Introduction
Esophageal cancer (EC) was the 7th most diagnosed
malignant tumor (572,000 new cases) and the 6th leading cause of
cancer-associated mortality (509,000 mortality cases) worldwide in
2018 (1). The incidence of EC varies
considerably among geographical regions and sexes. EC mostly occurs
in men (~70% of all cases), and there is a 2–3-fold difference in
the incidence and mortality rates between regions worldwide
(2). In the latest report from 2017,
EC ranked the 6th most common type of cancer diagnosed and the 4th
leading cause of cancer-associated mortality in China (3,4). At
present, esophagectomy is considered as the standard treatment for
patients with resectable EC (5).
However, ~50% of patients present with locally advanced disease at
the time of diagnosis (6).
Definitive radiation therapy with or without concurrent
chemotherapy has been recommended as the optimal treatment for
patients who are medically inoperable or have locally advanced
disease (7). Intensity-modulated
radiotherapy (IMRT) has been widely used for the treatment of EC,
because it can provide excellent dose coverage and conformity to
the target volume while minimizing excessive dose to healthy
tissues, compared with 3D conformal radiotherapy (8). In addition, the number of elderly
patients with EC has grown in the last decades due to the
increasing life expectancy and population aging. Elderly patients
with EC are more vulnerable to experience competing events,
including non-cancer-associated mortality (9). It is therefore crucial to clearly
evaluate the risk factors for cancer-specific survival in patients
with EC receiving IMRT.
Cancer patients are frequently exposed to >2
events different from the one of interest and are identified as
competing risk events (10). In the
presence of competing risks, conventional survival analysis may be
inappropriate as this type of analysis considers competing events
as censored events, which might overestimate the incidence of
cancer-associated mortality (11,12).
Recently, competing risk analysis has been widely used in cancer
research as it takes into account the informative nature of
censoring and may more effectively discriminate the effects of risk
factors on specific events (13,14). A
nomogram is a statistical model that generates individualized risk
prediction by combining risk factors (15). Competing risk nomograms have been
recently developed for various types of cancer, including renal
cell carcinoma (16), nasopharyngeal
carcinoma (17), breast cancer
(18), thyroid cancer (19) and lung cancer (14). However, to the best of our knowledge,
a competing risk nomogram for EC following IMRT is still lacking.
The present study therefore performed a competing risk analysis
based on a cohort of patients with EC undergoing IMRT.
Subsequently, competing nomograms were established to provide
clinicians with a quantitative tool that can be used to evaluate
the probability of EC-specific mortality (EC-SM) and non-esophageal
cancer specific mortality (NEC-SM) in order to better stratify the
risk and make the best clinical decision.
Materials and methods
Study population
The medical records of patients with EC who
underwent IMRT at the Shanghai Rui Jin Hospital Affiliated to
Shanghai Jiao Tong University School of Medicine between January
2014 and May 2017 were recovered. The inclusion criteria were as
follows: i) Diagnosis of EC was confirmed independently by two
pathologists through hematoxylin and eosin staining in a
retrospective review; ii) complete medical records containing the
clinicopathological characteristics of the patients were available;
iii) no history of previous or concurrent malignancy; and iv)
follow-up period of >3 months following IMRT. A total of 213
patients with EC undergoing IMRT in the Department of Radiation
Oncology of Rui Jin Hospital Affiliated Medicine School of Shanghai
Jiao Tong University were subsequently identified and included in
the present study. All patients received standard IMRT of 50.4 Gy
(28 fractions, 1.8 Gy per fraction, in 5.6 weeks) as the primary
treatment. When possible, salvage treatments, including surgery and
re-irradiation, were provided for patients with relapsed or
persistent disease. The present study was approved by the
Institutional Review Board at Shanghai Rui Jin Hospital Affiliated
to Shanghai Jiao Tong University School of Medicine. As the present
study was retrospective in nature, the requirement for written
informed consent from the patients was waived.
Clinical variables and
definitions
Clinicopathological characteristics of patients
included sex, age at diagnosis, date of diagnosis, tumor site,
performance status, tumor length, grade, pathological diagnosis,
tumor stage at the time of diagnosis, concurrent chemoradiotherapy,
primary tumor (T) stage, regional lymph nodes (N) stage, distant
metastasis (M) stage and surgery. All tumors were staged according
to the Tumor-Node-Metastasis (TNM) staging system of the American
Joint Committee on Cancer (AJCC, 7th edition, 2009) (20). Furthermore, the cut-off value of
tumor length affecting patient survival was determined using the
Cut-off Finder application version 1.0 (http://molpath.charite.de/cutoff/) (21).
The primary endpoints of the present study were
EC-SM and NEC-SM. EC-SM was defined as the mortality resulting from
recurrent, metastatic or residual EC. Patients who exhibited EC
progression at the last follow-up and patients who died from EC
without a documented specific reason were included in the
EC-specific group. NEC-SM was defined as the mortality resulting
from specific causes other than EC, including death without a
documented specific reason within 6 months of the last follow-up in
the absence of EC relapse. The data from the surviving patients and
from patients lost to follow-up were treated as censored data.
Patient follow-up
After IMRT treatment, all patients were followed at
3-month intervals for 2 years, at 6-month intervals for the next 3
years, and annually thereafter. The date of the last follow-up was
July 2018. The last follow-up was mainly made by telephone calls.
Recurrence was determined by clinical and radiological examination
or histological confirmation. The main pattern of recurrence was
recorded as the first site of detectable treatment failure during
the follow-up period. The research endpoint corresponded to the
overall survival (OS), which was calculated from the time of the
diagnosis of EC until death from all causes or until the end of the
follow-up period.
Statistical analysis
Clinicopathological characteristics were summarized
using descriptive statistics. Patients were divided into two groups
according to their age (<60 and ≥65 years). EC-SM and NEC-SM
were considered as two competing events, and the associations
between pretreatment variables and the risk of EC-SM were evaluated
using Fine and Gray's competing risk regression analysis (17). The cumulative incidence function
(CIF) was used to determine the probability of each event, and the
differences between the groups were estimated using Gray's test.
Predictors with a P<0.05 in the univariate analysis were used
for multivariate analysis based on proportional sub-distribution
hazard models (22). A nomogram
predicting EC-specific survival (EC-SS) was constructed for
patients with EC based on the identified independent risk factors.
Nomograms were created using the nomogram function of the ‘rms’
package in R software (Mathsoft; version 3.6.1), the prediction
performance was assessed using Harrell's concordance index
(C-index) (23) and calibration
curves were obtained by plotting the observed rates against the
nomogram-predicted probabilities via a Bootstrap method with 1,000
resamples (17). The package
‘partykit’ in R was used to generate a decision tree (http://cran.salud.gob.sv/web/packages/partykit/vignettes/ctree.pdf).
Overall survival was estimated using Kaplan-Meier method.
Comparison between risk groups was performed by using log-rank
test. Statistical analysis was performed using SPSS software
(version 16.0; SPSS Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline characteristics
The clinicopathological characteristics of the 213
patients with EC included in the present study are summarized in
Table I. The median age at diagnosis
was 63 years (range, 41–87 years). There were 183 men (85.9%) and
30 women (14.1%; male-to-female ratio, 6.1:1). The tumor
histological types included squamous cell carcinoma (89.7%) and
adenocarcinoma (8%) and other histological types (2.3%). The tumor
locations were thoracic (61.5%), abdominal (35.2%) and cervical
(3.3%). Concurrent chemoradiotherapy was administered to 172
patients (80.8%), whereas 42 patients (19.2%) were treated with
IMRT alone due to advanced disease, comorbidities or patient
requests. A total of 104 patients (51.2%) received radical
esophagectomy. The median length of the follow up was 19 months. In
addition, 25 patients (11.7%) presented with distant metastasis at
initial diagnosis (Table I).
 | Table I.Clinicopathological characteristics of
the 213 patients with esophageal cancer. |
Table I.
Clinicopathological characteristics of
the 213 patients with esophageal cancer.
| Clinical feature | Patient number
(n=213) (%) |
|---|
| Sex, n (%) |
|
| Male | 183 (85.9) |
|
Female | 30 (14.1) |
| Age (years), median
(range) | 63 (41–87) |
| Histological type, n
(%) |
|
| SCC | 191(89.7) |
|
Non-SCC | 22 (10.3) |
| Performance status, n
(%) |
|
| 0 | 82 (38.5) |
| 1-2 | 131 (61.5) |
| Tumor location, n
(%) |
|
|
Cervical | 7 (3.3) |
|
Thoracic | 131 (61.5) |
|
Abdominal | 75 (35.2) |
| Tumor length (cm),
median (range) | 5 (1–16) |
| Grade, n (%) |
|
|
Well | 17 (8.0) |
|
Moderate | 107 (50.2) |
|
Poor/undifferentiated | 89 (41.8) |
| T stage, n (%) |
|
| T1 | 8 (3.8) |
| T2 | 25 (11.7) |
| T3 | 135 (63.4) |
| T4 | 45 (21.1) |
| N stage, n (%) |
|
| N0 | 39 (18.3) |
| N1 | 63 (29.6) |
| N2 | 60 (28.2) |
| N3 | 51 (23.9) |
| M stage, n (%) |
|
|
Yes | 25 (11.7) |
| No | 188 (88.3) |
| AJCC stage, n
(%) |
|
| I | 8 (3.8) |
| II | 41 (19.2) |
|
III | 86 (40.4) |
| IV | 78 (36.6) |
| Concurrent
chemoradiotherapy |
|
|
Yes | 172 (80.8) |
| No | 41 (19.2) |
| Radical
surgery |
|
|
Yes | 104 (51.2) |
| No | 109 (48.8) |
A total of 23 patients (10.8%) were lost to
follow-up. In addition, 71 patients (33.3%) died during follow-up,
including 65 (91.5%) as a result of EC-SM and 6 (8.5%) as a result
of NEC-SM. Among the 6 NEC-SM patients, the most common causes were
treatment-associated complications (hemorrhage and pneumonia, 2
patients each; 66.7% account for NEC-SM) and cardiovascular and
respiratory diseases (one patient each, respectively; 33.3% account
for NEC-SM).
Survival outcomes of patients with EC
following IMRT
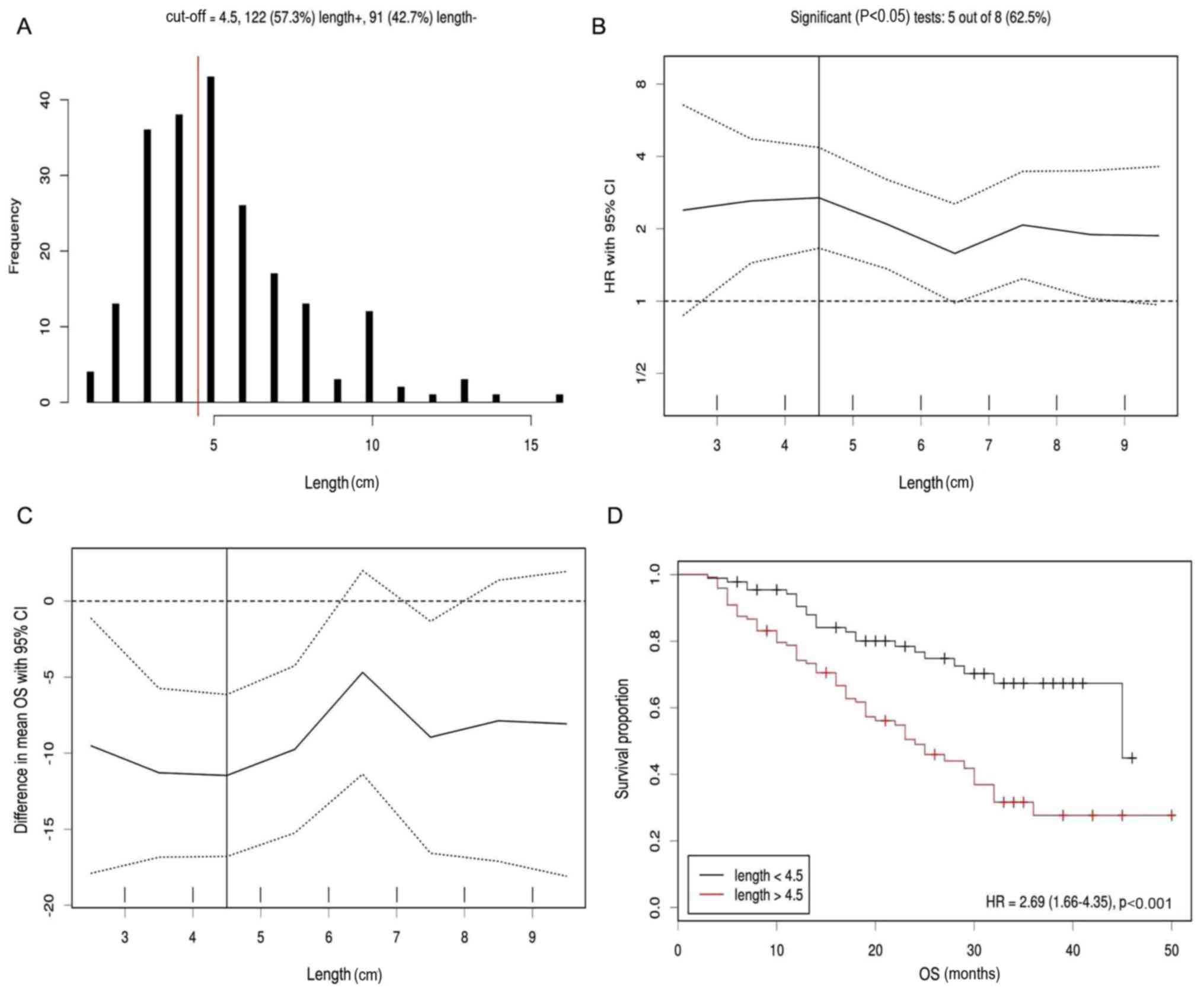
The results of the cut-off point determination for
primary tumor size indicated that 4.5 cm was the optimal point,
which was supported by multiple methods of Cut-off Finder,
including frequency (Fig. 1A),
hazard ratio (Fig. 1B), difference
in mean OS (Fig. 1C) and survival
proportion (Fig. 1D). Furthermore,
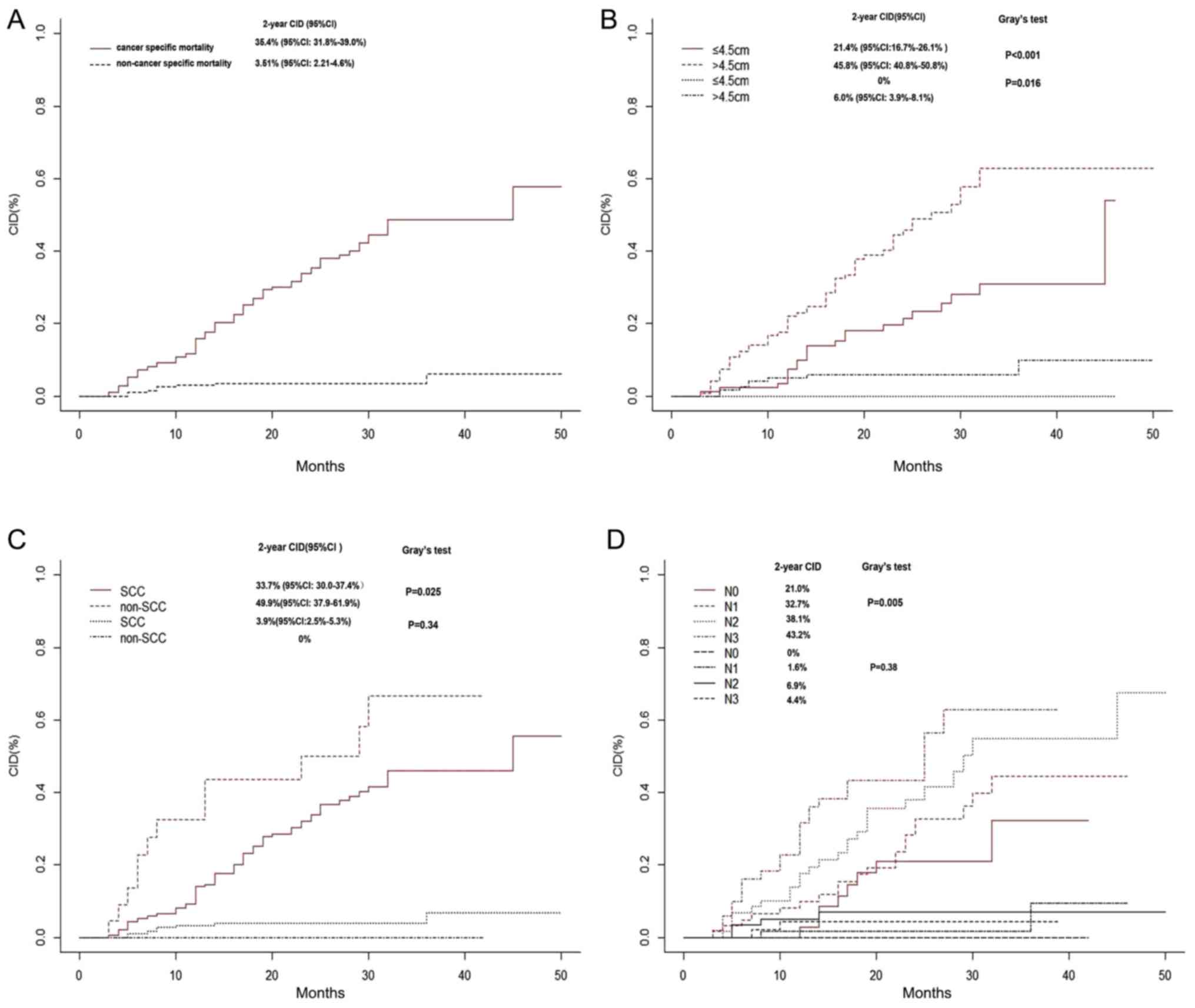
in patients with EC, the 2-year EC-SM and NEC-SM were 35.4 and
3.51%, respectively (Fig. 2A).
However, elevated 2-year EC-SM was observed in patients with tumor
length ≥4.5 cm compared with patients with tumors length <4.5 cm
(45.8 vs. 21.4%; P<0.001; Fig.
2B), in patients with non-squamous cell carcinoma compared with
patients with squamous cell carcinoma (49.9 vs. 33.7%; P=0.025;
Fig. 2C), and in patients with N3
stage in comparison with non-N3 stage (43.2%; P=0.005; Fig. 2D). The 2-year NEC-SM in patients with
tumor length ≥4.5 cm was 6% (vs. 0% in <4.5 cm group;
P=0.016).
Risk factors for EC-SM
The results from univariate competing risk
regression analysis demonstrated that performance status
[sub-distribution hazard ratio (SHR)=1.66; 95% confidence interval
(CI), 1.04–2.65; P=0.035, histological types SHR=2.03; 95% CI,
1.06–3.91; P=0.034], tumor grade (SHR=1.44; 95% CI, 1.01–2.08;
P=0.046), surgery (SHR=0.62; 95% CI, 0.40–0.96; P=0.033), T4 stage
(SHR=10.82; 95% CI, 1.40–83.59; P=0.022), N2/3 stage (SHR=2.26; 95%
CI, 1.08–4.73; P=0.03 and SHR=3.31; 95% CI, 1.54–7.09; P=0.02) and
metastasis status (SHR=2.12; 95% CI, 1.08–4.13; P=0.028) were
significantly associated with EC-SM (Table II). Multivariate analysis validated
that tumor length >4.5 cm (SHR=1.80; 95% CI, 1.03–3.16; P=0.04),
non-SCC (SHR=2.71; 95% CI, 1.48–4.93; P=0.001) and N3 stage
(SHR=2.52; 95% CI, 1.07–5.91; P=0.034) were independent predictors
for EC-SM (Table II).
 | Table II.Univariate and multivariate competing
risk analyses of patient clinicopathological characteristics for
the determination of esophageal cancer specific mortality. |
Table II.
Univariate and multivariate competing
risk analyses of patient clinicopathological characteristics for
the determination of esophageal cancer specific mortality.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | SHR | 95% CI | P-value | SHR | 95% CI | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
Male | 1 |
|
| – |
|
|
|
Female |
0.68 | 0.34–1.37 | 0.29 | – | – | – |
| Age, years |
|
|
|
|
|
|
|
<60 | 1 |
|
| – |
|
|
|
≥60 |
0.84 | 0.54–1.33 | 0.47 | – | – | – |
| Performance
status |
|
|
|
|
|
|
| 0 | 1 |
|
| 1 |
|
|
|
1-2 |
1.66 | 1.04–2.65 | 0.035 | 1.46 | 0.85–2.52 | 0.17 |
| Tumor location |
|
|
|
|
|
|
|
Cervical | 1 |
|
| – |
|
|
|
Thoracic/abdominal |
0.91 | 0.58–1.42 | 0.67 | – | – | – |
| Histological
types |
|
|
|
|
|
|
|
SCC | 1 |
|
| 1 |
|
|
|
Non-SCC |
2.03 | 1.06–3.91 | 0.034 | 2.71 | 1.48–4.93 |
0.001 |
| Grade |
|
|
|
|
|
|
|
High | 1 |
|
| 1 |
|
|
|
Moderate/poor |
1.44 | 1.01–2.08 | 0.046 | 1.14 | 0.75–1.74 | 0.61 |
| Surgery |
|
|
|
|
|
|
| No | 1 |
|
| 1 |
|
|
|
Yes |
0.62 | 0.40–0.96 | 0.033 | 1.52 | 0.85–2.72 | 0.15 |
| Tumor length,
cm |
|
|
|
|
|
|
|
<4.5 | 1 |
|
| 1 |
|
|
|
≥4.5 |
2.43 | 1.48–3.99 | <0.001 | 1.80 | 1.03–3.16 | 0.04 |
| T stage |
|
|
|
|
|
|
| T1 | 1 |
|
| 1 |
|
|
| T2 |
1.92 | 0.23–16.29 | 0.55 | 1.42 |
0.16–12.49 | 0.75 |
| T3 |
3.39 | 0.45–25.49 | 0.24 | 2.04 |
0.26–16.27 | 0.50 |
| T4 | 10.82 | 1.40–83.58 | 0.022 | 5.37 |
0.59–49.29 | 0.14 |
| N stage |
|
|
|
|
|
|
| N0 | 1 |
|
| 1 |
|
|
| N1 |
1.51 | 0.71–3.24 | 0.284 | 1.41 | 0.63–3.13 | 0.41 |
| N2 |
2.26 | 1.08–4.73 | 0.030 | 1.71 | 0.76–3.82 | 0.19 |
| N3 |
3.31 | 1.54–7.09 | 0.02 | 2.52 | 1.07–5.91 |
0.034 |
| Metastasis |
|
|
|
|
|
|
| No | 1 |
|
| 1 |
|
|
|
Yes |
2.12 | 1.08–4.13 | 0.028 | 1.41 | 0.66–3.00 | 0.37 |
| Concurrent
chemotherapy |
|
|
|
|
|
|
|
Yes | 1 |
|
| – | – | – |
| No |
1.20 | 0.68–2.12 | 0.52 | – | – | – |
| C-index (95%
CI) | – | – | – | – | 0.72
(0.66–0.77) | – |
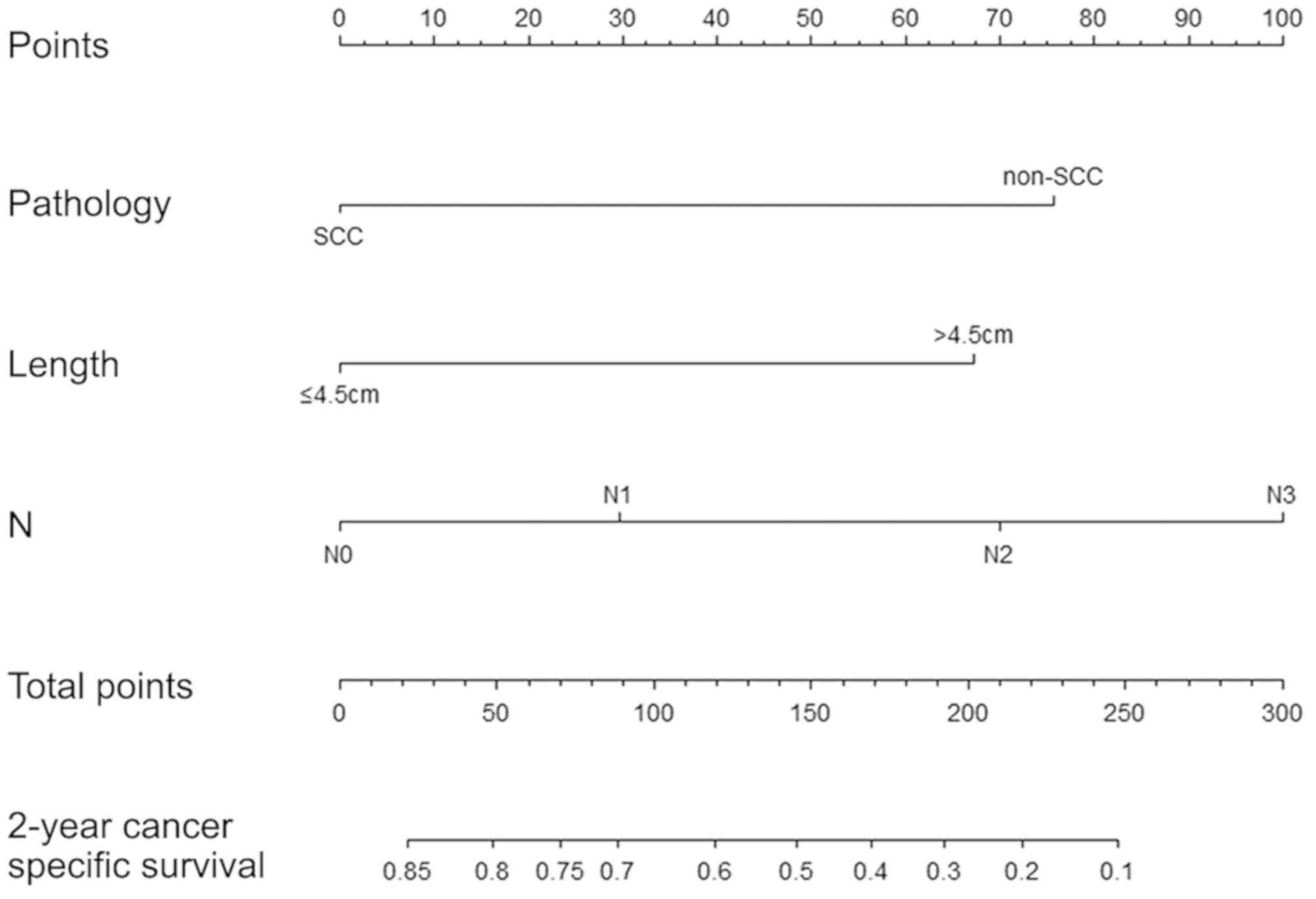
Construction and validation of
nomograms for EC-SS
All validated factors, including tumor length,
histological type and N stage, were integrated into the competing
nomograms in the EC-SS model (C-index=0.72; 95% CI, 0.66–0.77;
Fig. 3). The calibration plots for
the probabilities of 2-year EC-SS demonstrated reasonable
concordance between the nomogram-predicted survival rates and the
actual survival rates in the training sets (Fig. S1). In addition, the nomograms
exhibited improved discrimination power compared with the 7th
edition of TNM staging system of AJCC for predicting EC-SS (area
under the curve=0.707 vs. 0.634; Fig.
S2).
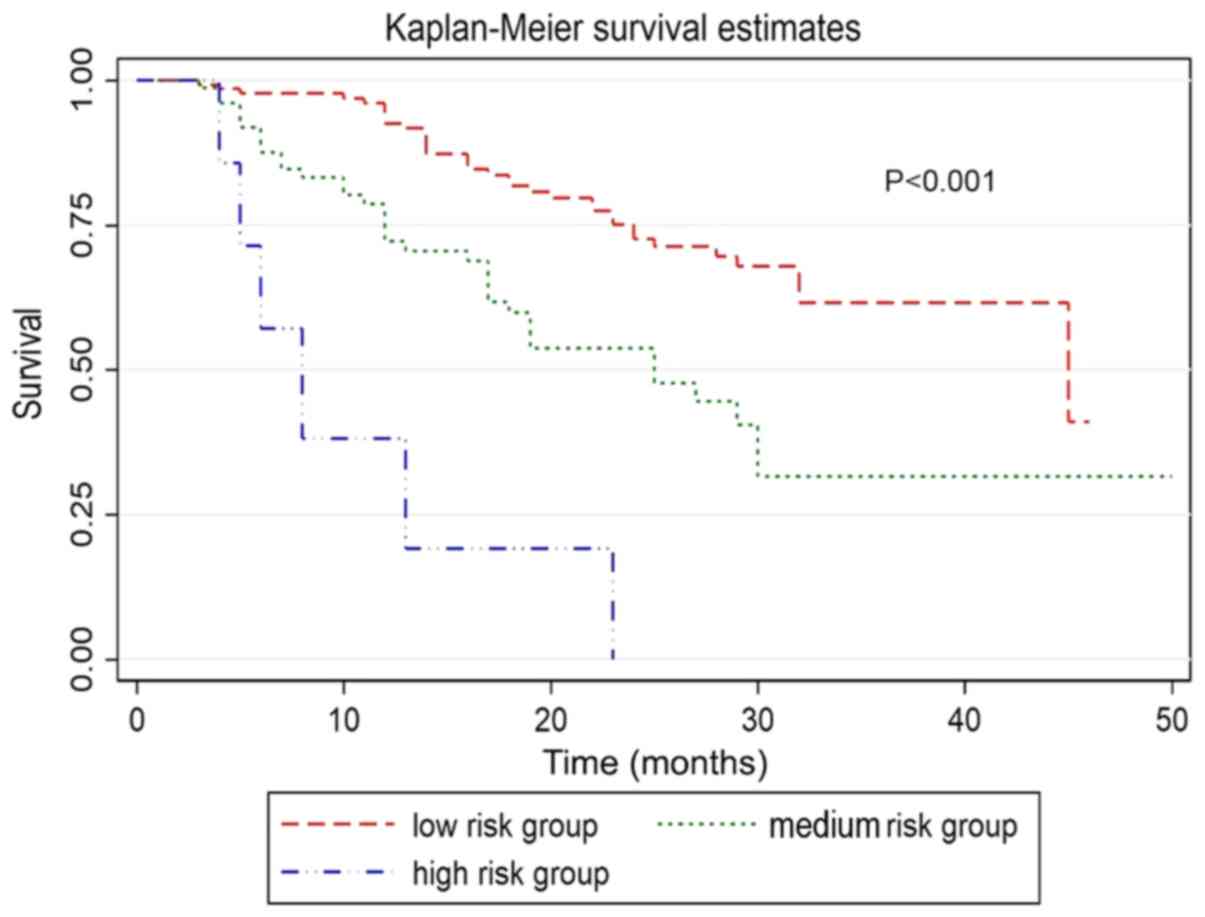
Comparison of prognosis based on risk
stratification
Each risk variable, including primary tumor size, N
stage and histological type, were assigned values according to the
nomogram for EC-SM (derived from the competing risk model). The
total score was obtained by adding the individual scores of all
significant parameters. Subsequently, three risk stratifications
were defined as follows: Low risk (total score ≤100), medium risk
(>100 total score ≤200) and high risk (total score >200). The
results of the prognosis according to the risk stratification were
as follows: 131 cases with low risk, 75 cases with medium risk and
7 cases with high risk. In addition, significant differences in OS
(P<0.001) and EC-SM (P<0.001) were observed in the three risk
groups (Fig. 4).
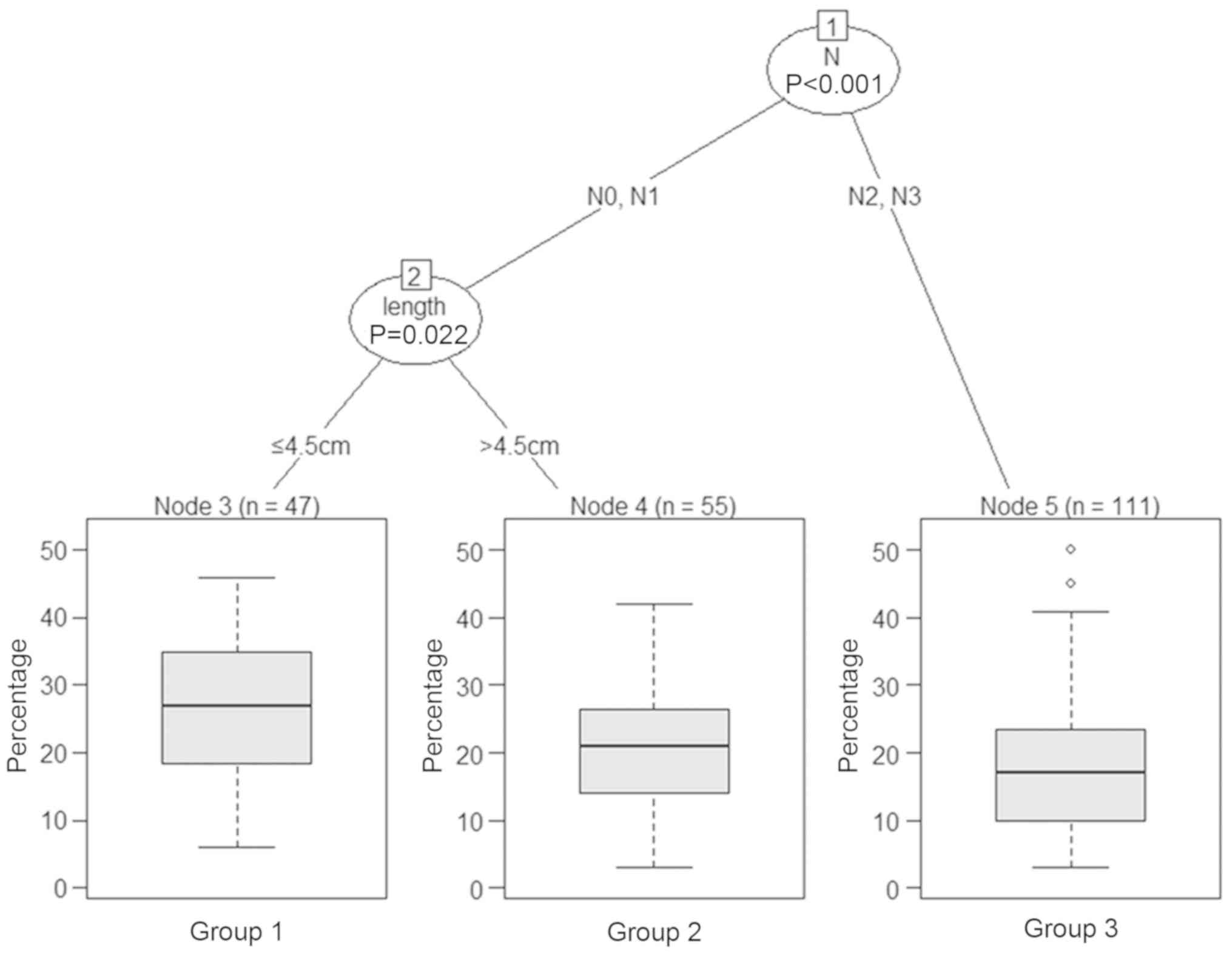
Classification tree for the factors
associated with EC-SM
The classification tree analysis method was used to
determine the factors associated with EC-SM following IMRT. The
results of the pruned tree presented in Fig. 5 demonstrated that N stage was the
initial node and that tumor length was also a determinant for EC-SM
of these patients.
Discussion
In the present study, the 2-year EC-SM was 35.4%,
the median length of the follow up was 19 months, and 3.5% of
patients died from causes other than EC. Since competing causes of
mortality might have an impact on the prognosis of patients with
EC, it is important to consider these competing risks when
evaluating the prognosis in order to determine the best treatment
option. To the best of our knowledge, the present study was the
first to develop competing risk nomograms based on the proportional
sub-distribution hazard method to predict EC-SM by using a cohort
of patients with EC. The AJCC system, which depends on TNM staging,
is the strongest clinical tool for the prognosis prediction of
patients with EC. However, T classification represents the invasion
depth of the tumor, which is different from the tumor length
(24). Previous studies have
reported existence of a link between tumor length and outcome in
patients with EC (25–30). Similarly, the present study
demonstrated that tumor length was an important prognostic factor
for the EC-SM of patients who underwent IMRT. Furthermore, although
most studies supported that tumor length is an important prognostic
factor for EC, no consensus was achieved on the tumor size cut-off
value, which ranges from 2 to 5 cm (31–33). The
results from the present study demonstrated that elevated 2-year
EC-SM was observed in patients with tumor length ≥4.5 cm (45.8% vs.
<4.5 cm group: 21.4%; P<0.001). The N stage reflects regional
lymph node metastasis, and previous studies reported that N stage
is an important factor influencing the prognosis of patients with
EC (23–25,34–36).
Similarly, the present study demonstrated that the N3 stage was the
strongest independent predictor for EC-SM, supporting the
prognostic significance of the AJCC staging system in EC.
Esophageal SCC and esophageal non-SCC have distinct
clinicopathological characteristics. The effects of the tumor
histological type on the outcome of patients with EC were therefore
investigated in the present study. The results demonstrated that
the 2-year ES-SM in patients with non-SCC was significantly higher
than in patients with SCC. This may be due to the fact that SCC
tends to be more radiosensitive than non-SCC, particularly
adenocarcinoma (37).
The prognostic factors identified in the present
study were integrated to develop nomograms that could predict the
2-year EC-SS of patients with EC. These nomograms displayed better
discrimination power than the TNM staging system for predicting the
EC-SS of patients with EC who received IMRT. Therefore, the
nomograms developed in the present study may be used by radiation
oncologists to precisely predict the individual patient's survival
probability at specific time intervals.
The present study had some limitations. Firstly,
only data of patients from one institution were included, which
might limit the applicability of the nomogram. Secondly, the sample
size was relatively small. Thirdly, the study was a retrospective
study, and inherent bias could therefore not be excluded.
Furthermore, different treatment strategies, including surgery and
chemotherapy, may also affect the prognosis of patients with EC
receiving IMRT. Finally, the results obtained in the present study
were not externally validated. External validation is therefore
required to determine whether the nomograms could be applied to
other patient groups. Further evaluation in prospective
multi-institute trials is therefore warranted.
In conclusion, the present study revealed that
NEC-SM may represent a significant competing event for patients
with EC and primary tumor length ≥4.5 cm. Furthermore, to the best
of our knowledge, the present study was the first to establish the
first competing risk nomogram for EC that could be used to predict
the probability of 2-year EC-SS. This nomogram may be considered as
a convenient individualized predictive tool for determining the
cancer-specific survival in patients with EC receiving IMRT.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present work was supported in part by Shanghai
Integrated Traditional Chinese and Western Medicine Project (grant
no ZHYY-ZXYJHZX-201913).
Availability of data and materials
The datasets used or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SZ and WQ designed the study. WQ, SZ and JC analyzed
and interpreted the patient data. SZ was a major contributor in
writing the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Institutional Review
Board at Shanghai Rui Jin Hospital Affiliated to Shanghai Jiao Tong
University School of Medicine. Written informed consent was
obtained from all individual participants included in the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Domper Arnal MJ, Ferrandez Arenas A and
Lanas Arbeloa A: Esophageal cancer: Risk factors, screening and
endoscopic treatment in Western and Eastern countries. World J
Gastroenterol. 21:7933–7943. 2015. View Article : Google Scholar
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng R, Zeng H, Zhang S and Chen W:
Estimates of cancer incidence and mortality in China, 2013. Chin J
Cancer. 36:662017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sohda M and Kuwano H: Current status and
future prospects for esophageal cancer treatment. Ann Thorac
Cardiovasc Surg. 23:1–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berry MF: Esophageal cancer: Staging
system and guidelines for staging and treatment. J Thorac Dis.
6:S289–S297. 2014.PubMed/NCBI
|
|
7
|
Xi M and Lin SH: Recent advances in
intensity modulated radiotherapy and proton therapy for esophageal
cancer. Expert Rev Anticancer Ther. 17:635–646. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fenkell L, Kaminsky I, Breen S, Huang S,
Van Prooijen M and Ringash J: Dosimetric comparison of IMRT vs. 3D
conformal radiotherapy in the treatment of cancer of the cervical
esophagus. Radiother Oncol. 89:287–291. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bollschweiler E, Plum P, Monig SP and
Holscher AH: Current and future treatment options for esophageal
cancer in the elderly. Expert Opin Pharmacother. 18:1001–1010.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Berry SD, Ngo L, Samelson EJ and Kiel DP:
Competing risk of death: An important consideration in studies of
older adults. J Am Geriatr Soc. 58:783–787. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Z, Geskus RB, Kattan MW, Zhang H and
Liu T: Nomogram for survival analysis in the presence of competing
risks. Ann Transl Med. 5:4032017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carmona R, Zakeri K, Green G, Hwang L,
Gulaya S, Xu B, Verma R, Williamson CW, Triplett DP, Rose BS, et
al: Improved method to stratify elderly patients with cancer at
risk for competing events. J Clin Oncol. 34:1270–1277. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Simpson MC, Massa ST, Boakye EA, Antisdel
JL, Stamatakis KA, Varvares MA and Osazuwa-Peters N: primary cancer
vs. competing causes of death in survivors of head and neck cancer.
JAMA Oncol. 4:257–259. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eguchi T, Bains S, Lee MC, Tan KS, Hristov
B, Buitrago DH, Bains MS, Downey RJ, Huang J, Isbell JM, et al:
Impact of increasing age on cause-specific mortality and morbidity
in patients with stage I non-small-cell lung cancer: A competing
risks analysis. J Clin Oncol. 35:281–290. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grobman WA and Stamilio DM: Methods of
clinical prediction. Am J Obstet Gynecol. 194:888–894. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kutikov A, Egleston BL, Wong YN and Uzzo
RG: Evaluating overall survival and competing risks of death in
patients with localized renal cell carcinoma using a comprehensive
nomogram. J Clin Oncol. 28:311–317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang XD, Zhou GQ, Lv JW, Zhou HQ, Zhong
CW, Wu CF, Zheng ZQ, He XJ, Peng L, Ma J and Sun Y: Competing risk
nomograms for nasopharyngeal carcinoma in the intensity-modulated
radiotherapy era: A big-data, intelligence platform-based analysis.
Radiother Oncol. 129:389–395. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo C, Zhong X, Deng L, Xie Y, Hu K and
Zheng H: Nomogram predicting locoregional recurrence to assist
decision-making of postmastectomy radiotherapy in patients with
T1-2N1 breast cancer. Int J Radiat Oncol Biol Phys. 103:905–912.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang L, Shen W and Sakamoto N:
Population-based study evaluating and predicting the probability of
death resulting from thyroid cancer and other causes among patients
with thyroid cancer. J Clin Oncol. 31:468–474. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li X, Wan J, Wu Z, Tu J, Hu Y, Wu S and
Lou L: Fatal adverse events with molecular targeted agents in the
treatment of advanced hepatocellular carcinoma: A meta-analysis of
randomized controlled trials. Drug Des Devel Ther. 12:3043–3049.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sfumato P, Filleron T, Giorgi R, Cook RJ
and Boher JM: Goftte: A R package for assessing goodness-of-fit in
proportional (sub) distributions hazards regression models. Comput
Methods Programs Biomed. 177:269–275. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Y, Jia Z, Mercola D and Xie X: A
gradient boosting algorithm for survival analysis via direct
optimization of concordance index. Comput Math Methods Med.
2013:8735952013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hong SM, Cho H, Moskaluk CA and Yu E:
Measurement of the invasion depth of extrahepatic bile duct
carcinoma: An alternative method overcoming the current T
classification problems of the AJCC staging system. Am J Surg
Pathol. 31:199–206. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang X, Wang Y, Qu P, Liu-Helmersson J,
Zhao L, Zhang L and Sang S: Prognostic value of tumor length for
cause-specific death in resectable esophageal cancer. Ann Thorac
Surg. 106:1038–1046. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang X, Wang Y, Jiang Y, Wang Z, Zhao L,
Xue X, Sang S and Zhang L: The prognostic impact of tumor length in
esophageal cancer: Protocol for a systematic review and
meta-analysis. Medicine (Baltimore). 97:e129022018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang BY, Liu CY, Lin CH, Hsu PK, Hsu WH,
Wu YC and Cheng CY: Endoscopic tumor length is an independent
prognostic factor in esophageal squamous cell carcinoma. Ann Surg
Oncol. 19:2149–2158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gaur P, Sepesi B, Hofstetter WL, Correa
AM, Bhutani MS, Watson TJ and Swisher SG; M. D. Anderson Esophageal
Cancer Group and University of Rochester School of Medicine and
Dentistry Foregut Group, : Endoscopic esophageal tumor length: A
prognostic factor for patients with esophageal cancer. Cancer.
117:63–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Koyanagi K, Kato F, Kanamori J, Daiko H,
Ozawa S and Tachimori Y: Clinical significance of esophageal
invasion length for the prediction of mediastinal lymph node
metastasis in Siewert type II adenocarcinoma: A retrospective
single-institution study. Ann Gastroenterol Surg. 2:187–196. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arigami T, Uchikado Y, Omoto I, Sasaki K,
Kita Y, Owaki T, Yanagita S, Mori S, Kurahara H, Okumura H, et al:
Primary tumor score based on tumor depth and length predicts
prognosis in esophageal squamous cell carcinoma. Anticancer Res.
38:5447–5452. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang X, Wang Y, Li C, Helmersson J, Jiang
Y, Ma G, Wang G, Dong W, Sang S and Du J: The prognostic value of
tumor length to resectable esophageal squamous cell carcinoma: A
retrospective study. Peer J. 5:e29432017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vadhwana B, Zosimas D, Lykoudis PM, Phen
HM, Martinou M and Khoo D: Tumour length as an independent
prognostic factor in resectable oesophageal carcinoma. Ann R Coll
Surg Engl. 22:1–6. 2019.
|
|
33
|
Xu H, Wu S, Luo H, Chen C, Lin L, Huang H
and Xue R: Prognostic value of tumor length and diameter for
esophageal squamous cell cancer patients treated with definitive
(chemo)radiotherapy: Potential indicators for nonsurgical T
staging. Cancer Med. 8:6326–6334. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yin H, Li D, Zhu C, Wang M and Wei N:
Factors relevant to the prognosis of patients with esophageal
cancer who received intensity-modulated radiotherapy. Thorac
Cancer. 9:1215–1219. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shiozaki H, Sudo K, Xiao L, Wadhwa R,
Elimova E, Hofstetter WL, Skinner HD, Lee JH, Weston B, Bhutani MS,
et al: Distribution and timing of distant metastasis after local
therapy in a large cohort of patients with esophageal and
esophagogastric junction cancer. Oncology. 86:336–339. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sakanaka K, Ishida Y, Itasaka S, Ezoe Y,
Aoyama I, Miyamoto S, Horimatsu T, Muto M and Hiraoka M:
Identification of a predictive factor for distant metastasis in
esophageal squamous cell carcinoma after definitive
chemoradiotherapy. Int J Clin Oncol. 21:899–908. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen GZ, Zhu HC, Dai WS, Zeng XN, Luo JH
and Sun XC: The mechanisms of radioresistance in esophageal
squamous cell carcinoma and current strategies in radiosensitivity.
J Thorac Dis. 9:849–859. 2017. View Article : Google Scholar : PubMed/NCBI
|