Introduction
Cancer is one of the most malignant diseases that
may affect different parts of the body and remains an extremely
serious life-threatening disease for humans (1). This disease is characterized by a rapid
and uncontrolled growth of abnormal cells, which may mass together
to form a tumor or proliferate throughout the body, initiating
abnormal growth at other sites (2).
Among the various types of cancer, lung cancer is known to be the
most common cause of cancer-associated mortality worldwide
(3). Central and Eastern Europe is
the region with highest mortality rate (3).
In general, treatment of cancer in humans uses
chemotherapeutic agents in addition to surgery and radiation
(2). Chemotherapeutic agents for
cancer, including breast cancer, often provide temporary relief of
symptoms, prolongation of life and occasionally cures (2). However, they often result in serious
side effects and can cause excessive damage to normal cells
(4). This has prompted continuous
efforts of researchers to identify novel anticancer compounds
through chemical synthesis, as well as isolation from plant
origins. A number of compounds derived from medicinal plants have
potential cytotoxicity against several types of cancer cells in
anticancer evaluation in vitro or in vivo (5–7). Plants
consumed by primates are assumed to be a promising source of
therapeutic agents for the management of human diseases and a
series of investigations have been conducted and provided novel
findings of their cytotoxicity against breast cancer cell lines
(1,8–10).
Kaempferol-3-O-rhamnoside isolated from the leaves of
Schima wallichii, a plant commonly consumed by primates,
exhibits inhibitory activity against MCF7 breast cancer cell
proliferation through the activation of the caspase cascade
signaling pathway (8). Subarnas
et al (9) evaluated
anti-proliferative effects of 42 species of primate-consumed plants
against MCF7 human breast cell lines using an MTT bioassay and
revealed that some plant extracts have strong inhibitory activity
against MCF7 cell proliferation. Furthermore,
2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone (ChalcEA),
isolated from the leaves of Eugenia aquea (E. aquea),
inhibits the proliferation of MCF7 cell lines and promotes
apoptosis via the activation of poly(adenosine diphosphate-ribose)
polymerase (PARP) protein (10).
Additionally, a friedolanostane triterpenoid from Garcinia
celebica leaves inhibits the growth of MCF7 cells through
induction of apoptosis and downregulation of the oncogene Akt
(1).
The present study was conducted to clarify the
inhibitory activity of ChalcEA against cell proliferation and
molecular pro-apoptotic activity through activation of the caspase
cascade of A549 lung cancer cells. Furthermore, a molecular
interaction of ChalcEA with caspase-3 was also evaluated using a
molecular docking simulation.
Materials and methods
Plant materials
The leaves of E. aquea were collected from
Pangandaran Beach Conservation Area (Pangandaran, West Java,
Indonesia). Determination of the plant species was performed by the
Department of Biology of Padjadjaran University (Bandung, West
Java, Indonesia). The leaves were dried in the open air for 4–5
days, away from direct sunlight.
Isolation of ChalcEA of E. aquea
ChalcEA was obtained from the leaves of E.
aquea. The isolation of the compound has been reported
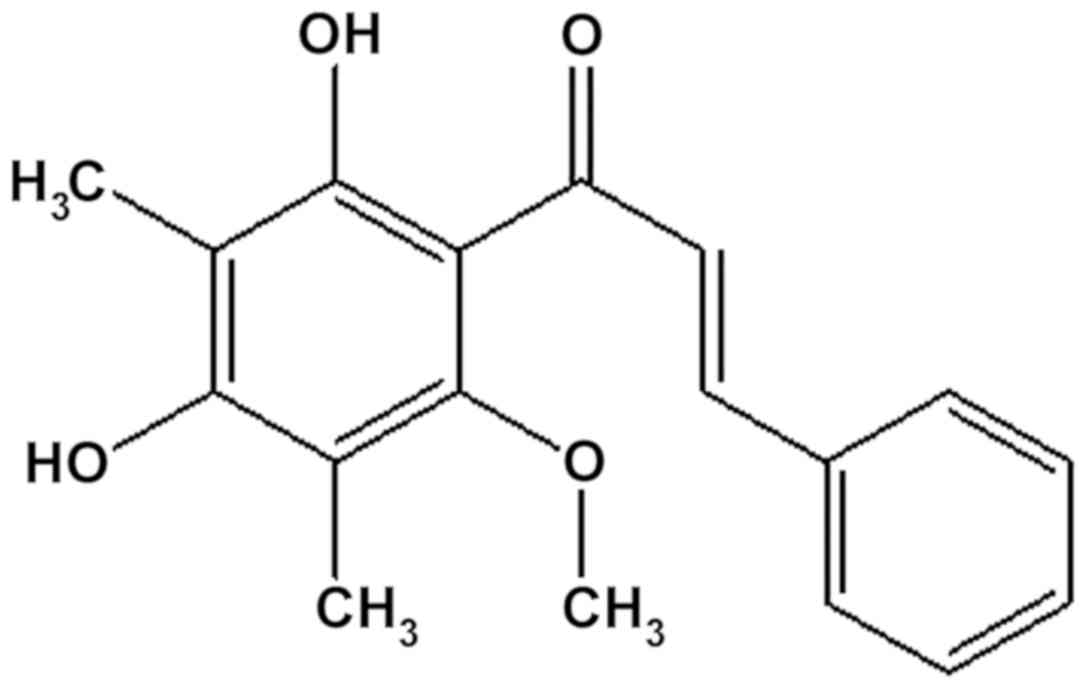
previously by Subarnas et al (10) and its structure was shown in Fig. 1. This compound was named
2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone (10).
Cell culture and treatment
The A549 lung cancer cell line was purchased from
the American Type Culture Collection. The cells were cultured in
RPMI-1640 medium (Sigma-Aldrich; Merck KGaA) supplemented with 10%
fetal bovine serum (Sigma-Aldrich; Merck KGaA) and 100 U/ml
penicillin and 100 µg/ml streptomycin (Sigma-Aldrich; Merck KGaA).
Cells were cultured under standard culture conditions in a
CO2 incubator with 5% CO2 at 37°C. The medium
was replaced once every 2 days. Cell condition were checked using
an Axio Vert A.1 for Biology (Zeiss AG) inverted microscope
(magnification, ×200).
Cell proliferation assay using Cell
counting Kit-8
Cell proliferation analysis was performed using an
MTS assay on cells in the presence of various concentrations of
ChalcEA (1.9–1,000 µg/ml). Cultured cells (1×104/well)
were plated into 96-well microtiter plates in a final volume of 100
µl/well. Subsequent to the initial cell seeding, various
concentrations of ChalcEA (1.9, 3.9, 7.8, 15.6, 31.25, 62.5, 125,
250, 500 and 1,000 µg/ml) were added and the cells were incubated
for 24 to 48 h at 37°C. After ChalcEA treatment was halted by the
replacing media, 10 µl/well of Cell Counting Kit-8 (Dojindo
Molecular Technologies, Inc.) were added into each well and
incubated for 3 h at 37°C in standard culture conditions according
to the manufacturer's protocol. The cell proliferation rate was
determined by measuring absorbance at a wavelength of 450 nm
(reference 620 nm) using an Infinite® 200 PRO microtiter
plate reader (Tecan Group, Ltd.). All samples were tested in
triplicate. CPI rate was calculated according to the method stated
in the manufacturer's protocol.
Cell extraction and western
blotting
A total of 1×106 A549 cells and
1×106 untreated control cells were treated with ChalcEA
(25 µM) for 12, 24, 36 and 48 h. Cells were washed twice using a
cold PBS buffer and the cell lysate was prepared using RIPA lysis
buffer (EMD Millipore; Sigma-Aldrich; Merck KGaA). The Pierce™
Modified Lowry Protein assay kit (Thermo Fisher Scientific Inc.)
was used to measured total protein that extracted from A549 cells
according to the manufacturer's protocol. A total of 25 µg/lane
A549 cell protein extracts were loaded on a 30% polyacrylamid gel
(Invitrogen; Thermo Fisher Scientific, Inc.) and electrotransferred
onto a Amersham™ Protran™ 0.45-µm nitrocellulose membrane (GE
Healthcare Life Sciences). Membranes were blocked using 5% skimmed
milk and agitated at 25°C for 30 min. Apoptosis-associated proteins
were analyzed using immunoblot analysis with caspase-3 (cat. no.
AF-605NA; 1:1,000; R&D Systems, Inc.) and caspase-9 antibodies
(cat. no. AF-8301; 1:1,000; R&D Systems, Inc.) incubated in 4°C
for 24 h. β-actin (cat. no. 4967; 1:15,000; Cell Signaling
Technology, Inc.) served as the loading control for 1 h incubation
in room temperature. Mouse anti-goat IgG horseradish
peroxidase-conjugated antibodies (cat. no. sc-2354; 1:10,000; Santa
Cruz Biotechnology, Inc.) served as secondary antibody for 90 min
incubation in room temperature. Visualization of protein bands was
conducted using chemiluminesence reagent (GE Healthcare). Bands on
the membrane were detected and measured using a C-DiGit®
Blot scanner (LI-COR Biosciences) with Image Studio Digits v. 5.2
(LI-COR Biosciences) for band density measurement. All samples were
tested in triplicate.
Molecular docking simulation
The X-ray crystallography derived caspase-3
(CAS329306) complex with 4-methyl-benzenesulfonamide (MB) was
obtained from Protein Data Bank (PDB ID: 2XYG; http://www.rcsb.org/structure/2XYG) (11). The macromolecule and ligand
structures were extracted using LigandScout version 4.2 Advanced
(Inte:Ligand GmbH). The molecular docking simulation methods were
modified according to a previous study (12). All ligands (Met61, Arg64, Ser120,
His121, Gly122, Glu123, Phe128, Ala162, Cys163, Thr166, Tyr204 and
Arg207) and the estrogen receptor α (ERα) receptor were prepared
for docking using AutoDockTools version 1.5.6. (The Scripps
Research Institute) The ligands and the receptor were protonated.
The default charges energy parameters were allocated to the protein
and ligand atoms. A grid box comprised 40×40×40 points spaced by
0.375 Å that was centered on the androgen receptor (AR) active site
(x=36.357; y=38.829; and z=32.088). Autogrid was used to calculate
grid box of binding affinity of each of the ligand atom types. The
resulting docked conformations were clustered using a
root-mean-square deviation tolerance of 1.0 Å. The ligand
conformation with the lowest free energy of binding, selected from
the most favored cluster as sorted by scores and by binding
position, was selected for further analysis. The ligand-interaction
features for each pose within the binding pocket were determined
automatically using LigandScout Advanced version 4.2.
Structure-based 3D-pharmacophore
modeling
A 3D structure-based pharmacophore model was derived
automatically from the X-ray derived structure of PDB ID: 2AX6 in
complex with hydroxyflutamide (HF), using Ligandscout version 4.2
Advanced based on a previous study (13).
Statistical analysis
Quantitative data was obtained using the band
scanner Image Studio Digits v. 5.2 (LI-COR Biosciences). Data are
presented as mean ± standard deviation. After calculation of the
normality of the data using a stem and leaf plot, one-way ANOVA
followed with Tukey's post hoc test was performed to assess the
statistical significance of more than two groups. The measurements
were assessed in triplicate. SPSS version 26 (IBM Corp.) was used
for all statistical analyses. P<0.05 was considered to indicate
a statistically significant difference.
Results
Inhibitory activity of ChalcEA against
proliferation of A549 cells
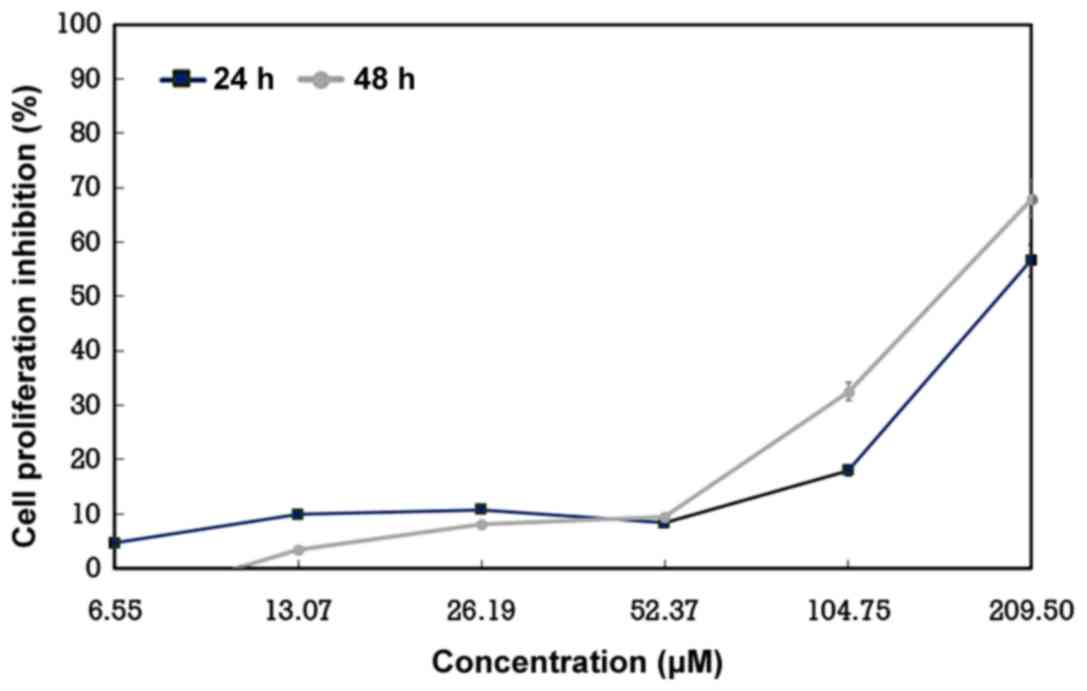
ChalcEA was evaluated for its effect on the
proliferation of A549 lung cancer cells in a proliferation assay.
The evaluation revealed a dose- and time-dependent inhibition of
cell proliferation by the compound. The compound strongly inhibited
the proliferation of A549 cells in 24 and 48 h examinations with an
IC50 value of 25.36 and 19.60 µM, respectively (Fig. 2).
Proapoptotic activity of ChalcEA
The proliferation assay results revealed strong
inhibitory activity of cell proliferation by ChalcEA against A549
cells demonstrated morphologically, for example cells broke into
small pieces (Fig. S1). Therefore,
caspase-inducing activity of the compound was examined in the A549
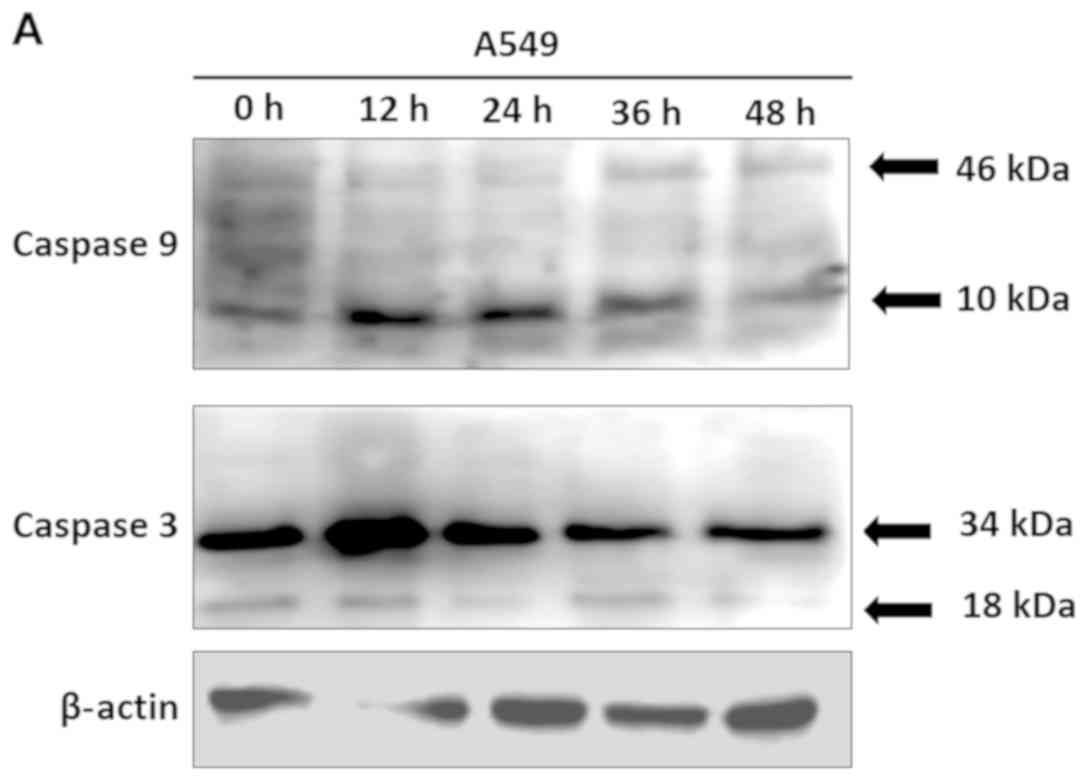
cell line. As shown in Fig. 3,
expression levels of active fragments of caspase-9 and caspase-3
were increased in A549 cells at 12 h, including caspase-9 fragments
with 46 kDa (P<0.0001) and 10 kDa (P=0.0001) and caspase 3
fragments with 34 kDa (P=0.0001) and 18 kDa (P=0.0001). These
results suggested that the inhibition of A549 human lung cancer
cell proliferation by ChalcEA was mediated by the induction of
apoptosis through activation of caspase-9 and caspase-3.
Molecular docking and pharmacophore
modelling of ChalcEA
Based on in vitro studies, molecular docking
was used to demonstrate ChalcEA interaction with caspase-3
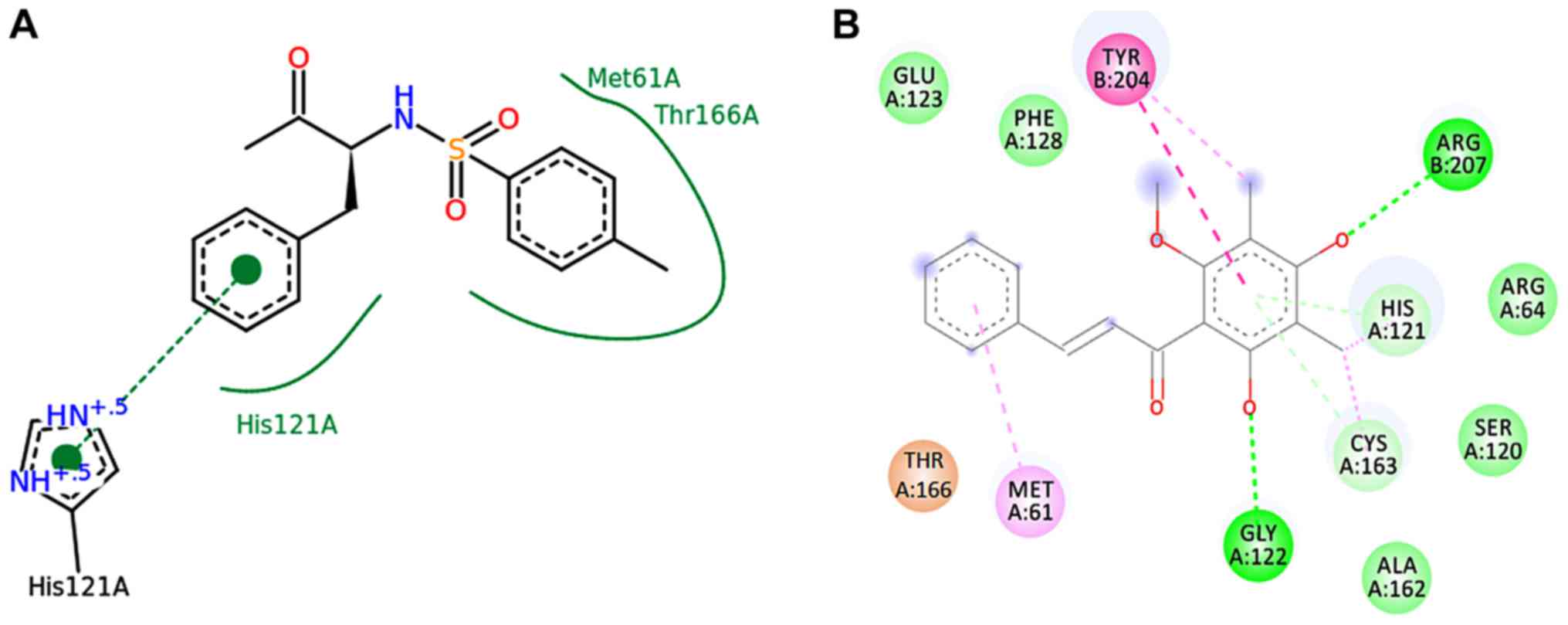
receptors by inhibiting ERα. As shown in Table I, ChalcEA had a lower binding energy
affinity than 4-methyl-benzenesulfonamide (MB). ChalcEA formed a
hydrogen bond interaction with amino acid residues of His121,
Cys163, Tyr166 and Arg207 (Fig. 4B and
D), whereas MB only had hydrogen bond interaction with His121
and Tyr166 (Fig. 4A and C).
Furthermore, Fig. 4C revealed that
MB induced conformation changes of Tyr204. ChalcEA also has
hydrogen bond interaction at the Cys163 and Arg207 sites that not
only conformationally changes Tyr204 but also stabilizes via
interaction with Glu123 and Gly122. Moreover the interactions were
stabilized by a π-π interaction between the aromatic rings of
ChalcEA and Phe128-Met61 (Fig. 4B and
D). The ChalcEA bond with caspase-3 can be seen in Fig. 4E. In the pharmacophore results,
ChalcEA was mapped well in two hydrophobics (yellow ball) with
pharmacophoric model of HF as indicated in Fig. 4D. However, ChalcEA has potential to
bind amino acid residues from receptors since this compound has
three hydrophobic regions, two hydrogen bond receptors (red) and
one hydrogen bond donor (green).
 | Table I.Molecular docking and pharmacophore
modelling results. |
Table I.
Molecular docking and pharmacophore
modelling results.
| Compounds | Free binding energy
(kcal/mol | Hydrogen bond
interaction | Pharmacophore
features |
|---|
|
4-methyl-benzenesulfonamide (MB) | −6.43 | His121, Tyr166 | 3HBA, 2Hy, NI |
|
2′,4′-dihydroxy-6′-methoxy-3′,
5′-dimethylchalcone | −6.53 | His121, Cys163,
Tyr166, Arg207 | 1 HBD, 2 HBA, 3
Hy |
Discussion
Previously, the compound ChalcEA obtained from the
leaves of E. aquea has been shown to have antiproliferation
activity in MCF7 cells and to promote proapoptotic activity via
PARP protein activation (10).
However, the present study investigated the effects of ChalcEA on
the growth of A549 lung cancer cells as lung cancer is known as a
malignant lung tumor characterized by uncontrolled cell growth in
tissues of the lung (14).
Worldwide, lung cancer is the most common cancer among men in terms
of both incidence and cancer-associated mortality, and among women
it has the third highest incidence in 2008 and 2012 (3,15). In
2012, there were 1.82 million new cases of lung cancer globally and
1.56 million cancer-associated deaths were due to lung cancer,
representing ~19.4% of all cancer-associated deaths (15). ChalcEA is also hypothesized to be
effective in inhibition of the growth of A549 lung cancer cells
(13). The present study
demonstrated that the compound showed significant inhibition of
A549 cell proliferation with IC50 values of 25.36 and
19.60 µM for 24 and 48 h treatments. The results of the present
study suggested that the underlying molecular mechanism of this
proapoptotic activity occurred through the activation of caspase-9
and caspase-3. Furthermore, a molecular interaction of ChalcEA with
caspase-3 was evaluated using molecular docking simulation.
The present study showed that ChalcEA isolated from
E. aquea leaves significantly inhibited A549 cell
proliferation. This evidence was in line with a previous study
which reported that the addition of ChalcEA isolated from the buds
of Cleistocalyx operculatus resulted in inhibition of lung
cancer GLC-82 ×enografts (13).
ChalcEA also significantly inhibits the growth of human liver
cancer SMMC-7721 cells and may induce apoptosis of SMMC-7721 cells
in vitro (16). The antitumor
effects of this compound have also been demonstrated in vivo
in a solid human tumor xenograft mouse model using human liver
cancer SMMC-7721 cells (17).
Additionally, ChalcEA inhibits subcutaneous tumor growth of human
hepatocarcinoma Be17402 cells (18);
however, reports of ChalcEA IC50 values in cancer cells
remain limited.
The results of the present study suggested that
ChalcEA triggered cell death intrinsically in A549 cells via the
mitochondrial caspase-9 signaling pathway and activation of
caspase-3 at 12 h. These results were in agreement with those of a
previous study which stated that ChalcEA activates Akt before 12 h
which then triggers cell death intrinsically through caspase-9
activation, in a xenograft model (13). Activation of caspase-3 marks the
occurrence of apoptosis and cell death (19). This evidence of the underlying
molecular mechanism may explain the effect of ChalcEA against
cancer angiogenesis and tumor growth in a solid tumor xenograft in
mouse model (13,17).
Based on previous in vitro studies, ChalcEA
interaction with caspase-3 receptors has been identified by
inhibiting ERα which then inhibits Akt to activate caspase-9
(12,17). Caspases function as mediators of
programmed apoptosis (19).
Caspase-3 is an activated death protease, catalyzing the specific
cleavage of a number of cellular proteins, for example PARP, DFF40,
DFF45, α-Fodrin and Gelsolin, that trigger cell DNA fragmentation
and blebbing (19). Caspase-9 can
directly cleave and activate caspase-3 and caspase-7 (20).
In the present study, ChalcEA was investigated as a
prospective drug candidate which should be further evaluated and
applied against caspase receptors for the treatment of lung cancer,
and it was revealed that ChalcEA serves as a caspase-3 inducer. The
3D structure of PDB ID: 2XYG was obtained from RCSB PDB (Fig. 4A). In a previous study, Ganesan et
al (21) clarified that
4-methyl-benzenesulfonamide (MB) (PDB ID: 2XYG) is an inhibitor
against caspase-3. MB was a rotation of the Tyr204 side chain,
which blocks the S2 subsite. S2 subsite is stabilized by
hydrophobic contacts with Cys163, Trp206 and Phe25. ChalcEA was
docked well toward caspase-3. Notably, ChalcEA (−6.53 kcal/mol)
could compete better than MB (−6.43 kcal/mol; Table I). That means that the bond between
ChalcEA and caspase-3 is stronger than MB, which functions as a
caspase-3 inhibitor (21). ChalcEA
is stabilized by water and accommodated by Gly122 and Gly165 as
well as MB (19). As shown in
Fig. 4B and D, ChalcEA formed
hydrogen bond interaction with His121, Cys163, Tyr166 and Arg207.
The interactions were stabilized by π-π interaction between
aromatic rings of ChalcEA and Phe128-Met61. Therefore, it can be
concluded that the hydrogen bonds formed with ChalcEA are stronger
than MB, so MB is unable to block the process of induction of
apoptosis by ChalcEA and cancer cells going to enter apoptotic
process.
Based on the current results, ChalcEA functions as a
potential activator of caspase-3. This conclusion was supported by
a previous study which indicated that four flavonoids (considering
that ChalcEA is itself a flavonoid compound) were estimated to be
promising candidates for further evaluation for lung cancer
prevention (22). Furthermore, Cui
et al (23) reported that
consumption of vegetables, tea and wine, all of which are rich
sources of flavonoids, are associated inversely with lung
cancer.
The present study suggested that ChalcEA obtained
from the leaves of E. aquea may be a compound with potential
for development as an anticancer treatment for lung cancer therapy.
The influence of ChalcEA on other pathways, such as malignancy, and
the ability of cells to invade to other organs, needs to be further
investigated.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Professor Unang
Supratman (Laboratorium Central, Universitas Padjadjaran West Java;
Indonesia), for providing all the necessary facilities to carry out
the present work. The authors would also like to thank Ms. Susianti
(Laboratorium Central; Universitas Padjadjaran; West Java;
Indonesia) for her excellent technical support and assistance.
Funding
The present study was supported by the Universitas
Padjadjaran Academic Leadership fund (grant no. 1-1-6).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YEH, NC, MM, RL and AS designed and performed the
experiments, analyzed the data and wrote the manuscript. TRu, AYC,
IS and TRo analyzed data and modified the paper. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ChalcEA
|
2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone
|
|
PARP
|
poly(adenosine diphosphate-ribose)
polymerase
|
|
MTS
|
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxylmethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
|
|
E. aquea
|
Eugenia aquea
|
|
MB
|
4-methyl-benzenesulfonamide
|
References
|
1
|
Subarnas A, Diantini A, Abdulah R,
Zuhrotun A, Nugraha PA, Hadisaputri YE, Puspitasari IM, Yamazaki
Ch, Kuwano H and Koyama H: Apoptosis-mediated antiproliferative
activity of friedolanostane triterpenoid isolated from the leaves
of Garcinia celebica against MCF-7 human breast cancer cell lines.
Biomed Rep. 4:79–82. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jaikumar B and Jasmine R: A Review on a
few medicinal plants possessing anticancer activity against human
breast cancer. Int J Pharm Tech Res. 9:333–365. 2016.
|
|
3
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sakarkar DM and Deshmukh VN:
Ethnopharmacological review of traditional medicinal plants for
anticancer activity. Int J Pharm Tech Res. 3:298–308. 2011.
|
|
5
|
Kinghorn AD, Farnsworth NR, Soejarto DD,
Geoffrey AC, John MP, George OU, Mansukh CW, Monroe EW, Hernán AN,
Rob AK, et al: Novel strategies for the discovery of plant-derived
anticancer agents. Pure Appl Chem. 71:1611–1618. 1999. View Article : Google Scholar
|
|
6
|
Kinghorn AD: The role of pharmacognosy in
modern medicine. Expert Opin Pharmacother. 3:77–79. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kinghorn AD, Farnsworth NR, Soejarto DD,
Geoffrey AC, Steven MS, John MP, Mansukh CW, Monroe EW, Nicholas
HO, David JK, et al: Novel strategies for the discovery of
plant-derived anticancer agents. Pharm Biol. 41 (Suppl):S53–S67.
2003. View Article : Google Scholar
|
|
8
|
Diantini A, Subarnas A, Lestari K, Halimah
E, Susilawati Y, Supriyatn a, Julaeha E, Achmad TH, Suradji EW,
Yamazaki C, et al: Kaempferol-3-O-rhamnoside isolated from the
leaves of Schima wallichii Korth. inhibits MCF-7 breast cancer cell
proliferation through activation of the caspase cascade pathway.
Oncol Lett. 3:1069–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Subarnas A, Diantini A, Abdulah R,
Zuhrotun A, Yamazaki C, Nakazawa M and Koyama H: Antiproliferative
activity of primates-consumed plants against MCF-7 human breast
cancer cell lines. E3 J Med Res. 1:38–43. 2012.
|
|
10
|
Subarnas A, Diantini A, Abdulah R,
Zuhrotun A, Hadisaputri YE, Puspitasari IM, Yamazaki Ch, Kuwano H
and Koyama H: Apoptosis induced in MCF-7 human breast cancer cells
by 2′,4′-dihydroxy-6-methoxy-3,5-dimethylchalcone isolated from
Eugenia aquea Burm f. leaves. Oncol Lett. 9:2303–2306. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bohl CE, Miller DD, Chen J, Bell CE and
Dalton JT: Structural basis for accommodation of nonsteroidal
ligands in the androgen receptor. J Biol Chem. 280:37747–37754.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Muchtaridi M, Syahidah HN, Subarnas A,
Yusuf M, Bryant SD and Langer T: Molecular docking and
3D-pharmacophore modeling to study the interactions of chalcone
derivatives with estrogen receptor alpha. Pharmaceuticals. 10:1–12.
2017. View Article : Google Scholar
|
|
13
|
Zhu XF, Xie BF, Zhou JM, Feng GK, Liu ZC,
Wei XY, Zhang FX, Liu MF and Zeng YX: Blockade of vascular
endothelial growth factor receptor signal pathway and antitumor
activity of ON-III
(2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone), a component
from Chinese herbal medicine. Mol Pharmacol. 67:1444–50. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
El-Awady RA, Hersi F, Al-Tunaiji H, Saleh
EM, Abdel-Wahab AH, Al Homssi A, Suhail M, El-Serafi A and Al-Tel
T: Epigenetics and miRNA as predictive markers and targets for lung
cancer chemotherapy. Cancer Biol Ther. 16:1056–70. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gutierrez RMP, Ramirez AM and Sauceda JV:
Review: Potential of chalcones as a source of drugs. Afr J Pharm
Pharmacol. 9:237–257. 2015. View Article : Google Scholar
|
|
17
|
Ye CL, Liu JW, Wei DZ, Lu YH and Qian F:
In vitro anti-tumor activity of
2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone against six
established human cancer cell lines. Pharmaco Res. 50:505–510.
2004.
|
|
18
|
Ye CL, Liu JW, Wei DZ, Lu YH and Qian F:
In vivo antitumor activity by
2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone in a solid human
carcinoma xenograft model. Cancer Chemother Pharmacol. 56:70–74.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Porter AG and Janicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brentnall M, Rodriguez-Menocal L, De
Guevara RL, Cepero E and Boise LH: Caspase-9, caspase-3 and
caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell
Biol. 14:32. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ganesan R, Jelakovic S, Mittl PR, Caflisch
A and Grütter MG: In silico identification and crystal structure
validation of caspase-3 inhibitors without a P1 aspartic acid
moiety. Acta Crystallogr Sect F Struct Biol Cryst Commun.
67:842–850. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Christensen KY, Naidu A, Parent ME, Pintos
J, Abrahamowicz M, Siemiatycki J and Koushik A: The risk of lung
cancer related to dietary intake of flavonoids. Nutr Cancer.
64:964–974. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cui Y, Morgenstern H, Greenland S, Tashkin
DP, Mao JT, Cai L, Cozen W, Mack TM, Lu QY and Zhang ZF: Dietary
flavonoid intake and lung cancer-a population-based Case-control
study. Cancer. 112:2241–2248. 2008. View Article : Google Scholar : PubMed/NCBI
|