibody was substituted
with antibody diluent buffer. In addition, positive control tissues
known to express the antigen in question were run in conjunction
with each batch of slides. The tissues were incubated with
mouse-derived primary antibody (Santa Cruz Biotechnology)
(dilution, 1:300) at room temperature for 1 h. After washing three
times with PBS, the tissues were incubated with the secondary
anti-mouse antibody (TaKaRa Bio) (dilution, 1:1,000) at room
temperature for 15 min. The staining for ZEB1, E-cadherin and
Vimentin was evaluated semi-quantitatively according to staining
intensity as previously reported (14).
miR-21 transfection
The cervical cancer cell lines (HeLa and SiHa) were
employed in the present study. Mimics control, miR-21 mimics, and
inhibitor control or miR-21 inhibitor were transfected into the
cells using Lipofectamine 2000 reagent as previously reported
(5). Quantitative real-time PCR was
used to confirm the expression levels of miR-21in the cell lines
after transfection.
Invasion assay
The cell invasion assay was performed using
Matrigel-coated upper chambers (BD Biosciences). Briefly, cells
(1×105) in 200 µl serum-free medium were seeded in the
upper chambers, and medium containing 10% serum as a
chemo-attractant was used in the lower chambers. After 48 h of
incubation, non-invading cells were gently removed, and cells at
the outer surface of the insert membranes were fixed with menthol
and, stained with 1% crystal violet. Images were captured under
200× magnification. Cells were counted from 5 random fields of
view. All experiments were performed three times independently.
Statistical analysis
The quantitative variables in symmetric
distributions were presented as means ± standard deviation
(SD) and comparisons between groups were performed through the
Student's t test. The miR-21 expression was expressed as median
(interquartile range, IQR) and comparisons between groups were
performed through the Wilcoxon rank sum test (two groups) or
Kruskal-Wallis test (multiple groups). If the Kruskal-Wallis test
was significant, Dunn's test was performed as a post-hoc analysis
to determine which levels of the independent variable differ from
each other. Protein expression was semi-quantified using an IHC
scoring system, and comparisons between groups on protein
expression were also performed through Wilcoxon rank sum test.
Correlations between laboratory indicators and clinical parameters
in specimens were calculated using Spearman's rank correlation
test. Data analysis was carried out with Intercooled Stata v.11 for
Windows; P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-21 expression in clinical
samples
Quantitative real-time PCR indicated that the
relative gene expression levels of miR-21 in cervical cancer and
lymphatic metastatic carcinoma tissues were significantly higher
than those in normal cervical tissues (P<0.05). Furthermore, the
expression levels of miR-21 were significantly higher in those
cervical cancer tissues with more severe and invasive
characteristics, including those with deeper muscular infiltration
depth, more severe parametrical invasion and more extensive lymph
node metastasis (P<0.05; Table
I), while no significant association was identified between the
expression levels of miR-21 and the tumor size or pathological type
(P>0.05; Table I). Further
correlation analysis revealed that the expression level of miR-21
was positively correlated with ZEB1 gene expression in cervical
cancer (Spearman's correlation test, rs=0.841, P<0.05).
 | Table I.Association between miR-21 expression
and pathological characteristics in cervical cancer. |
Table I.
Association between miR-21 expression
and pathological characteristics in cervical cancer.
| Parameters | Cases, n | miR-21a | P-valueb |
|---|
| Age, years |
|
|
|
| ≥50 | 32 | 3.636
(0.687–5.123) | 0.980 |
|
<50 | 13 | 2.166
(1.043–5.151) |
|
| Muscular
infiltration |
|
|
|
| ≥1/2 | 29 | 4.419
(1.562–6.062) | 0.001 |
|
<1/2 | 16 | 0.860
(0.484–2.166) |
|
| Tumor size, cm |
|
|
|
|
>4 | 20 | 4.301
(0.975–5.807) | 0.150 |
| ≤4 | 25 | 2.046
(0.561–4.187) |
|
| Parametrial
invasion |
|
|
|
| Yes | 10 | 4.514
(2.947–11.459) | 0.036 |
| No | 35 | 2.046
(0.712–4.434) |
|
| Pathologic types |
|
|
|
|
Squamous | 38 | 3.636
(0.961–5.289) | 0.316 |
|
Adenocarcinoma | 7 | 2.166
(0.503–3.574) |
|
| Lymph node
metastasis |
|
|
|
| Yes | 15 | 3.613
(1.005–4.860) | 0.045 |
| No | 30 | 2.123
(0.704–5.289) |
|
| HPV16 infection |
|
|
|
| Yes | 15 | 3.992
(2.166–4.434) | 0.027 |
| No | 30 | 1.451
(0.659–5.564) |
|
EMT-associated protein expression in
clinical samples
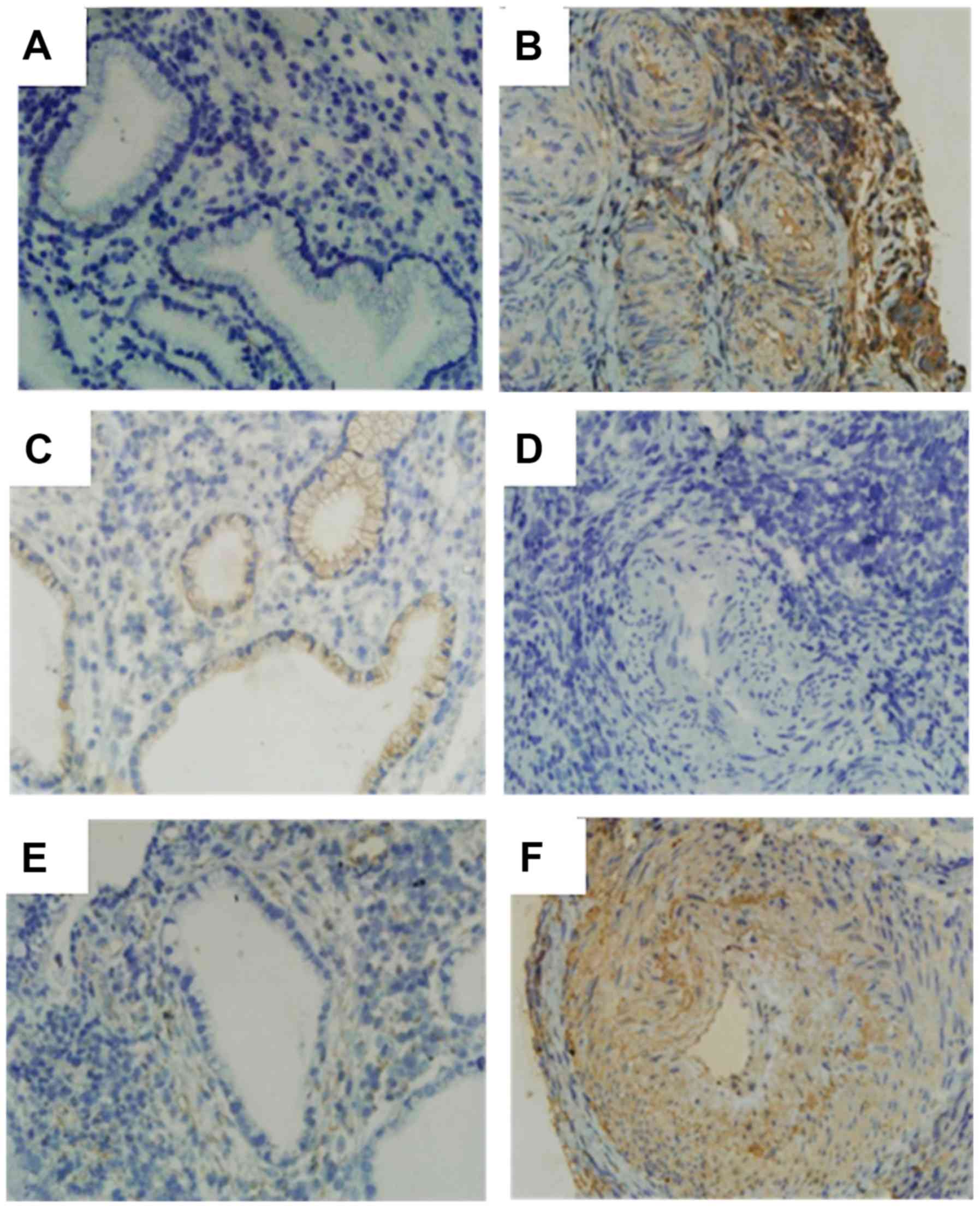
Immunohistochemistry assays indicated that the
expression level of ZEB1 in cervical cancer tissues was
significantly higher than that in normal tissues (P<0.05;
Fig. 1), and was also significantly
higher in cervical cancer with deeper muscular infiltration or more
extensive lymph node metastasis (P<0.05; Table SII). Similarly, the expression level
of Vimentin was also significantly higher in cervical cancer vs.
normal tissues (P<0.05; Fig. 1)
and was positively associated with lymph node metastasis (Table SIII) (P<0.05). In contrast, the
expression level of E-cadherin in cervical cancer tissues was
significantly lower than that in normal cervical tissue (P<0.05)
and was significantly decreased in cancer tissues with more
extensive lymph node metastasis (P<0.05) (Table SIV). Further statistical analysis
also revealed that the protein expression level of ZEB1 was
negatively associated with E-cadherin in cervical cancer tissues
(Spearman's correlation test, rs=−0.862, P<0.05).
miR-21 modulates EMT in cervical
cancer cells
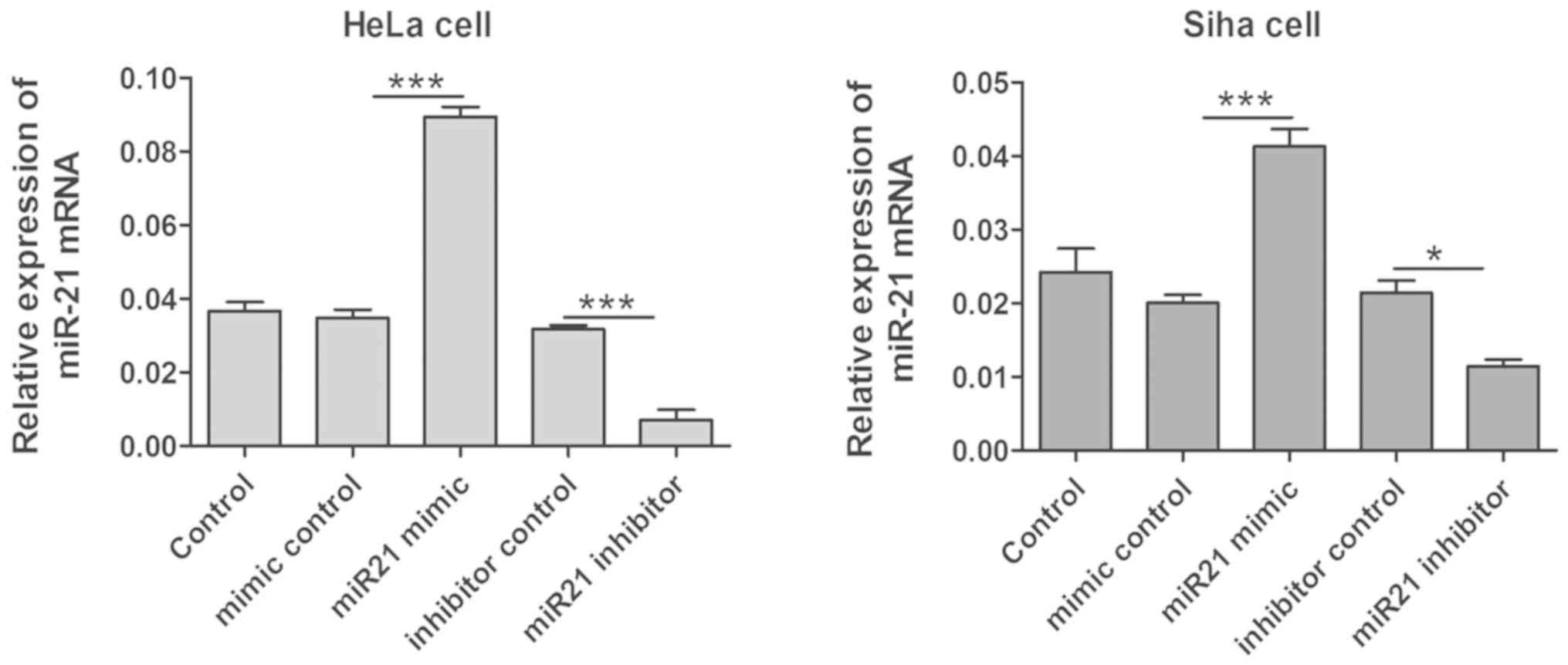
Quantitative real-time PCR analysis indicated that
the expression levels of miR-21 were significantly increased in
HeLa and SiHa cells after transfection with miR-21 mimics
(P<0.05; Fig. 2), and the
expression level of miR-21 significantly decreased after
transfection with miR-21 inhibitor (P<0.05; Fig. 2). Furthermore, the gene expression
level of ZEB1 and Snail in HeLa and SiHa cells also significantly
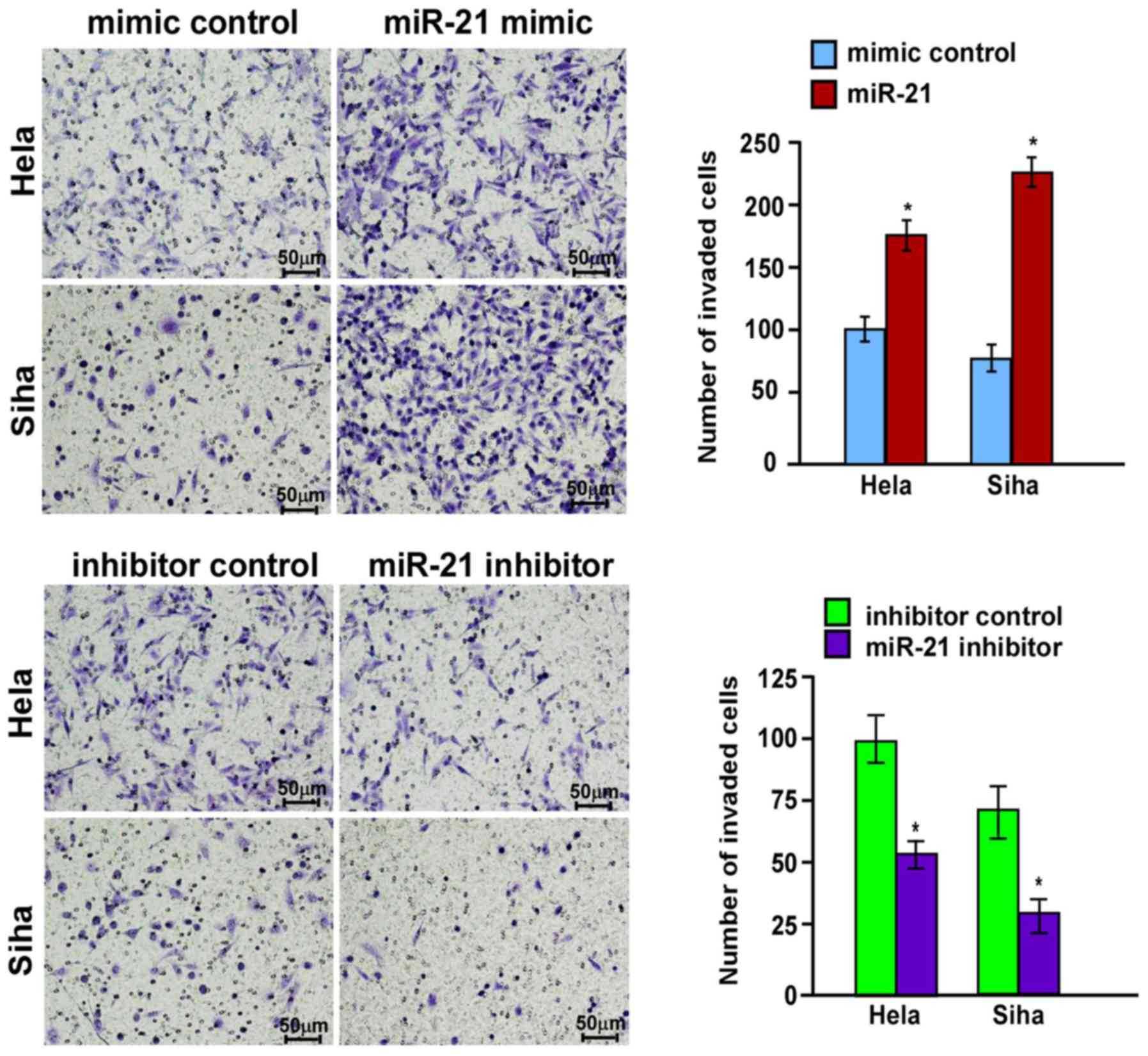
increased following transfection with miR-21 mimics. Transwell
assays also indicated that the invasiveness of HeLa and SiHa cells
was significantly enhanced after transfection with miR-21 mimics
(P<0.05; Fig. 3). In contrast,
the invasive ability of HeLa and SiHa cells significantly decreased
after transfection with the miR-21 inhibitor (P<0.05; Fig. 3).
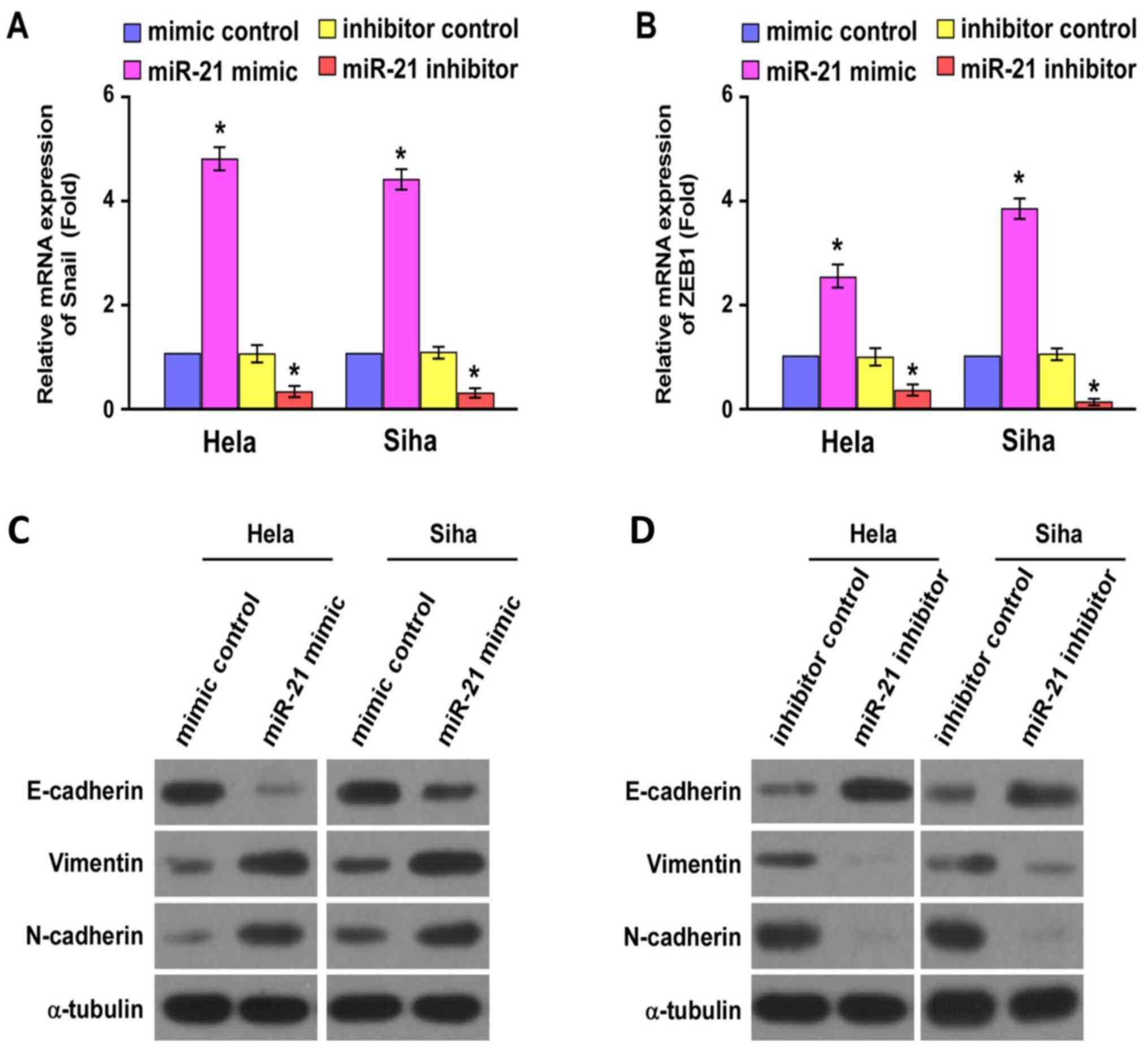
Quantitative real-time PCR analysis indicated that
the gene expression level of ZEB1 and Snail increased significantly
in HeLa and SiHa cells after transfection with miR-21 mimics
(P<0.05; Fig. 4). Significantly
decreased protein levels of E-cadherin and increased protein levels
of Vimentin and N-cadherin were observed in HeLa and SiHa cells
after transfection with miR-21 mimics by western blot analysis
(Fig. 4). On the contrary,
significantly increased protein levels of E-cadherin and decreased
protein levels of Vimentin and N-cadherin were observed in HeLa and
SiHa cells after transfection with miR-21 inhibitor (Fig. 4).
Discussion
MiR-21 is a well-known oncogenic miRNA involved in
the development of cervical cancer (4), and previous studies have indicated the
upregulation of miR-21 in cervical cancer cell lines and clinical
specimens (6,9). The present study also indicated that
the expression of miR-21 in cervical cancer and lymphatic
metastatic carcinoma tissues was significantly higher than that in
normal cervical tissues. Furthermore, the expression level of
miR-21 was positively correlated with muscular infiltration,
parametrical invasion and lymph node metastasis. Together with the
results of previous studies, these results demonstrated that miR-21
played a key role in the development and metastasis of cervical
cancer.
As the key transcription factor involved in EMT
(15), the gene expression of ZEB1
in cervical cancer tissues was significantly higher than in normal
cervical tissues. Furthermore, the gene expression level of ZEB1
was also positively correlated with muscular infiltration depth and
lymph node metastasis. Combined with results regarding increased
protein expression of Vimentin and decreased E-cadherin, it is
apparent that the EMT process is involved in the development and
biological behavior changes of cervical cancer. Furthermore,
primary cervical cancers with an EMT phenotype exhibit increased
tumor progression, invasion and metastasis in epithelial integrity
(10). Since EMT plays a major role
in metastasis and resistance to chemotherapy, it is necessary to
investigate the EMT process in cervical cancer.
The results of the present in vitro study
confirmed that the overexpression of miR-21 in cervical cancer cell
lines promoted their motility and invasiveness. Furthermore,
upregulation of the EMT-associated transcription factors ZEB1 and
Snail was observed in cervical cancer cells after overexpression of
miR-21. In addition, increased expression of Vimentin and
N-cadherin, and decreased expression of E-cadherin were observed.
From these results, it may be deduced that miR-21 promotes
metastasis of human cervical cancer by enhancing EMT. To date, the
direct targets of miR-21 involved in the EMT process had remained
elusive. The present study demonstrated that the gene expression
level of ZEB1 increased significantly in cervical cancer cell lines
after overexpression of miR-21. It is clear that miR-21 exerts its
gene regulation activity at the post-transcriptional level;
therefore, the increased gene expression of ZEB1 suggested that it
may not be the direct target of miR-21. MiR-21 probably targets
other signaling pathways, which then exert an influence on ZEB1
expression.
Previous studies have indicated that several signal
pathways are involved in EMT process, including NF-κB (16), Wnt/β-catenin (17), interleukin-6/signal transducer and
activator of transcription (STAT)3 (18) and AKT/glycogen synthase kinase
(GSK)-3β (19). STAT3 was indicated
to be a potential target of miR-21 in chronic lymphocytic leukemia
(20). Similarly, miR-21 may
negatively regulate the gene expression of STAT3 and promote the
EMT process in cervical cancer. Phosphatase and tensin homolog
(PTEN) was also reported to be a direct target of miR-21 in breast
phyllodes tumors (21). The ability
of miR-21 to induce myofibroblast differentiation in phyllodes
tumors was determined to be mediated via modulation of the
expression of Smad7 and PTEN, which regulate cell migration and
proliferation, respectively. In this way, activation of the
AKT/GSK-3β pathway by miR-21 may downregulate the gene expression
of PTEN and result in the upregulation of ZEB and Snail, which may
promote the EMT process in cervical cancer (19).
In summary, the present study indicated that miR-21
expression was upregulated in cervical cancer tissues and promoted
metastasis in cervical cancer through enhancing EMT, while the
direct targets of miR-21 still remain elusive. The present results
provide novel insight into the molecular mechanisms of miR-21 and
the EMT process, and suggest that miR-21 may serve as a potential
target in cervical cancer. Assessment of miRNA levels in cervical
cancer cells certainly opens novel possibilities for studying
molecular markers in the context of screening programs. Clearly,
future work should focus on clinically relevant samples and
predictive markers expressed in pre-cancerous tissue may be
identified for further validation.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Zhen Shen
(Department of Laboratory Medicine, Renji Hospital, School of
Medicine, Shanghai Jiaotong University, Shanghai, China) for his
critical review of the manuscript prior to submission.
Funding
This study was sponsored by Natural Science
Foundation of Fujian Province of China (grant no. 2017J01361). The
funders had no role in study design, data collection and analysis,
decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YT and YZ performed the experiments and prepared the
manuscript. JR collected and analyzed clinical data. YW
participated in the experimental design and revision of the
manuscript. YT and YW supervised the research work, participated in
designing the study and revised the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All study procedures were approved by the
Institutional Review Boards of the First Affiliated Hospital of
Xiamen University (approval no. KY2016-079) and informed written
consent was obtained from each participant.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Clifford GM, Tully S and Franceschi S:
Carcinogenicity of human papillomavirus (HPV) types in HIV-positive
Women: A meta-analysis from HPV infection to cervical cancer. Clin
Infect Dis. 64:1228–1235. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lui WO, Pourmand N, Patterson BK and Fire
A: Patterns of known and novel small RNAs in human cervical cancer.
Cancer Res. 67:6031–6043. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Farazi TA, Hoell JI, Morozov P and Tuschl
T: MicroRNAs in human cancer. Adv Exp Med Biol. 774:1–20. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang JY and Chen LJ: The role of miRNAs in
the invasion and metastasis of cervical cancer. Biosci Rep.
39:BSR201813772019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Z, Wang J, Wang X, Song W, Shi Y and
Zhang L: MicroRNA-21 promotes proliferation, migration, and
invasion of cervical cancer through targeting TIMP3. Arch Gynecol
Obstet. 297:433–442. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deftereos G, Corrie SR, Feng Q, Morihara
J, Stern J, Hawes SE and Kiviat NB: Expression of mir-21 and
mir-143 in cervical specimens ranging from histologically normal
through to invasive cervical cancer. PLoS One. 6:e284232011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yao Q, Xu H, Zhang QQ, Zhou H and Qu LH:
MicroRNA-21 promotes cell proliferation and down-regulates the
expression of programmed cell death 4 (PDCD4) in HeLa cervical
carcinoma cells. Biochem Biophys Res Commun. 388:539–542. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu J, Zhang W, Lv Q and Zhu D:
Overexpression of miR-21 promotes the proliferation and migration
of cervical cancer cells via the inhibition of PTEN. Oncol Rep.
33:3108–3116. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang L, Zhan X, Yan D and Wang Z:
Circulating microRNA-21 is involved in lymph node metastasis in
cervical cancer by targeting RASA1. Int J Gynecol Cancer.
26:810–816. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qureshi R, Arora H and Rizvi MA: EMT in
cervical cancer: Its role in tumour progression and response to
therapy. Cancer Lett. 356:321–331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cottonham CL, Kaneko S and Xu L: miR-21
and miR-31 converge on TIAM1 to regulate migration and invasion of
colon carcinoma cells. J Biol Chem. 285:35293–35302. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu CH, Huang Q, Jin ZY, Zhu CL, Liu Z and
Wang C: miR-21 and KLF4 jointly augment epithelial mesenchymal
transition via the Akt/ERK1/2 pathway. Int J Oncol. 50:1109–1115.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pecorelli S, Zigliani L and Odicino F:
Revised FIGO staging for carcinoma of the cervix. Int J Gynecol
Obstet. 105:107–182. 2009. View Article : Google Scholar
|
|
14
|
Suzuki T, Yano H, Nakashima Y, Nakashima O
and Kojiro M: Beta-eatenin expression in hepatocellular carcinoma:
Possible participation of beta-catenin in the dediferentiation
process. Gastmenterol Hepatol. 17:994–1000. 2002. View Article : Google Scholar
|
|
15
|
Sanchez-Tillo E, Lazaro A, Torrent R,
Cuatrecasas M, Vaquero EC, Castells A, Engel P and Postigo A: ZEB1
represses E-cadherin and induces an EMT by recruiting the SWI/SNF
chromatin-remodeling protein BRG1. Oncogene. 29:3490–3500. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu X, Wang D, Liu H, Feng Y, Zhu T, Zhang
L, Zhu B and Zhang Y: Knockdown of astrocyte elevated gene-1
(AEG-1) in cervical cancer cells decreases their invasiveness,
epithelial to mesenchymal transition, and chemoresistance. Cell
Cycle. 13:1702–1707. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang B, Kim J, Jeong D, Jeong Y, Jeon S,
Jung SI, Yang Y, Kim KI, Lim JS, Kim C and Lee MS: Klotho inhibits
the capacity of cell migration and invasion in cervical cancer.
Oncol Rep. 28:1022–1028. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miao JW, Liu LJ and Huang J:
Interleukin-6-induced epithelial-mesenchymal transition through
signal transducer and activator of transcription 3 in human
cervical carcinoma. Int J Oncol. 45:165–176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Z, Yu CP, Zhong Y, Liu TJ, Huang QD,
Zhao XH, Huang H, Tu H, Jiang S, Zhang Y, et al: Sam68 expression
and cytoplasmic localization is correlated with lymph node
metastasis as well as prognosis in patients with early-stage
cervical cancer. Ann Oncol. 23:638–646. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen N, Feng L, Qu H, Lu K, Li P, Lv X and
Wang X: Overexpression of IL-9 induced by STAT3 phosphorylation is
mediated by miR-155 and miR-21 in chronic lymphocytic leukemia.
Oncol Rep. 39:3064–3072. 2018.PubMed/NCBI
|
|
21
|
Gong C, Nie Y, Qu S, Liao JY, Cui X, Yao
H, Zeng Y, Su F, Song E and Liu Q: miR-21 induces myofibroblast
differentiation and promotes the malignant progression of breast
phyllodes tumors. Cancer Res. 74:4341–4352. 2014. View Article : Google Scholar : PubMed/NCBI
|