Introduction
Lung cancer is the most common cancer worldwide.
Although various types of therapy have been used for its treatment,
the mortality rate remains high; in 2018, there were 234,030 new
lung cancer cases and 154,050 lung cancer-associated deaths
reported in the United States (1).
It is therefore crucial to further investigate the underlying
mechanisms of lung cancer development.
Indoleamine-2,3-dioxygenase 1 (IDO1) is a
rate-limiting metabolic enzyme that converts tryptophan into
downstream catabolites known as kynurenines (2). It has been reported that kynurenines
can inhibit the activity and proliferation of T cells and other
immune cells, such as dendritic cells (3,4), leading
to successful tumor escape from immune surveillance. The tumor
microenvironment (TME) is a complicated environment of
proliferating tumor cells that also contains a number of immune
cells stimulating cancer progression by tumor immune escape. IDO1
is highly expressed in human lung cancer tissue (5) and has been reported to elicit
immunosuppressive effects and favor tumor progression in animal
models of lung cancer (6). Our
previous study has found that IDO1 is highly expressed in mouse LLC
cells. The immunosuppression effect of IDO1 in LLC cells is
associated with regulatory T-cell expansion, which serves a crucial
role in tumorigenesis and tumor development (7).
T-cell exhaustion is a process that occurs during
tumor progression and chronic viral infections, in which T cells
lose their ability to kill cancer or virus-infected cells upon
chronic antigen exposure (8,9). T-cell exhaustion is characterized by
the increased expression of numerous inhibitory receptors,
including programmed death-1 (PD-1) (10,11), B
and T lymphocyte attenuator (BTLA) (11), T-cell immunoglobulin domain and mucin
domain 3 (TIM3) (10),
lymphocyte-activation gene 3 (12),
CD244 (13) and cytotoxic T
lymphocyte antigen-4 (CTLA4) (13).
In addition, the secretion of certain cytokines, including
interleukin-2 (IL-2), tumor necrosis factor-α (TNF-α) and
interferon-γ (IFN-γ) (13–15), is decreased during T-cell exhaustion.
Recently, immunotherapy has demonstrated considerable promise in
the management of various types of malignancy, including NSCLC and
hepatocellular carcinoma (16,17).
Recent studies have reported that inhibition of T-cell exhaustion
is an important form of cancer immunotherapy (18,19). An
increasing number of recognized immune checkpoint inhibitors,
including those of PD-1 and CTLA4, affect the local tumor immune
environment by blocking the PD-1/PD-L1 and CTLA4 signaling pathways
and have been used to treat certain types of cancer, including
melanoma (20), non-small-cell lung
cancer (21) and urothelial cancer
(22). These immune checkpoint
inhibitors can restore T-cell antitumor activity by suppressing
T-cell exhaustion. At present, an increasing number of new immune
checkpoint inhibitors are being discovered, including LAG-3
(23), TIM-3 (24) and TIGIT (24).
The role of IDO1 in the immune escape mechanism of
cancer was first introduced in 2002 by Friberg et al
(25), who reported that LLC cells
stimulated a stronger allogeneic T-cell response when cultured in
the presence of an IDO1 inhibitor, leading to a delay in LLC tumor
growth following systemic treatment in vivo. Furthermore,
IDO-knockout mice presented with reduced tumor size and inhibited
expression of CD4+ and CD8+-PD1 in
tumor-infiltrating T-cells (26).
These studies suggested that IDO1 may serve a crucial role in
T-cell response during tumorigenesis; however, the association
between IDO1 and T-cell exhaustion in the tumor microenvironment
remains unknown and requires further investigation.
In the present study, the role of IDO1 in T-cell
exhaustion during tumor progression was investigated, with the aim
to clarify the association between IDO1 expression levels and lung
cancer. In addition, the present study investigated whether IDO1
can inhibit lung cancer growth by suppressing T-cell exhaustion in
the lung tumor-bearing mice which may inform the development of
IDO1-targeted molecular therapy to inhibit T-cell exhaustion of
lung cancer.
Materials and methods
Cell lines and mice
The Lewis Lung Carcinoma (LLC) cell line was
purchased from The Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences and was authenticated by conventional
tests of cell line quality control methods (morphology, isoenzymes,
mycoplasma). Cells were cultured in Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal
bovine serum, 2 mmol/l L-glutamine and 100 U/ml
penicillin/streptomycin (all Gibco; Thermo Fisher Scientific, Inc.)
placed at 37°C in a humidified incubator containing 5%
CO2.
A total of nine female C57BL/6 mice (aged 6–8 weeks,
weighing 15–20 g) were purchased from The Shanghai SLAC Laboratory
Animal Co., Ltd. The use of all mice in the present study followed
the Regulations for the Administration of Affairs Concerning
Experimental Animals of China, and Ethics approval from the
Institutional Animal Care and Use Committee of Jiangxi Academy of
Medical Sciences in China was obtained. The mice were housed in a
specific pathogen-free animal facility affiliated to Jiangxi
Academy of Medical Sciences with a controlled temperature of 25°C,
50–65% relative humidity and a 12-h light/dark cycle. The mice had
ad libitum access to sterile water and food.
IDO1 transfection with small
interfering (si)RNA
siRNA targeting IDO1 and luciferase gene glabra 2
(GL2; GL2-siRNA) were designed and synthesized by GE Healthcare
Dharmacon, Inc. The GL2-siRNA, which was not expressed in treated
cells (scrambled siRNA), was used as a negative control. The
sequences of the siRNA were as follows: IDO1 siRNA,
5′-GGGCUUCUUCCUCGUCUCUTT-3′ and GL2 siRNA,
5′-CGUACGCGGAAUACUUCGA-3′. These siRNAs were transfected into LLC
cells with Lipofectamine® 2000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). Briefly, LLC cells
(1×105/well) were seeded into 12-well plates until they
reached 50–70% confluence. Before transfection, medium was replaced
with 300 µl OptiMEM® serum-reduced medium (Gibco; Thermo
Fisher Scientific, Inc.). Subsequently, 1 µg IDO1-siRNA or
GL2-siRNA was incubated with 2 µl Lipofectamine® 2000
reagent in 200 µl OptiMEM® serum-reduced medium at room
temperature for 20 min, followed by addition of the mixture to the
cells that were gently agitated to distribute the mixture uniformly
for 24 h.
Extraction of IDO1 mRNA and reverse
transcription-quantitative (RT-q)PCR
Total RNA was extracted from cells using Invitrogen
TRIzol® Reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). For mRNA quantification, 1 µg total RNA was transcribed into
cDNA using MMLV Reverse Transcriptase kit (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturers'
instructions. The sequences of the primers were as follows: β-actin
forward 5′-AGGGAAATCGTGCGTGACAT-3′ and reverse,
5′-AACCGCTCGTTGCCAATAGT-3′; IDO1 forward,
5′-GTACATCACCATGGCGTATG-3′ and reverse, 5′-CGAGGAAGAAGCCCTTGTC-3′.
QPCR was performed using SYBR® Green PCR Master Mix
(Takara Bio, Inc.) in a final volume of 20 µl on the Bio-Rad
CFX96TM Real-Time System (Bio-Rad Laboratories, Inc.). The
amplification conditions were 95°C for 30 sec, 60°C for 30 sec and
72°C for 15 sec for 45 cycles. The expression levels of mRNA were
normalized to β-actin. The relative expressions level of IDO1 was
normalized to endogenous control and was expressed as
2−ΔΔCq (27).
Western blotting
LLC cells were lysed using RIPA buffer (Beijing
Solarbio Science & Technology Co., Ltd.) containing PMSF
protease inhibitor (1 mmol/l). Protein concentration was determined
by using the bicinchoninic acid protein assay (Bio-Rad
Laboratories, Inc.). Proteins (30 µg) were separated by 8% SDS-PAGE
and transferred to PVDF membranes. The membranes were blocked with
5% nonfat milk and 3% BSA in TBST (0.25% Tween-20) and subsequently
incubated overnight at 4°C with the following primary antibodies:
Mouse anti-human IDO1 mAb (cat. no. sc-53978; 1:200; Santa Cruz
Biotechnology, Inc.) and mouse anti-human β-actin mAb (cat. no.
sc-47778; 1:2,000; Santa Cruz Biotechnology, Inc.). The membranes
were washed three times with TBST and incubated with the secondary
antibody, goat anti-mouse IgG-HRP (cat. no. sc-358914; 1:5,000;
Santa Cruz Biotechnology, Inc.), at room temperature for 2 h.
Enhanced chemiluminescence reagent (OriGene Technologies, Inc.) was
used to detect the signal on the membrane. The relative expression
levels of the IDO1 protein were calculated using the gray scale
ratio of IDO1/β-actin using ImageJ version 1.46 software (National
Institutes of Health).
shRNA expression vector treatment
All mice were successfully modeled and randomly
divided into a control group (no treatment group) (n=3), a
scrambled-shRNA treatment group (n=3) and an IDO1-shRNA treatment
group (n=3) (28). Briefly, C57BL/6
mice were treated with 40 µg IDO1- or scrambled-shRNA (GE
Healthcare Dharmacon, Inc.) dissolved in 1 ml PBS by hydrodynamic
tail intravenous injection 3 days before cancer inoculation,
5×105 LLC cells resuspended in 0.1 ml PBS were injected
subcutaneously into the upper hind leg of all C57BL/6 mice
(28). On days 7, 14 and 21
following cancer cell inoculation, expect the control group mice
(no treatment), mice were injected with 40 µg IDO1-shRNA or
scrambled-shRNA dissolved in 1 ml PBS by hydrodynamic tail
intravenous injection. Tumor size was measured with a caliper every
other day, and the tumor volume was calculated as follows (29,30):
Tumor volume=0.5× (tumor length) × (tumor width)2. Tumor
onset was considered to have occurred when the tumor diameter
reached 5 mm. On day 21, all mice were sacrificed with 30%/min
carbon dioxide for ~180 sec prior to cervical dislocation (31,32) and
were confirmed dead based on cardiac arrest and respiratory arrest
40–60 sec later. Tumors, lymph nodes and spleens were collected,
tumors were weighed and tumor volumes were measured. These tissues
were stored at −80°C for further experiments. The shRNA sequences
were as follows: IDO1 shRNA, 5′-GGGCUUCUUCCUCGUCUCUTT-3′ and
scramble-shRNA, 5′-CGUACGCGGAAUACUUCGA-3′.
Co-culture of LLC cells and
lymphocytes in vitro
LLC cells were seeded in 12-well plates at a density
of 1×105 cells/well for 24 h and were transfected with
IDO1- or GL2-siRNA. After 24 h of transfection, lymphocytes
isolated from the spleen of LLC-bearing C57BL/6 mice (33). Briefly, spleens (~1 cm in length and
30 mm in width) were placed on a 40 µm Falcon Cell Strainer (VWR
International, LLC.) and gently squashed with a plunger. The cell
suspension was collected to centrifuge at 4°C, 250 g for 5 min,
then further isolated using ACK Lysing Buffer (Beijing Solarbio
Science & Technology Co., Ltd.) to lyse red blood cells.
Lymphocytes were added to the LLC cells at a density of
5×105 cells/well and were cultured at 37°C with 5%
CO2 for 48 h. Lymphocytes were subsequently collected
and stained with anti-CD4-FITC (cat. no. 553047; 1:200; BD
Pharmingen; BD Biosciences), anti-CD8-PE (cat. no. 12-0081-82;
1:200; eBioscience; Thermo Fisher Scientific, Inc.), anti-PD-1-APC
(cat. no. 17-9985-82; 1:200; eBioscience; Thermo Fisher Scientific,
Inc.) and anti-BTLA-PE (Cat. no. 12-5950-82; 1:200; eBioscience;
Thermo Fisher Scientific, Inc.), incubated at 4°C in dark for 30
min, then detected using flow cytometry (BD FACSCanto II; BD
Biosciences). The data were analyzed suing FlowJo version 10
software (Becton, Dickinson and Company).
Immunohistochemistry (IHC)
staining
Tumor tissues collected from lung tumor-bearing mice
on day 21 were fixed in 4% paraformaldehyde at 4°C for 24 h and
embedded in paraffin. The 4–7 µm sections were deparaffinized and
endogenous peroxidase activity was quenched using a 3%
H2O2 solution at 20°C for 20 min. The
antigens were retrieved using a sodium citrate buffer (0.01M, pH
6.0) in a pressure cooker (100°C) for 15 min and tissue sections
were incubated with an IDO1 primary antibody (cat. no. sc-53978;
1:50; Santa Cruz Biotechnology, Inc.) for 16 h at 4°C. The sections
were washed three times with PBS, incubated with ready to use
enzyme-conjugated streptavidin (cat. no. 14C12B01; Boster
Biological Technology) at 37°C for 30 min, washed three times with
PBS and stained with 3′-diaminobenzidine. Subsequently, the degree
of immunostaining was scored according to the proportion of
positively stained tumor cells and the intensity of staining. The
percentage of tumor cell staining was scored as 0 (negative), 1
(weak), 2 (moderate) and 3 (strong) for 0, <25, 26–50 and
>50% of positively stained cancer cells, respectively. The final
staining score was calculated as follows: Staining score=intensity
score × percentage score.
Phenotypic flow cytometry
analysis
To assess T-cell exhaustion, on day 21 following LLC
cell injection in C57BL/6 mice, lymphocytes from the lymph node and
the spleen were collected from mice. Single-cell suspensions were
prepared as aforementioned and analyzed using flow cytometry, the
detailed experimental procedures for cells prepared, stained and
analyzed were described in the ‘Co-culture of LLC cells and
lymphocytes in vitro’ section.
Secreted cytokine detection by
ELISA
The serum was obtained from the mice blood as
follows. After anesthesia, 0.5–1 ml blood was collected from the
eyeballs of mice, which was kept at room temperature for 2 h, then
centrifuged at 3,000 × g for 10 min to separate the serum. The
concentration of TNF-α and IL-2 in mouse serum was performed using
Quantikine Mouse TNF-α (cat. no. 88-7324-88; Invitrogen, Thermo
Fisher Scientific, Inc.) and IL-2 Immunoassay kits (cat. no.
88-7024-88; Invitrogen, Thermo Fisher Scientific, Inc.) according
to the manufacturer's instructions. Briefly, ELISA plates were
coated with capture antibody, blocked with ELISA/ELISPOT Diluent,
incubated with detection antibody and washed, serum cytokines were
detected by incubation with Avidin-HRP at room temperature for 30
min. Plates were then washed, substrate was added and plates were
read at 450 nm using microplate reader (Molecular Devices).
Statistical analysis
Data are presented as the mean ± standard deviation
and were analyzed using GraphPad Prism 5 (GraphPad Software, Inc.).
Student's t-test (two-tailed unpaired) was used to compare the
differences between two groups. One-way ANOVA was used to compare
≥3 groups followed by Tukey's post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
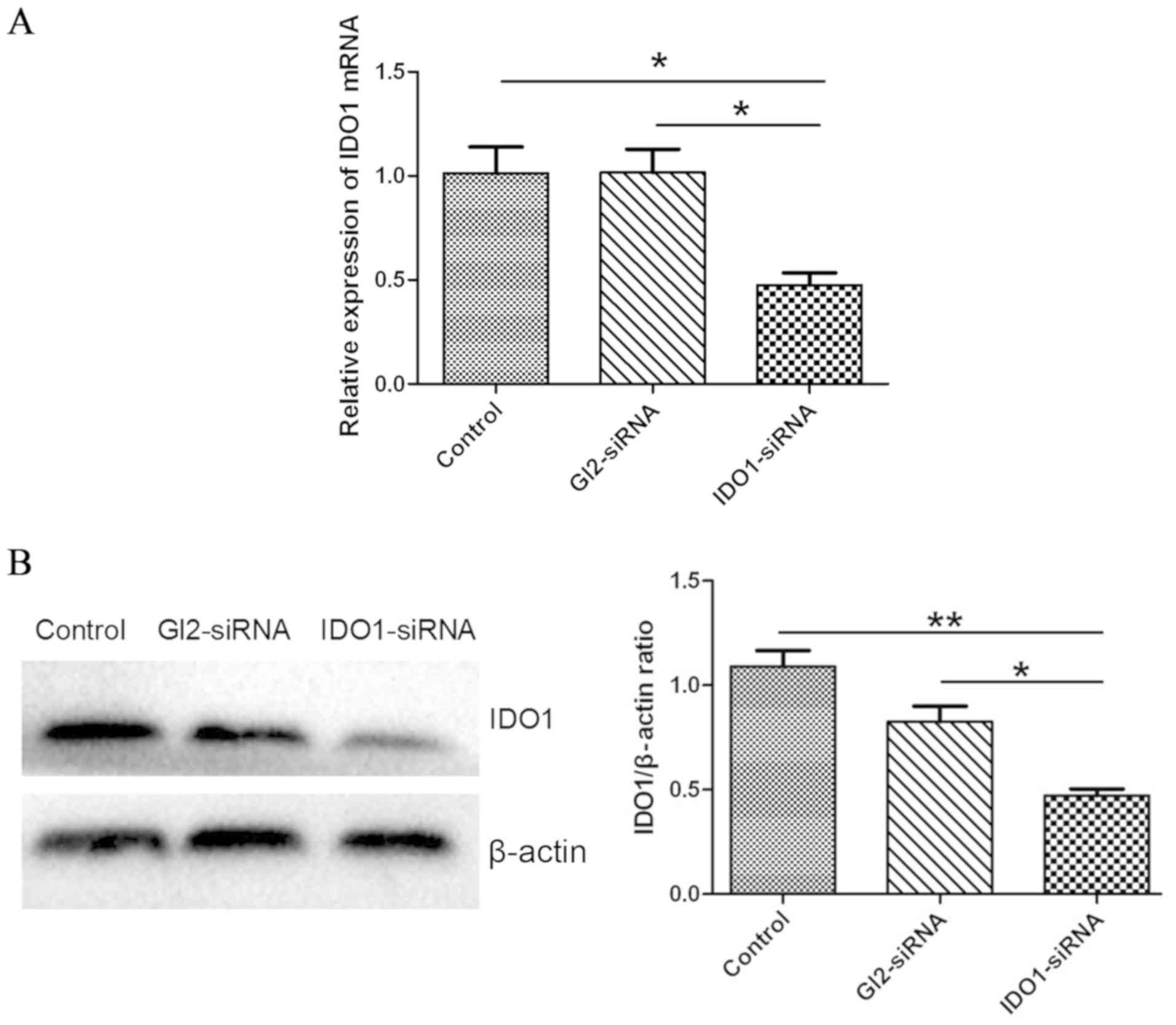
IDO1-siRNA decreases IDO1 expression
in vitro
To confirm IDO1 knockdown by IDO1-siRNA in LLC
cells, IDO1 mRNA and protein expression was assessed by RT-qPCR and
western blotting, respectively. As presented in Fig. 1A, IDO1 expression level in LLC cells
transfected with IDO1-siRNA was significantly decreased compared
with the control group (P<0.05). In addition, IDO1 protein
expression was significantly decreased following IDO1-siRNA
transfection compared with the control (Fig. 1B).
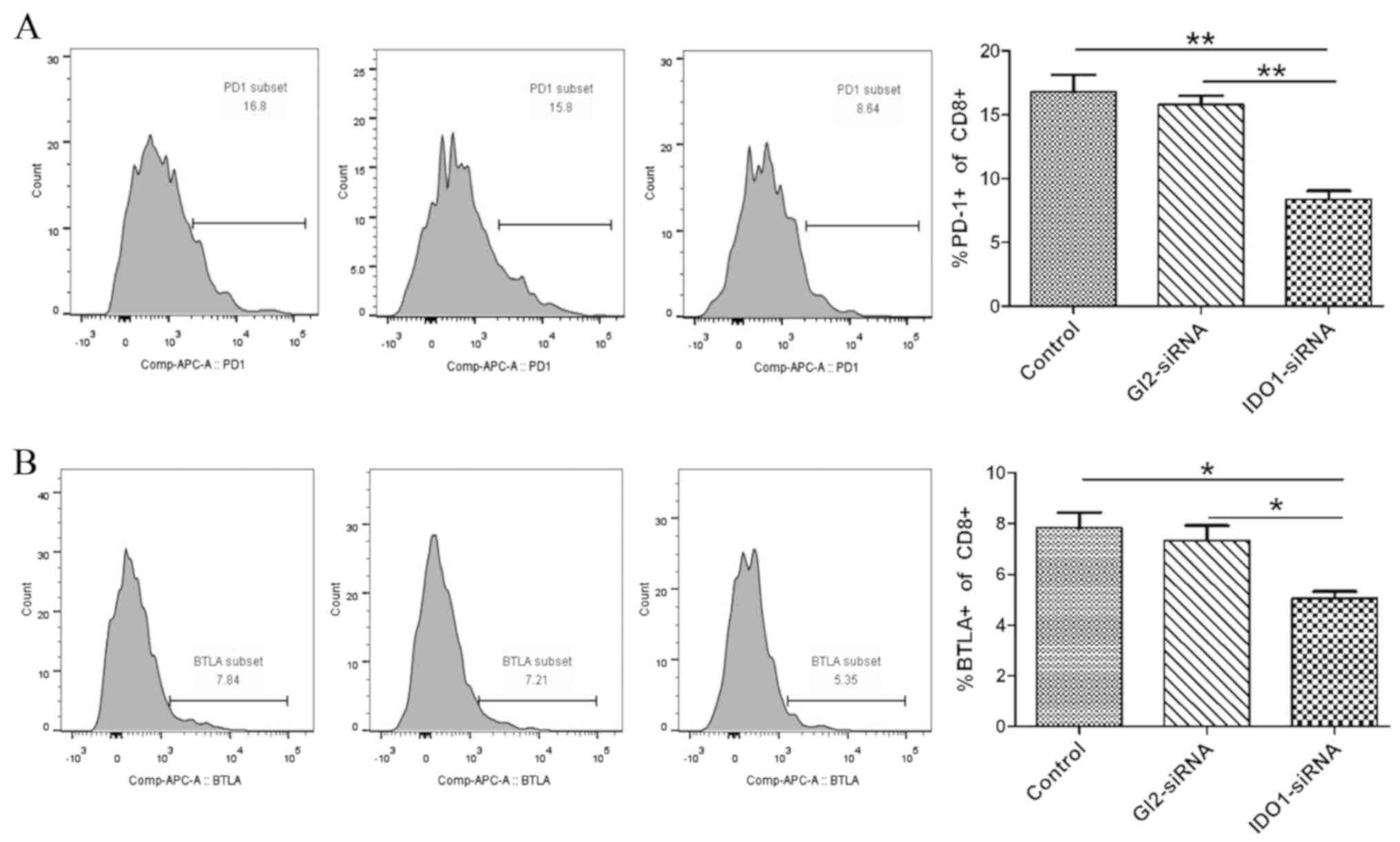
IDO1-siRNA inhibits T-cell exhaustion
in vitro
To determine the effect of IDO1 knockdown on T-cell
exhaustion, LLC cells treated with IDO1-siRNA, GL2-siRNA and the
untreated control group were co-cultured with T cells isolated from
the spleen of LLC tumor-bearing mice. T cells were collected, and
the expression of inhibitory receptors PD-1 and BTLA, which are
markers of T-cell exhaustion, was assessed by flow cytometry. The
results demonstrated that PD-1 and BTLA expression in
CD8+ T cells (PD-1, 8.37±1.17%; BTLA, 5.06±0.47%) in the
co-culture of IDO1-siRNA LLC cells and T cells was decreased
compared with the control (PD-1, 16.8±2.3%; BTLA, 7.83±1.07%) and
scrambled GL2-siRNA group (PD-1, 15.8±1.18%; BTLA, 7.34±1.02%;
Fig. 2A and B). These results
demonstrated that IDO1-siRNA in LLC cells inhibited T-cell
exhaustion in vitro.
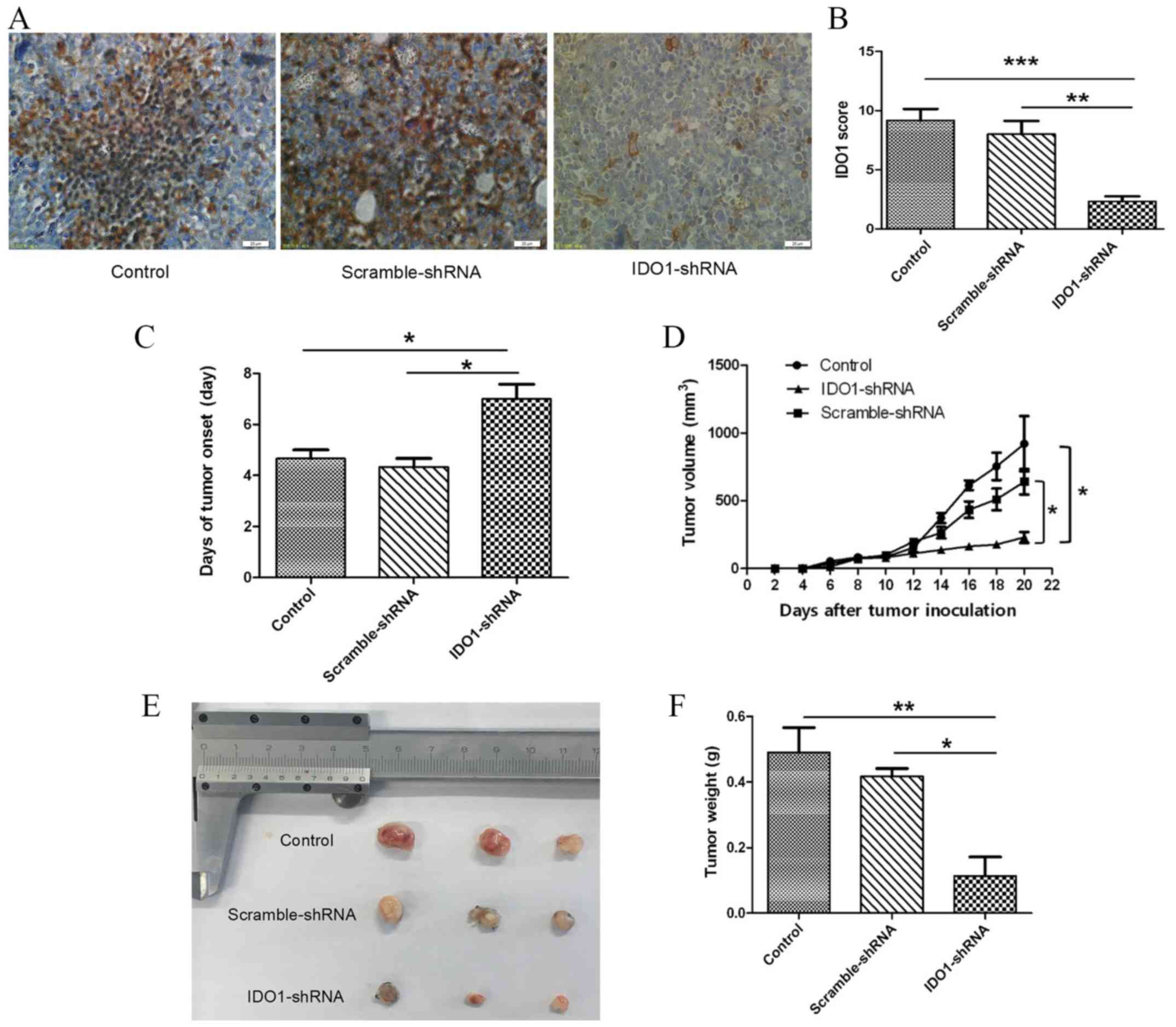
IDO1-shRNA transfection suppresses
lung cancer growth in vivo
The present study evaluated the therapeutic effect
of IDO1 knockdown by using IDO1-shRNA to treat tumor-bearing mice.
The IDO1 staining score of IDO1-shRNA-treated mice was decreased
compared with that of scrambled-shRNA-treated mice and the
untreated control group (Fig. 3A and
B), confirming the efficiency of IDO1 knockdown in tumor
tissues. Furthermore, in IDO1-shRNA-treated mice, the time of tumor
onset was delayed (Fig. 3C), and
tumor growth was significantly slower compared with that of
scrambled-shRNA-treated mice and control group (Fig. 3D). In addition, tumor size in
IDO1-shRNA-treated mice was decreased compared with
scrambled-shRNA-treated mice and the control group (Fig. 3E), and tumor weight was significantly
decreased in IDO1-shRNA-treated mice compared with the other two
groups (Fig. 3F). These results
suggested that intravenous injection of IDO1-shRNA may inhibit lung
cancer growth in mice.
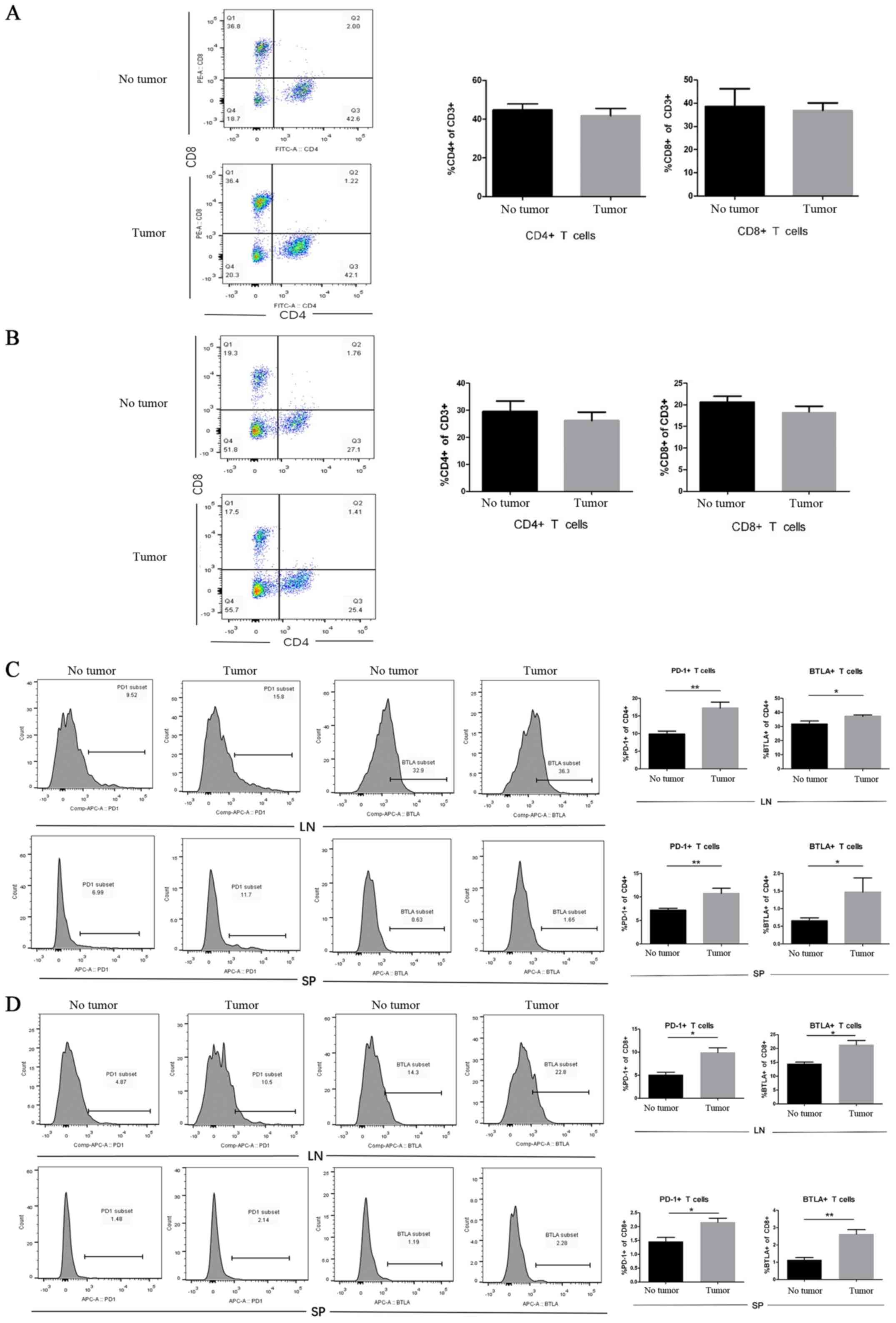
The presence of tumors increases the
expression of PD-1 and BTLA on CD4+ and CD8+ T cells in vivo
To determine the role of LLCs in the T-cell immune
response, C57BL/6 mice were inoculated subcutaneously with
5×105 LLC cells into the upper hind leg, whereas animals
were injected with PBS as the no tumor group. Lymphocytes from the
lymph nodes and the spleen were harvested and stained for
CD4+, CD8+ and the inhibitory receptors PD-1
and BTLA to analyze T cells in LLC tumor-bearing mice and no tumor
mice by flow cytometry. The results demonstrated that the
percentages of CD4+ and CD8+ T cells were
similar in tumor-bearing mice and mice without tumors (Fig. 4A and B). Furthermore, mice with
tumors presented with an increased percentage of PD-1+,
CD4+ and BTLA+ on CD4+ T cells
from the lymph nodes and the spleen compared with that in healthy
mice (Fig. 4C): in the lymph nodes,
the percentage of PD1+ cells was 17.2±0.9 vs. 9.9±0.5%,
and that of BTLA+ cells was 37.3±0.5 vs. 31.8±1.2% in
mice with tumor vs. mice without tumors, respectively. In the
spleen, the percentage of PD1+ cells was 10.7±0.6 vs.
7.2±0.2%, and that of BTLA+ cells was 1.5±0.2 vs.
0.7±0.05% in mice with tumor vs. mice without tumors, respectively.
Similar results were observed in CD8+ T cells (Fig. 4D), the percentage of PD1+
cells from the lymph node was 9.9±1.0 vs. 5.1±0.5% and that of
BTLA+ cells was 21.4±1.5 vs. 14.5±0.6% in mice with
tumors vs. mice without tumors, respectively. In the spleen, the
percentage of PD1+ cells was 2.2±0.1 vs. 1.5±0.1%, and
that of BTLA+ cells was 2.6±0.3 vs. 1.1±0.1% in mice
with tumors vs. mice without tumors, respectively. These results
demonstrated that tumors did not alter the number of
CD4+ and CD8+ T cells in the lymph nodes or
the spleen. However, the expression of PD1 and BTLA on
CD4+ and CD8+ T cells was increased in the
TME. Taken together, these results suggested that T-cell exhaustion
may occur in lung TME.
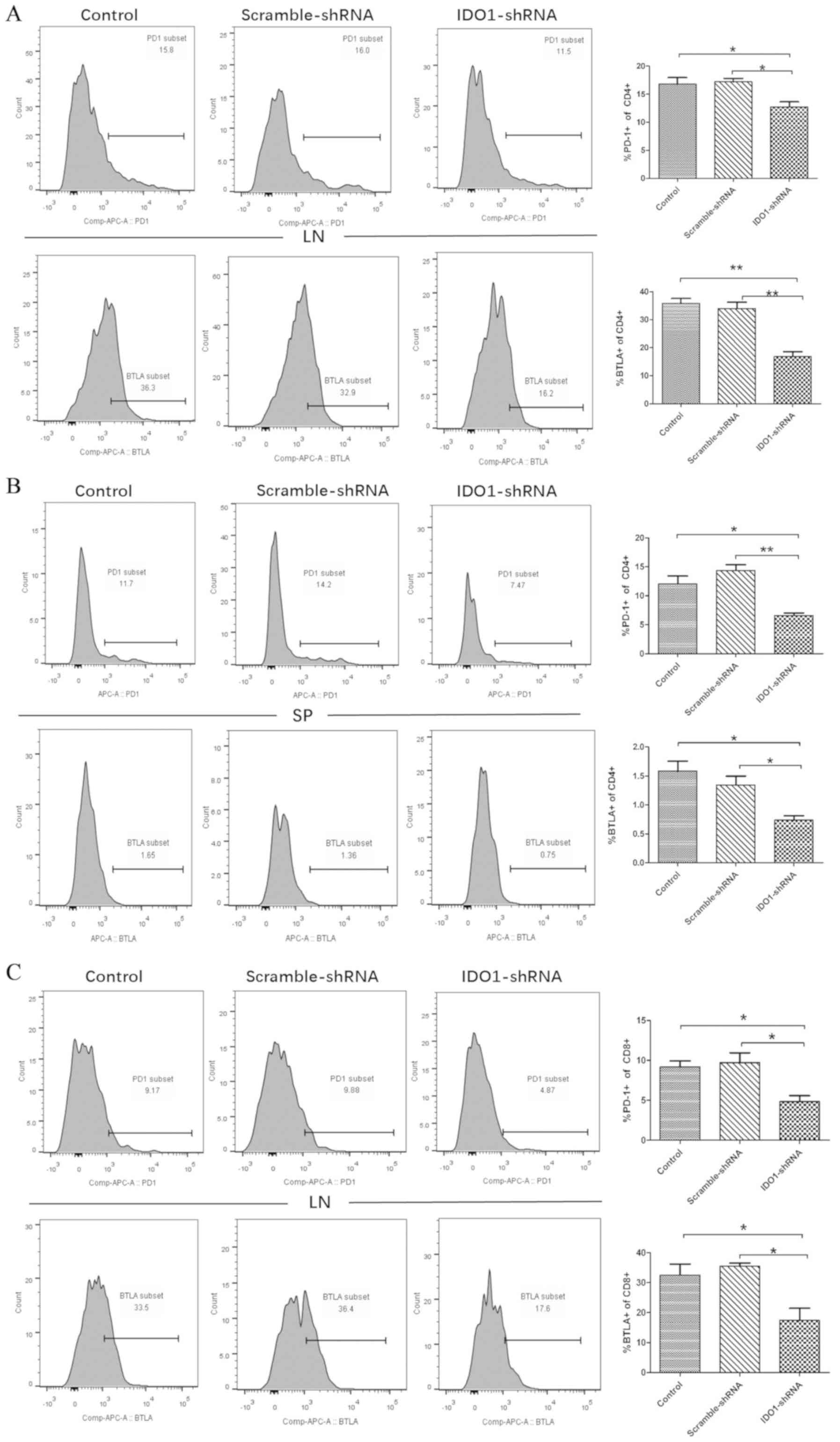
Treatment with IDO1-shRNA decreases
co-inhibitory receptor expression on
CD4+/CD8+ T cells in vivo
In the present study, IDO1-shRNA decreased the tumor
size of tumor-bearing mice. Subsequently, IDO1-shRNA treatment was
applied in vivo, as well as scrambled-shRNA treatment and
absence of treatment (control). Lymphocytes from the lymph nodes
and the spleen of LLC-bearing tumor mice were collected on day 21
to detect the expression of PD-1 and BTLA in CD4+ and
CD8+ T cells by flow cytometry. The expression of PD-1
and BTLA on CD4+ cells of lymph nodes from
IDO1-shRNA-treated mice was significantly decreased compared with
scrambled-shRNA-treated mice (16.8±0.6 to 12.7±0.9 and 35.8±2.3 to
16.8±1.8%, respectively; Fig. 5A).
The expression of PD-1 and BTLA on CD8+ T cells of lymph
nodes from IDO1-shRNA-treated mice was significantly lower compared
with that of mice treated with scrambled-shRNA (9.2±1.2 to 4.8±0.7
and 32.6±1.0 to 17.4±4.0%, respectively; far right-hand graph,
Fig. 5C). Furthermore, PD-1 and BTLA
expression on CD4+ T cells from the spleen was also
significantly decreased in IDO1-shRNA-treated mice compared with
scrambled-shRNA-treated mice and the control group (no treatment)
(Fig. 5B). These results
demonstrated that IDO1-shRNA suppressed T-cell exhaustion by
inhibiting the expression of co-inhibitory receptors.
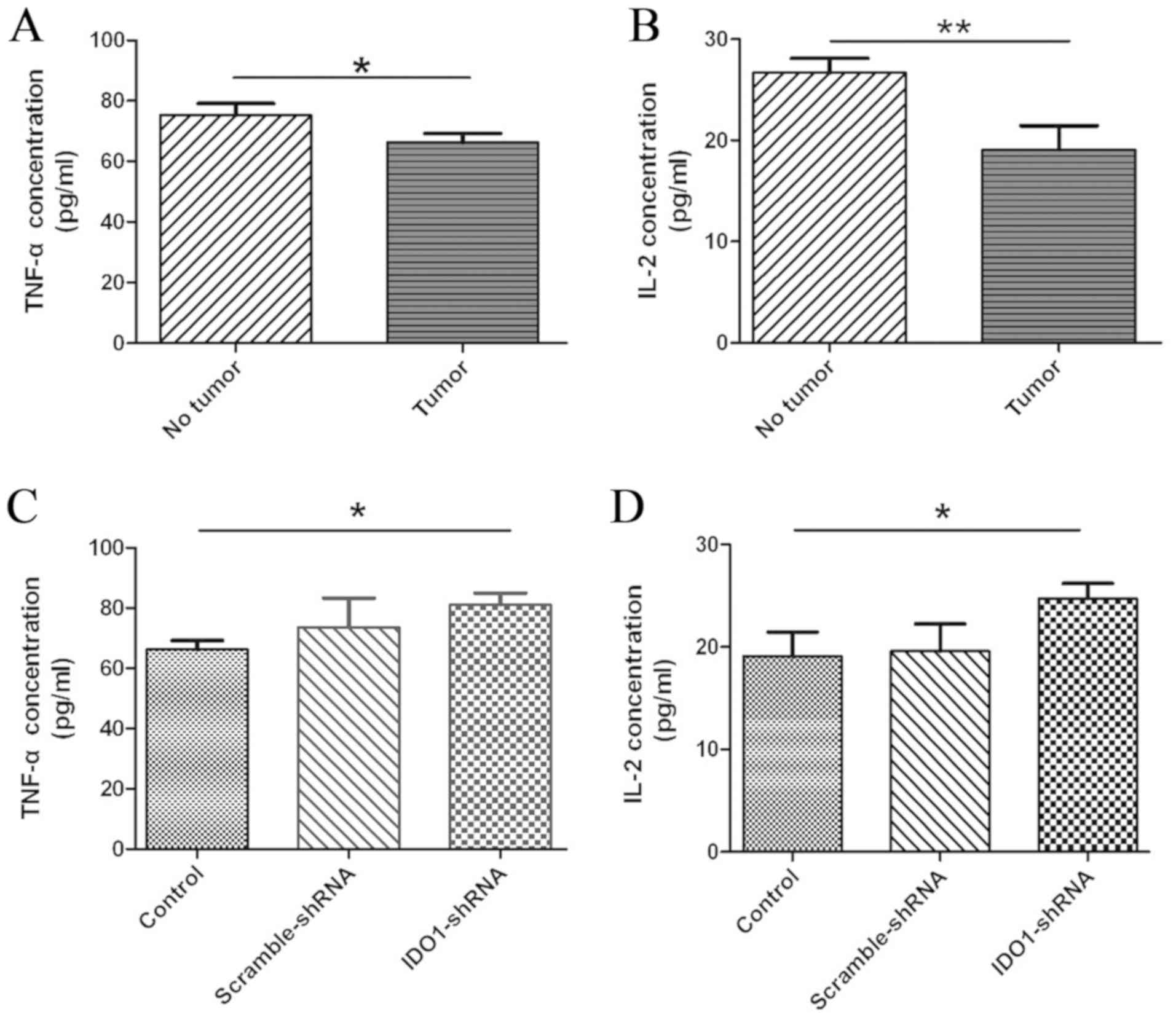
Treatment with IDO1-shRNA recovers
cytokine production in vivo
One characteristic of T-cell exhaustion is the
impaired production of cytokines (15). To determine whether treatment with
IDO1-shRNA in tumor-bearing mice increased cytokine production in
the TME, serum from untreated tumor-bearing mice (control),
scrambled-shRNA- or and IDO1-shRNA treated mice was collected.
Subsequently, serum TNF-α and IL-2 levels were analyzed by ELISA.
The results demonstrated that the serum TNF-α and IL-2 levels in
LLC-bearing mice were significantly lower compared with those in
healthy mice (Fig. 6A and B). In
addition, serum TNF-α and IL-2 levels in tumor-bearing mice treated
with IDO1-shRNA were significantly increased compared with the
control and scrambled-shRNA-treated mice (Fig. 6C and D). Furthermore, the TNF-α and
IL-2 concentrations of mice treated with IDO1-shRNA returned to the
level of mice without tumors (Fig. 6A, C
and B, D). These data suggested that treatment with IDO1-shRNA
recovered IL-2 and TNF-α production in vivo.
Discussion
IDO1 is an immunosuppressive regulatory factor in
the TME, which allows tumor cells to escape immune surveillance
(5). T-cell exhaustion is a state of
T-cell functional deficiency in tumors (34). At present, the effect of IDO1 on
T-cell exhaustion remains unclear. In the present study, T-cell
exhaustion was investigated in a murine lung cancer model. The
results demonstrated the importance of IDO1 in T-cell exhaustion,
and that IDO1 silencing may inhibit tumor growth by suppressing
T-cell exhaustion.
IDO1 is a rate-limiting enzyme involved in the
kynurenine pathway, which is a metabolic pathway that induces
immunosuppression by degrading tryptophan in the tumor-draining
lymph nodes and the TME (35). IDO1
is highly expressed in human lung cancer tissues (5) and a previous study reported that IDO1
downregulation in dendritic cells by gene silencing using an
immune-stimulatory nanosystem can suppress tumor growth in lung
cancer model (36). These studies
confirmed that IDO1 activity is involved in tumor growth. However,
only a limited number of studies have investigated the role of IDO1
derived from tumor tissues or cells in lung cancer progression
(26,28), which mostly focused on metabolic
reprogramming of immune cells or angiogenesis inhibition. In the
present study, we investigated the effect of IDO1 derived from lung
cancer on T-cells exhaustion. Our group has previously found that
IDO1 is highly expressed in LLC cells (data not published).
Ladomersky E reported inhibition of IDO1 activity by
1-methyl-tryptophan can increase patient survival (37); however, this treatment caused
numerous adverse effects, including toxic and autoimmunity disease
(38). The present study therefore
attempted to knock down IDO1 by siRNA in LLC cells and verify
whether IDO1-siRNA may silence IDO1 expression in vitro.
Furthermore, the effect of IDO1 derived from tumor tissues on tumor
growth was evaluated using shRNA interference, which is a mechanism
of silencing gene expression resulting in sequence-specific mRNA
interference (39). Hydrodynamic
tail vein injection of various shRNA plasmids has been used as a
treatment for prostate cancer, breast cancer and other diseases in
mouse models (40). In the present
study, IDO1 was knocked down in LLC tumor-bearing mice using shRNA.
Results demonstrated that IDO1-shRNA treatment significantly
delayed tumor onset and decreased tumor volume and weight compared
to scramble-RNA treatment negative control. Furthermore, the
results of the immunohistochemistry staining demonstrated that
IDO1-shRNA treatment successfully knocked down IDO1 in tumor
tissues. These results suggested that IDO1 may be silenced by
injection of IDO1-shRNA into mice, therefore inhibiting tumor
growth.
T cells that are reactive to tumor and viral
antigens lose their reactivity when exposed to the antigen-rich
environment of a larger tumor bed or viral load; such
non-responsive T cells are considered exhausted (13). T-cell exhaustion is characterized by
the upregulation of inhibitory molecules, including PD-1, BTLA and
TIM3 (41,42), and the reduced production of immune
signaling molecules and cytokines, including TNF-α, IL-2 and IFN-γ
(10,15). Therapeutic reinvigoration of
tumor-specific T cells has greatly improved clinical outcomes in
cancer (43). Previous studies in
murine and human cancers have reported that suppressing T-cell
exhaustion has a significant therapeutic effect on tumors by
restoring the anti-tumor function of T cells (34,44). In
numerous models of malignant tumors, including malignant T-lymphoma
and melanoma, the expression levels of PD-1 and BTLA were
demonstrated to be associated with the decrease of tumor-specific
T-cell effector function (45,46).
Blockage of both PD-1 and BTLA inhibitory pathways improves
tumor-specific antigen immune responses and reverses T-cell
exhaustion (11,47). A previous study has suggested that
the expression levels of PD-1, BTLA and 2B4 proteins in both
CD4+ and CD8+ T-cell compartments are
increased in mice with localized cancer compared with healthy
control (48). In the present study,
lymphocytes from lymph nodes and spleen were collected from mice.
These results demonstrated that tumors did not alter
CD4+ and CD8+ T-cell numbers in the lymph
nodes and the spleen in LLC-bearing tumor mice compared with mice
without tumors, but increased the expression of PD-1 and BTLA.
These results suggested that T-cell exhaustion may occur in the
lung TME. A previous study reported that IDO1 is highly expressed
in tumors and plays an important role in the T-cell response to
tumorigenesis (25). To further
investigate the association between IDO1 and T-cell exhaustion
in vitro, LLC cells transfected with IDO1-siRNA, Gl2-siRNA
or control were co-cultured with T cells from LLC tumor-bearing
mice. The results demonstrated that PD-1 and BTLA expression on
CD8+ T cells was decreased in LLC cells following IDO1
knockdown, suggesting that IDO1 knockdown in LLC cells may suppress
T-cell exhaustion during tumor progression. Furthermore, LLC
tumor-bearing mice were treated with IDO1-shRNA in order to knock
down IDO1 expression in tumors. The results demonstrated that IDO1
shRNA-treated mice presented with decreased expression of PD-1 and
BTLA on CD4+ and CD8+ T cells, and suggested
that IDO1-shRNA suppressed T-cell exhaustion by inhibiting the
expression of co-inhibitory receptors.
Cytokines serve a crucial role in tumor cell
proliferation. Exhausted T-cells progressively lose their
functional capacity to proliferate and to produce cytokines,
including TNF-α, IL-2 and IFN-γ (41). It has been reported that IL-2 can
reverse CD8+ T-cell exhaustion in malignant pleural
effusion of lung cancer (49). In
the present study, serum TNF-α and IL-2 level in LLC tumor-bearing
mice was decreased compared with healthy mice, suggesting TNF-α and
IL-2 secretion may be inhibited in tumors in vivo, which is
associated with T-cell exhaustion. In addition, the results of the
present study demonstrated that treatment with IDO1-shRNA in
LLC-bearing mice increased TNF-α and IL-2 production, compared with
scrambled shRNA-treated mice and untreated control mice, and the
concentrations of these cytokines after IDO1-shRNA treatment
returned to the level of mice without tumors (healthy mice), These
data suggested that treatment with IDO1-shRNA in vivo may
recover cytokine secretion.
Some limitations of this study are the small sample
size and the separation of T-cells. A total of nine female C57BL/6
mice in the present study were used and divided into three groups
(n=3). Although the small sample size was one of the limitations,
the differences between each group were observed and the sample
size was sufficient for statistical analysis to be performed,
suggesting that the conclusions of the present study were reliable.
A similar situation was previously reported, with a small sample
size in their mice experiments (n=3) (35). Regarding T-cells isolation, the
present study used T-cells isolated from lymph nodes and spleen
because extracting infiltrating T-cells from tumor tissues is
difficult and only results in a small number of extracted T-cells.
Therefore, extracting T-cells from lymph nodes and spleen was
sufficient for the present study as this method has met the
requirements of previous experiments (47,50).
In summary, the results of the present study
demonstrated that IDO1-shRNA delayed tumor onset and inhibited
tumor growth by impeding T-cell exhaustion in a murine lung cancer
model, which may be subsequent to the downregulation of T-cell
inhibitory receptors PD-1 and BTLA and the upregulation of IL-2 and
TNF-α secretion. These results suggested that IDO1-targeting
molecular therapy may be combined with suppressive T-cell
exhaustion therapy to treat patients with lung cancer, and that
IDO1 knockdown by gene silencing may be considered as a promising
treatment strategy for lung cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Youth Science
Foundation of Jiangxi Province (grant no. 20181BAB215020), Jiangxi
Provincial Department of Education Project (grant no. GJJ160268)
and Jiangxi Provincial Health Department Project (grant no.
201715520).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KS and YY conceived and designed the experiments. KS
and KY performed the experiments. YYH and YQH participated in the
model establishment. ZW conducted the statistical analysis. YY
drafted the manuscript. All authors read and approved the final
manuscript and agreed to be responsible for all aspects of the
study in ensuring the accuracy or integrity of any part of the work
are appropriately investigated and resolved.
Ethics approval and consent to
participate
Animal experiments in the present study conformed to
the Regulations on the Administration of Affairs Concerning
Experimental Animals of China and were approved by the Biomedical
Ethics Committee of Jiangxi Academy of Medical Sciences on Animal
experiments (Nanchang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Speeckaert R, Vermaelen K, van Geel N,
Autier P, Lambert J, Haspeslagh M, van Gele M, Thielemans K, Neyns
B, Roche N, et al: Indoleamine 2,3-dioxygenase, a new prognostic
marker in sentinel lymph nodes of melanoma patients. Eur J Cancer.
48:2004–2011. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Platten M, von Knebel Doeberitz N, Oezen
I, Wick W and Ochs K: Cancer immunotherapy by targeting IDO1/TDO
and their downstream effectors. Front Immunol. 5:6732014.PubMed/NCBI
|
|
4
|
Munn DH and Mellor AL: Indoleamine 2,3
dioxygenase and metabolic control of immune responses. Trends
Immunol. 34:137–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Munn DH and Mellor AL: IDO in the tumor
microenvironment: Inflammation, counter-regulation, and tolerance.
Trends Immunol. 37:193–207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Smith C, Chang MY, Parker KH, Beury DW,
DuHadaway JB, Flick HE, Boulden J, Sutanto-Ward E, Soler AP,
Laury-Kleintop LD, et al: IDO is a nodal pathogenic driver of lung
cancer and metastasis development. Cancer Discov. 2:722–735. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Que Z, Zou F, Zhang A, Zheng Y, Bi L,
Zhong J, Tian J and Liu J: Ganoderic acid Me induces the apoptosis
of competent T cells and increases the proportion of Treg cells
through enhancing the expression and activation of indoleamine
2,3-dioxygenase in mouse lewis lung cancer cells. Int
Immunopharmacol. 23:192–204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zajac AJ, Blattman JN, Murali-Krishna K,
Sourdive DJ, Suresh M, Altman JD and Ahmed R: Viral immune evasion
due to persistence of activated T cells without effector function.
J Exp Med. 188:2205–2213. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pauken KE and Wherry EJ: Overcoming T cell
exhaustion in infection and cancer. Trends Immunol. 36:265–276.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakuishi K, Apetoh L, Sullivan JM, Blazar
BR, Kuchroo VK and Anderson AC: Targeting Tim-3 and PD-1 pathways
to reverse T cell exhaustion and restore anti-tumor immunity. J Exp
Med. 207:2187–2194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fourcade J, Sun Z, Pagliano O, Guillaume
P, Luescher IF, Sander C, Kirkwood JM, Olive D, Kuchroo V and
Zarour HM: CD8(+) T cells specific for tumor antigens can be
rendered dysfunctional by the tumor microenvironment through
upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res.
72:887–896. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsuzaki J, Gnjatic S, Mhawech-Fauceglia
P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant
P, et al: Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are
negatively regulated by LAG-3 and PD-1 in human ovarian cancer.
Proc Natl Acad Sci USA. 107:7875–7880. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wherry EJ: T cell exhaustion. Nat Immunol.
12:492–499. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yi JS, Cox MA and Zajac AJ: T-cell
exhaustion: Characteristics, causes and conversion. Immunology.
129:474–481. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Balkhi MY, Ma Q, Ahmad S and Junghans RP:
T cell exhaustion and Interleukin 2 downregulation. Cytokine.
71:339–347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li S, Zhang S, Liu J, Yang C, Zhang L and
Cheng Y: The effect of PD-L1/PD-1 immunotherapy in the treatment of
squamous non-small-cell lung cancer: A meta-analysis of randomized
controlled clinical trials. J Thorac Dis. 11:4453–4463. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoon JS, Song JH, Yoon JH, Lee HY, Kim SW,
Chang Y, Lee YB, Cho EJ, Yu SJ, Sinn DH, et al: Adjuvant
cytokine-induced killer immunotherapy for hepatocellular carcinoma:
A propensity score-matched analysis of real-world data. BMC Cancer.
19:5232019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bu X, Yao Y and Li X: Immune checkpoint
blockade in breast cancer therapy. Adv Exp Med Biol. 1026:383–402.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mirzaei R, Sarkar S and Yong VW: T cell
exhaustion in glioblastoma: Intricacies of immune checkpoints.
Trends Immunol. 38:104–115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Robert C, Ribas A, Wolchok JD, Hodi FS,
Hamid O, Kefford R, Weber JS, Joshua AM, Hwu WJ, Gangadhar TC, et
al: Anti-programmed-death-receptor-1 treatment with pembrolizumab
in ipilimumab-refractory advanced melanoma: A randomised
dose-comparison cohort of a phase 1 trial. Lancet. 384:1109–1117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
O'Connor JM, Seidl-Rathkopf K, Torres AZ,
You P, Carson KR, Ross JS and Gross CP: Disparities in the use of
programmed death 1 immune checkpoint inhibitors. Oncologist.
23:1388–1390. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Teo MY and Rosenberg JE: Nivolumab for the
treatment of urothelial cancers. Expert Rev Anticancer Ther.
18:215–221. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harris-Bookman S, Mathios D, Martin AM,
Xia Y, Kim E, Xu H, Belcaid Z, Polanczyk M, Barberi T, Theodros D,
et al: Expression of LAG-3 and efficacy of combination treatment
with anti-LAG-3 and anti-PD-1 monoclonal antibodies in
glioblastoma. Int J Cancer. 143:3201–3208. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qin S, Xu L, Yi M, Yu S, Wu K and Luo S:
Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol
Cancer. 18:1552019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Friberg M, Jennings R, Alsarraj M,
Dessureault S, Cantor A, Extermann M, Mellor AL, Munn DH and
Antonia SJ: Indoleamine 2,3-dioxygenase contributes to tumor cell
evasion of T cell-mediated rejection. Int J Cancer. 101:151–155.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schafer CC, Wang Y, Hough KP, Sawant A,
Grant SC, Thannickal VJ, Zmijewski J, Ponnazhagan S and Deshane JS:
Indoleamine 2,3-dioxygenase regulates anti-tumor immunity in lung
cancer by metabolic reprogramming of immune cells in the tumor
microenvironment. Oncotarget. 7:75407–75424. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pan J, Yuan K, Peng S, Huang Y, Zhang Y,
Hu Y, Feng Y, Shi Y, Liu Y, Wang H, et al: Gene silencing of
indoleamin 2,3-dioxygenase hinders tumor growth through
angiogenesis inhibition. Int J Oncol. 50:2136–2144. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Naito S, von Eschenbach AC, Giavazzi R and
Fidler IJ: Growth and metastasis of tumor cells isolated from a
human renal cell carcinoma implanted into different organs of nude
mice. Cancer Res. 46:4109–4115. 1986.PubMed/NCBI
|
|
30
|
Escoffre JM, Novell A, Serrière S, Lecomte
T and Bouakaz A: Irinotecan delivery by microbubble-assisted
ultrasound: In vitro validation and a pilot preclinical study. Mol
Pharm. 10:2667–2675. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
American Veterinary Medical Association
(AVMA), . AVMA Guidelines for the Euthanasia of Animals: 2013
Edition. AVMA; Schaumburg, IL: 2013
|
|
32
|
Powell K, Ethun K and Taylor DK: The
effect of light level, CO2 flow rate, and anesthesia on
the stress response of mice during CO2 euthanasia. Lab
Anim (NY). 45:386–395. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Q, Johnston N, Zheng X, Wang H, Zhang
X, Gao D and Min W: miR-28 modulates exhaustive differentiation of
T cells through silencing programmed cell death-1 and regulating
cytokine secretion. Oncotarget. 7:53735–53750. 2016.PubMed/NCBI
|
|
34
|
Thommen DS and Schumacher TN: T cell
dysfunction in cancer. Cancer Cell. 33:547–562. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Muller AJ and Prendergast GC: Indoleamine
2,3-dioxygenase in immune suppression and cancer. Curr Cancer Drug
Targets. 7:31–40. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Y, Fu J, Shi Y, Peng S, Cai Y, Zhan
X, Song N, Liu Y, Wang Z, Yu Y, et al: A new cancer immunotherapy
via simultaneous DC-mobilization and DC-targeted IDO gene silencing
using an immune-stimulatory nanosystem. Int J Cancer.
143:2039–2052. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ladomersky E, Zhai L, Lenzen A, Lauing KL,
Qian J, Scholtens DM, Gritsina G, Sun X, Liu Y, Yu F, et al: IDO1
inhibition synergizes with radiation and PD-1 blockade to durably
increase survival against advanced glioblastoma. Clin Cancer Res.
24:2559–2573. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang T, Sun Y, Yin Z, Feng S, Sun L and
Li Z: Research progress of indoleamin 2,3-dioxygenase inhibitors.
Future Med Chem. 7:185–201. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fire A, Xu S, Montgomery MK, Kostas SA,
Driver SE and Mello CC: Potent and specific genetic interference by
double-stranded RNA in Caenorhabditis elegans. Nature. 391:806–811.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vakili S, Ebrahimi SS, Sadeghi A,
Gorgani-Firuzjaee S, Beigy M, Pasalar P and Meshkani R:
Hydrodynamic-based delivery of PTP1B shRNA reduces plasma glucose
levels in diabetic mice. Mol Med Rep. 7:211–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Amezquita RA and Kaech SM: Immunology: The
chronicles of T-cell exhaustion. Nature. 543:190–191. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Prall F and Hühns M: The PD-1 expressing
immune phenotype of T cell exhaustion is prominent in the
‘immunoreactive’ microenvironment of colorectal carcinoma.
Histopathology. 71:366–374. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yost KE, Spantidea PI, Wells DK, Qi Y,
Wang C, Kageyama R, McNamara KL, Granja JM, Sarin KY, Brown RA, et
al: Clonal replacement of tumor-specific T cells following PD-1
blockade. Nat Med. 25:1251–1259. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Paulos CM and June CH: Putting the brakes
on BTLA in T cell-mediated cancer immunotherapy. J Clin Invest.
120:76–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Karakatsanis S, Bertsias G, Roussou P and
Boumpas D: Programmed death 1 and B and T lymphocyte attenuator
immunoreceptors and their association with malignant
T-lymphoproliferative disorders: Brief review. Hematol Oncol.
32:113–119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hamid O, Robert C, Daud A, Hodi FS, Hwu
WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al:
Safety and tumor responses with lambrolizumab (anti-PD-1) in
melanoma. N Engl J Med. 369:134–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mittal R, Chen CW, Lyons JD, Margoles LM,
Liang Z, Coopersmith CM and Ford ML: Murine lung cancer induces
generalized T-cell exhaustion. J Surg Res. 195:541–549. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hu CY, Zhang YH, Wang T, Chen L, Gong ZH,
Wan YS, Li QJ, Li YS and Zhu B: Interleukin-2 reverses CD8(+) T
cell exhaustion in clinical malignant pleural effusion of lung
cancer. Clin Exp Immunol. 186:106–114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Khalil DN, Suek N, Campesato LF, Budhu S,
Redmond D, Samstein RM, Krishna C, Panageas KS, Capanu M, Houghton
S, et al: In situ vaccination with defined factors overcomes T cell
exhaustion in distant tumors. J Clin Invest. 129:3435–3447. 2019.
View Article : Google Scholar : PubMed/NCBI
|