Introduction
Pancreatic adenocarcinoma is the fourth and sixth
most common cause of cancer-associated mortality in the United
States (7.27%; 2018) and China (2.82%; 2015), respectively
(1,2). With the annual increase in the number
of pancreatic adenocarcinoma-associated mortality cases, it is
predicted that this malignancy will become the second leading cause
of cancer-associated mortality in the United States by 2030
(3). At present, complete surgical
resection remains the only therapeutic strategy for significantly
prolonging the survival of patients with pancreatic adenocarcinoma
(4). However, since pancreatic
adenocarcinoma is diagnosed at late and advanced stages, <20% of
newly diagnosed patients are eligible for potential curative
surgical resection (4,5). Despite recent advancements in
chemotherapy and radiotherapy, the 5-year survival rate of patients
who underwent curative resection remains poor (12-27%) (6–8). It is
therefore essential to develop an efficient prognostic system to
predict the survival of patients with pancreatic
adenocarcinoma.
The American Joint Committee on Cancer (AJCC)
Tumor-Node-Metastasis (TNM) staging system (9), which is based on the histological
analysis of tumor and metastasis, has been extensively used for the
prognostic evaluation of patients with pancreatic adenocarcinoma
(10,11). However, the current TNM staging
system for pancreatic adenocarcinoma does not include certain
clinicopathological factors that may affect the prognosis,
including age, sex, tumor grade and carbohydrate antigen 199
(CA199) level (11–13). CA199, as a measure of tumor burden,
is a diagnostic and prognostic marker (14,15).
Humphris et al (16) reported
that normal preoperative CA199 levels identified a good prognosis.
Furthermore, previous studies reported that the prognosis of
patients varies significantly within the same TNM stage (10,11). It
is therefore essential to develop a more efficient predictive model
that could be used to predict the survival of patients with
pancreatic adenocarcinoma.
Nomograms are multivariate predictive models that
have been extensively applied for clinical prognostic evaluation in
several types of malignancy (17–20).
Unlike the TNM staging system, nomograms are efficient in
incorporating additional prognostic factors, allowing therefore a
more accurate prediction (21,22). At
present, numerous nomograms have been established for predicting
the survival of patients with pancreatic adenocarcinoma; however,
most of these nomograms used the demographic and pathological data
from the United States, where a number of potential prognostic
factors, including tumor markers and surgical information, were not
included (13,23–27).
The present study aimed to develop and validate a
prognostic nomogram for patients with resected pancreatic
adenocarcinoma, by including additional prognostic factors based on
the clinical database from the China National Cancer Center.
Materials and methods
Patient dataset and study design
The present study was a retrospective study approved
by the Ethics Committee of National Cancer Center/Cancer Hospital,
Chinese Academy of Medical Sciences and Peking Union Medical
College (Beijing, China). A total of 368 patients with pancreatic
adenocarcinoma who underwent curative surgical resection (with an
R0 margin) at the China National Cancer Center between January 2008
and October 2018 were included in the present study. The cohort was
comprised of 208 (56.5%) male and 160 (43.5%) female patients with
a median age of 60 years (age range, 45–72 years). The study
inclusion criteria were as follows: i) Diagnosis of pancreatic
adenocarcinoma; ii) patients underwent curative surgical resection
with R0 resection, which was determined by no macroscopic or
microscopic residual carcinoma; and iii) based on the TNM staging
system, the stages of patients were T1-3N+M0 (9). The exclusion criteria were as follows:
i) Patients with T4 stage or distant metastasis; ii) patients that
underwent palliative surgery; iii) patients who had received
preoperative chemotherapy or radiotherapy; iv) patients who had
suffered from other malignancies before pancreatic adenocarcinoma
and (v) patients who died due to other reasons or unexpected
outcomes. Prior to radical pancreaticoduodenectomy or distal
pancreatectomy with splenectomy, patients were examined with
enhanced MRI and/or CT scanning to confirm the absence of locally
unresectable or distant metastases. Generally, there were more
people in the training set used to construct the nomogram and fewer
people in the validation set. In the present study, the patients
were randomly divided into study sets for model training (258
patients) and validation (110 patients) according to a ratio of
7:3.
All clinicopathological characteristics were
collected from the medical record database of the China National
Cancer Center and included the following: Age at the time of
diagnosis, sex, clinical symptoms (pain, jaundice, digestive
symptoms and weight loss), past medical history (diabetes and
hypertension) and life style (smoking status and alcohol
consumption). The laboratory data included CA199, carbohydrate
antigen 242 (CA242), neutrophil percentage (NP), alanine
aminotransferase, aspartate aminotransferase, total bilirubin,
prognostic nutritional index (PNI), platelet and lymphocyte ratio
(PLR), neutrophil and lymphocyte ratio (NLR), monocyte and
lymphocyte ratio (MLR), systemic inflammatory reaction index (SII),
systemic inflammatory response index (SIRI) and c reactive
protein/albumin (CRP/ALB), which were collected 2 weeks prior to
radical surgery. The PNI was calculated based on the serum albumin
and lymphocyte counts, by using the following equation: PNI=10 ×
albumin (g/dl) + 0.005 × lymphocyte count (/mm3). The
SII and SIRI were calculated based on neutrophil, lymphocyte,
monocyte and platelet counts, using the following equation:
SII=platelet count × neutrophil count/lymphocyte count;
SIRI=neutrophil count × monocyte count/lymphocyte count. The
remaining data were calculated as follows: PLR=platelet
count/lymphocyte count; NLR=neutrophil count/lymphocyte count;
MLR=monocyte count/lymphocyte count.
The American Society of Anesthesiologists
classification (ASA class) and information about blood transfusion,
total retrieved lymph nodes, tumor information (location, tumor
grade, and lymphovascular, perineural and capsule invasions) and
adjuvant therapy were also included in the present study. The tumor
stage and nodal involvement in the present study were defined
according to the 8th edition TNM staging system (28).
Follow-up analysis
The primary endpoint of the present study was the
overall survival (OS), which was measured from the date of radical
surgery to the mortality due to pancreatic adenocarcinoma, or to
the last follow-up (updated in May 2019). The follow-up procedure
was as follows: Once every three months within the first two years
following surgery and continual procedures every six months
thereafter, in which the postoperative treatment information and
survival conditions were recorded. Long-term prognostic data were
collected from the patients' clinical records or contact with the
patients' relatives via telephone. Patients who died after the
surgery (within 1 month following surgery) were excluded from the
present study. A total of three patients were lost due to
perioperative mortality.
Statistical analysis
X-tile software (v.3.6.1; Yale School of Medicine)
(29) was used to identify the
optimal cut-off values of the potential prognostic factors,
including NP, PNI, PLR, NLR, MLR, SII, SIRI and CRP/ALB. X-tile
software can divide marker data into two populations: low and high.
All possible divisions of the marker data were assessed by a
variety of standard statistical tests, including the log-rank test
for survival and means tests for associations between other marker
data. Then the program selected the optimal division of the data
(29). χ2 test was used
to compare the categorical characteristics between the two disjoint
sets. Cox proportional hazard univariate and multivariate
regression analyses were used to evaluate the independent
prognostic predictors for OS in the training set.
Multivariate analysis was performed to identify the
independent prognostic factors, in order to establish the nomogram
by using the rms package within R project software. Based on the
hazard ratio of the corresponding characteristics in the Cox
regression model, each independent prognostic factor was scored
using the ‘nomogram’ function within R project (21). Validations were performed in the
validation set using two parameters of the nomogram, discrimination
and calibration. The concordance (C)-index was implemented to
assess discrimination, whereas calibration curves were generated
using bootstrap resampling (1,000 resamples). The area under the
receiver operating characteristic curve (AUC) was determined to
assess the accuracy of survival predictions. The Kaplan-Meier
method was used to analyze the survival curves, and differences
were estimated using log-rank test.
Statistical analyses were performed using SPSS
software (version 25.0; IBM Corp.), GraphPad Prism software
(version 7.00; GraphPad Software Inc.) and R software (version
3.5.2; http://www.r-project.org). Two-tailed
P<0.05 was considered to indicate a significant difference.
Results
Patient characteristics
A total of 368 patients with resected pancreatic
adenocarcinoma were randomly divided into the training set (258
patients) and the validation set (110 patients). As presented in
Table I, the optimal cut-off values
identified using X-tile software were as follows: 0.700 for NP, 52
for PNI, 210.082 for PLR, 2.751 for NLR, 0.279 for MLP, 718.312 for
SII, 0.782 for SIRI and 0.142% for CRP/ALB. Based on these values,
patients were subsequently divided into low and high expression
groups. The mean follow-up period of the training set was 44.4
months (range, 2–104.9 months), while the 1-year, 3-year and 5-year
OS rates were 72.3, 41.4 and 26.4%, respectively. The mean
follow-up period of the validation set was 40.1 months (range,
1.5–102.8 months), while the 1-year, 3-year and 5-year OS rates
were 66.4, 38.8 and 25.6%, respectively. The clinicopathological
characteristics of patients from the training and validation sets
are presented in Table I. No
significant difference was observed between the two groups for each
characteristic.
 | Table I.Clinicopathological characteristics
of patients in the training and validation sets. |
Table I.
Clinicopathological characteristics
of patients in the training and validation sets.
|
| Training set | Validation set |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Patient, n | % | Patient, n | % | P-value |
|---|
| Sex |
|
|
|
| 0.675 |
|
Male | 144 | 55.8 | 64 | 58.2 |
|
|
Female | 114 | 44.2 | 46 | 41.8 |
|
| Age, years |
|
|
|
| 0.265 |
|
≤60 | 136 | 52.7 | 51 | 46.4 |
|
|
>60 | 122 | 47.3 | 59 | 53.6 |
|
| Symptom |
|
|
|
| 0.108 |
| No | 39 | 15.5 | 24 | 22.6 |
|
|
Yes | 212 | 84.5 | 82 | 77.4 |
|
| Pain |
|
|
|
| 0.195 |
| No | 104 | 41.6 | 52 | 49.1 |
|
|
Yes | 146 | 58.4 | 54 | 50.9 |
|
| Jaundice |
|
|
|
| 0.452 |
| No | 182 | 72.8 | 73 | 68.9 |
|
|
Yes | 68 | 27.2 | 33 | 31.1 |
|
| Digestive
symptoms |
|
|
|
| 0.023 |
| No | 202 | 80.8 | 74 | 69.8 |
|
|
Yes | 48 | 19.2 | 32 | 30.2 |
|
| Weight loss |
|
|
|
| 0.420 |
| No | 152 | 60.6 | 69 | 65.1 |
|
|
Yes | 99 | 39.4 | 37 | 34.9 |
|
| Diabetes |
|
|
|
| 0.342 |
| No | 189 | 73.3 | 85 | 78.0 |
|
|
Yes | 69 | 26.7 | 24 | 22.0 |
|
| Hypertension |
|
|
|
| 0.131 |
| No | 197 | 76.4 | 75 | 68.8 |
|
|
Yes | 61 | 23.6 | 34 | 31.2 |
|
| Smoke |
|
|
|
| 0.231 |
| No | 193 | 76.0 | 77 | 70.0 |
|
|
Yes | 61 | 24.0 | 33 | 30.0 |
|
| Alcohol |
|
|
|
| 0.218 |
| No | 204 | 80.3 | 82 | 74.5 |
|
|
Yes | 50 | 19.7 | 28 | 25.5 |
|
| ASA class |
|
|
|
| 0.405 |
| ≤2 | 188 | 74.6 | 85 | 78.7 |
|
|
>2 | 64 | 25.4 | 23 | 21.3 |
|
| Blood
transfusion |
|
|
|
| 0.372 |
| No | 155 | 61.5 | 61 | 56.5 |
|
|
Yes | 97 | 38.5 | 47 | 43.5 |
|
| Tumor location |
|
|
|
| 0.348 |
| Head
and neck | 124 | 48.1 | 47 | 42.7 |
|
| Body
and tail | 134 | 51.9 | 63 | 57.3 |
|
| T stage |
|
|
|
| 0.917 |
| T1 | 33 | 13.1 | 15 | 13.9 |
|
| T2 | 139 | 55.2 | 56 | 56.5 |
|
| T3 | 80 | 31.7 | 35 | 29.6 |
|
| N stage |
|
|
|
| 0.961 |
| N0 | 142 | 56.3 | 61 | 53.7 |
|
| N1 | 84 | 33.3 | 35 | 34.3 |
|
| N2 | 26 | 10.4 | 10 | 12.0 |
|
| Lymph nodes, n |
|
|
|
| 0.798 |
| ≤6 | 69 | 25.7 | 31 | 27.4 |
|
|
>6 | 171 | 74.3 | 82 | 72.6 |
|
| Tumor grade |
|
|
|
| 0.473 |
|
Poorly | 37 | 14.9 | 17 | 16.4 |
|
|
Moderately | 174 | 69.9 | 78 | 69.1 |
|
|
Well | 38 | 15.3 | 11 | 14.5 |
|
| Lymphovascular
invasion |
|
|
|
| 0.973 |
| No | 174 | 74.0 | 82 | 73.9 |
|
|
Yes | 61 | 26.0 | 29 | 26.1 |
|
| Perineural
invasion |
|
|
|
| 0.965 |
| No | 95 | 39.7 | 44 | 40.0 |
|
|
Yes | 144 | 60.3 | 66 | 60.0 |
|
| Capsule
invasion |
|
|
|
| 0.508 |
| No | 70 | 27.7 | 33 | 31.1 |
|
|
Yes | 183 | 72.3 | 73 | 68.9 |
|
| CEA, ng/ml |
|
|
|
| 0.370 |
| ≤5 | 158 | 63.7 | 64 | 58.7 |
|
|
>5 | 90 | 36.3 | 45 | 41.3 |
|
| CA199, U/ml |
|
|
|
| 0.907 |
|
≤37 | 56 | 22.6 | 24 | 22.0 |
|
|
>37 | 192 | 77.4 | 85 | 78.0 |
|
| CA242, IU/ml |
|
|
|
| 0.174 |
|
≤20 | 96 | 38.7 | 34 | 31.2 |
|
|
>20 | 152 | 61.3 | 75 | 68.8 |
|
| NP |
|
|
|
| 0.686 |
|
≤0.700 | 180 | 72.0 | 80 | 74.1 |
|
|
>0.700 | 70 | 38.0 | 28 | 25.9 |
|
| ALT, U/l |
|
|
|
| 0.712 |
|
≤40 | 145 | 63.9 | 66 | 66.0 |
|
|
>40 | 82 | 36.1 | 34 | 34.0 |
|
| AST, U/l |
|
|
|
| 0.960 |
|
≤40 | 156 | 68.7 | 69 | 69.0 |
|
|
>40 | 71 | 31.3 | 31 | 31.0 |
|
| TBIL, µmol/l |
|
|
|
| 0.232 |
|
≤17.1 | 143 | 63.0 | 56 | 56.0 |
|
|
>17.1 | 84 | 37.0 | 44 | 44.0 |
|
| PNI |
|
|
|
| 0.402 |
|
≤52 | 156 | 68.7 | 64 | 64.0 |
|
|
>52 | 71 | 31.3 | 36 | 36.0 |
|
| PLR |
|
|
|
| 0.234 |
|
≤210.082 | 222 | 88.8 | 91 | 84.3 |
|
|
>210.082 | 28 | 11.2 | 17 | 15.7 |
|
| NLR |
|
|
|
| 0.405 |
|
≤2.751 | 162 | 64.8 | 65 | 60.2 |
|
|
>2.751 | 88 | 35.2 | 43 | 39.8 |
|
| MLR |
|
|
|
| 0.341 |
|
≤0.279 | 163 | 65.2 | 76 | 70.4 |
|
|
>0.279 | 87 | 34.8 | 32 | 29.6 |
|
| SII |
|
|
|
| 0.770 |
|
≤718.312 | 198 | 79.2 | 87 | 80.6 |
|
|
>718.312 | 52 | 20.8 | 21 | 19.4 |
|
| SIRI |
|
|
|
| 0.120 |
|
≤0.782 | 110 | 43.0 | 38 | 35.2 |
|
|
>0.782 | 140 | 56.0 | 70 | 64.8 |
|
| CRP/ALB |
|
|
|
| 0.700 |
|
≤0.142% | 51 | 38.1 | 23 | 41.1 |
|
|
>0.142% | 83 | 61.9 | 33 | 58.9 |
|
| Adjuvant
therapy |
|
|
|
| 0.279 |
| No | 128 | 50.2 | 62 | 56.4 |
|
|
Yes | 127 | 49.8 | 48 | 43.6 |
|
Independent prognostic factors in the
training set
The results from univariate and multivariate
regression analyses for the training set are presented in Table II. Results from univariate analysis
demonstrated that OS was significantly associated with sex,
symptom, weight loss, ASA class, blood transfusion, tumor location,
T stage, N stage, tumor grade, capsule invasion, CA199, CA242, NP,
NLR, SII and adjuvant therapy (all P<0.05). Furthermore,
following multivariate analysis, blood transfusion, T stage, N
stage, tumor grade, capsule invasion, CA199, NP and adjuvant
therapy were identified as significant independent prognostic
factors for patients with resected pancreatic adenocarcinoma (all
P<0.05).
 | Table II.Univariate and multivariate analyses
on clinicopathological characteristics of patients in the training
set. |
Table II.
Univariate and multivariate analyses
on clinicopathological characteristics of patients in the training
set.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
|
Characteristics | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex |
|
|
|
|
|
Female | Reference |
| Reference |
|
|
Male | 1.370
(1.025–1.832) | 0.033 | 1.333
(0.903–1.966) | 0.148 |
| Symptom |
|
|
|
|
|
Yes | Reference |
| Reference |
|
| No | 0.654
(0.438–0.978) | 0.039 | 0.833
(0.489–1.420) | 0.501 |
| Weight loss |
|
|
|
|
|
Yes | Reference |
| Reference |
|
| No | 0.734
(0.548–0.983) | 0.038 | 0.804
(0.532–1.215) | 0.300 |
| ASA class |
|
|
|
|
|
>2 | Reference |
| Reference |
|
| ≤2 | 0.689
(0.485–0.978) | 0.037 | 1.119
(0.686–1.827) | 0.652 |
| Blood
transfusion |
|
|
|
|
|
Yes | Reference |
| Reference |
|
| No | 0.644
(0.469–0.884) | 0.007 | 0.594
(0.384–0.920) | 0.020 |
| Tumor location |
|
|
|
|
| Body
and tail | Reference |
| Reference |
|
| Head
and neck | 0.720
(0.539–0.961) | 0.026 | 0.853
(0.522–1.394) | 0.525 |
| T stage |
|
|
|
|
| T3 | Reference |
| Reference |
|
| T2 | 0.589
(0.431–0.807) | 0.001 | 0.513
(0.326–0.808) | 0.003 |
| T1 | 0.494
(0.300–0.814) | 0.006 | 0.451
(0.215–0.946) | 0.035 |
| N stage |
|
|
|
|
| N2 | Reference |
| Reference |
|
| N1 | 0.704
(0.425–1.167) | 0.174 | 0.592
(0.317–1.106) | 0.100 |
| N0 | 0.417
(0.254–0.685) | 0.001 | 0.400
(0.212–0.754) | 0.005 |
| Tumor grade |
|
|
|
|
|
Well | Reference |
| Reference |
|
|
Moderately | 2.464
(1.462–4.155) | 0.001 | 2.894
(1.383–6.057) | 0.005 |
|
Poorly | 4.102
(2.255–7.460) | 0.000 | 3.904
(1.671–9.121) | 0.002 |
| Capsule
invasion |
|
|
|
|
|
Yes | Reference |
| Reference |
|
| No | 0.585
(0.405–0.843) | 0.004 | 0.496
(0.297–0.829) | 0.007 |
| CA199, U/ml |
|
|
|
|
|
>37 | Reference |
| Reference |
|
|
≤37 | 0.477
(0.318–0.717) | 0.000 | 0.430
(0.230–0.802) | 0.008 |
| CA242, IU/ml |
|
|
|
|
|
>20 | Reference |
| Reference |
|
|
≤20 | 0.524
(0.362–0.758) | 0.001 | 0.961
(0.573–1.611) | 0.880 |
| NP |
|
|
|
|
|
>0.700 | Reference |
| Reference |
|
|
≤0.700 | 0.577
(0.402–0.827) | 0.003 | 0.361
(0.198–0.657) | 0.001 |
| NLR |
|
|
|
|
|
>2.751 | Reference |
| Reference |
|
|
≤2.751 | 0.653
(0.475–0.897) | 0.008 | 1.301
(0.723–2.343) | 0.380 |
| SII |
|
|
|
|
|
>718.312 | Reference |
| Reference |
|
|
≤718.312 | 0.665
(0.464–0.952) | 0.026 | 1.097
(0.614–1.960) | 0.754 |
| Adjuvant
therapy |
|
|
|
|
|
Yes | Reference |
| Reference |
|
| No | 1.433
(1.067–1.925) | 0.017 | 1.931
(1.297–2.875) | 0.001 |
Development and validation of the
nomogram
A nomogram predicting the 1-year, 3-year and 5-year
OS rates of patients with pancreatic adenocarcinoma was constructed
based on the independent prognostic factors identified from the Cox
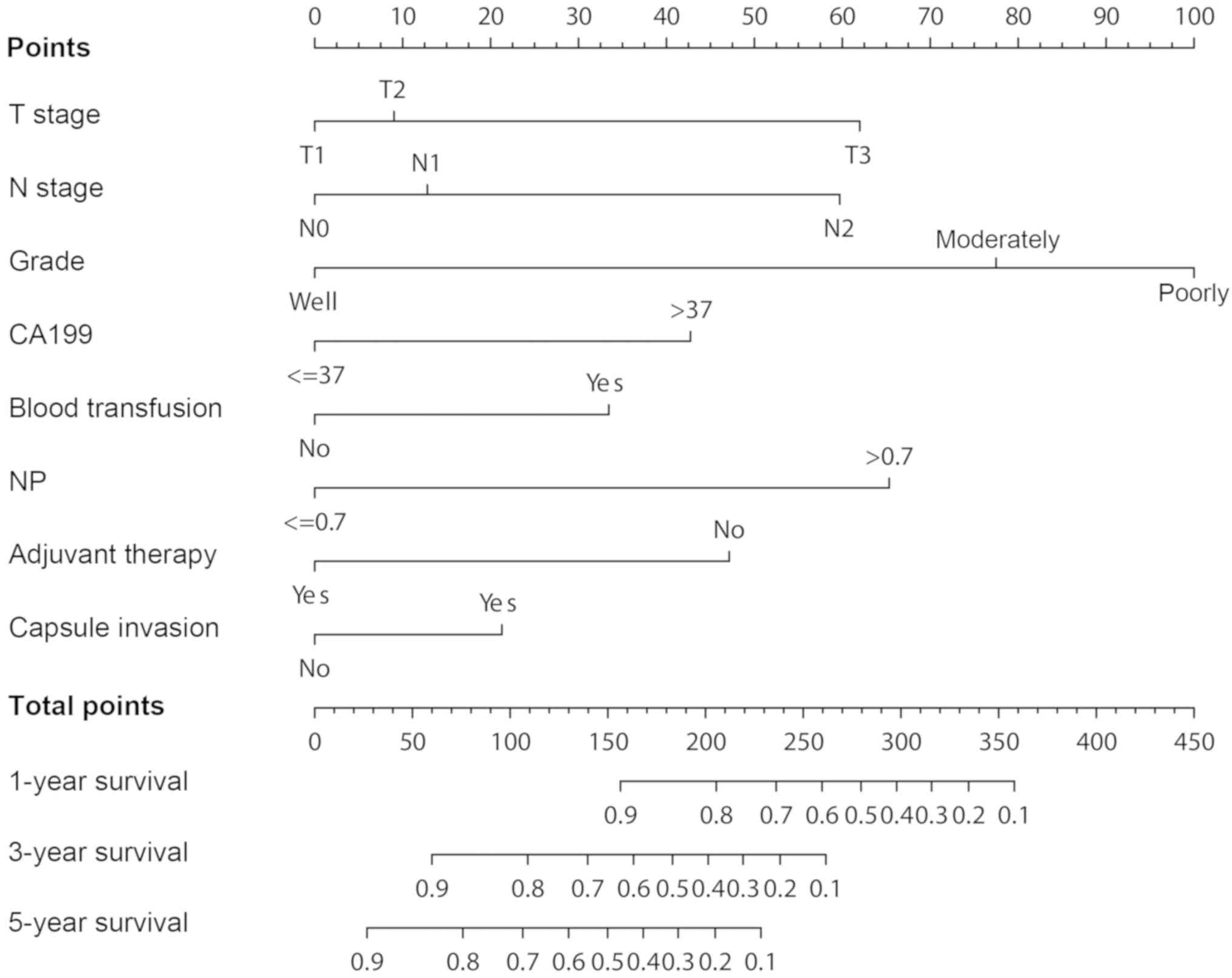
multivariate regression model in the training set (Fig. 1). Each subtype within these
indicators was allocated a score according to the ‘Points’ in the
nomogram. Next, the predicted survival probabilities for each
patient was calculated using the nomogram. For example, the total
points of patients were calculated by summing up the score for each
indicator first. Then the right position in the total points axis
was found and a perpendicular line drawn to the survival
probabilities axis. Finally, the predicted survival probabilities
at 1-year, 3-year and 5-year were obtained.
As presented in Table
III, the C-indices for the 1-year, 3-year and 5-year OS
prediction in the training set were 0.824 [95% confidential
interval (CI), 0.775–0.873], 0.782 (95% CI, 0.745–0.823) and 0.770
(95% CI, 0.731–0.810), respectively. The C-indices for the 1-year,
3-year and 5-year OS prediction in the validation set were 0.779
(95% CI, 0.705–0.853), 0.778 (95% CI, 0.718–0.838) and 0.766 (95%
CI, 0.709–0.823), respectively. The C-indices of both training and
validation sets were higher in the nomogram compared with the TNM
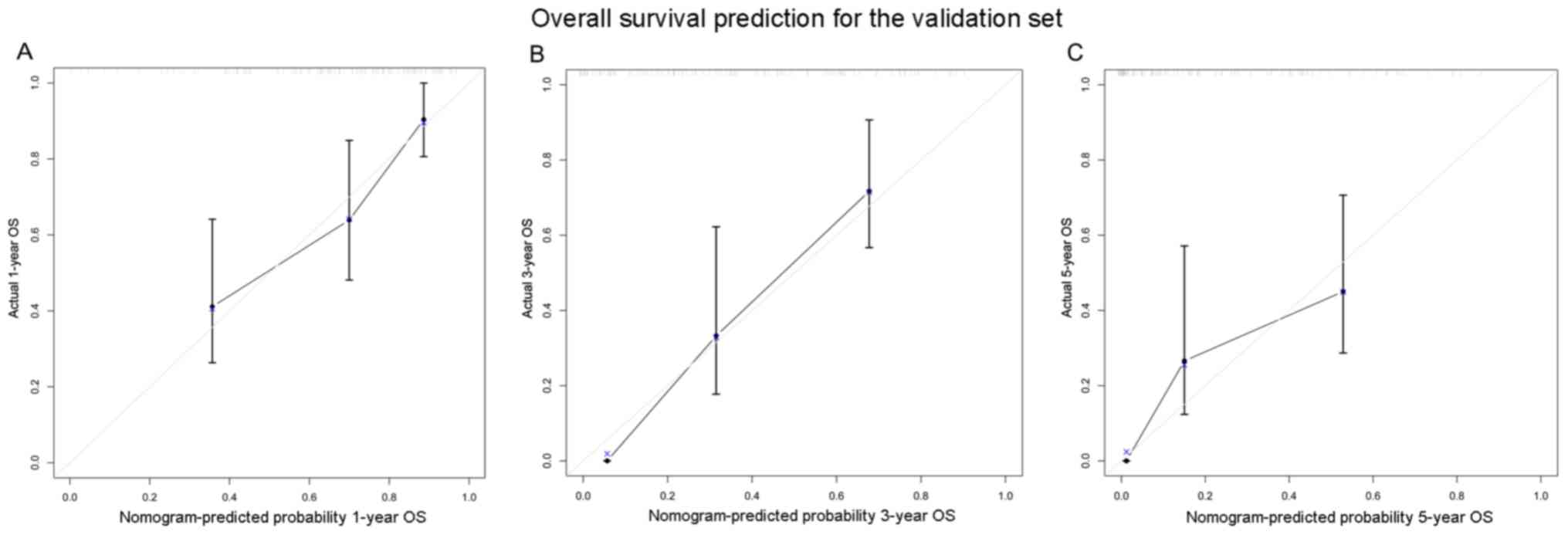
staging system. Furthermore, the calibration curves for the
probability of 1-year, 3-year and 5-year OS demonstrated a positive
association between the predicted and observed values in the
validation set (Fig. 2).
 | Table III.C-indexes for the nomogram and TNM
staging system. |
Table III.
C-indexes for the nomogram and TNM
staging system.
|
| 1-year OS | 3-year OS | 5-year OS |
|---|
|
|
|
|
|
|---|
|
Characteristics | C-index | 95% CI | C-index | 95% CI | C-index | 95% CI |
|---|
| Training set |
|
Nomogram | 0.824 | 0.775–0.873 | 0.782 | 0.742–0.823 | 0.770 | 0.731–0.810 |
| TNM
system | 0.667 | 0.591–0.742 | 0.648 | 0.589–0.706 | 0.642 | 0.585–0.699 |
| Validation set |
|
Nomogram | 0.779 | 0.705–0.853 | 0.778 | 0.718–0.838 | 0.766 | 0.709–0.823 |
| TNM
system | 0.695 | 0.603–0.787 | 0.672 | 0.595–0.749 | 0.669 | 0.594–0.744 |
Survival analysis according to the
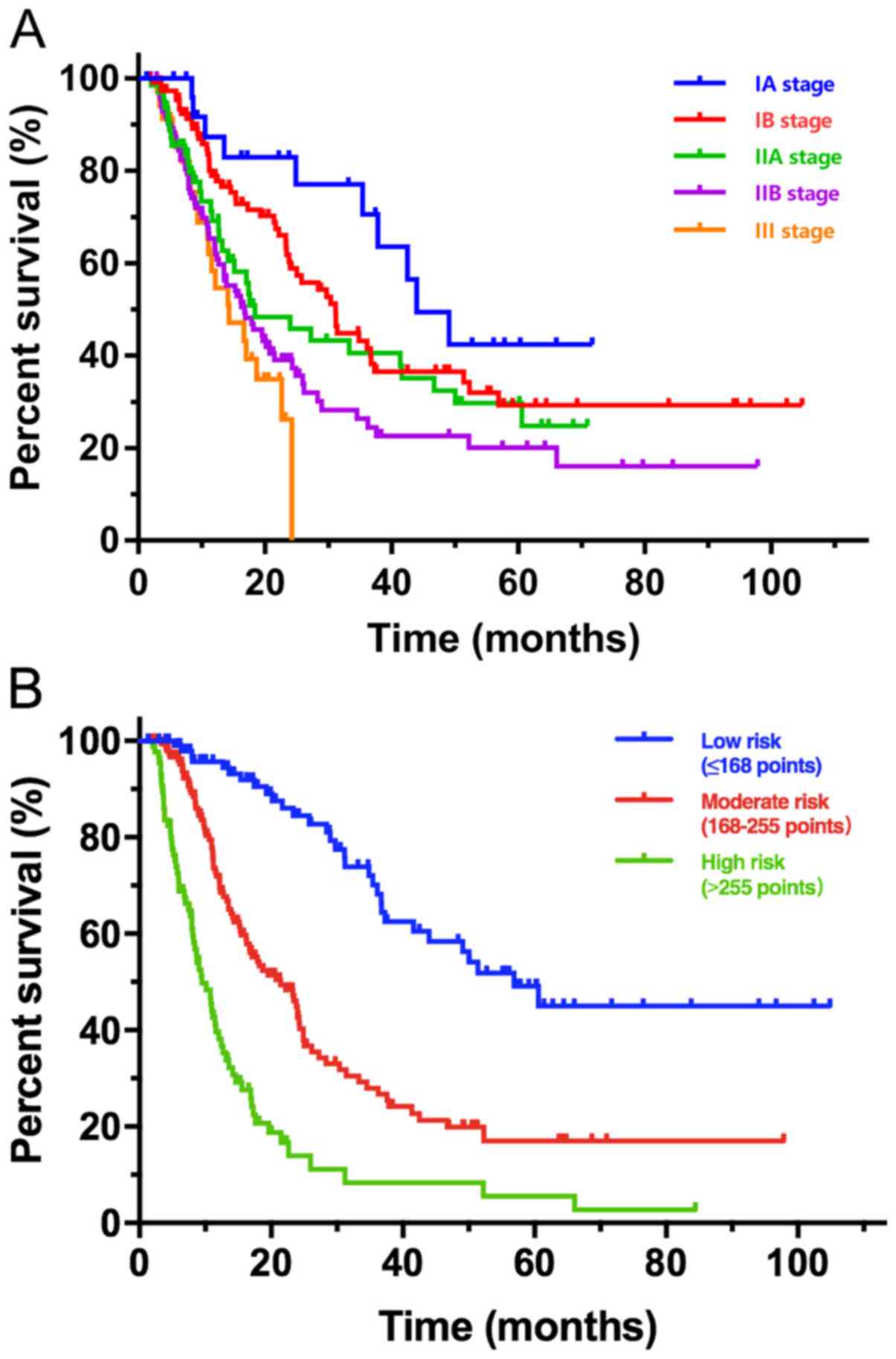
risk stratification system based on the nomogram
A clear risk stratification system of OS rates was
established using the predicted probabilities obtained from the
nomogram. The score for each independent prognostic factor is
presented in Table IV. Total scores
for each patient was defined as the sum of each score for each
indicator in the nomogram. According to the optimal cut-off values
of total scores, patients were classified into three groups as
follows: Low-risk group (≤168, n=124), moderate-risk group
(168-255, n=126) and high-risk group (>255, n=118), where each
group represented a distinct prognosis. Patients were categorized
according to the TNM staging system and the risk stratification
system, and the risk stratification system based on the nomogram
had the ability to delineate three different prognosis groups
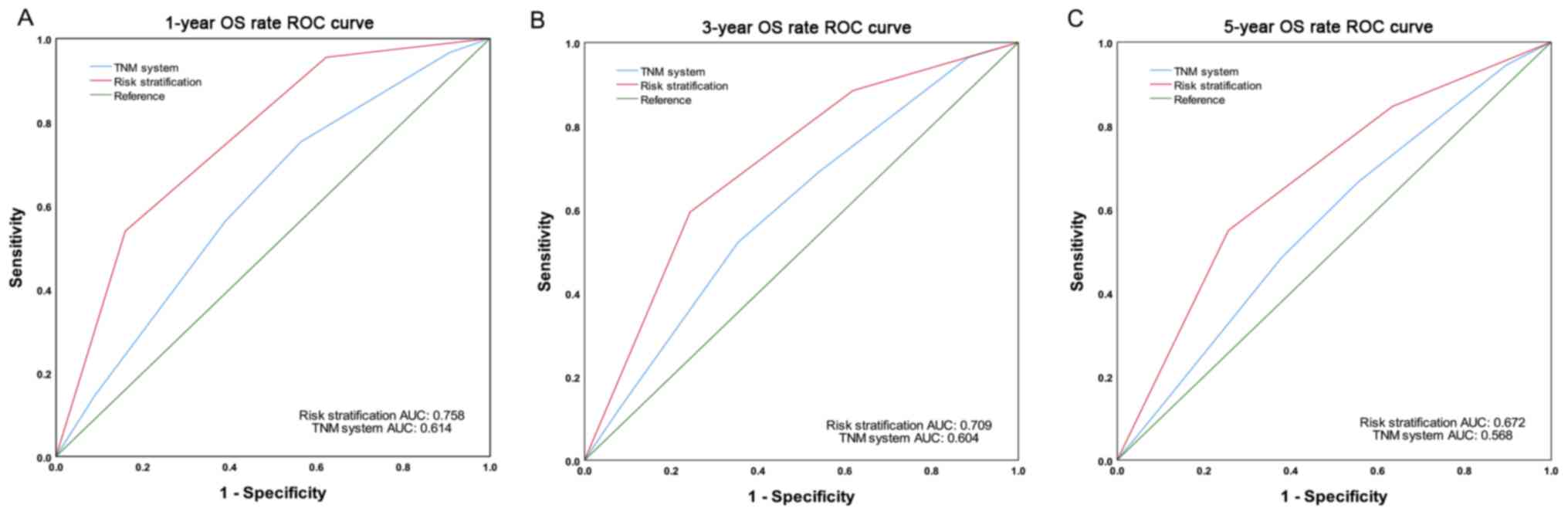
(P<0.01; Fig. 3). The AUC values
of the risk stratification system for predicting the 1-year, 3-year
and 5-year OS rates were 0.758, 0.709 and 0.672, respectively,
whereas the AUC values for the current TNM staging system are
0.614, 0.604 and 0.568, respectively (28) (Fig.
4).
 | Table IV.Score of characteristics in the
nomogram. |
Table IV.
Score of characteristics in the
nomogram.
| Characteristic | Score |
|---|
| Blood
transfusion |
|
| No | 0 |
|
Yes | 33.3 |
| NP |
|
|
≤0.7 | 0 |
|
>0.7 | 65.1 |
| CA199, U/ml |
|
|
≤37 | 0 |
|
>37 | 42.6 |
| T stage |
|
| T1 | 0 |
| T2 | 9 |
| T3 | 62.1 |
| N stage |
|
| N0 | 0 |
| N1 | 12.6 |
| N2 | 59.8 |
| Tumor grade |
|
|
Poorly |
100.0 |
|
Moderately | 77.5 |
|
Well | 0 |
| Adjuvant
therapy |
|
| No | 47.4 |
|
Yes | 0 |
| Capsule
invasion |
|
| No | 0 |
|
Yes | 21.2 |
Discussion
The AJCC TNM staging system is currently the most
extensively used system to predict the prognosis of several types
of cancer, including pancreatic adenocarcinoma (30). Numerous studies suggested that T and
N stages might not be the only clinical factors that can be used to
determine the prognosis of patients with pancreatic adenocarcinoma
(9,13,14,31).
Since implementing the traditional TNM staging system for patients
with resected pancreatic adenocarcinoma is considered as imprecise,
it is therefore essential to develop a more accurate survival
predictive model (13,14,31,32). In
order to address the limitations of the TNM staging system, the
present study developed and validated a survival predictive
nomogram for patients with resected pancreatic adenocarcinoma, by
including additional independent prognostic factors.
Nomogram is a graphical representation of a
statistical predictive model, which can predict patients'
individualized risk for a specific survival outcome (21). At present, a number of prognostic
nomograms have been developed to diagnose several types of cancer,
such as non-small-cell lung cancer and gastroenteropancreatic
neuroendocrine neoplasms (21,33,34). The
first nomogram for patients with pancreatic adenocarcinoma was
established in 2004 by Memorial Sloan-Kettering Cancer Center
(MSKCC) (23), which provided more
accurate survival predictions compared with the TNM staging system
when validated by external patient cohorts (13,35).
However, tumor markers, adjuvant therapy and other potential
prognostic factors were not considered in the MSKCC study.
Furthermore, in the MSKCC nomogram, patients with T1 stage were
assigned with higher scores than patients with T2 and T3 stages,
which is different from clinical data from patients, limiting the
popularization of MSKCC nomogram (31). Furthermore, other available nomograms
are not specific to patients with resected pancreatic
adenocarcinoma (24,26,33,36),
whereas the nomogram established in the present study was
specifically targeted for patients with resected pancreatic
adenocarcinoma. The present nomogram included T stage, N stage,
tumor grade, capsule invasion, CA199, NP, blood transfusion and
adjuvant therapy, which demonstrated an improved performance in
predicting the prognosis of patients compared with the TNM staging
system. To the best of our knowledge, the present study was the
first to describe a nomogram that included capsule invasion, NP and
blood transfusion to predict the OS of patients with resected
pancreatic adenocarcinoma.
T and N stages were included in the present
nomogram, as they have previously been considered as key
independent prognostic factors for pancreatic adenocarcinoma
(9–11,14). In
particular, T4 stage is associated with the presence of tumors
involved in the coeliac axis or superior mesenteric artery
(28). In addition, since patients
with T4 stage tumors may not be able to undergo surgery, patients
with T4 stage pancreatic adenocarcinoma were not included in the
study.
Consistent with previous findings, the results of
the present study demonstrated that, in addition to the T and N
stages, tumor grade may also act as an independent predictor for
patients with resected pancreatic adenocarcinoma (14,31,37). For
example, it has been reported that well-differentiated tumors are
significantly associated with longer survival rates (14,37,38). The
nomogram developed in the present study demonstrated the magnitude
of poor prognosis as tumor grade changed from well to poorly
differentiated. It has been reported that tumor grade incorporation
into the current TNM system enables accurate prognosis prediction
within particular clinical stages (30), which has been validated by two
subsequent studies (11,37). In the present study, patients with
distinct tumor grades were assigned to different points in the
nomogram, even if they were classified within the same TNM stage.
These observations partly illustrated the higher power of the
nomogram for predicting the survival of patients compared with the
TNM staging system.
Consistent with previous findings (39–41), the
results from the present study demonstrated that presence of
capsule invasion was an independent poor prognostic factor for OS
in patients with resected pancreatic adenocarcinoma. In another
study, Mannell et al (39)
reported that the malignant invasion of the pancreatic capsule was
significantly associated with poor prognosis. Furthermore, it has
been demonstrated that the incidence of capsule invasion has a
tendency to increase in relation to tumor size (40). This phenomenon may explain why
capsule invasion is considered a poor prognosis factor.
CA199 is a well-established marker used to determine
tumor burden, and the most frequently used tumor marker for
pancreatic adenocarcinoma (14,42,43).
CA199 has been reported as a diagnostic and a prognostic marker
(14–16). Evaluation of preoperative CA199 is
positively associated with tumor resectability and postoperative
prognosis (14,16,44,45).
Furthermore, postoperative CA199 levels have been reported to
predict OS and disease-free survival following cancer resection and
adjuvant chemotherapy (45–49). Consistent with the results from the
present study, a previous report demonstrated that CA199 can
predict the prognosis of patients with resected pancreatic
adenocarcinoma when combined with PLR (45). A 10-year follow-up study on a large
patient population indicated that CA199 is an independent
prognostic factor for advanced pancreatic adenocarcinoma (50).
In the nomogram developed in the present study,
blood transfusion was a prognostic factor that could have been
easily ignored. Previous studies reported that blood transfusion is
associated with the survival outcome of patients following
pancreatic resection (51–53). Furthermore, a previous study
identified blood transfusion as a significant negative predictor of
survival, whereas adjuvant chemotherapy is associated with
significantly longer survival (53).
These findings are consistent with the results from the present
study. Furthermore, intraoperative transfusion, lymph node
metastasis and lymph node ratio have been reported as independent
prognostic factors in predicting tumor recurrence (54). However, how intraoperative
transfusion may have a negative impact on the recurrence and OS of
patients with cancer remains unclear. It has been hypothesized that
blood transfusion may suppress the immune system of the recipient,
resulting therefore in early tumor recurrence (55). Conversely, it has been speculated
that patients requiring blood transfusion may have been associated
with perioperative complications, which may influence survival more
than the transfusion itself (56).
Prospective studies are therefore required to determine the
clinical impact and underlying mechanism of transfusion in patients
with pancreatic adenocarcinoma. However, blood transfusion should
be avoided by following the established operative procedures, in
order to minimize intraoperative bleeding.
Adjuvant therapy has been reported to be associated
with the prognosis of patients following pancreatic adenocarcinoma
resection (57–61). Although adjuvant therapy is not a
pathological factor, it is considered to significantly affect
survival (59–61). It was therefore included in the
nomogram developed in the present study. A systematic review
reported that despite curative-intent resection, the prognosis of
patients with recurrence remains poor, which leads therefore to
adjuvant therapy (61). Consistent
with the results from the present study, Corsini et al
(60) reported that patients with
pancreatic adenocarcinoma who receive adjuvant therapy following
successful resection have better survival rates. These findings
were described in numerous studies (57–59).
Subsequently, according to the present study and previous reports,
it is suggested that patients with pancreatic adenocarcinoma should
undergo postoperative chemotherapy.
It has been demonstrated that NP may act as a
prognostic factor for patients with resected pancreatic
adenocarcinoma. Previous studies reported the association between
inflammation and various types of malignancy, such as renal cell
carcinoma, pancreatic cancer (62–65), and
cancer-associated inflammation is ranked as the seventh most common
hallmark of tumor development (66).
Based on systemic inflammation analyses, several prognostic
factors, including NLR, MLR and PNI, have the ability to predict
the prognosis of patients with resectable pancreatic adenocarcinoma
(67–69). Consistent with these findings, the
present study identified NLR as a prognostic factor for pancreatic
cancer following univariate analysis; however, multivariate
analysis failed to validate NLR role as an independent prognostic
factor. Furthermore, the association between NP and the prognosis
of patients with pancreatic adenocarcinoma has been well
established. For example, pretreatment with NP has been reported to
act as an independent prognostic factor for patients with advanced
cancer who exhibit adverse outcomes (70). NP has also been considered as an
independent prognostic factor in patients with nasopharyngeal
carcinoma (71). Previous studies
suggested that neutrophils, which serve a crucial role in the
inflammatory tumor microenvironment, could mediate the pro-tumor
effects via different molecular mechanisms (72,73). In
addition, neutrophils are associated with increased tumor burden
and tumor aggressiveness, which may reflect the prognosis of
patients with various types of cancer (70). The results from the present study
demonstrated that NP may be considered as a novel prognostic
indicator for predicting survival outcome of patients with
pancreatic cancer. However, whether NP, as an immune index, may be
used as a prognostic marker for patients with resected pancreatic
adenocarcinoma requires further investigation using larger-scale
cohort studies.
Validation is essential to assure that the nomogram
is universally applicable (21). In
the present study, the calibration plots in the validation set
exhibited a positive association between the predicted nomogram and
the actual survival rate, which demonstrated the repeatability and
reliability of the present nomogram. Furthermore, the C-indices in
both training and validation sets were significantly higher in the
nomogram compared with the TNM staging system. In addition, a clear
risk stratification system of OS rates was established by using the
total scores obtained from the nomogram. In the present study, the
AUC values indicated that the risk stratification system based on
the nomogram demonstrated improved ability in predicting the
1-year, 3-year and 5-year OS rates compared with the TNM
system.
To the best of our knowledge, the present study was
the first to describe a nomogram that included tumor marker, immune
index, surgical information, pathological data and adjuvant therapy
to predict the OS of patients with resected pancreatic
adenocarcinoma. The present nomogram demonstrated favorable
discrimination and calibration in the training and validation sets.
It may therefore be used as a practical tool to predict the
prognosis of patients.
The present study presented some limitations.
Firstly, this study lacked an external validation. In the present
study, 70% of patients were randomly assigned into the training set
to develop the nomogram, whereas 30% of patients were assigned into
the validation set to validate the nomogram. Although this is a
generally accepted method for nomogram development and validation,
external validation based on other populations is required to
estimate the model accuracy. Secondly, although the validation
results demonstrated that the nomogram may be able to predict the
prognosis of patients, this nomogram did not include all potential
prognostic factors such as preoperative treatment, familial
pancreatic adenocarcinoma, the number of harvested lymph nodes etc.
Thirdly, due to the complexity of adjuvant therapy, specific
adjuvant therapy options were not subdivided in the present study,
which may have influenced the accuracy of the prediction.
In conclusion, the present study successfully
developed and validated a nomogram that could be used for the
prognosis prediction of patients with resected pancreatic
adenocarcinoma, based on the database from the China National
Cancer Center. Compared with the TNM staging system, the present
nomogram was more performant at predicting prognosis of patients.
This nomogram requires further external validation before its use
in clinical practice.
Acknowledgements
Not applicable.
Funding
The present study was funded by the CAMS Innovation
Fund for Medical Sciences (grant no. 2016-I2M-1-001).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
HR and CFW conceived and designed the present study.
HR and CRW drafted the initial manuscript and performed statistical
analysis. HR, CRW and SA acquired the data. SA performed the
statistical analysis. HR and CFW analyzed and interpreted the data.
All authors contributed to data analysis. All authors drafted and
critically revised the paper for important intellectual content.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of National Cancer Center/Cancer Hospital, Chinese
Academy of Medical Sciences and Peking Union Medical College
(Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AJCC
|
American Joint Committee on Cancer
|
|
ASA
|
American Society of
Anesthesiologists
|
|
AUC
|
area under the curve
|
|
CA199
|
carbohydrate antigen 199
|
|
CA242
|
carbohydrate antigen 242
|
|
CRP/ALB
|
c reactive protein/albumin
|
|
MLR
|
monocyte and lymphocyte ratio
|
|
NLR
|
neutrophil and lymphocyte ratio
|
|
NP
|
neutrophil percentage
|
|
OS
|
overall survival
|
|
PLR
|
platelet and lymphocyte ratio
|
|
PNI
|
prognostic nutritional index
|
|
SII
|
systemic inflammatory reaction
Index
|
|
SIRI
|
systemic inflammatory response
index
|
|
TNM
|
Tumor-Node-Metastasis
|
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rahib L, Smith BD, Aizenberg R, Rosenzweig
AB, Fleshman JM and Matrisian LM: Projecting cancer incidence and
deaths to 2030: The unexpected burden of thyroid, liver, and
pancreas cancers in the United States. Cancer Res. 74:2913–2921.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kamisawa T, Wood LD, Itoi T and Takaori K:
Pancreatic cancer. Lancet. 388:73–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heestand GM, Murphy JD and Lowy AM:
Approach to patients with pancreatic cancer without detectable
metastases. J Clin Oncol. 33:1770–1778. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferrone CR, Brennan MF, Gonen M, Coit DG,
Fong Y, Chung S, Tang L, Klimstra D and Allen PJ: Pancreatic
adenocarcinoma: The actual 5-year survivors. J Gastrointest Surg.
12:701–706. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Katz MH, Wang H, Fleming JB, Sun CC, Hwang
RF, Wolff RA, Varadhachary G, Abbruzzese JL, Crane CH, Krishnan S,
et al: Long-term survival after multidisciplinary management of
resected pancreatic adenocarcinoma. Ann Surg Oncol. 16:836–847.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cameron JL and He J: Two thousand
consecutive pancreaticoduodenectomies. J Am Coll Surg. 220:530–536.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Allen PJ, Kuk D, Castillo CF, et al:
Multi-institutional Validation Study of the American Joint
Commission on Cancer (8th Edition) Changes for T and N Staging in
Patients With Pancreatic Adenocarcinoma. Ann Surg. 265:185–191.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chatterjee D, Katz MH, Foo WC, Sundar M,
Wang H, Varadhachary GR, Wolff RA, Lee JE, Maitra A, Fleming JB, et
al: Prognostic Significance of New AJCC Tumor Stage in Patients
With Pancreatic Ductal Adenocarcinoma Treated With Neoadjuvant
Therapy. Am J Surg Pathol. 41:1097–1104. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rochefort MM, Ankeny JS, Kadera BE, Donald
GW, Isacoff W, Wainberg ZA, Hines OJ, Donahue TR, Reber HA and
Tomlinson JS: Impact of tumor grade on pancreatic cancer prognosis:
Validation of a novel TNMG staging system. Ann Surg Oncol.
20:4322–4329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan C, Morales-Oyarvide V, Babic A, Clish
CB, Kraft P, Bao Y, Qian ZR, Rubinson DA, Ng K, Giovannucci EL, et
al: Cigarette Smoking and Pancreatic Cancer Survival. J Clin Oncol.
35:1822–1828. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ferrone CR, Kattan MW, Tomlinson JS,
Thayer SP, Brennan MF and Warshaw AL: Validation of a postresection
pancreatic adenocarcinoma nomogram for disease-specific survival. J
Clin Oncol. 23:7529–7535. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barhli A, Cros J, Bartholin L and
Neuzillet C: Prognostic stratification of resected pancreatic
ductal adenocarcinoma: Past, present, and future. Dig Liver Dis.
50:979–990. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ballehaninna UK and Chamberlain RS: The
clinical utility of serum CA 19-9 in the diagnosis, prognosis and
management of pancreatic adenocarcinoma: An evidence based
appraisal. J Gastrointest Oncol. 3:105–119. 2012.PubMed/NCBI
|
|
16
|
Humphris JL, Chang DK, Johns AL, Scarlett
CJ, Pajic M, Jones MD, Colvin EK, Nagrial A, Chin VT, Chantrill LA,
et al NSW Pancreatic Cancer Network, : The prognostic and
predictive value of serum CA19.9 in pancreatic cancer. Ann Oncol.
23:1713–1722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Albert JM, Liu DD, Shen Y, Pan IW, Shih
YC, Hoffman KE, Buchholz TA, Giordano SH and Smith BD: Nomogram to
predict the benefit of radiation for older patients with breast
cancer treated with conservative surgery. J Clin Oncol.
30:2837–2843. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yi M, Meric-Bernstam F, Kuerer HM,
Mittendorf EA, Bedrosian I, Lucci A, Hwang RF, Crow JR, Luo S and
Hunt KK: Evaluation of a breast cancer nomogram for predicting risk
of ipsilateral breast tumor recurrences in patients with ductal
carcinoma in situ after local excision. J Clin Oncol. 30:600–607.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tendulkar RD, Agrawal S, Gao T, Efstathiou
JA, Pisansky TM, Michalski JM, Koontz BF, Hamstra DA, Feng FY,
Liauw SL, et al: Contemporary Update of a Multi-Institutional
Predictive Nomogram for Salvage Radiotherapy After Radical
Prostatectomy. J Clin Oncol. 34:3648–3654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nam RK, Kattan MW, Chin JL, Trachtenberg
J, Singal R, Rendon R, Klotz LH, Sugar L, Sherman C, Izawa J, et
al: Prospective multi-institutional study evaluating the
performance of prostate cancer risk calculators. J Clin Oncol.
29:2959–2964. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Iasonos A, Schrag D, Raj GV and Panageas
KS: How to build and interpret a nomogram for cancer prognosis. J
Clin Oncol. 26:1364–1370. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang ZY, Luo QF, Yin XW, Dai ZL, Basnet S
and Ge HY: Nomograms to predict survival after colorectal cancer
resection without preoperative therapy. BMC Cancer. 16:6582016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brennan MF, Kattan MW, Klimstra D and
Conlon K: Prognostic nomogram for patients undergoing resection for
adenocarcinoma of the pancreas. Ann Surg. 240:293–298. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song W, Miao DL and Chen L: Nomogram for
predicting survival in patients with pancreatic cancer. OncoTargets
Ther. 11:539–545. 2018. View Article : Google Scholar
|
|
25
|
Pu N, Li J, Xu Y, Lee W, Fang Y, Han X,
Zhao G, Zhang L, Nuerxiati A, Yin H, et al: Comparison of
prognostic prediction between nomogram based on lymph node ratio
and AJCC 8th staging system for patients with resected pancreatic
head carcinoma: A SEER analysis. Cancer Manag Res. 10:227–238.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He C, Zhang Y, Cai Z, Lin X and Li S:
Overall survival and cancer-specific survival in patients with
surgically resected pancreatic head adenocarcinoma: A competing
risk nomogram analysis. J Cancer. 9:3156–3167. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He C, Zhong L, Zhang Y, Cai Z and Lin X:
Development and validation of a nomogram to predict liver
metastasis in patients with pancreatic ductal adenocarcinoma: A
large cohort study. Cancer Manag Res. 11:3981–3991. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chun YS, Pawlik TM and Vauthey JN: 8th
Edition of the AJCC Cancer Staging Manual: Pancreas and
Hepatobiliary Cancers. Ann Surg Oncol. 25:845–847. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Camp RL, Dolled-Filhart M and Rimm DL:
X-tile: A new bio-informatics tool for biomarker assessment and
outcome-based cut-point optimization. Clin Cancer Res.
10:7252–7259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wasif N, Ko CY, Farrell J, Wainberg Z,
Hines OJ, Reber H and Tomlinson JS: Impact of tumor grade on
prognosis in pancreatic cancer: Should we include grade in AJCC
staging? Ann Surg Oncol. 17:2312–2320. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang L, Balavarca Y, van der Geest L,
Lemmens V, Van Eycken L, De Schutter H, Johannesen TB, Zadnik V,
Primic-Žakelj M, Mägi M, et al: Development and validation of a
prognostic model to predict the prognosis of patients who underwent
chemotherapy and resection of pancreatic adenocarcinoma: A large
international population-based cohort study. BMC Med. 17:662019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Diwakarla C, Hannan K, Hein N and Yip D:
Advanced pancreatic ductal adenocarcinoma - Complexities of
treatment and emerging therapeutic options. World J Gastroenterol.
23:2276–2285. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fang C, Wang W, Feng X, Sun J, Zhang Y,
Zeng Y, Wang J, Chen H, Cai M, Lin J, et al: Nomogram individually
predicts the overall survival of patients with
gastroenteropancreatic neuroendocrine neoplasms. Br J Cancer.
117:1544–1550. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liang W, Zhang L, Jiang G, Wang Q, Liu L,
Liu D, Wang Z, Zhu Z, Deng Q, Xiong X, et al: Development and
validation of a nomogram for predicting survival in patients with
resected non-small-cell lung cancer. J Clin Oncol. 33:861–869.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
de Castro SM, Biere SS, Lagarde SM, Busch
OR, van Gulik TM and Gouma DJ: Validation of a nomogram for
predicting survival after resection for adenocarcinoma of the
pancreas. Br J Surg. 96:417–423. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He C, Zhang Y, Cai Z, Duan F, Lin X and Li
S: Nomogram to Predict Cancer-Specific Survival in Patients with
Pancreatic Acinar Cell Carcinoma: A Competing Risk Analysis. J
Cancer. 9:4117–4127. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hlavsa J, Cecka F, Zaruba P, Zajak J,
Gurlich R, Strnad R, Pavlik T, Kala Z and Lovecek M: Tumor grade as
significant prognostic factor in pancreatic cancer: Validation of a
novel TNMG staging system. Neoplasma. 65:637–643. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Garcea G, Dennison AR, Ong SL, et al:
Tumour characteristics predictive of survival following resection
for ductal adenocarcinoma of the head of pancreas. Eur J Surg
Oncol. 33:892–897. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mannell A, van Heerden JA, Weiland LH and
Ilstrup DM: Factors influencing survival after resection for ductal
adenocarcinoma of the pancreas. Ann Surg. 203:403–407. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tsuchiya R, Oribe T and Noda T: Size of
the tumor and other factors influencing prognosis of carcinoma of
the head of the pancreas. Am J Gastroenterol. 80:459–462.
1985.PubMed/NCBI
|
|
41
|
Nagakawa T, Sanada H, Inagaki M, Sugama J,
Ueno K, Konishi I, Ohta T, Kayahara M and Kitagawa H: Long-term
survivors after resection of carcinoma of the head of the pancreas:
Significance of histologically curative resection. J Hepatobiliary
Pancreat Surg. 11:402–408. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Winter JM, Yeo CJ and Brody JR:
Diagnostic, prognostic, and predictive biomarkers in pancreatic
cancer. J Surg Oncol. 107:15–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Itzkowitz SH, Yuan M, Fukushi Y, Lee H,
Shi ZR, Zurawski V Jr, Hakomori S and Kim YS: Immunohistochemical
comparison of Lea, monosialosyl Lea (CA 19-9), and disialosyl Lea
antigens in human colorectal and pancreatic tissues. Cancer Res.
48:3834–3842. 1988.PubMed/NCBI
|
|
44
|
Brown EG, Canter RJ and Bold RJ:
Preoperative CA 19-9 kinetics as a prognostic variable in
radiographically resectable pancreatic adenocarcinoma. J Surg
Oncol. 111:293–298. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sakamoto T, Saito H, Amisaki M, Tokuyasu
N, Honjo S and Fujiwara Y: Combined preoperative
platelet-to-lymphocyte ratio and serum carbohydrate antigen 19-9
level as a prognostic factor in patients with resected pancreatic
cancer. Hepatobiliary Pancreat Dis Int. 18:278–284. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Landi F, Dopazo C, Sapisochin G, Beisani
M, Blanco L, Caralt M, Balsells J and Charco R: Long-term results
of pancreaticoduodenectomy with superior mesenteric and portal vein
resection for ductal adenocarcinoma in the head of the pancreas.
Cir Esp. 93:522–529. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Takagi C, Kikuchi Y, Shirakawa H,
Hoshimoto S, Tomikawa M, Ozawa I, Hishinuma S and Ogata Y:
Predictive Factors for Elevated Postoperative Carbohydrate Antigen
19-9 Levels in Patients With Resected Pancreatic Cancer. Anticancer
Res. 39:3177–3183. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Aoki S, Motoi F, Murakami Y, Sho M, Satoi
S, Honda G, Uemura K, Okada KI, Matsumoto I, Nagai M, et al;
Multicenter Study Group of Pancreatobiliary Surgery (MSG-PBS), .
Decreased serum carbohydrate antigen 19-9 levels after neoadjuvant
therapy predict a better prognosis for patients with pancreatic
adenocarcinoma: A multicenter case-control study of 240 patients.
BMC Cancer. 19:2522019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Imaoka H, Shimizu Y, Senda Y, et al:
Post-adjuvant chemotherapy CA19-9 levels predict prognosis in
patients with pancreatic ductal adenocarcinoma: A retrospective
cohort study. Pancreatology. 16:658–664. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Deng QL, Dong S, Wang L, Zhang CY, Ying
HF, Li ZS, Shen XH, Guo YB, Meng ZQ, Yu JM, et al: Development and
Validation of a Nomogram for Predicting Survival in Patients with
Advanced Pancreatic Ductal Adenocarcinoma. Sci Rep. 7:115242017.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mitra A, Pai E, Dusane R, Ranganathan P,
DeSouza A, Goel M and Shrikhande SV: Extended pancreatectomy as
defined by the ISGPS: Useful in selected cases of pancreatic cancer
but invaluable in other complex pancreatic tumors. Langenbecks Arch
Surg. 403:203–212. 2018.PubMed/NCBI
|
|
52
|
Homeyer RS, Roberts KJ, Sutcliffe RP,
Kaltenborn A, Mirza D, Qu Z, Klempnauer J and Schrem H: Ventilation
after pancreaticoduodenectomy increases perioperative mortality:
Identification of risk factors and their relevance in Germany that
do not apply in England. Hepatobiliary Pancreat Dis Int.
18:379–388. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Petrou A, Soonawalla Z, Silva MA, et al:
Prognostic indicators following curative pancreatoduodenectomy for
pancreatic carcinoma: A retrospective multivariate analysis of a
single centre experience. J BUON. 21:874–882. 2016.PubMed/NCBI
|
|
54
|
Hwang HK, Jung MJ, Lee SH, Kang CM and Lee
WJ: Adverse oncologic effects of intraoperative transfusion during
pancreatectomy for left-sided pancreatic cancer: The need for
strict transfusion policy. J Hepatobiliary Pancreat Sci.
23:497–507. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hoh H, Umpleby H, Cooper A and Taylor I:
Recurrence of colorectal cancer and perioperative blood
transfusion. Is blood storage time important? Dis Colon Rectum.
33:127–130. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Park SJ, Kim SW, Jang JY, Lee KU and Park
YH: Intraoperative transfusion: Is it a real prognostic factor of
periampullary cancer following pancreatoduodenectomy? World J Surg.
26:487–492. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fitzgerald TL, Hunter L, Mosquera C,
Jindal C, Biswas T, Zervos E and Efird JT: A simple matrix to
predict treatment success and long-term survival among patients
undergoing pancreatectomy. HPB (Oxford). 21:204–211. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ahmad NA, Lewis JD, Ginsberg GG, Haller
DG, Morris JB, Williams NN, Rosato EF and Kochman ML: Long term
survival after pancreatic resection for pancreatic adenocarcinoma.
Am J Gastroenterol. 96:2609–2615. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Morganti AG, Falconi M, van Stiphout RG,
Mattiucci GC, Alfieri S, Calvo FA, Dubois JB, Fastner G, Herman JM,
Maidment BW III, et al: Multi-institutional pooled analysis on
adjuvant chemoradiation in pancreatic cancer. Int J Radiat Oncol
Biol Phys. 90:911–917. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Corsini MM, Miller RC, Haddock MG, Donohue
JH, Farnell MB, Nagorney DM, Jatoi A, McWilliams RR, Kim GP, Bhatia
S, et al: Adjuvant radiotherapy and chemotherapy for pancreatic
carcinoma: The Mayo Clinic experience (1975–2005). J Clin Oncol.
26:3511–3516. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yegya-Raman N, Shah MM, Grandhi MS, Poplin
E, August DA, Kennedy TJ, Malhotra U, Spencer KR, Carpizo DR and
Jabbour SK: Adjuvant therapeutic strategies for resectable
pancreatic adenocarcinoma. Ann Pancreat Cancer. 1:12018. View Article : Google Scholar
|
|
62
|
Peng D, Li XS, Zhang CJ, et al: Prognostic
factors of patients with T3N0M0 renal cell carcinoma: a
single-center retrospective study of 182 patients. Beijing Da Xue
Xue Bao Yi Xue Ban. 48:806–811. 2016.(In Chinese). PubMed/NCBI
|
|
63
|
Demir IE, Friess H and Ceyhan GO: Neural
plasticity in pancreatitis and pancreatic cancer. Nat Rev
Gastroenterol Hepatol. 12:649–659. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Proctor MJ, Morrison DS, Talwar D, Balmer
SM, O'Reilly DS, Foulis AK, Horgan PG and McMillan DC: An
inflammation-based prognostic score (mGPS) predicts cancer survival
independent of tumour site: A Glasgow Inflammation Outcome Study.
Br J Cancer. 104:726–734. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Stotz M, Gerger A, Eisner F, Szkandera J,
Loibner H, Ress AL, Kornprat P, AlZoughbi W, Seggewies FS, Lackner
C, et al: Increased neutrophil-lymphocyte ratio is a poor
prognostic factor in patients with primary operable and inoperable
pancreatic cancer. Br J Cancer. 109:416–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ye S and Bai L: Comparison and validation
of the value of preoperative inflammation marker-based prognostic
scores in resectable pancreatic ductal adenocarcinoma. Cancer Manag
Res. 10:3405–3417. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhao W, Wang P, Jia H, Chen M, Gu X, Liu
M, Zhang Z, Cheng W and Wu Z: Neutrophil count and percentage:
Potential independent prognostic indicators for advanced cancer
patients in a palliative care setting. Oncotarget. 8:64499–64508.
2017.PubMed/NCBI
|
|
71
|
He JR, Shen GP, Ren ZF, Qin H, Cui C,
Zhang Y, Zeng YX and Jia WH: Pretreatment levels of peripheral
neutrophils and lymphocytes as independent prognostic factors in
patients with nasopharyngeal carcinoma. Head Neck. 34:1769–1776.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Powell DR and Huttenlocher A: Neutrophils
in the Tumor Microenvironment. Trends Immunol. 37:41–52. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Uribe-Querol E and Rosales C: Neutrophils
in Cancer: Two Sides of the Same Coin. J Immunol Res.
2015:9836982015. View Article : Google Scholar : PubMed/NCBI
|