Introduction
Breast cancer (BC) is the most commonly diagnosed
cancer and the leading cause of cancer-associated mortality in
women; according to data published by the Global Cancer Statistics
in 2018, there were >2.1 million new cases that year and 0.6
million BC-associated mortalities worldwide (1). Despite recent advances in surgery,
radiotherapy, chemotherapy, neoadjuvant chemotherapy and endocrine
therapy, the incidence of BC has increased in developed (54.4 per
100,000, female by age-standardized in 2018) and developing (31.3
per 100,000, female by age-standardized in 2018) countries
(1,2). The incidence of BC in China has
increased from 75.3 to 127.55 per 100,000 between 2005–2015,
respectively (3). Therefore, it is
important to identify novel specific targets to improve the
currently available therapeutic strategies and to clarify the
underlying molecular mechanisms of BC.
In the past decade, various studies have
investigated gene expression levels in BC. These studies have
screened numerous differentially expressed genes (DEGs) that may be
involved in the development and progression of BC (4,5).
However, the results have been largely inconsistent due to the
heterogeneity of specimens across experiments and the use of
different detection platforms and data processing methods (6,7). To
identify novel DEGs associated with BC, the Robust Rank Aggregation
(RRA) package was used to integrate multiple gene expression
profiles in the present study. The RRA package uses P-values to
compare each ranked gene against a randomly ranked gene and then
re-ranks the genes (8). Therefore,
the RRA package can be used to improve the current understanding of
the mechanism underlying BC.
The human discs large-associated protein 5 (DLGAP5)
gene, which is mapped to chromosome 14q22.3, is a cell cycle
regulator involved in carcinogenesis (9). Wang et al (10) reported that DLGAP5 was upregulated in
non-small cell lung cancer (NSCLC) and was associated with a
shorter survival time. In cytological experiments, the knockdown of
DLGAP5 caused inhibition of proliferation, migration and invasion
of NSCLC cells (10). Previous
studies have also shown that the expression levels of DLGAP5 are
upregulated in bladder (11),
prostate (12) and liver cancer
(13), as well as leukemia (14) were associated with poor prognosis. In
the present study, DLGAP5 was identified to be a hub gene in GEO
and Gene Expression-Based Outcome for BC Online (GOBO) databases.
Furthermore, DLGAP5 expression levels were confirmed in 24 paired
tumor and normal samples, and in 160 paraffin-embedded BC
specimens. In vitro functional analysis, a Cell Counting Kit
(CCK)-8 assay and cell cycle analysis were performed to determine
the underlying molecular mechanism of DLGAP5 in the progression of
BC. The present study aimed to identify potential novel prognosis
biomarkers and potential novel targets, to facilitate the
development of novel drugs for the treatment of BC.
Materials and methods
Identification of DEGs from the GEO
database
In total, three BC datasets, including GSE21422
(15), GSE29431 (Lopez et al,
unpublished data, 2011) and GSE61304 (16), were obtained from the GEO database
(http://www.ncbi.nlm.nih.gov/geo)
(Table I). These three datasets were
generated using the GPL570 platform of the (HG-U133_Plus_2)
Affymetrix Human Genome U133 Plus 2.0 array (http://www.affymetrix.com/index.affx). GSE21422
contained five healthy breast specimens and 14 BC specimens.
GSE29431 contained 12 healthy breast specimens and 54 primary BC
specimens. GSE61304 contained four healthy breast specimens of an
epithelial origin and 58 BC specimens of an epithelial origin.
Subsequently, the platform and matrix files were downloaded from
GEO. The dataset information is presented in Table I. The downloaded files were processed
with R software [version 2.6.2 (17)], calibrated, standardized and
log2-converted as necessary. To identify the DEGs in
each dataset, the limma package in R was used with the cutoff
criteria of |log2-fold-change (FC)|>1 and P<0.05
(18).
 | Table I.Details for Gene Expression Omnibus
breast cancer datasets. |
Table I.
Details for Gene Expression Omnibus
breast cancer datasets.
| Author, year | GEO | Platform | Normal | Tumor | (Refs.) |
|---|
| Kretschmer et
al, 2011 | GSE21422 | GPL570 | 5 | 14 | (15) |
| Lopez et al,
2011 | GSE29431 | GPL570 | 12 | 54 | Unpublished |
| Aswad et al,
2015 | GSE61304 | GPL570 | 4 | 58 | (16) |
Integration of the microarray
data
To identify significant and reliable DEGs, the
Robust Rank Aggregation (RRA) package (19) was used. The RRA package assumes that
each gene is randomly ordered within each dataset (20). A gene that was ranked high had a low
P-value, following correction and had a greater probability of
being considered a DEG. This was used to identify DEGs and to rank
them consistently and reliably without the influence of noise using
R (8). This approach allowed an
improved understanding of the molecular mechanisms involved in
cancer (21). Meanwhile, the heatmap
package [version 1.0.12 (22)] was
used to generate the heatmap of these DEGs.
GO and KEGG pathway analysis
To define the biological functions of the DEGs, GO
term enrichment and KEGG pathway analysis were performed using
DAVID (23). P<0.05 was
considered to indicate a statistically significant difference.
Establishment of the protein-protein
interaction (PPI) network
STRING (https://string-db.org/) is an online tool designed to
collect and integrate information from known and predicted PPI in a
large number of organisms (24). The
DEGs were analyzed using STRING to construct the PPI network. A
confidence score >0.4 was set as the cutoff value. Data was
subsequently visualized using Cytoscape software [version 3.7.1
(23)]. Molecular Complex Detection
(MCODE) was performed within Cytoscape to screen significant
modules of PPI network. The criteria default parameters were as
follows: Degree cut-off=10, node score cut-off=0.2, k-core=2 and
max. depth=100 (25).
Online database extraction
Kaplan-Meier (KM) plots were used to analyze the
overall survival time of patients with BC. The hazard ratios with
95% confidence interval and log-rank P-values were calculated using
the KM plotter (26). GOBO
(co.bmc.lu.se/gobo/), an online tool that
contains 1,881 BC specimens, was used to identify genes that
co-expressed with DLGAP5, and determine the clinical
characteristics of DLGAP5 in patients with BC (27,28).
Furthermore, GOBO also supports the applications of PAM50 gene
expression subtype analysis, which is recognized as a prognostic
gene signature assay by the National Comprehensive Cancer Network
(29). To analyze the associations
between DLGAP5 expression levels and the clinicopathological
characteristics (ER, PR, Her-2, basal-like status, and tumor
grade), the GEO datasets GSE12093, GSE7390, GSE6532 (30), GSE3494, GSE1456, GSE2603 (31), GSE2034 (32), GSE11121 (33), GSE4922 and GSE5327 (34) were combined. One-way ANOVA with
Tukey's post-hoc test was conducted when making comparisons in
datasets containing multiple groups (27,35).
Genes that correlated with DLGAP5 expression, with a Pearson
correlation coefficient >0.4 were also selected from the GOBO
database.
Tissue samples
The present study was approved by The Ethics
Committee of Tongji Hospital and written informed consent was
provided by all patients prior to the study start. A total of 24 BC
tissues and matched normal samples were obtained from patients with
BC (median age, 46 years; age range, 30–60 years), following
surgery at Tongji Hospital, Tongji Medical College of Huazhong
University of Science and Technology (Wuhan, China) between May
2019 and June 2019. All samples were preserved in RNAlater
Stabilization Solution (Qiagen GmbH) overnight at 4°C, and
subsequently stored at −80°C.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from tissues using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). RT was performed using HiScript®II RT SuperMix
(Vazyme Biotech Co., Ltd.). The protocol for RT was as follows:
42°C for 2 min, 50°C for 15 min and 85°C for 5 sec. qPCR was
performed using SYBR Green qPCR Mix (Toyobo Co., Ltd.). The
following primer sequences were used for qPCR: DLGAP5 forward,
5′-AAGTGGGTCGTTATAGACCTGA-3′ and reverse,
5′-TGCTCGAACATCACTCTCGTTAT-3′; GAPDH forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′. The following thermocycling
conditions were used for qPCR: Initial denaturation for 3 min at
95°C, 45 cycles of 10 sec at 95°C, 30 sec at 60°C and 15 sec at
95°C, and a final extension at 95°C for 15 sec and 65°C for 5 sec.
Melt curve analysis was subsequently performed. Relative mRNA
expression levels were calculated using the 2−ΔΔCq
method (36). All experiments were
performed in triplicate.
Immunohistochemistry (IHC)
A tissue microarray (cat. no. BR1921c; Alenabio) was
used to validate the expression levels of DLGAP5 in healthy breast
and BC specimens. A total of 192 breast specimens were analyzed, of
which 80 samples were invasive ductal carcinoma, 80 samples were
invasive lobular carcinoma, 21 samples were adjacent healthy
tissues, four samples were cancer adjacent breast tissue and seven
samples were healthy tissues. Additionally, 8 samples were missing
estrogen receptor (ER) status and progesterone receptor (PR)
status, while 12 samples were missing human epidermal growth factor
receptor 2 (HER2) status information. Tumors were staged based on
tumor-node-metastasis (TNM) system (7th edition) (37). Antigens were retrieved in citrate
buffer at 95°C (pH 6) for 15 min, and 3% hydrogen peroxide was used
for endogenous peroxidase blocking at 37°C for 30 min, followed by
incubation with 10% goat serum (Abcam) at room temperature for 1 h.
IHC was performed using a rabbit polyclonal anti-human DLGAP5
primary antibody (1:200; cat. no. A13575; Abclonal), and the slides
were incubated overnight at 4°C. The sections were subsequently
treated with a horseradish peroxidase-conjugated anti-rabbit
immunoglobulin G secondary antibody (1:500; cat. no. bs-0295D-HRP;
BIOSS), at 37°C for 30 min and the signal was visualized by
staining with 3,3′-diaminobenzidine at room temperature for 1 min.
After washing the slides with water, the sections were
counterstained with hematoxylin at room temperature for 2 min.
Corresponding negative control samples (cat. no. BR1921c Trail;
Alenabio) were incubated with PBS overnight at 4°C instead of the
primary antibody, and the subsequent steps were consistent with the
conditions of the DLGAP5 primary antibody test group. The samples
were examined using an Olympus BX-51 light microscope (Olympus
Corporation; magnification, ×400).
Scoring of the staining results
The staining intensity and the percentage of
positive cells were determined by two experienced pathologists who
were blinded to the experimental groups. The staining intensity was
scored as: i) 0, colorless; ii) 1, light yellow; iii) 2,
brown/yellow; and iv) 3, brown. The percentage of positive cells
was scored as: i) 0, no cell staining; ii) 1, >25% of cells
stained; iii) 2, 25–50% of cells stained; iv) 3, 51–75% of cells
stained; and v) 4, >75% of cells stained. The two scores were
multiplied to generate values ranging between 0 and 12. The strong
expression group (++/+++) was defined as a score ≥5. The weak
expression group (−/+) was defined as a score <5.
Cell culture and transfection
The human BC cell line MDA-MB-231 was obtained from
The Cell Bank of Type Culture Collection of Chinese Academy of
Sciences. Cells were cultured in L-15 medium (HyClone; GE
Healthcare Life Sciences) supplemented with 1%
penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.) and 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.) at 37°C with 5% CO2. Cells were seeded into 6-well
plates until they reached 50% confluence and
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was subsequently used to transfect the small
interfering RNAs (siRNAs) with 100 nM per well according to the
manufacturer's protocol. Subsequent experimentation was conducted
after transfection for 48 h. The DLGAP5 siRNA (si-DLGAP5-1 and
si-DLGAP5-2) and nontargeting negative control (si-NC) were
purchased from Guangzhou RiboBio Co., Ltd. The sequences were as
follows: si-DLGAP5-1, 5′-GAATCCAGATGGAGTCTTA-3′; si-DLGAP5-2,
5′-GAAGTCCCATCACTTGAAA-3′; and si-NC,
5′-TTCTCCGAACGTGTCACGTdTdT-3′. Knockdown efficiency of DLGAP5 siRNA
was assessed using RT-qPCR.
CCK-8 and cell cycle assays
For the CCK-8 assay (cat. no. CK04, Dojindo
Molecular Technologies, Inc.), after transfection for 48 h,
~3×103 MDA-MB-231 cells were resuspended in 100 µl L-15
medium and plated into 96-well plates. After cultured for 24, 48,
72 and 96 h, cells were incubated with CCK-8 solution (10 µl/well)
for 2 h at 37°C. The optical density was measured at 450 nm using a
microplate reader (http://www.moleculardevices.com).
For the cell cycle assay, cells were harvested after
transfection for 72 h and fixed with pre-cooled 70% ethanol at
−20°C overnight. Cells were washed twice with PBS to remove all
ethanol. RNase A enzyme (Thermo Fisher Scientific, Inc.) and
propidium iodide (0.05 mg/ml) were added and incubated for 30 min
at room temperature in the dark before analyzing the samples using
a FACSCalibur system (Beckman Coulter). FlowJo software version 7.6
(FlowJo LLC) was used for the cell cycle analysis. Each experiment
was performed >2 times.
Prediction of mitophagy receptors
GeneCards is an integrative database that provides
comprehensive information on all annotated and predicted human
genes; it can also provide information on the localization of the
protein of interest (38). In
addition, the iLIR database is an online tool that is used to
identify microtubule-associated proteins 1A/1B light chain 3B
(LC3)-interacting region (LIR) motifs within eukaryotic proteins
(39,40). These two databases were used to
determine whether DLGAP5 may function in mitophagy as a potential
autophagy receptor.
Statistical analysis
The GSE29431 and GSE61304 datasets were selected in
the following analysis due to a larger sample size and unpaired
Student's t-test was used. Paired Student's t-test was applied to
detect DLGAP5 expression in BC tissues and matched normal samples.
χ2 test were performed to analyze the IHC results.
One-way analysis of variance (ANOVA), followed by Tukey's post-hoc
test were performed when making comparisons in the GOBO datasets
containing multiple groups. One-way ANOVA and Dunnett's post-hoc
test were applied when multiple groups were compared with the si-NC
group. The receiver operating characteristic curves were plotted to
identify the diagnostic value of DLGAP5 in BC. Statistical analyses
were performed using SPSS version 22.0 (IBM Corp.) and GraphPad
Prism 8 (GraphPad Software, Inc.). All data are presented as the
mean ± standard deviation of three independent experiments and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Identification of DEGs in BC
The BC expression microarray datasets GSE21422,
GSE29431 and GSE61304 were standardized, and the results are
presented in Fig. S1. In the
GSE21422 dataset, 946 upregulated and 1,380 downregulated genes
were identified by the limma package using an adjusted P<0.05
and |logFC|>1 as the cutoff criteria; in the GSE29431 dataset,
497 upregulated and 948 downregulated genes were identified; in the
GSE61304 dataset, 326 upregulated and 511 downregulated genes were
identified (Fig. S2).
Identification of DEGs in BC using
integrated bioinformatics
The three datasets were screened using the limma
package and analyzed using the RRA package (P<0.05 after
correction; |logFC|>1). Using this approach, 85 DEGs that
included 40 upregulated and 45 downregulated genes were identified
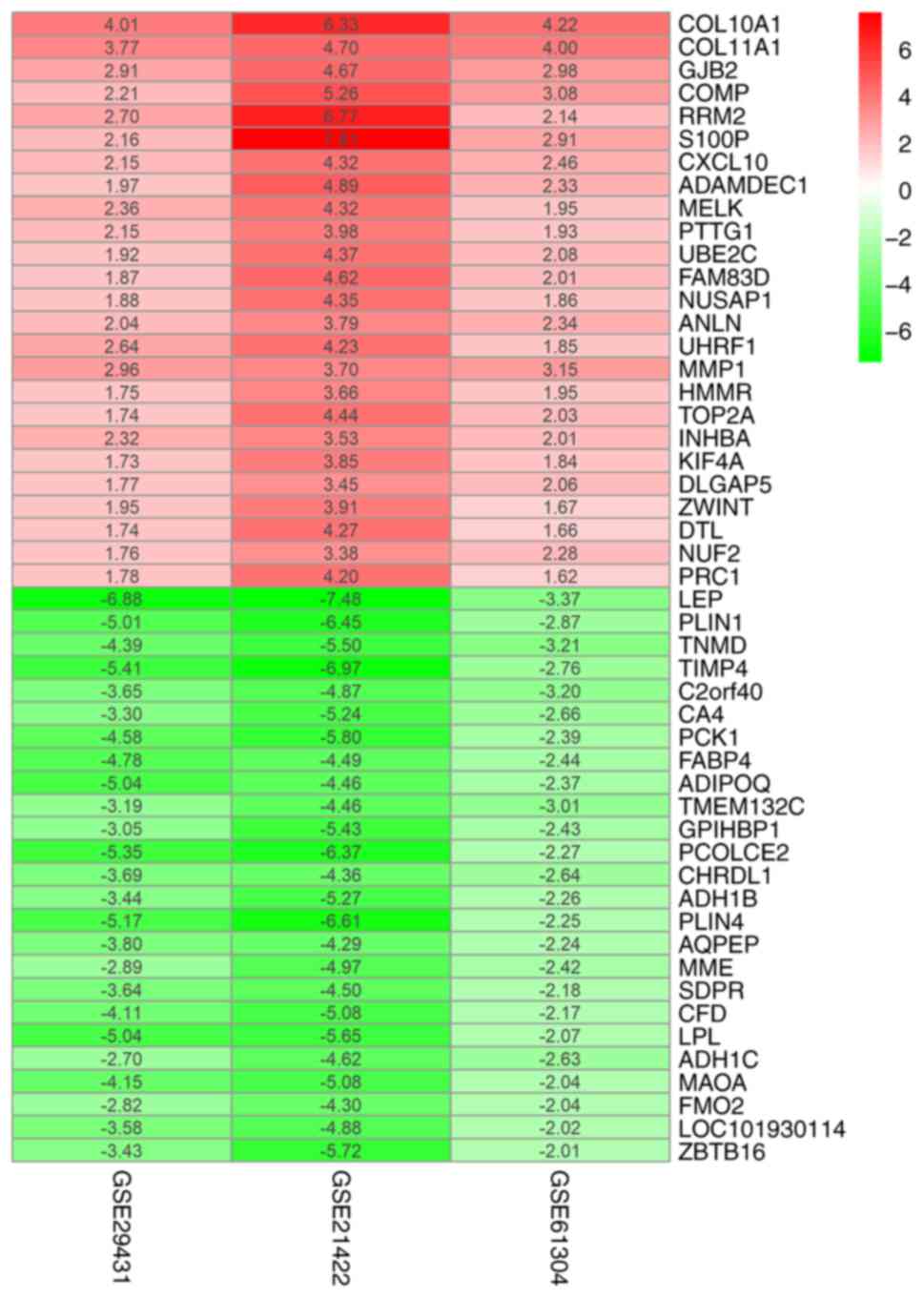
(Table SI). The heatmap package was
used to generate the heatmap of the top 25 upregulated and
downregulated genes (Fig. 1).
GO term enrichment and KEGG pathway
analysis
GO term enrichment and KEGG pathway analyses were
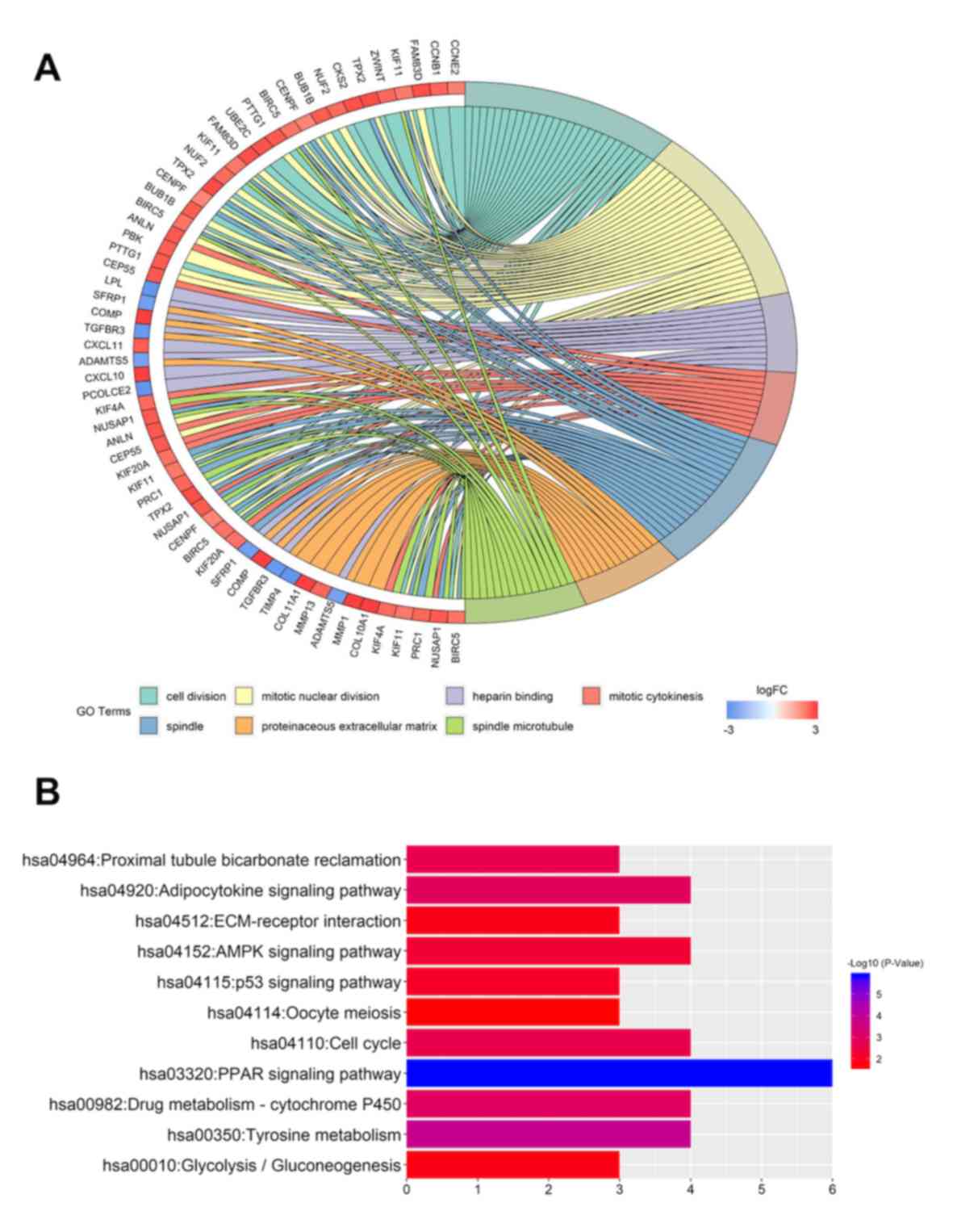
performed using DAVID. In the biological function group, the
upregulated DEGs were involved in ‘cell division’, ‘mitotic nuclear
division’, ‘cell proliferation’ and ‘mitotic cytokinesis’ (Table SII), whereas the downregulated DEGs
were involved in ‘cellular response to tumor necrosis factor’ and
‘response to linoleic acid’ (Table
SIII). In the cell component group, the upregulated DEGs were
associated with processes that involved the ‘spindle’, the ‘spindle
microtubule’ and the ‘midbody’ (Table
SII), whereas the downregulated genes were associated with
processes involved in the ‘cell surface’ and ‘extracellular space’
(Table SIII). In the molecular
function group, the upregulated DEGs were involved in ‘microtubule
binding’ and ‘protein kinase binding’ (Table SII), whereas the downregulated DEGs
were involved in ‘heparin binding’ (Table SIII) (Fig. 2A).
A total of 11 KEGG pathways were associated with
these DEGs. The upregulated genes were particularly enriched in the
‘cell cycle’, ‘p53 signaling pathway’, ‘ECM-receptor interaction’
and ‘oocyte meiosis’ (Fig. 2B). The
downregulated genes were enriched in the ‘PPAR signaling pathway’,
‘tyrosine metabolism’, ‘drug metabolism-cytochrome P450’,
‘adipocytokine signaling pathway’, ‘proximal tubule bicarbonate
reclamation’, ‘AMPK signaling pathway’ and
‘glycolysis/gluconeogenesis’ (Fig.
2B).
Analysis of the DEGs in BC using the
PPI network
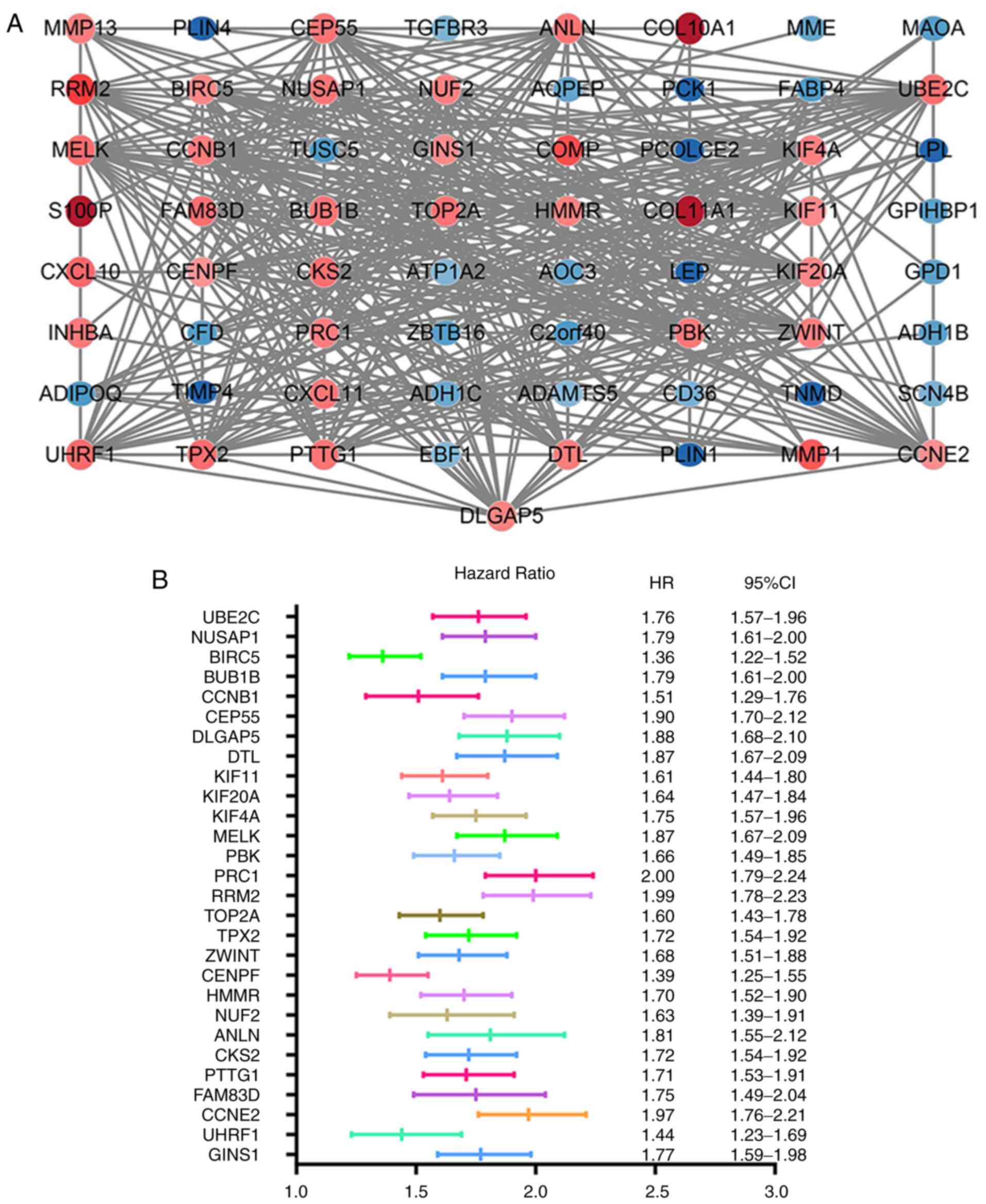
The STRING database was used to construct the PPI
network of 85 DEGs that included 40 upregulated and 45
downregulated genes, and the results were analyzed using the
Cytoscape software (Fig. 3A). A
total of 30 hub genes were identified, including
ubiquitin-conjugating enzyme E2 C (UBE2C), nucleolar and
spindle-associated protein 1 (NUSAP1), baculoviral IAP
repeat-containing protein 5 (BIRC5), mitotic checkpoint
serine/threonine-protein kinase BUB1 beta (BUB1B),
G2/mitotic-specific cyclin-B1 (CCNB1), centrosomal protein of 55
kDa (CEP55), disks large-associated protein 5 (DLGAP5),
denticleless protein homolog (DTL), kinesin-like protein KIF11
(KIF11) and kinesin-like protein KIF20A (KIF20A), which were the
top 10 DEGs with the highest degrees of protein-protein
connectivity.
Using MCODE, the two most significant functional
modules were investigated (Fig.
S3). KEGG pathway analysis was performed using DAVID software.
The genes in module 1 were mainly involved in the ‘cell cycle
control’, ‘p53 signaling’ and ‘oocyte meiosis’, whereas the genes
in module 2 were mainly involved in ‘PPAR signaling’,
‘adipocytokine signaling’, ‘AMPK signaling’ and ‘ECM-receptor
interaction’ (Table II).
 | Table II.Kyoto Encyclopedia of Genes and
Genomes pathway analysis of two key modules selected from the
protein-protein interaction network. |
Table II.
Kyoto Encyclopedia of Genes and
Genomes pathway analysis of two key modules selected from the
protein-protein interaction network.
| A, Module 1 |
|---|
|
|---|
| Term | Description | Count | P-value |
|---|
| hsa04110 | Cell cycle | 4 |
3.00×10−04 |
| hsa04115 | p53 signaling
pathway | 3 |
2.52×10−03 |
| hsa04114 | Oocyte meiosis | 3 |
6.78×10−03 |
|
| B, Module
2 |
|
| Term |
Description | Count | P-value |
|
| hsa03320 | PPAR signaling
pathway | 6 |
1.83×10−08 |
| hsa04920 | Adipocytokine
signaling pathway | 4 |
1.15×10−04 |
| hsa04152 | AMPK signaling
pathway | 4 |
6.11×10−04 |
| hsa04512 | ECM-receptor
interaction | 3 |
6.66×10−03 |
Survival analysis
Information of the 30 hub genes was available in the
KM plotter database. Among these, 28 genes were identified as
indicators of BC prognosis. The random survival forest map
comparing the genes is presented in Fig.
3B.
Analysis of DLGAP5 expression levels
using RT-qPCR and online databases
To investigate the role of DLGAP5 in BC, the
expression levels of DLGAP5 were examined in BC tissues. The
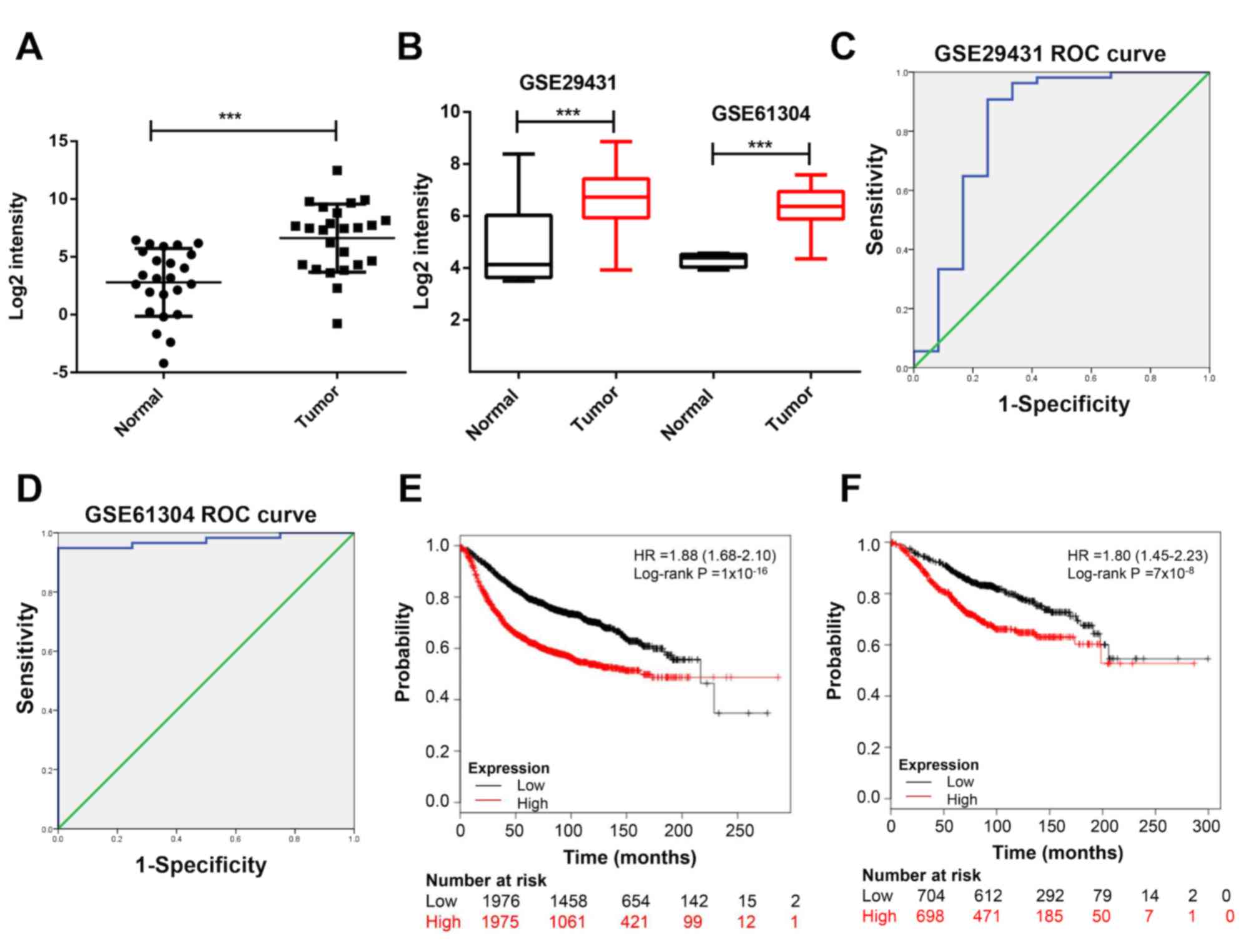
results of the RT-qPCR analysis suggested that DLGAP5 was
upregulated in BC tissues compared with normal tissues (P<0.001;
Fig. 4A). Due to a relative larger
sample size compared with GSE21422, two datasets, GSE29431 and
GSE61304 were used in the following analysis. The mRNA expression
levels of DLGAP5 in patients with BC were analyzed in the GSE29431
and GSE61304 datasets in order to expand the sample size; the
results in these datasets also suggested that DLGAP5 was
significantly upregulated in tumor specimens compared with normal
breast tissues (P<0.001; Fig.
4B). Furthermore, the receiver operating characteristic (ROC)
curves were plotted to identify the diagnostic value of DLGAP5 in
BC. DLGAP5 expression levels were associated with tumor
classification as assessed by an area under the curve (AUC) >0.8
(GSE29431, AUC=0.821; GSE61304, AUC=0.974; Fig. 4C and D). In detail, the cut-off point
for the expression of DLGAP5 to distinguish BC and non-malignant
groups using the ROC curve was 5.572, with a sensitivity of 83.3%
and a specificity of 75.0% in GSE29431. The cut-off point was
4.573, with a sensitivity of 94.8% and a specificity of 100% in
GSE61304. Therefore, high expression of DLGAP5 was associated with
a less favorable progression-free (Fig.
4E) and overall survival time (Fig.
4F) using the Kaplan-Meier Plotter online database.
Association between DLGAP5 expression
levels and the clinicopathological features of BC
To further investigate the association between
DLGAP5 expression and the clinicopathological characteristics of
the patients with BC, the GOBO online database was used. DLGAP5
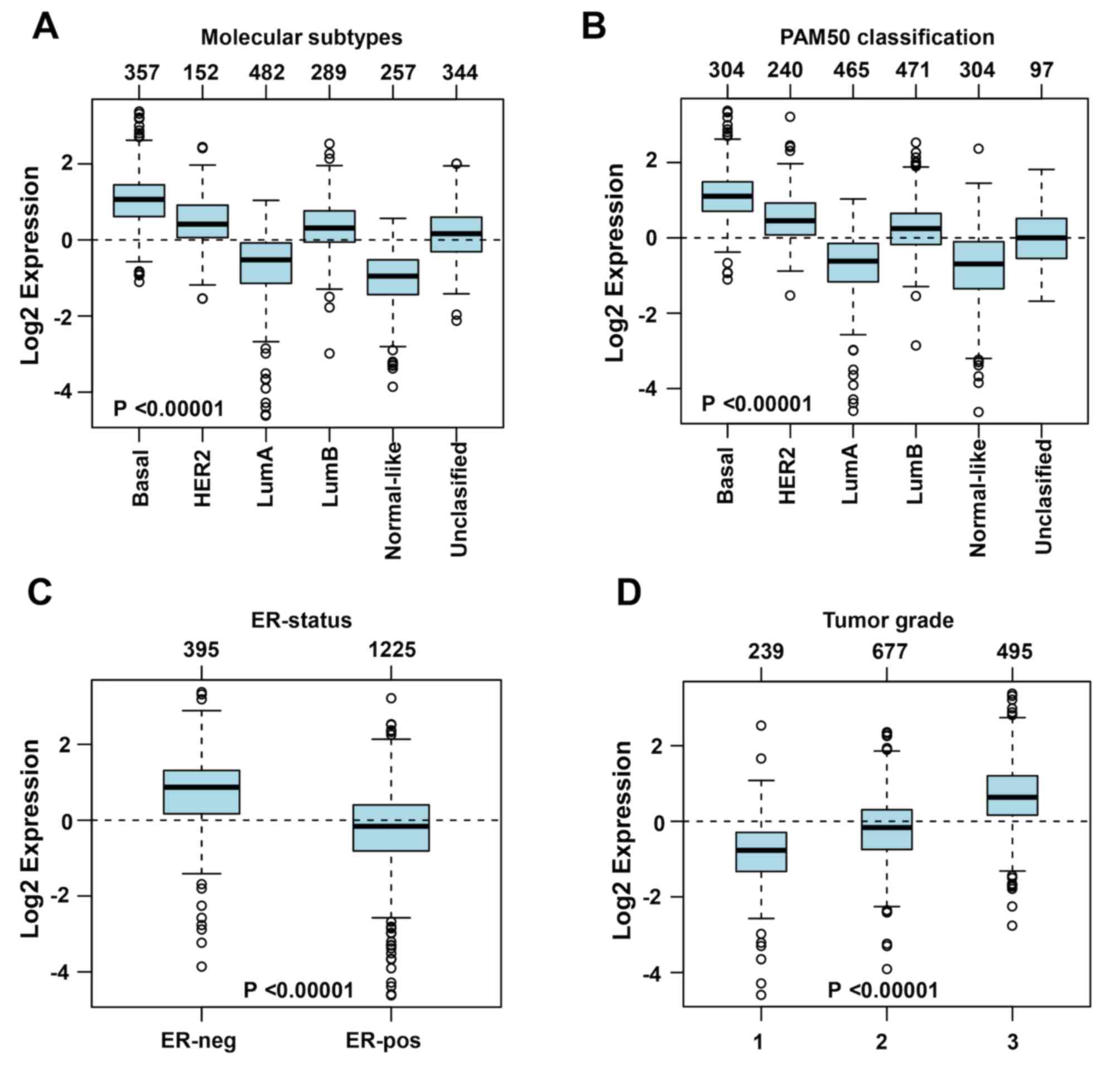
expression levels were higher in the basal subtype (n=357) and
lower in the normal-like (n=257) and luminal A subtypes (n=482)
(Fig. 5A). Similar results were
obtained following PAM50 subtype analysis (29) (Fig.
5B). A negative association was observed between the expression
levels of DLGAP5 and the ER (Fig.
5C). In addition, DLGAP5 expression levels increased with
increasing tumor grade (Fig.
5D).
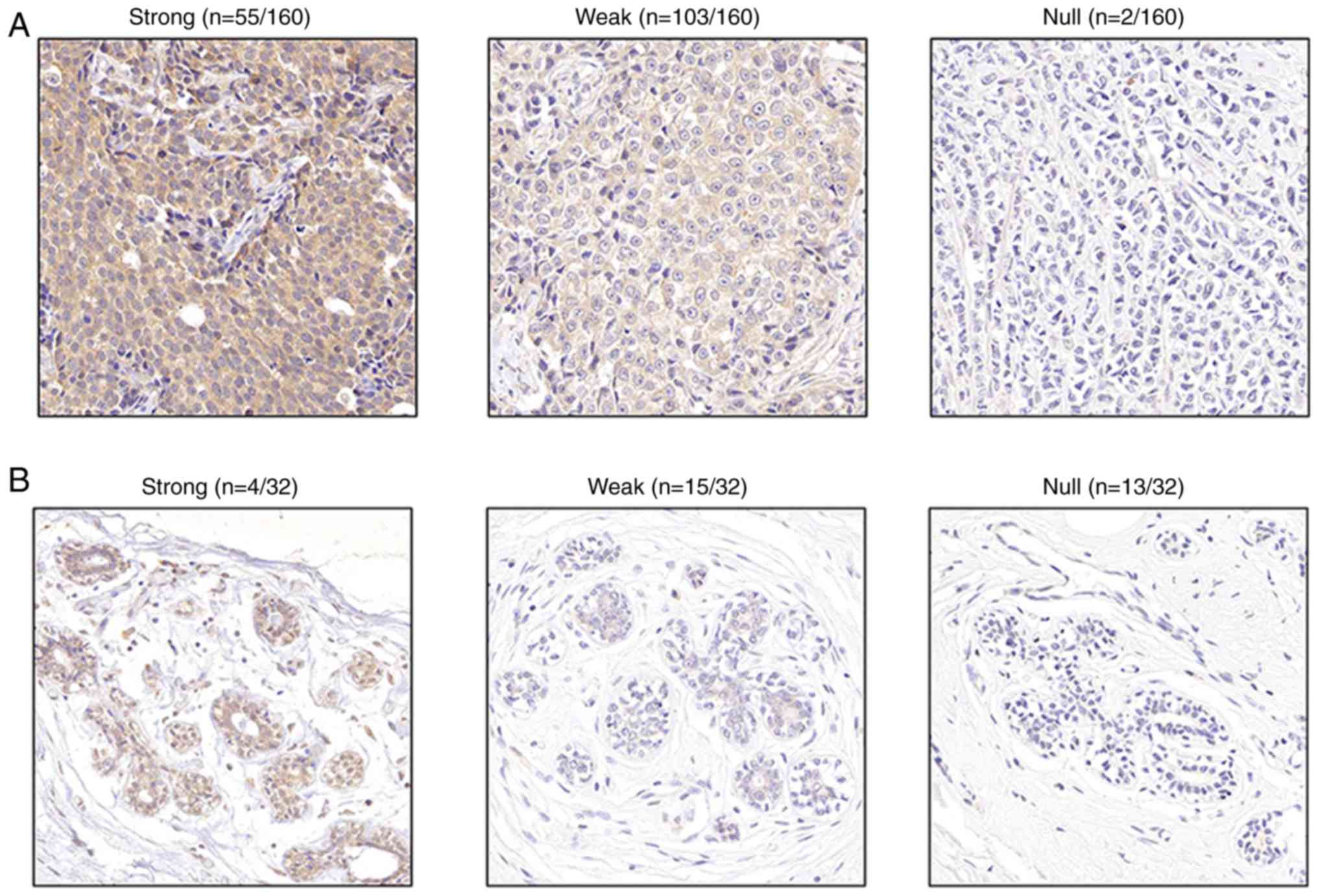
IHC was performed to verified the results of online
datasets. DLGAP5 staining was brown/yellow in invasive ductal and
lobular carcinoma specimens, with expression rate of 98.8% and
strong expression rate of 34.4%, which were significantly higher
compared with healthy breast tissues (59.4 and 12.5%, respectively;
with both comparison P<0.05; Fig.
6). The representative IHC images for DLGAP5 expression levels
in BC are presented in Fig. 6, and
the information of tissue samples included in the microarray is
presented in Table SIV. The 160
cancer specimens were divided into strong (++/+++) or weak (−/+)
expression groups, and the association between IHC scores and
patient clinicopathological characteristics was investigated. The
results revealed that high DLGAP5 expression levels in BC were
associated with clinical stage (χ2=4.002; P=0.045) and
lymph node status (χ2=5.806; P=0.016) (Table III). No significant associations
were present between DLGAP5 expression levels and age, tumor stage,
ER, progesterone receptor (PR) or human epidermal growth factor
receptor-2 (Her-2) expression (Table
III).
 | Table III.Association of DLGAP5 expression
levels with clinical characteristics of patients with breast
cancer. |
Table III.
Association of DLGAP5 expression
levels with clinical characteristics of patients with breast
cancer.
|
| DLGAP5 expression
levels |
|
|
|---|
|
|
|
|
|
|---|
| Clinical
characteristics | Strong, n | Weak, n | χ2 | P-value |
|---|
| Age, years |
|
| 0.639 | 0.420 |
|
≤50 | 34 | 58 |
|
|
|
>50 | 21 | 47 |
|
|
| T stage |
|
| 0.420 | 0.520 |
|
1-2 | 41 | 83 |
|
|
|
3-4 | 14 | 22 |
|
|
| Lymph node |
|
| 5.806 | 0.016a |
| − | 27 | 72 |
|
|
| + | 28 | 33 |
|
|
| Clinical stage |
|
| 4.002 | 0.045a |
|
I–IIB | 38 | 87 |
|
|
|
IIIA-IIIB | 17 | 18 |
|
|
| ER status |
|
| 0.347 | 0.556 |
| + | 43 | 84 |
|
|
| − | 10 | 15 |
|
|
| PR status |
|
| 0.022 | 0.882 |
| + | 32 | 61 |
|
|
| − | 21 | 38 |
|
|
| Her-2 status |
|
| 3.061 | 0.080 |
| 3+ | 8 | 6 |
|
|
|
0-1 | 45 | 89 |
|
|
Biological functions of
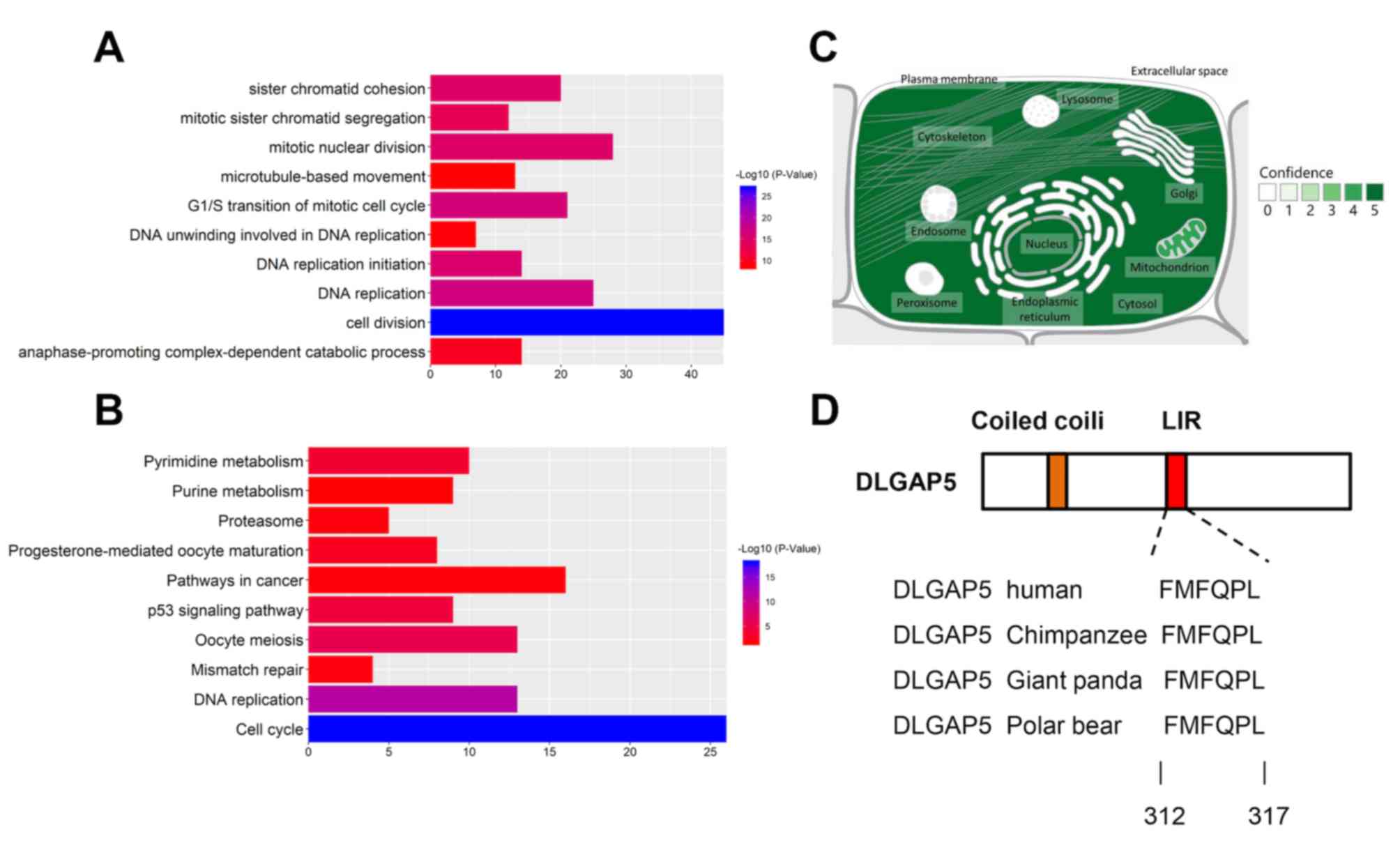
DLGAP5-associated genes
To further investigate the clinical relevance of
DLGAP5 in BC, genes that correlated with DLGAP5 expression, with a
Pearson correlation coefficient >0.4 were selected from the GOBO
database. A total of 292 genes were identified with functions in
‘cell division’, ‘DNA replication’, ‘G1/S transition of
mitotic cell cycle’, ‘DNA replication initiation’ and ‘mitotic
nuclear division’ (Fig. 7A). KEGG
pathways analysis demonstrated that DLGAP5-associated genes were
involved in the ‘cell cycle’, ‘DNA replication’, ‘oocyte meiosis’,
‘p53 signaling’ and ‘pyrimidine metabolism’ (Fig. 7B).
Mitophagy receptor prediction
The predicted subcellular location of DLGAP5 was
identified in the GeneCards database. DLGAP5 is primarily localized
in the cytosol, cytoskeleton, nuclei and mitochondria (Fig. 7C). Using the iLIR online tool, DLGAP5
was identified to possess a LIR motif, which is conserved in
several mammalian species (41)
(Fig. 7D).
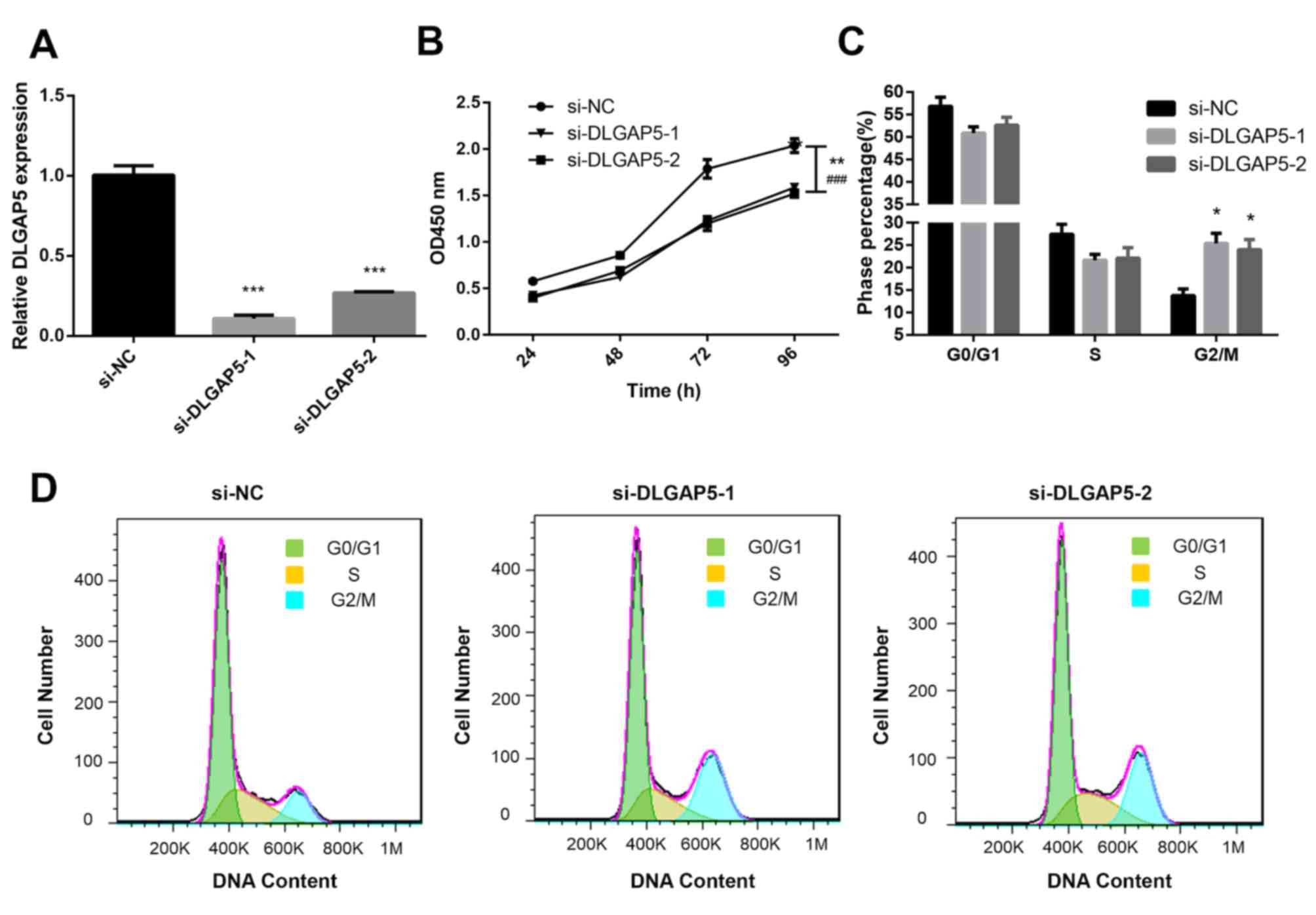
Effects of DLGAP5 on the proliferation
of MDA-MB-231 cells
To further determine the biological functions of
DLGAP5 in BC, the MDA-MB-231 cell line was used. siRNA was
transfected into BC cells to establish DLGAP5-knockdown cells.
RT-qPCR was used to verify the expression levels of DLGAP5 in the
transfected cells. Compared with the si-NC-transfected cells,
DLGAP5 was significantly downregulated in si-DLGAP5-transfected
cells (P<0.001; Fig. 8A). CCK-8
and flow cytometry assays were performed to determine the effects
of DLGAP5 knockdown in cell proliferation. The CCK-8 assay
demonstrated that knockdown of DLGAP5 repressed the proliferation
of MDA-MB-231 cells compared with the si-NC group (P<0.01 at 96
h si-DLGAP5-1 vs. si-NC; P<0.001 at 96 h si-DLGAP5-2 vs. si-NC;
Fig. 8B). Cell cycle analysis
demonstrated that the cells were predominantly distributed in the
G2/M stage in the DLGAP5 knockdown group (si-DLGAP5-1
and si-DLGAP5-2 group, 24.8 and 24.0%, respectively; negative
control group, 13.7%; P<0.05; Fig. 8C
and D).
Discussion
In the present study, bioinformatics analyses were
used to integrate and analyze three BC datasets containing gene
expression profiles, GSE21422, GSE29431 and GSE61304. Using the RRA
package, 85 DEGs were identified, including 40 upregulated and 45
downregulated genes, which were used to investigate the regulatory
mechanisms underlying BC and to generate a PPI network. Using
MCODE, two highly connected networks were identified. The first
network comprised 27 DEGs that were primarily enriched in ‘cell
cycle control’ and ‘p53 signaling and oocyte meiosis’, whereas the
second network comprised 13 DEGs that were mainly enriched in ‘PPAR
signaling’, ‘adipocytokine signaling’, ‘AMPK signaling’ and
‘ECM-receptor interaction’. Using PPI and KEGG pathway enrichment
analysis, various key signaling pathways and hub genes that may
serve important roles in the development of BC were identified. The
identified pathways and hub genes may represent novel targets to be
used in therapy.
In the present study, the STRING database was used
to construct the PPI network, in order to detect the hub genes. The
genes associated with breast cancer prognosis were selected for
further experimentation. Among these 30 hub genes, 28 genes were
indicators of BC prognosis, including DLGAP5. The genes with a high
degree of protein-protein connectivity in the PPI network play a
key role in the network (42).
DLGAP5 was one of the top 10 genes with highest degree of
protein-protein connectivity. DLGAP5, a microtubule-associated
protein, is phosphorylated by Aurora kinase A (AURKA) (43). In addition, DLGAP5 upregulation is
associated with poor prognosis of patients with colorectal cancer
(44), lung cancer (10), bladder cancer (11) and prostate cancer (12), indicating that DLGAP5 plays an
important role in cancer prognosis. Branchi et al (44) reported that DLGAP5 was associated
with the nodal status, and high DLGAP5 expression levels were
associated with a less favorable overall survival rate in distinct
molecular colorectal cancer subtypes. Schneider et al
(45) and Shi et al (46) demonstrated that DLGAP5 was a
promising diagnostic and prognostic biomarker for lung cancer; high
expression levels of DLGAP5 were significantly associated with age,
sex, clinical stage, pathological T stage, new tumor event and
therapeutic outcome. Tagal et al (47) reported that DLGAP5 was
AURKA-dependent and essential for the survival and proliferation of
transcription activator BRG1 (SMARCA4/BRG1) mutant NSCLC cells.
Furthermore, targeting mitosis-associated genes, including DLGAP5,
TPX2 and RAN, has been reported to induce apoptosis of the BRG1
mutant NSCLC cells, both in vitro and in vivo
(47). Espinoza et al
(12) demonstrated that DLGAP5 was a
predictive biomarker for the prognosis of high-risk prostate cancer
in addition to functioning as a resistance factor. Furthermore,
hypoxia-inducible factor 1-alpha (HIF-1α) binding sites were
revealed on the promoter of DLGAP5, indicating that hypoxia plays a
modulatory role on DLGAP5 expression (11). These results were further confirmed
by Yamamoto et al (48).
Eissa et al (11)
demonstrated that DLGAP5 was a reliable and promising biomarker for
the detection of bladder cancer, and sensitivity of urine cytology
was improved when combined with the mRNA expression of DLGAP5. Kim
and Cho (49) reported that DLGAP5
was a stem-cell proliferation biomarker.
The age of the patient, size of the tumor and
molecular biological factors such as ER, PR or Her-2 expression are
notable factors affecting the prognosis of breast cancer (50,51). In
addition, whether the tumor was associated with lymph nodes and
distant metastases also had an important impact on the patient's
prognosis (52). According to the
Surveillance, Epidemiology and End Results Program, patients with
BC with regional lymph node metastasis exhibit a 5-year survival
rate of 85.5%, which is lower compared with the 5-year survival
rate in patients with a localized tumor (98.8%) (53). In addition, late-stage breast cancer
was also associated with a less favorable prognosis (54). To investigate the association between
DLGAP5 expression and the clinicopathological characteristics, the
GOBO database was investigated and IHC analysis was performed in
the present study. The results demonstrated that high DLGAP5
expression levels in BC were associated with the clinical stage and
the lymph node status. These results were consistent with the
online survival analysis and suggested that high DLGAP5 expression
levels were associated with a poor prognosis in patients with
breast cancer.
In the present study, to confirm the biological
function of DLGAP5 in BC, 282 DLGAP5-associated genes were selected
from the GOBO database. KEGG pathway analysis demonstrated that
these genes were mainly involved in ‘cell cycle control’, ‘DNA
replication’, ‘oocyte meiosis’, ‘p53 signaling’ and ‘pyrimidine
metabolism’. A number of these biological processes have been
reported in previous studies. For example, Kuo et al
(55) reported that DLGAP5 knockdown
inhibited the proliferation of hepatocellular carcinoma cells due
to the accumulation of p53 and the downregulation of gankyrin.
Zhang et al (56) reported
that DLGAP5 was a downstream molecule of NUSAP1 and regulated the
cell cycle; in addition, si-NUSAP1 suppressed invasive BC cell
proliferation and invasion and enhanced susceptibility to
epirubicin by affecting the cell cycle progression. In the present
study, a CCK-8 assay and cell cycle analysis were used, and the
results demonstrated that knockdown of DLGAP5 induced cell cycle
arrest at the G2/M phase and inhibited cell
proliferation. The aforementioned processes are important for the
functions of normal and tumorigenic cells, suggesting that further
studies are required to confirm the role of DLGAP5 in
carcinogenesis.
Previously, the clinical significance of autophagy
in cancer has been investigated (57,58).
Mitochondrial autophagy, or mitophagy, is defined as selective
mitochondrial degradation through autophagy (59). Increasing evidence has demonstrated
that abnormal mitophagy is associated with cancer development and
progression and may be associated with an altered response to
cancer therapy (60,61). Meanwhile, mitophagy receptors contain
two key features, the mitochondrial localization and the LIR motif,
which is required to bind the LC3 ligand (41). Given these characteristics, DLGAP5
may be considered a mitophagy receptor. Of note, DLGAP5 has been
reported to be localized in the cytosol, cytoskeleton, nuclei and
mitochondria (13). In the present
study, DLGAP5 was identified to contain a LIR motif. Although the
present results suggested that DLGAP5 may be involved in mitophagy,
further studies are required to define the precise role and
molecular mechanism of DLGAP5 in BC.
The present study was not without limitations.
First, only one cell line was used to detect the role of DLGAP5 in
BC. Secondly, the role of DLGAP5 in mitophagy was only predicted
using online data instead of validation via patient samples. Thus,
further experiments using more cancer cell lines and xenograft
models are required, in order to determine the underlying molecular
mechanisms of DLGAP5 and other hub genes in the prognosis of
BC.
In conclusion, the present study provided evidence
to suggest that DLGAP5 acts as a potential oncogene in breast
cancer. Knockdown of DLGAP5 largely repressed the proliferation of
breast cancer MDA-MB-231 cells and induced cell cycle arrest.
Furthermore, the present results provided novel insight into the
clinical relevance of DLGAP5 as a biomarker for prognosis and its
underlying molecular mechanisms in BC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by The National Natural
Science Foundation of China (grant no. 81802676) and The Wuhan
Youth Cadre Project (grant nos. 2017zqnlxr01 and 2017zqnlxr02).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
TX collected and analyzed the data and was a major
contributor of the manuscript. MD acquired and analyzed the
clinical characteristics of the breast cancer specimens. TX, HL and
RZ performed the experiments. XL designed the present study and
critically revised the manuscript. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Tongji Hospital and written informed consent was
provided by all patients prior to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeSantis CE, Ma J, Gaudet MM, Newman LA,
Miller KD, Goding Sauer A, Jemal A and Siegel RL: Breast cancer
statistics, 2019. CA Cancer J Clin. 69:438–451. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mubarik S, Malik SS, Wang Z, Li C, Fawad M
and Yu C: Recent insights into breast cancer incidence trends among
four Asian countries using age-period-cohort model. Cancer Manag
Res. 11:8145–8155. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tang L, Chen Y, Tang X, Wei D, Xu X and
Yan F: Long noncoding RNA DCST1-AS1 promotes cell proliferation and
metastasis in triple-negative breast cancer by forming a positive
regulatory loop with miR-873-5p and MYC. J Cancer. 11:311–323.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gruosso T, Gigoux M, Manem VSK, Bertos N,
Zuo D, Perlitch I, Saleh SMI, Zhao H, Souleimanova M, Johnson RM,
et al: Spatially distinct tumor immune microenvironments stratify
triple-negative breast cancers. J Clin Invest. 129:1785–1800. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen DQ, Kong XS, Shen XB, Huang MZ, Zheng
JP, Sun J and Xu SH: Identification of differentially expressed
genes and signaling pathways in acute myocardial infarction based
on integrated bioinformatics analysis. Cardiovasc Ther.
2019:84907072019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Võsa U, Kolde R, Vilo J, Metspalu A and
Annilo T: Comprehensive meta-analysis of microRNA expression using
a robust rank aggregation approach. Methods Mol Biol. 1182:361–373.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kolde R, Laur S, Adler P and Vilo J:
Robust rank aggregation for gene list integration and
meta-analysis. Bioinformatics. 28:573–580. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hewit K, Sandilands E, Martinez RS, James
D, Leung HY, Bryant DM, Shanks E and Markert EK: A functional
genomics screen reveals a strong synergistic effect between
docetaxel and the mitotic gene DLGAP5 that is mediated by the
androgen receptor. Cell Death Dis. 9:10692018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Q, Chen Y, Feng H, Zhang B and Wang
H: Prognostic and predictive value of HURP in non-small cell lung
cancer. Oncol Rep. 39:1682–1692. 2018.PubMed/NCBI
|
|
11
|
Eissa S, Matboli M, Mansour A, Mohamed S,
Awad N and Kotb YM: Evaluation of urinary HURP mRNA as a marker for
detection of bladder cancer: Relation to bilharziasis. Med Oncol.
31:8042014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Espinoza I, Sakiyama MJ, Ma T, Fair L,
Zhou X, Hassan M, Zabaleta J and Gomez CR: Hypoxia on the
expression of hepatoma upregulated protein in prostate cancer
cells. Front Oncol. 6:1442016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsou AP, Yang CW, Huang CYF, Yu RC, Lee
YC, Chang CW, Chen BR, Chung YF, Fann MJ, Chi CW, et al:
Identification of a novel cell cycle regulated gene, HURP,
overexpressed in human hepatocellular carcinoma. Oncogene.
22:298–307. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hatfield KJ, Reikvam H and Bruserud Ø:
Identification of a subset of patients with acute myeloid leukemia
characterized by long-term in vitro proliferation and altered cell
cycle regulation of the leukemic cells. Expert Opin Ther Targets.
18:1237–1251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kretschmer C, Sterner-Kock A, Siedentopf
F, Schoenegg W, Schlag PM and Kemmner W: Identification of early
molecular markers for breast cancer. Mol Cancer. 10:152011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aswad L, Yenamandra SP, Ow GS, Grinchuk O,
Ivshina AV and Kuznetsov VA: Genome and transcriptome delineation
of two major oncogenic pathways governing invasive ductal breast
cancer development. Oncotarget. 6:36652–36674. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
R Development Core Team, . R: A Language
and Environment for Statistical Computing, Version 3.0.1
(2013-05-16). R Foundation Statistical Compututing; Vienna,
Austria: 2013, Available online. https://www.R-project.orgNovember 1–2013
|
|
18
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: Limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu S, Zhao R, Shen J, Zhang Y, Shi J, Xu
C, Chen J, Lin R, Han W and Luo D: Integrated bioinformatics
analysis to screen hub genes in the lymph node metastasis of
thyroid cancer. Oncol Lett. 19:1375–1383. 2020.PubMed/NCBI
|
|
20
|
Yang J, Han S, Huang W, Chen T, Liu Y, Pan
S and Li S: A meta-analysis of microRNA expression in liver cancer.
PLoS One. 9:e1145332014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li C, Yin Y, Liu X, Xi X, Xue W and Qu Y:
Non-small cell lung cancer associated microRNA expression
signature: Integrated bioinformatics analysis, validation and
clinical significance. Oncotarget. 8:24564–24578. 2017.PubMed/NCBI
|
|
22
|
Zhang C, Hu X, Qi F, Luo J and Li X:
Identification of CD2, CCL5 and CCR5 as potential therapeutic
target genes for renal interstitial fibrosis. Ann Transl Med.
7:4542019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43:(Database Issue). D447–D452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bader GD and Hogue CW: An automated method
for finding molecular complexes in large protein interaction
networks. BMC Bioinformatics. 4:22003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nagy Á, Lánczky A, Menyhárt O and Győrffy
B: Validation of miRNA prognostic power in hepatocellular carcinoma
using expression data of independent datasets. Sci Rep. 8:92272018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ringnér M, Fredlund E, Häkkinen J, Borg Å
and Staaf J: GOBO: Gene expression-based outcome for breast cancer
online. PLoS One. 6:e179112011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lemler DJ, Lynch ML, Tesfay L, Deng Z,
Paul BT, Wang X, Hegde P, Manz DH, Torti SV and Torti FM: DCYTB is
a predictor of outcome in breast cancer that functions via
iron-independent mechanisms. Breast Cancer Res. 19:252017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Parker JS, Mullins M, Cheang MC, Leung S,
Voduc D, Vickery T, Davies S, Fauron C, He X, Hu Z, et al:
Supervised risk predictor of breast cancer based on intrinsic
subtypes. J Clin Oncol. 27:1160–1167. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Loi S, Haibe-Kains B, Desmedt C, Lallemand
F, Tutt AM, Gillet C, Ellis P, Harris A, Bergh J, Foekens JA, et
al: Definition of clinically distinct molecular subtypes in
estrogen receptor-positive breast carcinomas through genomic grade.
J Clin Oncol. 25:1239–1246. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu
W, Giri DD, Viale A, Olshen AB, Gerald WL and Massagué J: Genes
that mediate breast cancer metastasis to lung. Nature. 436:518–524.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Klijn JG, Zhang Y, Sieuwerts AM,
Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME, Yu
J, et al: Gene-expression profiles to predict distant metastasis of
lymph-node-negative primary breast cancer. Lancet. 365:671–679.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schmidt M, Böhm D, von Törne C, Steiner E,
Puhl A, Pilch H, Lehr HA, Hengstler JG, Kölbl H and Gehrmann M: The
humoral immune system has a key prognostic impact in node-negative
breast cancer. Cancer Res. 68:5405–5413. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Minn AJ, Gupta GP, Padua D, Bos P, Nguyen
DX, Nuyten D, Kreike B, Zhang Y, Wang Y, Ishwaran H, et al: Lung
metastasis genes couple breast tumor size and metastatic spread.
Proc Natl Acad Sci USA. 104:6740–6745. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu S, Jiang X, Li J, Li C, Guo M, Ye F,
Zhang M, Jiao Y and Guo B: Comprehensive analysis of the GATA
transcription factor gene family in breast carcinoma using gene
microarrays, online databases and integrated bioinformatics. Sci
Rep. 9:44672019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee SC, Jain PA, Jethwa SC, Tripathy D and
Yamashita MW: Radiologists' role in breast cancer staging:
Providing key information for clinicians. Radiographics.
34:330–342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stelzer G, Rosen N, Plaschkes I, Zimmerman
S, Twik M, Fishilevich S, Stein TI, Nudel R, Lieder I, Mazor Y, et
al: The genecards suite: From gene data mining to disease genome
sequence analyses. Curr Protoc Bioinformatics. 54:1.30.1–1.30.33.
2016. View
Article : Google Scholar
|
|
39
|
Kalvari I, Tsompanis S, Mulakkal NC,
Osgood R, Johansen T, Nezis IP and Promponas VJ: iLIR: A web
resource for prediction of Atg8-family interacting proteins.
Autophagy. 10:913–925. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jacomin AC, Samavedam S, Promponas V and
Nezis IP: iLIR database: A web resource for LIR motif-containing
proteins in eukaryotes. Autophagy. 12:1945–1953. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang Y, Yao Y, Qiu X, Wang G, Hu Z, Chen
S, Wu Z, Yuan N, Gao H, Wang J, et al: Listeria hijacks host
mitophagy through a novel mitophagy receptor to evade killing. Nat
Immunol. 20:433–446. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jian L and Yang G: Identification of key
genes involved in diabetic peripheral neuropathy progression and
associated with pancreatic cancer. Diabetes Metab Syndr Obes.
13:463–476. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yu CT, Hsu JM, Lee YC, Tsou AP, Chou CK
and Huang CY: Phosphorylation and Stabilization of HURP by
Aurora-A: Implication of HURP as a Transforming Target of Aurora-A.
Mol Cell Biol. 25:5789–5800. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Branchi V, García SA, Radhakrishnan P,
Győrffy B, Hissa B, Schneider M, Reißfelder C and Schölch S:
Prognostic value of DLGAP5 in colorectal cancer. Int J Colorectal
Dis. 34:1455–1465. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schneider MA, Christopoulos P, Muley T,
Warth A, Klingmueller U, Thomas M, Herth FJ, Dienemann H, Mueller
NS, Theis F and Meister M: AURKA, DLGAP5, TPX2, KIF11 and CKAP5:
Five specific mitosis-associated genes correlate with poor
prognosis for non-small cell lung cancer patients. Int J Oncol.
50:365–372. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shi YX, Yin JY, Shen Y, Zhang W, Zhou HH
and Liu ZQ: Genome-scale analysis identifies NEK2, DLGAP5 and ECT2
as promising diagnostic and prognostic biomarkers in human lung
cancer. Sci Rep. 7:80722017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tagal V, Wei S, Zhang W, Brekken RA,
Posner BA, Peyton M, Girard L, Hwang T, Wheeler DA, Minna JD, et
al: SMARCA4-inactivating mutations increase sensitivity to Aurora
kinase A inhibitor VX-680 in non-small cell lung cancers. Nat
Commun. 8:140982017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yamamoto S, Takayama K, Obinata D,
Fujiwara K, Ashikari D, Takahashi S and Inoue S: Identification of
new octamer transcription factor 1-target genes upregulated in
castration-resistant prostate cancer. Cancer Sci. 110:3476–3485.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim D and Cho JY: NQO1 is required for
β-lapachone-mediated downregulation of breast-cancer stem-cell
activity. Int J Mol Sci. 19(pii): E38132018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Freedman RA, Keating NL, Lin NU, Winer EP,
Vaz-Luis I, Lii J, Exman P and Barry WT: Breast cancer-specific
survival by age: Worse outcomes for the oldest patients. Cancer.
124:2184–2191. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Del Prete S, Caraglia M, Luce A, Montella
L, Galizia G, Sperlongano P, Cennamo G, Lieto E, Capasso E,
Fiorentino O, et al: Clinical and pathological factors predictive
of response to neoadjuvant chemotherapy in breast cancer: A single
center experience. Oncol Lett. 18:3873–3879. 2019.PubMed/NCBI
|
|
52
|
Hueman MT, Wang H, Yang CQ, Sheng L,
Henson DE, Schwartz AM and Chen D: Creating prognostic systems for
cancer patients: A demonstration using breast cancer. Cancer Med.
7:3611–3621. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Howlader N, Noone AM, Krapcho M, Miller D,
Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, et al:
SEER Cancer Statistics Review, 1975–2016. National Cancer
Institute; Bethesda, MD, USA:
|
|
54
|
Nechuta S, Lu W, Zheng Y, Cai H, Bao PP,
Gu K, Zheng W and Shu XO: Comorbidities and breast cancer survival:
A report from the Shanghai breast cancer survival study. Breast
Cancer Res Treat. 139:227–235. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kuo TC, Chang PY, Huang SF, Chou CK and
Chao CC: Knockdown of HURP inhibits the proliferation of
hepacellular carcinoma cells via downregulation of gankyrin and
accumulation of p53. Biochem Pharmacol. 83:758–768. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang X, Pan Y, Fu H and Zhang J:
Nucleolar and spindle associated protein 1 (NUSAP1) inhibits cell
proliferation and enhances susceptibility to epirubicin in invasive
breast cancer cells by regulating cyclin D kinase (CDK1) and DLGAP5
expression. Med Sci Monit. 24:8553–8564. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhong Z, Sanchez-Lopez E and Karin M:
Autophagy, inflammation, and immunity: A Troika governing cancer
and its treatment. Cell. 166:288–298. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Rybstein MD, Bravo-San Pedro JM, Kroemer G
and Galluzzi L: The autophagic network and cancer. Nat Cell Biol.
20:243–251. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Palikaras K, Lionaki E and Tavernarakis N:
Coordination of mitophagy and mitochondrial biogenesis during
ageing in C. Elegans. Nature. 521:525–528. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lu H, Li G, Liu L, Feng L, Wang X and Jin
H: Regulation and function of mitophagy in development and cancer.
Autophagy. 9:1720–1736. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chourasia AH and Macleod KF: Tumor
suppressor functions of BNIP3 and mitophagy. Autophagy.
11:1937–1938. 2015. View Article : Google Scholar : PubMed/NCBI
|