Introduction
Colorectal cancer (CRC) is a familial malignancy of
the alimentary canal (1). CRC is the
second most common tumor of the gastrointestinal tract, the third
most frequent malignancy and the fourth most common cause of
cancer-associated mortality worldwide (2,3). The
metastasis and recurrence rates of CRC are high, and its morbidity
and mortality are increasing each year (4,5).
According to the histopathologic malignant degree grade of the
World Health Organization (WHO) criteria, CRC can be divided into
low (I and II) and high (III and IV) grades (6). Research into the pathobiology and
underlying molecular mechanisms of CRC metastasis is limited
(7). Further exploration of these
molecular mechanisms is required.
Increasing evidence has indicated that microRNAs
(miRNAs/miRs) regulate the occurrence and development of a variety
of cancer cell types (8). miRNAs can
modulate cell proliferation, differentiation, apoptosis and
carcinogenesis by combining with the 3′ untranslated region
(3′-UTR) of target mRNAs in cancer (9–11).
miR-331-3p, located on 12q22n and a member of the miR-331 family,
functions as a tumor suppressor in CRC; marked downregulation of
miR-331-3p in CRC is linked to increased cell proliferation and
decreased apoptosis (12,13). This aberrant expression of miR-331-3p
is also linked to cell proliferation and migration in various other
cancers, such as acute lymphocytic leukemia, lung cancer,
glioblastoma, gastric cancer, prostate cancer, non-small cell lung
cancer and pancreatic cancer (14–20). The
functions of miR-331-3p in CRC cell migration, as well as the up-
and downstream regulatory mechanisms of miR-331-3p, are
unclear.
Circular RNAs (circRNAs) are competitive endogenous
RNAs which modulate gene expression and biological function via
miRNA sponging (21,22). CircRNAs are highly expressed in
multiple tumor tissues, including CRC (23,24).
CircRNAs participate in cancer-associated physiological and
pathological processes, such as cell proliferation, migration and
invasion, cell cycle progression, metastasis and carcinogenesis
(25). Numerous studies show that
circRNAs or long non-coding RNAs promote the progression of cancer
by sponging miR-331-3p (26–28). Bioinformatics analysis predicted the
presence of a binding site between hsa_circ_0038646 and miR-331-3p
(26–29). In the present study, bioinformatics
analysis was used to predict the binding sites for miR-331-3p in
hsa_circ_0038646 and glutamate receptor ionotropic kainate 3
(GRIK3). To the author's best knowledge, no reports regarding
hsa_circ_0038646 have been published to date. GRIK3 is shown to be
a novel oncogenic factor in different types of cancer, including
breast cancer (30) and gastric
cancer (31). Thus far, the
interaction between GRIK3 and miR-331-3p in CRC has not been
reported.
In the present study, the expression and function of
hsa_circ_0038646 and miR-331-3p in CRC were assessed, and the role
of the hsa_circ_0038646-miR-331-3p-GRIK3 axis in CRC progression
was determined.
Materials and methods
Human CRC tissue collection
Human CRC tumor tissues and adjacent normal control
tissues were acquired from 62 enrolled patients with CRC who were
diagnosed based on pathology, and had undergone curative surgery
resection at Tianjin Baodi Hospital between May and October 2018.
The cohort of patients with CRC was comprised of 25 females and 37
males, aged 52–73 years, with an average age of 59.48±6.36 years.
According to the Tumor Node Metastasis (TNM) classification
standard of the Union for International Cancer Control (32), the cohort included TNM stage I
(n=20), TNM stage II (n=23), TNM stage III (n=12) and TNM stage IV
(n=7). None of the patients had received anticancer treatments such
as radiotherapy or chemotherapy prior to surgery. Based on WHO
grading (6), the CRC tumor samples
were divided into low- and high-grade groups. The present study was
approved by the Clinical Ethical Committee of Tianjin Baodi
Hospital and written informed consent was provided by all
participants prior to the study start.
Cell culture
The human CRC cell lines SW480, HT29, DLD-1, SW620
and HCT116, as well as the normal human colon epithelial cell line
NCM460, were provided by the Cell Bank of Type Culture Collection
of Chinese Academy of Sciences. After cells were tested and
authenticated via STR profiling, SW480 and SW620 cells were seeded
in Leibovitz's L-15 medium (Gibco, Invitrogen; Thermo Fisher
Scientific, Inc.). HT29 and HCT116 cells were grown in McCoy's 5A
medium (Gibco, Invitrogen; Thermo Fisher Scientific, Inc.) with 10%
fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific,
Inc.). DLD-1 cells were cultured in RPMI 1640 medium (Invitrogen;
Thermo Fisher Scientific, Inc.) containing 5% FBS, and 1%
penicillin and streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.). Notably, all cells were cultured in a humidified atmosphere
of 5% CO2 at 37°C. Experiments were performed after 72 h
of incubation, when the cells achieved 60–70% confluency.
Cell transfection
SW620 and HCT116 cells were seeded into 24-well
plates at a density of 5×104 for 24 h at 37°C. When the
cells reached 70–80% confluence, they were transfected with 120 nM
small interfering (si)RNAs, with the following sequences:
hsa_circ_0038646, 5′-GUAUGGACUCAUCCACCAGGGGA-3′, and NC,
5′-GGGUAUUCACAUACCCGGGCAGA-3′; and GRIK3,
5′-GGUCUCUGGGCCAUUCAGGGGAU-3′, and NC,
5′-GUGCUUGGGCACCUCGGUAUGGA-3′. Recombinant plasmids of
pcDNA3.1-hsa_circ_0038646 and pcDNA3.1-GRIK3 (120 nM) were
purchased from Shanghai GenePharma Co., Ltd., which were cloned
into the pcDNA3.1 plasmid and the corresponding empty pcDNA3.1
plasmid (Invitrogen; Thermo Fisher Scientific, Inc.), which was
used as the negative control. miR-331-3p mimics (120 nM)
(5′-GCCCCUGGGCCUAUCCUAGAA-3′) and mimics NC
(5′-UUCUCCGAACGUGUCACGUTT-3′), miR-331-3p inhibitors (120 nM)
(5′-UUCUAGGAUAGGCCCCAGGGGC-3′) and inhibitors NC
(5′-CAGUACUUUUGUGUAGUACAA-3′), as well as the corresponding
negative controls (NCs) were purchased from Applied Biosystems;
Thermo Fisher Scientific, Inc., and performed for at least 5 h at
37°C with Lipofectamine® 2000 transfection reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions. After 72 h, cells were harvested.
Cell proliferation assay
Following 48 h of transfection and at 70%
confluency, the cells were incubated for an additional 24, 48, 72
or 96 h. Then, 12 µl of Cell Counting Kit-8 (CCK-8) solution (7sea
Biotech Co., Ltd.) was added into each well for 24, 48 and 72 h,
respectively, and maintained at 37°C for 2 h. For quantification,
cell viability was detected at 450 nm using the SUNRISE Microplate
Reader (Tecan Group, Ltd.), as previously described (33).
Cell migration assay
A Transwell assay was performed to assess cell
migration. After cultured SW620 and HCT116 cells reached 80%
confluence, cells (5×104) were plated in the upper
chamber of Transwell plates in serum-free medium without FBS, for
24 h. Leibovitz's L-15 medium (for SW620) (Gibco; Thermo Fisher
Scientific, Inc.) or McCoy's 5A medium (for HCT116) (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS were pre-added
in the lower chambers, respectively. and the temperature were
maintained at 37°C for 24 h. Subsequently, cells in the upper
chamber were discarded, and the migratory cells were fixed with 4%
formaldehyde for 30 min at room temperature, prior to staining with
0.5% crystal violet solution for 30 min at room temperature. The
migratory cells were blindly counted in five randomly selected
fields, under a light microscope (magnification, ×200) and using
Image J software (version 1.42; National Institutes of Health). The
experiment was performed in triplicate.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from human CRC tissues or
cells with TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
cDNA was synthesized using the RevertAid™ First Strand cDNA
Synthesis kit (DBI Bioscience) at 95°C. NanoDrop™ ND 1000
microspectrophotometer (NanoDrop Technologies; Thermo Fisher
Scientific, Inc.) was used to quantify the extracted RNA. RT-qPCR
was carried out on an ABI 7900 system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) using SYBR Select Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The following primer
sequences (Generay Biotech Co., Ltd.) were used for qPCR:
hsa_circ_0038646 forward, 5′-TATGGTGGAGAAGCGGGTGTT-3′ and reverse,
5′-CCAGGGCAGGAGAATGTGA-3′; GRIK3 forward,
5′-GCTGGTCTGCACTGAACTCT-3′ and reverse, 5′-AAAGGGCATCCCCTGAATGG-3′;
GAPDH forward, 5′-AGGTGAAGGTCGGAGTCAAC-3′ and reverse,
5′-CGCTCCTGGAAGATGGTGAT-3′; miR-331-3p forward,
5′-GAGCTGAAAGCACTCCCAA-3′ and reverse, 5′-CACACTCTTGATGTTCCAGGA-3′;
and U6 forward, 5′-AGAGCCTGTGGTGTCCG-3′ and reverse,
5′-CATCTTCAAAGCACTTCCCT-3′. The following thermocycling conditions
were used for qPCR: Initial denaturation at 95°C for 1 min,
followed by 28 cycles of denaturation at 95°C for 10 sec, annealing
at 58°C for 15 sec, elongation at 72°C for 5 sec and a final
extension at 72°C for 5 sec. GAPDH and human U6 RNA were used as
internal controls for quantification of hsa_circ_0038646 and GRIK3,
and miR-331-3p, respectively. The 2−∆∆Cq method
(34) was used for quantification
and a 2-fold change was considered significant. All RT-qPCR assays
were performed in triplicate. Furthermore, the correlation between
expression of hsa_circ_0038646 and miR-331-3p, or between the
expression of miR-331-3p and GRIK3 was calculated.
Western blotting
Total protein was obtained from CRC cells with RIPA
lysis buffer (Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. Protein concentrations were determined
using a BCA protein assay kit (Beyotime Institute of
Biotechnology). Proteins (40 µg) were isolated via 10% SDS-PAGE and
transferred to PVDF membranes (EMD Millipore). After blocking with
5% milk prepared in Tris-buffered saline with 0.05% Tween-20 (TBST)
at room temperature for 1 h, membranes were incubated at 4°C
overnight with primary antibodies against GRIK3 (1:1,000; cat. no.
ab183035; Abcam) and GAPDH (1:2,500; cat. no. ab9845; Abcam). The
membranes were rinsed four times with TBST. A secondary antibody
conjugated to horseradish peroxidase (1:2,000; cat. no. ab6721;
Abcam) was added, and the membranes were incubated for 1 h at room
temperature. Protein bands were visualized using the enhanced
chemiluminescence kit (EMD Millipore). Image Lab Software (version
2.0; Bio-Rad Laboratories, Inc.) in the ChemiDoc XRS System (Thermo
Fisher Scientific, Inc.) was used for quantification.
Luciferase reporter assay
Bioinformatics software Circular RNA Interactome
(https://circinteractome.nia.nih.gov/index.html) was
used to predict the presence of binding sites for miR-331-3p in
hsa_circ_0038646 and GRIK3, and a luciferase reporter assay was
performed to validate the association between hsa_circ_0038646,
miR-331-3p and GRIK3. SW620 and HCT116 cells were seeded into
24-well plates at the density of 5×104 and
co-transfected with wild-type (WT) or mutated (Mut)
hsa_circ_0038646, which were inserted into the reporter vector
pmirGLO (600 ng; Promega Corporation) and GRIK3 3′-UTR reporter
plasmid sequences, which were inserted into the pGL3 vector (600
ng; Shanghai GenePharma, Co., Ltd.) with either miR-331-3p mimics
or NC (120 nM), using Lipofectamine® 2000 transfection
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). After 24 h of
transfection, luciferase activity was measured with the Dual
Luciferase Reporter Assay System (Promega Corporation) and
normalized to Renilla luciferase activity.
Statistical analysis
Data were analyzed with SPSS 16.0 software (SPSS,
Inc.) and presented as the mean ± standard deviation. Student's
t-tests and one-way ANOVAs followed by Tukey's test were used for
the analysis of differences between two and multiple groups,
respectively. Correlation analysis was conducted using Pearson
correlation test where appropriate. Statistical significance was
considered as P<0.05. All experiments were performed in
triplicate.
Results
Expression and function of
hsa_circ_0038646 in human CRC
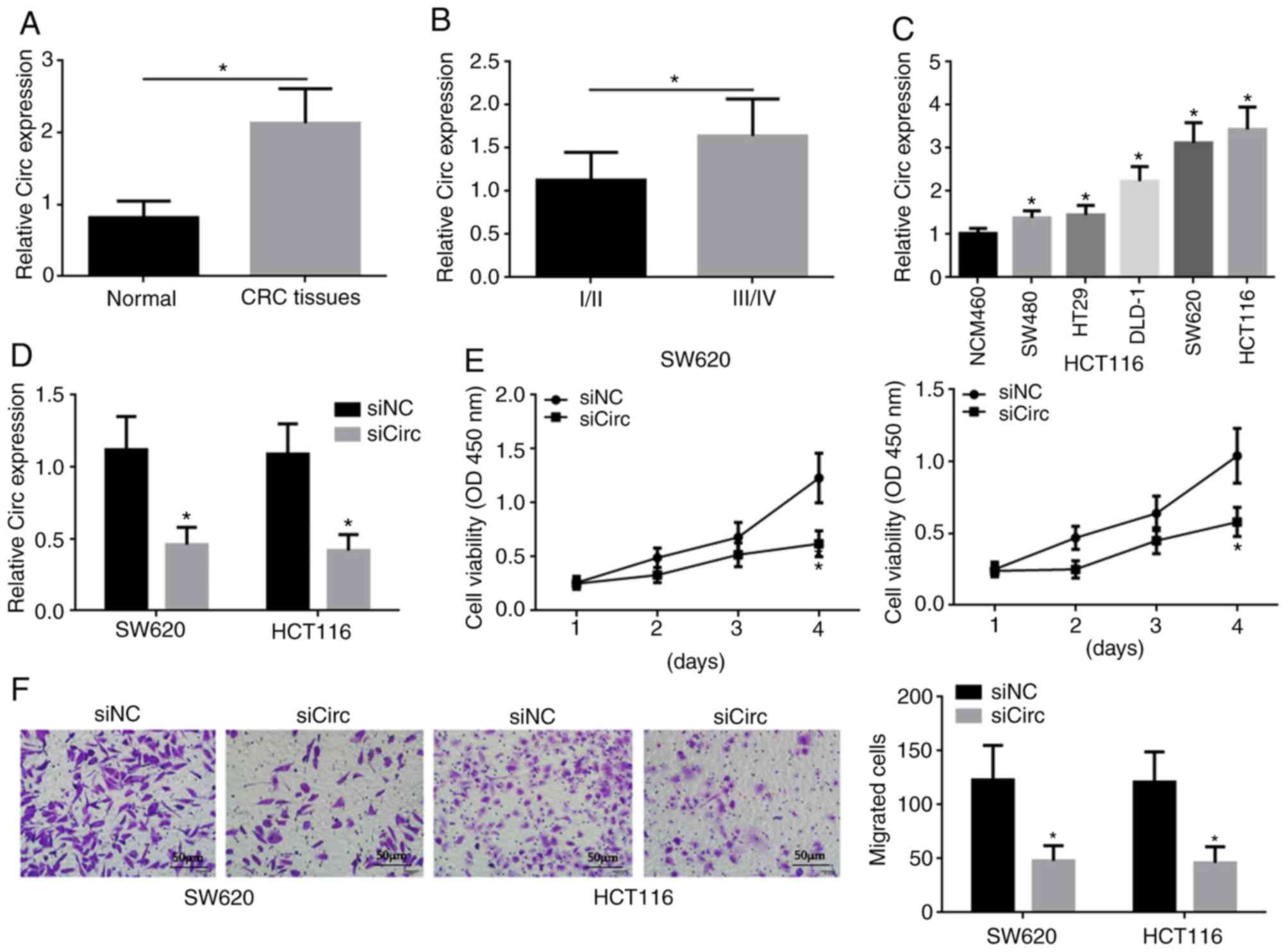
Hsa_circ_0038646 exhibited increased expression in
human CRC tissue compared to normal tissue (Fig. 1A). This aberrant expression was
positively associated with a higher tumor grade (III/IV) in CRC
(Fig. 1B). Hsa_circ_0038646 also had
increased expression, compared to a control cell line, in various
human CRC cell lines, including SW480, HT29, DLD-1, SW620 and
HCT116, and was particularly highly expressed in SW620 and HCT116
cells (Fig. 1C). These findings
indicated that increased hsa_circ_0038646 expression might be
related to CRC progression.
SW620 and HCT116 cells with reduced expression of
hsa_circ_0038646 were generated using siRNA targeting
hsa_circ_0038646 (siCirc), and hsa_circ_0038646 expression levels
were detected by RT-qPCR (Fig. 1D).
Reduced expression of hsa_circ_0038646 reduced the proliferative
capacity of SW620 and HCT116 cells, and showed a significant
difference on day 4 of incubation as determined using a CCK-8 assay
(Fig. 1E). Moreover, Transwell
assays also revealed that reduced expression of hsa_circ_0038646
inhibited the migration of SW620 and HCT116 cells (Fig. 1F).
Hsa_circ_0038646 regulates CRC cell
proliferation and migration by targeting miR-331-3p
Bioinformatics analysis predicted the presence of a
binding site between hsa_circ_0038646 and miR-331-3p (Fig. 2A). However, the expression levels of
hsa_circ_0038646 in CRC with low tumor grades (I and II) are not
currently available in public databases. WT and Mut luciferase
reporter plasmids were designed (Fig.
2A). Additionally, to validate the targeting relationship
between hsa_circ_0038646 and miR-331-3p, oe of hsa_circ_0038646 was
conducted using plasmids encoding WT or Mut hsa_circ_0038646 cDNA
(oeCirc-WT and oeCirc-Mut, respectively), with hsa_circ_0038646
upregulated in cells transfected with the WT vector compared with
the Mut vector (Fig. 2B).
Subsequently, miR-331-3p expression was upregulated or inhibited
via transfection with miR-331-3p mimics or inhibitors, respectively
(Fig. 2C). It was then found that
upregulation of miR-331-3p reduced the luciferase activity in SW620
and HCT116 cells transfected with the WT reporter plasmid.
Interestingly, no effect was observed in the luciferase reporter
assay with cells transfected with the Mut plasmid (Fig. 2D). RT-qPCR and Pearson correlation
analysis also indirectly indicated that miR-331-3p is a target of
hsa_circ_0038646 (Fig. 2E-G).
Downregulation of hsa_circ_0038646 expression resulted in a
significant increase in miR-331-3p levels in SW620 and HCT116 cells
(Fig. 2E). In addition, compared
with the oeVector group, the expression of miR-331-3p was
significantly suppressed by oeCirc-WT, but not by oeCirc-Mut
(Fig. 2F). Pearson correlation
analysis showed that miR-331-3p levels were also negatively
correlated with hsa_circ_0038646 levels in human CRC tissues
(Fig. 2G).
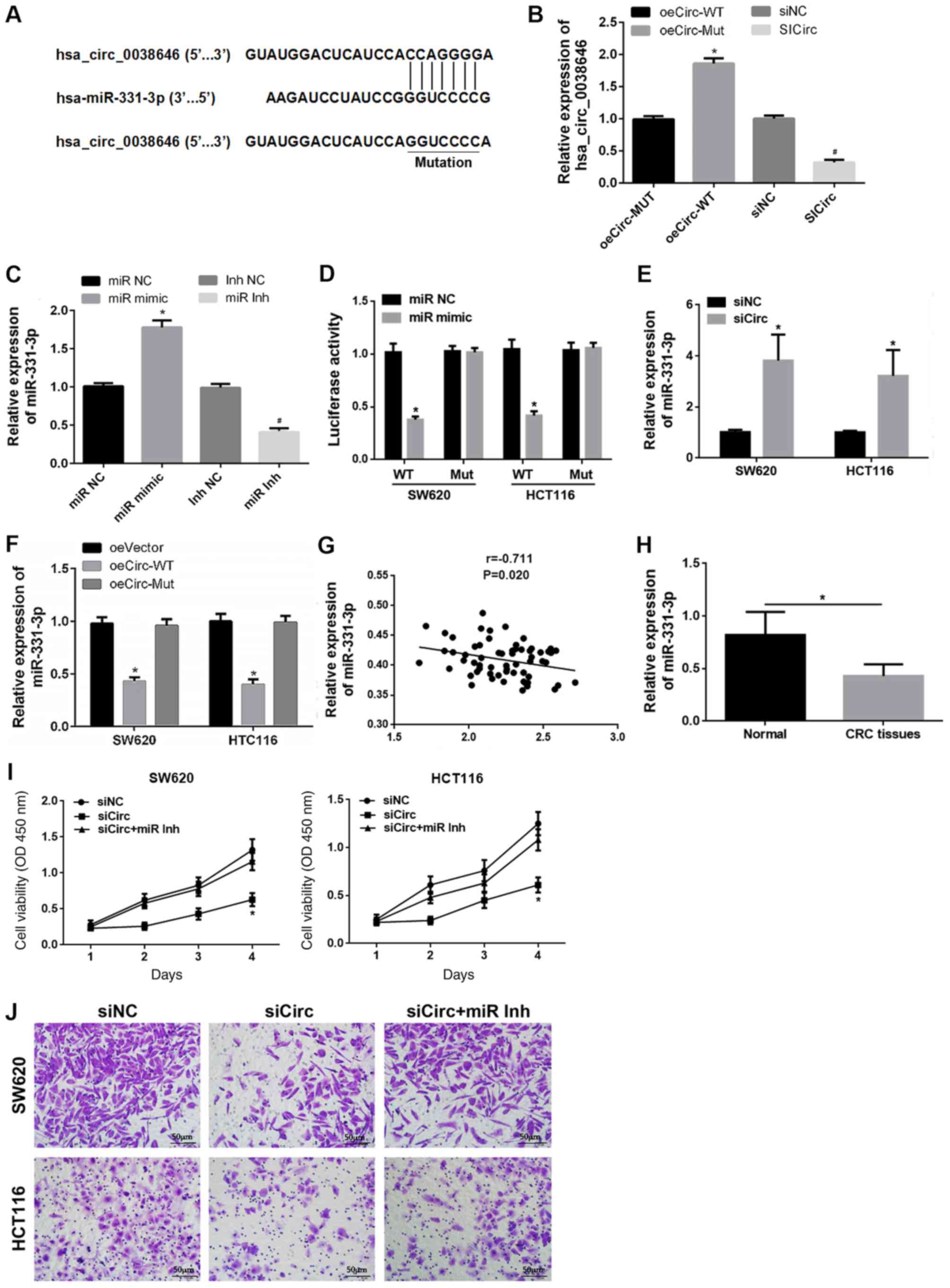 | Figure 2.Hsa_circ_0038646 modulates cell
proliferation and migration via sponging of miR-331-3p. (A)
Predicted binding site between hsa_circ_0038646 and miR-331-3p. (B)
Expression of hsa_circ_0038646 after transfection with oeCirc or
siCirc. (C) Overexpression or inhibition of miR-331-3p by mimics or
inhibitors, respectively. (D) miR-331-3p is a target of
hsa_circ_0038646, as shown by dual-luciferase reporter assays in
SW620 and HCT116 cells. (E) Hsa_circ_0038646 negatively regulated
miR-331-3p expression in vitro. (F) Overexpression of WT
hsa_circ_0038646 inhibited miR-331-3p expression in CRC cells
compared to oeVector group. (G) Relationship between
hsa_circ_0038646 and miR-331-3p expression in CRC tissues. (H)
Relative miR-331-3p mRNA expression in CRC tissues. Inhibition of
miR-331-3p rescued the suppressed CRC cell. (I) proliferation and
(J) migration mediated by hsa_circ_0038646 knockdown. *P<0.05
vs. oeCirc-Mut group, miR NC, oeVector group and siNC group,
respectively; #P<0.05 vs. siNC group and Inh NC
group, respectively. All experiments were performed in triplicate.
CRC, colorectal cancer; NC, negative control; si, small
interfering; miR, miR-331-3p; WT, wild-type; Mut, mutated; oe,
overexpression vector; Inh, inhibitor; OD, optical density. |
RT-qPCR revealed that miR-331-3p was downregulated
in human CRC tissues (Fig. 2H).
Reduced expression of hsa_circ_0038646 resulted in reduced human
CRC cell proliferation and migration in vitro. However,
following co-transfection with both siCirc and miR-331-3p
inhibitors, there was no reduction in cell proliferation and
migration, indicating that decreased expression of miR-331-3p
attenuated the effects of hsa_circ_0038646 siRNA (Fig. 2I and J).
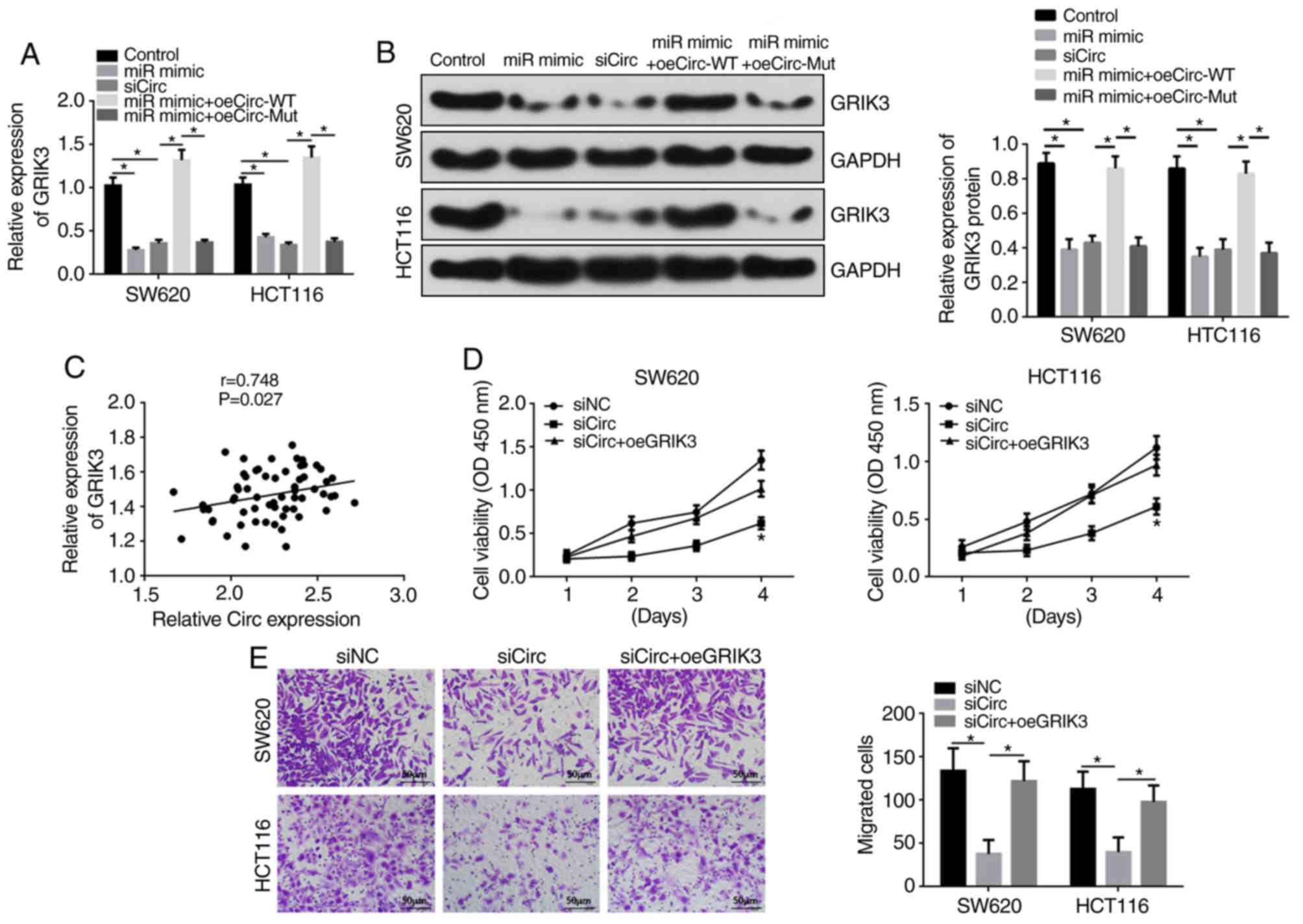
GRIK3 is a target of miR-331-3p
Bioinformatics analysis revealed that GRIK3 contains
a binding site for miR-331-3p (Fig.
3A). GRIK3 was upregulated or downregulated using oeGRIK3 or
siRNA, respectively (Fig. 3B). The
luciferase reporter assays were then repeated, and this confirmed
that transfection of miR-331-3p mimics decreased the luciferase
activity of SW620 and HCT116 cells transfected with the
WT-GRIK3-3′-UTR, but had no effect in SW620 and HCT116 cells
transfected with the Mut-GRIK3-3′-UTR (Fig. 3C). RT-qPCR and western blotting
analyses revealed that upregulation of miR-331-3p decreased GRIK3
mRNA and protein levels (Fig. 3D and
E). GRIK3 levels were inversely correlated with miR-331-3p in
CRC tissues (Fig. 3F). To the
author's best knowledge, the present study has shown the first
evidence suggesting that the downregulation of GRIK3 expression
significantly inhibits SW620 and HCT116 cell proliferation and
migration (Fig. 3G and H).
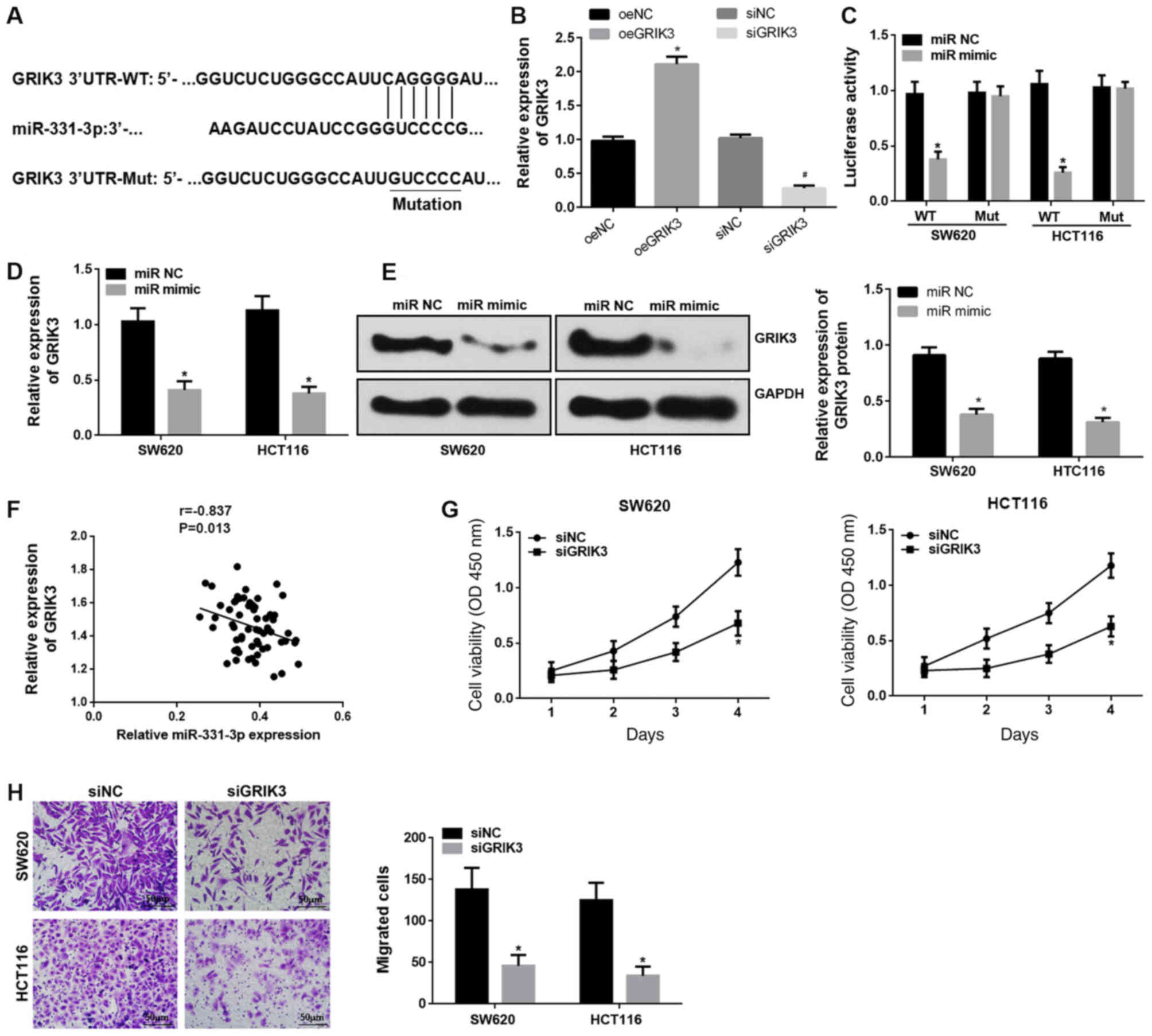 | Figure 3.GRIK3 is a target of miR-331-3p. (A)
Predicted binding site between miR-331-3p and GRIK3. (B) Expression
of GRIK3 following transfection with oeGRIK3 or siRNA. (C) GRIK3 is
a target of miR-331-3p, as shown by dual-luciferase reporter assay
in vitro. (D) RT-qPCR and (E) western blotting show that
upregulation of miR-331-3p repressed GRIK3 mRNA and protein levels
in human CRC cells, respectively. (F) Association between
miR-331-3p and GRIK3 expression in CRC tissues. Roles of GRIK3 in
CRC cell (G) proliferation and (H) migration. *P<0.05 vs. oeNC
group, miR NC and siNC group, respectively; #P<0.05
vs. siNC group. All experiments were performed in triplicate. CRC,
colorectal cancer; NC, negative control; si, small interfering;
miR, miR-331-3p; WT, wild-type; Mut, mutated; oe, overexpression
vector; OD, optical density; GRIK3, glutamate receptor ionotropic
kainate 3. |
Hsa_circ_0038646 accelerates human CRC
cell proliferation and migration by upregulating GRIK3 via sponging
of miR-331-3p
Compared to the control group, miR-331-3p
upregulation or hsa_circ_0038646 knockdown decreased mRNA and
protein levels of GRIK3 in SW620 and HCT116 cells, as shown by
RT-qPCR and western blotting (Fig. 4A
and B). Furthermore, transfection with oeCirc-WT, but not
oeCirc-Mut, abolished the effects of miR-331-3p mimics on GRIK3
levels compared with the siCirc group. GRIK3 expression was also
positively correlated with hsa_circ_0038646 in human CRC tissues
(Fig. 4C).
CCK-8 and Transwell assays showed that compared to
the siNC group, cell proliferation and migration were significantly
decreased in the siCirc group, whilst oeGRIK3 markedly rescued the
suppression effect of siCirc on SW620 and HCT116 cell proliferation
and migration (Fig. 4D and E).
Discussion
CRC is a fatal illness with high mortality and
morbidity (35), and high rates of
metastasis and recurrence (6).
Despite the use of surgical and adjuvant therapy, the survival rate
has not improved dramatically, and ~50% of patients die from local
recurrence or metastasis following surgery (36). Novel biomarkers and effective
therapeutic targets are urgently required. A single report has
demonstrated that miR-331-3p is significantly downregulated in both
human CRC cells and tissues (28).
This suppression of miR-331-3p expression accelerated cell
proliferation and inhibited apoptosis (14). miR-331-3p has also been reported to
be downregulated in cervical cancer (CC) (37). Upregulation of miR-331-3p restrained
migration and invasion, and facilitated apoptosis in CC cells
(28). Downregulation of miR-331-3p
has also been shown to inhibit apoptosis and promote cell
proliferation in human papillomavirus-positive CC (38), as well as facilitate proliferation,
invasion and migration in gastric cancer (39). The role of miR-331-3p in CRC cell
migration is unclear, and the potential up- and downstream
regulatory mechanisms of miR-331-3p are unknown. In the present
study, it was shown that miR-331-3p was downregulated in human CRC
tissues, and reduced expression of miR-331-3p resulted in increased
cell proliferation. Reduced expression of miR-331-3p also inhibited
cell migration in human CRC cells. It was predicted using
bioinformatic analysis that hsa_circ_0038646 and GRIK3 contain
miR-331-3p binding sites, and may be up- and downstream of
miR-331-3p, respectively. Based on this, it was hypothesized that
the hsa_circ_0038646/miR-331-3p/GRIK3 axis may play a pivotal role
in CRC progression.
The expression and functions of hsa_circ_0038646 in
CRC tissues and cells were assessed by RT-qPCR, and CCK-8 and
Transwell assays, respectively. To the author's knowledge, there
has been no report on the role of hsa_circ_0038646 in any disease.
It was determined in the present study that hsa_circ_0038646
exhibited increased expression compared to controls in human CRC
tissues, as well as various human CRC cell lines (SW480, HT29,
DLD-1, SW620 and HCT116). Hsa_circ_0038646 was especially highly
expressed in SW620 and HCT116 cells. This aberrant hsa_circ_0038646
expression was positively associated with higher tumor grade
(III/IV) in CRC tissues. Next, SW620 and HCT116 cells with reduced
expression of hsa_circ_0038646 were constructed. Decreased
expression of hsa_circ_0038646 inhibited CRC cell proliferation and
migration. These data indicated that hsa_circ_0038646 acts as an
oncogene in CRC progression. Previous reports have shown that
circRNAs are differentially expressed in various cancers, including
CRC (39). Increasing evidence has
indicated that circRNAs modulate multiple physiological and
pathological processes, including cell survival, proliferation,
metastasis, tumorigenesis and tumor progression, and may be
significant biomarkers in cancers (40,41). The
present study demonstrated that hsa_circ_0038646 is upregulated in
CRC, which may result in increased cell proliferation and
migration.
CircRNAs exert their functions by acting as miRNA
sponges (42). To further confirm
whether hsa_circ_0038646 exerts its role in CRC via sponging of
miR-331-3p, hsa_circ_0038646 reporter plasmids sequences were
generated containing the WT and Mut 3′-UTR, according to the
predicted binding site. Using luciferase reporter assays and
RT-qPCR, it was found that miR-331-3p was a potential target of
hsa_circ_0038646, and that hsa_circ_0038646 could negatively
regulate miR-331-3p expression. Furthermore, CCK-8 and Transwell
assays suggested that suppression of miR-331-3p inhibits the
effects of reduced hsa_circ_0038646 expression on CRC cell
proliferation and migration. These outcomes indicated that
miR-331-3p functions as a tumor inhibitor in CRC. The present study
is the first to report that hsa_circ_0038646 exerts its functions
in CRC via sponging of miR-331-3p.
miRNAs play roles in cancer by transcriptionally and
post-transcriptionally suppressing the expression of target genes
via complementation of the 3′-UTR of mRNAs (43). In the present study, a downstream
target of miR-331-3p was investigated. GRIK3 was identified as a
target of miR-331-3p in vitro, and inhibition of GRIK3
repressed CRC cell proliferation and migration. GRIK3 has been
reported to function as an oncogenic protein, and has served as a
prognostic and therapeutic target in several cancers, including
glioma (44), lung adenocarcinoma
(45), gastric cancer (31) and breast cancer (30). To the author's best knowledge, the
role of GRIK3 in CRC has not been reported, and the present study
is the first to demonstrate that GRIK3 functions as an oncogenic
protein in CRC.
In the present study, RT-qPCR and western blotting
were used to determine that GRIK3 expression was modulated by
hsa_circ_0038646 in human SW620 and HCT116 cells. This demonstrated
that hsa_circ_0038646 accelerates human CRC cell proliferation and
migration by upregulating GRIK3 via sponging of miR-331-3p.
Overall, the present study suggested that
hsa_circ_0007534 and GRIK3 act as tumor promoters in CRC
progression, and miR-331-3p can inhibit human CRC cell migration.
This demonstrates that the hsa_circ_0007534/miR-331-3p/GRIK3 axis
may be a promising therapeutic target for CRC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HD and JZ made substantial contributions to the
conception of the study, designing the experiments, interpretation
of the data and writing the paper. ZH and FF performed the
experiments and collected data. DC, LZ and EH acquired and analyzed
the data. JB and HW analyzed and interpreted the data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Clinical
Ethical Committee of Tianjin Baodi Hospital (approval no. 2018-6),
and performed in accordance with The Declaration of Helsinki.
Written informed consent was provided by all participants prior to
the study start.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lipton LR: Familial colorectal cancer and
polyposis genes, pathways and predictions (unpublished PhD thesis).
University of London. 2006.
|
|
2
|
Kahouli I, Tomaro-Duchesneau C and Prakash
S: Probiotics in colorectal cancer (CRC) with emphasis on
mechanisms of action and current perspectives. J Med Microbiol.
62:1107–1123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chua YJ and Zalcberg JR: Progress and
challenges in the adjuvant treatment of stage II and III colon
cancers. Expert Rev Anticancer Ther. 8:595–604. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hamilton SR and Aaltonen LA: Pathology and
genetics. Tumours of the digestive system. WHO Classification of
Tumours. 2:IARC Press. (Lyon). 103–119. 2000.
|
|
7
|
Wei W, Yang Y, Cai J, Cui K, Li RX, Wang
H, Shang X and Wei D: miR-30a-5p suppresses tumor metastasis of
human colorectal cancer by targeting ITGB3. Cell Physiol Biochem.
39:1165–1176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singh SR and Rameshwar P: MicroRNA in
development and in the progression of cancer. Springer. (New York,
NY). 2014. View Article : Google Scholar
|
|
10
|
Ryan BM, Robles AI and Harris CC: Genetic
variation in microRNA networks: The implications for cancer
research. Nat Rev Cancer. 10:389–402. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang L, Tang H, Thayanithy V, Subramanian
S, Oberg AL, Cunningham JM, Cerhan JR, Steer CJ and Thibodeau SN:
Gene networks and microRNAs implicated in aggressive prostate
cancer. Cancer Res. 69:9490–9497. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao D, Sui Y and Zheng X: MiR-331-3p
inhibits proliferation and promotes apoptosis by targeting HER2
through the PI3K/Akt and ERK1/2 pathways in colorectal cancer.
Oncol Rep. 35:1075–1082. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zanette DL, Rivadavia F, Molfetta GA,
Barbuzano FG, Proto-Siqueira R, Silva WA Jr, Falcão RP and Zago MA:
miRNA expression profiles in chronic lymphocytic and acute
lymphocytic leukemia. Braz J Med Biol Res. 40:1435–1440. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nymark P, Guled M, Borze I, Faisal A,
Lahti L, Salmenkivi K, Kettunen E, Anttila S and Knuutila S:
Integrative analysis of microRNA, mRNA and aCGH data reveals
asbestos- and histology-related changes in lung cancer. Genes
Chromosomes Cancer. 50:585–597. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Epis MR, Giles KM, Candy PA, Webster RJ
and Leedman PJ: miR-331-3p regulates expression of neuropilin-2 in
glioblastoma. J Neurooncol. 116:67–75. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo X, Guo L, Ji J, Zhang J, Zhang J, Chen
X, Cai Q, Li J, Gu Q, Liu B, et al: miRNA-331-3p directly targets
E2F1 and induces growth arrest in human gastric cancer. Biochem
Biophys Res Commun. 398:1–6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Epis MR, Giles KM, Kalinowski FC, Barker
A, Cohen RJ and Leedman PJ: Regulation of expression of
deoxyhypusine hydroxylase (DOHH), the enzyme that catalyzes the
activation of eIF5A, by miR-331-3p and miR-642-5p in prostate
cancer cells. J Biol Chem. 287:35251–35259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li X, Zhu J, Liu Y, Duan C, Chang R and
Zhang C: MicroRNA-331-3p inhibits epithelial-mesenchymal transition
by targeting ErbB2 and VAV2 through the Rac1/PAK1/β-catenin axis in
non-small-cell lung cancer. Cancer Sci. 110:1883–1896.
2019.PubMed/NCBI
|
|
20
|
Chen X, Luo H, Li X, Tian X, Peng B, Liu
S, Zhan T, Wan Y, Chen W, Li Y, et al: miR-331-3p functions as an
oncogene by targeting ST7L in pancreatic cancer. Carcinogenesis.
39:1006–1015. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ebbesen KK, Kjems J and Hansen TB:
Circular RNAs: Identification, biogenesis and function. Biochim
Biophys Acta. 1859:163–168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhong Y, Du Y, Yang X, Mo Y, Fan C, Xiong
F, Ren D, Ye X, Li C, Wang Y, et al: Circular RNAs function as
ceRNAs to regulate and control human cancer progression. Mol
Cancer. 17:792018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Salzman J: Circular RNA expression: Its
potential regulation and function. Trends Genet. 32:309–316. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ge J, Jin Y, Lv X, Liao Q, Luo C, Ye G and
Zhang X: Expression profiles of circular RNAs in human colorectal
cancer based on RNA deep sequencing. J Clin Lab Anal.
33:e229522019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao ZJ and Shen J: Circular RNA
participates in the carcinogenesis and the malignant behavior of
cancer. RNA Biol. 14:514–521. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang L, Song X, Chen X, Wang Q, Zheng X,
Wu C and Jiang J: Circular RNA CircCACTIN promotes gastric cancer
progression by sponging MiR-331-3p and regulating TGFBR1
expression. Int J Biol Sci. 15:1091–1103. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen HH, Zong J and Wang SJ: LncRNA
GAPLINC promotes the growth and metastasis of glioblastoma by
sponging miR-331-3p. Eur Rev Med Pharmacol Sci. 23:262–270.
2019.PubMed/NCBI
|
|
28
|
Luan X and Wang Y: LncRNA XLOC_006390
facilitates cervical cancer tumorigenesis and metastasis as a ceRNA
against miR-331-3p and miR-338-3p. J Gynecol Oncol. 29:e952018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu T, Song Z and Gai Y: Circular RNA
circ_0001649 acts as a prognostic biomarker and inhibits NSCLC
progression via sponging miR-331-3p and miR-338-5p. Biochem Biophys
Res Commun. 503:1503–1509. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xiao B, Kuang Z, Zhang W, Hang J, Chen L,
Lei T, He Y, Deng C, Li W, Lu J, et al: Glutamate ionotropic
receptor kainate type subunit 3 (GRIK3) promotes
epithelial-mesenchymal transition in breast cancer cells by
regulating SPDEF/CDH1 signaling. Mol Carcinog. 58:1314–1323.
2019.PubMed/NCBI
|
|
31
|
Gong B, Li Y, Cheng Z, Wang P, Luo L,
Huang H, Duan S and Liu F: GRIK3: A novel oncogenic protein related
to tumor TNM stage, lymph node metastasis, and poor prognosis of
GC. Tumour Biol. 39:10104283177043642017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Novák J and Fabian P: Comments on the TNM
classification of malignant tumours-7th edition. Klin Onkol.
24:149–150. 2011.(In Czech). PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li GF, Li L, Yao ZQ and Zhuang SJ:
Hsa_circ_0007534/miR-761/ZIC5 regulatory loop modulates the
proliferation and migration of glioma cells. Biochem Biophys Res
Commun. 499:765–771. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yin Q, Wang PP, Peng R and Zhou H: MiR-19a
enhances cell proliferation, migration, and invasiveness through
enhancing lymphangiogenesis by targeting thrombospondin-1 in
colorectal cancer. Biochem Cell Biol. 97:731–739. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang H, Wang R and Wang M: miR-331-3p
suppresses cell invasion and migration in colorectal carcinoma by
directly targeting NRP2. Oncol Lett. 18:6501–6508. 2019.PubMed/NCBI
|
|
37
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang M, Song Y and Zhai F: ARFHPV E7
oncogene, lncRNA HOTAIR, miR-331-3p and its target, NRP2, form a
negative feedback loop to regulate the apoptosis in the
tumorigenesis in HPV positive cervical cancer. J Cell Biochem.
119:4397–4407. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiang W, Zhang X, Chu Q, Lu S, Zhou L, Lu
X, Liu C, Mao L, Ye C, Timko MP, et al: The circular RNA profiles
of colorectal tumor metastatic cells. Front Genet. 9:342018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang WJ, Wang Y, Liu S, Yang J, Guo SX,
Wang L, Wang H and Fan YF: Silencing circular RNA hsa_circ_0000977
suppresses pancreatic ductal adenocarcinoma progression by
stimulating miR-874-3p and inhibiting PLK1 expression. Cancer Lett.
422:70–80. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang S, Zeng X, Ding T, Guo L, Li Y, Ou S
and Yuan H: Microarray profile of circular RNAs identifies
hsa_circ_0014130 as a new circular RNA biomarker in non-small cell
lung cancer. Sci Rep. 8:28782018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li G, Yang H, Han K, Zhu D, Lun P and Zhao
Y: A novel circular RNA, hsa_circ_0046701, promotes carcinogenesis
by increasing the expression of miR-142-3p target ITGB8 in glioma.
Biochem Biophys Res Commun. 498:254–261. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen K and Rajewsky N: The evolution of
gene regulation by transcription factors and microRNAs. Nat Rev
Genet. 8:93–103. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yuan Y, Xiang W, Yanhui L, Ruofei L, Yunhe
M, Jiewen L and Qing M: Dysregulation of microRNA-128 expression in
WHO grades 2 glioma is associated with glioma-associated epilepsy:
Down-regulation of miR-128 induces glioma-associated seizure.
Epilepsy Res. 127:6–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pradhan MP, Desai A and Palakal MJ:
Systems biology approach to stage-wise characterization of
epigenetic genes in lung adenocarcinoma. BMC Syst Biol. 7:1412013.
View Article : Google Scholar : PubMed/NCBI
|