Introduction
Cisplatin is an important chemotherapy drug that was
approved by the Food and Drug Administration 40 years ago (1). It has since been widely used in the
clinical treatment of various types of solid tumor including
breast, bladder, head and neck, lung, ovarian and testicular
cancers (2–4). However, its use has been limited due to
inherent and acquired resistance (5).
The pathways involved in the acquired cisplatin
resistance are complex. The main resistance mechanisms include DNA
damage repair and cell cycle interference (6), mitochondrial dysfunction (7), inhibition of cell apoptosis (8), formation of an anoxic microenvironment
(9) and epithelial-mesenchymal
transition (10). Numerous
strategies can be used to avoid cisplatin resistance by influencing
the molecular mechanisms of resistance in tumor cells. These
strategies include the development of novel platinum antitumor
drugs, the promotion of platinum-based drug transport into tumor
cells, the development of inhibitors that regulate the mechanisms
of resistance, and the synergistic use of cisplatin with other
drugs presenting specific and targeted effects on tumor cells
(11,12).
SET and MYND domain containing 3 (SMYD3) is a
protein that catalyzes the methylation of histones at H3K4, H4K5
and H4K20, and which elicits oncogenic effects by activating the
transcription of downstream target genes in hepatocellular,
colorectal, cervical and breast cancers (13). For example, SMYD3 can promote
epithelial-mesenchymal transition (14), interfere with the cell cycle
(15), promote cell proliferation
(16), increase the activity of
telomerase and promote cell immortalization (17). These processes are also closely
associated with chemotherapy-resistance (18). In addition, SMYD3 deficiency induces
DNA-damage hypersensitivity, decreases the number of repair foci
and leads to impaired homologous recombination, all of which are
also associated with chemotherapy resistance (19). At present, research around SMYD3
signaling has mainly focused on its downstream pathways. Studies
have found that hepatitis C virus core proteins can inactivate
miR-124 through DNA methylation, thereby up-regulating SMYD3, while
miR-124 can promote cancer cell sensitivity to cisplatin (20–22). In
addition, previous studies from our laboratory demonstrated that
SMYD3 can increase cellular resistance to dexamethasone (23) and regulate the ATM-CHK2/p53-cdc25c
pathway (15), suggesting that SMYD3
may also be involved in the development of chemotherapy resistance.
However, the direct effect of cisplatin on SMYD3 and the underlying
mechanisms are unclear. Therefore, the present study assessed these
issues. Research on this topic is of vital importance, and may
serve the development of novel treatment strategies that would
overcome cisplatin resistance and allow the clinical treatment of
tumors.
Materials and methods
Cell lines and transfection
MCF-7 and T47D human breast cancer cells (The Cell
Bank of Type Culture Collection of the Chinese Academy of Sciences)
were cultured in RPMI 1640 medium (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% fetal bovine serum (Hangzhou Sijiqing
Biological Engineering Materials Co., Ltd.), 100 U/ml penicillin G
and 0.1 mg/ml streptomycin and placed at 37°C in a humidified
incubator containing 5% CO2. The overexpression plasmid
of SMYD3 was a gift from Professor Philip Tucker (Institute for
Cellular and Molecular Biology, University of Texas, Austin, TX,
USA), and the empty vector was used as a control. The small
interfering (si) RNAs were synthesized by Guangzhou RiboBio Co.,
Ltd. The sequences of these siRNAs were as follows: siRNA targeting
SMYD3 (si-SMYD3), sense, 5′-CAAGGAUGCUGAUAUGCUAdTdT-3′ and
antisense, 3′-dTdTGUUCCUACGACUAUACGAU-5′. The sequence of the
control siRNA was 5′-ACTGTTCTATGACTTGTCGTGAATA-3′. Transient
transfection was performed using TurboFect reagent (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Briefly, 1 µg plasmid or siRNA were transfected into MCF-7 or T47D
cells with 2 µl TurboFect reagent. Cells were collected 24 or 48 h
later for subsequent testing.
Reverse transcription quantitative
(RT-q)PCR and semi-qPCR
Total RNA was isolated from MCF-7 and T47D cells
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
under RNase-free conditions. After quantification of RNA using a
photometer, cDNA was synthesized using M-MLV reverse transcriptase
(Invitrogen, Thermo Fisher Scientific, Inc.) from 2 µg total RNA,
according to the manufacturer's instructions.
For semi-qPCR, primers specific for the cDNAs of the
SMYD3 gene and the constitutive GAPDH gene were used (Genewiz
Inc.). For SMYD3, the forward primer positions were 647–670
(5′-CCCAGTATGTCTTTGCTGAATCAC-3′) and the reverse primer positions
were 935–956 (5′-ACTTCCAGTGCGCCTTCAGCTC-3′). For GAPDH, the forward
primer positions were 217–236 (5′-ATTCAACGGCACAGTCAAGG-3′) and the
reverse primer positions were 411–429 (5′-GCAGAAGGGGCGGAGATGA-3′).
The PCR reactions were as follows: 95°C for 5 min, then 30 cycles
at 95°C for 1 min, at 56°C for 1 min, and at 72°C for 30 sec;
extension was carried out at 72°C for 10 min. PCR products were
electrophoretically separated by 1.5% agarose gels and expression
observed using ethidium bromide staining. The densities of the
bands were analyzed using Gel/Chemi Doc (Bio-Rad Laboratories,
Inc.) and quantified using Quantity One software v 4.6.6 (Bio-Rad
Laboratories, Inc.).
qPCR was performed using Fast SYBR Green Master Mix
(Applied Biosystems; Thermo Fisher Scientific Inc.) and detected
using an ABI Step One system (Thermo Fisher Scientific, Inc.). The
sequences of the primers (Invitrogen; Thermo Fisher Scientific,
Inc.) were as follows: GAPDH forward, 5′-ATTCAACGGCACAGTCAAGG-3′
and reverse, 5′-GCAGAAGGGGCGGAGATGA-3′; SMYD3 forward,
5′-CCCAGTATCTCTTTGCTCAATCAC-3′ and reverse,
5′-ACTTCCAGTGTGCCTTCAGTTC-3′; miR-124 forward,
5′-ACACTCCAGCTGGGTAAGGCACGCGG-3′ and reverse,
5′-TGGTGTCGTGGAGTCG-3′. qPCR was performed as follows: 95°C for 2
min, followed by 40 cycles of 95°C for 10 sec, 65°C for 30 sec and
72°C for 30 sec. The 2−ΔΔCq method was used to calculate
relative transcription levels and all experiments were repeated at
least three times (24).
Western blotting
A total of 48 h after transfection, MCF-7 and T47D
cells were washed twice with ice-cold PBS and lysed at 4°C with
RIPA lysis buffer containing protease and phosphatase inhibitors
(cat. no. R0010; Beijing Solarbio Science & Technology Co.,
Ltd.) for 30 min. The lysates were centrifuged at 17,000 × g for 10
min, the supernatants were quantified using the BCA protein
concentration assay kit (Beyotime Institute of Biotechnology) and
mixed with SDS sample buffer. Subsequently, proteins (50 µg/lane)
were separated using 12% SDS-PAGE and transferred onto
nitrocellulose membranes. Membranes were blocked using 5% non-fat
dry milk dissolved in PBS for 90 min at 20°C. Membranes were then
incubated with primary antibodies against SMYD3 (rabbit anti-human
monoclonal antibody; 1:1,000; cat. no. ab187149; Abcam) and GAPDH
(mouse anti-human; dilution; 1:5,000; cat. no. sc-365402; Santa
Cruz) at 4°C overnight. Subsequently, membranes were incubated with
infrared fluorescent goat anti-rabbit (1:10,000; cat. no.
926-68071; LI-COR Biosciences;) and goat anti-mouse (1:10,000; cat.
no. 926-32210; LI-COR Biosciences;) secondary antibodies for 2 h in
the dark at room temperature. Signals were visualized using an
Odyssey Imaging System (LI-COR Biosciences).
Luciferase reporter assay
The SMYD3 3′-untranslated region (3′UTR) was
subcloned into pGL3 Basic vector (Promega Corporation). MCF-7 or
T47D cells were seeded into 24-well plates at a density of
1×108 cells/ml and then treated with different
concentrations of cisplatin (0, 2, 4, 8, 16, 32 or 64 µM) for 24 h
and transfected with pGL3-SMYD3 3′UTR and pEGFP-C3 plasmids using
TurboFect reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. The luciferase activity was measured
using the Luciferase assay system (Promega Corporation) according
to the manufacturer's instructions. The values were normalized to
the signal of enhanced green fluorescent protein and the 0 µM
cisplatin group was used as control group (25).
MTT assay
MCF-7 and T47D cells were seeded into 96-well plates
at the density of 5×104 cells/ml. After serum-free
medium starvation for 24 h, cells were transfected with siRNA or
overexpression SMYD3 and corresponding controls as aforementioned.
Cells transfected with overexpression or small interference SMYD3
and co-treated with cisplatin for 24, 48 and 72 h were incubated
with 5 mg/ml MTT (Beijing Solarbio Science & Technology Co.,
Ltd.) at 37°C for 4 h. Subsequently, medium was removed, 150 µl
DMSO was added in each well to dissolve formazan crystals and
optical density (OD) was measured at 570 nm using a Synergy4
microplate reader (BioTek Instruments, Inc.; Agilent Technologies,
Inc.). Cell viability was calculated as follows: Cell viability
(%)=[OD (treated)-OD (blank)]/[OD (untreated)-OD (blank)] ×100.
Each sample was examined in duplicate, and the experiment was
repeated three times.
Clonogenic assay
Following transfection with siRNA or treatment with
0, 2, 4, 8, 16 and 32 µM cisplatin at 37°C for 24 h, cells were
seeded in a 6-well plate at a density of 1,000 cells/well. Cells
were subsequently cultured for 10 days at 37°C to obtain visible
colonies. Cells were washed twice with PBS, fixed with methanol for
15 min at room temperature and stained with Giemsa for 30 min at
room temperature. An inverted fluorescence microscope, ECLIPSE
TE2000 (20×) was used to count colonies containing at least 50
cells (Nikon Corporation).
Analysis of cell mitochondrial
membrane potential
Previous studies demonstrated that cisplatin can
promote apoptosis by reducing mitochondrial membrane potential
(26,27). In the present study, MCF-7 cells were
treated with 0 or 8 µM cisplatin and/or siRNA targeting SMYD3 mRNA
for 48 h at 37°C. Then cells were harvested with trypsin and
resuspended in medium at a density of 1×106 cells/ml.
Subsequently, cells were mixed with 50 µg/ml rhodamine 123 dye
solution (Sigma-Aldrich; Merck KGaA), which is a positively charged
pigment that can bind to mitochondria with a high negative membrane
potential (28). After being
incubated at 37°C and 5% CO2 for 30 min. Cells were
collected by centrifugation at 1,700 × g for 10 min at room
temperature, washed twice with PBS and analyzed in the Accuri C6
flow cytometer (Becton Dickinson Biosciences, USA) using FlowJo v
10.2 (FlowJo LLC).
Statistical analysis
Data are presented as the means ± standard deviation
(mean ± SD). Significant differences were evaluated using two-way
ANOVA. Statistical analyses were performed using GraphPad Prism
version 5.00 (GraphPad Software, Inc.). All experiments were
carried out at least three times. P<0.05 was considered to
indicate a statistically significant difference.
Results
Cisplatin sensitivity may be inversely
related to endogenous SMYD3 expression
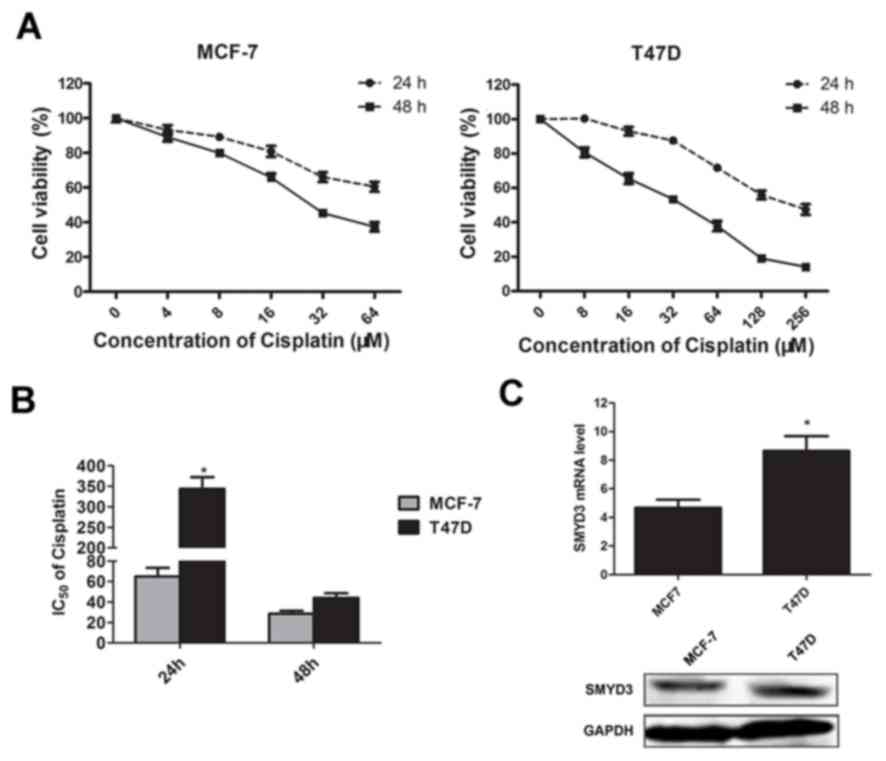
MTT assays were performed to assess the effect of
cisplatin on MCF-7 and T47D cell viability after 24 and 48 h
treatment. As presented in Fig. 1A,
following cisplatin treatment, viability of T47D cells was lower
compared with MCF-7 cells. This indicates that MCF-7 cells may be
more sensitive to cisplatin. Furthermore, the expression of SMYD3
in these two cell lines was further investigated by RT-qPCR and
western blotting. The results demonstrated that both mRNA and
protein level of SMYD3 in the T74D cells were higher compared with
MCF-7 cells (Fig. 1C). To
quantitatively assess the effects of cisplatin on these two cell
lines, the half maximal inhibitory concentration (IC50)
values of cisplatin were calculated. As presented in Fig. 1B, following 24 h of cisplatin
treatment, the observed IC50 values for MCF-7 and T47D
cells were 62.13±3.58 and 342.76±17.66 µM, respectively. These
results indicated that the differential expression of SMYD3
observed may be associated with the different cisplatin
sensitivities of these tumor cell lines.
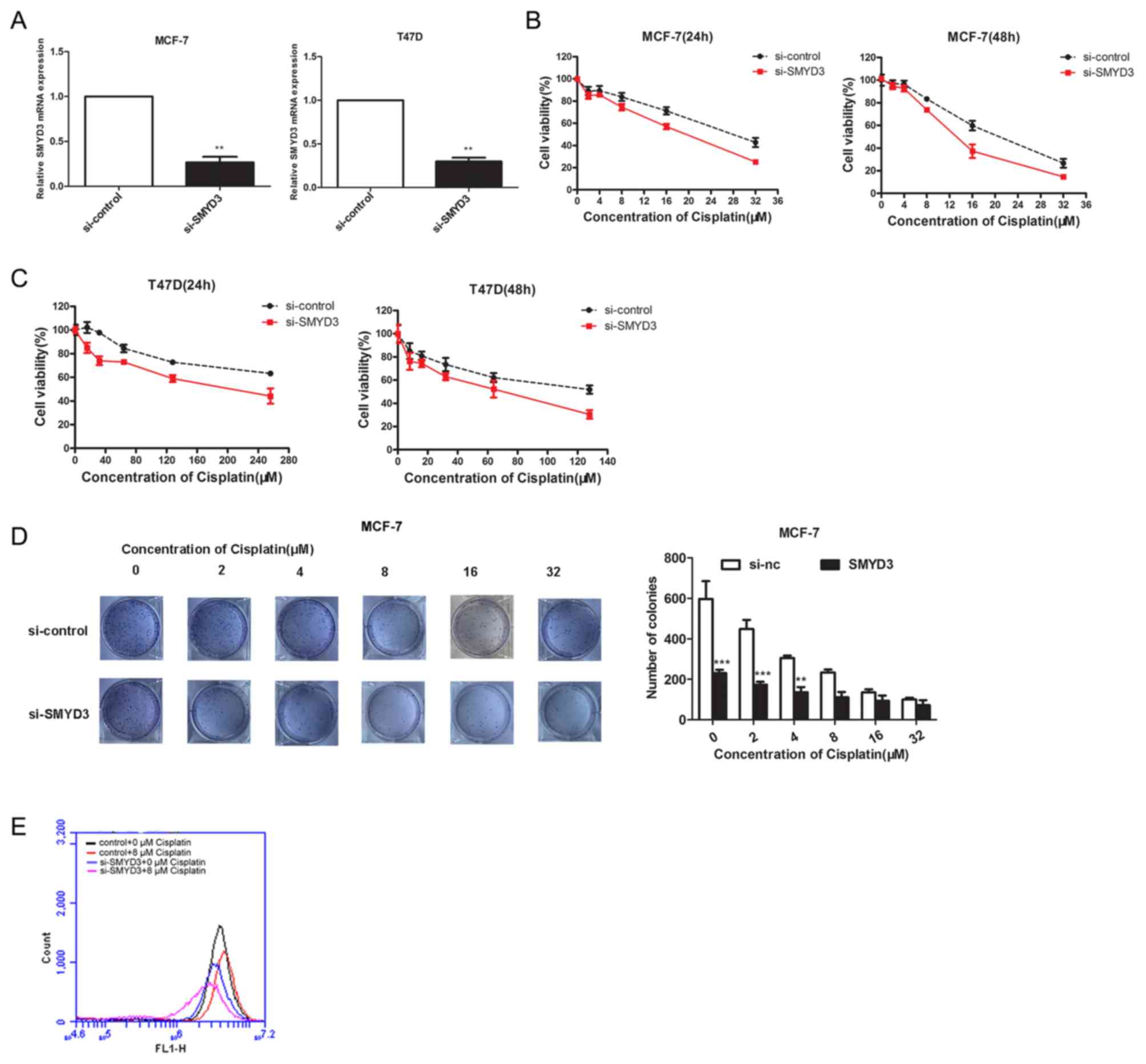
SMYD3 knockdown increases cisplatin
sensitivity in tumor cells
SMYD3 is often highly expressed in cancer tissues
and is closely associated with malignancy and poor prognosis of
patients (29,30). However, the association between SMYD3
expression, chemotherapy and its outcomes remains unclear. In the
present study, SMYD3 was significantly knocked down by siRNA
transfection in MCF-7 and T47D cell lines (Fig. 2A), and MTT and colony-formation
assays were performed to assess the cisplatin sensitivity in these
two cell lines. The results demonstrated that SMYD3 knockdown
significantly reduced the cell viability following cisplatin
treatment (Fig. 2B and C),
suggesting that SMYD3 may be involved in the development of cell
resistance to cisplatin. To verify this hypothesis, further
colony-formation assays were performed using MCF-7 cells (Fig. 2D) and the sensitivity of these cells
to cisplatin was enhanced after SMYD3 knockdown. In addition, to
investigate whether SMYD3 can affect the effect of cisplatin on the
mitochondrial membrane potential of cells, rhodamine 123 staining
was performed. As presented in Fig.
2E, SMYD3 knockdown decreased the mitochondrial membrane
potential, which was enhanced in the presence of cisplatin. These
results demonstrated that SMYD3 knockdown and cisplatin treatment
may cooperatively promote the loss of the mitochondrial membrane
potential in cancer cells.
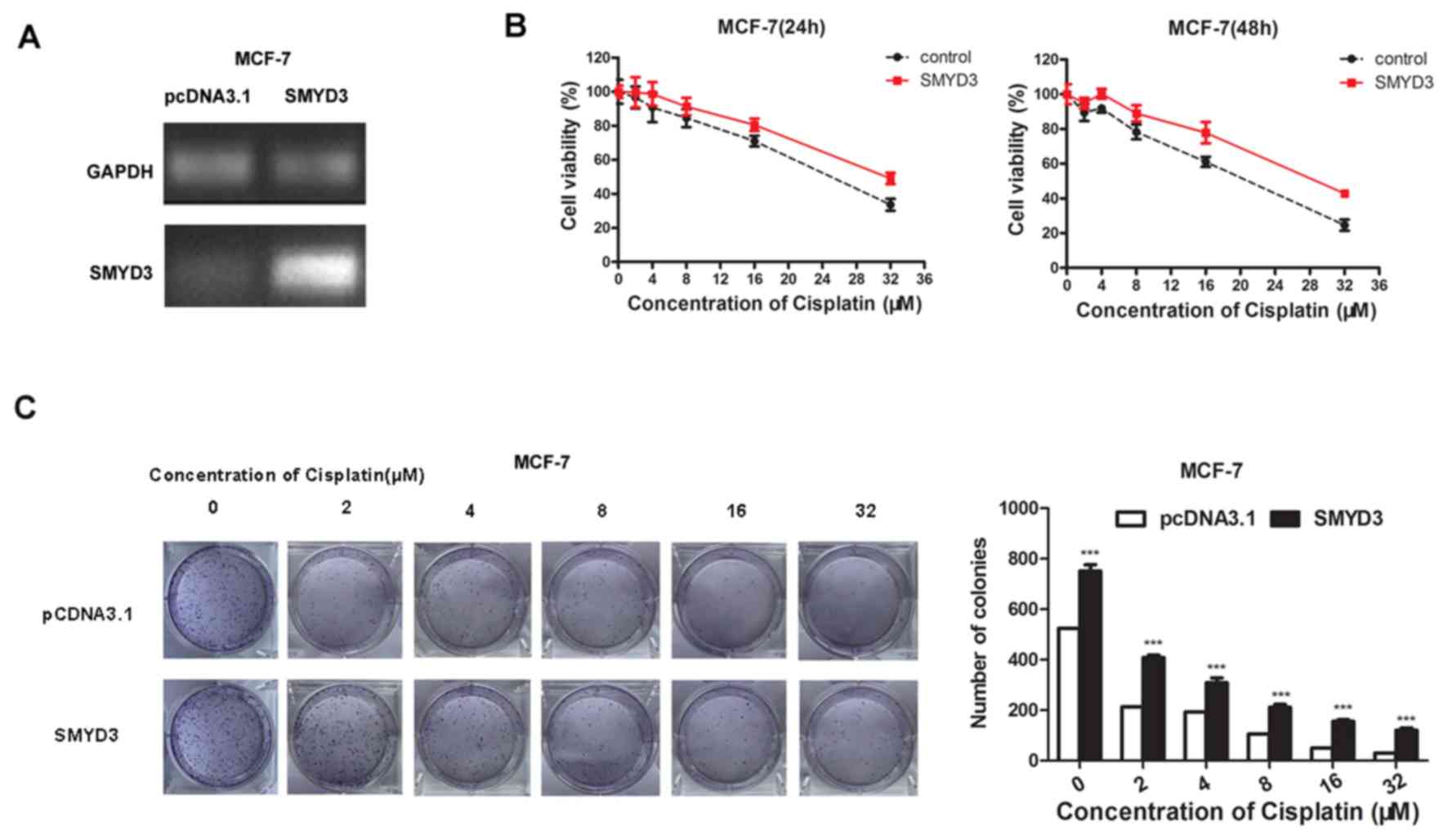
SMYD3 overexpression reduces cisplatin
sensitivity
MCF-7 cells were successfully transfected with the
overexpression plasmid of SMYD3 or a control empty plasmid.
(Fig. 3A). Following transfection
for 24 h, SMYD3-overexpressing cells exhibited increased cell
viability and increased tolerance to cisplatin compared with
control (Fig. 3B). Similar results
were also observed following transfection of cells with the SMYD3
overexpression plasmid for 48 h (Fig.
3B). The results of the colony-formation assay also
demonstrated that overexpression of SMYD3 could significantly
increase the tolerance of cells to cisplatin (Fig. 3C).
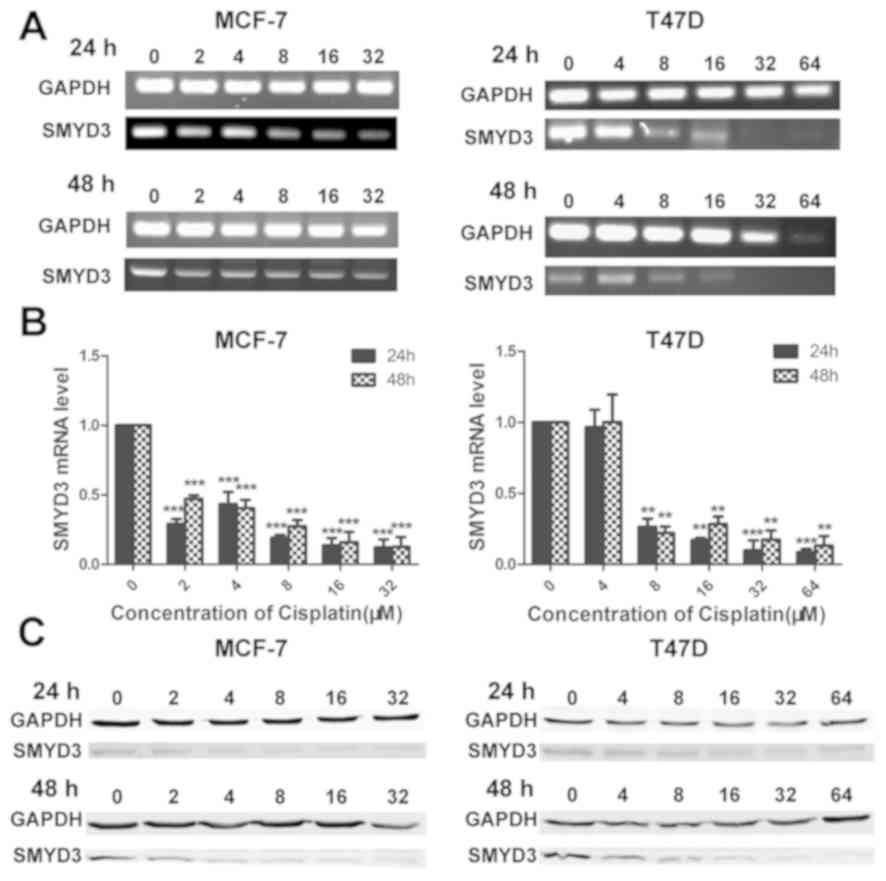
Cisplatin inhibits SMYD3
expression
The aforementioned results demonstrated that cell
sensitivity to cisplatin may be associated with SMYD3 expression.
To determine the underlying mechanisms of cisplatin-mediated
cytotoxicity, MCF-7 and T47D cells were treated with cisplatin at
different concentrations for 24 or 48 h, and SMYD3 expression level
was assessed by RT-PCR and western blotting. Following treatment
with cisplatin for 24 and 48 h, SMYD3 mRNA level was significantly
downregulated, suggesting that cisplatin can inhibit the
transcription of SMYD3 (Fig. 4A and
B). These results of western blotting analysis also
demonstrated that SMYD3 expression was decreased following
cisplatin treatment in a concentration-dependent manner (Fig. 4C).
miR-124 may be involved in the
cisplatin-mediated inhibition of SMYD3 expression
Since miRNAs can combine with the 3′UTRs of mRNAs to
inhibit transcription (31). In the
present study, a luciferase reporter assay was used to test whether
cisplatin-mediated inhibition of SMYD3 expression may occur via the
regulation of a specific miRNA that mediates SMYD3 transcription.
This assay assessed the transcriptional activity of SMYD3 3′UTR
following treatment with different concentrations of cisplatin
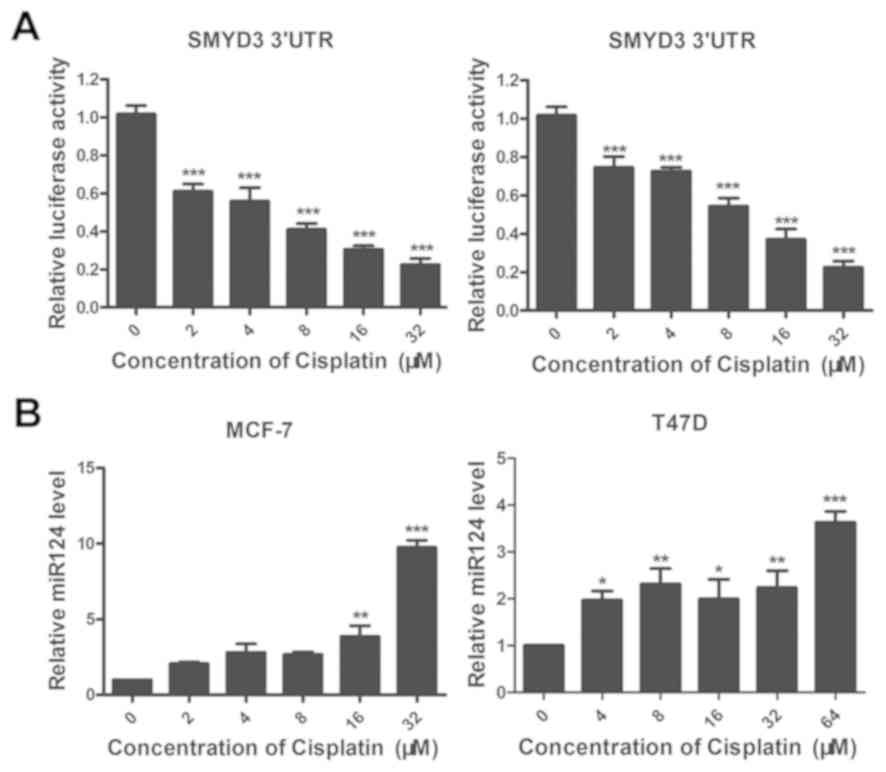
(Fig. 5). Following cisplatin
treatment, the luciferase activity of the SMYD3 3′UTR was
significantly downregulated (Fig.
5A), suggesting that cisplatin may inhibit SMYD3 expression by
upregulating the transcription of a miRNA that could promote the
degradation of SMYD3 mRNA.
As previous studies reported that miR-124 is an
upstream regulator of SMYD3 (20,32), the
present study hypothesized that miR-124 may be involved in the
inhibitory effect of cisplatin on the expression of SMYD3. To
verify this hypothesis, MCF-7 and T47D cells were treated for 24 h
with different concentrations of cisplatin and the level of miR-124
was assessed by RT-qPCR. For both cell lines, miR-124 level was
significantly increased following cisplatin treatment in
concentration-dependent manner in MCF-7 and T47D cell lines,
respectively (Fig. 5B), suggesting
that cisplatin may inhibit SMYD3 expression by upregulating
miR-124.
Discussion
The present study demonstrated that SMYD3 expression
may affect cell sensitivity to cisplatin, SMYD3 overexpression or
knockdown could decreased or enhanced the sensitivity of MCF-7 and
T47D cells to cisplatin. The results demonstrated that the use of
SMYD3-targeting siRNA may reduce cell resistance to cisplatin,
providing therefore a novel approach to the treatment of
cancer.
Cisplatin causes cytotoxicity by inducing apoptosis,
which involves the induction of exogenous and endogenous death
receptors through mitochondrial pathways (33). The main mechanism of cisplatin
resistance involves the inactivation of apoptotic protein p53
(34). Dai et al (35) reported that SMYD3 regulates p53
protein expression, which is essential in SMYD3-induced glioma cell
proliferation. MAPK family members are also involved in the
cisplatin resistance mechanism. The N-terminal kinases of c-JUN and
MAPK1 cannot be activated in cisplatin-resistant cells, leading to
an inability to initiate the FAS signaling pathway and therefore
cell apoptosis (36). In addition,
previous studies demonstrated that SMYD3-mediated MAP3K2
methylation prevents MAP3K2 from binding to the protein phosphatase
2, inhibiting the effect of this negative regulator on Ras-ERK1/2
signals, which could lead to the development of lung and pancreatic
adenocarcinomas (37,38). The results from the present study
demonstrated that the combination of SMYD3 knockdown and
cisplatin-mediated SMYD3 expression inhibition may promote the
mitochondrial membrane potential collapse and subsequently decrease
the viability of MCF-7 cells.
In the present study, cisplatin treatment could
downregulate SMYD3 expression and inhibit the transcriptional
regulatory activity of the SMYD3 3′UTR. Specific interactions
between miRNAs and 3′UTRs are known to promote the degradation of
target mRNA (31). Furuta et
al (21) reported that miR-124
can inhibit the growth of cancer by targeting SMYD3. Furthermore,
it has been reported that miR-124 can promote cancer cell
sensitivity to cisplatin (22), and
the findings of the present study demonstrated that the expression
of SMYD3 has a negative regulatory relationship with miR-124. In
addition, sulforaphane can enhance the effects of cisplatin by
activating miR-124 (39). Our
previous study also demonstrated that SMDY3 may have a crucial role
in the anti-tumor effect of sulforaphane (15). Taken together, these findings
suggested that SMYD3 may impair cell sensitivity to cisplatin, and
that miR-124 may serve a crucial role in this process. These
results may help future investigation on SMYD3 expression
interference by cisplatin, contribute to the development of
therapeutic options to circumvent cisplatin resistance and
therefore allow cancer treatment.
Acknowledgements
Not applicable.
Funding
The current study was supported by the National
Natural Science Foundation of China (grant nos. 31470816 and
31300642), the Natural Science Foundation of Tianjin (grant no.
18JCZDJC33800) and the Young Teachers' Innovation Fund of Tianjin
University of Science and Technology (grant no. 2016LG06).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
LW, XL, NW, HH and TZ conceived and designed the
experiments. LW, MX and CW performed the experiments and analyzed
the data, and were major contributors in writing the manuscript.
QD, ZM and XC provided technical guidance and participated in data
acquisition and analysis. All the authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rosenberg B: Cisplatin: Its history and
possible mechanisms of action. Prestayko AW, Crooke ST and Carter
SK: Cisplatin: Current Status and New Developments. Academic Press;
NYC: pp. 9–21. 1980, View Article : Google Scholar
|
|
2
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Joss RA, Bürki K, Dalquen P, Schatzmann E,
Leyvraz S, Cavalli F, Ludwing C, Siegenthaler P, Allberto P, Stahel
R, et al: Combination chemotherapy with mitomycin, vindesine, and
cisplatin for non-small cell lung cancer association of antitumor
activity with initial tumor burden and treatment center. Cancer.
65:2426–2434. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Garutti M, Pelizzari G, Bartoletti M,
Malfatti MC, Gerratana L, Tell G and Puglisi F: Platinum salts in
patients with breast cancer: A focus on predictive factors. Int J
Mol Sci. 20:E33902019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eljack ND, Ma HY, Drucker J, Shen C,
Hambley TW, New EJ, Friedrich T and Clarke RJ: Mechanisms of cell
uptake and toxicity of the anticancer drug cisplatin. Metallomics.
6:2126–2133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li C, Liu M, Yan A, Liu W, Hou J, Cai L
and Dong X: ERCC1 and the efficacy of cisplatin in patients with
resected non-small cell lung cancer. Tumour Biol. 35:12707–1212.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cocetta V, Ragazzi E and Montopoli M:
Mitochondrial Involvement in Cisplatin Resistance. Int J Mol Sci.
20:E33842019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bao A, Li Y, Tong Y, Zheng H, Wu W and Wei
C: 1,25-Dihydroxyvitamin D3 and cisplatin
synergistically induce apoptosis and cell cycle arrest in gastric
cancer cells. Int J Mol Med. 33:1177–1184. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hrzenjak A, Fischer C, Leithner K,
Wohlkoenig C, Quehenberger F, Olschewski A and Olschewski H: 838:
Panobinostat reduces hypoxia-related cisplatin resistance of
non-small cell lung carcinoma cells via HIF-1alpha destabilization.
Eur J Cancer. 50 (Suppl 5):S203–S204. 2014. View Article : Google Scholar
|
|
10
|
Latifi A, Abubaker K, Castrechini N, Ward
AC, Liongue C, Dobill F, Kumar J, Thompson EW, Quinn MA, Findlay JK
and Ahmed N: Cisplatin treatment of primary and metastatic
epithelial ovarian carcinomas generates residual cells with
mesenchymal stem cell-like profile. J Cell Biochem. 112:2850–2864.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sahu BD, Kalvala AK, Koneru M, Mahesh
Kumar J, Kuncha M, Rachamalla SS and Sistla R: Ameliorative effect
of fisetin on cisplatin-induced nephrotoxicity in rats via
modulation of NF-κB activation and antioxidant defence. PLoS One.
9:e1050702014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liao HY, Wang GP, Gu LJ, Huang SH, Chen
XL, Li Y and Cai SW: HIF-1α siRNA and cisplatin in combination
suppress tumor growth in a nude mice model of esophageal squamous
cell carcinoma. Asian Pac J Cancer Prev. 13:473–477. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hamamoto R, Silva FP, Tsuge M, Nishidate
T, Katagiri T, Nakamura Y and Furukawa Y: Enhanced SMYD3 expression
is essential for the growth of breast cancer cells. Cancer Sci.
97:113–118. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zou JN, Wang SZ, Yang JS, Luo XG, Xie JH
and Xi T: Knockdown of SMYD3 by RNA interference down-regulates
c-Met expression and inhibits cells migration and invasion induced
by HGF. Cancer Lett. 280:78–85. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang L, Wang QT, Liu YP, Dong QQ, Hu HJ,
Miao Z, Li S, Liu Y, Zhou H, Zhang TC, et al: ATM signaling pathway
is implicated in the SMYD3-mediated proliferation and migration of
gastric cancer cells. J Gastric Cancer. 17:295–305. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu P, Chu GC, Zhu G, Yang H, Luthringer D,
Prins G, Habib F, Wang Y, Wang R, Chung LW and Zhau HE: Multiplexed
quantum dot labeling of activated c-Met signaling in
castration-resistant human prostate cancer. PLoS One. 6:e286702011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu C, Fang X, Ge Z, Jalink M, Kyo S,
Bjorkholm M, Gruber A, Sjoberg J and Xu D: The telomerase reverse
transcriptase (hTERT) gene is a direct target of the histone
methyltransferase SMYD3. Cancer Res. 67:2626–2631. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hassan KA, Wang L, O'Dowd P, Kim G,
Korkaya H, Davis A, Liu S, Kalemkerian GP and Wicha MS: Abstract
3309: Blocking the Notch pathway inhibits the
epithelial-mesenchymal transition (EMT) status in lung cancer and
alters chemoresistance. Cancer Res. 72:3309. 2012.
|
|
19
|
Chen YJ, Tsai CH, Wang PY and Teng SC:
SMYD3 promotes homologous recombination via regulation of
H3K4-mediated gene expression. Sci Rep. 7:38422017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeng B, Li Z, Chen R, Guo N, Zhou J, Zhou
Q, Lin W, Cheng D, Liao Q, Zheng L and Gong Y: Epigenetic
regulation of miR-124 by hepatitis C virus core protein promotes
migration and invasion of intrahepatic cholangiocarcinoma cells by
targeting SMYD3. FEBS Lett. 586:3271–3278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Furuta M, Kozaki KI, Tanaka S, Arii S,
Imoto I and Inazawa J: miR-124 and miR-203 are epigenetically
silenced tumor-suppressive microRNAs in hepatocellular carcinoma.
Carcinogenesis. 31:766–776. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiang S, Fanyi K, Zhenfeng Z, Mingming R,
Qingjun M, Yanguang LI and Zhen S: miR-124 and miR-142 enhance
cisplatin sensitivity of non-small cell lung cancer cells through
repressing autophagy via directly targeting SIRT1. RSC Adv.
9:5234–5243. 2019. View Article : Google Scholar
|
|
23
|
Luo XG, Ding Y, Zhou QF, Ye L, Wang SZ and
Xi T: SET and MYND domain-containing protein 3 decreases
sensitivity to dexamethasone and stimulates cell adhesion and
migration in NIH3T3 cells. J Biosci Bioeng. 103:444–450. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak K and Schmittgen T: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dandekar DH, Kumar M, Ladha JS, Ganesh KN
and Mitra D: A quantitative method for normalization of
transfection efficiency using enhanced green fluorescent protein.
Anal Biochem. 342:341–344. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Del Bello B, Valentini MA, Zunino F,
Comporti M and Maellaro E: Cleavage of Bcl-2 in oxidant- and
cisplatin-induced apoptosis of human melanoma cells. Oncogene.
20:4591–4595. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Isonishi S, Saitou M, Yasuda M, Ochiai K
and Tanaka T: Enhancement of sensitivity to cisplatin by orobol is
associated with increased mitochondrial cytochrome c release in
human ovarian carcinoma cells. Gynecol Oncol. 90:413–420. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Johnson LV, Walsh ML and Chen LB:
Localization of mitochondria in living cells with rhodamine 123.
Proc Natl Acad Sci USA. 77:990–994. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sarris ME, Moulos P, Haroniti A,
Giakountis A and Talianidis I: Smyd3 is a transcriptional
potentiator of multiple cancer-promoting genes and required for
liver and colon cancer development. Cancer Cell. 29:354–366. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fei X, Ma Y, Liu X and Meng Z:
Overexpression of SMYD3 is predictive of unfavorable prognosis in
hepatocellular carcinoma. Tohoku J Exp Med. 243:219–226. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fang Z and Rajewsky N: The impact of miRNA
target sites in coding sequences and in 3′UTRs. PLoS One.
6:e180672011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bottino C, Peserico A, Simone C and
Caretti G: SMYD3: An oncogenic driver targeting epigenetic
regulation and signaling pathways. Cancers (Basel). 12:E1422020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sprowl JA, Lancaster CS, Pabla N, Hermann
E, Kosloske AM, Gibson AA, Li L, Zeeh D, Schlatter E, Janke LJ, et
al: Cisplatin-induced renal injury is independently mediated by
OCT2 and p53. Clin Cancer Res. 20:4026–4035. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abedini MR, Muller EJ, Brun J, Bergeron R,
Gray DA and Tsang BK: Cisplatin induces p53-dependent FLICE-like
inhibitory protein ubiquitination in ovarian cancer cells. Cancer
Res. 68:4511–4517. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dai B, Wan W, Zhang P, Zhang Y, Pan C,
Meng G, Xiao X, Wu Z, Jia W, Zhang J and Zhang L: SET and MYND
domain-containing protein 3 is overexpressed in human glioma and
contributes to tumorigenicity. Oncol Rep. 34:2722–2730. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brozovic A, Fritz G, Christmann M,
Zisowsky J, Jaehde U, Osmak M and Kaina B: Long-term activation of
SAPK/JNK, p38 kinase and fas-L expression by cisplatin is
attenuated in human carcinoma cells that acquired drug resistance.
Int J Cancer. 112:974–985. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Colon-Bolea P and Crespo P: Lysine
methylation in cancer: SMYD3-MAP3K2 teaches us new lessons in the
Ras-ERK pathway. Bioessays. 36:1162–1169. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Riera TV, Wigle TJ, Gureasko J,
Boriack-Sjodin PA and Copeland RA: Abstract 2144: Kinetic mechanism
of the lysine methyltransferase SMYD3 using MAP3K2 protein
substrate. Cancer Res. 75 (15 Suppl):S21442015.
|
|
39
|
Wang X, Li Y, Dai Y, Liu Q, Ning S, Liu J,
Shen Z, Zhu D, Jiang F, Zhang J and Li Z: Sulforaphane improves
chemotherapy efficacy by targeting cancer stem cell-like properties
via the miR-124/IL-6R/STAT3 axis. Sci Rep. 6:367962016. View Article : Google Scholar : PubMed/NCBI
|