Introduction
In extramedullary hemopoiesis, normal blood cells
are formed outside of the bone marrow when the bone marrow is
functioning normally (1).
Extramedullary hemopoiesis is associated with congenital
hemoglobinopathies, such as spherocytosis, sickle cell anemia and
thalassemia or with bone marrow replacement conditions, such as
myelofibrosis, lymphoma, leukemia and myelodysplasia (2). Extramedullary hemopoiesis is primarily
observed in the spleen and liver, but is less common in the
thoracic region and it is important to diagnose, as it can lead to
pulmonary hemorrhage (3,4). In radiography, extramedullary
hemopoiesis is identified as a bilateral lobulated fatty dense mass
(5). The spleen and liver are the
most common sites for involvement of extramedullary hemopoiesis;
however, the whole body is affected, whereby ~5% of all cases are
reported as thoracic extramedullary hemopoiesis (3). Cytopathological diagnosis lacks
efficiency (6), thus novel
strategies for accurate diagnosis are required for effective
treatment.
Thoracic extramedullary hemopoiesis is located in
the pulmonary parenchyma, pleural spaces, pulmonary arteries and
the lower paravertebral area (7). It
is diagnosed using chest X-ray, CT and MRI (8). Currently, MRI is the gold standard used
to diagnosis patients with thoracic extramedullary hemopoiesis
(9). When thoracic extramedullary
hemopoiesis presents as posterior mediastinum masses in specific
clinical contexts, it is easy to diagnose by MRI (10). The other radiological presentations,
such as those observed in intrathoracic mass, may be more difficult
to diagnose by MRI and CT as they are not associated with adjacent
bone destruction and require biopsy (11). Needle biopsy is typically preferred
as a reference standard for the diagnosis of extramedullary
hemopoiesis however, high vascularization of tissues is one of the
complications of this method thus, it is avoided and in addition
improved imaging techniques are required for effective diagnosis
(2). Antitumor therapy is essential
for the survival of patients with leukemia (12) and in the management of extramedullary
hemopoiesis; of which, irradiation is the primary treatment of
choice (13). The diffuse pattern,
such as infiltration and fleeting in densities of thoracic
extramedullary hemopoiesis is uncommon for X-ray, MRI and CT images
(4) compared with biopsy.
The objective of the present study was to compare
the diagnostic parameters of chest CT with MRI for the diagnosis of
thoracic extramedullary hemopoiesis in patients with leukemia, with
an open lung biopsy as a reference standard. Open lung biopsy has
high diagnostic yields and an acceptable level of safety for
respiratory distress disease (14).
Materials and methods
Subjects
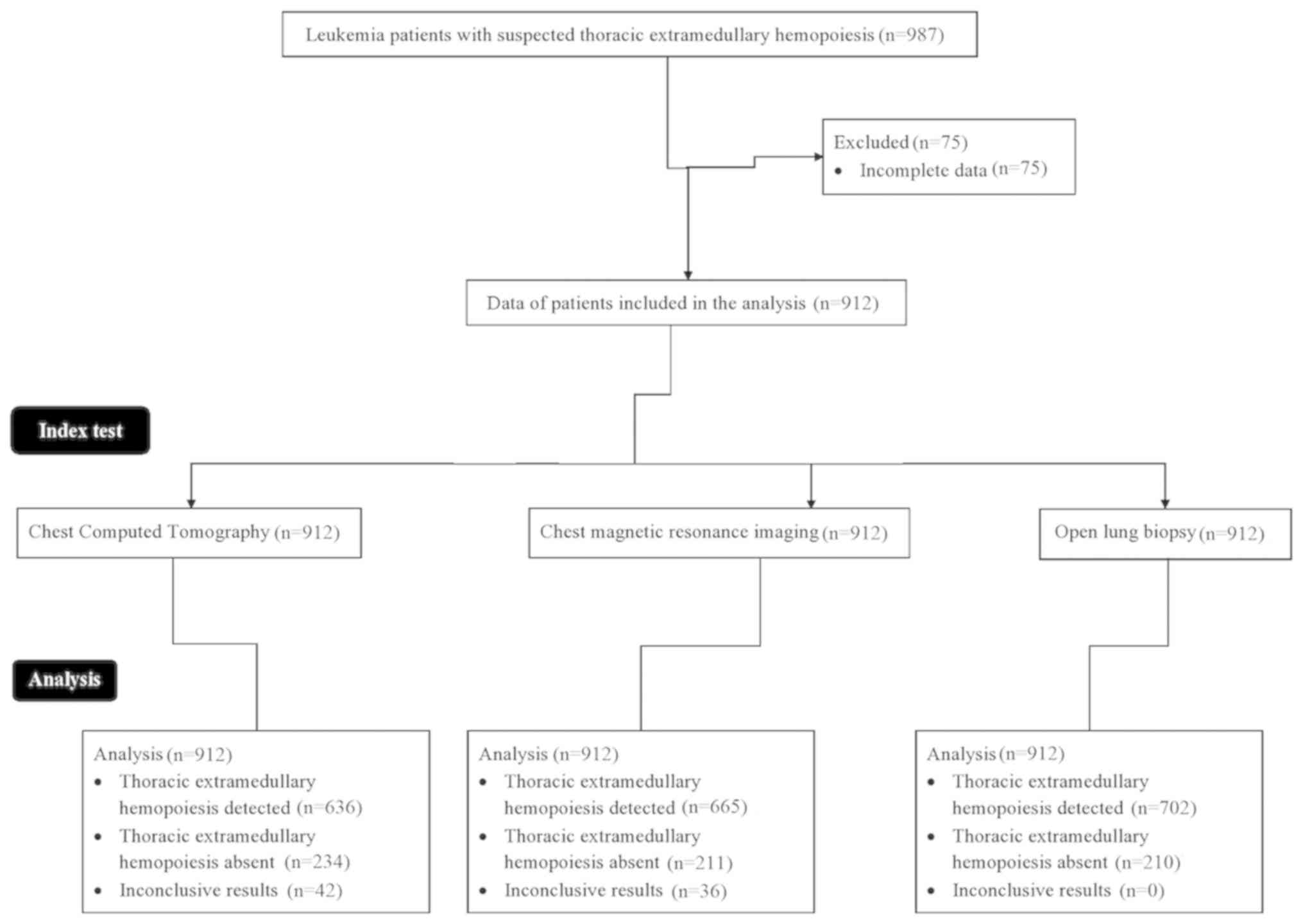
A total of 987 patients with leukemia with the
sign(s) and symptoms of suspected paravertebral and/or pulmonary
extramedullary hemopoiesis at the First People's Hospital of
Yongkang (Yongkang, Zhejiang, China) and Tongji Medical College
Huazhong University of Science and Technology (Wuhan, Hubei, China)
were recruited between 3rd January 2015 and 1st March 2019. Among
them, 75 patients were excluded from the analysis, as their data in
their respective medical records was incomplete. Thus, chest CT,
MRI and open lung biopsy data of a total of 912 patients with
leukemia were included in the analysis of the present study. The
present study was approved by the review board of the First
People's Hospital of Yongkang (Yongkang, Zhejiang, China) and
written informed consent was provided from all the patients.
Details of patient recruitment and workflow are demonstrated in
Fig. 1.
Inclusion criteria
Patients with leukemia aged ≥18 years old with
epistaxis, hemoptysis, pulmonary hypertension, severe tricuspid
regurgitation, complaints of chest pain, enlargement of the chest,
abdominal bloating, shortness of breath and/or the other sign(s)
and symptoms of suspected paravertebral and/or pulmonary
extramedullary hemopoiesis (suspected of having extramedullary
hemopoiesis) who required diagnosis at admission were included in
the study.
Chest CT
Static anterior and posterior CT images of the chest
were obtained by shielding the liver and spleen (as per protocol)
using the Light speed VCT CT 96 (GE Healthcare) at 120 kVp and 240
mA/sec (radiation exposure factors). The images were obtained from
the apices to the lung bases under a deep inspiration (2) by three radiologists (minimum 3 years of
experience) at The First People's Hospital of Yongkang and The
Tongji Medical College Huazhong University of Science and
Technology. The reconstructed image thickness was 1.5-mm.
Chest MRI
Chest MRIs were performed using a 1.5-T SIGNA™
Artist (GE Healthcare) by three radiologists with full parallel
imaging capabilities and a minimum of 3-years of experience. The
breath-hold imaging protocol was utilized. MRI workstation SIGNA™
Artist (GE Healthcare) was used as the imaging platform. Coronal
and axial T2 and T1-weighted images with a 2-mm slice thickness,
192×256 matrix size and 44 cm field of view were evaluated for
image analysis (8). Apparent
transverse relaxation time was 2 msec.
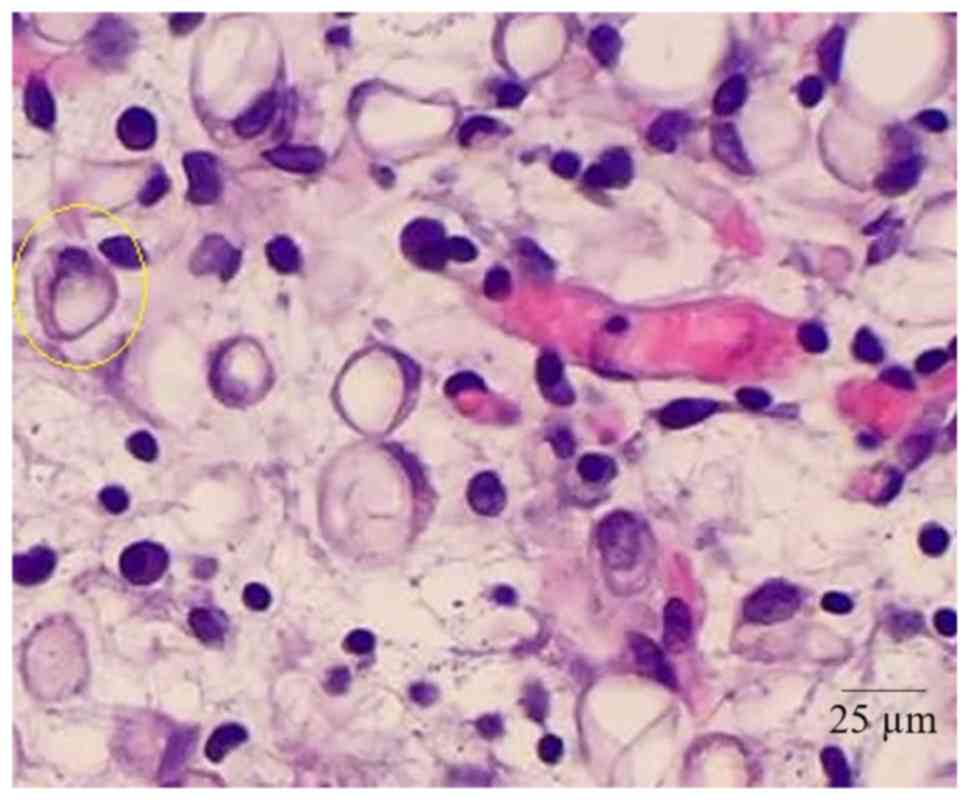
Open lung biopsy
The open lung biopsies (not video-assisted
thoracoscopic biopsy) were performed when the patients were
arranged in the supine position by three physicians (minimum 3
years of experience). The samples were collected and sent to the
laboratory for examination purposes. All laboratory analyses were
performed as previously described (15) by pathologists (minimum 3 years of
experience) at The First People's Hospital of Yongkang and The
Tongji Medical College Huazhong University of Science and
Technology. Atypical cellular infiltration and presence of
megakaryocyte was considered as thoracic extramedullary
hemopoiesis.
Image analysis
All the images were analyzed by a total of 5
radiologists (minimum 5-years of experience in cancer imaging
analysis) of the aforementioned hospitals. The differences of
opinion in diagnosis were solved by consensus of all radiologists.
Thoracic extramedullary hemopoiesis was defined as diffusivity of
both lung fields being increased compared with the blood pool and
no other abnormal focal being increased compared with the blood
pool (5). If the microscopic septa
were below the resolution of images, then it was considered as
normal (16). Image analysis was
included in the evaluation of the distribution, location and
density of masses (the adipose tissue; Table I) (8).
 | Table I.Image analysis of thoracic
extramedullary hemopoiesis. |
Table I.
Image analysis of thoracic
extramedullary hemopoiesis.
| Observations |
|
|---|
|
|
|---|
| Chest CT | Chest MRI | Prediction |
|---|
| Hypodense tissue | T1 weighted images:
homogeneous/heterogeneous hypointense signals | Suspicious for
thoracic extramedullary hemopoiesis |
| Dense soft parts | T2 weighted images:
homogeneous/heterogeneous hyperintense signals, hyperintense
foci |
|
| Septal
thickening |
|
|
| Ground-glass
opacities |
|
|
| aAlveolar/ground-glass
consolidation | – | – |
Decision making of irradiation
The beneficial score was calculated for CT and MRI
scans, for each patient, as per the equation below (1) and plotted for decision making of
irradiation.
Beneficial score=True thoracic
extramedullary hemopoiesis detectedData of leukemia patients
included-(False thoracic extramedullary hemopoiesis detectedData of
leukemia patients included×Level of diagnostic confidence above
which decision of irradiation was taken1-level of diagnostic
confidence above which decision of irradiation was taken)
Statistical analysis
InStat v.Window 3.0.1 (GraphPad Software, Inc.) was
used for statistical analysis. Categorial parameters are presented
as the frequency (%) and continuous parameters are presented as the
mean ± standard deviation. Categorial parameters were analyzed
using χ2 independence test and continuous parameters
(accuracy and sensitivity) were analyzed using the Wilcoxon sum
rank test following Tukey-Kramer multiple comparisons tests
(considering critical value q>3.14 as significant) for
sensitivity and accuracy. Multivariate regression analyses were
performed for predictive features of thoracic extramedullary
hemopoiesis. Sensitivity was determined as the ratio of numbers of
true thoracic extramedullary hemopoiesis detected by imaging
modality to those detected by the open lung biopsy. Accuracy was
determined as the ratio of numbers of true thoracic extramedullary
hemopoiesis absent detected by imaging modality to those detected
by the open lung biopsy. P<0.05 and a 95% confidence interval
were considered to indicate a statistically significant
difference.
Results
Demographic and clinical
characteristics in patients with leukemia
The demographic and clinical characteristics of
patients, at the time of admission are presented in Table II. Chest pain was the most
frequently recorded sign/symptom of suspected paravertebral and/or
pulmonary extramedullary hemopoiesis in the hospitalized patients.
The patient cohort consisted of more male patients compared with
female patients (77 vs. 23%) and the majority of the patients had a
4–6-year history of leukemia (78%). All of the patients were
treated with hydroxyurea to control splenomegaly and a raised total
white cell count.
 | Table II.Demographic and clinical
characteristics of the patients with leukemia at the time of
admission. |
Table II.
Demographic and clinical
characteristics of the patients with leukemia at the time of
admission.
| Characteristic | Number |
|---|
| Patients with
leukemia included | 912 |
| in study, n |
| Age, years |
|
|
Minimum | 18 |
|
Maximum | 65 |
| Mean ±
SD |
48.5400±11.1300 |
| Sex, n (%) |
|
|
Male | 699 (77) |
|
Female | 213 (23) |
| Ethnicity |
|
| Han
Chinese | 837 (92) |
|
Mongolian | 65 (7) |
|
Tibetan | 10 (1) |
| History of
leukemia, n (%) |
|
| ≤3
years | 102 (11) |
| 4–6
years | 712 (78) |
| >6
years | 98
(11) |
| Mean hemoglobin
content ± SD, mg/dl |
10.1200±2.1500 |
| Epistaxis, n
(%) | 312 (34) |
| Chest pain, n
(%) | 445 (49) |
| Enlargement of
chest, n (%) | 245 (27) |
| Mean cardiac output
± SD, l/min |
4.5100±1.2100 |
| Mean pulmonary
vascular resistance ± SD, WU |
3.6100±0.5200 |
| Mean pulmonary
arterial pressure ± SD, mmHg |
76.1200±8.8900 |
| Mean tricuspid
regurgitation ± SD, cm |
2.7200±0.2100 |
| Mean white cell
count, cell/litre |
40.09×109±1.12×109 |
| Mean platelet
count, cell/litre |
369.76±x109±1.01×109 |
| Diabetes mellitus,
n (%) | 255 (28) |
| Chronic renal
failure, n (%) | 45 (5) |
| Mechanically
ventilated, n (%) | 2
(0.2) |
| Mean airway
pressure ± SD, cm H2O |
16.8900±2.8100 |
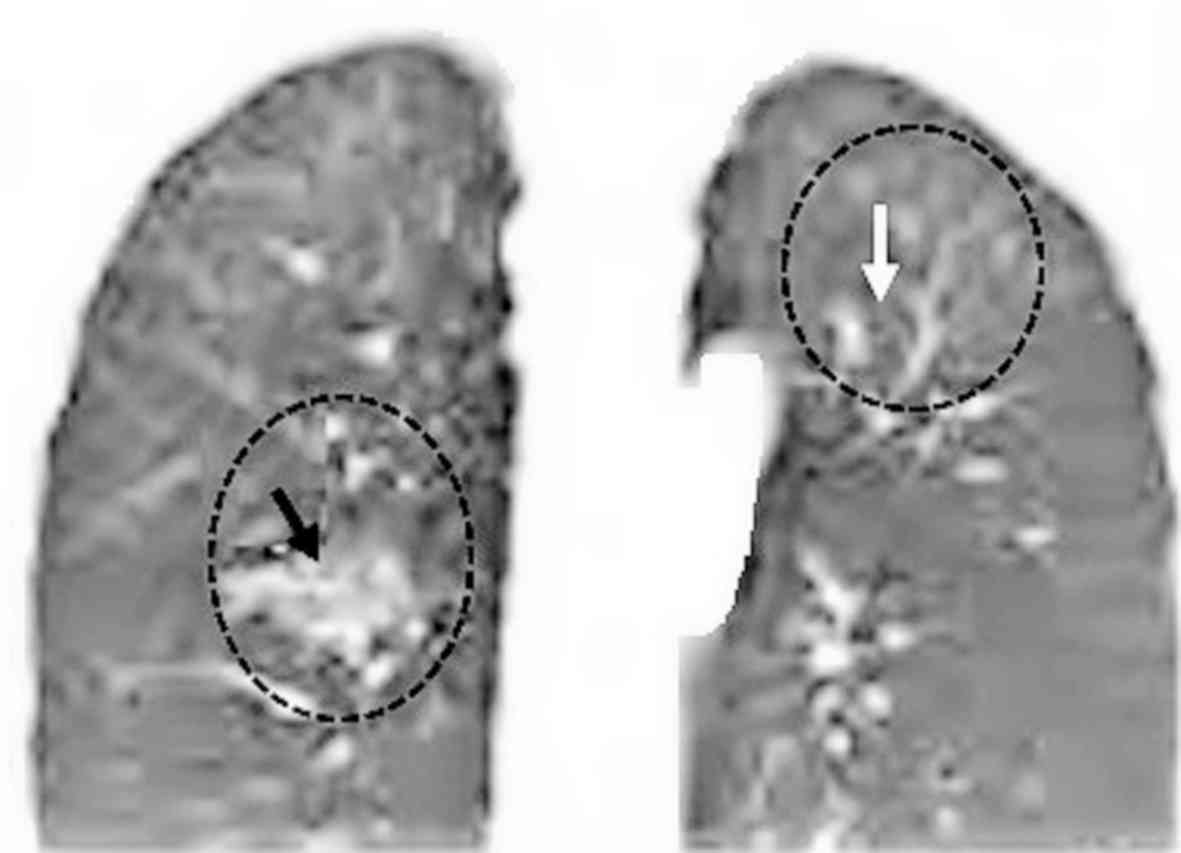
Characteristics of the masses observed
using chest CT
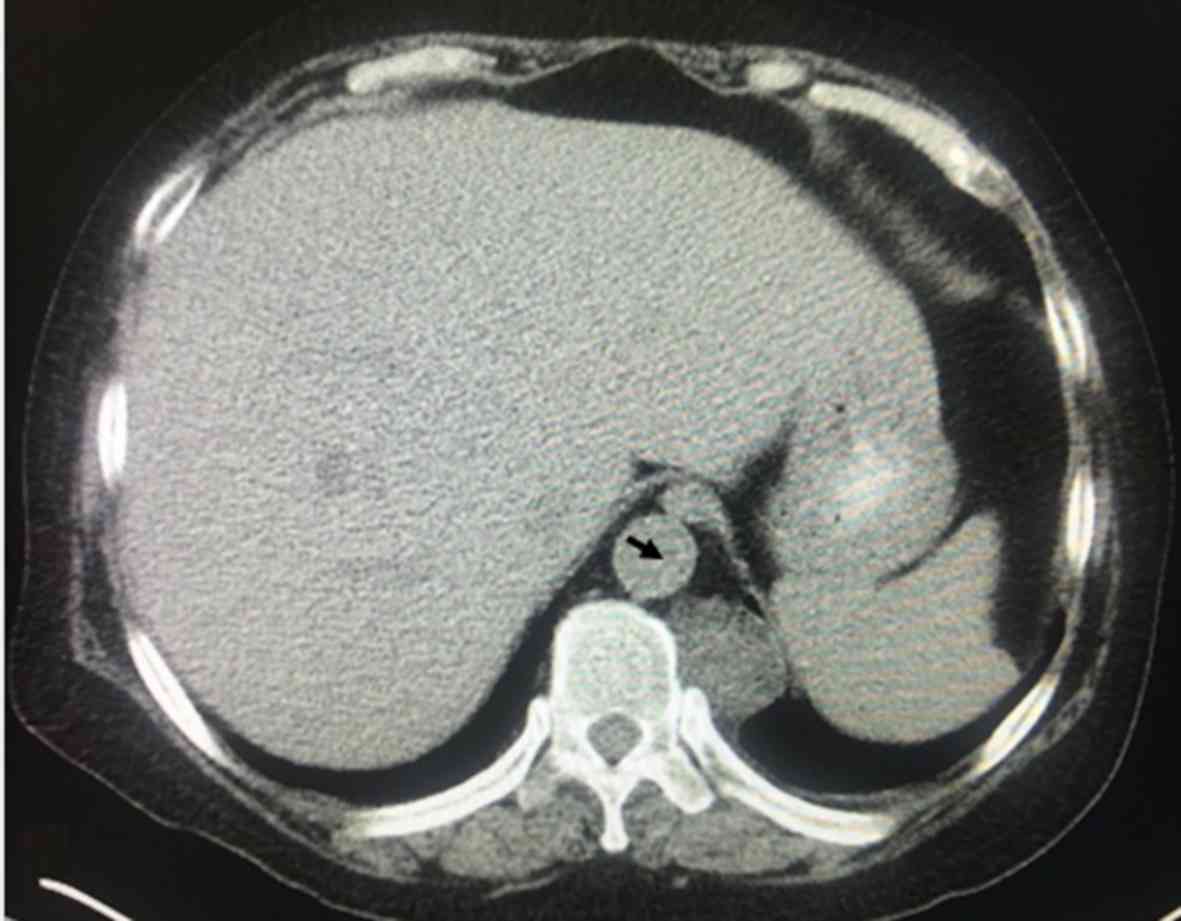
A total of 568 patients had lower paravertebral
masses, 68 patients had septal thickening and 276 patients had
decreased diffusivity of both lung fields compared with the blood
pool. An example of a lower paravertebral mass is presented in
Fig. 2, which had the bilateral
masses (adipose tissue) in the paravertebral regions. In 455
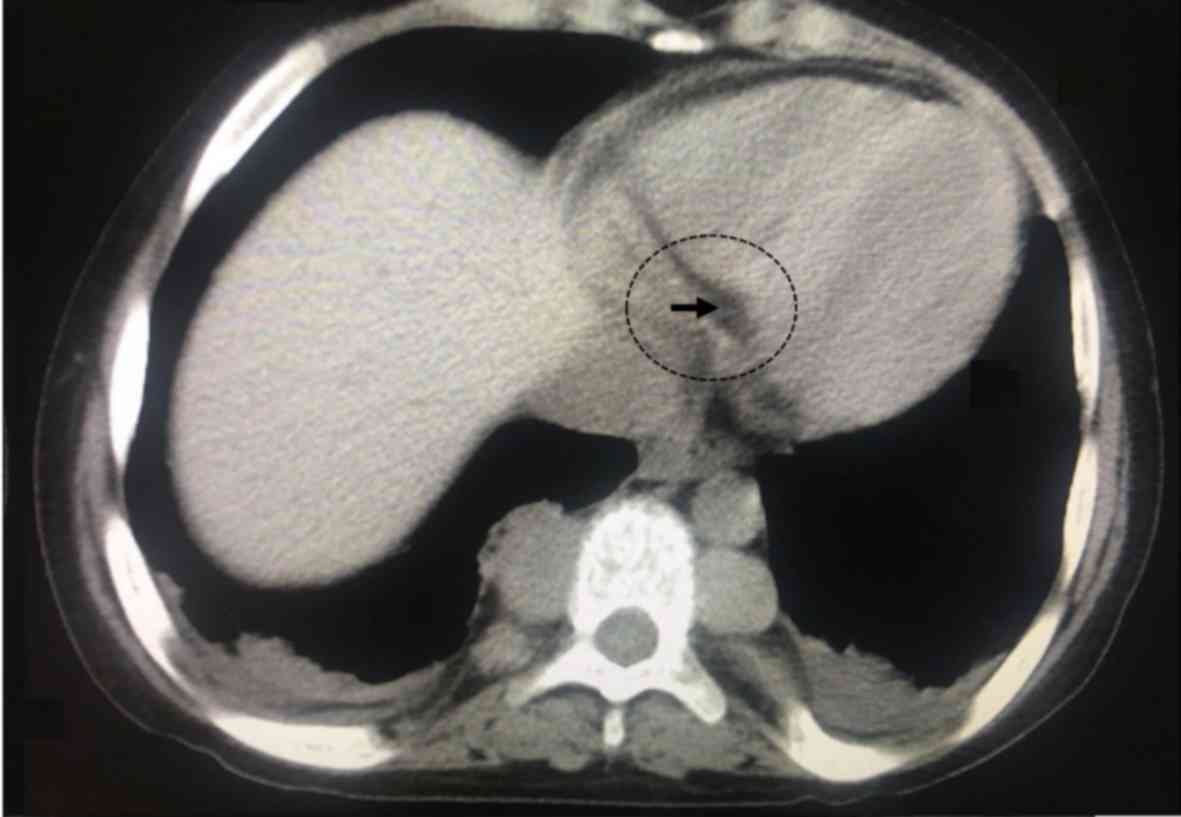
patients, the masses were relatively symmetrical and bilateral. In
62 patients, the mass was unilateral on the right side and in 51
patients the mass was unilateral on the left side. A representative
image of a unilateral homogeneous mass in the left side is
presented in Fig. 3. Of the 568
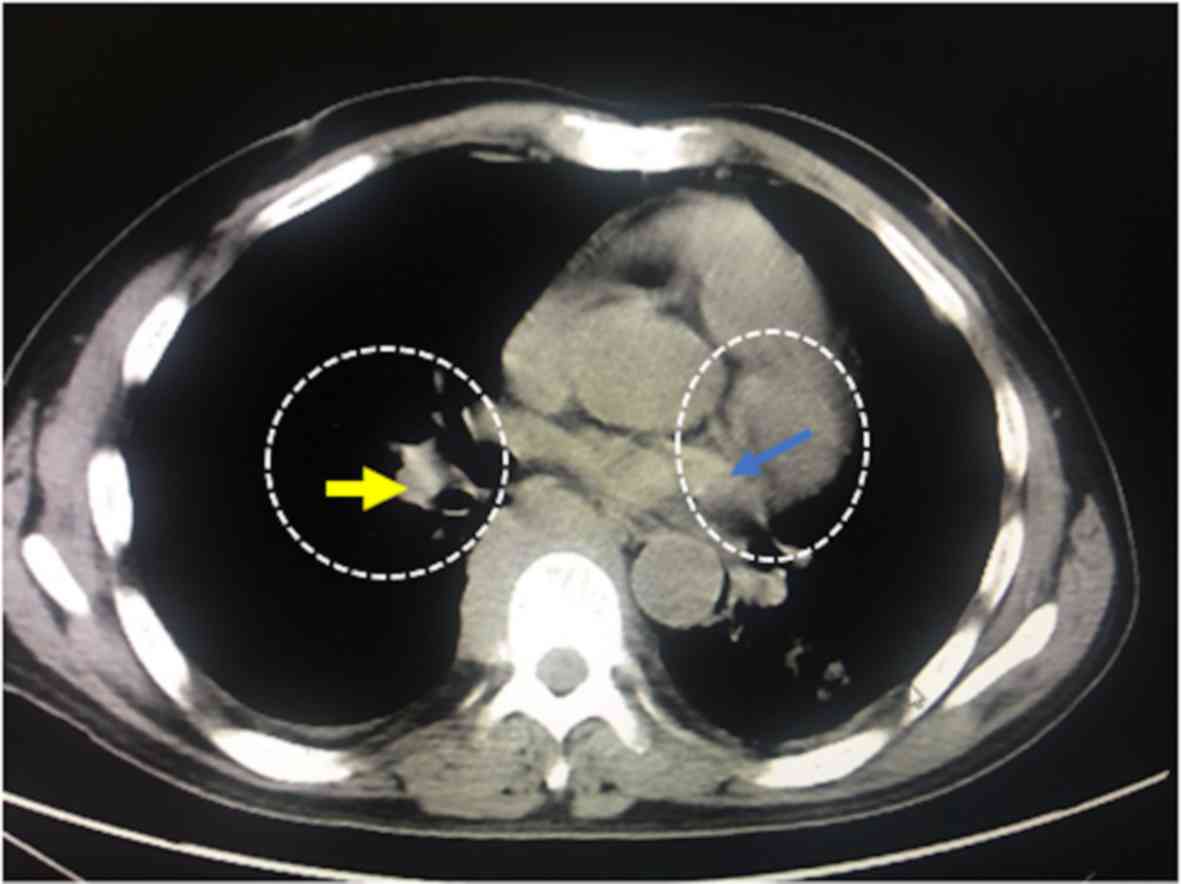
patients with detected masses; the presence of adipose tissue was
confirmed in 445 patients, significant masses were found in 71
patients and homogeneous masses, with iso dense/iso attenuating
soft parts were found in 52 patients (Fig. 4).
Characteristics of images in patients
with leukemia using MRI
Extramedullary hemopoiesis was observed in 607 of
patients with leukemia, while 305 patients exhibited no
heterogenous and/or mixed type signals (Table III). Of those, 231 patients had
hyperintense foci within the lung lesion, 131 patients had
homogeneous hyperintense signals and 245 had heterogeneous
hyperintense signals in lung lesions (Fig. 5).
 | Table III.Diagnostic parameters of adopted
imaging modalities. |
Table III.
Diagnostic parameters of adopted
imaging modalities.
| Parameters | Open lung
biopsy | Chest CT | Chest MRI |
|---|
| Number of patients
with leukemia, n | 912 | 912 |
P-valuea | 912 |
P-valuea |
| True thoracic
extramedullary hemopoiesis detected, n (%) | 702 (77) | 568 (62) | <0.0001 | 607 (67) | <0.0001 |
| True thoracic
extramedullary hemopoiesis absent, n (%) | 210 (23) | 175 (19) | 0.051 | 154 (17) | 0.001 |
| False thoracic
extramedullary hemopoiesis detected, n (%) | 0
(0) | 68 (7) | <0.0001 | 58 (6) | <0.0001 |
| False thoracic
extramedullary hemopoiesis absent, n (%) | 0
(0) | 59 (7) | <0.0001 | 57 (6) | <0.0001 |
| Inconclusive
results, n (%) | 0
(0) | 42 (5) | <0.0001 | 36 (4) | <0.0001 |
| Mean
sensitivity | 1 | 0.8090 | <0.0001 | 0.8650 | <0.0001 |
| Mean accuracy | 1 | 0.8330 | <0.0001 | 0.7330 | <0.0001 |
Thoracic extramedullary hemopoiesis
detection in patients with leukemia
Open lung biopsies detected features of thoracic
extramedullary hemopoiesis in 702 patients, and absence in 210
patients (Table III; Fig. 6).
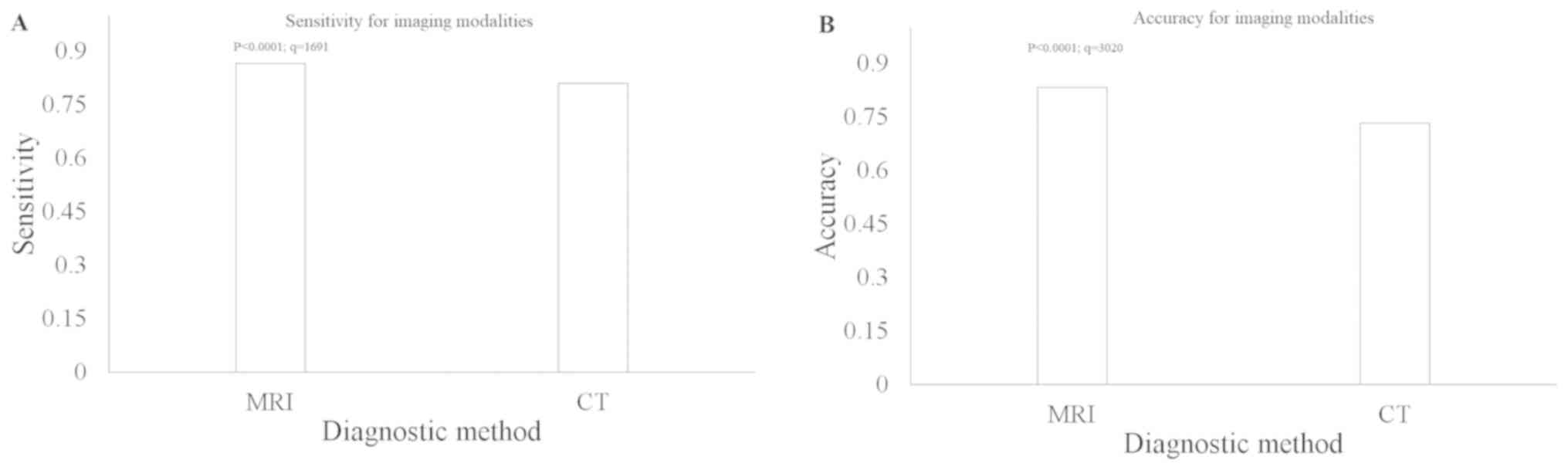
MRI has greater sensitivity and CT
higher accuracy compared with open lung biopsy
With respect to open lung biopsy, chest MRI had a
higher sensitivity compared with that in chest CT (0.865 vs. 0.809;
P<0.0001; q=1691; Fig. 7A).
However, chest CT had a higher accuracy compared with that in MRI
(0.833 vs. 0.733; P<0.0001; q=3020, Fig. 7B). The other diagnostic parameters of
imaging modalities are presented in Table III. A total of 68 and 58 scans of
false positive thoracic extramedullary hemopoiesis were reported by
chest CT and MRI, respectively. Furthermore, 59 and 57 cases of
absent false thoracic extramedullary hemopoiesis were reported by
chest CT and MRI, respectively.
Predictive factors for extramedullary hemopoiesis in
patients with leukemia. Univariate regression analysis was
performed prior to multivariate to identify independent factors.
Age (P=0.041), history of leukemia (P<0.05), hemoglobin content
(P=0.038), enlargement of chest (P=0.028), tricuspid regurgitation
(P=0.049), white cell count (P=0.048) and platelet count (P=0.047)
were associated with thoracic extramedullary hemopoiesis (Table IV).
 | Table IV.Multivariate regression analyses for
predictive features of thoracic extramedullary hemopoiesis in
patients with leukemia (n=702). |
Table IV.
Multivariate regression analyses for
predictive features of thoracic extramedullary hemopoiesis in
patients with leukemia (n=702).
|
Characteristics | Risk ratio | 95% CI | P-value |
|---|
| Age, years | 1.5000 | 2.2000–3.5000 | 0.0410a |
| Sex (male) | 1.3000 | 1.5100–1.7000 | 0.0520 |
| Ethnicity (Han
Chinese) | 0.9000 | 1.3000–1.9000 | 0.1230 |
| History of
leukemia |
|
|
|
| ≤3
years | 1.1000 | 1.5000–2.1000 | 0.0520 |
| 4–6
years | 1.5000 | 1.3000–2.5000 | 0.0420a |
| >6
years | 1.9000 | 1.2000–4.8000 | 0.0210a |
| Hemoglobin content,
mg/dl | 1.5000 | 1.3000–2.3000 | 0.0380a |
| Epistaxis | 1.3000 | 1.4000–2.2000 | 0.0530 |
| Chest pain | 0.8500 | 0.0900–1.0100 | 0.0900 |
| Enlargement of
chest | 1.7000 | 1.1000–2.3000 | 0.0280a |
| Cardiac output,
l/min | 1.1000 | 1.2000–2.2000 | 0.0520 |
| Pulmonary vascular
resistance, WU | 1.2100 | 1.3700–2.2300 | 0.0530 |
| Pulmonary arterial
pressure, mmHg | 1.3000 | 1.3800–2.2500 | 0.0590 |
| Tricuspid
regurgitation, cm | 1.4000 | 1.1100–2.3300 | 0.0490a |
| White cell count,
cell/litre | 1.7000 | 1.1200–2.2400 | 0.0480a |
| The platelet count,
cell/litre | 1.6000 | 1.2300–2.5200 | 0.0470a |
| Diabetes mellitus
(% HbA1C) | 1.6000 | 1.1000–1.9500 | 0.0850 |
| Chronic renal
failure (serum creatinine content) | 1.5000 | 1.2200–1.8200 | 0.0790 |
| Mechanically
ventilated (presence of support) | 1.7000 | 1.1300–1.7500 | 0.0560 |
| Mean airway
pressure, cm H2O | 1.2000 | 1.2200–1.3500 | 0.1560 |
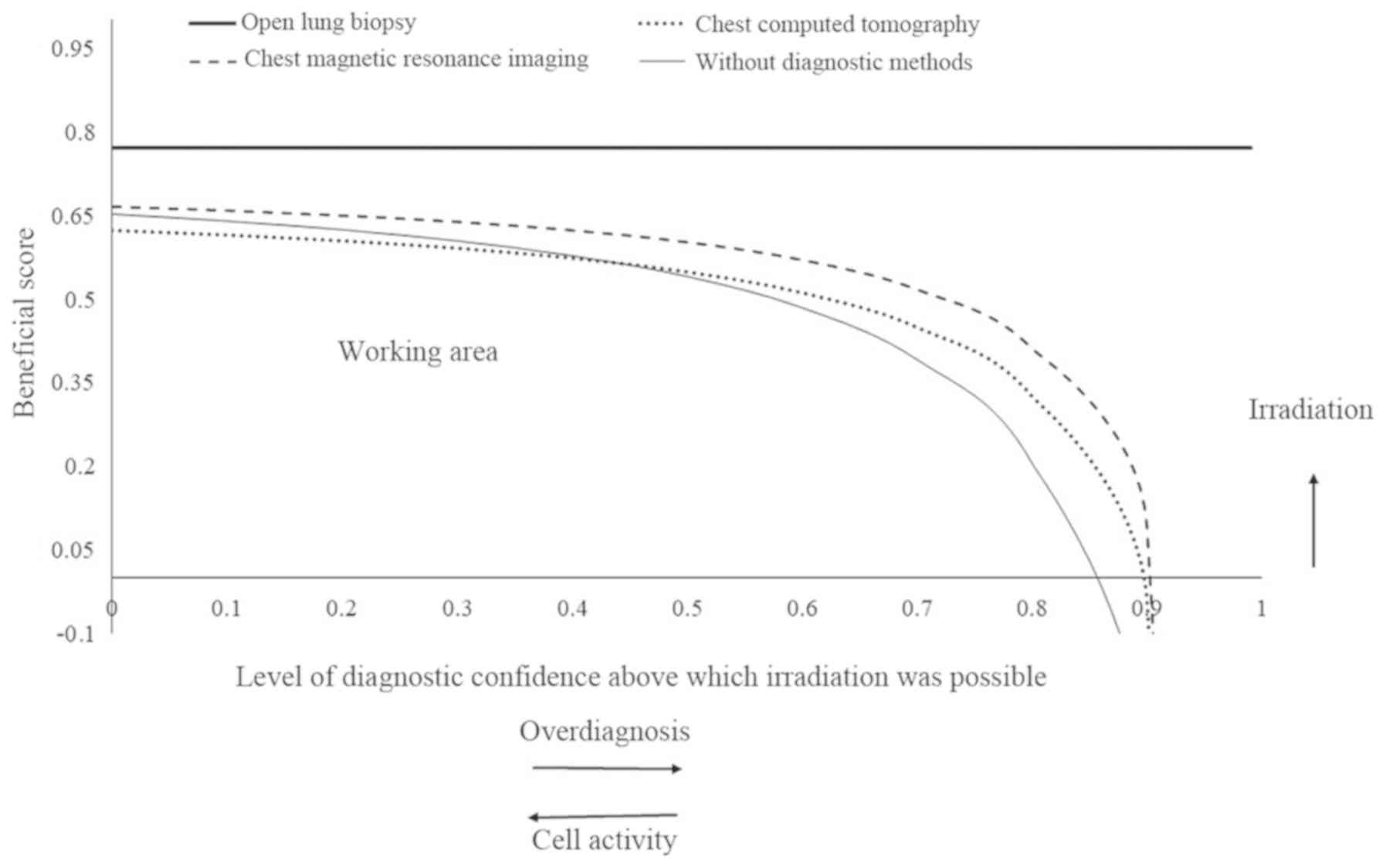
Decision making of irradiation in
patients with leukemia
There was a low rate of overdiagnosis using chest CT
and MRI for the detection of thoracic extramedullary hemopoiesis in
patients with leukemia, compared with open lung biopsy (except for
a high rate of overdiagnosis in the lungs). However, the working
area that detects thoracic extramedullary hemopoiesis at least once
in images was higher for the chest MRI compared with CT (0–0.905
vs. 0.430–0.900; Fig. 8).
Discussion
In the present study with respect to open lung
biopsies, chest MRI had a greater sensitivity compared with CT,
which is inconsistent with previous case reports (8,17). The
absence of diffusion restriction on MRI favors diagnosis (17). The diagnostic differences are not
large between open lung biopsy and chest MRI (T1- or T2-weighted
images) or the chest CT, as lesions possess dense soft parts on the
CT scans and signal intensities on MRI (2). No definitive imaging pattern is
pathognomonic and a biopsy is required for the diagnosis of
thoracic extramedullary hemopoiesis (8). Open lung biopsy has the risk of
persistent air leakage and hemorrhagic complications due to
pulmonary hypertension (2,18,19). The
results of the present study demonstrated that the chest MRI or the
CT is a non-invasive and reliable method for the diagnosis of
thoracic extramedullary hemopoiesis in patients with leukemia.
The results from the present study demonstrated that
chest CT had a higher accuracy compared with that in chest MRI,
which is inconsistent with previous case reports (8,13,16,20–22).
These case reports had inadequate sample sizes, thus introduced
statistical errors. The chest CT is the main imaging modality for
the diagnosis of thoracic extramedullary hemopoiesis as it provides
a specific finding for nodules and masses (16). For scattered foci of internal fat, CT
reveals a low-density mass whereas MRI demonstrates variable
enhancements (23). MRI is the
examination of choice and provides valuable diagnostic information
(9) however, it can be variable and
influenced by fibrous structure, fatty components and iron
deposition on the cellular surface of the hematopoietic tissue
(8). MRI signals for thoracic
extramedullary hemopoiesis appear with higher signal intensity
similar to those of tumors (13,24).
Therefore, MRI misdiagnoses thoracic extramedullary hemopoiesis.
The results of the present study indicated that unlike the chest
MRI, the chest CT can confirm thoracic extramedullary hemopoiesis
with/without adipose tissue.
In the present study, there were 68 and 58 scans of
false positive thoracic extramedullary hemopoiesis, using chest CT
and MRI, respectively. CT detects thoracic extramedullary
hemopoiesis by septal thickening and ground-glass opacities,
however lung infections and congestive cardiac failure also
demonstrate ground-glass opacities under the chest CT (19,21,25). In
addition, CT scans detect pleural metastases, which are originally
pleural soft tissues (13). Chest
MRI detects intense signals due to iron deposition on adipose
tissues (1) and patients with
leukemia are regularly treated with blood transfusions (26), which leads to higher iron deposition
on lung medullary stroma and to false thoracic extramedullary
hemopoiesis detection using chest MRI (1).
The present study reported that there were 59 and 57
cases of absent false thoracic extramedullary hemopoiesis using
chest CT and MRI, respectively. Low cellular activity or little
medullary stroma present in the lungs may not be detected by chest
CT (16). In addition, older lesions
with iron deposition or fatty infiltration may not be present as a
hypointense signal on T1-weighted images or as a hyperintense
signal on T2-weighted images (2,8).
There are several limitations to the present study,
for example it is a retrospective in design and not a prospective
study. Diffusion-weighted imaging has a diagnostic role in the
detection of thoracic extramedullary hemopoiesis, as it does not
demonstrate diffusion restriction in the images (17). Diffusion-weighted images was not
performed in the present study despite previous recommendation to
do so over T1- or T2-weighted images of the chest MRI, in order to
decrease inconclusive and false positive results. In the present
study, follow-up conditions and treatment were not described for
the enrolled patients, which may have affected the validity of the
results as treatment is affected by the diagnosis accuracy of the
respective disease. Sensitivities, accuracies and the decision
making of irradiation for paravertebral and pulmonary
extramedullary hemopoiesis evaluation were not provided
separately.
In conclusion, the present non-inferiority
retrospective diagnostic study revealed that chest CT and MRI both
have diagnostic importance in the detection of thoracic
extramedullary hemopoiesis in patients with leukemia with
manageable complications. The chest CT could confirm thoracic
extramedullary hemopoiesis with/without fat involvement. The chest
MRI had high sensitivity due to the absence of diffusion
restriction. MRI misdiagnoses thoracic extramedullary hemopoiesis
and CT confirmed thoracic extramedullary hemopoiesis. The chest CT
is recommended for the diagnosis of suspected paravertebral and/or
pulmonary extramedullary hemopoiesis in patients with leukemia.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
ZL acquired the data, performed the literature
review, contributed to methodology, investigation, and drafted,
reviewed and edited the initial manuscript for intellectual
content. YX contributed to the analysis and validation, performed
the literature review and acquired the data. QW contributed to the
conceptualization of the present study, performed software
analysis, acquired data and performed the literature review. All
authors have read and approved the manuscript.
Ethics approval and consent to
participate
The original study protocol (YKYY/CL/15/19 dated 30
March 2019) was approved by the First People's Hospital of Yongkang
review board (Yongkang, Zhejiang, China). The study reporting
adhered to the law of China, the results from the study
strengthened the use of observational studies, and the Declaration
of Helsinki v.2008. Written informed consent was provided from all
participating patients. As this was a retrospective study,
registration into the clinical trial registry was waived by the
institutional review board.
Patient consent for publication
Informed consent was signed by all participating
patients for the publication of the study, including personal data
and images in all formats.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
References
|
1
|
Solazzo A, D'Auria V, Moccia LG, Vatrella
A, Bocchino M and Rea G: Posterior mediastinal extramedullary
hematopoiesis secondary to hypoxia. Transl Med UniSa. 14:1–4.
2016.PubMed/NCBI
|
|
2
|
Marchiori E, Escuissato DL, Irion KL,
Zanetti G, Rodrigues RS, Meirelles GS and Hochhegger B:
Extramedullary hematopoiesis: Findings on computed tomography scans
of the chest in 6 patients. J Bras Pneumol. 34:812–816. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lalueza A, Silva CP, Alcalá-Galiano A,
Arrieta E, Cánovas JM, Calero MR and Álvarez C: Presacral and
intrathoracic extramedullary hematopoiesis in a patient with β
thalassemia minor. Clin Respir J. 12:322–326. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ozbudak IH, Shilo K, Hale S, Aguilera NS,
Galvin JR and Franks TJ: Alveolar airspace and pulmonary artery
involvement by extramedullary hematopoiesis: A unique manifestation
of myelofibrosis. Arch Pathol Lab Med. 132:99–103. 2008.PubMed/NCBI
|
|
5
|
Yang M, Covington MF, Nguyen BD, Johnson
GB, Mesa RA and Roarke MC: 99mTc-Sulfur colloid bone
marrow scintigraphy in diagnosis of diffuse pulmonary
extramedullary hematopoiesis secondary to myelofibrosis. J Nucl Med
Technol. 46:368–732. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singhal N, Tahlan A, Bansal C, Handa U and
D'Cruz S: Coexistence of leukemic infiltration and extramedullary
hematopoeisis in a lymph node: A cytological diagnosis. J Cytol.
28:138–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Monga V and Silverman M: Pulmonary
extramedullary hematopoiesis involving the pulmonary artery.
Hematol Rep. 7:57142015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
La Fianza A, van der Byl G, Maccabelli G,
Torretta L and Calliada F: CT and MR findings in extramedullary
haematopoiesis with biliary system encasement: A case report. J
Radiol Case Rep. 4:1–8. 2010.PubMed/NCBI
|
|
9
|
Mattei TA, Higgins M, Joseph F and Mendel
E: Ectopic extramedullary hematopoiesis: Evaluation and treatment
of a rare and benign paraspinal/epidural tumor. J Neurosurg Spine.
18:236–242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Occhipinti M, Heidinger BH, Franquet E,
Eisenberg RL and Bankier AA: Imaging the posterior mediastinum: A
multimodality approach. Diagn Interv Radiol. 21:293–306. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park JB, Lee SA, Kim YH, Lee WS and Hwang
JJ: Extramedullary hematopoiesis mimicking mediastinal tumor in a
patient with hereditary spherocytosis: Case report. Int J Surg Case
Rep. 41:223–225. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pardanani A, Brown P, Neben-Wittich M,
Tobin R and Tefferi A: Effective management of accelerated phase
myelofibrosis with low-dose splenic radiotherapy. Am J Hematol.
85:715–716. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bao Y, Liu Z, Guo M, Li B, Sun X and Wang
L: Extramedullary hematopoiesis secondary to malignant solid
tumors: A case report and literature review. Cancer Manag Res.
10:1461–1470. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Philipponnet C, Cassagnes L, Pereira B,
Kemeny JL, Devouassoux-Shisheboran M, Lautrette A, Guerin C and
Souweine B: Diagnostic yield and therapeutic impact of open lung
biopsy in the critically ill patient. PLoS One. 13:e01967952018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Depuydt OE, Daeze C, Benoit D, Praet M,
Vermassen E and Decruyenaere M: Diagnostic potential of open lung
biopsy in mechanically ventilated patients with diffuse pulmonary
infiltrates of unclear aetiology. Anaesth Intensive Care.
41:610–617. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Singh I, Mikita G, Green D, Risquez C and
Sanders A: Pulmonary extra-medullary hematopoiesis and pulmonary
hypertension from underlying polycythemia vera: A case series. Pulm
Circ. 7:261–267. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Panda A, Chandrashekhara SH, Nambirajan A
and Mishra P: Idiopathic myelofibrosis with disseminated
hepatosplenic, mesenteric, renal and pulmonary extramedullary
haematopoeisis, portal hypertension and tuberculosis: Initial
presentation and 2 years follow-up. BMJ Case Rep. 2016(pii):
bcr20162178542016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wong AK and Walkey AJ: Open lung biopsy
among critically Ill, mechanically ventilated patients. A
metaanalysis. Ann Am Thorac Soc. 12:1226–1230. 2015.PubMed/NCBI
|
|
19
|
Ali SZ, Clarke MJ, Kannivelu A, Chinchure
D and Srinivasan S: Extramedullary pulmonary hematopoiesis causing
pulmonary hypertension and severe tricuspid regurgitation detected
by technetium-99m sulfur colloid bone marrow scan and single-photon
emission computed tomography/CT. Korean J Radiol. 15:376–380. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qiu D, Hu X, Xu L and Guo X:
Extramedullary hematopoiesis on 18F-FDG PET/CT in a patient with
thalassemia and nasopharyngeal carcinoma: A case report and
literature review. J Cancer Res Ther. 11:10342015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mubarak V, Fanning S, Windsor M, Duhig E
and Bowler S: Pulmonary extramedullary haematopoiesis. BMJ Case
Rep. 2011(pii): bcr06201143922011.PubMed/NCBI
|
|
22
|
Bajwa AA, Usman F, Wolfson D, Laos LF and
Cury JD: A 62-year-old woman with dyspnea, leukocytosis, and
diffuse ground-glass opacities. Chest. 137:1470–1473. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ho AK, Girgis S and Low G: Uncommon liver
lesions with multimodality imaging and pathology correlation. Clin
Radiol. 7:191–204. 2018. View Article : Google Scholar
|
|
24
|
Zhou PP, Clark E and Kapadia MR: A
systematic review of presacral extramedullary haematopoiesis: A
diagnosis to be considered for presacral masses. Colorectal Dis.
18:1033–1040. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Trow TK, Argento AC, Rubinowitz AN and
Decker R: A 71-year-old woman with myelofibrosis, hypoxemia, and
pulmonary hypertension. Chest. 138:1506–1510. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gu Y, Estcourt LJ, Doree C, Hopewell S and
Vyas P: Comparison of a restrictive versus liberal red cell
transfusion policy for patients with myelodysplasia, aplastic
anaemia, and other congenital bone marrow failure disorders.
Cochrane Database Syst Rev. 2015:CD0115772015.
|