Introduction
Osteosarcoma (OS) is one of the most common and
destructive primary bone malignancies, with an annual incidence of
~5 cases per 1,000,000 individuals worldwide (1,2). The
incidence of OS exhibits bimodal distribution, with the primary
peak occurring in adolescence, while the incidence of OS in the
elderly is relatively low (3,4). OS
originates from mesenchymal tissues and is characterized by
malignant osteogenesis and osteoblast differentiation, which
confers a high degree of malignancy and strong metastatic ability.
The incidence of common pulmonary metastasis through the blood is
currently >85%, and due to therapeutic limitations in previous
years, amputation was the principal method of treatment for OS.
However, the survival rate of patients treated by surgery alone is
15–17%, and the appearance and function of the limbs after
amputation can negatively impact patient welfare (5). However, with rapid developments in
medicine and technology, limb-salvage surgery following OS has
become possible. To inhibit the growth and metastasis of tumors, OS
treatment has progressed from surgery alone to a combination of
surgery and adjuvant chemotherapy (6). However, the overall survival rate of
patients with OS remains unsatisfactory, with ~50% developing fatal
lung metastases in the advanced stages of disease (7). Due to the limitations of current
diagnostic techniques, only 15–20% of patients with metastases can
be diagnosed by auxiliary examination, thus metastasis remains the
leading mortality-associated factor for patients with OS (8). Therefore, to identify novel biomarkers
and therapeutic targets, it is of great significance to study the
mechanisms of OS occurrence and metastasis at the molecular
level.
Bioinformatics tools are widely used to explore
genes involved in the development of OS. Using differential gene
expression analysis, Xie et al (9) identified ZNRD1, GPR68, CAT, FUT3, ANPEP
and CDK1 as key genes associated with chemotherapy resistance in
OS. Analyzing the DNA methylation profiles of OS, Chen et al
(10) revealed that the abnormal
methylation of NMU and NMUR1 may contribute to the development of
OS. Furthermore, based on integrated bioinformatics analysis, Wang
et al (11) showed that
microRNA-203 may be a suppressor of OS. Through WGCNA, Tian et
al (12) showed that IGFBP5,
IGFBP6, WISP3, and MYL2 which involved in insulin-like growth
factor binding may play key roles in the metastasis of
osteosarcoma. Similarly, through WGCNA analysis, Wang et al
(13) indicated that MEPE, BPIFB1,
HBA2, and SERPINB3 were key genes involved in the metastasis of
osteosarcoma. However, the majority of these findings were only
obtained using prediction tools, and lacked experimental
validation.
Mitogen-activated protein kinases (MAPKs) are a
class of serine/threonine protein kinases expressed in almost all
cell types, which regulate evolutionarily conserved signal
transduction pathways and various cellular functions, including
proliferation, migration, apoptosis and differentiation (14). Abnormal MAPK signaling plays a key
role in the occurrence and development of different types of cancer
(15). MAPK15 is the most recently
identified member of the MAPK family (16), and it is widely accepted to be
upregulated in a variety of cancer types. Studies have shown that
unlike other members of the MAPK family, the phosphorylation of
MAPK15 is mainly self-phosphorylation, so its total protein level
is positively correlated with the phosphorylation level (17,18).
Additionally, the overexpression of MAPK15 promoted gastric cancer
cell proliferation by stabilizing the expression of c-Jun (19). MAPK15 is also regarded as an oncogene
in male germ cell tumors (20), and
was revealed as a core gene involved in the radio-resistance of
nasopharyngeal carcinoma cells (21). However, there are few studies focused
on the relationship between phosphorylation location and protein
activity of MAPK15. Similarly, the role of MAPK15 in OS remains
unknown.
In present study, a combination of bioinformatics
and related experimental methods were used to identify
metastasis-associated genes in OS. The present study revealed that
MAPK15 may be a novel biomarker for the diagnosis of OS, as well as
an effective target for clinical treatment.
Materials and methods
Data source
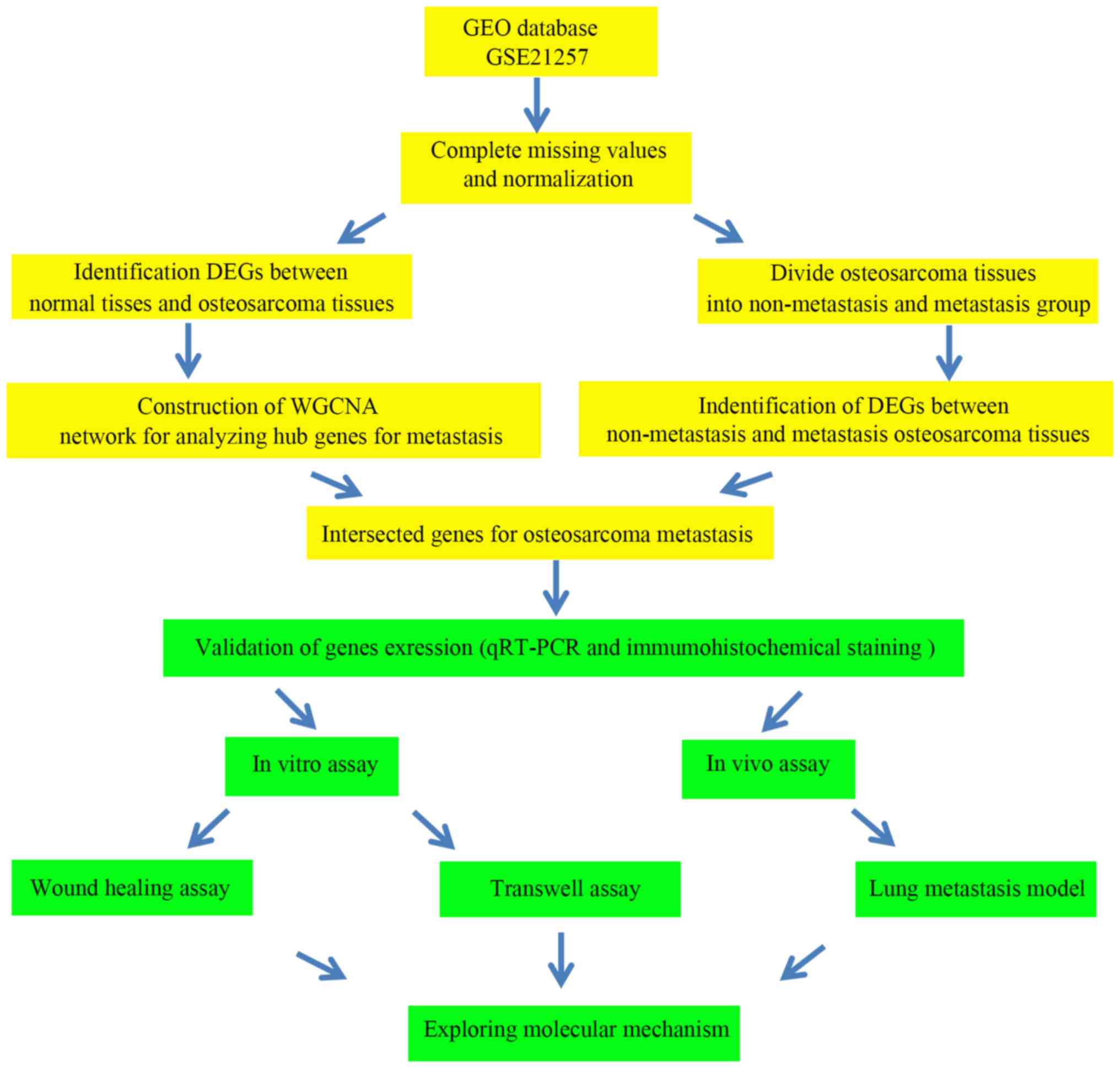
The analysis and experimental procedures of the
present study are outlined in Fig.
1. To identify key genes associated with the metastasis of OS,
human gene expression data from OS tissues (dataset GSE87624) were
downloaded from the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/), and subsequently
used to perform WGCNA and DEG analysis. The GSE87624 dataset,
comprising 44 human patient OS samples and 3 normal bone samples,
was first published by Scott et al (22).
Identification of DEGs
EdgeR is a Bioconductor software package (version
number: 3.9; http://www.bioconductor.org/packages/release/bioc/html/edgeR.html)
with the capacity to determine the differential expression of genes
(23). Therefore, in the present
study, the level of differential gene expression between OS and
normal bone tissues was assessed using the EdgeR package
(version number: 3.9; http://www.bioconductor.org/packages/release/bioc/html/edgeR.html)
in R. The DEGs between metastatic and non-metastatic OS tissues
were also analyzed. An over-dispersed Poisson model was used to
explain biological and technological variability, and the Empirical
Bayesian method was used to mitigate the excessive dispersion of
transcription and improve the reliability of reasoning. A
combination of adjusted P<0.05 and |log2 fold change (FC)|≥1 was
used as the stringent cutoff to determine the significance of the
differences in gene expression. Then, WGCNA was conducted to
determine the DEGs between normal bone and OS tissues.
WGCNA
In the present study, the R package WGCNA
(v.1.66; http://cran.r-project.org/web/packages/WGCNA/index.html)
was employed to conduct WGCNA for all DEGs between normal bone and
OS tissues. First, the DEG expression profiles and their associated
clinical information were imported. Pearson's correlation analysis
was then performed to cluster the samples and detect outliers; the
outlier threshold was set as 10,000. Then, all genes-pairs were
analyzed using Pearson's correlation analysis and a matrix of
similarity was constructed. In addition, to identify specific
modules, WGCNA uses a soft-thresholding procedure to avoid the
selection of an arbitrary cut-off. The β value represented a
soft-thresholding parameter that could emphasize strong
correlations between genes and penalize weak correlations to ensure
a scale-free network. The network connectivity (k) of the gene was
defined as the sum of its adjacency with all the other genes for
network generation. The decision value of the threshold power was
determined on the basis of the scale-free topology criterion, which
aims to mimic a network structure commonly found in nature. In the
present study, β=3 was selected based on the scale-free topology
criterion >0.85. The cutreeDynamic function was used for
adaptive branch pruning of hierarchical clustering dendrograms and
the dynamicTreeCut package (v.1.66; http://cran.r-project.org/web/packages/WGCNA/index.html)
was adopted to generate co-expression modules. Subsequently, to
further analyze the gene modules, the dissimilarity of the module
eigengenes (ME) was calculated using the moduleEigengenes function
in the R WGCNA package, which was defined as the first principal
component of a given module and considered to be representative of
the gene expression profiles in a module. A cut-off line for the
module dendrogram was selected and the module was merged.
Eventually, the adjacency was converted into a topological overlap
matrix (TOM), and modules were subjected to hierarchical cluster
analysis according to the TOM-based dissimilarity measure with the
mini-size set as 20.
Screening of metastasis-related hub
genes
The correlation between the co-expression modules
and the metastasis of OS was analyzed. Firstly, gene significance
(GS) was defined as the log10 transformation of the corresponding
P-value (GS=lgP) of the correlation between gene expression and
pathological stage. Secondly, module eigengenes (MEs) were
determined by principal component analysis and selected to
represent the mean measure of the overall co-expression module.
Finally, the degree of correlation between MEs and clinical traits
was determined using Pearson's correlation analysis. Significant
modules were identified according to the absolute value of moderate
intensity correlation (>0.3), with a significance threshold of
P<0.05. All genes in the significant modules related to the
significance of metastatic traits were screened to identify the
core genes, according to GS>0.2 and MM>0.8. To obtain
credible metastasis-related hub genes, Bioinformatics and
Evolutionary Genomics (http://bioinformatics.psb.ugent.be/webtools/Venn/) was
used to identify genes which were not only hub genes in the
metastasis-associated gene modules in WGCNA, but also
differentially expressed genes in differential expression analysis.
The metastasis-related hub genes were then employed for
experimental evaluation.
Tissue ethics
All OS tissues used in the current study were
obtained from the Zhujiang Hospital affiliated South Medical
University (Guangzhou, China) between July 2014 and September 2019.
Patients were enrolled using the advanced procedures if they met
the following criteria: i) The tissues were obtained from operation
and two pathologists diagnosed as osteosarcoma; ii) patients
diagnosed and treated for the first time; and iii) patients willing
to participate. The exclusion criteria were as follows: i) Patients
complicated with other malignancies; ii) patients with other
systemic disease; iii) patients received treatment prior to
admission; and iv) patients and/or their families refused to
participate. A total of 26 pairs of OS and adjacent non-tumor
tissues were obtained from extremities of OS patients, of which 11
pairs were obtained from patients without, and 15 pairs were
provided from patients with OS metastasis at diagnosis. The
procedures of the current study were approved by the Clinical
Ethics Management Committee of Zhujiang Hospital, South Medical
University, and all patients provided informed consent in
writing.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was extracted from OS tissues using
TRIzol® reagent (Takara Bio, Inc.), and an ultraviolet
spectrophotometer (Bio-Rad Laboratories, Inc.) was used to assess
the concentration and quality; samples with OD260/OD280 of 1.8–2.1
were considered to qualify for subsequent experimentation. cDNA
synthesis was conducted using the 1st Strand cDNA Synthesis kit
(Shanghai Yeasen Biotech Co., Ltd.,) according to the
manufacturer's instructions, and HieffTM qPCR SYBR®
Green Master Mix (Shanghai Yeasen Biotech Co., Ltd.,) was used to
performed RTq-PCR. The thermocycling conditions were as follows:
95°C for 5 min, 40 cycles of 95°C for 30 sec, annealing at 60°C for
30 sec, and a final elongation step at 72°C for 30 sec. The
2−ΔΔCt method (24) was
employed to measure the relative expression levels of target genes,
and GAPDH was used as the loading control. The primers used in the
present study were as follows: Deleted in lung and esophageal
cancer protein 1 (DLEC1) forward, 5′-CCAAAACGCGGAGGTCTTTAG-3′ and
reverse, 5′-GGGAGGAATACAAGGAGGACT-3′; forkhead box J1 (FOXJ1)
forward, 5′-GCCTCCCTACTCGTATGCCA-3′ and reverse,
5′-GCCGACAGGGTGATCTTGG-3′; MAPK15 forward,
5′-GGGCCTATGGCATTGTGTG-3′ and reverse,
5′-TCTCTGGGCATCTGTCTTATCC-3′; and GAPDH forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′.
Immunohistochemical staining
(IHC)
The OS tissues were dehydrated and embedded in
paraffin (Wuhan Servicebio Technology Co., Ltd.); the tissues were
subsequently cut into 4-µm slices for use in the present study,
where they were deparaffinized using xylene and rehydrated in a
descending alcohol series under room temperature. After restoration
with sodium citrate the samples were treated with 3%
H2O2 to block endogenous peroxidase activity,
and then blocked using 5% bovine serum albumin (BSA; Servicebio,
Wuhan, China) for 30 min under room temperature. The specimens were
subsequently incubated with a primary anti-MAPK15 antibody
(dilution: 1:400; cat. no. ab137619; Abcam, USA) for 12 h at 4°C,
followed by a second incubation with horseradish
peroxidase-conjugated secondary antibodies (dilution: 1:200; cat.
no. G1210-2-A-100; Wuhan Servicebio Technology Co., Ltd.) for 2 h
at room temperature. After subsequent development using the Cell
and Tissue Staining HRP-DAB kit (Beyond) according to the
manufacturer's protocol, images were captured with an orthophoto
microscope (magnification, ×400).
Cell culture and transfection
The human OS MG63 and 143B cell lines were purchased
from the American Type Culture Collection. Cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc.) supplemented with 100 U/ml penicillin and 100
µg/ml streptomycin (Wuhan Boster Biological Technology, Ltd.,), and
10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.,) at 37°C with 5% CO2. Human MAPK15-targeting short
hairpin RNA (shRNA) and the corresponding scramble shRNA
oligonucleotide sequences were cloned into the pSuper-retro-puro
vector to generate pSuper-retro-MAPK15-RNAi(s) and
pSuper-retro-scramble-RNAi(s). The shRNA sequences were as follows:
scramble shRNA,
5′-CCGGAATTCTCCGAACGTGTCACGTCTCGAGACGTGACACGTTCGGAGAATTTTTTTG-3′;
and MAPK15 shRNA,
5′-CCGGGCTTGGAGGCTACTCCCCTCGAGGGGAGTAGCCTCCAAGCTTTTTG-3′. In order
to obtain cell lines that stably exhibited low-level MAPK15
expression (MAPK15-knockdown), the cells were treated with
puromycin (0.5 µg/ml) for 12 days following transfection. MAPK15
(NM_139021.3) was used as backbone and subcloned into pcDNA3.1
vector to construct MAPK15 overexpression plasmid (Guangzhou
GeneCopoeia Co., Ltd.). The transfection processes of MAPK15
overexpression plasmid or pcDNA3.1 empty vector were preformed
using Lipo2000 (Invitrogen; Thermo Fisher Scientific, Inc), and
cells transfected pcDNA3.1 empty vector were seted as negative
control (NC).
Wound-healing assay
After transfection, total 1×106 MG63 and
143B cells were plated into per well of 6-well plates and incubated
at 37°C for 24 h. At 100% confluence, the cells were serum starved
for 24 h. Then, the cell monolayer was then scraped with a 200-µl
sterile pipette tip to form a central linear wound. After washed by
PBS for three times, cells were treated with serum free DMEM medium
with or without a c-Jun inhibitor SP600125 (MCE) and cultured at
37°C with 5% CO2. Then, the cells were photographed
under an optical microscope (magnification, ×40) and the wound
closure rate was recorded after 24 h. At 0 h, the wound was
regarded as 100% of the average clearance. The relative migration
rate was calculated as follows: Relative migration
rate=(Streat-0 h -Streat-24 h)/(SNC-0
h-SNC-24 h) ×100%, where Streat-0 h and
Streat-24 h was the area of the scratch at 0 h and 24 h
in the treatment group, SNC-0 h and SNC-24 h
was the area of the scratch at 0 h and 24 h in the NC group.
Transwell invasion assay
A total of 2×104 MG63 and 143B cells were
resuspended in serum-free DMEM and placed into the upper chamber of
a Transwell insert (Invitrogen; Thermo Fisher Scientific, Inc.)
with 8-µm pores, which had been pre-coated with Matrigel at 37°C
(Becton, Dickinson and Company); DMEM containing 10% FBS was placed
in the lower chamber as a chemical attractant, and the inserts were
incubated at 37°C (5% CO2) for 24 h. After removing the
non-invasive cells on the top of the membrane, the cells were fixed
with 4% paraformaldehyde for 15 min, stained with 0.5% crystal
violet for a further 30 min, counted and photographed under an
optical microscope equipped with a digital camera (magnification,
×40). The number of invasive cells was counted in five random
fields per sample.
In vivo lung metastasis model
A total of 10 4-week-old female nude mice (Beijing
HuaFukang Biological Technology Co. Ltd) were housed in a facility
at 23–24°C, and the light-dark cycle was set at 12-h intervals.
After one week of adaptive feeding, mice were randomly divided into
the sh-scramble (n=5) and sh-MAPK groups (n=5). A total of
1×107 MAPK15-knockdown 143B cells and the corresponding
control 143B cells were injected into the tail veins of the mice in
the corresponding groups. Animal health and behavior after
injecting were monitored each day. While the mouse had the features
of hard breathe and limitation of motion, the mouse was sacrificed
in order to reduce animal suffering. When half of mice in any group
(n≥3) was sacrificed due to the above reasons, this experiment
should be ended. Cervical dislocation was performed manually in all
mice and resulted in euthanasia within ~10 sec in 5 weeks
post-injection, and the death of mice was verified by the absence
of a heart beat and the onset of rigor mortis. Ensuring the death
of mice, the lungs were removed and the tissues were fixed in 4%
paraformaldehyde for 30 min under room temperature, and
subsequently stained with hematoxylin and eosin prior for 1 min
under room temperature to image capture. The animal care and
experimental procedures used in the present study were approved by
the Institutional Animal Care and Use Committee of the Southern
medical university. In addition, all mouse procedures, euthanasia
and surgery, including injections of 143B cells, were conducted
painlessly or under anesthesia using a combination of hydrochloric
acid medetonidine 0.3 mg/kg + midazolam 4 mg/kg + butorphanol
tartrate 5 mg/kg through tail vein injection (25). In the present study, the success of
anesthesia is evaluated as the decrease of respiratory rate, the
increase of respiratory depth and the disappearance of eyelid and
corneal reflexes.
Western blot analysis
The samples were homogenized in RIPA (including 1%
PMSF) to extract the total protein, according to the manufacturer's
instructions. Equal amounts of protein (25 µg) were loaded into
each lane of a 10% gel, separated by SDS-PAGE and transferred onto
PVDF membranes (Merck KGaA). The membranes were then blocked with
5% non-fat milk (Wuhan Servicebio Technology Co., Ltd.,) at room
temperature for 2 h, and incubated overnight at 4°C with primary
antibodies (dilution: 1:1000) against GAPDH (cat. no. 60004-1-Ig;
ProteinTech Group, Inc.), MAPK15 (cat. no. 13452-2-AP; ProteinTech
Group, Inc.), c-Jun (cat. no. 66313-1-Ig; ProteinTech Group, Inc.),
p-c-Jun (cat. no. 5464; Cell Signaling Technology, Inc.), MMP-9
(cat. no. 10375-2-AP; ProteinTech Group, Inc.,) and MMP-2 (cat. no.
10373-2-AP; ProteinTech Group, Inc.). The membranes were washed
three times with TBST and incubated with a horseradish
peroxidase-conjugated goat anti-mouse or goat anti-rabbit IgG
secondary antibody (dilution: 1:3000; Wuhan Servicebio Technology
Co., Ltd.). The protein bands were visualized with an enhanced
chemiluminescent (ECL) kit (Wuhan Servicebio Technology Co., Ltd.)
using Bio-rad Chemidoc image software (v.5.2.1; Bio-Rad
Laboratories, Inc.). In the present study, GAPDH was used as a
reference gene for normalizing the expression of MAPK15, MMP2 and
MMP9, while relative expression of p-C-Jun was calculated according
to formula (p-c-Jun/GAPDH)/(total c-Jun/GAPDH).
Statistical analysis
All statistical analyses were carried out using
GraphPad Prism (v.5.0; GraphPad Software, Inc.). One-way analysis
of variance and the LSD-t test were performed to analyze the
statistical differences between≥3 groups, while the independent
sample t-test was used to detect differences between two groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Co-expression module construction by
WGCNA
Prior to the construction of a co-expression module,
DEG analysis was performed for normal bone and OS tissue data
retrieved from the GSE87624 dataset, and 1,043 DEGs were identified
(Table SI). After removing outlier
samples using cluster analysis, the DEGs and corresponding clinical
information of 32 OS tissue were used to construct a co-expression
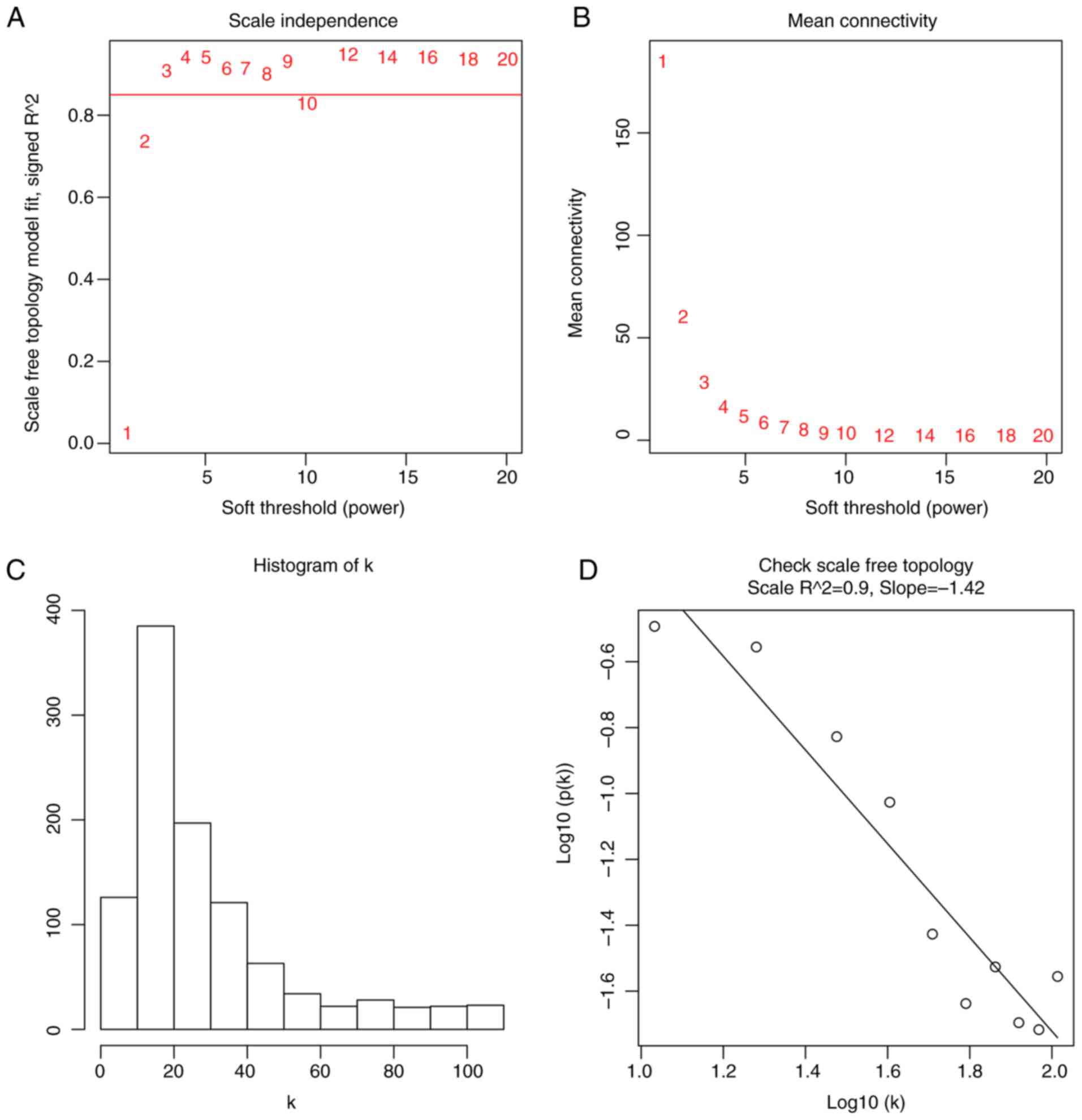
module by WGCNA (Fig. S1). When the
soft power β was set as 3, the scale independence of the topology
network reached higher than 0.85 (Fig.
2A) with the mean connectivity lower than 50 (Fig. 2B). So a soft power of β=3 was
selected as the soft threshold for performing subsequent analyses.
The result showed that when β=3, the topological overlap matrix was
able to meet the scale-free topology criterion with
R2=0.9 (Fig. 2C and D).
The dynamic tree cut function was used to prune the branches in
hierarchical clustering dendrograms that determined the generation
of the co-expression modules. Then, the MEs were calculated by the
moduleEigengenes function to quantify the co-expression similarity
of the modules and the clustered modules were merged based on the
similarity. A total of 16 co-expressed modules were identified,
while unclustered genes were distributed in the grey module
(Fig. 3). The 16 co-expression
modules were then used for subsequent analysis.
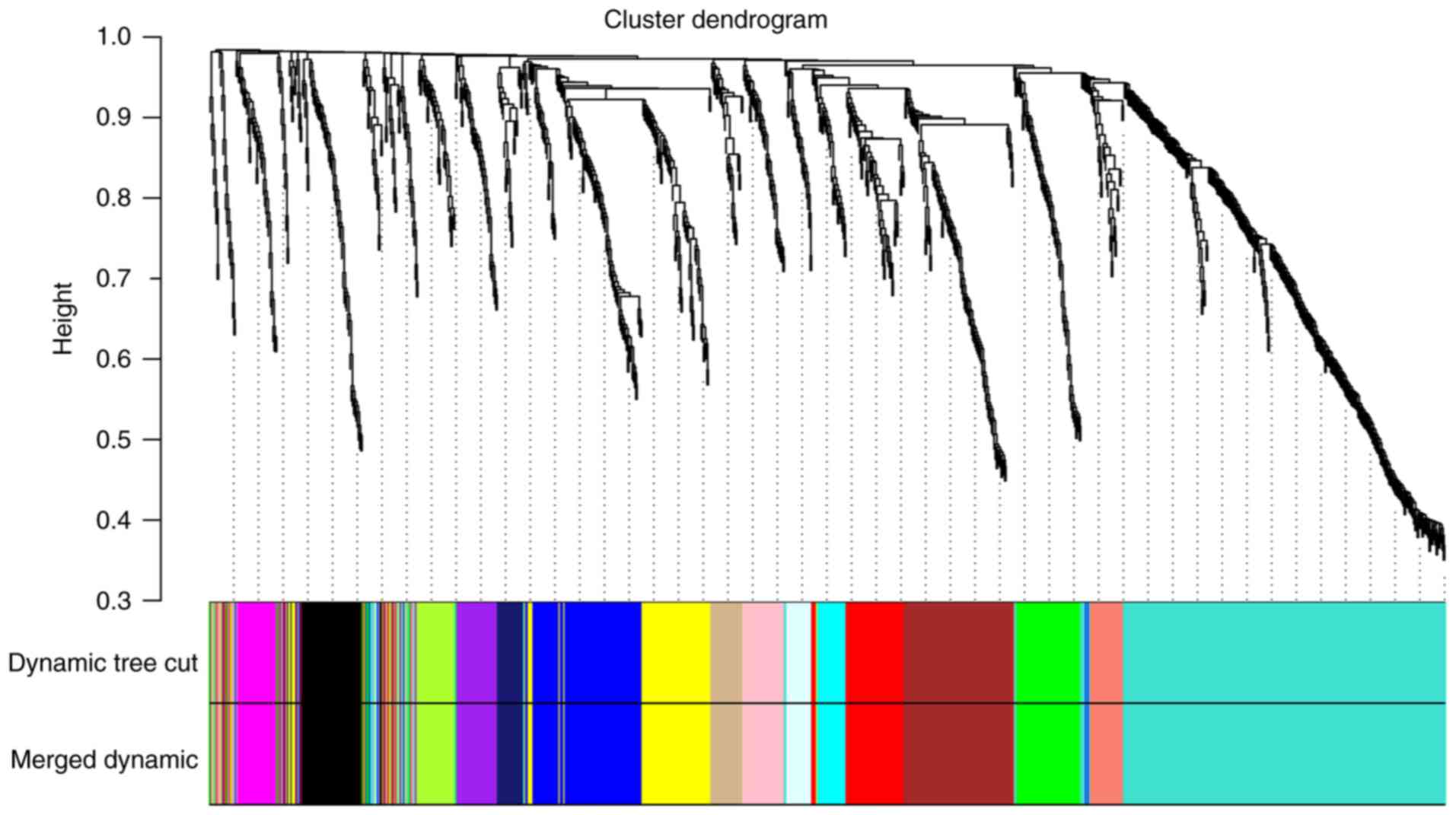 | Figure 3.Clustering dendrograms of all DEGs
between normal bone tissues and osteosarcoma tissues, with
dissimilarity based on topological overlap, together with assigned
module colors. Dynamic tree cut algorithm was applied to the
dendrogram for module identification, and the closed modules
(difference of feature vectors <0.2) were merged into new
modules. Different colors represent different gene modules and
there are 16 co-expressed modules in the weighted gene
co-expression network analysis network, including tan, black,
magenta, midnight blue, salmon, pink, green, turquoise, purple,
light cyan, brown, cyan, red, green yellow, blue and yellow. DEG,
differentially expressed gene. |
Identification of
metastasis-associated modules and screening of metastasis-related
hub genes
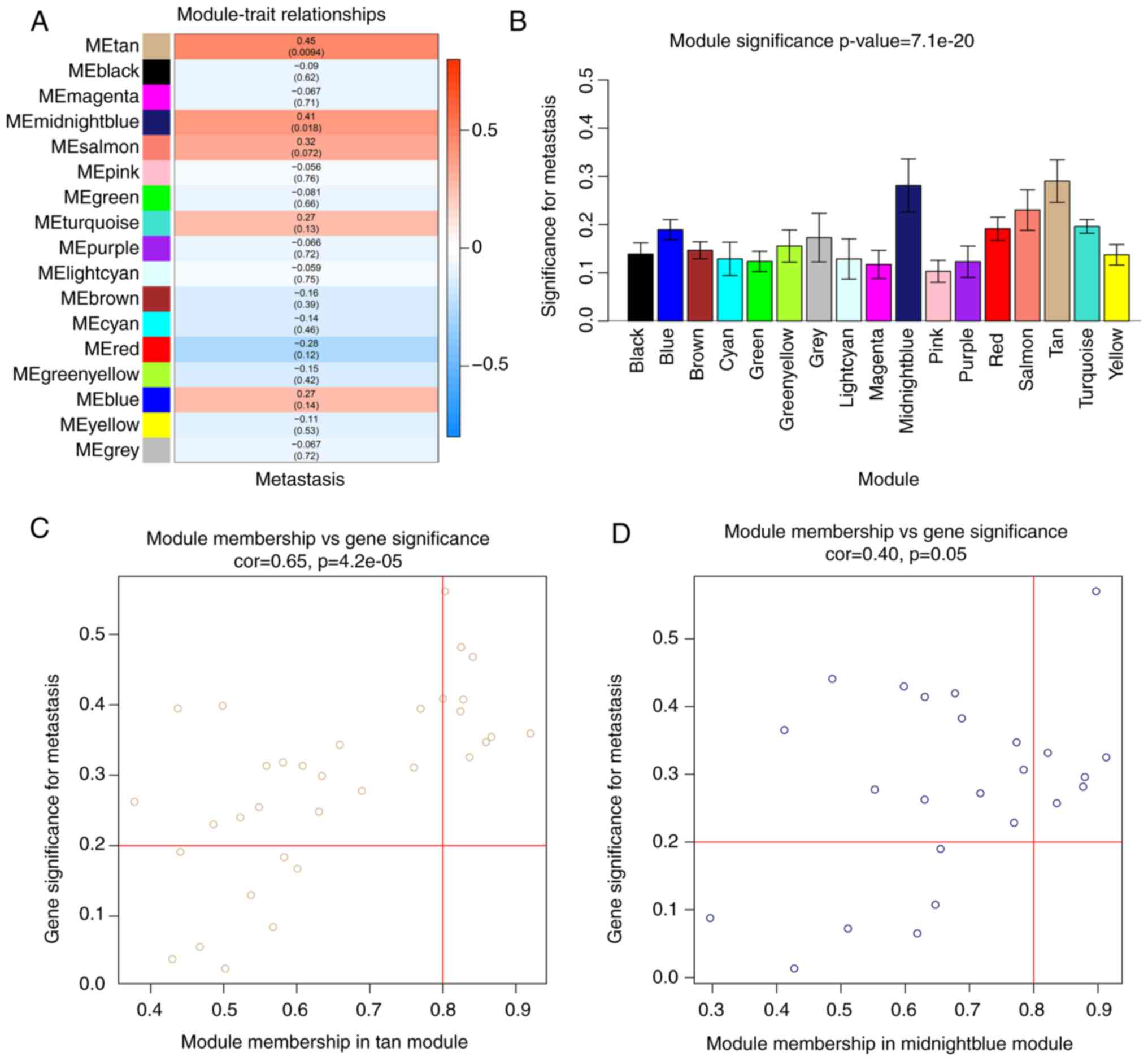
Using WGCNA, the relationship between modules and
tumor metastasis was investigated, and the MEtan (cor=0.45;
P=0.0094) and MEmidnightblue modules (cor=0.41; P=0.018) (Fig. 4A and B), containing 33 and 24 genes,
respectively, were found to be associated with OS metastasis. All
significantly associated genes were screened according to GS>0.2
and MM>0.8 and ultimately, 16 module core genes were obtained
from the metastasis-related modules, including PRR15, PLEKHH1,
MIR200A, OVOL1, PCSK6, MIR200B, RHOV, TRPM1, MAPK15, DLEC1, GAGE2A,
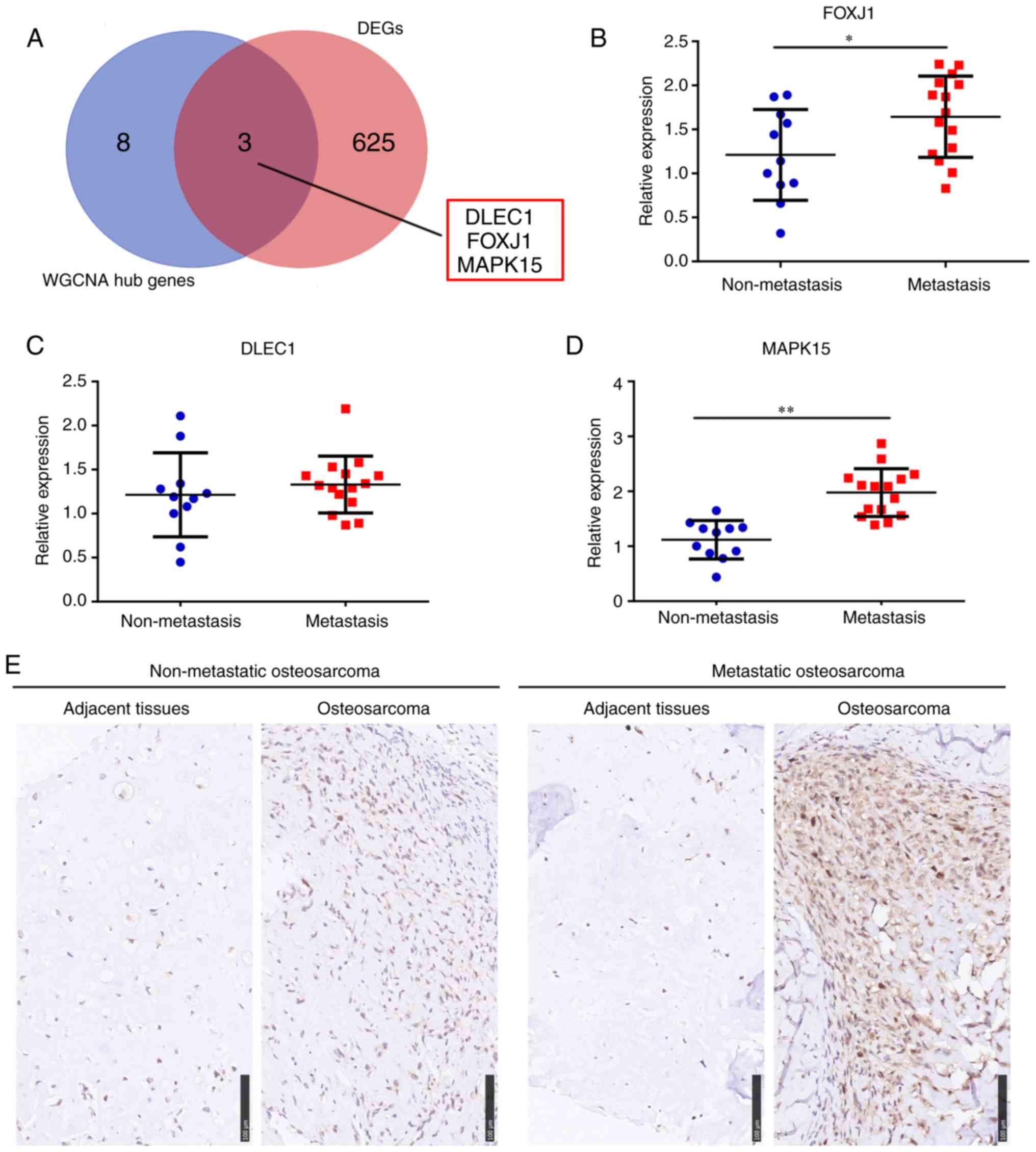
FOXJ1, FOXA3, PAGE1, C7orf57 and GZMB (Fig. 4C and D). DEG analysis was also
performed between metastatic and non-metastatic OS tissue data and
628 DEGs were identified (Table
SII). The intersection of the core genes in the metastasis
related-modules, and DEGs between metastatic and non-metastatic OS
tissues was then determined. The expression change fold of 16
module core genes obtained from the metastasis-related modules
between metastatic and non-metastatic OS tissues were also showed
in Table I. According to the
significance difference of FDR<0.05, the results indicated
DLEC1, FOXJ1 and MAPK15 as core genes in the metastasis
related-modules, which were also highly expressed in metastatic OS
tissues (Fig. 5A).
 | Table I.Expression change of 16 module core
genes obtained from the metastasis-related modules between
metastatic and non-metastatic osteosarcoma tissues. |
Table I.
Expression change of 16 module core
genes obtained from the metastasis-related modules between
metastatic and non-metastatic osteosarcoma tissues.
| Gene | logFC | FDR |
|---|
| FOXJ1 | 4.35 | <0.01 |
| MAPK15 | 2.44 | <0.01 |
| DLEC1 | 1.78 | 0.01 |
| RHOV | 1.36 | 0.07 |
| GZMB | 1.74 | 0.10 |
| OVOL1 | 1.69 | 0.12 |
| GAGE2A | 3.43 | 0.17 |
| MIR200B | 2.48 | 0.21 |
| PRR15 | 1.52 | 0.21 |
| FOXA3 | 1.65 | 0.23 |
| PAGE1 | 2.21 | 0.26 |
| TRPM1 | 1.45 | 0.28 |
| MIR200A | 1.98 | 0.30 |
| C7orf57 | 1.08 | 0.32 |
| PCSK6 | 1.07 | 0.34 |
| PLEKHH1 | 0.82 | 0.38 |
Validating the expression of DLEC1,
FOXJ1 and MAPK15 in OS tissues
To verify that the DLEC1, FOXJ1 and MAPK15 hub genes
were associated with OS metastasis, RT-qPCR was performed to
quantify the expression of these genes in OS tissues from 26
patients. The results revealed that the mRNA expression levels of
MAPK15 and FOXJ1, but not DLEC1, were significantly increased in
the OS tissues of patients who exhibited tumor metastasis at
diagnosis (Fig. 5B-D); the increased
expression level was most significant for MAPK15. Therefore, IHC
was also performed and the results showed that the MAPK15 protein
was also highly expressed in OS tissues from patients with
metastasis at diagnosis (Fig.
5E).
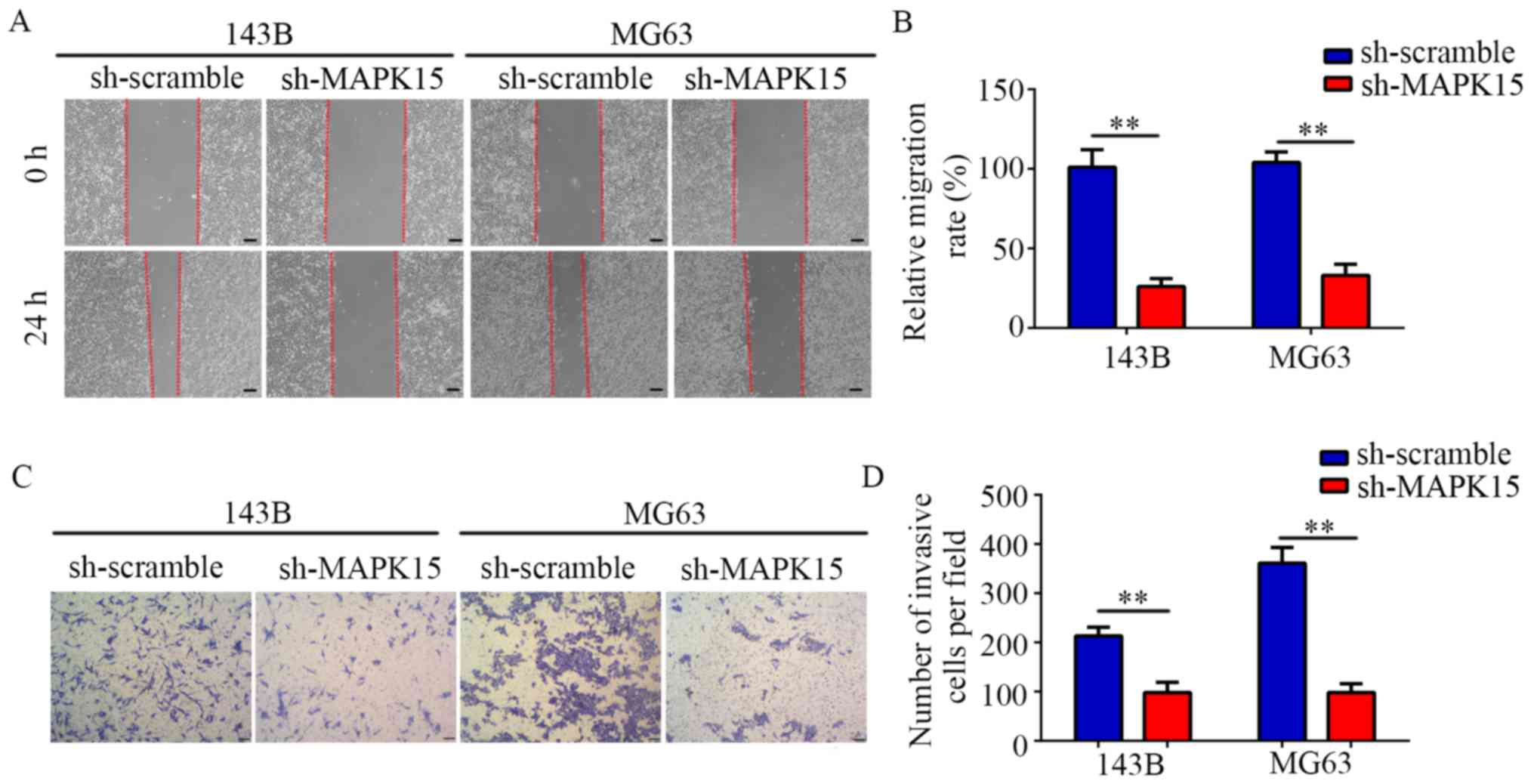
MAPK15-knockdown suppresses OS cell
migration and invasion in vitro
Targeting MAPK15 shRNAs were used to construct MG63
and 143B cell lines stably expression low levels of MAPK15
(MAPK15-knockdown). The results of the wound-healing assays showed
that the migration rate of the sh-MAPK15 group was significantly
decreased compared with that of the sh-scramble group (Fig. 6A and B). Furthermore, the results of
the Transwell assays demonstrated that inhibiting the expression of
MAPK15 decreased the number of invasive MG63 and 143B cells per
field (Fig. 6C and D). Collectively,
these results suggest that MAPK15-knockdown reduces the migratory
and invasive abilities of OS cells.
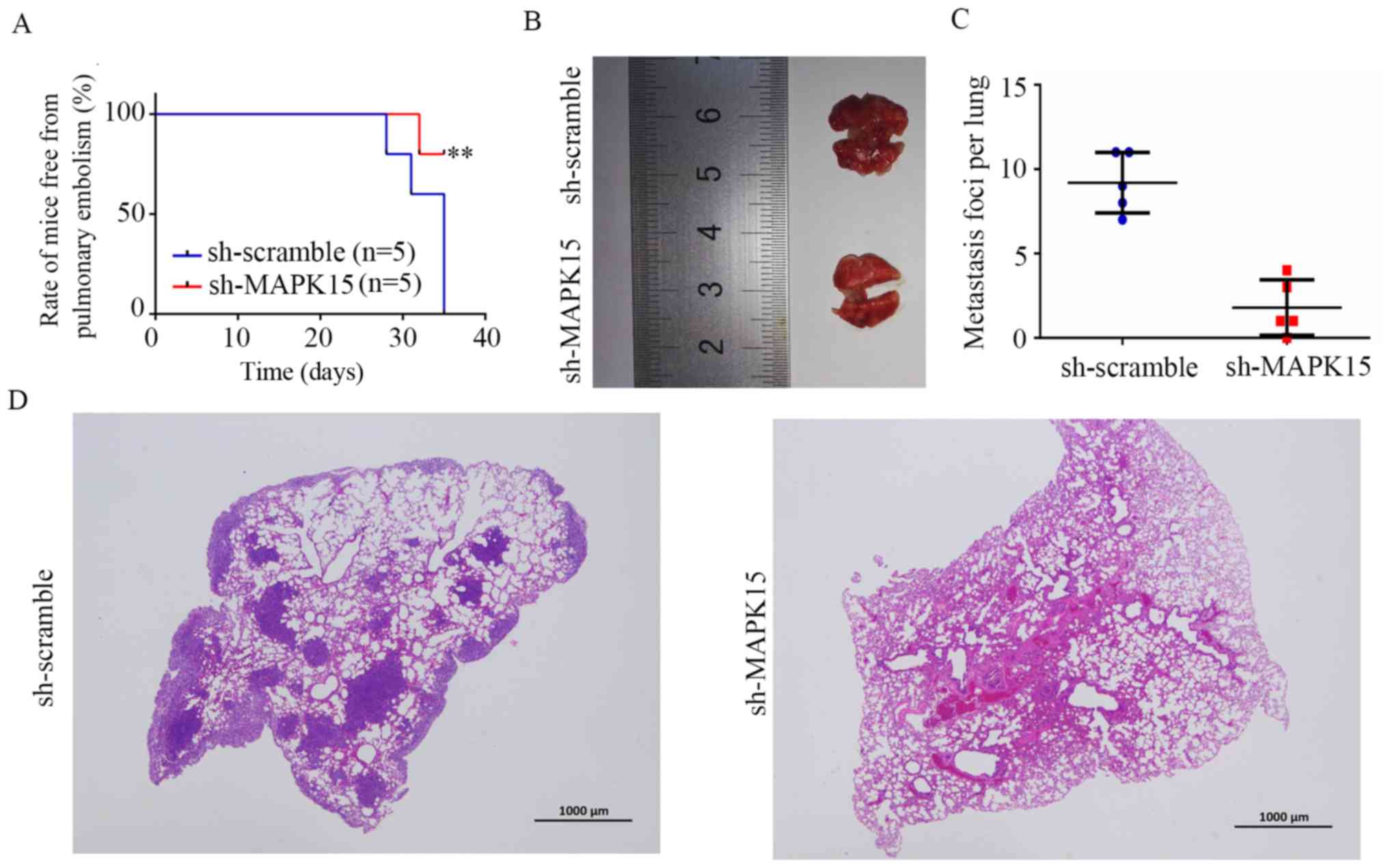
MAPK15 inhibition decreases the
metastasis of 143B-cell tumors in vivo
Studies have shown that about 15–20% of patients
have evidence of metastases at diagnosis, mostly in the lungs
(26,27). Therefore, A mouse model of lung
metastasis was used to detect the effect of MAPK15 inhibition on
the metastasis of OS cells in vivo. 143B cells transfected
with sh-MAPK15 and sh-scramble lentivirus were injected into the
tail vein of 4-week-old female nude mice. The time of mice in
sh-scramble and sh-MAPK15 group free from pulmonary embolism
induced breathing and movement difficultly was recorded (Fig. 7A). The results of lung tissues
diagram (Fig. 7B and C) and HE stain
(Fig. 7D) both indicated that
metastasis was significantly decreased in the lungs of mice
inoculated with MAPK15-knockdown 143B cells, suggesting that MAPK15
promotes the metastatic potential of OS.
MAPK15 significantly regulated the
c-Jun/MMPs pathway in OS cells
Previous studies have indicated that MAPK15
increases the phosphorylation level of c-Jun and activated Jun, and
therefore the levels of c-Jun and p-c-Jun were detected after
MAPK15 inhibition or overexpression. The results showed that
following MAPK15 inhibition, the expression of p-c-Jun was
significantly decreased in both MG63 and 143B cells. Then, the
expression of MMP2 and MMP9, downstream proteins of c-Jun, were
also detected, revealing that both MMP2 and MMP9 expression was
significantly decreased following MAPK15-knockdown (Fig. 8A and B). Similarly, we found that
p-C-Jun was significantly increased while MAPK15 overexpression, as
well as increasing the expression of MMP2 and MMP-9 (Fig. 8C and D).
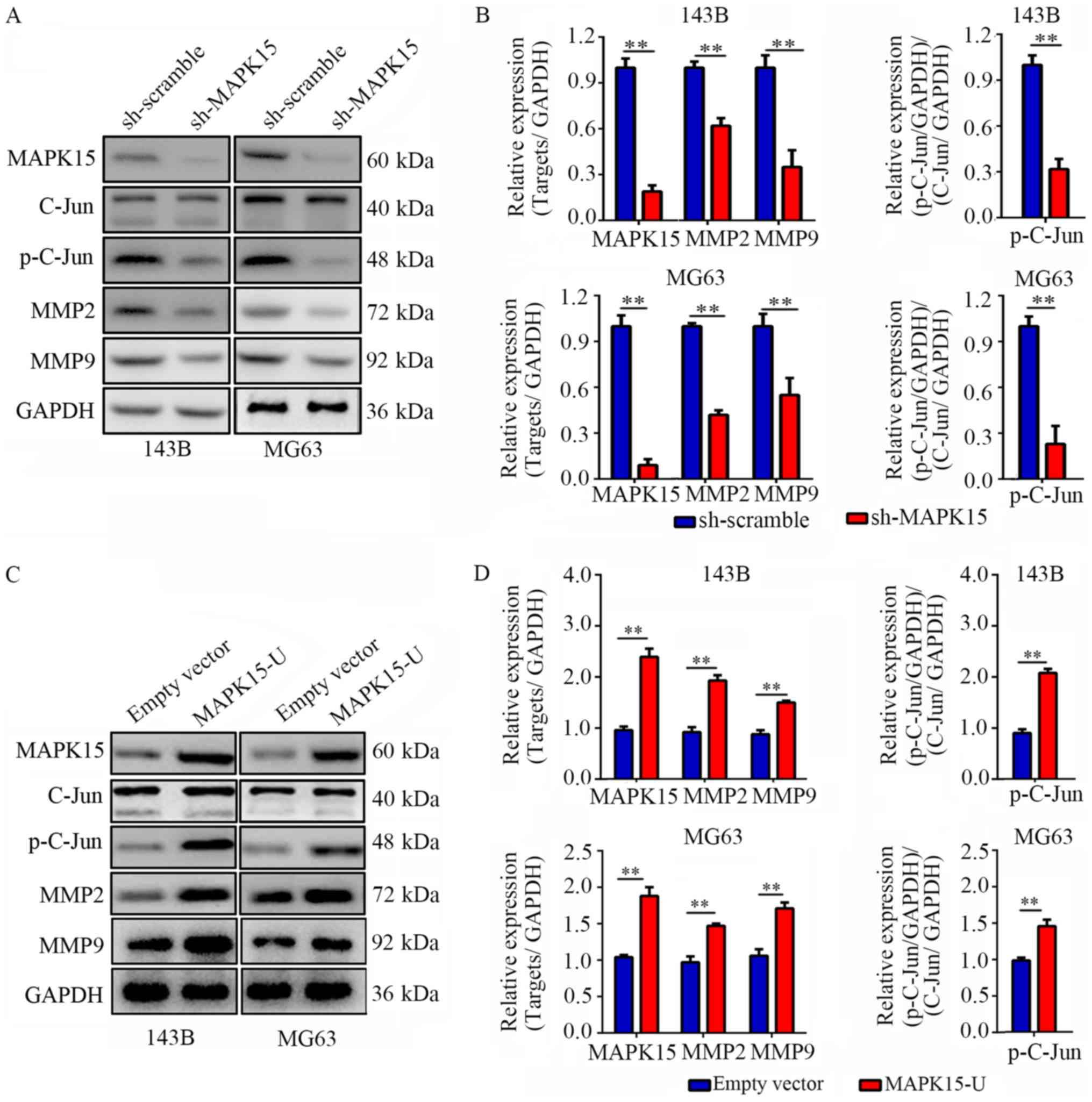 | Figure 8.MAPK15 significantly regulates
C-Jun/MMPs signaling pathway. (A) Western blotting was used to
detect the expression levels of MAPK15, c-Jun, P-c-Jun, MMP2 and
MMP9. MAPK15 expression was inhibited. (B) Statistical analysis for
MAPK15, c-Jun, p-c-Jun, MMP2 and MMP9. (C) Western blotting was
used to detect the expression of MAPK15, c-Jun, P-c-Jun, MMP2 and
MMP9. (D) Statistical analysis for MAPK15, c-Jun, p-c-Jun, MMP2 and
MMP9. **P<0.01. MAPK15, mitogen-activated protein kinase 15;
MMP, matrix metalloproteinase; NC, normal control; p-,
phosphorylated-. |
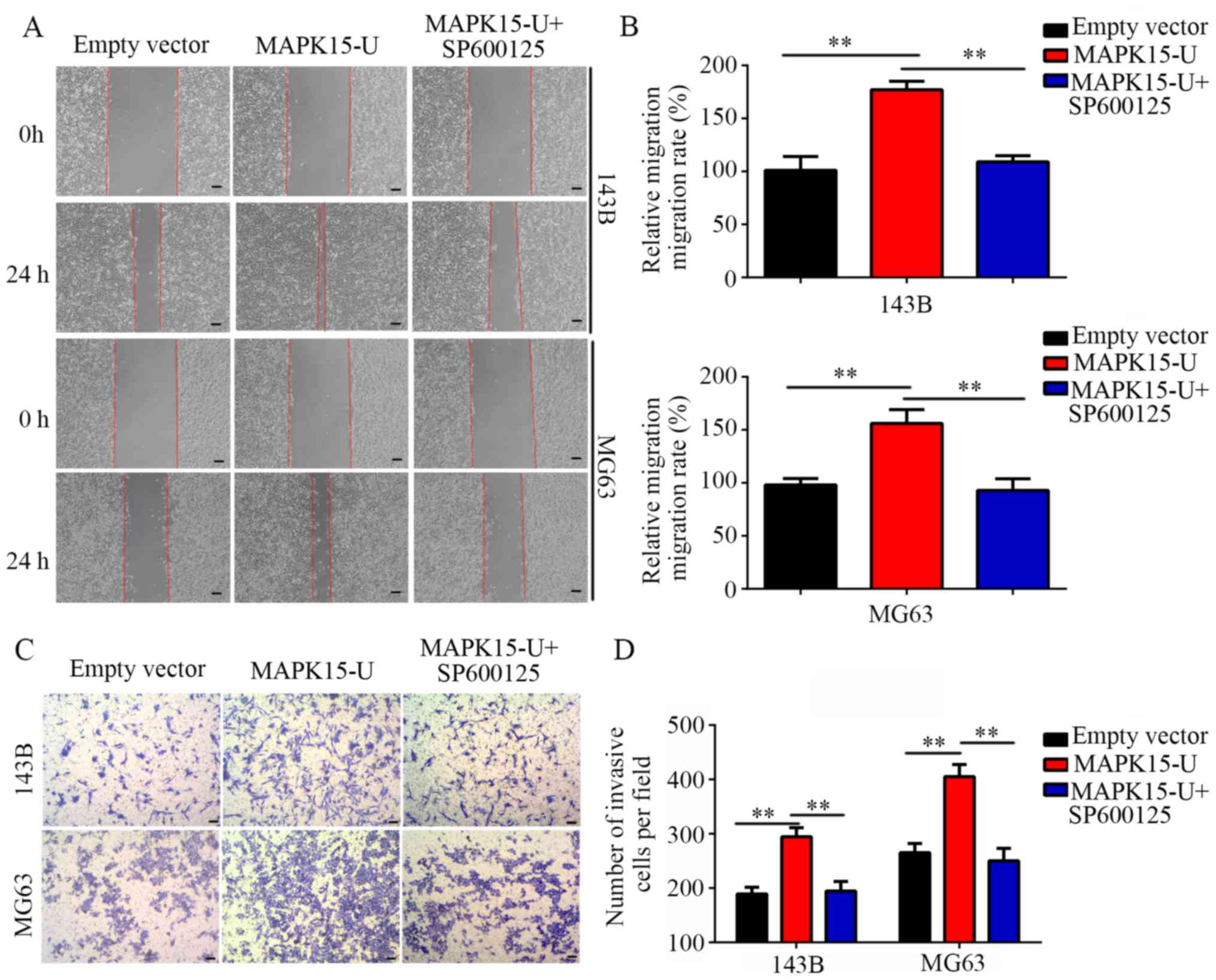
Inhibition of c-Jun reverses the
effects of MAPK15-overexpression on the migration and invasion of
OS cells
To determine whether the c-Jun/MMP pathways are
involved in the migration and invasion induced by MAPK15, a c-Jun
inhibitor SP600125 was used. Wound-healing assays showed that the
overexpression of MAPK15 increased the migratory ability of OS
cells, while SP600125 blocked this effect (Fig. 9A and B). Similarly, Transwell assays
showed that the number of invasive cells was significantly
increased by MAPK15 overexpression, which was subsequently blocked
by SP600125 (Fig. 9C and D).
Discussion
OS is the most common primary malignant tumor in the
skeletal system with strong characteristics of invasion, metastasis
and recurrence. Current clinical treatments are not effective for
patients with OS-associated metastasis and recurrence, thus the
prognosis of patients with end-stage OS with metastasis has not
improved (28). In addition,
epidemiological studies have shown that metastasis is the leading
cause of death in patients with OS (29). Therefore, exploring the molecular
mechanism of OS and discovering novel therapeutic targets has
important clinical significance for improving the survival and
prognosis of patients with OS.
In the present study, two bioinformatics methods
(WGCNA and DEG analysis) were used to analyze data from the
GSE87624 dataset, in order to identify key oncogenes associated
with the metastasis of OS. According to WGCNA, 16 genes including
DLEC1, FOXJ1 and MAPK15 was predicted as core genes in
metastasis-related modules. Furthermore, through DEG analysis for
non-metastatic and metastatic OS tissues, we showed that DLEC1,
FOXJ1 and MAPK15 were also upregulated DEGs in metastatic OS
tissues. Then, the mRNA and protein level of DLEC1, FOXJ1 and
MAPK15 in OS tissues was detected. We found that both the mRNA and
protein expression levels of FOXJ1 and MAPK15 were significantly
upregulated in the OS tissues of patients with tumor metastasis at
diagnosis.
MAPK15 is one of the most recently discovered MAPKs,
but has been researched in a series of different tumor types
(30). Wu et al (31) reported that MAPK15 was highly
expressed in several human lung cancer cell lines; this study also
demonstrated that siRNA-silencing of MAPK15, or NF-kB inhibition,
reduced the sensitivity of lung cancer cells to arsenic trioxide,
suggesting that MAPK15 regulates the drug resistance of cancer
cells via the NF-kB pathway. Xu et al (32) found that the overexpression of MAPK15
could further increase the trans-activation of activator protein-1
by promoting the phosphorylation of proto-oncogene c-Jun, thus
inducing the proliferation and transformation of cancer cells.
Similarly, Jin DH (and a number of others) have also found that the
MAPK15 copy number and level of expression in gastric cancer
tissues were significantly increased, compared with non-gastric
cancer tissues; additionally, siRNA knockdown of MAPK15 inhibited
the proliferation of gastric cancer cells, leading to cell cycle
arrest G1/S phase (19).
Consistently, the present study confirmed that MAPK15 also plays an
oncogenic role in OS, and that its inhibition significantly
decreased OS cell metastasis in vitro and in
vivo.
MMPs are involved in matrix degradation and play key
roles in tumor growth, invasion and angiogenesis (33,34).
c-Jun is a major transcription factor, which regulates the
expression of MMPs (35). The role
of the c-Jun/MMP pathways in OS has been widely reported; by
activating JNK/c-Jun/MMP-2 signaling, the oncogene Astrocyte
elevated gene-1 promotes the development of OS (36). Cryptochrome 2, an OS suppressor,
inhibited the proliferation of OS cells by decreasing the
expression of c-Jun/MMPs (37).
Similarly, c-Jun/MMPs were also the target for OS therapy; by
inhibiting c-Jun-associated pathways, glabridin effectively
inhibited the proliferation of OS cells (38). Likewise, nobiletin inhibited the
proliferation, migration and invasion capacities of OS cells by
blocking the c-Jun/ MMP signaling pathways (39). In colorectal cancer, MAPK15 binds to
and increases the level of c-Jun phosphorylation. Consistently, the
present study illustrated that MAPK15 inhibition significantly
decreased the level of c-Jun phosphorylation, as well as that of
its target genes, including MMP2 and 9; c-Jun inhibition blocked
the effects of MAPK15 overexpression on the migration and invasion
of OS cells.
In the present study, WGCNA and DEG analysis of the
GSE87624 dataset revealed that DLEC1, FOXJ1 and MAPK15 were
predicted hub genes for the metastasis of OS, and that MAPK15 was
most significantly upregulated in OS tissues. A series of in
vitro and in vivo experiments subsequently confirmed
that MAPK15 promoted the proliferation and metastasis of OS cells
and tumors, respectively, by activating the c-Jun/MMP signaling
pathways. Therefore, MAPK15 may be a novel biomarker for the
diagnosis of OS, as well as an effective therapeutic target.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the Science and
Technology Program of Guangzhou, China (grant no.
201704020129).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZS and LL conceived and designed the study. ZS, BY
and ZZ collected the data, performed the associated related assays
and wrote the manuscript. SZ, SL and CW performed data analysis and
validation. YJ contributed to the study design and proofread the
manuscript, and all authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The OS tissue related study was approved by the
Clinical Ethics Management Committee of Zhujiang Hospital, South
Medical University, and all patients provided informed consent in
writing. The animal care and experimental procedures used in the
present study were approved by the Institutional Animal Care and
Use Committee of the Southern Medical University.
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tang H, Tang Z, Jiang Y, Wei W and Lu J:
Pathological and therapeutic aspects of matrix metalloproteinases:
Implications in osteosarcoma. Asia Pac J Clin Oncol. 15:218–224.
2019.PubMed/NCBI
|
|
2
|
Limaiem F, Kuhn J and Khaddour K: Cancer,
Telangiectatic Osteosarcoma. StatPearls. StatPearls Publishing,
Treasure Island (FL). Online. 2019
|
|
3
|
Valery PC, Laversanne M and Bray F: Bone
cancer incidence by morphological subtype: A global assessment.
Cancer Causes Control. 26:1127–1139. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roberts RD, Lizardo MM, Reed DR, Hingorani
P, Glover J, Allen-Rhoades W, Fan T, Khanna C, Sweet-Cordero EA,
Cash T, et al: Provocative questions in osteosarcoma basic and
translational biology: A report from the Children's oncology group.
Cancer. 25:3514–3525. 2019. View Article : Google Scholar
|
|
5
|
Bernthal NM, Federman N, Eilber FR, Nelson
SD, Eckardt JJ, Eilber FC and Tap WD: Long-term results (>25
years) of a randomized, prospective clinical trial evaluating
chemotherapy in patients with high-grade, operable osteosarcoma.
Cancer. 118:5888–5893. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carina V, Costa V, Sartori M, Bellavia D,
De Luca A, Raimondi L, Fini M and Giavaresi G: Adjuvant biophysical
therapies in osteosarcoma. Cancers. 11:E3482019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Daw NC, Chou AJ, Jaffe N, Rao BN, Billups
CA, Rodriguez-Galindo C, Meyers PA and Huh WW: Recurrent
osteosarcoma with a single pulmonary metastasis: A
multi-institutional review. Br J Cancer. 112:278–282. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick R: Osteosarcoma: Current treatment and a collaborative
pathway to success. J Clin Oncol. 33:3029–3035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie B, Li Y, Zhao R, Xu Y, Wu Y, Wang J,
Xia D, Han W and Chen D: Identification of key genes and miRNAs in
osteosarcoma patients with chemoresistance by bioinformatics
analysis. Biomed Res Int. 2018:47610642018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen XG, Ma L and Xu JX: Abnormal DNA
methylation may contribute to the progression of osteosarcoma. Mol
Med Rep. 17:193–199. 2018.PubMed/NCBI
|
|
11
|
Wang JS, Duan MY, Zhong YS, Li XD, Du SX,
Xie P, Zheng GZ and Han JM: Investigating ageinduced differentially
expressed genes and potential molecular mechanisms in osteosarcoma
based on integrated bioinformatics analysis. Mol Med Rep.
19:2729–2739. 2019.PubMed/NCBI
|
|
12
|
Tian H, Guan D and Li J: Identifying
osteosarcoma metastasis associated genes by weighted gene
co-expression network analysis (WGCNA). Medicine (Baltimore).
97:e107812018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang JS, Wang YG, Zhong YS, Li XD, Du SX,
Xie P, Zheng GZ and Han JM: Identification of co-expression modules
and pathways correlated with osteosarcoma and its metastasis. World
J Surg Oncol. 17:462019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Najafi M, Ahmadi A and Mortezaee K:
Extracellular-signal- regulated kinase/mitogen-activated protein
kinase signaling as a target for cancer therapy: An updated review.
Cell Biol Int. 43:1206–1222. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Papa S, Choy PM and Bubici C: The ERK and
JNK pathways in the regulation of metabolic reprogramming.
Oncogene. 38:2223–2240. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abe MK, Saelzler MP, Espinosa R III, Kahle
KT, Hershenson MB, Le Beau MM and Rosner MR: ERK8, a new member of
the mitogen-activated protein kinase family. J Biol Chem.
277:16733–16743. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lau ATY and Xu YM: Regulation of human
mitogen-activated protein kinase 15 (extracellular signal-regulated
kinase 7/8) and its functions: A recent update. J Cell Physiol.
234:75–88. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cargnello M and Roux PP: Activation and
function of the MAPKs and their substrates, the MAPK-activated
protein kinases. Microbiol Mol Biol Rev. 75:50–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin DH, Lee J, Kim KM, Kim S, Kim DH and
Park J: Overexpression of MAPK15 in gastric cancer is associated
with copy number gain and contributes to the stability of c-Jun.
Oncotarget. 6:20190–20203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rossi M, Colecchia D, Ilardi G, Acunzo M,
Nigita G, Sasdelli F, Celetti A, Strambi A, Staibano S, Croce CM
and Chiariello M: MAPK15 upregulation promotes cell proliferation
and prevents DNA damage in male germ cell tumors. Oncotarget.
7:20981–20998. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Z, Li N, Shen L and Fu J: Quantitative
proteomic analysis identifies MAPK15 as a potential regulator of
radioresistance in nasopharyngeal carcinoma cells. Front Oncol.
8:5482018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Scott MC, Temiz NA, Sarver AE, LaRue RS,
Rathe SK, Varshney J, Wolf NK, Moriarity BS, O'Brien TD, Spector
LG, et al: Comparative transcriptome analysis quantifies immune
cell transcript levels, metastatic progression, and survival in
osteosarcoma. Cancer Res. 78:326–337. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Robinson MD, McCarthy DJ and Smyth GK:
edgeR: A bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shimazui T, Yoshikawa K, Ishitsuka R,
Kojima T, Kandori S, Yoshino T, Miyazaki J, Uchida K and Nishiyama
H: Systemic transduction of p16(INK4a) antitumor peptide inhibits
lung metastasis of the MBT-2 bladder tumor cell line in mice. Oncol
Lett. 17:1203–1210. 2019.PubMed/NCBI
|
|
26
|
Meazza C and Scanagatta P: Metastatic
osteosarcoma: A challenging multidisciplinary treatment. Expert Rev
Anticancer Ther. 16:543–556. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aljubran AH, Griffin A, Pintilie M and
Blackstein M: Osteosarcoma in adolescents and adults: Survival
analysis with and without lung metastases. Ann Oncol. 20:1136–1141.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Y, Yang J, Zhao N, Wang C, Kamar S,
Zhou Y, He Z, Yang J, Sun B, Shi X, et al: Progress in the
chemotherapeutic treatment of osteosarcoma. Oncol Lett.
16:6228–6237. 2018.PubMed/NCBI
|
|
29
|
Ahmed G, Zamzam M, Kamel A, Ahmed S,
Salama A, Zaki I, Kamal N and Elshafiey M: Effect of timing of
pulmonary metastasis occurrence on the outcome of metastasectomy in
osteosarcoma patients. J Pediatr Surg. 54:775–779. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Coulombe P and Meloche S: Atypical
mitogen-activated protein kinases: Structure, regulation and
functions. Biochim Biophys Acta. 1773:1376–1387. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu DD, Lau ATY, Yu FY, Cai NL, Dai LJ, Ok
Kim M, Jin DY and Xu YM: Extracellular signal-regulated kinase
8-mediated NF-KB activation increases sensitivity of human lung
cancer cells to arsenic trioxide. Oncotarget. 8:49144–49155.
2017.PubMed/NCBI
|
|
32
|
Xu YM, Zhu F, Cho YY, Carper A, Peng C,
Zheng D, Yao K, Lau AT, Zykova TA, Kim HG, et al: Extracellular
signal-regulated kinase 8-mediated c-Jun phosphorylation increases
tumorigenesis of human colon cancer. Cancer Res. 70:3218–3227.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shay G, Lynch CC and Fingleton B: Moving
targets: Emerging roles for MMPs in cancer progression and
metastasis. Matrix Biol 44–46. 200–206. 2015. View Article : Google Scholar
|
|
34
|
Merchant N, Nagaraju GP, Rajitha B,
Lammata S, Jella KK, Buchwald ZS, Lakka SS and Ali AN: Matrix
metalloproteinases: Their functional role in lung cancer.
Carcinogenesis. 38:766–780. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ge HX, Zou FM, Li Y, Liu AM and Tu M: JNK
pathway in osteoarthritis: Pathological and therapeutic aspects. J
Recept Signal Transduct Res. 37:431–436. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang F, Ke ZF, Wang R, Wang YF, Huang LL
and Wang LT: Astrocyte elevated gene-1 (AEG-1) promotes
osteosarcoma cell invasion through the JNK/c-Jun/MMP-2 pathway.
Biochem Biophys Res Commun. 452:933–939. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu Y, Li Y, Zhou L, Yang G, Wang M and
Hong Y: Cryptochrome 2 (CRY2) suppresses proliferation and
migration and regulates clock gene network in osteosarcoma cells.
Med Sci Monit. 24:3856–3862. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jie Z, Xie Z, Zhao X, Sun X, Yu H, Pan X,
Shen S, Qin A, Fang X and Fan S: Glabridin inhibits osteosarcoma
migration and invasion via blocking the p38- and JNK-mediated
CREB-AP1 complexes formation. J Cell Physiol. 234:4167–4178. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cheng HL, Hsieh MJ, Yang JS, Lin CW, Lue
KH, Lu KH and Yang SF: Nobiletin inhibits human osteosarcoma cells
metastasis by blocking ERK and JNK-mediated MMPs expression.
Oncotarget. 7:35208–35223. 2016.PubMed/NCBI
|