Introduction
Prostate cancer (PCa) has become the most frequently
diagnosed cancer in the male genitourinary system in the United
States, in 2013 (1). The main
methods for the early diagnosis of PCa include transrectal
ultrasound, PCa-specific antigen determination and digital rectal
examination (2,3). In addition, increasing evidence has
indicated that inflammation serves a key role in the pathogenesis
of PCa via regulation of the tumor microenvironment by releasing
growth factors and proinflammatory cytokines, and, therefore,
influencing tumor development (4–6).
Therefore, an improved understanding of the detailed molecular
mechanism underlying metastasis is urgently required in order to
develop therapies and prevent PCa.
Zinc finger protein 24 (ZNF24; also known as KOX17,
Zfp191 or ZNF191) is a member of the Kruppel-like zinc finger
transcription factor family (7). Its
N terminus harbors a SCAN domain that primarily functions as a
dimerization domain in zinc finger proteins (8), and its C terminus contains four C2H2
zinc finger motifs that serve as DNA binding domains (9). ZNF24 is ubiquitously expressed during
embryonic development and in adult tissues (10,11),
suggesting that it serves a key role in multiple different cell
types. Independent studies have demonstrated that knockout of ZNF24
results in premature death at different time points during
development (12,13), suggesting that ZNF24 serves a crucial
role in regulating organ development. In addition, ZNF24 has been
revealed to regulate proliferation, differentiation, migration and
invasion in several different types of cancer, such as
hepatocellular carcinoma and gastric cancer (14,15).
Upregulation of ZNF24 in neural progenitor cells maintains these
cells in an actively proliferating state and suppresses neuronal
differentiation (16). Furthermore,
the role of ZNF24 in regulating cell migration and invasion has
been investigated in aortic vascular smooth muscle cells, where
ZNF24 promotes cell migration (17).
However, to the best of our knowledge, the molecular role and
clinical effects of ZNF24 in PCa remain unclear.
The epithelial-to-mesenchymal transition (EMT) is
the initial step of migration (18).
Loss of epithelial cell-cell adhesion and the gain of
mesenchymal-like morphology are the main characteristics of the EMT
(19,20). EMT can enhance the invasive ability
of tumor cells, and enable them to leave their primary sites, which
leads to metastasis (21). Several
studies have revealed that EMT could be activated by EMT-related
transcription factors, including Twist, Slug and Snail (22,23).
The present study evaluated the gene and protein
expression levels of ZNF24 in PCa tissue samples and cancer cell
lines. Subsequently, gain and loss of function assays were
performed to specifically up- or downregulate ZNF24 in PCa cell
lines (PC-3 and DU-145) to assess its effect on PCa migration and
invasion, as well as growth. Furthermore, the present study
examined whether Twist1 was a downstream target of ZNF24. The
results revealed a novel mechanism of ZNF24 in PCa migration and
invasion.
Materials and methods
Patient tissue specimens
A total of 68 human PCa (mean, 65.3 years; range,
24–74 years) and 43 matched adjacent non-tumor tissues were
obtained from patients who underwent surgical resection at Shenzhen
People's Hospital (Shenzhen, China) between May 2008 and December
2012 for reverse transcription-quantitative PCR (RT-qPCR) and
western blotting analyses. None of the patients had undergone
chemotherapy or radiotherapy prior to surgery. Gleason score and
pathological stage of prostate patients were determined as
described previously (24,25). All tissues were immediately frozen in
liquid nitrogen and stored at −80°C. The present study was approved
by the Clinical Research Ethics Committee of Shenzhen People's
Hospital. All patients provided written informed consent prior to
experiments.
Cell culture
The human PCa cell lines PC-3 and DU-145, and the
human normal prostate cell line RWPE-1 were purchased from The Cell
Bank of Type Culture Collection of Chinese Academy of Sciences and
cultured in RPMI-1640 medium (Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 mg/ml streptomycin
in a humidified atmosphere at 37°C with 5% CO2.
Transfection
ZNF24 short hairpin RNA (shRNA) plasmid was
purchased from Hanbio Biotechnology Co., Ltd. The sequences were as
follows: ZNF24 shRNA,
5′-CCGGGAGGATTTGGAGAGTGAACTTCTCGAGAAGTTCACTCTCCAAATCCTCTTTTTG-3′;
and control shRNA (shControl),
5′-CCGGGCTGACCCTGAAGTTCATCCTCGAGGATGAACTTCAGGGTCAGCTTTTTG-3′. The
pcDNA3.1 (vector) and pcDNA-3.1-ZNF24 plasmid were purchased from
Vigene Biosciences. The plasmids (2.5 µg) were transfected using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Following transfection
for 48 h, cells were collected and prepared for further
experiments.
Western blotting
Tissue samples and cells were lysed using RIPA
buffer (Beyotime Institute of Biotechnology). The protein
concentration was measured using a BCA kit (Pierce; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Protein
samples (~40 µg) were separated via SDS-PAGE (10% gel) and the
protein was then transferred onto a PVDF membrane, followed by
incubation with 5% skimmed milk in TBS with 0.1% Tween-20 at 4°C
overnight. Subsequently, the membranes were incubated with primary
antibodies at 4°C overnight. The primary antibodies were: ZNF24
(1:2,000 dilution; cat. no. ab176589; Abcam), E-cadherin (1:1,000
dilution; cat. no. 3195; Cell Signaling Technology, Inc.),
N-cadherin (1:1,000 dilution; cat. no. 13116; Cell Signaling
Technology, Inc.) and β-actin (1:5,000 dilution; cat. no. ab179467;
Abcam). Following three washes with TBST at room temperature for 5
min, membranes were incubated in with secondary horseradish
peroxidase-conjugated goat anti-rabbit antibody (1:5,000 dilution;
cat. no. ab205718; Abcam) for 1 h at room temperature. Following
three washes with TBST at room temperature for 5 min, the blots
were visualized using an ECL solution kit (Pierce; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Each
experiment was repeated independently three times.
RT-qPCR
RNA was harvested from tissue specimens and cells
using TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. RNA (2 µg) was used to
synthesize cDNA using the High-Capacity cDNA Reverse Transcription
kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The reaction conditions used were as
follows: 42°C for 60 min and 70°C for 5 min. Subsequently, SYBR
Green Master (Roche Diagnostics GmbH) was utilized for the qPCR
using the ABI PRISM 7500 system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The reaction conditions used were as follows:
Pre-denaturation at 95°C for 4 min, 30 cycles of denaturation at
94°C for 30 sec, annealing at 59°C for 30 sec and extension at 72°C
for 1 min. The thermocycling conditions were as follows: 5 min at
65°C, 5 min at 42°C, and 1 min at 85°C. GAPDH was used as the
internal reference for other genes. The 2−ΔΔCq method
was utilized to calculate the relative expression of target genes
(26). The primers were as follows:
ZNF24: Forward, 5′-GTTCCTGAGCCGGAGGTTT-3′, reverse
5′-CTCAGGGCAATACCCCGTTT-3′; E-cadherin: Forward,
5′-AGTGACTGATGCTGATGCCC-3′; reverse, 5′-AATGTACTGCTGCTTGGCCT;
N-cadherin: Forward, 5′-GTGCATGAAGGACAGCCTCT-3′; reverse,
5′-TGGAAAGCTTCTCACGGCAT-3′; Twist1: Forward,
5′-TCAAGAGGTCGTGCCAATCA-3′; reverse, 5′-TTGCAGGCCAGTTTGATCCC-3′;
Slug: Forward, 5′-GCTACCCAATGGCCTCTCTC-3′; reverse,
5′-CTTCAATGGCATGGGGGTCT-3′; Snail: Forward,
5′-CGAGTGGTTCTTCTGCGCTA-3′; reverse, 5′-GGGCTGCTGGAAGGTAAACT-3′;
GAPDH: Forward, 5′-CCATGGGGAAGGTGAAGGTC-3′; and reverse,
5′-GCGCCCAATACGACCAAATC-3′. Each experiment was repeated
independently three times.
Colony-formation assay
PC-3 and DU-145 cells were placed into 6-well plates
at a density of 5×103 cells/well. Cells were cultured
for 2 weeks with serum-free RPMI-1640 medium. Subsequently, the
cells were fixed with 100% methanol at room temperature for 5 min
and stained with 0.5% crystal violet at room temperature for 10
min. Each experiment was repeated independently three times.
Wound healing assay
A total of 5×105 PC-3 and DU-145 cells
were plated in a 6-well plate. When the cell confluence reached
85–100%, a 10-µl pipette was used to make scratches on the cells
covering the bottom of the plate of the same width. The cells were
then washed three times with PBS and then cultured with serum-free
RPMI-1640 medium. At 0 and 48 h after scratching, the migration
distance in the scratched area was examined, and images were
captured under a light microscope (magnification, ×40). The
relative distance of migration=(width at 0 h-width at 48 h)/width
at 48 h. Each experiment was repeated independently three
times.
Transwell invasion assay
Invasion assays were performed as previously
described by using Matrigel invasion chambers (Thermo Fisher
Scientific, Inc.) (27,28). In brief, a total of 2×104
PC-3 and DU-145 cells were transfected with ZNF24 or ZNF24 shRNA,
respectively. After transfection for 48 h, cells were counted and
re-suspended in serum-free RPMI-1640 medium. Cells
(~4×104) were seeded in 24-well Matrigel invasion
chambers (Thermo Fisher Scientific, Inc.). The upper chamber was
filled with serum-free RPMI-1640 medium and the bottom chamber was
filled with RPMI-1640 medium supplemented with 10% FBS. Cells were
incubated at 37°C for 20 h. The cells were stained with 0.5%
crystal violet at room temperature for 10 min and then washed with
PBS. The cells on the surface of the chamber were removed using a
cotton-tipped swab. The cells that had invaded to the bottom
chamber were quantified using a light microscope (magnification,
×40). Each experiment was repeated independently three times.
Dual luciferase reporter assay
A dual luciferase reporter assay was utilized to
further verify the targeting association of ZNF24 and Twist1.
Briefly, the promoter region of Twist1 (−2000, +200) was cloned
into pGL3-basic plasmid (Vigene Biosciences). A mixture of 1 µg
pGL3-Twist1, 2 µg ZNF24 or ZNF24 shRNA and 20 ng Renilla
plasmid was transfected into a total of 5×105 PC-3 and
DU-145 cells using Lipofectamine® 2000 (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Following transfection for 24 h, the cells were collected and
lysed. Firefly and Renilla luciferase activities were
measured with a Dual-Luciferase Reporter Assay system (Promega
Corporation) according to the manufacturer's protocol.
Renilla luciferase activities were used as an internal
control for transfection efficiency. Each experiment was repeated
independently three times.
Chromatin immunoprecipitation (ChIP)
and quantitative (q) ChIP assay
ChIP and qChIP analyses were performed using an
EZ-ChIP kit (EMD Millipore) according to the manufacturer's
protocol. In brief, PC-3 and DU-145 cells were cultured to 80–100%
confluence, and the chromatin was cross-linked by 1% formaldehyde
at 37°C for 15 min. Subsequently, cross-linked chromatin was
sonicated (1 sec on, 1 sec off; 3×20 times) at 4°C to generate
200–1,000 bp fragments. Next, 5 µg anti-immunoglobulin G (IgG)
antibody (cat. no. ab171870; Abcam) or anti-ZNF24 (cat. no.
ab176589; Abcam) were used to immunoprecipitate chromatin fragments
at 4°C overnight. IgG antibody was used as the control. After
purifying the antibody-interact DNA, RT-qPCR was conducted to
analyze the precipitated chromatin DNA, as aforementioned. The
primer sequences were as follows: Twist1: Forward,
5′-AAGGGATGGACCTGAAACGG-3′; and reverse,
5′-GGCAAACTGGAAGCAGCAAA-3′. The qPCR conditions were as follows: 5
min at 98°C, denaturation at 98°C for 30 sec, annealing at 56°C for
30 sec and extension at 72°C for 20 sec, performed for 32
cycles.
Cell Counting Kit-8 (CCK-8) assay
Cells (~3×103) in 200 µl RPMI-1640 medium
were placed in 96-well plates. A total of six parallel wells were
prepared for each group. CCK-8 solution (20 µl; Beyotime Institute
of Biotechnology) was added into each well at 0, 24, 48 and 72 h,
and incubated for 1 h at 37°C. The optical density value was
measured at 450 nm in each well. Each experiment was repeated
independently three times.
Bioinformation analysis
The expression of ZNF24 in prostate tissue was
obtained from The Human Protein Atlas database (https://www.proteinatlas.org). The details are as
follows: Antibody number, CAB025642 and HPA024062; male, age 76;
Patient ID: 2932.
Statistical analysis
SPSS v19.0 software (IBM Corp.) was employed for
data analysis. Values in all graphs are presented as the mean ±
standard deviation. For Table I, the
mean value of ZNF24 mRNA content (6.4) in tumor cells was set as
the standard. Therefore, higher values than the standard value were
defined as high expression, and lower values than the standard
value were defined as low expression. The χ2 test was
applied for comparisons between ZNF24 expression and clinical
information of patients with PCa. Student's t-test was applied for
comparisons between two groups, and one-way ANOVA followed by
Tukey's post-hoc test was applied for comparisons among multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
 | Table I.Clinicopathological variables of 68
patients with prostate cancer. |
Table I.
Clinicopathological variables of 68
patients with prostate cancer.
|
|
| ZNF24
expression |
|
|---|
|
|
|
|
|
|---|
| Variables | Number, n (%)
(n=68) | Low, n (%)
(n=26) | High, n (%)
(n=42) | P-value |
|---|
| Age, years |
|
|
|
|
|
<66 | 30 (44.1) | 13 (50.0) | 17 (40.5) | 0.442 |
|
≥66 | 38 (55.9) | 13 (50.0) | 25 (59.5) |
|
| Tumor volume,
% |
|
|
|
|
|
<30 | 26 (38.2) | 14 (53.8) | 12 (28.6) | 0.037 |
|
≥30 | 42 (61.8) | 12 (46.2) | 30 (71.4) |
|
| Gleason score
(24) |
|
|
|
|
|
<6 | 32 (47.1) | 17 (65.4) | 15 (35.7) | 0.017 |
|
>7 | 36 (52.9) | 9 (34.6) | 27 (64.3) |
|
| Pathological stage
(25) |
|
|
|
|
|
I–II | 31 (45.6) | 16 (61.5) | 15 (35.7) | 0.038 |
|
III–IV | 37 (54.4) | 10 (38.5) | 27 (64.3) |
|
| Metastasis |
|
|
|
|
|
Yes | 29 (42.6) | 7 (26.9) | 22 (52.4) | 0.039 |
| No | 39 (57.4) | 19 (70.1) | 20 (47.6) |
|
Results
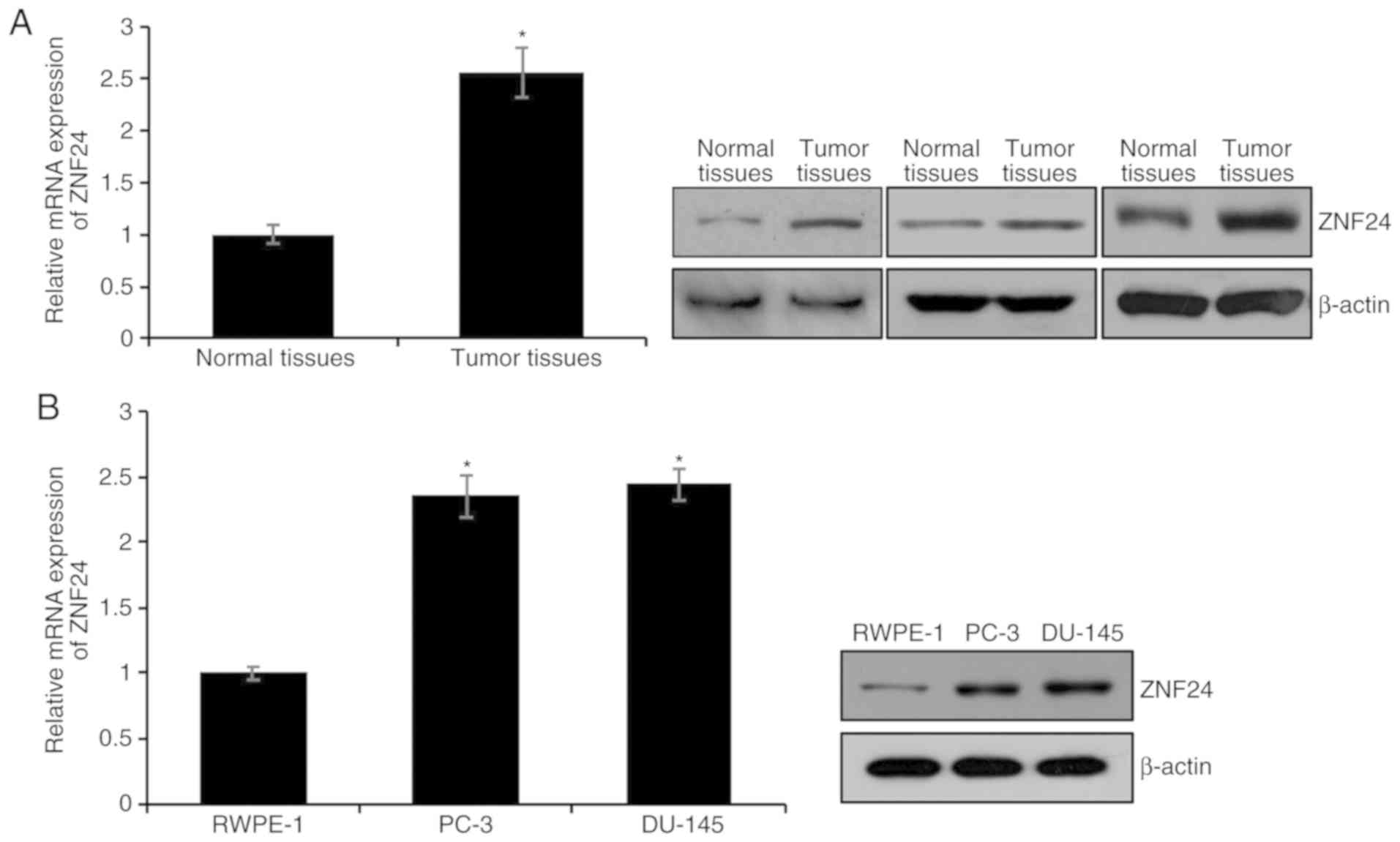
ZNF24 is upregulated in PCa tissues
and cell lines
To decipher the function of ZNF24 in PCa, the
present study first detected ZNF24 expression in 68 human PCa and
43 adjacent non-tumor tissues using RT-qPCR and western blotting
analyses. mRNA and protein expression levels of ZNF24 in tumor
tissues were markedly higher compared with those in adjacent
non-tumor tissues (P<0.05; Fig.
1A). Additionally, ZNF24 expression in PCa cell lines,
including PC-3 and DU-145, was detected, and the human normal
prostate RWPE-1 cell line served as the control. Consistent with
the results in the tissue samples, the expression levels of ZNF24
in the PCa cell lines were significantly higher than those in
RWPE-1 cells (P<0.05; Fig. 1B).
In order to further investigate the function of ZNF24 in PCa, the
association between ZNF24 expression and pathological
characteristics of patients with PCa was evaluated. ZNF24
expression was positively associated with tumor volume, Gleason
score, pathological stage and metastasis (Table I). Additionally, the present study
analyzed the expression levels of ZNF24 in prostate tissues using
data from The Human Protein Atlas database (https://www.proteinatlas.org; data not shown).
Consistent with the aforementioned findings, ZNF24 was upregulated
in PCa. These findings suggest that ZNF24 may serve an essential
role in PCa.
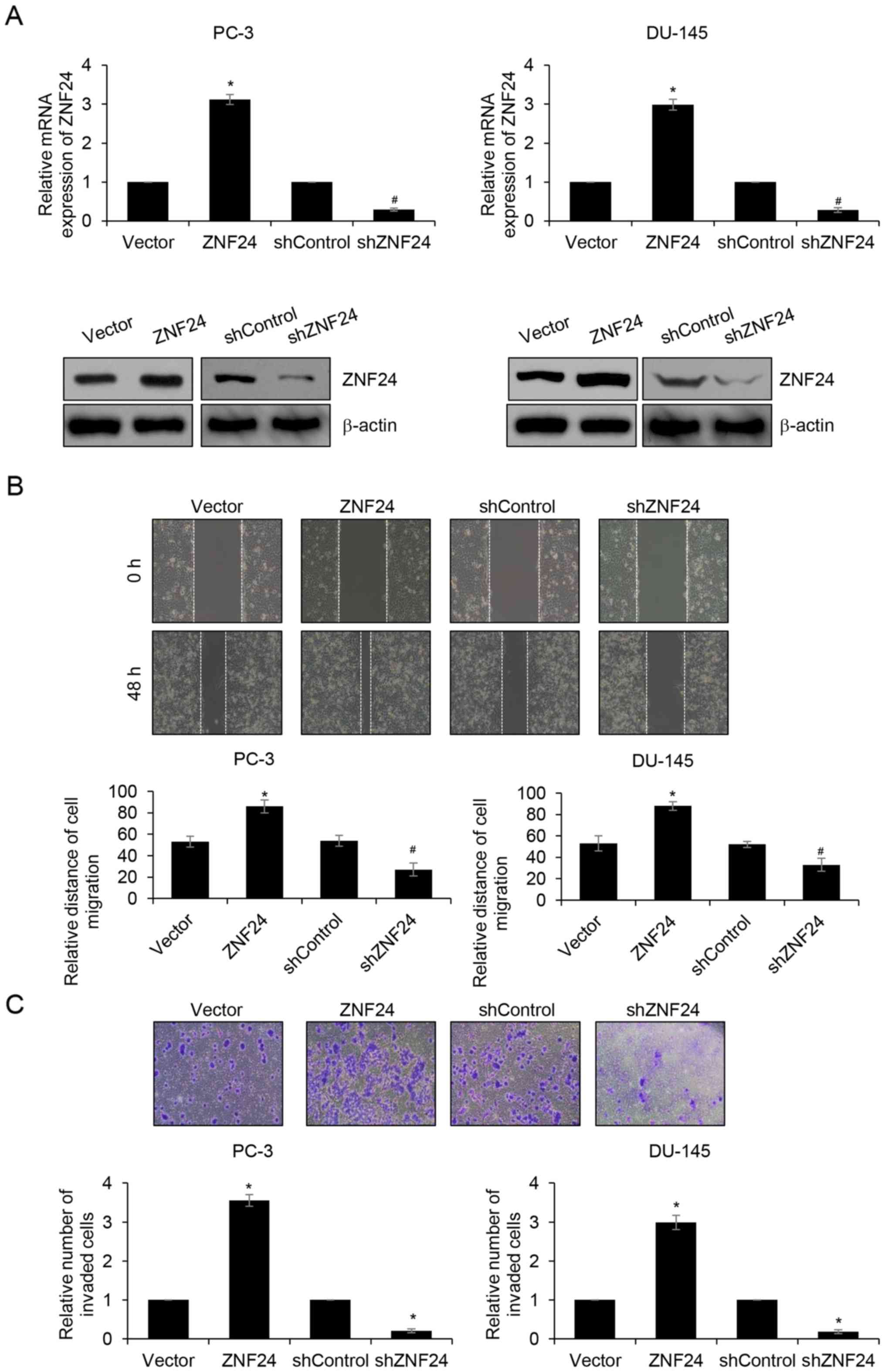
ZNF24 promotes PCa cell migration and
invasion in vitro
In order to further investigate the role of ZNF24
overexpression in carcinogenesis and progression of PCa, the
present study induced overexpression or knockdown of ZNF24 in PC-3
and DU-145 cells. The transfection efficiency of ZNF24 was
confirmed by western blotting and RT-qPCR analyses (P<0.05;
Fig. 2A). Subsequently, wound
healing and Transwell invasion assays were performed to determine
the effects of ZNF24 on cell migration and invasion in PCa. The
results of the wound healing assay indicated that overexpression of
ZNF24 markedly promoted the relative migratory distance compared
with that in the vector group; however, inhibition of ZNF24
resulted in the opposite result, and the relative migratory
distance was lower than that in shControl group (P<0.05;
Fig. 2B). Additionally, similar
results were observed in Transwell invasion assays. The numbers of
invasive cells were increased when cells overexpressed ZNF24, and
decreased when ZNF24 was knocked-down (P<0.05; Fig. 2C). Overall, the results demonstrated
that ZNF24 promoted PCa cell migration and invasion in
vitro.
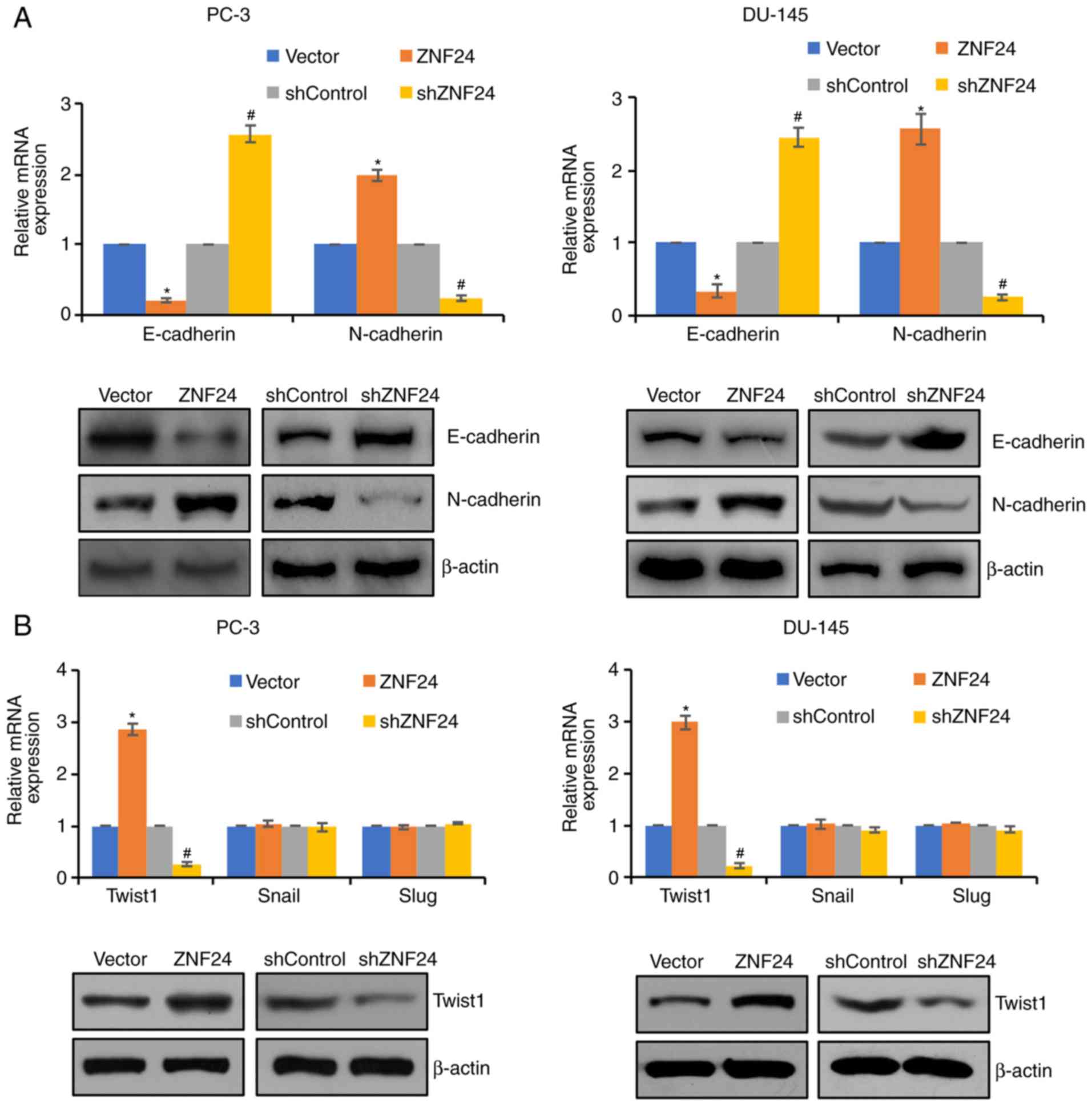
ZNF24 facilitates PCa cell invasion
and metastasis through regulation of EMT
In order to further investigate the underlying
mechanism of enhanced migration and invasion by ZNF24 in PCa, the
effect of ZNF24 on EMT was investigated. The typical EMT phenotype
is downregulation of E-cadherin and upregulation of N-cadherin
(29). The present study revealed
that overexpression of ZNF24 resulted in elevated expression of
N-cadherin and decreased expression of E-cadherin (P<0.05;
Fig. 3A). Conversely, knockdown of
ZNF24 led to the opposite result, N-cadherin expression was
decreased, and E-cadherin expression was increased (P<0.05;
Fig. 3A). Furthermore, based on the
analysis of ZNF24 expression in the prostate adenocarcinoma
database (Fred Hutchinson CRC, Nat Med 2016) (30), it was identified that ZNF24 was
upregulated in PCa. Additionally, the EMT-associated transcription
factor, Twist1, was upregulated in PCA. However, ZNF24 expression
had no effect on other EMT-associated transcription factors,
including Slug and Snail (Fig. 3B).
Therefore, ZNF24 may promote EMT through regulation of Twist1 in
PCa. These results suggest that ZNF24 facilitated PCa cell invasion
and metastasis through regulation of EMT.
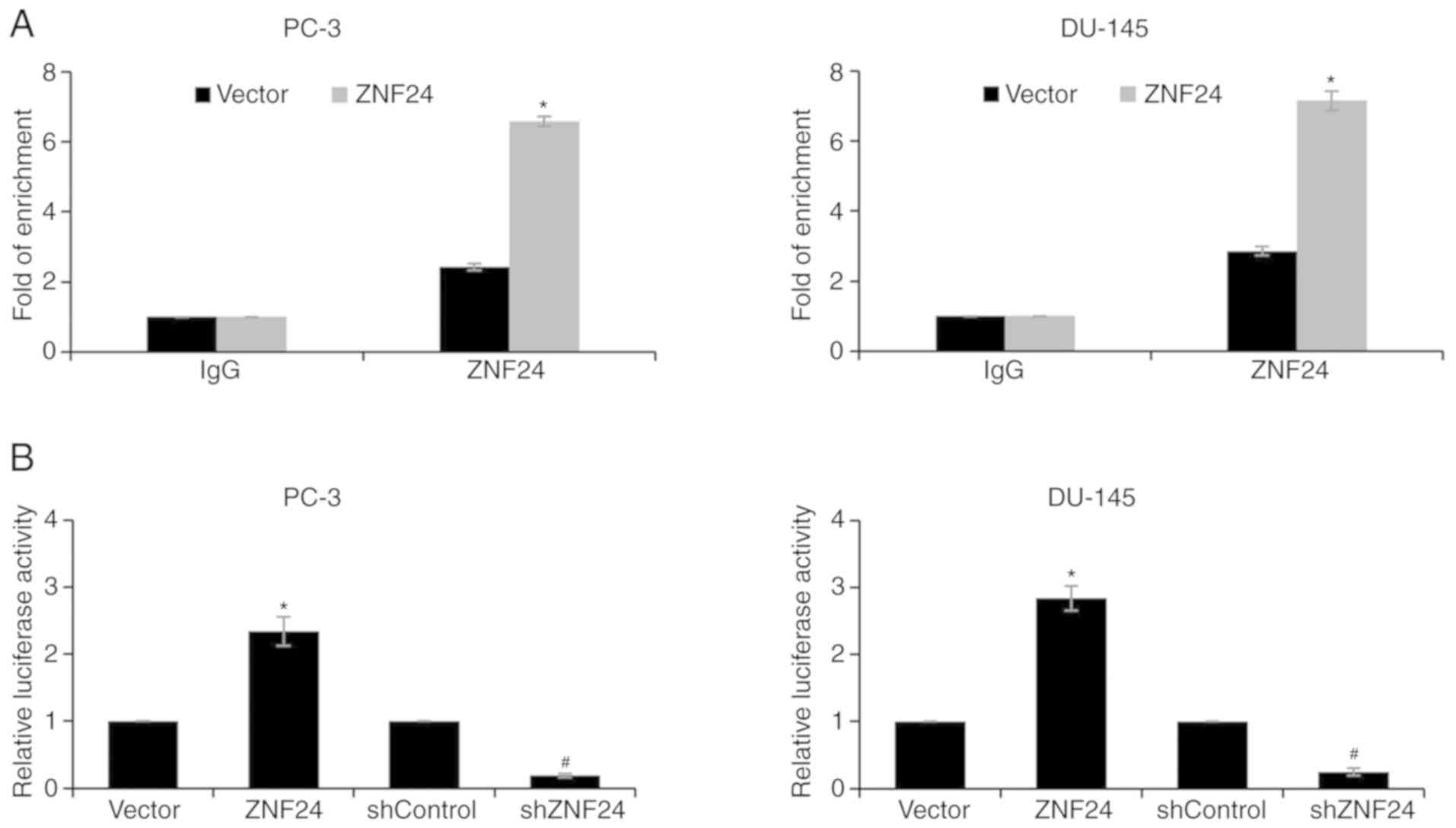
Twist1 is a target gene of ZNF24
In order to ascertain whether ZNF24 could directly
regulate Twist1 expression, ChIP and qChIP assays, and a dual
luciferase activity assay were performed. The fold of enrichment of
Twist1 was increased almost 7-fold when ZNF24 was overexpressed,
suggesting that ZNF24 could bind the promoter region of Twist1
(P<0.05; Fig. 4A). Subsequently,
a dual luciferase reporter assay was performed. The results
demonstrated that the luciferase activity was significantly
increased when ZNF24 was overexpressed, compared with the vector
group. Conversely, shZNF24 transfection resulted in decreased
luciferase activity, indicating that ZNF24 transcriptionally
activated Twist1 expression (P<0.05; Fig. 4B). The aforementioned results suggest
that Twist1 is a target gene of ZNF24.
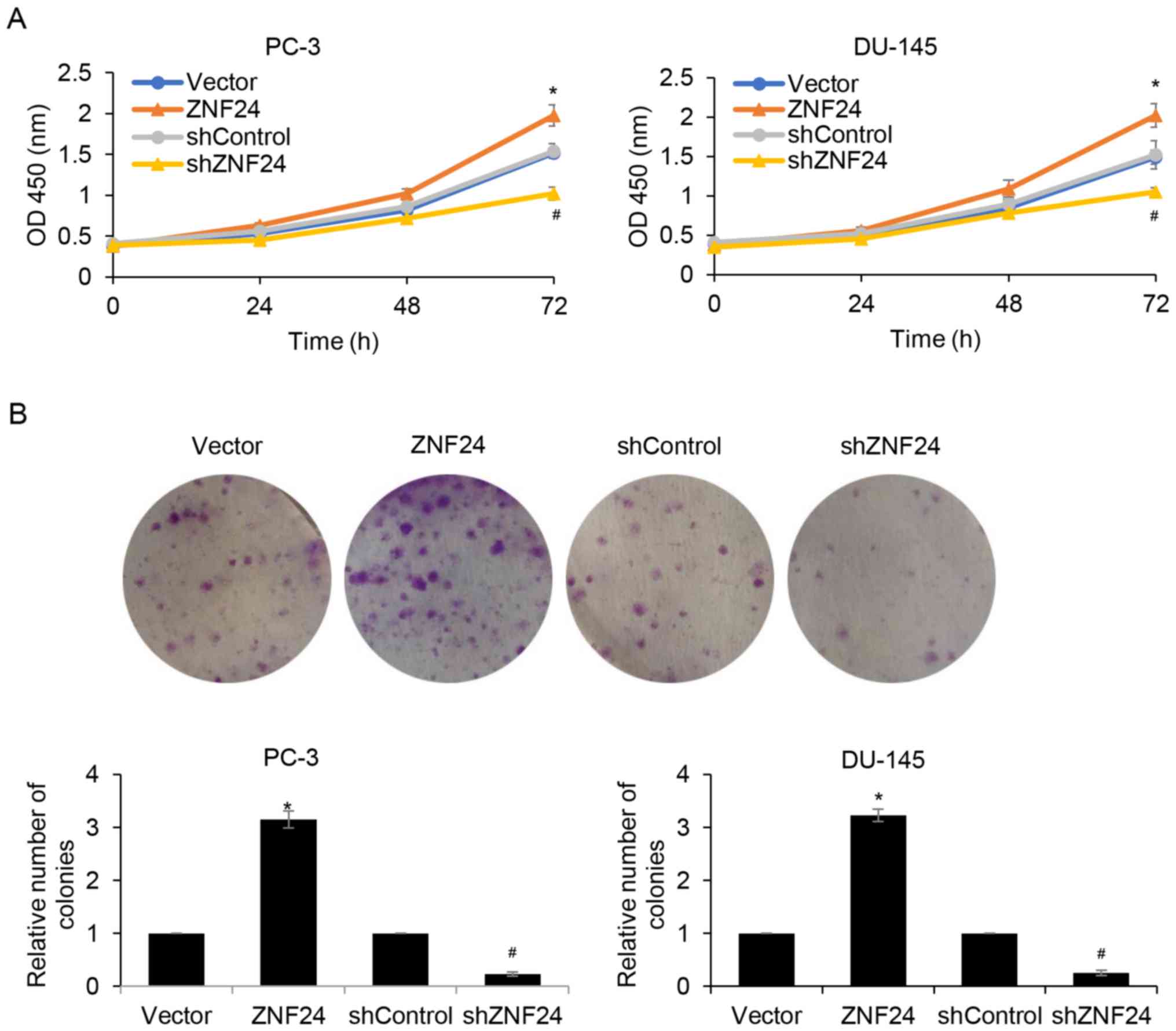
Upregulation of ZNF24 promotes PCa
cell proliferation
Since ZNF24 expression was associated with tumor
volume, it was assumed that ZNF24 may serve a role in cell
proliferation. In order to verify this hypothesis, in vitro
experiments were used to examine the effects of ZNF24 on cell
proliferation. A CCK-8 assay was used to detect cell proliferation
at 0, 24, 48 and 72 h. The cell proliferation rate was markedly
increased when ZNF24 was overexpressed compared with the vector
group (P<0.05; Fig. 5A).
Conversely, ZNF24 knockdown decreased the cell proliferation rate
(P<0.05; Fig. 5A). For the DU-145
cells, the change of proliferation rate at 24, 48 and 72 h after
culture was determined to be in conformity with the PC-3 cells
(P<0.05; Fig. 5A). Subsequently,
colony formation assays confirmed that ectopic expression of ZNF24
promoted cell proliferation and inhibition of ZNF24 suppressed cell
proliferation in PC-3 and DU-145 cells (P<0.05; Fig. 5B). These results indicated that ZNF24
promoted PCa cell proliferation.
Discussion
ZNF24, also known as ZNF191 and KOX17 (7,31),
contains four zinc finger motifs that encode putative DNA binding
domains. In previous years, multiple studies have revealed that
ZNF24 is involved in embryonic development (10,13,14) and
hematopoiesis (32), and negatively
regulates vascular endothelial growth factor (VEGF) expression
(33). In hepatocellular carcinoma,
ZNF24 directly binding to the β-catenin promoter promotes cell
proliferation (14). Liu et
al (15) reported that
microRNA-940 promotes tumor cell invasion and metastasis by
downregulating ZNF24 in gastric cancer (GC), suggesting that ZNF24
serves as a tumor suppressor in GC. The contradicting results of
previous studies indicate that ZNF24 plays different roles in
different organizations. However, to the best of our knowledge, the
detailed function of ZNF24 in PCa remains unknown.
The present study demonstrated that ZNF24 was
frequently overexpressed in human PCa tissues and cell lines.
Furthermore, the results revealed that overexpression of ZNF24
facilitated PCa cell migration and invasion in vitro through
promoting EMT. Additionally, ZNF24 could transcriptionally regulate
Twist1, a key transcription factor of the EMT process. The present
study revealed that upregulation of ZNF24 increased Twist1
expression and inhibition of ZNF24 decreased Twist1 expression in
PC-3 and DU-145 cells. Taken together, the data revealed that ZNF24
served as a novel oncogene in PCa and acted as a potential
therapeutic target. However, a previous study indicated that ZNF24
functions as a negative regulator of developmental and tumor
angiogenesis by directly binding to an 11-bp fragment of the VEGF
proximal promoter and thus inhibiting VEGF transcription (33). Additionally, ZNF24 serves as a
potential tumor suppressor and is inversely associated with
microRNA-940 expression in GC (15).
The opposing functions of ZNF24 in PCa and GC may be due to ZNF24
interacting with different proteins in different types of
cancer.
Upregulation of ZNF24 was associated with tumor
volume. Additionally, the present study revealed that ectopic
expression or inhibition of ZNF24 could promote or suppress cell
growth of the human PCa cell lines PC-3 and DU-145 in vitro.
These results demonstrated that ZNF24 may be associated with cell
proliferation of human PCa. Consistent with previous studies, ZNF24
may serve a key role in cell proliferation during embryonic
development (10,13,14).
However, there were several limitations to the
present study. First, it is preferable to perform
immunohistochemistry assays to determine the expression of ZNF24 in
PCa tissues. Furthermore, the effect of ZNF24 in vivo
requires further investigation. In addition, the upstream genes of
ZNF24 in PCa remain unknown. It would be useful to investigate
whether the stages of PCa can also influence ZNF24 expression.
There may be multiple proteins that up- or downregulate ZNF24
expression during different stages of PCa. An assay for
RNA-sequencing and transposase-accessible chromatin using
sequencing may be performed using diverse stages of PCa tissue
samples, and potential transcription factors could be predicted.
This may further decipher PCa development.
In summary, the present study demonstrated that
ZNF24 served as an oncogene in PCa. Mechanistically, ZNF24 directly
regulated Twist1 expression to promote the EMT process, and
contributed to the promotion of cell migration and invasion ability
of PCa cells. In addition, ZNF24 also promoted cell proliferation.
Therefore, the present study provided a strong rationale for ZNF24
as a therapeutic target in PCa.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XH and NL conceived and designed the study. NL and
XX performed the experiments. NL and XX wrote the paper. XH, NL and
XX reviewed the manuscript. All authors read and approved the
manuscript, and agreed to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The Ethics Committee of Shenzhen People's Hospital
approved the present study (approval no. LA2019013). All patients
provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Panebianco V, Sciarra A, Marcantonio A,
Forte V, Biondi T, Laghi A and Catalano C: Conventional imaging and
multiparametric magnetic resonance (MRI, MRS, DWI, MRP) in the
diagnosis of prostate cancer. Q J Nucl Med Mol Imaging. 56:331–342.
2012.PubMed/NCBI
|
|
3
|
Cusan L: Prostate cancer screening with
PSA, DRE and TRUS. Can J Oncol. 4 (Suppl 1):S63–S64. 1994.
|
|
4
|
Crowell PD and Goldstein AS: Functional
evidence that progenitor cells near sites of inflammation are
precursors for aggressive prostate cancer. Mol Cell Oncol.
4:e12797232017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shafique K, Proctor MJ, McMillan DC,
Qureshi K, Leung H and Morrison DS: Systemic inflammation and
survival of patients with prostate cancer: Evidence from the
glasgow inflammation outcome study. Prostate Cancer Prostatic Dis.
15:195–201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sfanos KS and De Marzo AM: Prostate cancer
and inflammation: The evidence. Histopathology. 60:199–215. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han ZG, Zhang QH, Ye M, Kan LX, Gu BW, He
KL, Shi SL, Zhou J, Fu G, Mao M, et al: Molecular cloning of six
novel Krüppel-like zinc finger genes from hematopoietic cells and
identification of a novel transregulatory domain KRNB. J Biol Chem.
274:35741–35748. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Edelstein LC and Collins T: The SCAN
domain family of zinc finger transcription factors. Gene. 359:1–17.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang H, Sun R, Liu G, Yao M, Fei J and
Shen H: Characterization of the target DNA sequence for the
DNA-binding domain of zinc finger protein 191. Acta Biochim Biophys
Sin (Shanghai). 40:704–710. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khalfallah O, Faucon-Biguet N, Nardelli J,
Meloni R and Mallet J: Expression of the transcription factor
Zfp191 during embryonic development in the mouse. Gene Expr
Patterns. 8:148–154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prost JF, Nègre D, Cornet-Javaux F, Cortay
JC, Cozzone AJ, Herbage D and Mallein-Gerin F: Isolation, cloning,
and expression of a new murine zinc finger encoding gene. Biochim
Biophys Acta. 1447:278–283. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Howng SY, Avila RL, Emery B, Traka M, Lin
W, Watkins T, Cook S, Bronson R, Davisson M, Barres BA and Popko B:
ZFP191 is required by oligodendrocytes for CNS myelination. Genes
Dev. 24:301–311. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Chen X, Yang H, Wang S, Guo B, Yu L,
Wang Z and Fu J: The zinc finger transcription factor 191 is
required for early embryonic development and cell proliferation.
Exp Cell Res. 312:3990–3998. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu G, Jiang S, Wang C, Jiang W, Liu Z,
Liu C, Saiyin H, Yang X, Shen S, Jiang D, et al: Zinc finger
transcription factor 191, directly binding to β-catenin promoter,
promotes cell proliferation of hepatocellular carcinoma.
Hepatology. 55:1830–1839. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu X, Ge X, Zhang Z, Zhang X, Chang J, Wu
Z, Tang W, Gan L, Sun M and Li J: MicroRNA-940 promotes tumor cell
invasion and metastasis by downregulating ZNF24 in gastric cancer.
Oncotarget. 6:25418–25428. 2015.PubMed/NCBI
|
|
16
|
Khalfallah O, Ravassard P, Lagache CS,
Fligny C, Serre A, Bayard E, Faucon-Biguet N, Mallet J, Meloni R
and Nardelli J: Zinc finger protein 191 (ZNF191/Zfp191) is
necessary to maintain neural cells as cycling progenitors. Stem
Cells. 27:1643–1653. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lv L, Zhang J, Wang P, Meng Q, Liang W and
Zhang L: Zinc finger protein 191 deficiency attenuates vascular
smooth muscle cell proliferation, migration, and intimal
hyperplasia after endovascular arterial injury. J Vasc Surg.
59:500–509. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial- mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thiery JP: Epithelial-mesenchymal
transitions in development and pathologies. Curr Opin Cell Biol.
15:740–746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yao D, Dai C and Peng S: Mechanism of the
mesenchymal-epithelial transition and its relationship with
metastatic tumor formation. Mol Cancer Res. 9:1608–1620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Ling MT, Guan XY, Tsao SW, Cheung
HW, Lee DT and Wong YC: Identification of a novel function of
TWIST, a bHLH protein, in the development of acquired taxol
resistance in human cancer cells. Oncogene. 23:474–482. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Epstein JI, Egevad L, Amin MB, Delahunt B,
Srigley JR and Humphrey PA; Grading Committee, : The 2014
international society of urological pathology (ISUP) consensus
conference on gleason grading of prostatic carcinoma: Definition of
grading patterns and proposal for a new grading system. Am J Surg
Pathol. 40:244–252. 2016.PubMed/NCBI
|
|
25
|
Buyyounouski MK, Choyke PL, McKenney JK,
Sartor O, Sandler HM, Amin MB, Kattan MW and Lin DW: Prostate
cancer-major changes in the American joint committee on cancer
eighth edition cancer staging manual. CA Cancer J Clin. 67:245–253.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roy R, Rodig S, Bielenberg D, Zurakowski D
and Moses MA: ADAM12 transmembrane and secreted isoforms promote
breast tumor growth: A distinct role for ADAM12-S protein in tumor
metastasis. J Biol Chem. 286:20758–20768. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang J, Bielenberg DR, Rodig SJ, Doiron R,
Clifton MC, Kung AL, Strong RK, Zurakowski D and Moses MA:
Lipocalin 2 promotes breast cancer progression. Proc Natl Acad Sci
USA. 106:3913–3918. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kumar A, Coleman I, Morrissey C, Zhang X,
True LD, Gulati R, Etzioni R, Bolouri H, Montgomery B, White T, et
al: Substantial interindividual and limited intraindividual genomic
diversity among tumors from men with metastatic prostate cancer.
Nat Med. 22:369–378. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rousseau-Merck MF, Huebner K, Berger R and
Thiesen HJ: Chromosomal localization of two human zinc finger
protein genes, ZNF24 (KOX17) and ZNF29 (KOX26), to 18q12 and
17p13-p12, respectively. Genomics. 9:154–161. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Noll L, Peterson FC, Hayes PL, Volkman BF
and Sander T: Heterodimer formation of the myeloid zinc finger 1
SCAN domain and association with promyelocytic leukemia nuclear
bodies. Leuk Res. 32:1582–1592. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Harper J, Yan L, Loureiro RM, Wu I, Fang
J, D'Amore PA and Moses MA: Repression of vascular endothelial
growth factor expression by the zinc finger transcription factor
ZNF24. Cancer Res. 67:8736–8741. 2007. View Article : Google Scholar : PubMed/NCBI
|