Introduction
Gastrointestinal (GI) lymphomas account for ~5–20%
of all cases of extranodal lymphomas (1). However, primary (P)GI lymphoma is
considered rare, accounting for only 1–4% of all types of GI cancer
(2). In the last decades, the
incidence of PGI has exhibited an increasing trend (3). PGI may present throughout the
gastrointestinal tract; most commonly in the stomach, followed by
the small intestine, ileocecal region and rectum (4,5). Diffuse
large B-cell lymphoma (DLBCL) is the most common pathological
subtype of GI lymphoma, followed by mucosa-associated lymphoid
tissue (MALT) lymphoma (5). DLBCL is
typically diagnosed at an advanced stage due to non-specific
symptoms, such as abdominal pain, loss of appetite and
unintentional weight loss (6).
However, the pathological and neoplastic molecular mechanisms
underlying the progression of PGI-DLBCL are yet to be elucidated.
Increasing evidence suggest that the aberrant expression of
microRNAs (miRNAs/miRs) may regulate cancer progression. Thus,
miRNAs may serve as promising diagnostic and prognostic biomarkers
for different types of cancer (7–9).
Previous studies have reported that miRNAs serve as tumor
suppressor genes or oncogenes in the context of DLBCL (10–14).
However, to the best of our knowledge, the molecular mechanism
underlying the influence of miRNAs in the pathogenesis of PGI-DLBCL
is not yet fully understood.
miRNAs are a conserved class of long non-coding RNAs
(~19–24 nucleotides in length) and regulate posttranscriptional
gene repression via direct binding to the 3′-untranslated region of
downstream mRNAs (15,16). miRNAs play a key role in several
biological pathways, including cell proliferation, stress
resistance, cell apoptosis and fat metabolism (7,13).
miR-150 influences the development of different types of solid
tumors, such as colorectal, lung, breast, pancreatic and esophageal
cancer (17–21). For example, downregulation of miR-150
in patients with esophageal squamous-cell carcinoma is associated
with poor prognosis (19).
Conversely, high miR-150 expression has been reported to promote
clonogenicity and cell proliferation, while suppressing apoptosis
in breast cancer cells (22).
Furthermore, high miR-150 expression in patients with non-small
cell lung cancer (NSCLC) is associated with shorter overall
survival (OS) time, suggesting the prognostic relevance of
upregulation of miR-150 in NSCLC tissues (23). Increased miR-150 expression has also
been reported in other carcinomas, such as thyroid and prostate
cancer (24,25).
Furthermore, miR-150 has been demonstrated to be
associated with hematopoietic specificity in malignant lymphoma
(26). Monticelli et al
(27) studied miRNA expression
profiles of hematopoietic lineage cells and reported low miR-150
expression in progenitor B- and T-cells, but high miR-150
expression in mature cells. Notably, downregulation of miR-150 was
demonstrated to be associated with poor prognosis in chronic
lymphocytic leukemia (28). In a
related clinical study, significantly repressed miR-150 expression
in Burkitt lymphoma tissues was indicated to inhibit the
proliferation of Raji cells, suggesting its potential role as a
novel therapeutic target (29).
Conversely, Gebauer et al (30) found that the increased expression of
miR-150 was observed in malt marginal zone lymphoma, which
indicates that miR-150 may represent a potential treatment for MALT
lymphoma.
Despite the aforementioned contradictory results,
miR-150 expression level is associated with the progression of
lymphoma and influences survival outcomes. However, to the best of
our knowledge, the association between miR-150 and PGI-DLBCL has
not yet been fully investigated. Thus, the present study aimed to
determine whether miR-150 expression significantly influenced the
pathogenesis and prognosis of patients with PGI-DLBCL.
Materials and methods
Study participants
The present study was approved by the Ethics
Committee of Tianjin Medical University Cancer Institute (Tianjin,
China) and written informed consent was obtained from all patients
prior to the study start. A total of 84 PGI-DLBCL tissues and
paired adjacent non-tumor tissues were collected from 44 men and 40
women (mean age, 54 years; age range, 35–70 years) who underwent
radical surgery at the Tianjin Medical University Cancer Institute
and Hospital, between January 2011 to December 2015. Tissue samples
were stored at −80°C prior to subsequent experimentation. All
patients were randomly selected, with a balanced distribution of
age and sex, no selection bias and no external validation.
Diagnosis of PGI-DLBCL was confirmed by pathologists (Department of
Pathology, Tianjin Medical University Cancer Institute and
Hospital) and based on morphological, immunophenotypic and
molecular tests, according to the World Health Organization (WHO)
classification criteria (31). The
inclusion criteria were as follows: Patients were diagnosed with
PGI-DLBCL according to the WHO classification criteria, patients
did not receive radiotherapy or chemotherapy prior to surgery, no
previous tumors or associated tumors were reported and complete
clinical data and long-term follow-up data were available. All
patients were staged according to the Lugano staging system for
gastrointestinal non-Hodgkin's lymphoma (32).
RNA and cDNA preparation and Reverse
transcription (RT)-PCR
Total RNA was extracted from 10-µm-thick
formalin-fixed paraffin-embedded PGI-DLBCL tissues and adjacent
non-tumor tissue sections using TRIzol® reagent
(Invitrogen, Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions. The concentrations and purity of total
RNA were measured using NanoDrop 2000 (NanoDrop Technologies;
Thermo Fisher Scientific, Inc.) and total RNA was reverse
transcribed into cDNA using the cDNA synthesis kit (Takara Bio,
Inc.), according to the manufacturer's protocol, and subsequently
stored at −20°C. Subsequently, qPCR was performed using the CM9600
Sequence Detection System (Bio-Rad Laboratories, Inc.). According
to the manufacturer's instructions, DNA was amplified with 10 µl of
kit-supplied QuantiTect SYBR Green RT-PCR Master mix, 0.4 µl of
each primer (10 µM), 2 µl of cDNA (50 ng RNA), and 7.2 µl of double
distilled H2O (all Applied Biosystems; Thermo Fisher
Scientific, Inc.). The following primer sequences were used for
qPCR: miR-150 forward, 5′-TCTCCCAACCCTTGTACCAGTG-3′ and reverse,
5′-TCTCCCAACCCTTGTACCAGTG-3′; and U6 forward,
5′-GCGCGTCGTGAAGCGTTC-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′. The
following thermocycling conditions were used for qPCR: initial
denaturation at 95°C for 10 min, followed by 40 cycles of
denaturation at 95°C for 15 sec and annealing at 60°C for 1 min.
Relative miRNA levels were quantified using the 2−ΔΔCq
method and normalized to U6 snRNA (RNU6B; Invitrogen; Thermo Fisher
Scientific, Inc.). All experiments were performed in
triplicate.
Immunohistochemical staining and
scoring
Expression of B-cell lymphoma 6 (BCL-6), CD10 and
multiple myeloma antigen 1 (MUM1) was determined via
immunohistochemical staining. The slices were stored at room
temperature for 60 min, then soaked twice in xylene for 10 min and
rehydrated in decreasing concentrations of ethanol for 5 min at a
time (100, 95, 85, 75 and 50%), prior to incubation in pure water
for 5 min. Following deparaffinization and rehydration, the
sections were incubated with 3% H2O2 for 5–10
min at room temperature to inhibit endogenous peroxidase activity,
and subsequently washed with PBS 3 times. The slices were immersed
in 0.01 mol citrate buffer, heated to boiling in a medical
microwave oven and baked for 8 min. When the buffer temperature
dropped to room temperature, the slices were soaked in PBS twice
for 5 min. Subsequently, 10% normal goat serum (Cell Signaling
Technology, Inc.) was used to block the samples at room temperature
for 60 min. Tissue sections were incubated with primary antibodies
(all 1:100) directed against BCL-6 (cat. no. ZM-0011; OriGene
Technologies, Inc.), CD10 (cat. no. ZM0283; OriGene Technologies,
Inc.) and MUM1 (cat. no. ZA-0583; OriGene Technologies, Inc.)
overnight at 4°C. Subsequently incubated with secondary antibodies
(cat. no. 31926; Cell Signaling Technology, Inc.) and incubated at
room temperature for 30 min. The slides were stained with
3,3′-diaminobenzidine at room temperature for 15 min. The specimens
were visualized under a light microscope (magnification, ×40).
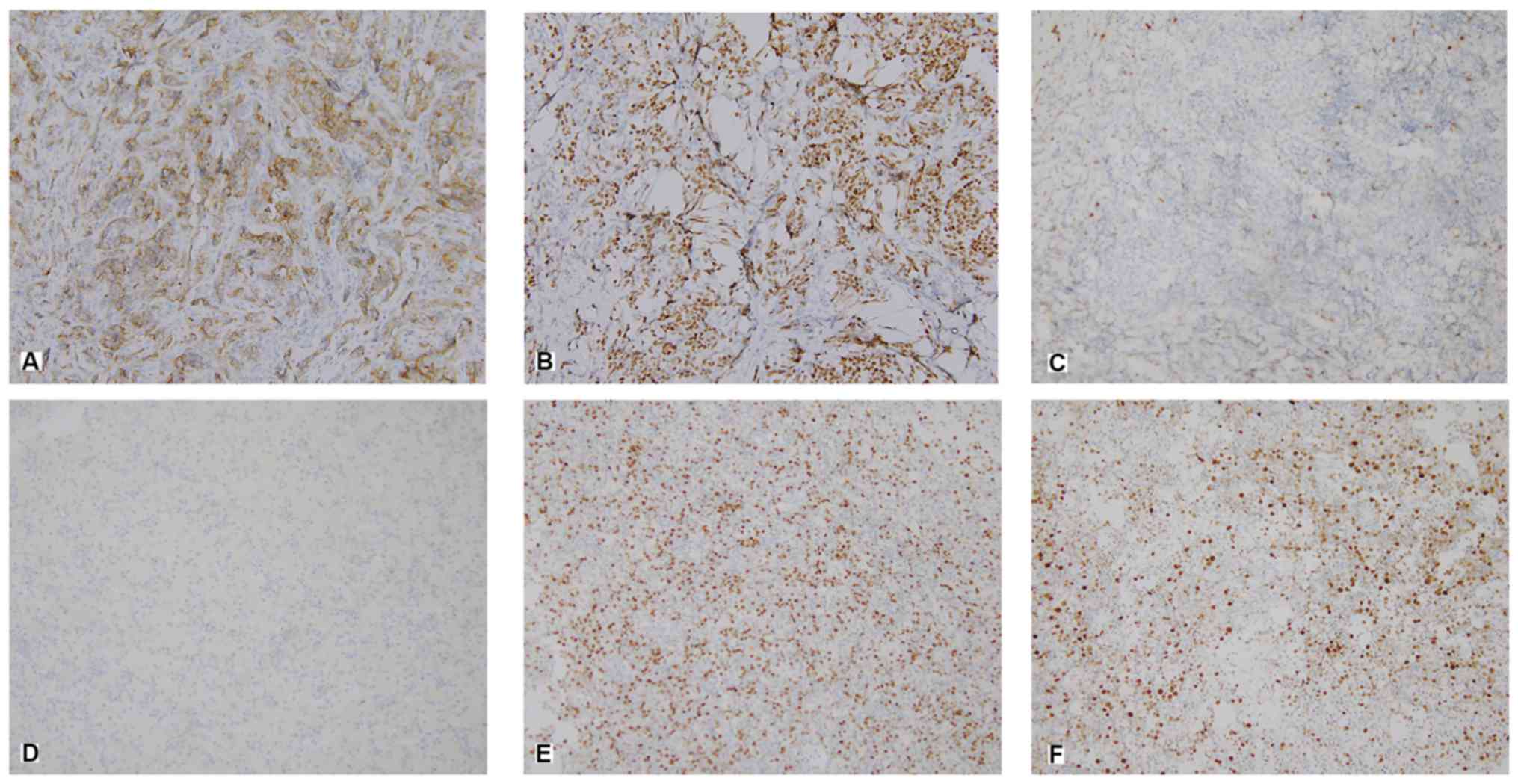
A semi-quantitative scoring system was used to
assess the percentage of positive tumor cells and the staining
intensity. The experimental group and the control group were
photographed, and the relevant results were obtained by using Image
Pro Plus image analysis software (version 7.0, Meyer Instruments,
Inc.). Staining intensity was scored as follows: 0, no staining;
1+, weak staining; 2+, moderate staining; and 3+, strong staining
(Fig. 1).
Treatment and response assessment
According to the National Comprehensive Cancer
Network treatment guidelines (33),
all patients were treated with CHOP [cyclophosphamide (750
mg/m2, day 1), doxorubicin (50 mg/m2, day 1),
vincristine (1.4 mg/m2, day 1) and prednisone (100 mg,
days 1–5)] or CHOP-like regimen with rituximab (375
mg/m2 at day 1) (R-CHOP). Patients were treated for 6–8
cycles (each cycle, 21 days). The treatment response at the end of
every two cycles was assessed based on results from computed
tomography, magnetic resonance imaging, color ultrasound or
18F-fluorodeoxyglucose positron emission tomography,
according to the International Working Group response criteria as
either: Complete response, partial response, stable disease and
progressive disease (34).
Statistical analysis
Statistical analysis was performed using SPSS
(version 20.0; IBM Corp.) and GraphPad Prism (version 7.0; GraphPad
Software, Inc.) software. Data were presented as the mean ±
standard deviation. The association between miR-150 expression and
clinicopathological characteristics was assessed using Pearson's
χ2 test. The paired Student's t-test was used to compare
the miR-150 expression between tumor and adjacent non-tumor tissue
samples, while unpaired Students' t-test was used to assess miR-150
expression in the International Prognostic Index (IPI) subgroups.
Survival curves were generated using the Kaplan-Meier method and
compared using the log-rank test. OS time was measured from the
date of diagnosis to the date of mortality or the last follow-up.
Progression-free survival (PFS) time was measured from the date of
diagnosis to the date of disease progress, recurrence, mortality or
last follow-up. In the multivariate and univariate analyses, the
prognostic value of miR-150 in patients with PGI-DLBCL was
evaluated using Cox regression. Receiver operating characteristic
(ROC) curve analysis was performed and the area under the curve
(AUC) was measured to assess miR-150 specificity and sensitivity.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-150 expression and
clinicopathological characteristics in PGI-DLBCL
Data on the association between miR-150 expression
and clinicopathological characteristics in patients with PGI-DLBCL
are presented in Table I. Of the 84
assessed patients, 63 (75.00%) exhibited stomach involvement, 47
(55.95%) had advanced disease (clinical stages IIE-IV) and 65
(77.38%) presented with a good performance status (0–1). All tissue
samples were stained with CD10, BCL6 and MUM1, which classified 31
patients (36.90%) into the germinal center B-cell like (GCB)
subgroup and 53 patients (63.10%) into the non-GCB subgroup.
Elevated lactate dehydrogenase (LDH) expression was observed in 31
patients (36.90%), while B symptoms (Systemic symptoms such as: i)
Fever of unknown origin exceeding 38°C for 3 consecutive days; ii)
unexplained weight loss of >10% within 6 months; iii) night
sweats) were identified in 60 patients (71.43%) and low IPI scores
(0–2) were observed in 41 patients (48.81%). A total of 54 patients
(64.29%) were treated with the R-CHOP regimen. In all, lymph node
and bone marrow metastasis were observed in 32 (38.10%) and 21
(25.00%) patients, respectively. According to receiver operating
characteristic (ROC) curve analysis, the most notable cut-off point
for miR-150 was 8.965, with 79.8% sensitivity and 77.1%,
specificity, respectively. Patients were divided into two subgroups
according to this cut-off value, as follows: Low miR-150 group
(≤8.965; n=66) and high miR-150 group (>8.965; n=18). Low
miR-150 expression was significantly associated with clinical stage
(P=0.029), IPI score (P=0.025), ECOG score (P=0.002) and treatment
regime (P=0.049). However, no significant association was observed
between low miR-150 expression and sex, age, tumor origin,
pathological type, LDH level, B symptoms, lymph node metastasis and
bone marrow metastasis.
 | Table I.Association between miR-150
expression and clinicopathological characteristics in patients with
primary gastrointestinal diffuse large B-cell lymphoma (n=84). |
Table I.
Association between miR-150
expression and clinicopathological characteristics in patients with
primary gastrointestinal diffuse large B-cell lymphoma (n=84).
|
|
| miR-150 expression
level |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Patients, n=84 | Low, n=66 | High, n=18 | P-value |
|---|
| Sex |
|
|
| 0.761 |
|
Male | 44 | 34 | 10 |
|
|
Female | 40 | 32 | 8 |
|
| Age, years |
|
|
| 0.969 |
|
≤60 | 33 | 26 | 7 |
|
|
>60 | 51 | 40 | 11 |
|
| Tumor origin |
|
|
| 0.759 |
|
Stomach | 63 | 50 | 13 |
|
|
Intestinal | 21 | 16 | 5 |
|
| Pathological
type |
|
|
| 0.844 |
|
GCB | 31 | 24 | 7 |
|
|
Non-GCB | 53 | 42 | 11 |
|
| Lugano staging
status |
|
|
| 0.029a |
|
I–II | 37 | 25 | 12 |
|
|
IIE-IV | 47 | 41 | 6 |
|
| IPI score |
|
|
| 0.025a |
|
0–2 | 41 | 28 | 13 |
|
|
3–5 | 43 | 38 | 5 |
|
| ECOG score |
|
|
| 0.002a |
|
0–1 | 65 | 56 | 9 |
|
|
2–4 | 19 | 10 | 9 |
|
| B symptoms |
|
|
| 0.274 |
|
Negative | 24 | 17 | 7 |
|
|
Positive | 60 | 49 | 11 |
|
| LDH level |
|
|
| 0.064 |
|
Normal | 53 | 45 | 8 |
|
|
Elevated | 31 | 21 | 10 |
|
| Treatment |
|
|
| 0.047a |
|
CHOP | 30 | 20 | 10 |
|
|
R-CHOP | 54 | 46 | 8 |
|
| Lymph node
metastasis |
|
|
| 0.309 |
|
Negative | 52 | 39 | 13 |
|
|
Positive | 32 | 27 | 5 |
|
| Bone marrow
metastasis |
|
|
| 0.759 |
|
Negative | 63 | 49 | 14 |
|
|
Positive | 21 | 17 | 4 |
|
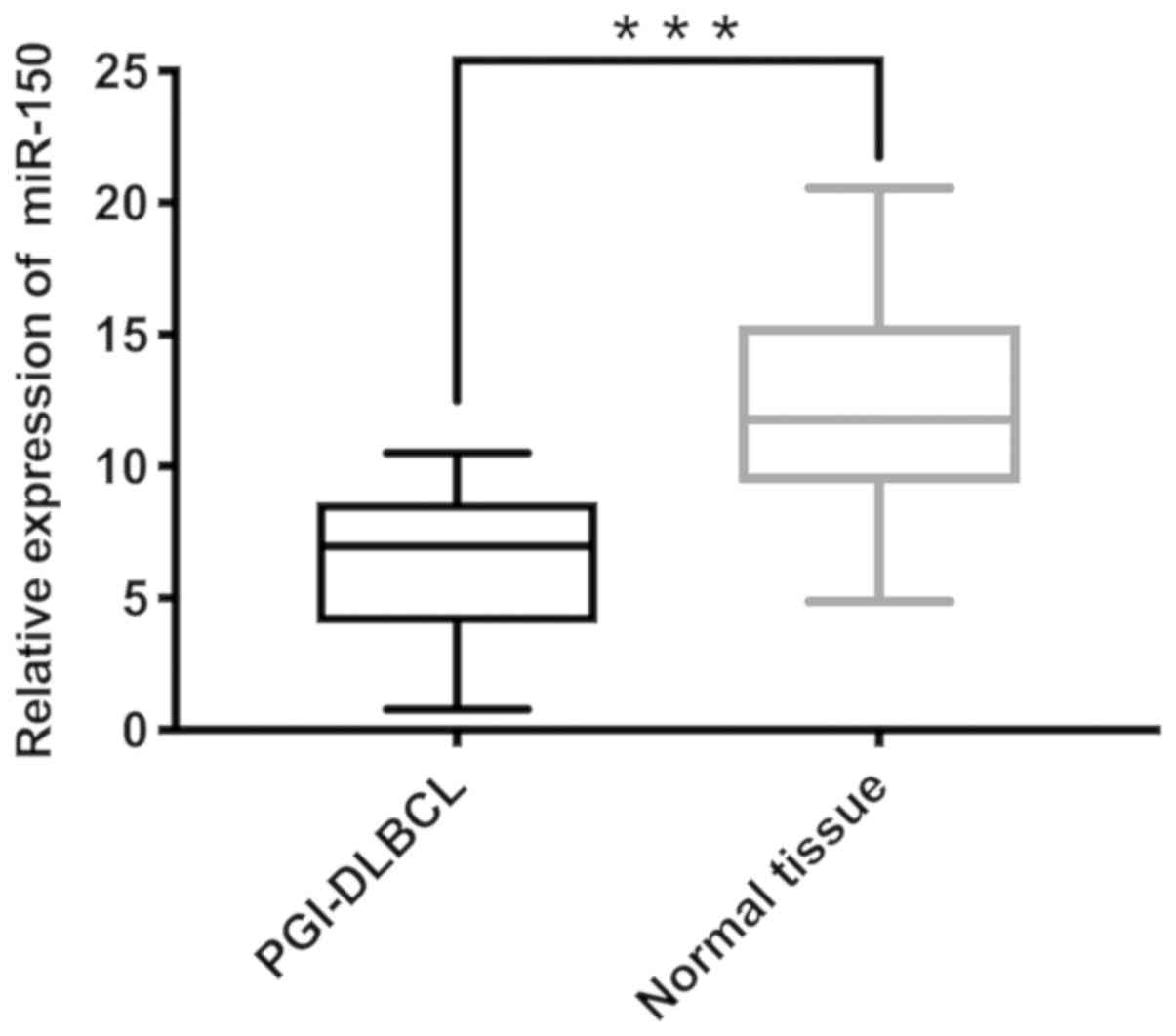
miR-150 expression is significantly
lower in PGI-DLBCL tissues compared with non-tumor tissues
The mean miR-150 expression in tumor tissues was
6.11 and the median was 5.74 (minimum, 0.29; maximum, 10.52), while
the average miR-150 expression in adjacent non-tumor tissues was
12.00 and the median was 11.45 (minimum, 2.23; maximum, 20.56).
RT-qPCR analysis demonstrated significantly lower miR-150
expression in PGI-DLBCL tissues compared with adjacent non-tumor
tissues (P<0.001; Fig. 2).
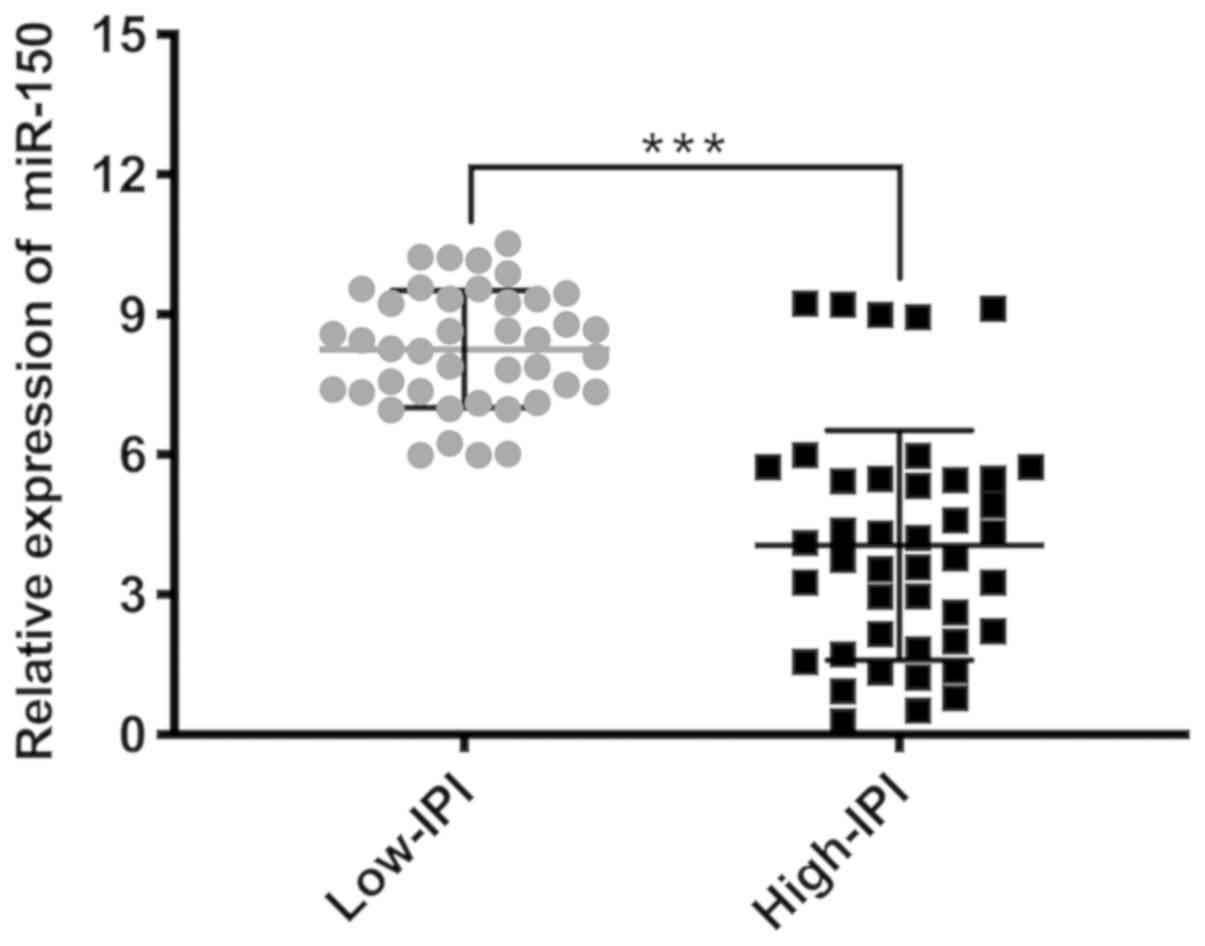
Subgroup analysis was subsequently performed after patients with
PGI-DLBCL were divided into two groups: Low IPI score (0–2) and
high IPI score (3–5). miR-150 expression was significantly
elevated in patients with low-IPI scores compared with patients in
the high-IPI group (P<0.001; Fig.
3).
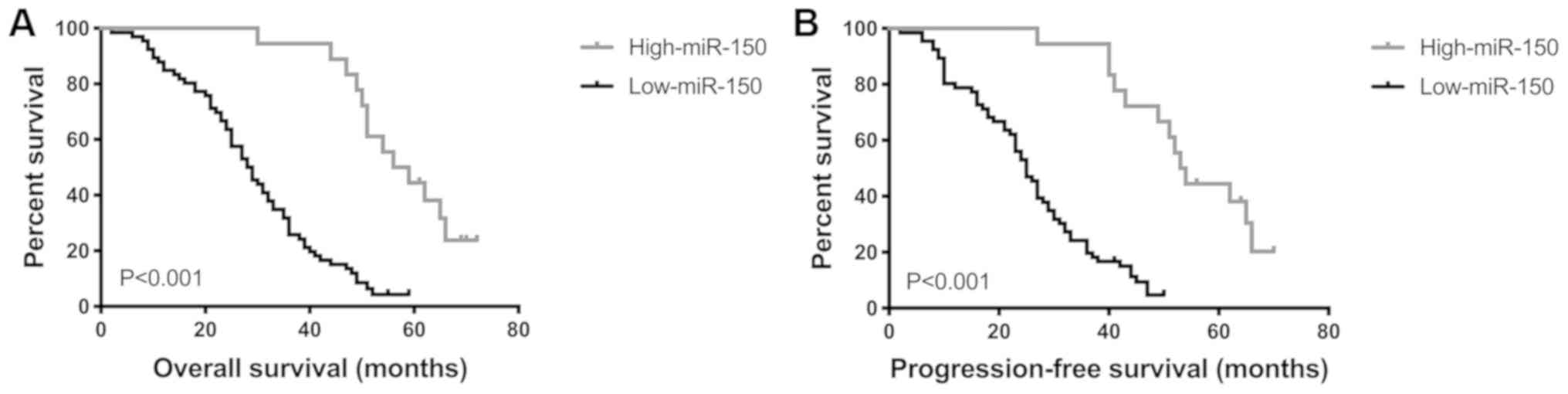
Low miR-150 expression is associated
with shorter OS and PFS time
Survival analysis of all 84 patients with PGI-DLBCL
was performed using the Kaplan-Meier method, in order to determine
the prognostic value of miR-150 in PGI-DLBCL. OS and PFS time were
significantly shorter in patients with low miR-150 expression
compared with patients with high miR-150 expression (both
P<0.001; Fig. 4). Univariate
analysis demonstrated that the prognosis of PGI-DLBCL was
significantly associated with miR-150 level, treatment and IPI
score (Table II). Multivariate
analysis indicated that miR-150 level and IPI score were two
prognostic indicators of OS and PFS time in patients with PGI-DLBCL
(miR-150 OS, P=0.015 and PFS, P=0.015; IPI OS, P=0.004 and PFS,
P=0.002; Table III). Taken
together, these results suggest that miR-150 may be used as a
prognostic marker in patients with PGI-DLBCL.
 | Table II.Univariate analysis of the prognostic
characteristics for primary gastrointestinal diffuse large B-cell
lymphoma. |
Table II.
Univariate analysis of the prognostic
characteristics for primary gastrointestinal diffuse large B-cell
lymphoma.
| Characteristic | OS, HR (95%
CI) | P-value | PFS, HR (95%
CI) | P-value |
|---|
| miR-150 expression
(Low vs. high) | 1.761
(1.101–2.814) | 0.018a | 1.873
(1.170–2.998) | 0.009a |
| Treatment (CHOP vs.
R-CHOP) | 1.972
(1.251–3.108) | 0.003a | 2.704
(1.314–3.274) | 0.002a |
| IPI score (0–2 vs.
3–5) | 1.984
(1.236–3.185) | 0.005a | 2.054
(1.279–3.300) | 0.003a |
| ECOG (0–1 vs.
2–4) | 1.298
(0.822–2.049) | 0.263 | 1.358
(0.860–2.143) | 0.189 |
| Lugano staging
status (I–II vs. IIE-IV) | 1.165
(0.737–1.842) | 0.513 | 1.230
(0.778–1.944) | 0.376 |
 | Table III.Multivariate analysis of the
independent prognostic characteristics for primary gastrointestinal
diffuse large B-cell lymphoma. |
Table III.
Multivariate analysis of the
independent prognostic characteristics for primary gastrointestinal
diffuse large B-cell lymphoma.
| Characteristic | OS, HR (95%
CI) | P-value | PFS, HR (95%
CI) | P-value |
|---|
| miR-150 expression
(Low vs. high) | 2.043
(1.147–3.638) | 0.015a | 2.074
(1.154–3.730) | 0.015a |
| IPI score (0–2 vs.
3–5) | 2.030
(1.262–3.264) | 0.004a | 2.082
(1.295–3.346) | 0.002a |
| Treatment (CHOP vs.
R-CHOP) | 1.305
(0.552–3.085) | 0.544 | 1.499
(0.633–3.549) | 0.357 |
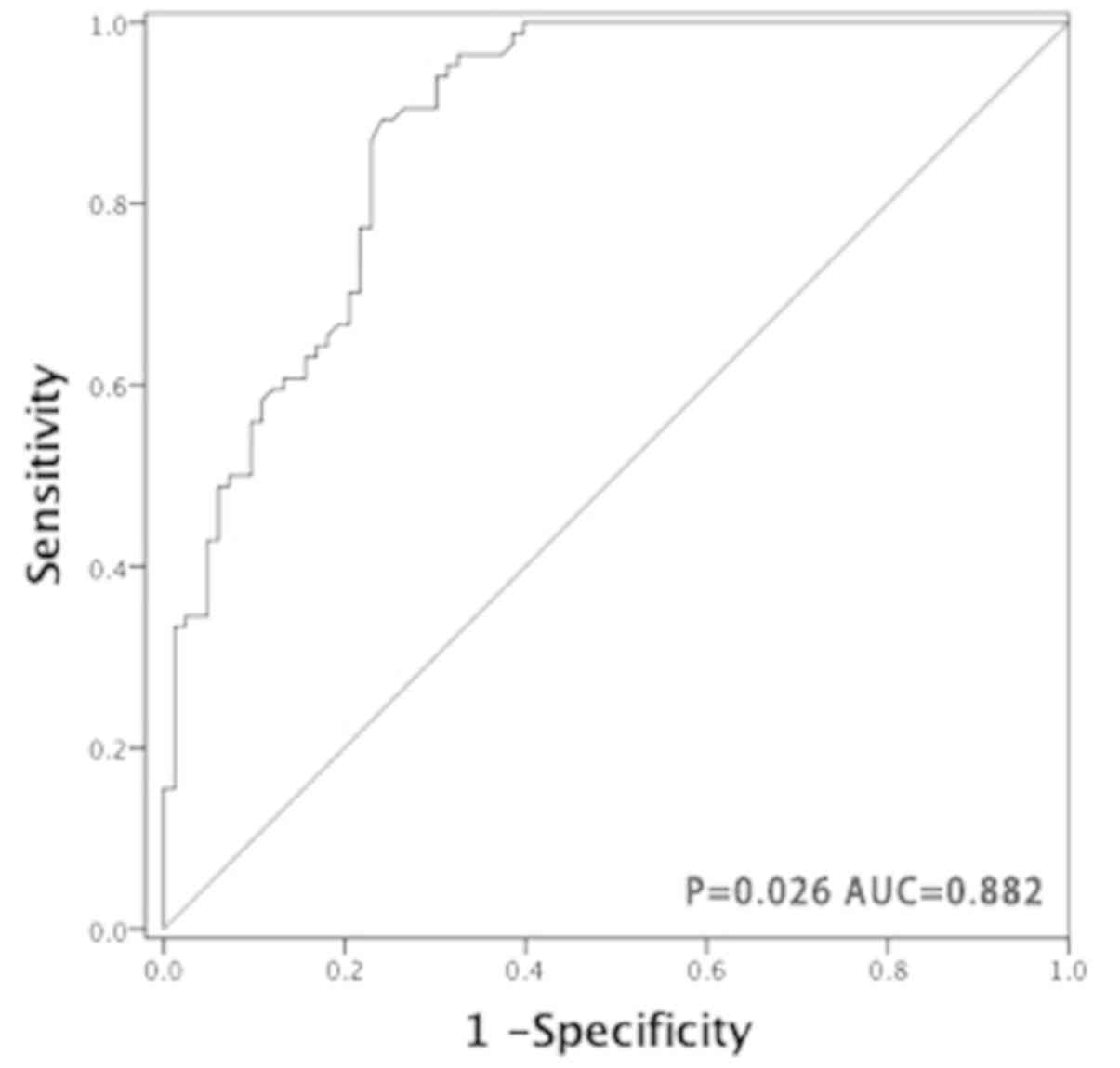
miR-150 has a potential diagnostic
value for PGI-DLBCL
The ROC curve was generated by dividing all tumor
and non-tumor tissue samples into one class, in order to determine
whether miR-150 may be used as a biomarker to distinguish PGI-DLBCL
from adjacent non-tumor tissues. The AUC was 0.882 (P=0.026;
Fig. 5), suggesting that miR-150 has
a potential diagnostic value for patients with PGI-DLBCL.
Discussion
PGI lymphoma originates in the stomach, with or
without peri-gastric or abdominal lymph node invasion (35). DLBCL is the most frequently diagnosed
histopathological subtype of PGI lymphoma; however, PGL-DLBCL
accounts for ~2% of all lymphomas (36). Thus, studies specifically focused on
PGI-DLBCL are limited. Although the pathogenesis of PGI-DLBCL
remains unclear, its increased incidence may be associated with
genetic and epigenetic molecular mechanisms (37,38).
Previous studies have demonstrated that the imbalance of miRNA
expression seems to be a common feature in several types of cancer.
Emerging evidence suggests that these miRNAs act as promising
biomarkers and therapeutic targets (39), and thus may be used as a tool to
develop novel anticancer cell strategies.
A previous study have implicated aberrant miR-150
expression in tumorigenesis. Additionally, miR-150 may have a
potential therapeutic role against cancer by regulating the
expression of oncogenes and/or tumor suppressor genes (40). A number of studies have demonstrated
dynamic changes in miR-150 expression in several solid tumors, and
also during the development of hematological malignancies. For
example, Zhang et al (41)
reported that miR-150 regulates NSCLC progression by inducing the
proliferation and migration of NSCLC cells. Furthermore, malignant
hematopoiesis and leukemia development are associated with aberrant
miR-150 expression (42,43). Elevated miR-150 expression was
demonstrated to be associated with decreased postoperative survival
of patients with gastric cancer (44). However, to the best of our knowledge,
little is known about the expression pattern and clinical
significance of miR-150 in PGI-DLBCL. Thus, the present study aimed
to investigate miR-150 expression in patients with PGI-DLBCL. Taken
together, the current results demonstrated that miR-150 may
influence the pathogenesis of PGI-DLBCL and act as a key
independent prognostic factor for this disease.
The results of the present study indicated that
miR-150 expression was significantly lower in PGI-DLBCL tissues
compared with adjacent non-tumor tissues. Furthermore, miR-150
expression was also decreased in the high-IPI subgroup compared
with the low-IPI subgroup. Statistical analysis demonstrated that
low miR-150 expression was significantly associated with stage
(IIE-IV), IPI score (3–5), ECOG status (2–4) and
treatment (R-CHOP). ROC curve analysis indicated that the optimal
cut-off concentration of miR-150 was 8.965, with an AUC value of
0.882, and high sensitivity (79.8%) and specificity (77.1%). The
present results suggest that miR-150 expression level may help
distinguish between patients with PGI-DLBCL and healthy
individuals. The OS and PFS time were significantly shorter in
patients with low miR-150 expression compared with those with high
miR-150 expression. Furthermore, miR-150 was demonstrated to be an
independent prognostic factor during both univariate and
multivariate analyses.
The IPI score is the most commonly used prognostic
evaluation tool in DLBCL, and the prognosis of patients with a high
IPI score is generally poor, while that of patients with a low IPI
score is relatively favorable (33,45). In
the present study, statistical analysis demonstrated significant
differences between the miR-150 and IPI subgroups, suggesting that
both may be used as a powerful index to determine the prognosis of
patients. Furthermore, a previous study reported that the prognosis
of patients with DLBCL, with GCB expression profiles is more
favorable compared with patients with non-GCB (46). However, the present study failed to
demonstrate a significant difference between miR-150 expression
level and pathological subtype. Regarding treatment, a significant
association was observed between miR-150 expression and the R-CHOP
treatment regime, which is consistent with a previous finding that
the CHOP regimen with rituximab (CD20 antibody) significantly
improves the prognosis of patients (47).
A number of limitations exist within the present
study. First, inadequate samples containing other extranodal
lymphomas were obtained from patients, limiting assessment to only
PGI-DLBCL specimens. This made it difficult to determine the effect
of miR-150 expression in other extranodal lymphomas. Secondly,
although the present study demonstrated the ability of miR-150 to
predict the prognosis of patients with PGI-DLBCL, due to population
size limitations, it failed to independently verify the sample
settings and the existence of the same expression results in
different samples. Future studies should aim to increase the
population size and include patients with other extranodal
lymphomas, in order to overcome these shortcomings and verify the
current results.
Taken together, the results of the present study
indicate that that miR-150 serves a vital role in the pathogenesis
and progression of PGI-DLBCL, whereby low miR-150 expression is
associated with poor predicted clinical outcomes. Furthermore,
miR-150 expression was investigated in PGI-DLBCL tissues, which
consolidated its role as a valuable diagnostic and prognostic
biomarker, and potential therapeutic target in patients with
PGI-DLBCL.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant nos. 81100337 and 81470283).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HaZ and YW designed the study and reviewed the final
manuscript. XinW performed the experiments, analyzed the
experimental data and drafted the initial manuscript. PG, LC and YK
helped perform the experiments. XiaW, ZZ, YY and HY acquired
patient information and specimens. HuZ, XL, LQ, LL, QZ, ZQ and TD
helped acquire the specimens. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Tianjin Medical University Cancer Institute (Tianjin,
China) and written informed consent was provided by all patients
prior to the study start. All procedures were performed in
accordance with the ethical standards of the Institutional Review
Board and The Declaration of Helsinki, and its later amendments or
comparable ethical standards.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AUC
|
area under the curve
|
|
BCL-6
|
B-cell lymphoma 6
|
|
ECOG
|
Eastern Cooperative Oncology Group
|
|
GCB
|
germinal center B-cell like
|
|
IPI
|
International Prognostic Index
|
|
LDH
|
lactate dehydrogenase
|
|
MALT
|
mucosa-associated lymphoid tissue
|
|
miRNA
|
microRNA
|
|
miR-150
|
microRNA-150
|
|
MUM1
|
multiple myeloma antigen 1
|
|
NSCLC
|
non-small cell lung cancer
|
|
NSCLC
|
non-small cell lung cancer
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
PGI-DLBCL
|
primary gastrointestinal diffuse large
B-cell lymphoma
|
|
ROC
|
receiver operating characteristic
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
References
|
1
|
Peng JC, Zhong L and Ran ZH: Primary
lymphomas in the gastrointestinal tract. J Dig Dis. 16:169–176.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang T, Gui W and Shen Q: Primary
gastrointestinal non-Hodgkin's lymphoma: Clinicopathological and
prognostic analysis. Med Oncol. 27:661–666. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cogliatti SB, Schmid U, Schumacher U,
Eckert F, Hansmann ML, Hedderich J, Takahashi H and Lennert K:
Primary B-cell gastric lymphoma: A clinicopathological study of 145
patients. Gastroenterology. 101:1159–1170. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koch P, del Valle F, Berdel WE, Willich
NA, Reers B, Hiddemann W, Grothaus-Pinke B, Reinartz G, Brockmann
J, Temmesfeld A, et al: Primary gastrointestinal non-Hodgkin's
lymphoma: I. Anatomic and histologic distribution, clinical
features, and survival data of 371 patients registered in the
German multicenter study GIT NHL 01/92. J Clin Oncol. 19:3861–3873.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Papaxoinis G, Papageorgiou S, Rontogianni
D, Kaloutsi V, Fountzilas G, Pavlidis N, Dimopoulos M, Tsatalas C,
Xiros N and Economopoulos T: Primary gastrointestinal non-Hodgkin's
lymphoma: A clinicopathologic study of 128 cases in Greece. A
hellenic cooperative oncology group study (HeCOG). Leuk Lymphoma.
47:2140–2146. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suresh B, Asati V, Lakshmaiah KC, Babu G,
Lokanatha D, Jacob LA, Lokesh KN, Rudresh AH, Rajeev LK, Smitha S,
et al: Primary gastrointestinal diffuse large B-cell lymphoma: A
prospective study from South India. South Asian J Cancer. 8:57–59.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen K and Rajewsky N: The evolution of
gene regulation by transcription factors and microRNAs. Nat Rev
Genet. 8:93–103. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng CJ, Bahal R, Babar IA, Pincus Z,
Barrera F, Liu C, Svoronos A, Braddock DT, Glazer PM, Engelman DM,
et al: MicroRNA silencing for cancer therapy targeted to the tumour
microenvironment. Nature. 518:107–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu FQ, Zeng L, Tang N, Tang YP, Zhou BP,
Li FF, Wu WG, Zeng XB and Peng SS: MicroRNA-155 downregulation
promotes cell cycle arrest and apoptosis in diffuse large B-cell
lymphoma. Oncol Res. 24:415–427. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhuang H, Shen J, Zheng Z, Luo X, Gao R
and Zhuang X: MicroRNA-146a rs2910164 polymorphism and the risk of
diffuse large B cell lymphoma in the Chinese Han population. Med
Oncol. 31:3062014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Berglund M, Hedström G, Amini RM, Enblad G
and Thunberg U: High expression of microRNA-200c predicts poor
clinical outcome in diffuse large B-cell lymphoma. Oncol Rep.
29:720–724. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ivey KN and Srivastava D: microRNAs as
developmental regulators. Cold Spring Harb Perspect Biol.
7:a0081442015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Larrabeiti-Etxebarria A, Lopez-Santillan
M, Santos-Zorrozua B, Lopez-Lopez E and Garcia-Orad A: Systematic
review of the potential of MicroRNAs in diffuse large B cell
lymphoma. Cancers (Basel). 11(pii): E1442019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koscianska E and Krzyzosiak WJ: Current
understanding of the role of microRNAs in spinocerebellar ataxias.
Cerebellum Ataxias. 1:72014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bi N, Cao J, Song Y, Shen J, Liu W, Fan J,
He J, Shi Y, Zhang X, Lu N, et al: A microRNA signature predicts
survival in early stage small-cell lung cancer treated with surgery
and adjuvant chemotherapy. PLoS One. 9:e913882014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang W, Xu P, Wang H, Niu Z, Zhu D, Lin Q,
Tang L and Ren L: MicroRNA-150 suppresses triple-negative breast
cancer metastasis through targeting HMGA2. Onco Targets Ther.
11:2319–2332. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yokobori T, Suzuki S, Tanaka N, Inose T,
Sohda M, Sano A, Sakai M, Nakajima M, Miyazaki T, Kato H and Kuwano
H: miR-150 is associated with poor prognosis in esophageal squamous
cell carcinoma via targeting the EMT inducer ZEB1. Cancer Sci.
104:48–54. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma Y, Zhang P, Wang F, Zhang H, Yang J,
Peng J, Liu W and Qin H: miR-150 as a potential biomarker
associated with prognosis and therapeutic outcome in colorectal
cancer. Gut. 61:1447–1453. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee KH, Lee JK, Choi DW, Do IG, Sohn I,
Jang KT, Jung SH, Heo JS, Choi SH and Lee KT: Postoperative
prognosis prediction of pancreatic cancer with seven microRNAs.
Pancreas. 44:764–768. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang S, Chen Y, Wu W, Ouyang N, Chen J,
Li H, Liu X, Su F, Lin L and Yao Y: miR-150 promotes human breast
cancer growth and malignant behavior by targeting the pro-apoptotic
purinergic P2X7 receptor. PLoS One. 8:e807072013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yin QW, Sun XF, Yang GT, Li XB, Wu MS and
Zhao J: Increased expression of microRNA-150 is associated with
poor prognosis in non-small cell lung cancer. Int J Clin Exp
Pathol. 8:842–846. 2015.PubMed/NCBI
|
|
24
|
Dezhong L, Xiaoyi Z, Xianlian L, Hongyan
Z, Guohua Z, Bo S, Shenglei Z and Lian Z: miR-150 is a factor of
survival in prostate cancer patients. J BUON. 20:173–179.
2015.PubMed/NCBI
|
|
25
|
Dettmer MS, Perren A, Moch H, Komminoth P,
Nikiforov YE and Nikiforova MN: MicroRNA profile of poorly
differentiated thyroid carcinomas: New diagnostic and prognostic
insights. J Mol Endocrinol. 52:181–189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang W, Wang X, Zhang Y, Wang D, Gao H,
Wang L and Gao S: Prognostic role of microRNA-150 in various
carcinomas: A meta-analysis. Onco Targets Ther. 9:1371–1379. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Monticelli S, Ansel KM, Xiao C, Socci ND,
Krichevsky AM, Thai TH, Rajewsky N, Marks DS, Sander C, Rajewsky K,
et al: MicroRNA profiling of the murine hematopoietic system.
Genome Biol. 6:R712005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Szurián K, Csala I, Piurkó V, Deák L,
Matolcsy A and Reiniger L: Quantitative miR analysis in chronic
lymphocytic leukaemia/small lymphocytic lymphoma-proliferation
centres are characterized by high miR-92a and miR-155 and low
miR-150 expression. Leuk Res. 58:39–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang M, Yang W, Li M and Li Y: Low
expression of miR-150 in pediatric intestinal Burkitt lymphoma. Exp
Mol Pathol. 96:261–266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gebauer N, Kuba J, Senft A, Schillert A,
Bernard V and Thorns C: MicroRNA-150 Is up-regulated in extranodal
marginal zone lymphoma of MALT type. Cancer Genomics Proteomics.
11:51–56. 2014.PubMed/NCBI
|
|
31
|
Tomonaga M: Outline and direction of
revised WHO classification of tumors of haematopoietic and lymphoid
tissues. Rinsho Ketsueki. 50:1401–1406. 2009.(In Japanese).
PubMed/NCBI
|
|
32
|
Huang J, Jiang W, Xu R, Huang H, Lv Y, Xia
Z, Sun X, Guan Z, Lin T and Li Z: Primary gastric non-Hodgkin's
lymphoma in Chinese patients: Clinical characteristics and
prognostic factors. BMC Cancer. 10:3582010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Horwitz SM, Zelenetz AD, Gordon LI, Wierda
WG, Abramson JS, Advani RH, Andreadis CB, Bartlett N, Byrd JC,
Fayad LE, et al: NCCN guidelines insights: Non-Hodgkin's lymphomas,
version 3.2016. J Natl Compr Canc Netw. 14:1067–1079. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheson BD, Pfistner B, Juweid ME, Gascoyne
RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca
E, et al: Revised response criteria for malignant lymphoma. J Clin
Oncol. 25:579–586. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ferreri AJ and Montalban C: Primary
diffuse large B-cell lymphoma of the stomach. Crit Rev Oncol
Hematol. 63:65–71. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dawson IM, Cornes JS and Morson BC:
Primary malignant lymphoid tumours of the intestinal tract. Report
of 37 cases with a study of factors influencing prognosis. Br J
Surg. 49:80–89. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nakamura S and Iida M: Molecular genetics
in primary gastrointestinal lymphomas. Fukuoka Igaku Zasshi.
99:123–130. 2008.(In Japanese). PubMed/NCBI
|
|
38
|
Nakamura S, Matsumoto T, Nakamura S, Jo Y,
Fujisawa K, Suekane H, Yao T, Tsuneyoshi M and Iida M: Chromosomal
translocation t(11;18)(q21;q21) in gastrointestinal mucosa
associated lymphoid tissue lymphoma. J Clin Pathol. 56:36–42. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lan H, Lu H, Wang X and Jin H: MicroRNAs
as potential biomarkers in cancer: Opportunities and challenges.
Biomed Res Int. 2015:1250942015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Watanabe A, Tagawa H, Yamashita J, Teshima
K, Nara M, Iwamoto K, Kume M, Kameoka Y, Takahashi N, Nakagawa T,
et al: The role of microRNA-150 as a tumor suppressor in malignant
lymphoma. Leukemia. 25:1324–1334. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang L, Lin J, Ye Y, Oba T, Gentile E,
Lian J, Wang J, Zhao Y, Gu J, Wistuba II, et al: Serum MicroRNA-150
predicts prognosis for early-stage non-small cell lung cancer and
promotes tumor cell proliferation by targeting tumor suppressor
gene SRCIN1. Clin Pharmacol Ther. 103:1061–1073. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
He Y, Jiang X and Chen J: The role of
miR-150 in normal and malignant hematopoiesis. Oncogene.
33:3887–3893. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fayyad-Kazan H, Bitar N, Najar M, Lewalle
P, Fayyad-Kazan M, Badran R, Hamade E, Daher A, Hussein N, ElDirani
R, et al: Circulating miR-150 and miR-342 in plasma are novel
potential biomarkers for acute myeloid leukemia. J Transl Med.
11:312013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Katada T, Ishiguro H, Kuwabara Y, Kimura
M, Mitui A, Mori Y, Ogawa R, Harata K and Fujii Y: microRNA
expression profile in undifferentiated gastric cancer. Int J Oncol.
34:537–542. 2009.PubMed/NCBI
|
|
45
|
Song JL, Wei XL, Zhang YK, Hao XX, Huang
WM, Wei Q, Wei YQ and Feng R: The prognostic value of the
international prognostic index, the national comprehensive cancer
network IPI and the age-adjusted IPI in diffuse large B cell
lymphoma. Zhonghua Xue Ye Xue Za Zhi. 39:739–744. 2018.(In
Chinese). PubMed/NCBI
|
|
46
|
van Imhoff GW, Boerma EJ, van der Holt B,
Schuuring E, Verdonck LF, Kluin-Nelemans HC and Kluin PM:
Prognostic impact of germinal center-associated proteins and
chromosomal breakpoints in poor-risk diffuse large B-cell lymphoma.
J Clin Oncol. 24:4135–4142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Coiffier B, Lepage E, Briere J, Herbrecht
R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G,
Gaulard P, et al: CHOP chemotherapy plus rituximab compared with
CHOP alone in elderly patients with diffuse large-B-cell lymphoma.
N Engl J Med. 346:235–242. 2002. View Article : Google Scholar : PubMed/NCBI
|