Introduction
Oral squamous cell carcinoma (OSCC) is characterized
as squamous cell carcinoma originated from lips, buccal mucosa,
anterior tongue, floor of the mouth, hard palate, gingiva and
retromolar trigone (1). It is the
eighth most fatal malignancy worldwide, accounting for 3.01% of all
cancer cases and 1.79% of cancer-associated mortalities in 2019 in
the United States (2,3). Despite considerable progress in
clinical treatment, the overall 5-year survival rate of OSCC was
~60% in United States in 2005 to 2011 (4). Tumor recurrence and metastasis are
considered the most common causes of mortality with OSCC (5). A number of tumor suppressor genes and
oncogenes, including DNA-binding protein inhibitor ID-1 (ID-1) are
currently used to predict the prognosis of different types of
malignant tumor. For example, the expression levels of ID-1 were
correlated with poor prognosis in epithelial ovarian tumors,
nasopharyngeal carcinoma and head and neck squamous cell carcinoma
(6–8).
ID-1 plays a vital role in the development of cancer
and is associated with poor patient outcome (9). ID-1 predominantly elevated in mRNA and
protein levels in several types of cancer, such as prostate, breast
and ovarian cancer (10),
contributing to tumorigenesis by inhibiting cellular
differentiation, stimulating proliferation and facilitating tumor
neoangiogenesis (10). A previous
study demonstrated that overexpression of ID-1 protein was
associated with tumor angiogenesis in OSCC; however, due to a short
follow-up time, the association between ID-1 expression and
survival rate was unable to be determined (11).
To the best of our knowledge, few studies have
investigated the association between ID-1 expression and survival
rate in OSCC (11–13). Thus, the present study analyzed
long-term follow-up data to determine the association between ID-1
expression and survival rate, and establish its role as a potential
prognostic marker for patients with OSCC.
Materials and methods
Patient information and follow-up
data
The present research was a retrospective study. A
total of 128 patients with OSCC, who underwent tumor resection and
neck dissection at the Qilu Hospital of Shandong University
(Shandong, China) were enrolled into the present study from January
2004 to October 2008. The inclusion criterion was OSCC without
distant metastasis that the tumor had not undergone distant
metastasis. A total of 128 oral cancer tissues and 60 adjacent
normal tissues were collected, and the margin of non-neoplastic
tissues was 1–1.5 cm away from tumor edge with biopsy-negative. The
surgical procedure selected the range of neck dissection according
to the primary lesions and metastatic lymph nodes in the neck of
the patient. Supraomohyoid neck dissection was used to select
patients in clinical stage I, while radical neck dissection (RND)
or modified RND (in which the sternocleidomastoid muscle, internal
jugular vein, spinal accessory nerve were retained) were used to
select patients in clinical stages II and III. The clinical stages
of OSCC were classified according to the American Joint Committee
on Cancer staging manual (14).
Patients with primary lesions >4 cm in length or with cervical
lymphatic metastasis received postoperative radiotherapy. The
patients were in stage II or III of OSCC. There were total 67
patients. The treatment was 2 Gy per day duration for one month
with a total dose of 60 Gy. The clinicopathological characteristics
of the patients with OCSS are presented in Table I. Excluding those lost to follow-up,
each patient was followed-up for 10 years, until October 2018.
Disease-specific survival (DSS) time was considered to be the
interval between surgery and time of mortality due to OSCC. Data
were censored for patients who died from other causes (such as
heart disease, chronic lower respiratory disease or diabetes
mellitus) or at the time of the last follow-up. The deaths directly
caused by OSCC were counted as death cases, while deaths caused by
other reasons or lost to follow-up during treatment were not
included. Other causes of death included heart disease, chronic
lower respiratory diseases and diabetes mellitus etc.
 | Table I.Clinicopathological characteristics of
patients with oral squamous cell carcinoma (n=128). |
Table I.
Clinicopathological characteristics of
patients with oral squamous cell carcinoma (n=128).
| Characteristic | Patient, n (%) |
|---|
| Age, years |
|
| ≤60 | 68 (53.1) |
|
>60 | 60 (46.9) |
| Sex |
|
| Male | 90 (70.3) |
|
Female | 38 (29.7) |
| Tumor size, mm |
|
|
<31 | 65 (50.8) |
| ≥31 | 63 (49.2) |
| Tumor location |
|
|
Tongue | 45 (35.2) |
|
Gingival | 29 (22.7) |
| Mouth
floor | 16 (12.5) |
| Lip | 10 (7.8) |
|
Cheek | 16 (12.5) |
| Soft
palate | 12 (9.4) |
| Pathological
differentiation grade |
|
|
Well | 74 (57.8) |
|
Moderate | 42 (32.8) |
|
Poor | 12 (9.4) |
| Clinical stage |
|
|
I+II | 71 (55.5) |
|
III+IV | 57 (44.5) |
Immunohistochemistry (IHC)
IHC was performed on 128 oral cancer tissues and 60
adjacent normal tissues (the margin of tissues with
biopsy-negative, 1–1.5 cm away from tumor edge). There were just 60
adjacent normal tissues, so 60 adjacent normal tissues were
assessed. Paraffin-embedded sample sections (4-µm thickness) were
dewaxed and rehydrated in xylene and graded ethanol (100, 95, 90,
70% and each grade for 5 min). The slides were then subjected to
antigen retrieval using 0.01 M citric buffer (pH 6.0) for 20 min,
followed by incubation in 3% H2O2 at room
temperature for 10 min. Non-specific binding was blocked by
incubation in 10% goat serum for 10 min at room temperature. Slides
were then washed and incubated with primary antibodies of ID-1
(rabbit polyclonal antibody, cat. no. PA5-101010, dilution 1:400,
Santa Cruz Biotechnology, Inc.) at 4°C overnight. The next day, the
slides were washed in phosphate-buffered saline (PBS) three rinses
(each for 5 min) and incubated with multimer anti-rabbit/mouse
horseradish peroxidase(HPR)-conjugated secondary antibodies (cat.
no. SV0004, dilution 1:300, Wuhan Boster Biological Technology,
Ltd.) for 30 min at 37°C. Then slides were washed and visualized by
adding the substrate DAB. The slides were then rinsed in distilled
water and counterstained with haematoxylin for 3 min at room
temperature. The slides were observed, and images were captured
using a light phase-contrast microscope at ×400 magnification
(Leica, Microsystems). Immunoreactivity was graded based on the
density and degree of positively-stained cells (15). Patients were subsequently divided
into two groups, according to the ID-1 expression level median
value: Low expression group (absent/weak, <3.7) and high
expression group (median/strong, >3.7).
Prognostic indicators
The difference in ID-1 expression was compared
between patients with and without tumor recurrence. Univariate
survival analysis was performed to assess the association between
ID-1 expression and survival rate, while multivariate analysis was
performed to determine whether ID-1 may act as an independent
prognostic factor for DSS time in patients with OSCC. Previous
research showed that there was significant correlation between ID-1
expression levels and tumor size, clinical stage, lymph node
involvement (11). Pathologic
differentiation grade has also been shown to be an important
indicator of clinical prognosis in squamous cell carcinoma
(16). Therefore, in multivariate
survival analysis, the following characteristics were assessed
according to previously established grading systems (11,16):
Tumor size, pathological differentiation grade, clinical stage,
lymph node metastasis and ID-1 expression. The overall survival
rate which was calculated through the total survival cases/total
cases. Pathological differentiation grades were classified as
follows: Well, well-differentiated; moderate, moderately
differentiated; poor, poorly differentiated (17). Clinical stage were classified as
follows: Stage I, T1N0M0; Stage II, T2N0M0; Stage III, T3N0M0,
T(1–3)N1M0; Stage IV, T4aN(0,1)M0, T(1-4a)N2M0, TN3M0 and T4bNM0
(14). Lymph node metastasis was
classified as negative or positive.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 23.0; IBM Corp). Data are presented as mean ±
standard deviation. An unpaired t-test was used to compare the
expression of ID-1 in nonneoplastic oral tissues and OSCC. The
Mann-Whitney test was used to assess the association between ID-1
expression and recurrence. The Kaplan-Meier method was used to
assess DSS times, while the log-rank test was implemented to
compare survival curves. Five and ten-year survival descriptive
data are presented of the cases, and no further statistical
analysis was performed on these data. Multivariate Cox proportional
hazards analysis was performed to determine the prognostic value of
ID-1. P<0.05 was considered to indicate a statistically
significant difference.
Results
Follow-up parameters
Follow-up data were received from all patients, with
a median survival time of 68.00±14.89 months (range, 4–120 months).
Compared with the previous statistics (11), there were 65 new cases of local
recurrence and 34 new cases of lymph node metastasis. A total of 93
patients (72.7%) died during the follow-up period, of which 83
(64.8%) died of OSCC and 10 (7.8%) died of other causes, such as
heart disease, chronic lower respiratory diseases and diabetes
mellitus. A further 19 patients (14.8%) were lost to follow-up,
thus only 16 patients (12.5%) survived the entire follow-up period.
These data are presented in Table
I.
ID-1 expression in OSCC tissues
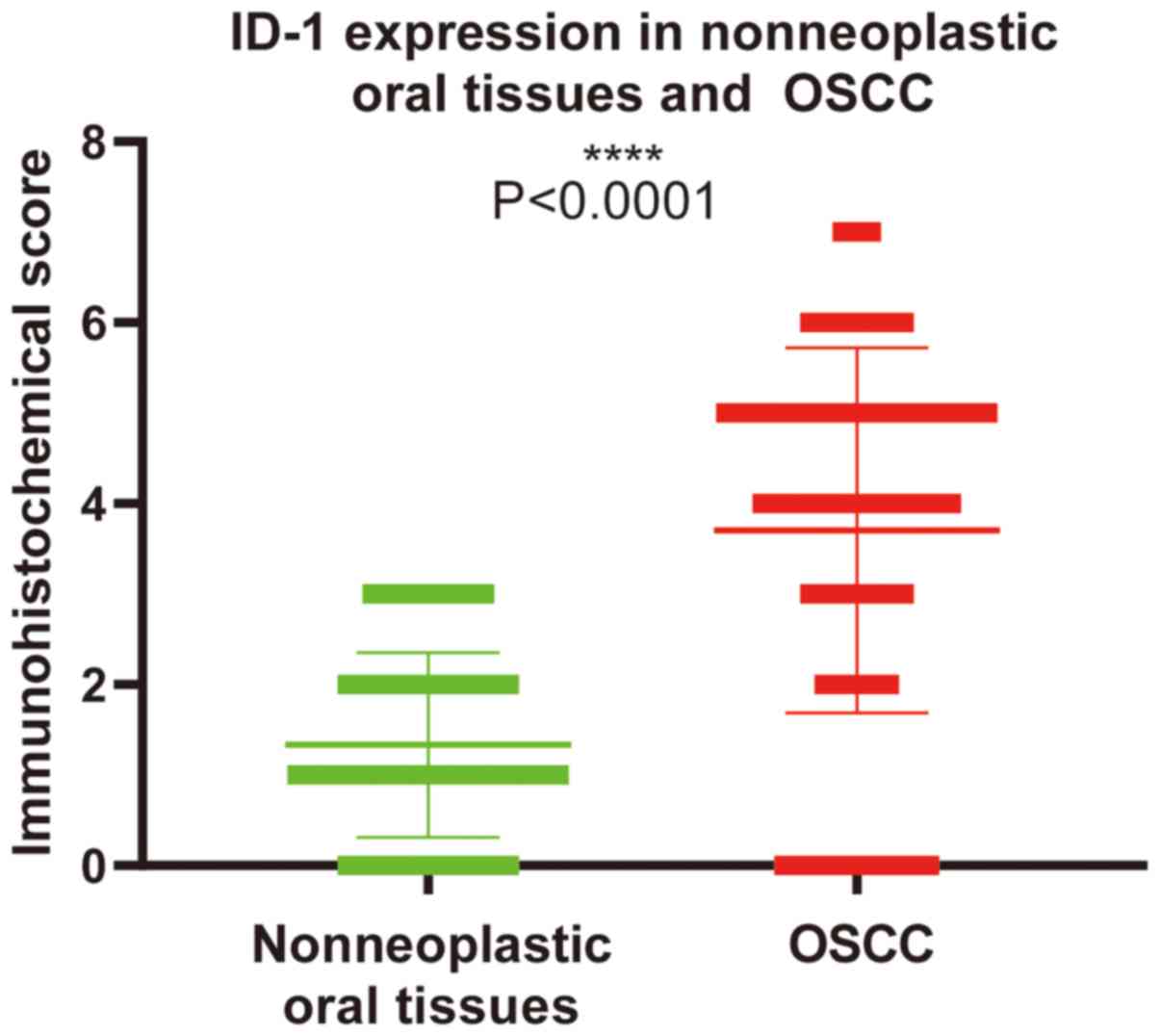
ID-1 expression was significantly higher in OSCC
tissues compared with that in nonneoplastic oral tissues
(P<0.0001; Fig. 1). Among the 60
adjacent normal tissues, ID-1 expression was absent in 36 cases and
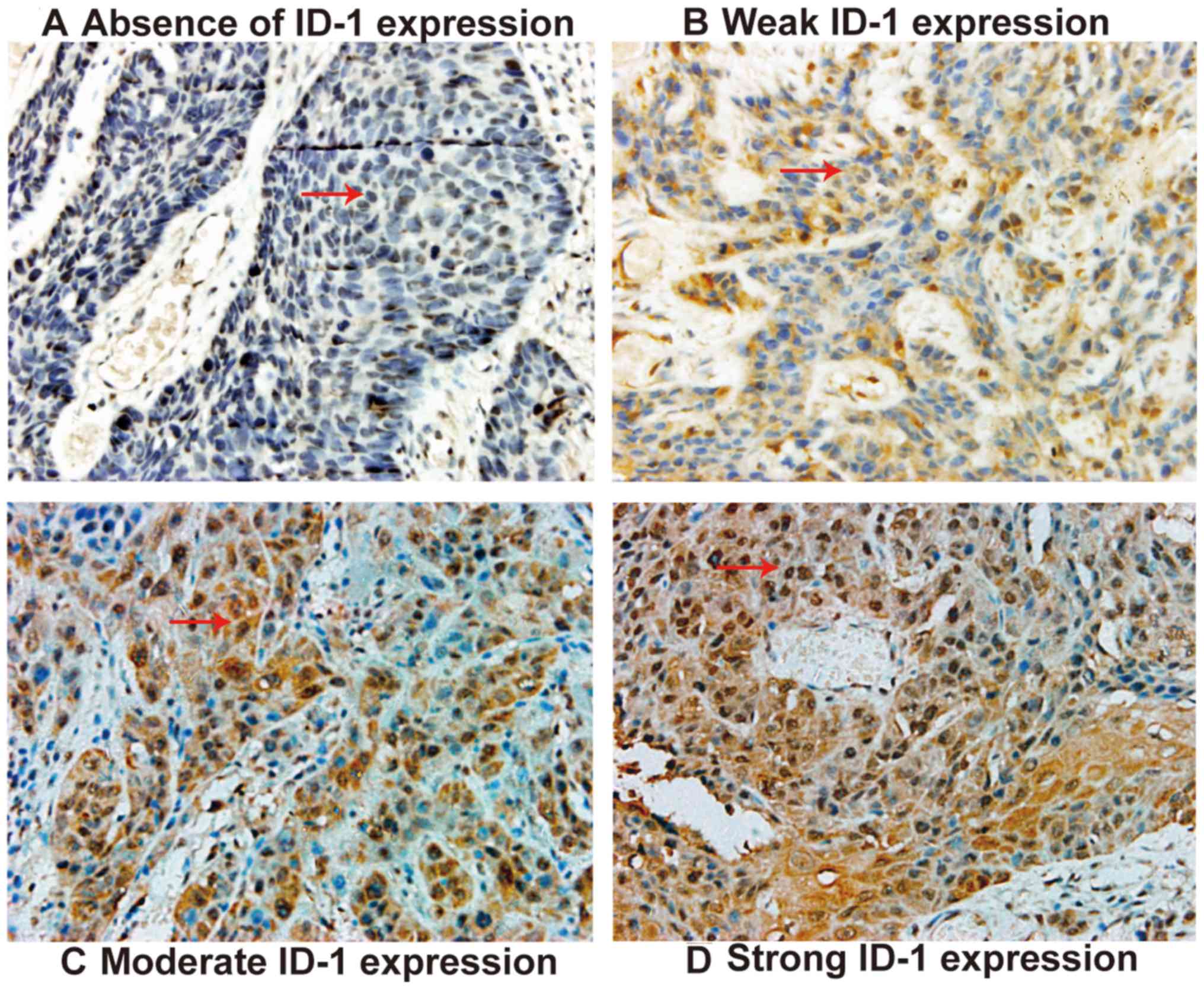
weakly expressed in 24 cases. Among the 128 OSCC tissues,
cytoplasmic and nuclear ID-1 protein expression level was negative
in 21 cases (Fig. 2A), and 24 cases
exhibited weak ID-1 expression levels, with weak nuclear staining
and partial cytoplasmic expression (Fig.
2B). Moderate expression levels of ID-1 were exhibited in 64
cases, with areas of intense staining in the cytoplasm and nucleus
(Fig. 2C). Strong ID-1 expression
was observed in 19 cases, where the majority of the cytoplasm and
nucleus were positively stained (Fig.
2D). The low ID-1 expression group (absent/weak) consisted of
45 cases and the high ID-1 expression group (median/strong)
consisted of 83 cases.
High ID-1 expression is associated
with tumor recurrence
ID-1 expression was significantly higher in patients
with local tumor and lymph node metastasis recurrence compared with
those without recurrence, 4.01±1.92 vs. 2.92±2.06 (P=0.007) and
4.08±1.90 vs. 2.98±2.06 (P=0.001), respectively (Table II).
 | Table II.Associations between ID-1 expression
levels and recurrence in patients with oral squamous cell
carcinoma. |
Table II.
Associations between ID-1 expression
levels and recurrence in patients with oral squamous cell
carcinoma.
| Classification | Patient, n (%) | ID-1 expression
(mean ± SD) |
P-valuea |
|---|
| Local tumor
recurrence |
|
| 0.007 |
|
Negative | 36
(28.1) | 2.92±2.06 |
|
|
Positive | 92
(71.9) | 4.01±1.92 |
|
| Lymph node
metastasis recurrence |
|
| 0.001 |
|
Negative | 44
(34.4) | 2.98±2.06 |
|
|
Positive | 84
(65.6) | 4.08±1.90 |
|
| Total | 128 (100.0) | 3.67±1.94 |
|
High ID-1 expression is associated
with poor survival
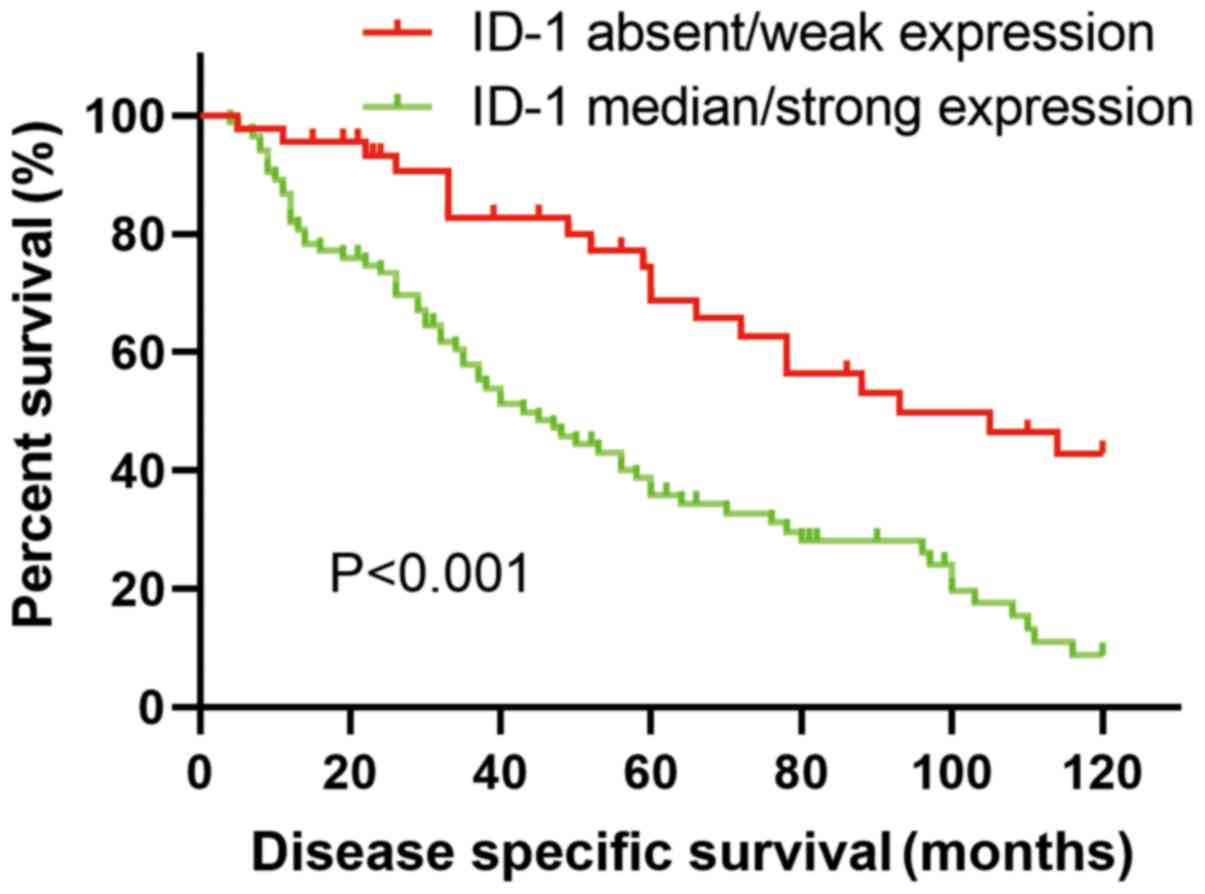
The median DSS times in the low and high ID-1
protein expression groups were 93.00±22.94 months and 43.00±6.84
months, respectively. Patients with higher ID-1 protein expression
levels exhibited significantly shorter DSS times compared with
those with lower ID-1 expression levels (P<0.001; Table III). The 5- and 10-year
disease-specific survival curve (univariate survival analysis) is
shown in Fig. 3. The 5- and 10-year
overall survival rates between the two groups are presented in
Table IV.
 | Table III.Kaplan-Meier survival analysis in
patients with oral squamous cell carcinoma, according to ID-1
expression. |
Table III.
Kaplan-Meier survival analysis in
patients with oral squamous cell carcinoma, according to ID-1
expression.
|
|
| Disease-specific
survival, months |
|
|---|
|
|
|
|
|
|---|
| Classification | Patient number,
n | Mean ± SD | Median ± SD |
P-valuea |
|---|
| Absent/weak ID-1
expression | 45 | 86.08±6.02 | 93.00±22.94 | <0.001 |
| Median/strong ID-1
expression | 83 | 54.97±4.43 | 43.00±6.84 |
|
| Total | 128 | 70.53±5.23 | 68.00±14.89 |
|
 | Table IV.Survival rates in patients with oral
squamous cell carcinoma. |
Table IV.
Survival rates in patients with oral
squamous cell carcinoma.
| Classification | 5-year survival
rate, % | 10-year survival
rate, % |
|---|
| Absent/weak ID-1
expression | 75 | 55 |
| Median/strong ID-1
expression | 40 | 23 |
| Total | 52 | 34 |
ID-1 is an independent prognostic
factor for DSS time in patients with OSCC
Multivariate survival analysis identified that ID-1
protein expression levels (P=0.002) and lymph node metastasis
(P=0.034) are independent prognostic factors for DSS time in
patients with OSCC (Table V).
 | Table V.Multivariate survival analysis for
disease-specific survival in patients with oral squamous cell
carcinoma. |
Table V.
Multivariate survival analysis for
disease-specific survival in patients with oral squamous cell
carcinoma.
| Characteristic | 95% CI | OR |
P-valuea |
|---|
| Tumor size | 0.99–1.04 | 1.102 | 0.394 |
| Pathologic
differentiation grade | 0.94–1.82 | 1.305 | 0.114 |
| Clinical stage | 0.99–2.12 | 1.464 | 0.054 |
| Lymph node
metastasis | 1.04–2.93 | 1.750 | 0.034 |
| ID-1
expression | 1.08–1.42 | 1.239 | 0.002 |
Discussion
ID-1 plays a number of key roles in cellular
differentiation, cell cycle progression, tumorigenesis and
metastasis (18). ID-1 predominantly
acts as an oncogene in several types of tumor, including gastric
(19), pancreatic (20), colorectal (21) and lung cancer (22). Furthermore, a previous study
demonstrated that higher protein levels of ID-1 are associated with
tumor recurrence and lymph node metastasis in patients with OSCC
(11). However, the association
between ID-1 expression levels and the survival status of
postoperative patients has not investigated. To the best of our
knowledge, the present study was the first to analyze long-time
follow-up data, to investigate the association between ID-1
expression levels and survival rates in patients with OSCC.
The present study identified 65 new cases of local
tumor recurrence and 34 new cases of recurring lymph node
metastasis, and the results demonstrated a significant association
between ID-1 expression levels and tumor recurrence. These results
were consistent with those of a previous study (11), which provides support that high ID-1
expression levels are associated with poor prognosis in patients
with OSCC, thus suggesting that ID-1 may serve as an indicator of
patient outcome.
The results of the present study were also
consistent with a previous study on oral squamous cell carcinoma
demonstrating that high ID-1 expression levels were associated with
poor prognosis (12). However, the
results from a recent report on primary esophageal squamous cell
carcinoma (ESCC) contradicted the results in the present study,
suggesting that strong ID-1 expression levels in the metastatic
lymph nodes from patients with ESCC were associated with improved
prognosis (23). Conversely, another
study on ESCC reported that ID-1 was a predictor of poor patient
survival (24). A reason for this
discrepancy may be due to the fact that metastases results from the
survival and proliferation of primary tumor cells, tumor evolution
and progression, which can result in biological diversity between
different patients (25). A number
of previous studies have demonstrated that some gene expression
profiles and biomarkers between primary and metastatic tumors are
differentially expressed, such as programmed cell death protein-1
and zeste homolog-2 (26–28). In renal cell carcinoma, the
expression levels of PD-1, programmed cell death-ligand 1 (PD-L1)
and PD-L2 between primary and metastatic sites are different
(26). Tissue microarray and cDNA
microarray analysis revealed differential gene and protein
expression levels in primary breast tumors and corresponding lymph
node metastases (27). In metastatic
breast cancer, the expression levels of the zeste homolog-2,
estrogen receptor, progesterone receptor and human epidermal growth
factor receptor 2 were different between primary and metastatic
lesions (28). In the present study,
ID-1 expression was not detected in metastatic lymph nodes. The
comparison of ID-1 expression between the primary tumor and
metastatic lesion in the same case is more convincing. Thus,
investigations into the differential expression pattern of ID-1 in
metastatic paired lymph nodes, and the association between ID-1 and
prognosis should be investigated in prospective studies.
Despite improvements in therapeutic strategies, the
overall 5-year survival rate of OSCC was ~60% in United States
between 2005 and 2011 (4). The
results of the present study indicated that the 5-year survival
rate of OSCC was 52%, while the 10-year survival rate was 34%. This
is consistent with the results of an epidemiological survey on OSCC
(29). Local tumor recurrence and
lymph node metastasis are considered the primary factors
contributing to poor prognosis and low 5-year survival rates of
patients with OSCC (30). Thus,
long-term patient follow-up was investigated in the present study,
and ID-1 expression level was demonstrated to be associated with
survival rate. Univariate survival analysis indicated that the
median DSS times of patients in the low ID-1 expression level group
were significantly longer compared with those with high ID-1
expression levels. Furthermore, multivariate analysis demonstrated
that ID-1 expression levels and lymph node metastasis were
independent prognostic factors for DSS time in patients with OSCC,
which was consistent with findings from previous studies on
different types of tumors, such as non-small cell lung cancer and
glioblastoma (22,31). In non-small cell lung cancer, high
ID-1 expression levels were associated with a shorter survival time
(22), and genetic knockdown of ID-1
led to a significant increase in survival time in a nude mice with
glioblastoma (31).
The molecular mechanism by which ID-1 promotes tumor
recurrence and lymph node metastasis has not yet been fully
investigated. It is hypothesized that ID-1 may promote
lymphangiogenesis by upregulating the expression of vascular
endothelial growth factor C in OSCC (32). Previous studies have reported that
ID-1 interacts with different signaling molecules and pathways,
such as the PI3K/AKT and Wnt/β-Catenin signaling pathways, which
regulate the proliferation, migration, metastasis and
chemoresistance of the tumor (18).
ID-1 activated the NF-κB signaling pathway to promote migration and
invasion in non-small cell lung cancer cells (22).
MicroRNAs (miRNAs) have also been reported to play a
key role in the regulation of ID1 expression. Through targeting
bone morphogenetic protein receptor type 1A and blocking
BMP/Smad/ID-1 signaling, miRNA-885-3p was demonstrated to inhibit
growth of HT-29 colon cancer cell xenografts in nude mice via
angiogenetic disruption (33). These
previous studies laid the foundation for clinical and mechanistic
studies of ID-1 in OSCC; however, further research is required to
fully understand its role therein.
The present study was not without limitations.
First, the present study was a retrospective study with a single
center cohort, which comprised a limited heterogeneous sample
group. Furthermore, the effect of postoperative radiotherapy was
not considered. The limitations might have a bias effect on the
results; however, the present study featured long-term follow-up of
128 patients, therefore the results should be reliable and
reproducible.
In conclusion, the present study demonstrated that
high ID-1 expression levels were significantly associated with
local tumor recurrence and lymph node metastasis, suggesting that
ID-1 may serve as an independent prognostic factor for DSS time in
patients with OSCC. An initial assessment of the impact of ID-1 on
patients with OSCC has been provided; however, validation analysis
of a large-scale prospective homogeneous sample cohort is still
required to fully determine the role of ID-1 in OSCC.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Key Technology
Research and Development Program of Shandong, China (grant no.
2017GSF221008).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
JC designed the present study and wrote the
manuscript. DW acquired the data. FZ contributed to data analysis
and interpretation. ZY contributed to the data collection and
analysis. SL participated in drafting the initial manuscript and
performed statistical analysis. ZD designed the study and revised
the manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of Qilu Hospital of Shandong University (Jinan, China)
and informed consent was provided by all the patients prior to the
start of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chow LQM: Head and neck cancer. N Engl J
Med. 382:60–72. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shield KD, Ferlay J, Jemal A,
Sankaranarayanan R, Chaturvedi AK, Bray F and Soerjomataram I: The
global incidence of lip, oral cavity, and pharyngeal cancers by
subsite in 2012. CA Cancer J Clin. 67:51–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chinn SB and Myers JN: Oral cavity
carcinoma: Current management, controversies, and future
directions. J Clin Oncol. 33:3269–3276. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schindl M, Schoppmann SF, Ströbel T,
Heinzl H, Leisser C, Horvat R and Birner P: Level of Id-1 protein
expression correlates with poor differentiation, enhanced malignant
potential, and more aggressive clinical behavior of epithelial
ovarian tumors. Clin Cancer Res. 9:779–785. 2003.PubMed/NCBI
|
|
7
|
Sun W, Guo MM, Han P, Lin JZ, Liang FY,
Tan GM, Li HB, Zeng M and Huang XM: Id-1 and the p65 subunit of
NF-κB promote migration of nasopharyngeal carcinoma cells and are
correlated with poor prognosis. Carcinogenesis. 33:810–817. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin J, Guan Z, Wang C, Feng L, Zheng Y,
Caicedo E, Bearth E, Peng JR, Gaffney P and Ondrey FG: Inhibitor of
differentiation 1 contributes to head and neck squamous cell
carcinoma survival via the NF-kappaB/survivin and phosphoinositide
3-kinase/Akt signaling pathways. Clin Cancer Res. 16:77–87. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Benezra R, Davis RL, Lockshon D, Turner DL
and Weintraub H: The protein Id: A negative regulator of
helix-loop-helix DNA binding proteins. Cell. 61:49–59. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Perk J, Iavarone A and Benezra R: Id
family of helix-loop-helix proteins in cancer. Cancer. 5:603–614.
2005.PubMed/NCBI
|
|
11
|
Dong Z, Liu S, Zhou C, Sumida T, Hamakawa
H, Chen Z, Liu P and Wei F: Overexpression of Id-1 is associated
with tumor angiogenesis and poor clinical outcome in oral squamous
cell carcinoma. Oral Oncol. 46:154–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nishimine M, Nakamura M, Mishima K, Kishi
M, Kirita T, Sugimura M and Konishi N: Id proteins are
overexpressed in human oral squamous cell carcinomas. J Oral Pathol
Med. 32:350–357. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao J, Wang S, Liu N and Tang X:
Correlation between the expression of Id-1 and
hyperthermia-associated molecules in oral squamous cell carcinoma.
J Clin Pathol. 66:758–763. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lydiatt WM, Patel SG, O'Sullivan B,
Brandwein MS, Ridge JA, Migliacci JC, Loomis AM and Shah JP: Head
and neck cancers-major changes in the American joint committee on
cancer eighth edition cancer staging manual. CA Cancer J Clin.
67:122–137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schoppmann SF, Schindl M, Bayer G, Aumayr
K, Dienes J, Horvat R, Rudas M, Gnant M, Jakesz R and Birner P:
Overexpression of Id-1 is associated with poor clinical outcome in
node negative breast cancer. Int J Cancer. 104:677–682. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kyrgidis A, Tzellos TG, Kechagias N,
Patrikidou A, Xirou P, Kitikidou K, Bourlidou E, Vahtsevanos K and
Antoniades K: Cutaneous squamous cell carcinoma (SCC) of the head
and neck: Risk factors of overall and recurrence-free survival. Eur
J Cancer. 46:1563–1572. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu JM and Montgomery E: Classification and
pathology. Surg Clin North Am. 88:483–520. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ke J, Wu R, Chen Y and Abba ML: Inhibitor
of DNA binding proteins: Implications in human cancer progression
and metastasis. Am J Transl Res. 10:3887–3910. 2018.PubMed/NCBI
|
|
19
|
Li L, Wei X, Wu B, Xiao Y, Yin M and Yang
Q: siRNA-mediated knockdown of ID1 disrupts Nanog- and
Oct-4-mediated cancer stem cell-likeness and resistance to
chemotherapy in gastric cancer cells. Oncol Lett. 13:3014–3024.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Georgiadou D, Sergentanis TN, Sakellariou
S, Filippakis GM, Zagouri F, Vlachodimitropoulos D, Psaltopoulou T,
Lazaris AC, Patsouris E and Zografos GC: VEGF and Id-1 in
pancreatic adenocarcinoma: Prognostic significance and impact on
angiogenesis. Eur J Surg Oncol. 40:1331–1337. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao ZR, Zhang ZY, Zhang H, Jiang L, Wang
MW and Sun XF: Overexpression of Id-1 protein is a marker in
colorectal cancer progression. Oncol Rep. 19:419–424.
2008.PubMed/NCBI
|
|
22
|
Li J, Li Y, Wang B, Ma Y and Chen P: Id-1
promotes migration and invasion of non-small cell lung cancer cells
through activating NF-κB signaling pathway. J Biomed Sci.
24:952017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu Y, Luo KJ, Wen J and Zhu ZH: Strong
expression of Id-1 in metastatic lymph nodes from esophageal
squamous cell carcinoma is associated with better clinical outcome.
J Thorac Dis. 10:5499–5507. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yuen HF, Chan YP, Chan KK, Chu YY, Wong
ML, Law SY, Srivastava G, Wong YC, Wang X and Chan KW: Id-1 and
Id-2 are markers for metastasis and prognosis in oesophageal
squamous cell carcinoma. Br J Cancer. 97:1409–1415. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fidler IJ and Hart IR: Biological
diversity in metastatic neoplasms: Origins and implications.
Science. 217:998–1003. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang X, Yin X, Zhang H, Sun G, Yang Y,
Chen J, Zhu X, Zhao P, Zhao J, Liu J, et al: Differential
expressions of PD-1, PD-L1 and PD-L2 between primary and metastatic
sites in renal cell carcinoma. BMC Cancer. 19:3602019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hao X, Sun B, Hu L, Lähdesmäki H, Dunmire
V, Feng Y, Zhang SW, Wang H, Wu C, Wang H, et al: Differential gene
and protein expression in primary breast malignancies and their
lymph node metastases as revealed by combined cDNA microarray and
tissue microarray analysis. Cancer. 100:1110–1122. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Inari H, Suganuma N, Kawachi K, Yoshida T,
Yamanaka T, Nakamura Y, Yoshihara M, Nakayama H, Yamanaka A, Masudo
K, et al: Expression of enhancer of zeste homolog 2 correlates with
survival outcome in patients with metastatic breast cancer:
Exploratory study using primary and paired metastatic lesions. BMC
Cancer. 17:1602017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Patel SG, Amit M, Yen TC, Liao CT,
Chaturvedi P, Agarwal JP, Kowalski LP, Ebrahimi A, Clark JR, Cernea
CR, et al: Lymph node density in oral cavity cancer: Results of the
international consortium for outcomes research. Br J Cancer.
109:2087–2095. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tabatabaeifar S, Larsen MJ, Larsen SR,
Kruse TA, Thomassen M and Sørensen JA: Investigating a case of
possible field cancerization in oral squamous cell carcinoma by the
use of next-generation sequencing. Oral Oncol. 68:74–80. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Soroceanu L, Murase R, Limbad C, Singer E,
Allison J, Adrados I, Kawamura R, Pakdel A, Fukuyo Y, Nguyen D, et
al: Id-1 is a key transcriptional regulator of glioblastoma
aggressiveness and a novel therapeutic target. Cancer Res.
73:1559–1569. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dong Z, Wei F, Zhou C, Sumida T, Hamakawa
H, Hu Y and Liu S: Silencing Id-1 inhibits lymphangiogenesis
through down-regulation of VEGF-C in oral squamous cell carcinoma.
Oral Oncol. 47:27–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xiao F, Qiu H, Cui H, Ni X, Li J, Liao W,
Lu L and Ding K: MicroRNA-885-3p inhibits the growth of HT-29 colon
cancer cell xenografts by disrupting angiogenesis via targeting
BMPR1A and blocking BMP/Smad/Id1 signaling. Oncogene. 34:1968–1978.
2015. View Article : Google Scholar : PubMed/NCBI
|