Introduction
In total, ~429,800 new cases of bladder cancer and
165,100 cancer-associated mortalities occurred in 2012, worldwide
(1). Bladder cancer is the ninth
most commonly-occurring cancer worldwide (2), and is the most common type of
urothelial cancer (3,4). The highest incidence rates were
observed in men in Southern (age-standardized rate=21.8) and
Western Europe (age-standardized rate=19.7), North America
(age-standardized rate=19.5), Northern Africa (age-standardized
rate=15.1) and Western Asia (age-standardized rate=19.0), and the
incidence rates are evidently lower in women than men (2). Chemotherapy is an important method for
postoperative treatment of bladder cancer (5). However, some patients exhibit poor
sensitivity to chemotherapy, leading to poor therapeutic effects
(6). Adriamycin is the first line
chemotherapy drug for bladder cancer, and primary and secondary
resistance of Adriamycin has been observed in bladder cancer
(7). Multiple mechanisms are
involved in Adriamycin resistance, including increased cancer cell
proliferation and epithelial-to-mesenchymal transition (EMT)
(8,9).
Oxysterols such as 24S-hydroxycholesterol and
25-hydroxycholesterol constitute a family of oxidized derivatives
of cholesterol (10); these
metabolites are under investigation as risk markers for multiple
outcomes, from cardiovascular disease to cancer (11–24).
24S-hydroxycholesterol has been proposed as a marker for the
developmental and pathological changes in the brain (16,25,26). For
example, increased circulating 24S-hydroxycholesterol levels is
associated with the early stages of late onset Alzheimer's disease
(18), and higher concentrations of
circulating 24S-hydroxycholesterol levels have been observed in
individuals with Alzheimer's disease (18,25).
25-hydroxycholesterol has been investigated with respect to
outcomes including breast, colon, and hepatocellular cancer
(27). To the best of our knowledge,
there are no prior data regarding the role of
24S-hydroxycholesterol and 25-hydroxycholesterol in bladder
cancer.
The present study hypothesized that
25-hydroxycholesterol may affect the expression of EMT-associated
genes and promote Adriamycin resistance in bladder cancer cells.
Thus, it may be a novel prognostic marker for bladder cancer
progression and overall patient survival.
Materials and methods
Analysis of study population and tumor
samples
A total of 157 patients with primary bladder cancer
were recruited from Shanghai Tianyou Hospital Affiliated to Tongji
University and Jinling Hospital between January 1995 and December
2008. The present study enrolled women who were ≥18 years of age
and who were diagnosed with primary bladder cancer. Patients with
cancer recurrence or with incomplete medical records or inadequate
follow-up were excluded. The cohort consisted of 57 female and 100
male patients. The median age of the patients was 69 years (range,
41–92 years). Follow-up information was available in all cases.
Tumor samples were obtained directly from surgery
following the removal of the necessary amount of tissue for routine
pathology examination. All tissue specimens were snap-frozen
immediately following collection and stored at −80°C. Tumors were
graded by the Bergkvist classification system (28). The corresponding adjacent normal
tissue sample was obtained >3 cm away from the site at which the
primary tumor was sampled (29). All
tumor tissues and adjacent normal tissues were blindly reviewed by
two pathologists from the Department of Urology, Shanghai Tianyou
Hospital Affiliated to Tongji University (Shanghai, China). For
each patient, comprehensive clinical and pathological data were
collected and entered into a Shanghai Tianyou Hospital approved
database. Pathological details and UICC TNM classification were
also collected (30). The Ethics
Committee of Jinling Hospital approved the present study. Written
informed consent was obtained from each patient according to The
Helsinki Declaration.
Reagents
24S-hydroxycholesterol and 25-hydroxycholesterol
were purchased from Yanke, Inc. (http://xmykswjs.china.herostart.com), and Adriamycin
was obtained from Kangbeibio, Inc. (http://www.kangbeibio.com). 24S-hydroxycholesterol
(10−6 M), 25-hydroxycholesterol (10−6 M) and
Adriamycin were solubilized in DMSO (Beyotime Institute of
Biotechnology).
Bladder cancer cell lines
Human invasive bladder cancer cell lines (T24 and
RT4 cells) were obtained from Tiangen Biotech Co., Ltd. T24 and RT4
cell lines have been previously described (31,32).
Cells were grown in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin (Sigma-Aldrich
Merck KGaA). All cell lines were maintained at 37°C in a humidified
atmosphere with 5% CO2.
24S-hydroxycholesterol and
25-hydroxycholesterol quantification
24S-hydroxycholesterol and 25-hydroxycholesterol
were quantified in tissue samples using Liquid chromatography-mass
spectrometry (LC/MS). Tissues were extracted by crushing in liquid
nitrogen, followed by the stepwise addition of distilled H2O. LC/MS
was performed as previously described (33,34).
Quantitative analysis of 24S-hydroxycholesterol and
25-hydroxycholesterol was performed using Shimadzu LC-20XR HPLC
system (Shimadzu Scientific Instruments; http://www.ssi.shimadzu.com), equipped with an
ultrasonic degasser, column oven and autosampler.
MTT assay
The effect of 24S-hydroxycholesterol and
25-hydroxycholesterol on the proliferation of bladder cancer cells
was assessed via an MTT assay (Sigma-Aldrich, Merck KGaA). The MTT
analysis was performed as previously described (35,36).
Cells were plated at a density of 8×103 cells/well in
96-well plates in DMEM supplemented with 10% FBS and incubated at
37°C in a 5% CO2 incubator for 12 h. Cells were treated
with 24S-hydroxycholesterol (10−6 M),
25-hydroxycholesterol (10−6 M) and vehicle DMSO
(control), for 48 h. Subsequently, MTT (5 mg/ml) was added to the
wells (20 µl/well) and the plates were incubated at 37°C for 4 h in
a 5% CO2 incubator. The supernatant was discarded, and
the purple formazan crystals were dissolved using 150 ml of DMSO.
Following incubation for 10 min at room temperature, cell viability
was measured using a Synergy™ 4 Multi-Detection Microplate Reader
(BioTek Instruments, Inc.), at a wavelength of 570 nm.
Western blot analysis
Western blotting was performed as previously
described (35,36). Total protein was extracted from T24
and RT4 cells using extraction buffer comprising NaCl/Pi containing
0.5% Triton X-100, 1 mM EDTA, 1 mM phenylmethyl sulfonyl fluoride
and complete protease inhibitors (Roche Diagnostics). Total protein
was quantified using a bicinchoninic acid assay kit (Thermo Fisher
Scientific, Inc.) and 5 μg protein/lane was separated via SDS-PAGE
on a 12% gel. Samples were then transferred onto a nitrocellulose
membrane and blocked for 1 h at room temperature with 5% skimmed
milk powder (w/v) in NaCl/Pi. The membranes were incubated with the
following primary antibodies: anti-E-cadherin (1:500; cat. no.
ab40772), anti-N-cadherin (1:500; cat. no. ab18203;),
anti-fibronectin (1:500; cat. no. ab2413) and anti-SOCS3 (1:500;
cat. no. ab16030) (all purchased from Abcam), overnight at 4°C.
Following the primary incubation, membranes were incubated with
horseradish peroxidase-conjugated anti-rabbit secondary antibodies
(1:10,000; cat. no. ab191866; Abcam) for 30 min at room
temperature. Protein bands were visualized using Odyssey™ Infrared
Imaging System (LI-COR Biosciences). β-actin (1:500; cat. no.
ab8226; Abcam) was used as the loading control.
Statistical analysis
Samples were analyzed via Student's t-test for
comparison of two groups. One-way Analysis of variance (ANOVA),
followed by Bonferroni adjustment was used for the comparison among
multiple groups. Data are presented as the mean ± standard error of
the mean. Overall survival was analyzed by Kaplan-Meier methods
(37,38). Survival was compared in terms of
25-hydroxycholesterol concentrations. Survival curves were compared
via log-rank tests. Hazard ratios with 95% confidence interval (CI)
and P-values are presented. P<0.05 was considered to indicate a
statistically significant difference. Statistical analyses were
conducted using SAS® software (version 9.4; SAS
Institute, Inc.).
Results
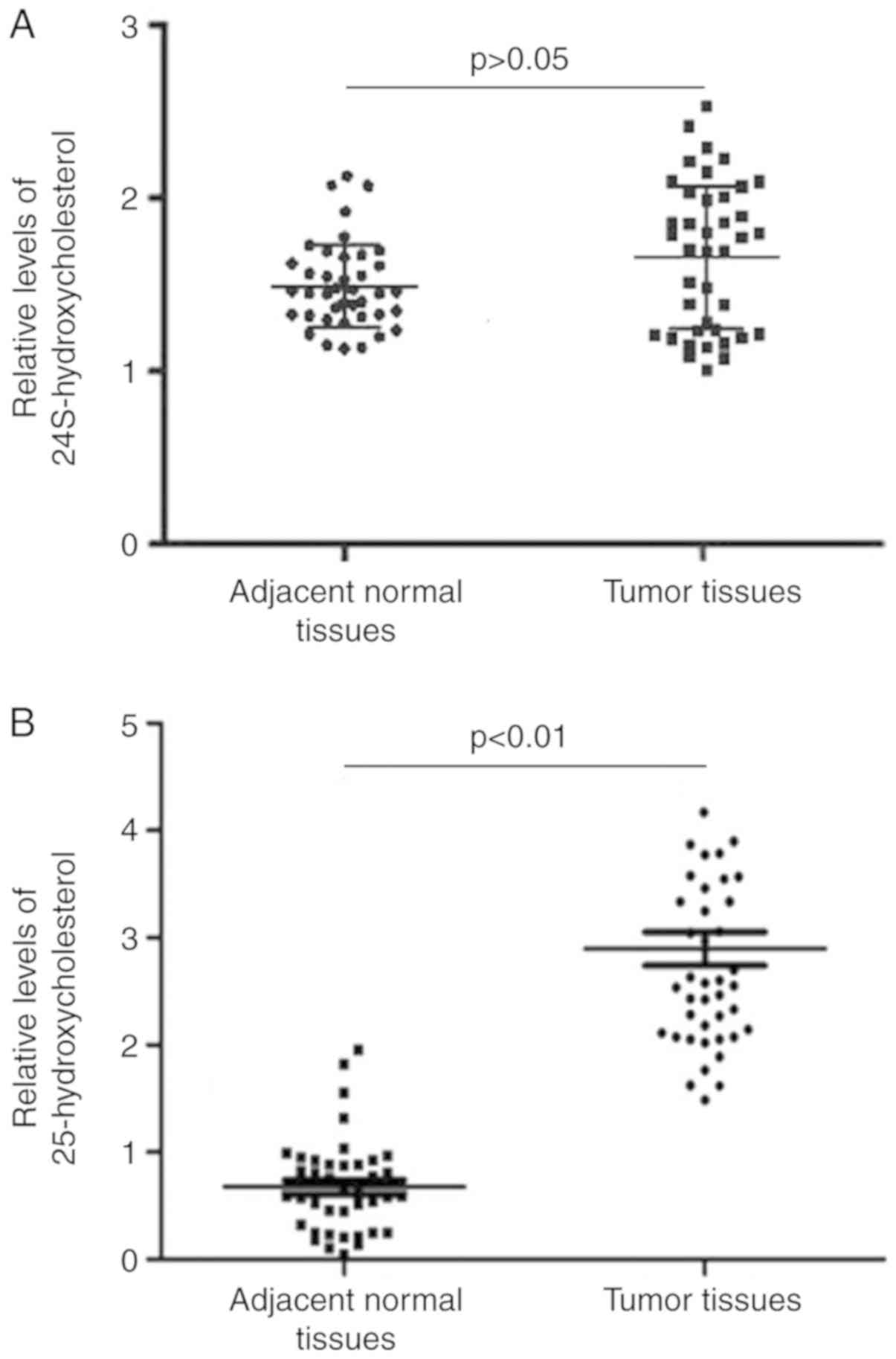
25-hydroxycholesterol is aberrantly
upregulated in bladder cancer
In an attempt to identify 24S-hydroxycholesterol and
25-hydroxycholesterol concentrations between bladder cancer tissues
and adjacent normal tissues, LC/MS was performed. There was no
significant difference in 24S-hydroxycholesterol concentrations
between cancer tissues and adjacent normal tissues (Fig. 1A). However, it was observed that the
relative levels of 25-hydroxycholesterol were significantly
increased in bladder cancer tissues (P<0.01; Fig. 1B).
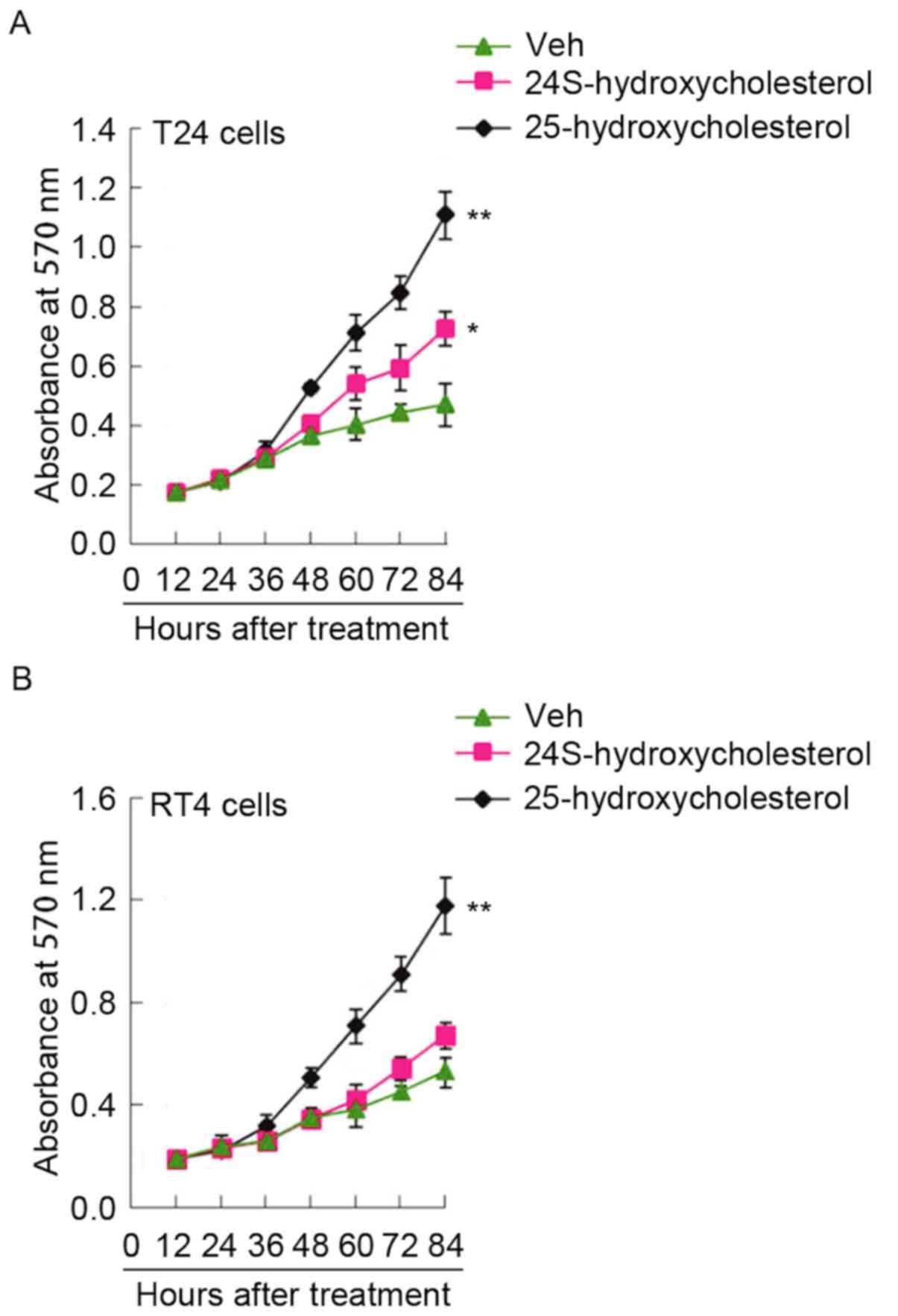
25-hydroxycholesterol promotes
proliferation in T24 and RT4 bladder cancer cells
The capacity of 24S-hydroxycholesterol and
25-hydroxycholesterol to stimulate bladder cancer cell
proliferation was evaluated in T24 and RT4 cells with the MTT
assay. The results indicated that 24S-hydroxycholesterol weakly
promoted proliferation in T24 cells (Fig. 2A). 25-hydroxycholesterol
significantly promoted proliferation in T24 and RT4 cells (Fig. 2A and B).
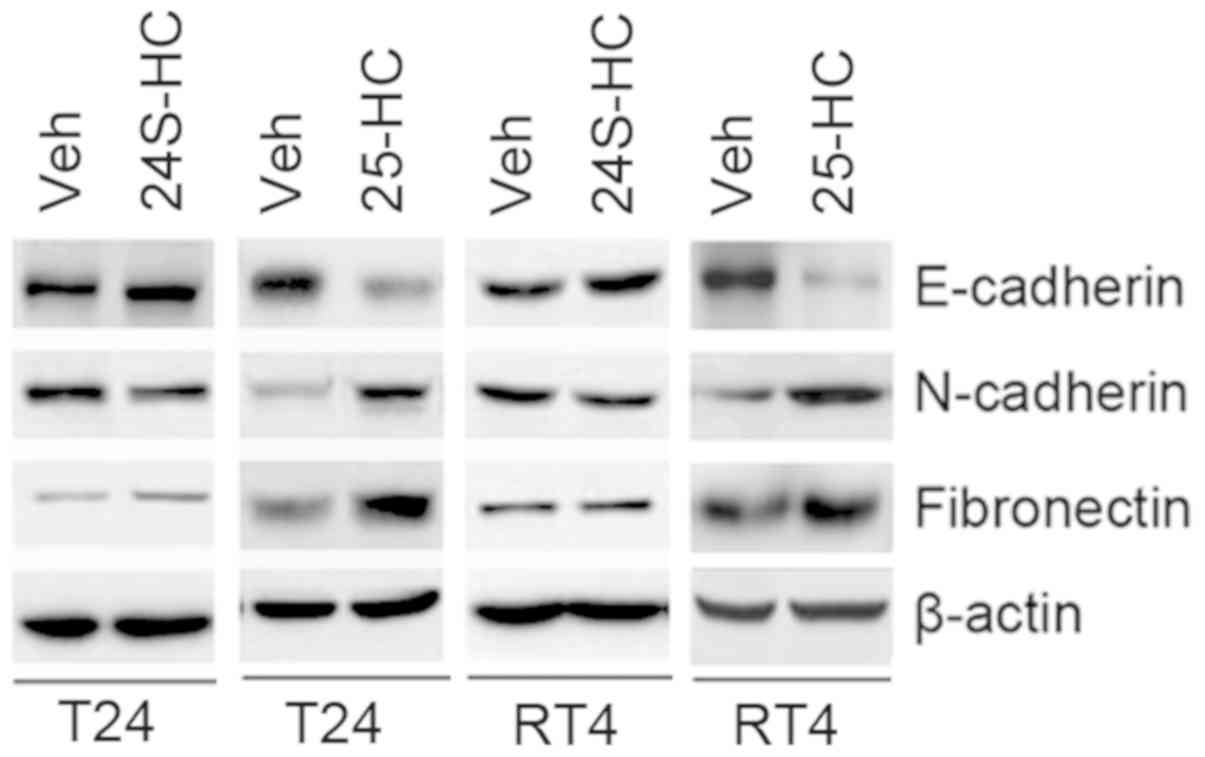
25-hydroxycholesterol affects the
expression of EMT-associated genes in T24 and RT4 cells
In order to identify whether 25-hydroxycholesterol
could affect EMT in bladder cancer, the present study investigated
3 EMT-associated genes, including E-cadherin, N-cadherin and
Fibronectin, and western blot analyses were performed in order to
analyze their expression levels. 25-hydroxycholesterol led to a
decreased expression level of E-cadherin and increased expressions
of N-cadherin and Fibronectin in T24 and RT4 cells (Fig. 3). However, 24S-hydroxycholesterol did
not affect E-cadherin, N-cadherin and Fibronectin expression in T24
and RT4 cells (Fig. 3).
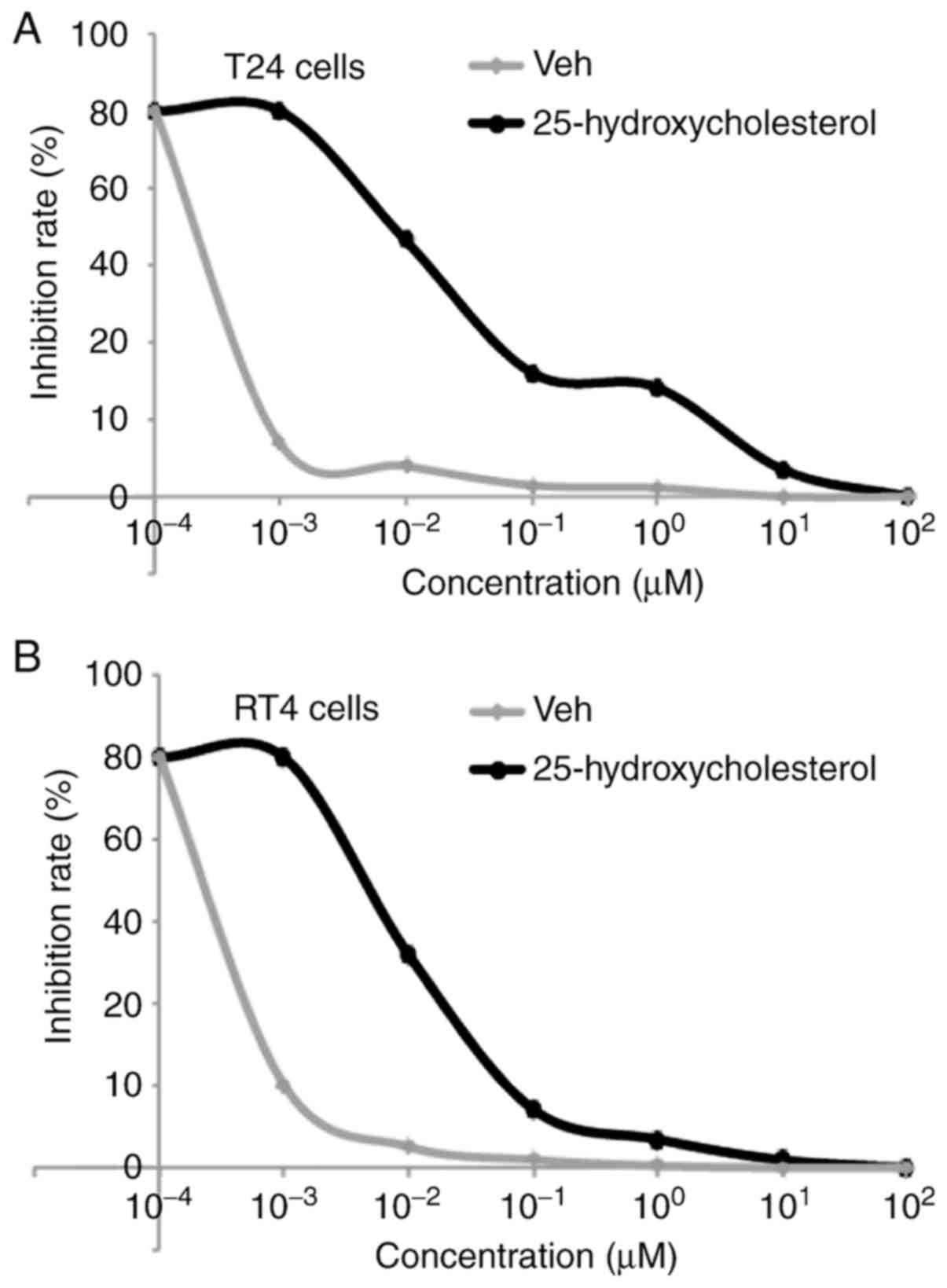
25-hydroxycholesterol promotes
Adriamycin resistance in T24 and RT4 cells
In order to further identify whether
25-hydroxycholesterol could affect Adriamycin efficacy, the present
study performed an MTT assay in T24 and RT4 cells treated as
indicated (Fig. 4A and B). It was
observed that increased 25-hydroxycholesterol promoted Adriamycin
resistance in a dose-dependent manner in T24 and RT4 cells
(Fig. 4A and B). The present study
also performed western blotting to determine SOCS3 protein
expression in T24 and RT4 cells. The results demonstrated that
SOCS3 protein expression was increased following treatment with
25-hydroxycholesterol in T24 and RT4 cells (Fig. 4C and D).
25-hydroxycholesterol is a novel
prognostic marker for bladder cancer progression and overall
patient survival
Kaplan-Meier curves were applied to assess overall
survival time for 157 human primary bladder cancers, stratified
based on concentrations of tumor 25-hydroxycholesterol. Using a
log-rank test, the two overall survival curves were significantly
different (P<0.01; Fig. 4E).
Survival time among patients with high 25-hydroxycholesterol
concentrations was much poorer than survival time among patients
with low 25-hydroxycholesterol concentrations (Fig. 4E). It is well established that stage
and grade are important prognostic factors for bladder cancer
(39). In the present study,
patients with high-grade tumors had markedly worse survival
(P<0.05; data not shown). Adjustment for tumor stage and grade
did not impact the associations between 25-hydroxycholesterol
concentrations and overall survival.
Discussion
Dietary cholesterol is positively associated with
the risk of cancers of the breast (primarily postmenopausal), lung,
stomach, pancreas, rectum, colon, kidney and bladder (40). In an experimental model,
intracellular cholesterol was revealed to be an important component
of the cell membrane (41), which in
turn is necessary for cancer cell proliferation and metastasis
(42,43). In addition, intracellular cholesterol
biosynthesis has been proposed as an important mechanism for
chemotherapy resistance in bladder cancer cells (44). 25-hydroxycholesterol is synthesized
from cholesterol by the enzyme -CYP7B1 (45). A single serum measurement of
25-hydroxycholesterol can reliably estimate its average levels over
a 1-year period (33). The present
study details an evaluation of the role of 24S-hydroxycholesterol
and 25-hydroxycholesterol in bladder cancer. The results obtained
suggest that 25-hydroxycholesterol, a primary metabolite of
cholesterol, may serve an important role in the progression of
bladder cancer.
It has previously been reported that
25-hydroxycholesterol activates ERα in breast cancer MCF-7 cells;
it promotes proliferation in MCF-7 and BG-1 ovarian cancer cells;
25-hydroxycholesterol upregulates diverse estrogen target genes;
modulates ERα and Cyclin D1 protein levels; stimulates growth in
breast and ovarian cancer cells and induces an ER-mediated
prevention of hypoxia-dependent apoptosis (46). Consistent with these results of
experimental models (46), it was
observed in the present study that 25-hydroxycholesterol is
aberrantly upregulated in bladder cancer and it promotes
proliferation in T24 and RT4 bladder cancer cells.
EMT is a process that exerts pivotal roles in
development and wound healing that is characterized by loss of
homotypic adhesion and cell polarity, and increased aggressive
phenotypes such as invasion and migration (47). At the molecular level, EMT is
characterized by loss of epithelial proteins (e.g., E-cadherin and
keratin 8) and increased expression of mesenchymal proteins [e.g.,
fibronectin and N-cadherin (47)].
To the best of our knowledge, the present study is the first to
demonstrate that 25-hydroxycholesterol regulates the expressions of
EMT-associated genes in bladder cancer cells. These results suggest
that increased 25-hydroxycholesterol promotes EMT in bladder
cancer. EMT is associated with bladder cancer progression and
metastasis, and also monitors drug efficacy (47). Loss of epithelial markers and
increased expression of mesenchymal markers have been observed in
Adriamycin-resistant cancer cell lines (48). The present study demonstrated that
25-hydroxycholesterol promotes Adriamycin resistance in T24 and RT4
cells and increased 25-hydroxycholesterol is associated with
shorter overall survival of bladder cancer patients. In addition,
SOCS3 overexpression enhances Adriamycin resistance in T24 bladder
cancer cells (6). It was also
observed that the protein expression of SOCS3 was increased by
25-hydroxycholesterol in T24 and RT4 cells. Taken together, these
results indicate that 25-hydroxycholesterol may promote Adriamycin
resistance by regulating SOCS3 expression.
Overall, the present study provided evidence towards
the underlying molecular mechanisms and clinical implications for
the role of 25-hydroxycholesterol in bladder cancer.
25-hydroxycholesterol is significantly upregulated in human bladder
cancer samples, and may act as a crucial regulator, which promotes
cells proliferation and EMT in bladder cancer. In addition,
25-hydroxycholesterol is associated with shorter overall survival
time and may be a prognostic biomarker for monitoring Adriamycin
efficacy and early detection of Adriamycin resistance in bladder
cancer.
Acknowledgements
Not applicable.
Funding
The present study was funded by Tianyou Hospital
Affiliated to Tongji University and Jinling Hospital.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
CW performed the majority of the experimental work,
designed the study and wrote the initial draft of the manuscript.
HH and WF performed the remainder of the experimental work and
assisted with the preparation of the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Jinling Hospital (approval no.20150708) and written
informed consent was obtained from all patients at the time of
enrollment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Antoni S, Ferlay J, Soerjomataram I, Znaor
A, Jemal A and Bray F: Bladder cancer incidence and mortality: A
global overview and recent trends. Eur Urol. 71:96–108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F
and Liu Y: Long non-coding RNA UCA1 increases chemoresistance of
bladder cancer cells by regulating Wnt signaling. FEBS J.
281:1750–1758. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lacey JV Jr, Devesa SS and Brinton LA:
Recent trends in breast cancer incidence and mortality. Environ Mol
Mutagen. 39:82–88. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sternberg CN, Donat SM, Bellmunt J,
Millikan RE, Stadler W, De Mulder P, Sherif A, von der Maase H,
Tsukamoto T and Soloway MS: Chemotherapy for bladder cancer:
Treatment guidelines for neoadjuvant chemotherapy, bladder
preservation, adjuvant chemotherapy, and metastatic cancer.
Urology. 69 (Suppl):62–79. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li MZ, Lai DH, Zhao HB, Chen Z, Huang QX
and Situ J: SOCS3 overexpression enhances ADM resistance in bladder
cancer T24 cells. Eur Rev Med Pharmacol Sci. 21:3005–3011.
2017.PubMed/NCBI
|
|
7
|
Meng Q, Lei T and Zhang M, Zhao J, Zhao
X-H and Zhang M: Identification of proteins differentially
expressed in adriamycin-resistant (pumc-91/ADM) and parental
(pumc-91) human bladder cancer cell lines by proteome analysis. J
Cancer Res Clin Oncol. 139:509–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsuruo T, Iida H, Nojiri M, Tsukagoshi S
and Sakurai Y: Circumvention of vincristine and Adriamycin
resistance in vitro and in vivo by calcium influx blockers. Cancer
Res. 43:2905–2910. 1983.PubMed/NCBI
|
|
9
|
Li Q-Q, Xu J-D, Wang W-J, Cao XX, Chen Q,
Tang F, Chen ZQ, Liu XP and Xu ZD: Twist1-mediated
adriamycin-induced epithelial-mesenchymal transition relates to
multidrug resistance and invasive potential in breast cancer cells.
Clin Cancer Res. 15:2657–2665. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smith LL: Cholesterol autoxidation
1981–1986. Chem Phys Lipids. 44:87–125. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nelson ER, Wardell SE, Jasper JS, Park S,
Suchindran S, Howe MK, Carver NJ, Pillai RV, Sullivan PM, Sondhi V,
et al: 27-Hydroxycholesterol links hypercholesterolemia and breast
cancer pathophysiology. Science. 342:1094–1098. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leoni V, Masterman T, Mousavi FS, Wretlind
B, Wahlund LO, Diczfalusy U, Hillert J and Björkhem I: Diagnostic
use of cerebral and extracerebral oxysterols. Clin Chem Lab Med.
42:186–191. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saito Y and Noguchi N: 7-Hydroxycholestrol
as a possible biomarker of cellular lipid peroxidation: Difference
between cellular and plasma lipid peroxidation. Biochem Biophys Res
Commun. 446:741–744. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hahn C, Reichel C and von Bergmann K:
Serum concentration of 7 alpha-hydroxycholesterol as an indicator
of bile acid synthesis in humans. J Lipid Res. 36:2059–2066.
1995.PubMed/NCBI
|
|
15
|
Kuroki S, Okamoto S, Naito T, Oda H,
Nagase S, Sakai H, Nawata H, Yamashita H, Chijiiwa K and Tanaka M:
Serum 7 α-hydroxycholesterol as a new parameter of liver function
in patients with chronic liver diseases. Hepatology. 22:1182–1187.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koschack J, Lütjohann D, Schmidt-Samoa C
and Irle E: Serum 24S-hydroxycholesterol and hippocampal size in
middle-aged normal individuals. Neurobiol Aging. 30:898–902. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bandaru VVR and Haughey NJ: Quantitative
detection of free 24S-hydroxycholesterol, and 27-hydroxycholesterol
from human serum. BMC Neurosci. 15:1372014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zuliani G, Donnorso MP, Bosi C, Passaro A,
Dalla Nora E, Zurlo A, Bonetti F, Mozzi AF and Cortese C: Plasma
24S-hydroxycholesterol levels in elderly subjects with late onset
Alzheimer's disease or vascular dementia: A case-control study. BMC
Neurol. 11:1212011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leoni V, Long JD, Mills JA, Di Donato S
and Paulsen JS; PREDICT-HD study group, : Plasma
24S-hydroxycholesterol correlation with markers of Huntington
disease progression. Neurobiol Dis. 55:37–43. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Babiker A, Dzeletovic S, Wiklund B,
Pettersson N, Salonen J, Nyyssönen K, Eriksson M, Diczfalusy U and
Björkhem I: Patients with atherosclerosis may have increased
circulating levels of 27-hydroxycholesterol and cholestenoic acid.
Scand J Clin Lab Invest. 65:365–375. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ziedén B, Kaminskas A, Kristenson M,
Kucinskienê Z, Vessby B, Olsson AG and Diczfalusy U: Increased
plasma 7 β-hydroxycholesterol concentrations in a population with a
high risk for cardiovascular disease. Arterioscler Thromb Vasc
Biol. 19:967–971. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Murakami H, Tamasawa N, Matsui J, Yasujima
M and Suda T: Plasma oxysterols and tocopherol in patients with
diabetes mellitus and hyperlipidemia. Lipids. 35:333–338. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Linseisen J, Wolfram G and Miller AB:
Plasma 7β-hydroxycholesterol as a possible predictor of lung cancer
risk. Cancer Epidemiol Biomarkers Prev. 11:1630–1637.
2002.PubMed/NCBI
|
|
24
|
Prunet C, Petit JM, Ecarnot-Laubriet A,
Athias A, Miguet-Alfonsi C, Rohmer JF, Steinmetz E, Néel D, Gambert
P and Lizard G: High circulating levels of 7β- and
7α-hydroxycholesterol and presence of apoptotic and oxidative
markers in arterial lesions of normocholesterolemic atherosclerotic
patients undergoing endarterectomy. Pathol Biol (Paris). 54:22–32.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Noguchi N, Saito Y and Urano Y: Diverse
functions of 24(S)-hydroxycholesterol in the brain. Biochem Biophys
Res Commun. 446:692–696. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lütjohann D, Papassotiropoulos A, Björkhem
I, Locatelli S, Bagli M, Oehring RD, Schlegel U, Jessen F, Rao ML,
von Bergmann K, et al: Plasma 24S-hydroxycholesterol
(cerebrosterol) is increased in Alzheimer and vascular demented
patients. J Lipid Res. 41:195–198. 2000.PubMed/NCBI
|
|
27
|
Kloudova A, Guengerich FP and Soucek P:
The Role of Oxysterols in Human Cancer. Trends Endocrinol Metab.
28:485–496. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bergkvist A, Ljungqvist A and Moberger G:
Classification of bladder tumours based on the cellular pattern.
Preliminary report of a clinical-pathological study of 300 cases
with a minimum follow-up of eight years. Acta Chir Scand.
130:371–378. 1965.PubMed/NCBI
|
|
29
|
Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F
and Liu Y: TGF-β-induced upregulation of malat1 promotes bladder
cancer metastasis by associating with suz12. Clin Cancer Res.
20:1531–1541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Uicc T: Classification of malignant
tumours. Wiley-Liss; New York, NY: 2002
|
|
31
|
Huang L, Luo J, Cai Q, Pan Q, Zeng H, Guo
Z, Dong W, Huang J and Lin T: MicroRNA-125b suppresses the
development of bladder cancer by targeting E2F3. Int J Cancer.
128:1758–1769. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yoshino H, Chiyomaru T, Enokida H,
Kawakami K, Tatarano S, Nishiyama K, Nohata N, Seki N and Nakagawa
M: The tumour-suppressive function of miR-1 and miR-133a targeting
TAGLN2 in bladder cancer. Br J Cancer. 104:808–818. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu DL, Sookthai D, Le Cornet C, et al:
Reproducibility of serum oxysterols and lanosterol among
postmenopausal women: Results from EPIC-Heidelberg. Clin Biochem.
52:117–122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu Q, Ishikawa T, Sirianni R, Tang H,
McDonald JG, Yuhanna IS, Thompson B, Girard L, Mineo C, Brekken RA,
et al: 27-Hydroxycholesterol promotes cell-autonomous, ER-positive
breast cancer growth. Cell Rep. 5:637–645. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liao XH, Lu DL, Wang N, Liu LY, Wang Y, Li
YQ, Yan TB, Sun XG, Hu P and Zhang TC: Estrogen receptor α mediates
proliferation of breast cancer MCF-7 cells via a
p21/PCNA/E2F1-dependent pathway. FEBS J. 281:927–942. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Varol N, Konac E and Bilen CY: Does
Wnt/β-catenin pathway contribute to the stability of DNMT1
expression in urological cancer cell lines? Exp Biol Med (Maywood).
240:624–630. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Metz CE: Basic principles of ROC analysis.
Semin Nucl Med. 8:283–298. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zweig MH and Campbell G:
Receiver-operating characteristic (ROC) plots: A fundamental
evaluation tool in clinical medicine. Clin Chem. 39:561–577. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wallace DM, Bryan RT, Dunn JA, Begum G and
Bathers S; West Midlands Urological Research Group, : Delay and
survival in bladder cancer. BJU Int. 89:868–878. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hu J, La Vecchia C, de Groh M, Negri E,
Morrison H and Mery L; Canadian Cancer Registries Epidemiology
Research Group, : Dietary cholesterol intake and cancer. Ann Oncol.
23:491–500. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mouritsen OG and Bagatolli LA: Lipid
domains in model membranes: A brief historical perspective. Essays
Biochem. 57:1–19. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chichili GR and Rodgers W:
Cytoskeleton-membrane interactions in membrane raft structure. Cell
Mol Life Sci. 66:2319–2328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
George KS and Wu S: Lipid raft: A floating
island of death or survival. Toxicol Appl Pharmacol. 259:311–319.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hamm R, Chen Y-R, Seo E-J, Zeino M, Wu CF,
Müller R, Yang NS and Efferth T: Induction of cholesterol
biosynthesis by archazolid B in T24 bladder cancer cells. Biochem
Pharmacol. 91:18–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bauman DR, Bitmansour AD, McDonald JG,
Thompson BM, Liang G and Russell DW: 25-Hydroxycholesterol secreted
by macrophages in response to Toll-like receptor activation
suppresses immunoglobulin A production. Proc Natl Acad Sci USA.
106:16764–16769. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lappano R, Recchia AG, De Francesco EM,
Angelone T, Cerra MC, Picard D and Maggiolini M: The cholesterol
metabolite 25-hydroxycholesterol activates estrogen receptor
α-mediated signaling in cancer cells and in cardiomyocytes. PLoS
One. 6:e166312011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
McConkey DJ, Choi W, Marquis L, Martin F,
Williams MB, Shah J, Svatek R, Das A, Adam L, Kamat A, et al: Role
of epithelial-to-mesenchymal transition (EMT) in drug sensitivity
and metastasis in bladder cancer. Cancer Metastasis Rev.
28:335–344. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sommers CL, Heckford SE, Skerker JM,
Worland P, Torri JA, Thompson EW, Byers SW and Gelmann EP: Loss of
epithelial markers and acquisition of vimentin expression in
adriamycin- and vinblastine-resistant human breast cancer cell
lines. Cancer Res. 52:5190–5197. 1992.PubMed/NCBI
|