Introduction
Renal cell carcinoma (RCC) has attracted increasing
attention over the past decades (1,2), and the
lack of early symptoms or signals makes timely diagnosis difficult
for patients with RCC (3,4). Of the patients diagnosed with RCC, 30%
present with distant metastasis following diagnosis. Once malignant
metastasis occurs in patients with RCC, the 5-year survival rate is
<10% (1). Due to the tendency of
malignant metastasis and insensitivity to chemotherapy, the
prognosis of patients with RCC is poor (5). Metastasis of malignant tumors is a
complicated biological program. Malignant tumor cells migrate away
from the primary tumor location, into the surrounding tissues and
reach the distal organs through circulation or lymphatic channels
to develop metastatic foci (6). The
epithelial-mesenchymal transition (EMT) serves as an important
mechanism during the metastatic process (7). EMT promotes epithelial cells to undergo
dedifferentiation, resulting in a loss of polarization and fewer
cell-cell junctions, whilst enhancing interstitial transformation,
tumor migration and invasion (8).
Zinc finger E-box binding homeobox 1 (ZEB1) is a key activator of
EMT, which upregulates the plasticity of tumor cells, resulting in
tumor cells displaying similar characteristics to stem cells
(9). Recent studies have
demonstrated that ZEB1 serves a key role in the EMT process in lung
adenocarcinoma (10), colorectal
cancer and breast cancer (11,12).
During the development of malignant cancer,
significant metabolic disorders occur (8,13–15).
Therefore, it is important to determine whether molecules and
proteins associated with energy regulation may serve as potential
biomarkers for the early diagnosis of RCC. Nucleobindin-2 (NUCB-2)
is a neuropeptide that serves an important role in regulating food
intake and energy homeostasis (7). A
number of previous reports have described NUCB-2 abnormalities in
metabolic diseases (16,17), and there is considerable evidence
suggesting that NUCB-2 serves an important role during the
development and metastasis of breast cancer (18), prostate cancer (19–22) and
colon cancer (23), and that
increased NUCB-2 expression was positively correlated with
metastasis of RCC and a low postoperative survival rate (24). The AMP-dependent protein kinase
(AMPK)/mammalian target of rapamycin (mTOR) signaling pathway is a
central pathway involved in energy metabolism and tumor development
(25,26).
To the best of our knowledge, the specific role and
mechanism of NUCB-2 in RCC remain unknown. In the present study,
NUCB-2 expression was analyzed in the serum and tumor tissues from
patients with RCC, and was correlated with metastasis and clinical
pathological typing. At the cellular level, knocking out NUCB-2
reduced proliferation, migration and invasion of SK-RC-52 cells,
which are derived from human RCC. Furthermore, lower NUCB-2
expression in murine kidney cancer Renca cells inhibited tumor
growth and metastasis in mice.
Materials and methods
Tissue sample collection
Between October 2016 and October 2017, 68 adult
patients with RCC treated at The First Hospital of Jiamusi
University (Jiamusi, China) were examined. These patients included
37 men and 31 women. Patients were aged as follows: 8 patients
<40 years, 40 patients between 40 and 60 years and 20 patients
>60 years. Furthermore, 18, 19 and 31 patients had stage I, II
and III RCC, respectively, according to the Tumor-Node-Metastasis
(TNM) staging system (27). After
obtaining written informed consent from patients, 10 ml blood was
collected in EDTA anticoagulant tube. Serum was obtained following
blood centrifugation at 1,000 × g for 15 min at 4°C, and was stored
at −80°C for <2 months. All the 10 donors who underwent
nephrectomy suffered from hydronephosis, and the intravenous
pyelogram and computed tomography urography revealed that the
kidneys lost function. These patients included six men and four
women aged between 20 and 60 years. Tissue samples were collected
after surgery and stored at −80°C. The present study was approved
by The Ethics Committee of the First Affiliated Hospital of Jiamusi
University.
Human RCC cell lines
The SK-RC-1 cell line was derived from a primary
clear cell RCC specimen and the SK-RC-52 cell line was derived from
a clear cell RCC metastatic lesion in the mediastinum (28). Both cell lines were obtained from the
Memorial Sloan-Kettering Cancer Center. The mouse renal carcinoma
cell line Renca was purchased from the American Type Culture
Collection. The cells were cultured in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine
serum (Sigma-Aldrich; Merck KGaA) and 2 mM glutamine (Gibco; Thermo
Fisher Scientific, Inc.) in an atmosphere of 5% CO2 at
37°C. Knockout of the NUCB-2 gene in SK-RC-52 cells was performed
using the CRISPR-Cas9 system, and gene-editing services were
provided by Beyotime Institute of Biotechnology (Haimen, China).
The guide RNA sequence 5′-TCTATCTTCGCACTTTCCAC-3′ targeting exon5
of NUCB −2 gene locus (NM_001352661.1) was designed by a CRISPR
gRNA design tool (ATUM). ACCG was added to the 5′ end of the sgRNA
sense strand, and AAAC was added to the 5′ end of the antisense
strand to form a cohesive end to digest pGL3-U6-sgRNA-PGK-Puro with
Bsa-I. The NUCB-2 knockout selected cell clones were confirmed by
sequencing and the target gene expression was validated by western
blotting. Cas9 nuclease was provided by Aldevron (cat. no.
9212).
Compounds and antibodies
Rapamycin, dorsomorphin and MTT were purchased from
Sigma-Aldrich; Merck KGaA. SK-RC-52 cells were treated with
dorsomorphin at 40 µM for 100 min at 37°C. SK-RC-52 cells were
treated or not with rapamycin at 100 nmol/l for 12 h at 37°C. The
antibodies used in the present study were purchased from EMD
Millipore, BD Biosciences and Sigma-Aldrich; Merck KGaA.
Immunohistochemistry (IHC)
Tissues were fixed with 10% formaldehyde for 24 h at
room temperature, and tissue sections were cut into sections of
4-µm thickness and subsequently used for IHC. Tissues were blocked
at room temperature for 1 h in a humidified chamber with 5% bovine
serum albumin (Invitrogen; Thermo Fisher Scientific, Inc.)
dissolved in PBS. Sections were incubated with a rabbit polyclonal
antibody against NUCB-2 (cat. no. ab224348; Abcam; 1:100) at 4°C
overnight, and with an HRP conjugated goat anti-rabbit IgG H&L
secondary antibody (cat. no. ab205718; Abcam; 1:2,000) at 37°C for
1 h. The degree of immunostaining was semi-quantitatively evaluated
blindly by two independent expert pathologists. The pathologists
scored the number of positively stained cells per field in five
fields under a light microscope (magnification, ×200). NUCB-2
expression was calculated based on the intensity of staining and
the percentage of positively stained cells. To determine the
percentage of stained cells, the number of stained and unstained
cells was averaged across five fields at ×200 magnification.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA covering four disease stages and normal
tissues from 68 patients and 10 normal donors was extracted with
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Complementary DNA (cDNA) was reverse transcribed from the
RNA using PrimeScript RT Reagent kit (Clontech Laboratories, Inc.)
at 42°C for 15 min and 85°C for 5 sec. The sequences of the primers
used were: Human NUCB-2 forward, 5′-GAGTCAACGGATTTGGTCGT-3′ and
reverse, 5′-TTGATTTTGGAGGGATCTCG-3′ (29); mouse NUCB-2 forward,
5′-GGAGCCAAGTCCTGATCTCTAC-3′ and reverse,
5′-TTCAGACAGGCCAAGGTTTT-3′; human GAPDH forward,
5′-GAGTCAACGGATTTGGTCGT-3′ and reverse 5′-TTGATTTTGGAGGGATCTCG-3′;
and mouse GAPDH forward, 5′-AACTTTGGCATTGTGGAAGG-3′ and reverse,
5′-ACACATTGGGGGTAGGAACA-3′.
RNA was quantified by qPCR assay with Quantities
SYBR Green Master mix (MedChemExpress). The PCR reactions were as
follows: Stage 1, 95°C for 15 sec; stage 2, 40 cycles of 15 sec at
95°C and 30 sec at 60°C; stage 3, melting curve analysis. PCR data
were analyzed using the 2−ΔΔCq method (30) with the GAPDH gene as a control.
Spearman's rank-order correlation was used for correlation
analysis. The system used to grade tissues was the TNM staging
system (27). The healthy donor
tissue and I, II, III, IV stage tissue were ranked as 0, 1, 2, 3
and 4, respectively, and the Spearman's correlation coefficient was
calculated.
Invasion and migration assays
For the cell proliferation assay, 5×104
SK-RC-1, SK-RC-52 and NUCB-2-knockout SK-RC-52 cells were seeded
into 96-well plates. The proliferative rate of the cells was
determined with premixed WST-1 Cell Proliferation Reagent (Clontech
Laboratories, Inc.) 24 h after incubation, according to the
manufacturer's protocol.
A monolayer wound-healing assay was performed to
compare the migratory ability of SK-RC-1, SK-RC-52 and
NUCB-2-knockout SK-RC-52 cells. Cell lines were cultured until
confluent, scratched and imaged using a phase-contrast microscope
(magnification, ×50) at 0 and 24 h. In certain experiments, MCM was
added at different concentrations at the 0 h time point. The
minimum distance in mm between the wound edges of the scratch area
was analyzed using Adobe Photoshop version 7.0 (Adobe Systems,
Inc.). All experiments were performed in triplicate.
For Transwell migration and invasion assays, a QCM™
24-well cell migration assay and invasion system (EMD Millipore)
was used. A total of 2×105 cells were seeded into the
inserts in 300 µl serum-free medium, and 500 µl medium supplemented
with 10% FBS was placed in the lower chamber. Cells were incubated
in an atmosphere of 5% CO2 at 37°C for 48 h. The
migrated and invaded cells were stained with CyQuant GR dye
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturers' instructions. Migration and invasion were assessed
on a fluorometer (FLx800 Microplate Fluorescence Reader; BioTek
Instruments, Inc.) using a 480/520 nm filter.
Western blotting
Cells were lysed using radioimmunoprecipitation
assay buffer (EMD Millipore) on ice for 30 min. The total cell
lysate was collected after centrifugation at 12,000 × g for 15 min
at 4°C. For extraction of nuclear proteins, a Nuclear Extract kit
(Active Motif) was used. The concentration of protein was
determined using a bicinchoninic acid protein assay kit (EMD
Millipore). Proteins (50 µg) were separated by 6–12% SDS-PAGE and
transferred onto polyvinylidene difluoride membranes. After
transfer, 5% non-fat dry milk was used to block the membranes for 1
h. The membranes were incubated with rabbit anti-human NUCB-2
polyclonal antibody (cat. no. ab224348, 1:100, Abcam) and
recombinant GAPDH antibody (cat. no. ab181602, 1:100, Abcam) at 4°C
for overnight. Membranes were washed three times in TBST for 15 min
and incubated with an HRP goat anti-rabbit IgG H&L (cat. no.
ab205718; 1:200; Abcam) and goat anti-mouse IgG H&L (cat. no.
ab150117; 1:200; Abcam) at room temperature for 1 h. Signals were
visualized using enhanced chemiluminescence substrate (EMD
Millipore) on an imaging capture system (Alpha Imaging).
In vivo experiments
Short hairpin (sh)RNAs targeting human NUCB-2, mouse
NUCB-2 and a control vector construct were obtained from Beyotime
Institute of Biotechnology. Cells were transfected with each shRNA
plasmid using Lipofectamine 2000 reagent (Invitrogen Thermo Fisher
Scientific, Inc.). One day before transfection, cells were seeding
at the density of 2×106 cells in 10 cm dish, so that
they can reach 30–50% confluence at the time of transfection. shRNA
duplex (300 pmol) and Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.; 30 µl) were added to each dish containing cells
for 24 h at 37°C in a 5% CO2 incubator before cells were
injected into mice.
All procedures were performed in an animal facility
using protocols approved by The Institutional Animal Care and Use
Committee of The Jiamusi University Affiliated No. 1 Hospital
(Jiamusi, China). A total of 12 six-week-old male BALB/c mice
weighing 18–22 g were obtained from the animal center of Jiamusi
University, and housed under controlled illumination (12:12 h
light/dark cycle; lights on/off, 6/18 h) and temperature (22±2°C)
for 7 days with food and water available ad libitum. Each
group contained ten mice, and animals were injected subcutaneously
into the back with 0.1 ml containing 1×106 Renca cells
or shRNA-Renca cells. Every other day, the size of the tumor
nodules was measured. The mice were sacrificed by cervical
dislocation after anesthesia when the health of the mouse continued
to deteriorate and the intake of food intake continued to decrease,
the weight of the mice decreased for 3 days, the mice weighed
<19 grams and when the mouse tumor volume was >2,000
mm3.
Statistical analysis
Spearman's rank-order correlation was used for
correlation analysis. All data are expressed as the mean ± standard
deviation. Statistical analyses were conducted using SPSS version
17.0 (SPSS, Inc.). A Student's t-test was used to compare the mean
values between two experimental groups. One-way ANOVA was used to
compare the mean values among three experimental groups with a
least significant difference post-hoc test assuming equal variance
or a Tambane's T2 post-hoc test when equal variance was not
assumed. The adjusted significance of the P-value was set at 0.05.
A population standard deviation to test the normal distribution of
samples was used. P<0.05 was considered to indicate a
statistically significant difference.
Results
Increased expression of NUCB-2 in
RCC
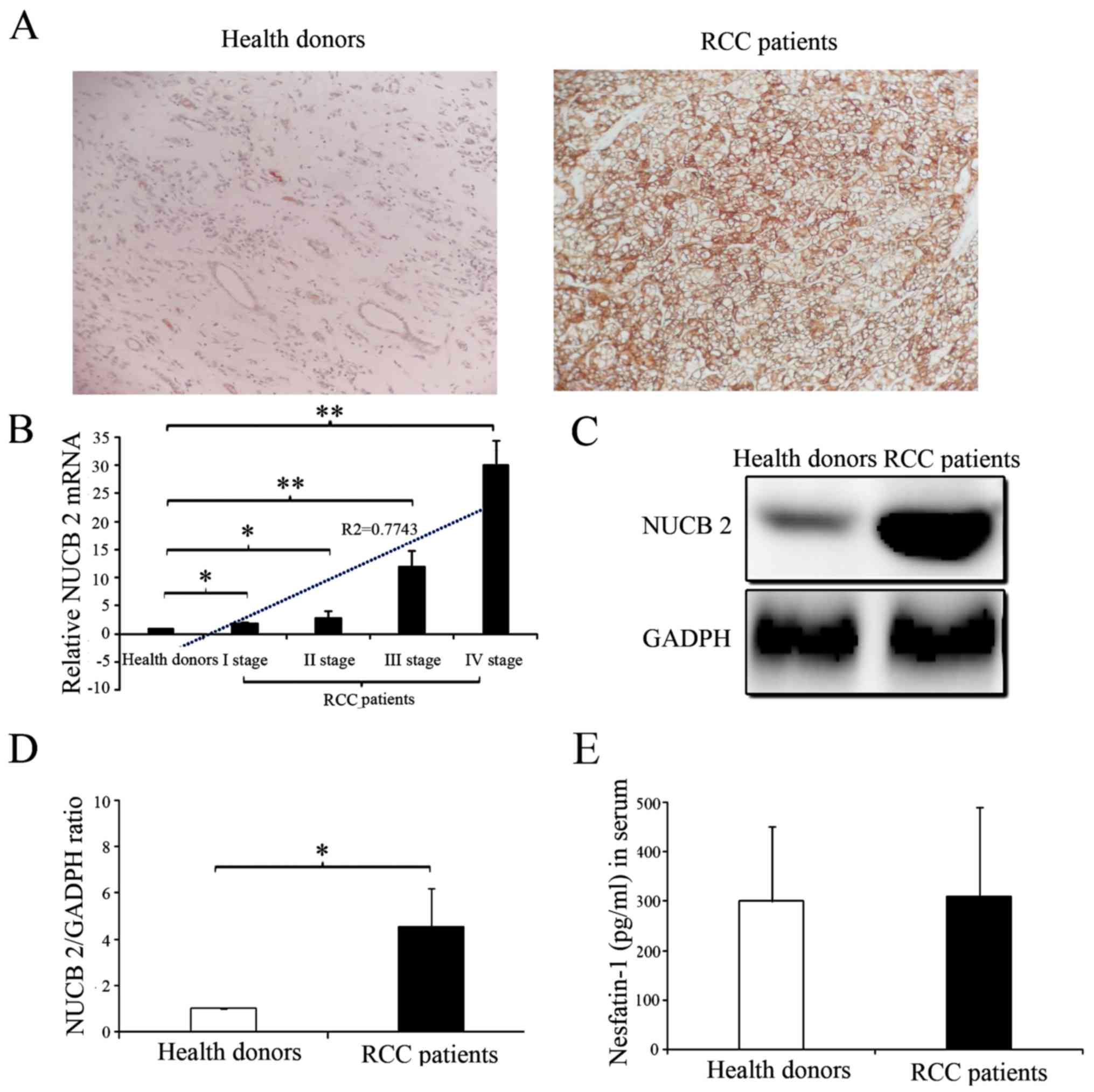
The presence and distribution of NUCB-2 in the
tissues were assessed via IHC. The NUCB-2 protein was primarily
localized in the cytoplasm. High expression of NUCB-2 was detected
in the tumor tissues of patients with RCC, whereas low expression
of NUCB-2 was detected in the kidney tissues of healthy donors
(Fig. 1A). The NUCB-2 mRNA and
protein expression levels in the kidney tissues of 68 RCC patients
and 10 normal donors were analyzed with RT-qPCR and western blot
analysis. The results showed that NUCB-2 mRNA and protein
expression were significantly higher in the tissue samples from
patients with RCC compared with normal group (Fig. 1B-D; P<0.05). Furthermore, the
expression of NUCB-2 mRNA was positively correlated with RCC stage
(Fig. 1B; r2=0.7743;
P<0.05). To further determine whether NUCB-2 overexpression was
systemic or local, the serum concentrations of the N-terminally
active protein nesfatin-1 of NUCB-2 in both samples were determined
and no significant difference was identified between the two groups
(Fig. 1E).
NUCB-2 knockout reverses EMT
phenotypes in RCC
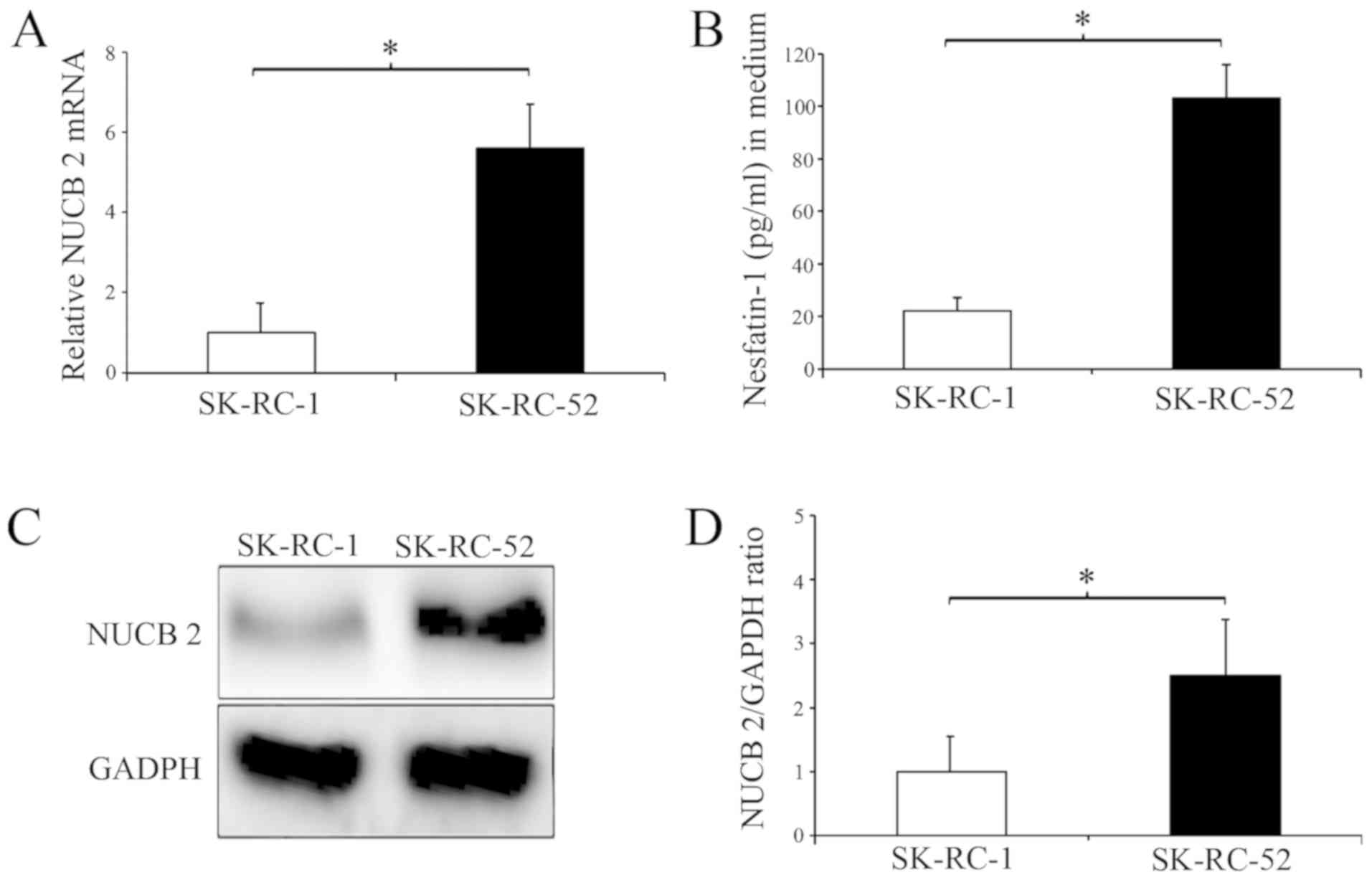
In the present study, two human RCC cell lines were
selected for study, SK-RC-1 and SK-RC-52 cells. These two cell
lines were used to determine the effect of NUCB-2 on RCC migration
and invasion, which are key steps in the initial progression of
cancer metastasis (31). Higher
NUCB-2 mRNA and protein expression was identified in SK-RC-52 cells
compared with the SK-RC-1 cells (Fig.
2A, C and D) and higher concentrations of nesfatin-1 were
identified in the cell culture medium of the SK-RC-52 cells
(Fig. 2B). The SK-RC-1 cell line is
derived from a primary clear cell RCC specimen, whereas the
SK-RC-52 cell line is derived from a clear cell RCC metastatic
lesion in the mediastinum (28);
therefore, the results presented in Fig.
2 suggest that NUCB-2 may be involved in metastasis of RCC.
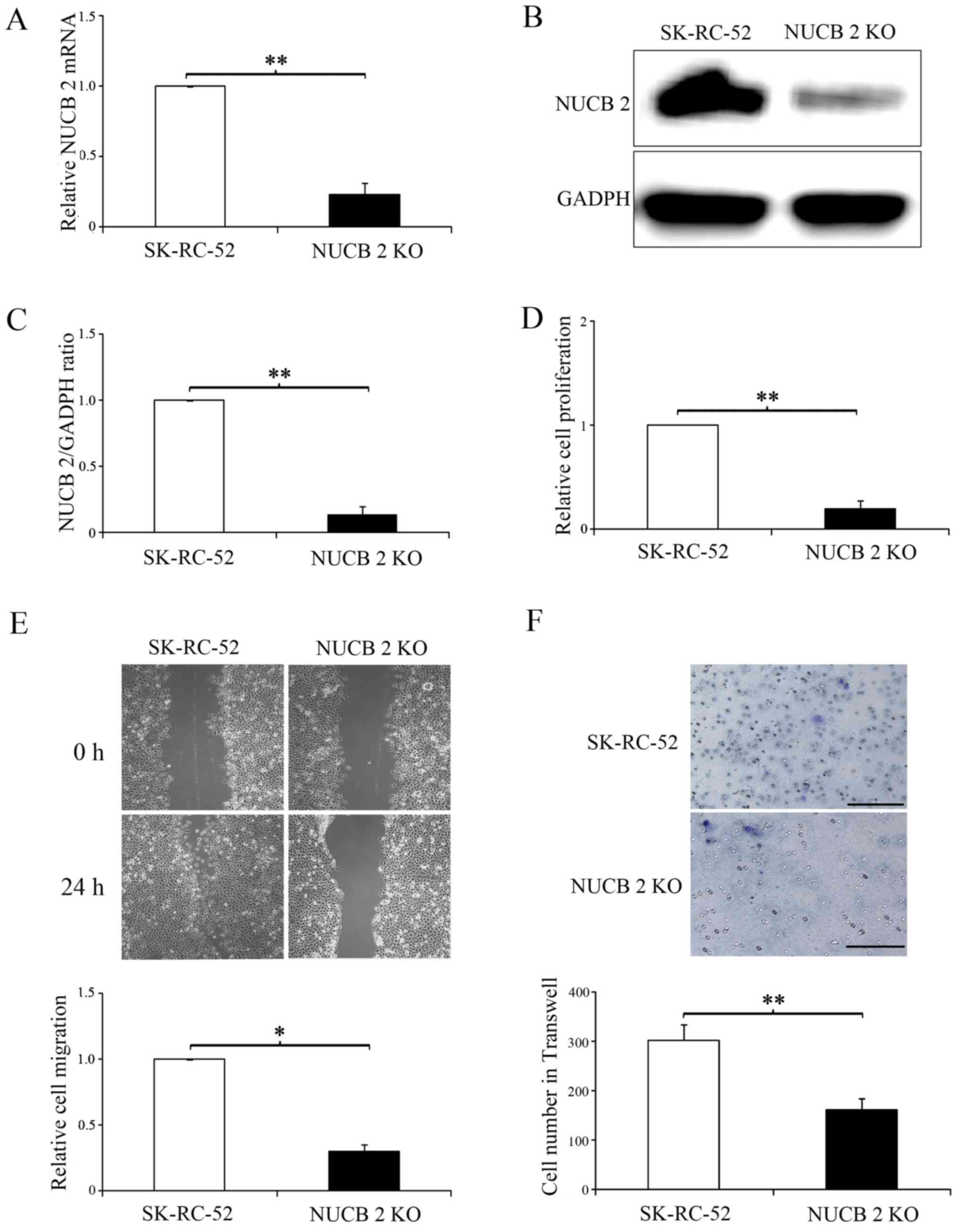
Based on the results of NUCB-2 expression in the two
cell lines, SK-RC-52 cells were used for all subsequent
experiments. The NUCB-2 gene in SK-RC-52 cells was knocked out via
the CRISPR-Cas9 system, and the data demonstrated that NUCB-2
knockout was stable (Fig. 3A-C).
Based on the cell proliferation (Fig.
3D), and the migration (Fig. 3E)
and invasion assays (Fig. 3F),
NUCB-2 knockout significantly attenuated these behaviors compared
with SK-RC-52 cells. These results further demonstrate that NUCB-2
serves an important role in the metastasis of RCC.
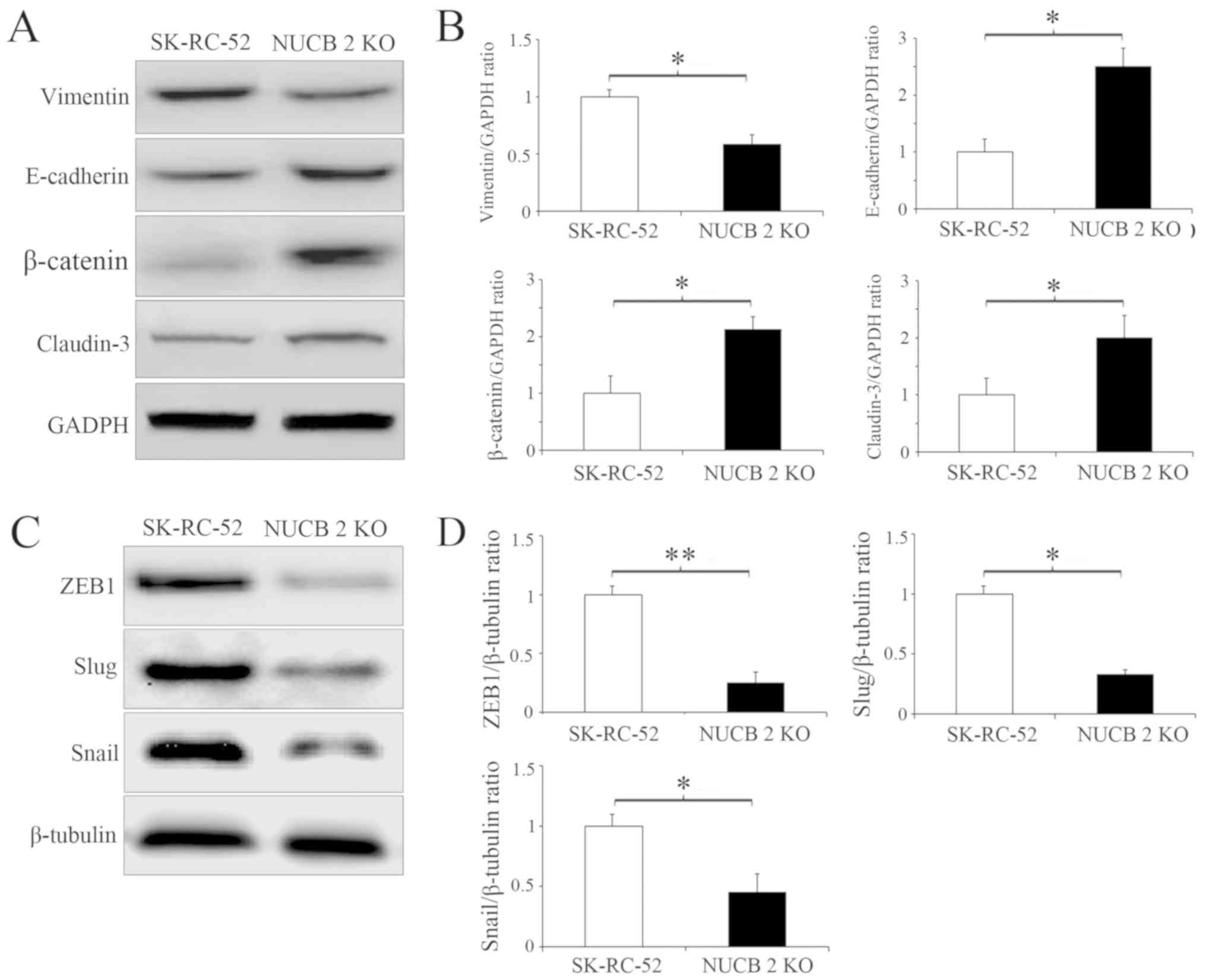
Several biochemical markers are used to characterize
EMT, for example, epithelial cells primarily express E-cadherin and
claudin-3, whereas mesenchymal cells express vimentin and
N-cadherin (32). Since the targeted
inhibition of NUCB-2 attenuated migration and invasion, it was next
determined whether this inhibition was sufficient to suppress or
attenuate EMT in RCC by examining the expression of the
aforementioned EMT markers. The stable knockout of NUCB-2 in
SK-RC-52 cells significantly increased E-cadherin and claudin-3
levels whilst significantly decreasing expression of vimentin (all
P<0.05; Fig. 4A and B). ZEB1 is a
major regulator of EMT in malignant tumors (32,33), and
Snail and Slug are important transcription factors involved in EMT
regulation (34). ZEB1 (P<0.01),
Snail and Slug (both P<0.05) expression was significantly
decreased in the NUCB-2-knockout cells, suggesting that NUCB-2 may
be involved in EMT through the ZEB1 signaling (Fig. 4C and D).
NUCB-2 promotes EMT in RCC via the
AMPK/mTOR signaling pathway
To further clarify which signaling pathway is
involved in the NUCB-2-mediated EMT in RCC, the association between
NUCB-2 and the AMPK/mTOR signaling pathway was assessed. Previous
studies have reported that NUCB-2 affects AMPK activation and
promotes mTOR phosphorylation, which leads to the upregulation of
protein synthesis, cell proliferation, cell cycle progression and
angiogenesis (35–37); the latter of which serves an
important role in tumor metastasis. Acetyl-CoA carboxylase (ACC) is
a downstream target of energy-sensitive AMPK both of which are
phosphorylated in their active form. The data in Fig. 5 shows that phosphorylated AMPK and
phosphorylated ACC expression was significantly increased by
knocking out NUCB-2 expression in SK-RC-52 cells. These results
suggest that the AMPK pathways are involved in NUCB-2-regulated EMT
properties, migration and invasion in RCC. The mTOR signaling
pathway is downstream of AMPK. mTOR contains two subunits of
functionally and biochemically distinct multiprotein complexes
called mTOR complex (mTORC)1 and mTORC2 (38–40), in
which mTORC1 serves a central role in cell growth and regulation of
metabolism (41). eIF4E-binding
protein 1 (4E-BP1) and ribosomal S6 kinase (S6K) are direct
substrates of mTORC1 activity (42).
As shown in Fig. 5, the
phosphorylation of 4E-BP1 and S6K decreased when the NUCB-2 gene
was knocked out in SK-RC-52 cells, suggesting that the mTOR pathway
is also involved in the NUCB-2-regulated EMT in RCC.
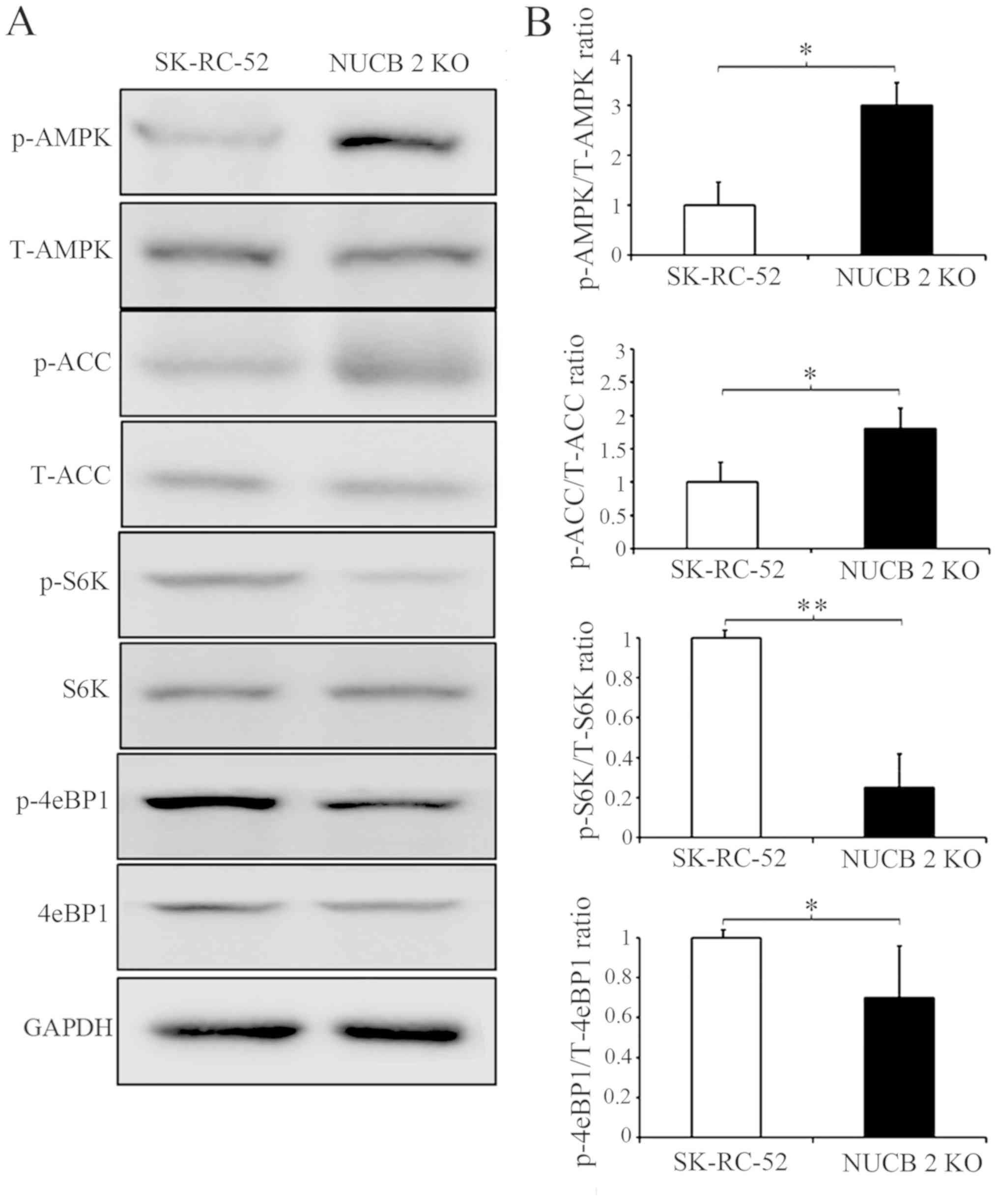 | Figure 5.AMPK and TORC1 pathways are critical
for regulating the NUCB-2-mediated inhibition of migration and
invasion. (A) Effect of NUCB-2-KO on proteins involved in the AMPK
and TORC1 pathways. (B) Densitometry analysis of the ratio of the
phosphorylated variant to the total expression. Expression was
first normalized to GAPDH. *P<0.05, **P<0.01. NUCB-2,
nucleobindin 2; AMPK, AMP-dependent protein kinase; TORC1, target
of rapamycin complex; ACC, acetyl-CoA carboxylase; S6, ribosomal S6
kinases; 4eBP1, 4E-BP1, eIF4E-binding protein 1; p-, phospho; t-,
total; KO, knockout. |
To further clarify the results described above,
competitive AMPK inhibitor was used (43) to inhibit the AMPK signaling pathway
in NUCB-2-knockout SK-RC-52 cells. After treatment with
dorsomorphin (40 µM, 100 min), AMPK and ACC phosphorylation
decreased, suggesting that dorsomorphin inhibition was successful.
However, S6K and 4eBP1 showed the opposite results (Fig. 6A). Cell migration and invasion
following treatment with dorsomorphin was also assessed. After
treatment with dorsomorphin, the migratory and invasive
capabilities of RCC cells were restored in the NUCB-2-knockout
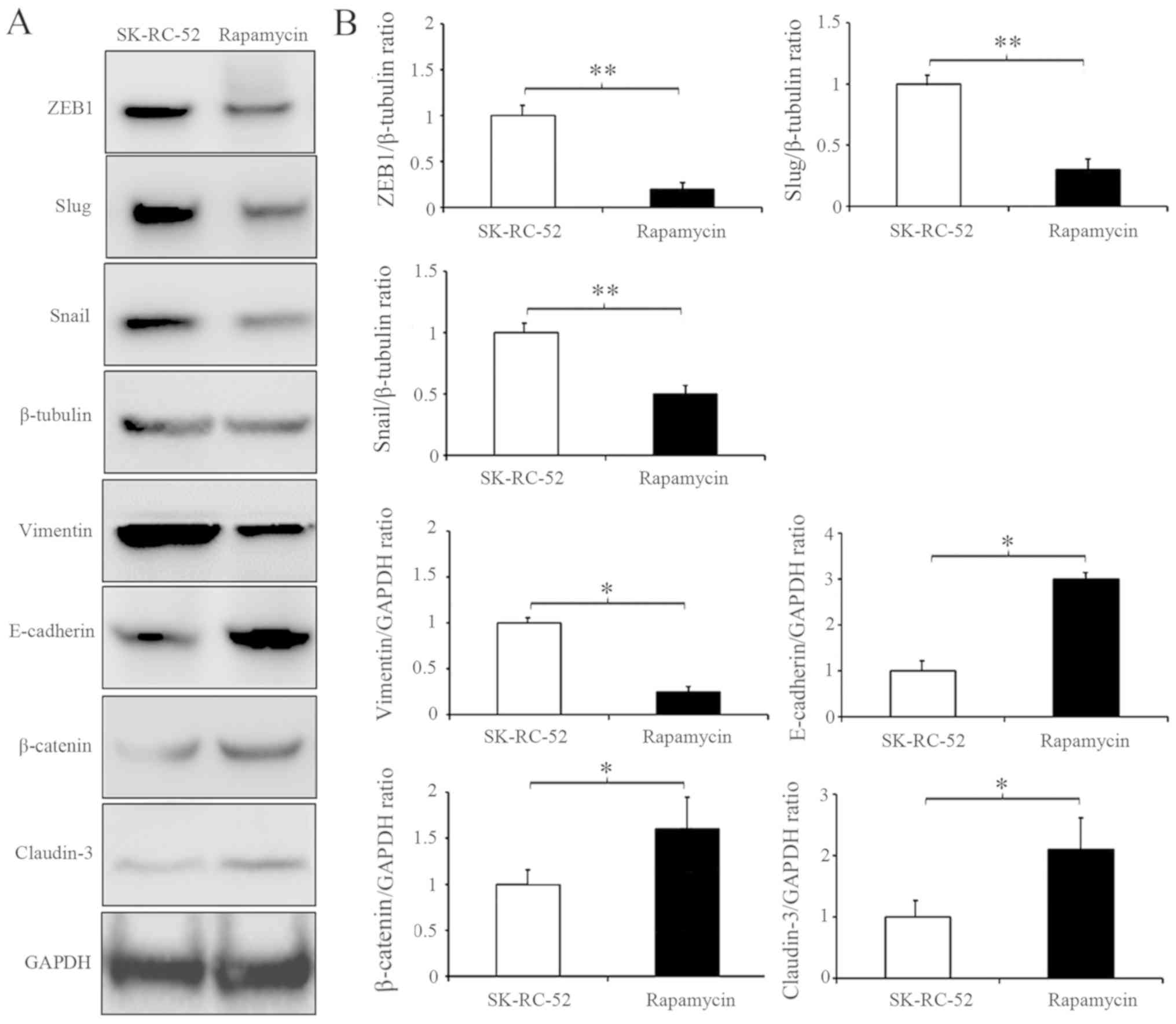
cells (Fig. 6B and C). Rapamycin is
a commonly used TORC1 inhibitor. SK-RC-52 cells were treated
without or with rapamycin (100 nmol/l) for 12 h, and EMT biomarkers
were examined by western blot analysis. TORC1 inhibition resulted
in significantly decreased expression of ZEB1, Snail and Slug (All
P<0.01), which are involved in EMT regulation in SK-RC-52 cells,
and several EMT biochemical markers experienced opposite changes
(Fig. 7; P<0.01). These results
suggest that NUCB-2 may promote EMT in RCC via the AMPK/mTOR
signaling pathway.
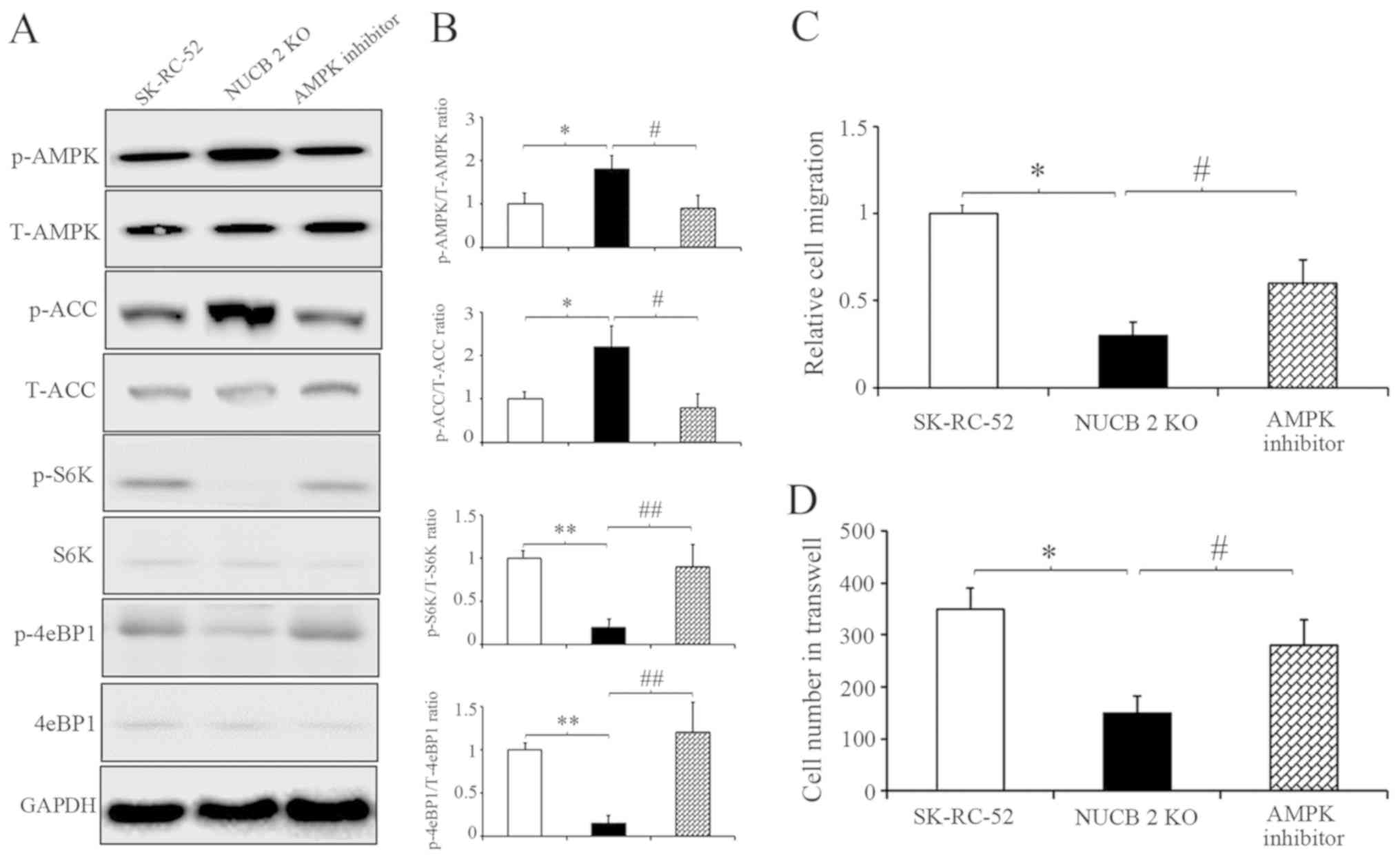 | Figure 6.NUCB-2 upregulates
epithelial-mesenchymal transition in renal cell carcinoma through
the AMPK/TORC1 pathway. (A) SK-RC-52 cells with NUCB-2-KO were
treated with 40-µM dorsomorphin or control for 100 min. (B) Effect
of the inhibitor on members of the AMPK or TORC1 pathways.
Densitometry analysis of the western blots in the left panel. The
relative levels of protein levels normalized to those of GAPDH.
*P<0.05, **P<0.01 SK-RC-52 vs. NUCB-2 KO cells;
#P<0.05, ##P<0.01 NUCB-2 KO vs. NUCB-2
KO cells treated with an AMPK inhibitor. (C) Relative cell
migration in the three groups. *P<0.05, #P<0.05.
(D) Number of invaded cells transferred in the Transwell assay in
the three groups. When cells were treated with an AMPK inhibitor,
the migratory and invasive abilities of NUCB-2-KO stable clones was
increased. *P<0.05, #P<0.05. NUCB-2, nucleobindin
2; AMPK, AMP-dependent protein kinase; TORC1, target of rapamycin
complex; ACC, acetyl-CoA carboxylase; S6, ribosomal S6 kinases;
4eBP1, 4E-BP1, eIF4E-binding protein 1; p-, phospho; t-, total; KO,
knockout. |
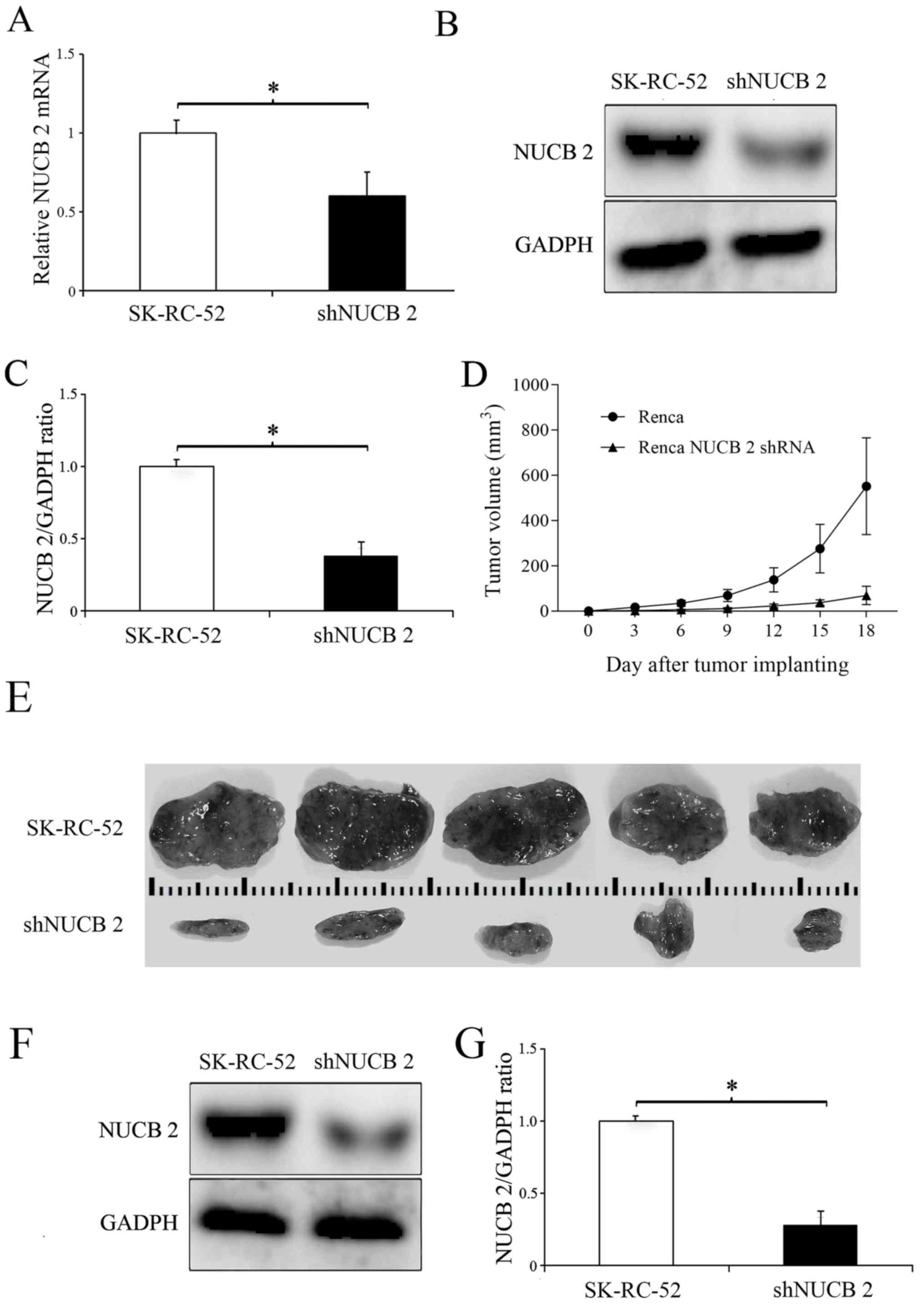
In vivo experiment
To determine the role of NUCB-2 in vivo, the
expression of NUCB-2 in the murine kidney cancer cell line Renca
was inhibited by transfection with a NUCB-2-targeting shRNA
(experimental group), and non-transfected Renca cells were used as
a control. The results showed that the levels of NUCB-2 mRNA and
protein were significantly decreased following transfection with
shRNA (P<0.05; Fig. 8A-C), and
tumor growth was significantly slower in the NUCB-2 inhibition
group compared with the control group (Fig. 8D-G), suggesting that NUCB-2 serves an
important role in RCC growth.
Discussion
The present study is the first to describe the role
and mechanisms of NUCB-2 in RCC metastasis, to the best of our
knowledge. NUCB-2 was highly expressed in patients with RCC, and
the expression of NUCB-2 was strongly associated with clinical
stage. NUCB-2 upregulated EMT through the AMPK/TORC1/ZEB1 signaling
pathway. Finally, inhibition of NUCB-2 expression can inhibit the
growth of RCC tumors in animals. These data suggest that NUCB-2 may
be a potential marker for the diagnosis of RCC.
NUCB-2 is widely expressed throughout the body and
is primarily expressed in the hypothalamic nucleus (24). NUCB-2 participates in a variety of
pathophysiological processes, primarily serving an important role
in maintaining the energy and nutrient balance (16,17).
NUCB-2 has also been demonstrated to serve an important role in
tumor development. Suzuki et al (18) found that NUCB-2 acts as a tumor
promoter during breast cancer development and metastasis. Zhang
et al (20–22) reported that increased NUCB-2
expression is associated with prostate cancer recurrence and a poor
prognosis. In a study by Qi et al (23), NUCB-2 was highly expressed in RCC. A
retrospective clinical study by Fu et al (44) found that high NUCB-2 expression
levels were positively correlated with Fuhrman grade. Together,
these studies have concluded that NUCB-2 is associated with poor
tumor prognosis. In the present study, similar results regarding
NUCB-2 function in promoting RCC cell proliferation, invasion and
metastasis were described. Based on the previously mentioned
findings, the underlying mechanism of NUCB-2 in RCC was
investigated.
The NUCB-2 gene was knocked out in SK-RC-52 cells
using the CRISPR-Cas9 system and cell proliferation, invasion and
migration assays were performed. The results showed that cell
proliferation and metastasis were suppressed in the NUCB-2-knockout
cells. EMT is a key reversible step that facilitates tumor
migration, invasion and metastasis (7). Metabolic reprogramming is a distinct
hallmark in EMT development (45–47). The
findings of the present study implicate NUCB-2 as a key regulator
of EMT in RCCs based on the observation that the increased
expression of NUCB-2 in the metastatic human RCC cell line,
SK-RC-52 altered expression of a number of biochemical markers
(decreased E-cadherin, increased vimentin and N-cadherin). However,
these markers exhibited the opposite trend in expression in
SK-RC-52 cells when NUCB-2 was knocked out by genetic editing. ZEB1
is a transcription factor and a master regulator of EMT in several
types of cancer (32,33). ZEB1 was highly expressed in SK-RC-52
cells, a cell line derived from RCC mediastinal metastases,
suggesting that NUCB-2 may promote the malignant behaviors of RCC
by upregulating ZEB1.
In various cancer cells, the activation of AMPK
stimulates the tumor suppressor gene p53, which has been reported
to control apoptosis and the cell cycle in the induction of the EMT
and in metastasis (45–47). To investigate the mechanism
underlying the high expression of NUCB-2 and renal cell
proliferation and mesenchymal transition, the association between
NUCB-2 and the AMPK/mTOR signaling pathways was examined. The
results demonstrated that knockdown of NUCB-2 expression in
SK-RC-52 cells increased the phosphorylation of AMPK and decreased
mTOR phosphorylation, consistent with numerous previous studies
(48–50). ZEB1, is a major mediator of tumor
migration, and exerts its effects by activating the mTOR signaling
pathway, and ZEB1 activity was effectively inhibited when an mTOR
inhibitor was used (51,52). Furthermore, the abrogation of AMPK
expression by dorsomorphin restored NUCB-2-induced invasion,
migration and the EMT in RCC in the NUCB-2-knockout SK-RC-52 cells.
At the same time, the phosphorylation of two important downstream
substrates of mTORC1, S6K and 4eBP1 was observed, suggesting their
activation.
In addition, xenograft mouse experiments confirmed
that the knockdown of NUCB-2 using a specific shRNA decreased renal
cell tumor nodule formation compared with mice injected with cells
expressing levels of high NUCB-2.
In conclusion, patient samples, cell lines and mouse
models were analyzed, and NUCB-2 was identified as a valid marker
associated with RCC malignant metastasis and a poor prognosis. The
results of the present study suggest that NUCB-2 promotes EMT in
RCC via the AMPK/TORC1/ZEB1 pathway. These findings suggest that
NUCB2 may serve as a potential diagnostic marker and therapeutic
target for treating patients with RCC.
Acknowledgements
Not applicable.
Funding
This study was funded by the National Natural
Science Foundation of China (Grant Nos. 81171996 and 81272289).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RT, WN, CZ and ZL conceived and designed the
experiments. RT, WN, PD, YY and LC performed the experiments. SN
collected and organized the experimental data. RT, XW and SL
collected tissue samples, analyzed and interpreted the data and RT
wrote the manuscript. CZ and ZL revised the manuscript. CZ and ZL
revised and approved the final version of the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethical
Committee of The First Affiliated Hospital of Jiamusi University
(Jiamusi, China; approval no. HREC06FEB2016). In all cases, written
informed consent was obtained, and the experiments were conducted
in accordance with the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
4E-BP1
|
eIF4E-binding protein 1
|
|
ACC
|
acetyl-CoA carboxylase
|
|
AMPK
|
AMP-dependent protein kinase
|
|
EMT
|
epithelial-mesenchymal transition
|
|
IHC
|
immunohistochemistry
|
|
mTOR
|
mammalian target of rapamycin
|
|
mTORC
|
mTOR complex
|
|
NUCB-2
|
nucleobindin 2
|
|
RCC
|
renal cell carcinoma
|
|
S6K
|
ribosomal S6 kinases
|
|
shRNA
|
short hairpin RNA
|
|
ZEB1
|
zinc finger E-box binding to homeobox
1
|
References
|
1
|
Pichler M, Hutterer GC, Chromecki TF,
Jesche J, Kampel-Kettner K, Pummer K and Zigeuner R: Renal cell
carcinoma stage migration in a single European centre over 25
years: Effects on 5- and 10-year metastasis-free survival. Int Urol
Nephrol. 44:997–1004. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Athar U and Gentile TC: Treatment options
for metastatic renal cell carcinoma: A review. Can J Urol.
15:3954–3966. 2008.PubMed/NCBI
|
|
5
|
Ljungberg B, Bensalah K, Canfield S,
Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L,
Merseburger AS, et al: EAU guidelines on renal cell carcinoma: 2014
update. Eur Urol. 67:913–924. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oh-I S, Shimizu H, Satoh T, Okada S,
Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, et
al: Identification of nesfatin-1 as a satiety molecule in the
hypothalamus. Nature. 443:709–712. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Massari F, Ciccarese C, Santoni M,
Brunelli M, Piva F, Modena A, Bimbatti D, Fantinel E, Santini D,
Cheng L, et al: Metabolic alterations in renal cell carcinoma.
Cancer Treat Rev. 41:767–776. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang P, Sun Y and Ma L: ZEB1: At the
crossroads of epithelial mesenchymal transition, metastasis and
therapy resistance. Cell Cycle. 14:481–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu W, Huang YJ, Liu C, Yang YY, Liu H,
Cui JG, Cheng Y, Gao F, Cai JM and Li BL: Inhibition of TBK1
attenuates radiation-induced epithelial-mesenchymal transition of
A549 human lung cancer cells via activation of GSK-3β and
repression of ZEB1. Lab Invest. 94:362–370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Z, Sun B, Qi L, Li H, Gao J and Leng
X: Zinc finger E-box binding homeobox 1 promotes vasculogenic
mimicry in colorectal cancer through induction of
epithelial-to-mesenchymal transition. Cancer Sci. 103:813–820.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li H, Song S, Xu Y, Zhao J and Liu H:
Knockdown of ZEB1 suppresses the formation of vasculogenic mimicry
in breast cancer cell line MDA-MB- 231 through downregulation of
Flk-1. Minerva Med. 108:191–193. 2017.PubMed/NCBI
|
|
13
|
Pavlides S, Tsirigos A, Migneco G,
Whitaker-Menezes D, Chiavarina B, Flomenberg N, Frank PG, Casimiro
MC, Wang C, Pestell RG, et al: The autophagic tumor stroma model of
cancer: Role of oxidative stress and ketone production in fueling
tumor cell metabolism. Cell Cycle. 9:3485–3505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Martinez-Outschoorn UE, Balliet RM,
Rivadeneira DB, Chiavarina B, Pavlides S, Wang C, Whitaker-Menezes
D, Daumer KM, Lin Z, Witkiewicz AK, et al: Oxidative stress in
cancer associated fibroblasts drives tumor-stroma co-evolution: A
new paradigm for understanding tumor metabolism, the field effect
and genomic instability in cancer cells. Cell Cycle. 9:3256–3276.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kang J, Shakya A and Tantin D: Stem cells,
stress, metabolism and cancer: A drama in two Octs. Trends Biochem
Sci. 34:491–499. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li QC, Wang HY, Chen X, Guan HZ and Jiang
ZY: Fasting plasma levels of nesfatin-1 in patients with type 1 and
type 2 diabetes mellitus and the nutrient-related fluctuation of
nesfatin-1 level in normal humans. RegulPept. 159:72–77. 2010.
|
|
17
|
Gonzalez R, Reingold BK, Gao X, Gaidhu MP,
Tsushima RG and Unniappan S: Nesfatin-1 exerts a direct,
glucose-dependent insulinotropic action on mouse islet β-and MIN6
cells. J Endocrinol. 208:R9–R16. 2011.PubMed/NCBI
|
|
18
|
Suzuki S, Takagi K, Miki Y, Onodera Y,
Akahira J, Ebata A, Ishida T, Watanabe M, Sasano H and Suzuki T:
Nucleobindin 2 in human breast carcinoma as a potent prognostic
factor. Cancer Sci. 103:136–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao X, Liu XM and Zhou LH: Recent progress
in research on the distribution and function of NUCB-2/nesfatin-1
in peripheral tissues. Endocr J. 60:1021–1027. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang H, Qi C, Wang A, Yao B, Li L, Wang Y
and Xu Y: Prognostication of prostate cancer based on NUCB-2
protein assessment: NUCB-2 in prostate cancer. J Exp Clin Cancer
Res. 32:772013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang H, Qi C, Li L, Luo F and Xu Y:
Clinical significance of NUCB-2 mRNA expression in prostate cancer.
J Exp Clin Cancer Res. 32:562013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang H, Qi C, Wang A, Li L and Xu Y: High
expression of nucleobindin 2 mRNA: An independent prognostic factor
for overall survival of patients with prostate cancer. Tumour Biol.
35:2025–2028. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qi C, Ma H, Zhang HT, Gao JD and Xu Y:
Nucleobindin 2 expression is an independent prognostic factor for
clear cell renal cell carcinoma. Histopathology. 66:650–657. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stengel A, Goebel M, Yakubov I, Wang L,
Witcher D, Coskun T, Taché Y, Sachs G and Lambrecht NW:
Identification and characterization of nesfatin-1 immunoreactivity
in endocrine cell types of the rat gastric oxyntic mucosa.
Endocrinology. 150:232–238. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
William WN, Kim JS, Liu DD, Solis L,
Behrens C, Lee JJ, Lippman SM, Kim ES, Hong WK and Lee HY: The
impact of phosphorylated AMP-activated protein kinase expression on
lung cancer survival. Ann Oncol. 23:78–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zong H, Yin B, Zhou H, Cai D, Ma B and
Xiang Y: Inhibition of mTOR pathway attenuates migration and
invasion of gallbladder cancer via EMT inhibition. Mol Biol Rep.
41:4507–4512. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lam JS, Klatte T and Breda A: Staging of
renal cell carcinoma: Current concepts. Indian J Urol. 25:446–454.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ebert T, Bander NH, Finstad CL, Ramsawak
RD and Old LJ: Establishment and characterization of human renal
cancer and normal kidney cell lines. Cancer Res. 50:5531–5536.
1990.PubMed/NCBI
|
|
29
|
Gregersen I, Skjelland M, Holm S, Holven
KB, Krogh-Sørensen K, Russell D, Askevold ET, Dahl CP, Ørn S,
Gullestad L, et al: Increased systemic and local interleukin 9
levels in patients with carotid and coronary atherosclerosis. PLoS
One. 8:e727692013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brodaczewska KK, Szczylik C, Fiedorowicz
M, Porta C and Czarnecka AM: Choosing the right cell line for renal
cell cancer research. Mol Cancer. 15:832016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takeyama Y, Sato M, Horio M, Hase T,
Yoshida K, Yokoyama T, Nakashima H, Hashimoto N, Sekido Y, Gazdar
AF, et al: Knockdown of ZEB1, a master epithelial-to-mesenchymal
transition (EMT) gene, suppresses anchorage-independent cell growth
of lung cancer cells. Cancer Lett. 296:216–224. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Y, El-Naggar S, Darling DS, Higashi Y
and Dean DC: Zeb1 links epithelial-mesenchymal transition and
cellular senescence. Development. 135:579–588. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sánchez-Tilló E, Liu Y, de Barrios O,
Siles L, Fanlo L, Cuatrecasas M, Darling DS, Dean DC, Castells A
and Postigo A: EMT-activating transcription factors in cancer:
Beyond EMT and tumor invasiveness. Cell Mol Life Sci. 69:3429–3456.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu Y, Pang XY, Dong M, Wen F and Zhang Y:
Nesfatin-1 inhibits ovarian epithelial carcinoma cell proliferation
in vitro. Biochem Biophys Res Commun. 440:467–472. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang ML, Zhang ZH, Wang C, Li K, Li SB,
Boden G, Li L and Yang GY: Nesfatin-1 action in the brain increases
insulin sensitivity through Akt/AMPK/TORC2 pathway in diet-induced
insulin resistance. Diabetes. 61:1959–1968. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Z, Xu G, Li Y, Zhao J, Mulholland MW
and Zhang W: mTOR-dependent modulation of gastric
nesfatin-1/NUCB-2. Cell PhysiolBiochem. 29:493–500. 2012.
|
|
38
|
Saxton RA and Sabatini DM: mTOR signaling
in growth, metabolism, and disease. Cell. 9:960–976. 2017.
View Article : Google Scholar
|
|
39
|
Wullschleger S and Loewith R: TOR
signaling in growth and metabolism. Cell. 124:471–484. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Laplante M and Sabatini DM: mTOR signaling
in growth control and disease. Cell. 149:274–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang K and Fingar DC: Growing knowledge
of the mTOR signalling network. Semin Cell Dev Biol. 36:79–90.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ma XM and Blenis J: Molecular mechanisms
of mTOR-mediated translational control. Nat Rev Mol Cell Biol.
10:307–318. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Viollet B, Horman S, Leclerc J, Lantier L,
Foretz M, Billaud M, Giri S and Andreelli F: AMPK inhibition in
health and disease. Crit Rev Biochem Mol Biol. 45:276–295. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fu H, Zhu Y, Wang Y, Liu Z, Zhang J, Wang
Z, Xie H, Dai B, Xu J and Ye D: High NUCB2 expression level
represents an independent negative prognostic factor in Chinese
cohorts of non-metastatic clear cell renal cell carcinoma patients.
Oncotarget. 15:9188–9194. 2016.
|
|
45
|
Pinheiro C, Garcia EA, Morais-Santos F,
Moreira MA, Almeida FM, Jubé LF, Queiroz GS, Paula ÉC, Andreoli MA,
Villa LL, et al: Reprogramming energy metabolism and inducing
angiogenesis: Co-expression of monocarboxylate transporters with
VEGF family members in cervical adenocarcinomas. BMC Cancer.
15:8352015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Saeidi N, Meoli L, Nestoridi E, Gupta NK,
Kvas S, Kucharczyk J, Bonab AA, Fischman AJ, Yarmush ML and
Stylopoulos N: Reprogramming of intestinal glucose metabolism and
glycemic control in rats after gastric bypass. Science.
341:406–410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Soga T: Cancer metabolism: Key players in
metabolic reprogramming. Cancer Sci. 104:275–281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rattan R, Giri S, Singh AK and Singh I:
5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside inhibits
cancer cell proliferation in vitro and in vivo via AMP-activated
protein kinase. J Biol Chem. 280:39582–39593. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dong D, Cai GY, Ning YC, Wang JC, Lv Y,
Hong Q, Cui SY, Fu B, Guo YN and Chen XM: Alleviation of senescence
and epithelial-mesenchymal transition in aging kidney by short-term
caloric restriction and caloric restriction mimetics via modulation
of AMPK/mTOR signaling. Oncotarget. 8:16109–16121. 2017.PubMed/NCBI
|
|
50
|
Liu T, Sun Q, Li Q, Yang H, Zhang Y, Wang
R, Lin X, Xiao D, Yuan Y, Chen L and Wang W: Dual PI3K/mTOR
inhibitors, GSK2126458 and PKI-587, suppress tumor progression and
increase radiosensitivity in nasopharyngeal carcinoma. Mol Cancer
Ther. 14:429–439. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kan JY, Yen MC, Wang JY, Wu DC, Chiu YJ,
Ho YW and Kuo PL: Nesfatin-1/Nucleobindin-2 enhances cell
migration, invasion, and EMT via LKB1/AMPK/TORC1/ZEB1 pathways in
colon cancer. Oncotarget. 7:31336–31349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sun S, Hang T, Zhang B, Zhu L, Wu Y, Lv X,
Huang Q and Yao H: miRNA-708 functions as a tumor suppressor in
colorectal cancer by targeting ZEB1 through Akt/mTOR signaling
pathway. Am J Transl Res. 11:5338–5356. 2019.PubMed/NCBI
|