Introduction
Dasatinib is a multikinase inhibitor, which has
potent effects against chronic leukemia and used in cases of
imatinib resistance as a second-generation targeted drug approved
by the Food and Drug Administration (1,2). It has
also been shown to act as potentiator of immunotherapy when used
with an anti-PD-1 inhibitor and as a pharmacologic on/off switch
for immune CAR-T cell activity (3,4).
Moreover, dasatinib has been shown to promote the clearance of
senescent cells, indicating its potential application in improving
the overall health of older adults (5,6). Several
molecular targets for dasatinib have been identified, including
BCR-ABL fusion protein, Src and the collagen receptor discoidin
domain receptor 2 (2,7,8).
Accumulating data has demonstrated the mechanisms by which
dasatinib actions are associated with the induction of apoptosis
(9), autophagy (10) and necroptosis (11), depending on the cell type being
targeted. However, it remains unclear whether dasatinib can evoke
pyroptosis in tumor cells.
Pyroptosis, a mode of necrotic cell death, is
characterized by cell swelling and the release of pro-inflammatory
molecules due to pore formation in the cell membrane (12). One of the main family of proteins
involved in pyroptosis are gasdermin proteins, cleavage fragments
of these proteins insert into the cell membrane. Gasdermin D
(GSDMD) mediates protection against bacterial infections, whereas
gasdermin E (GSDME) is involved in chemotherapy-induced pyroptosis
in tumor cells (13,14), which requires the activation of
caspase-3. The expression of the GSDME gene is promoted by
anti-oncogene p53 (15). Research
has shown that targeted drugs can induce pyroptosis in cancer
cells, such as trametinib in lung carcinoma cells, and cytotoxic
antitumor agent 5-fluorouracil in gastric cancer cells (16,17).
In order to determine whether dasatinib can induce
pyroptosis in tumor cells, two GSDME-expressing cell lines were
chosen for the present study. These cell lines have previously been
used to study chemotherapy-induced pyroptosis (13). To the best of our knowledge the
present study demonstrates for the first time that dasatinib
induces typical pyroptosis in human SH-SY5Y and A549 tumor
cells.
Materials and methods
Drugs and chemicals
Dasatinib was purchased from Selleck Chemicals. A 40
mM solution was prepared in dimethyl sulfoxide and stored at −20°C
until use. Doxorubicin (DOX), specific caspase-3 inhibitor
Z-DEVD-FMK (zDEVD) and pan-caspase inhibitor Z-VAD (OMe)-FMK (zVAD)
were obtained from MedChemExpress. Cell counting kit-8 (CCK-8) was
purchased from Bimake. Annexin V-propidium iodide (PI) apoptosis
kit was purchased from Beijing 4A Biotech Co., Ltd. Lactate
dehydrogenase (LDH) assay kit was obtained from Nanjing Jiancheng
Bioengineering Institute.
Cell lines and cell culture
Human neuroblastoma SH-SY5Y cell line and human
non-small cell lung cancer A549 cell line were purchased from The
Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences. The SH-SY5Y cell line was authenticated at 100% with an
ATCC profile by Meide Huasheng Detection Technology Co (584164.b2bname.com/), using the short tandem repeat
DNA profile method. SH-SY5Y cells were cultured in DMEM/F12 medium
(HyClone; GE Healthcare Life Sciences), and A549 cells were
maintained with F12 medium (HyClone; GE Healthcare Life Sciences).
The two cell lines were supplemented with 10% fetal bovine serum
(PAN-Biotech GmbH). The cells were incubated at 37°C in a
humidified atmosphere with 5% CO2.
CCK-8 assay
CCK-8 assay was determined according to the
manufacturer's protocol. Cells were seeded into a 96-well plate at
a density of 3,000 cells/well, incubated for 24 h at 37°C, and then
exposed to dasatinib or DOX for 72 h at 37°C. After co-incubation
with CCK-8 for 2 h at 37°C, the optical density (OD) values were
read by a microplate reader (Bio-Ra Laboratories, Inc.) at 450 nm.
Viability of the control group without drug was considered as 100%.
Cell survival rates was calculated as follows: (%)=(OD of the
drug-treated groups-OD of background)/(OD of the control group-OD
of background) ×100%. These rates were then plotted in GraphPad
Prism 5 (GraphPad Software, Inc.).
Western blot analysis
Cells were lysed with lysis buffer, which contained
50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, 50 mM sodium
fluoride, 1 mM dithiothreitol, 1% Triton X-100, 1 mM sodium
orthovanadate and protease inhibitors. The protein concentration
was measured using Quick Start™ Bradford 1× Dye Reagent (cat. no.
500-0205; Bio-Rad Laboratories, Inc.) with a microplate reader at
590 nm. A total of 20 µg protein/lane was separated via SDS-PAGE on
a 10 or 12.5% gel. The proteins were transferred to a PVDF membrane
(EMD Millipore), and then blocked with 5% skimmed milk at 4°C for 1
h. The membrane was incubated with primary antibodies overnight at
4°C, and incubated with the appropriate horseradish
peroxidase-conjugated secondary antibody for 1 h at room
temperature. The immunoreactive bands were visualized using the ECL
Plus Western Blotting Detection System (Bio-Rad Laboratories,
Inc.), and detected by an Amersham Imager 600 (GE Healthcare). The
GSDME (1:2,000; cat. no. ab215191) and GSDMD (1:1,000; cat. no.
ab210070) antibodies were obtained from Abcam. The antibodies
against poly (ADP-ribose) polymerase 1 (PARP-1; 1:1,000; cat. no.
9532) and cleaved caspase-3 (1:500; cat. no. 9664) were purchased
from Cell Signaling Technology, Inc. The p53 (1:2,000; cat. no.
sc-126) and β-actin (1:50,000; cat. no. sc-47778) antibodies were
purchased from Santa Cruz Biotechnology, Inc. The secondary
antibodies goat anti-rabbit IgG conjugated with HRP Conjugate
(1:1,000, cat. no. HS101-01) and goat anti-mouse IgG conjugated
with HRP (1:1,000, cat. no. HS201-01) were purchased from Beijing
Transgen Biotech Co., Ltd.
Determination of apoptotic cells via
Annexin V/PI staining
Cells were collected after exposure to the drugs for
24 h (SY5Y cells) and 48 h (A549 cells) and then stained with 5 µl
Annexin V and final concentration of 2 µg/ml PI for 10 min at room
temperature in the dark according to the manufacturer's protocols.
The fluorescent intensities of the various groups were detected by
a BD FACSCalibur flow cytometer (BD Biosciences) and were analyzed
by CellQuest Pro software version 5.1 (BD Biosciences).
Identification of dead cells stained
with PI
The PI-stained assay was used for the identification
of dead cells. The cells were stained with 2 µg/ml PI in the dark
for 10 min at room temperature after collection. The proportion of
PI-positive cells was detected by a BD FACSCalibur flow cytometer
and analyzed by CellQuest Pro software version 5.1.
LDH release assay
The LDH release assay was performed following the
manufacturer's protocols. The total LDH present in the culture
medium from the control group was set as 100%, and LDH release was
calculated as follows: LDH release (%)=(OD of the drug-treated
group-OD of blank control group)/(OD of the maximum group-OD of
blank control group) ×100%.
RNA interference
Small interfering (si)RNA against p53 was
synthesized by Invitrogen; Thermo Fisher Scientific, Inc. The
sequences of p53 were as follows: p53 siRNA: Forward,
5′-CAGCACATGACGGAGGTTGT-3′ and reverse,
3′-TCATCCAAATACTCCACACGC-5′; p53 siRNA: Forward,
5′-GAGGTTGGCTCTGACTGTACC-3′ and reverse,
3′-TCCGTCCCAGTAGATTACCAC-5′. siRNA (100 pmol) was transfected into
A549 cells using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) following the manufacturer's protocols.
After 6 h transfection the cell were then maintained with refreshed
culture medium for 18 h (the total transfection time was 24 h), the
cells were then used for subsequent experiments. The p53 protein
level was detected by western blot analysis.
Cell recovery assay
A549 cells were seeded into a 96-well plate at a
density of 3,000 cells/well, incubated for 24 h at 37°C. After 24 h
of exposure to dasatinib at 37°C, the drug was washed out. The
cells were washed with phosphate buffer saline solution three times
and then refreshed with new F12 culture medium. After incubation
for 48 h at 37°C, floating cells were transferred to a
centrifugation tube, and the adherent cells were digested for 2 min
with 0.1% trypsinase solution. Cells were then centrifuged for 5
min at 800 × g at room temperature, and then suspended in phosphate
buffer. The cells were counted using a Coulter counter (Beckman
Coulter, Inc.). The percentage of adherent cells was calculated as
follows: %=Adherent cell number/(adherent cell number + floating
cell number) ×100%.
Statistical analysis
Data are presented as the mean ± SD from three
independent experiments. Statistical analysis was calculated using
ANOVA followed by Tukey's test using SSPS 19.0 (IBM Corp.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Dasatinib has distinct effects on the
survival rates of SH-SY5Y and A549 cells
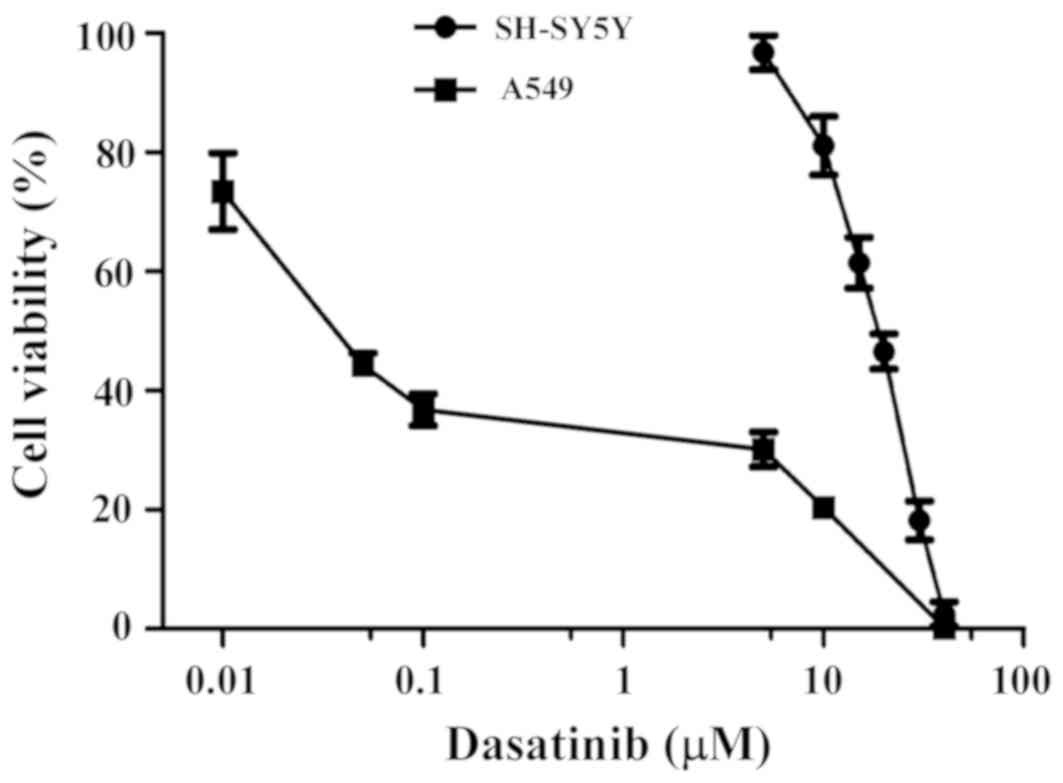
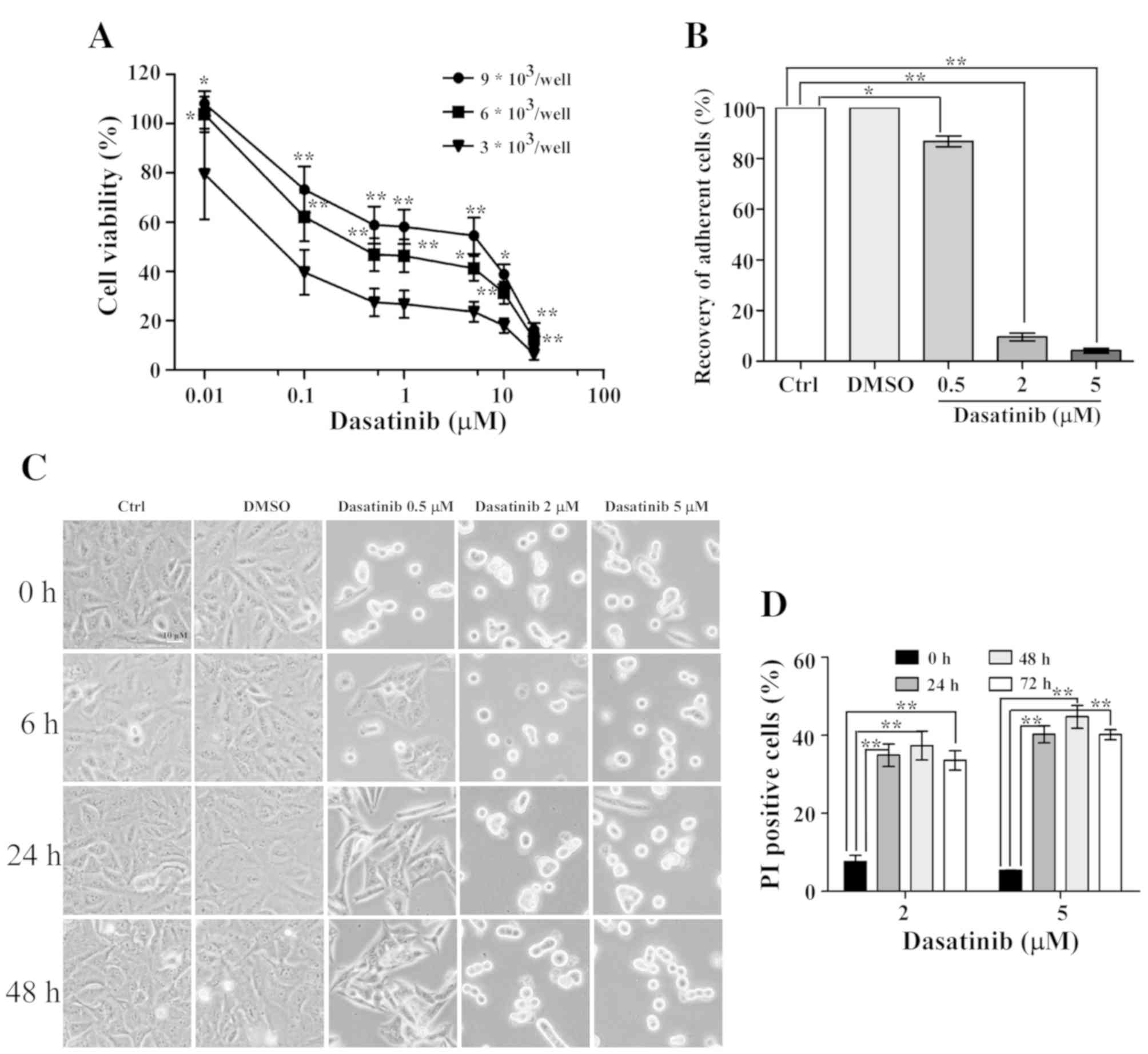
The effects of dasatinib treatment on cell survival
rates were detected using the CCK-8 method. As shown in Fig. 1, SH-SY5Y cells were less sensitive to
dasatinib than the A549 cells, and the IC50 value was
17.9 µM. Moreover, the range of viable concentrations of dasatinib
was narrow in SH-SY5Y cells. Although the cell viability of SH-SY5Y
cells was 81.1% with 10 µM dasatinib treatment, almost all of the
cells died after exposure to 40 µM of the drug. In contrast to
SH-SY5Y cells, A549 cells were more sensitive to dasatinib. The
inhibition rate was 63.2% when the cells were treated with 0.01 µM
dasatinib. However, the survival rates declined very slowly from
0.5 to 5 µM dasatinib treatment (Fig.
1).
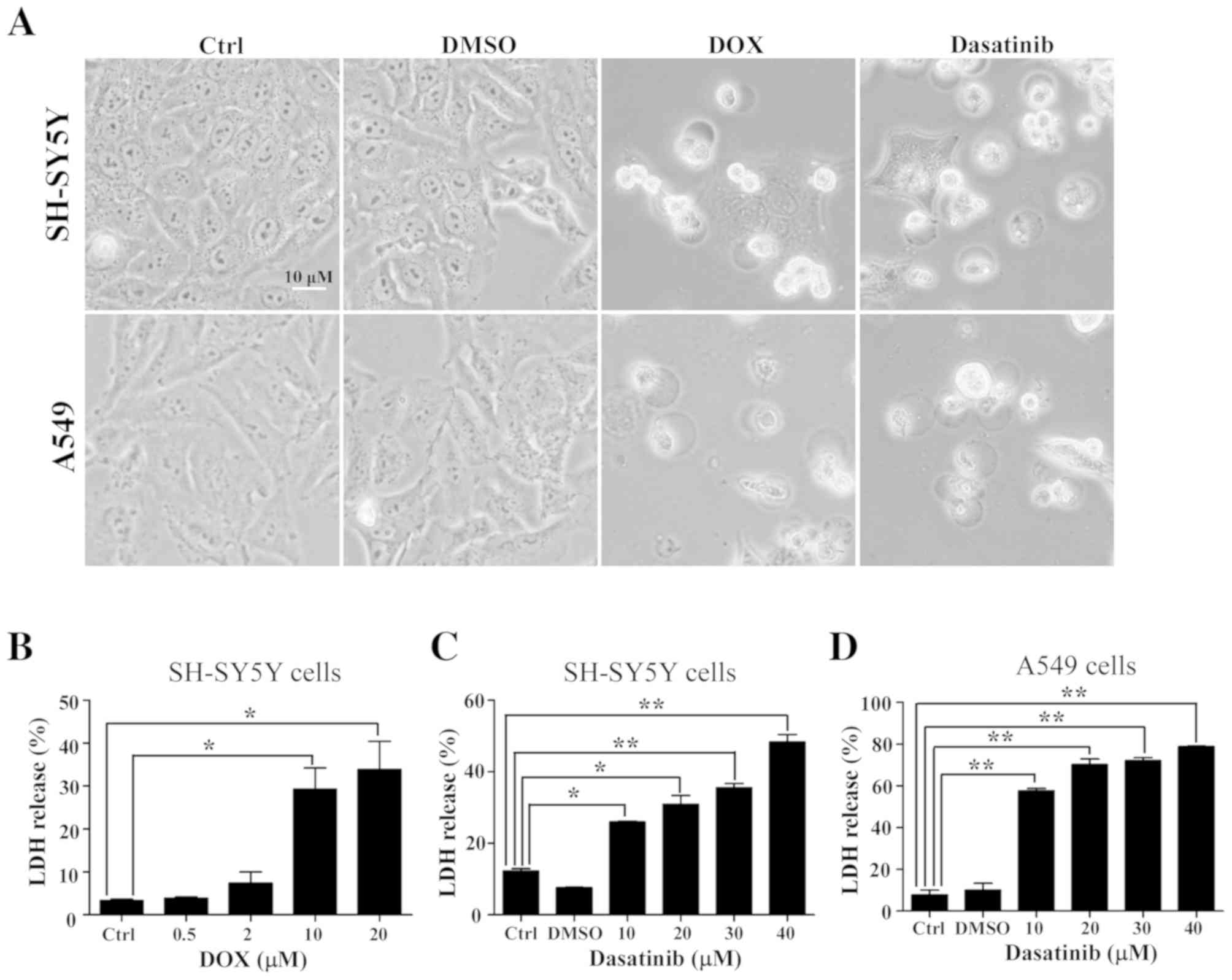
Dasatinib treatment induces typical
pyroptosis in SH-SY5Y and A549 cells
The typical characteristics of pyroptosis, including
the large bubbled morphology, the generation of GSDME-N terminal
fragments and the release of LDH (13), were significantly induced in the
SH-SY5Y and A549 cells by DOX, a positive control drug for tumor
chemotherapy (Figs. 2A and B and
3A). This is consistent with results
from a previous study (13). Similar
characteristics were observed in SH-SY5Y and A549 cells after
exposure to 30 or 40 µM dasatinib for 48 h (Figs. 2 and 3B
and C). Interestingly, cleavage of GSDMD was detected in
SH-SY5Y cells after treatment with either dasatinib or DOX
(Fig. 3A and B). While there was a
high level of LDH release in A549 cells following treatment with 10
µM dasatinib, very few GSDME-N fragments were detected (Figs. 2C and 3C).
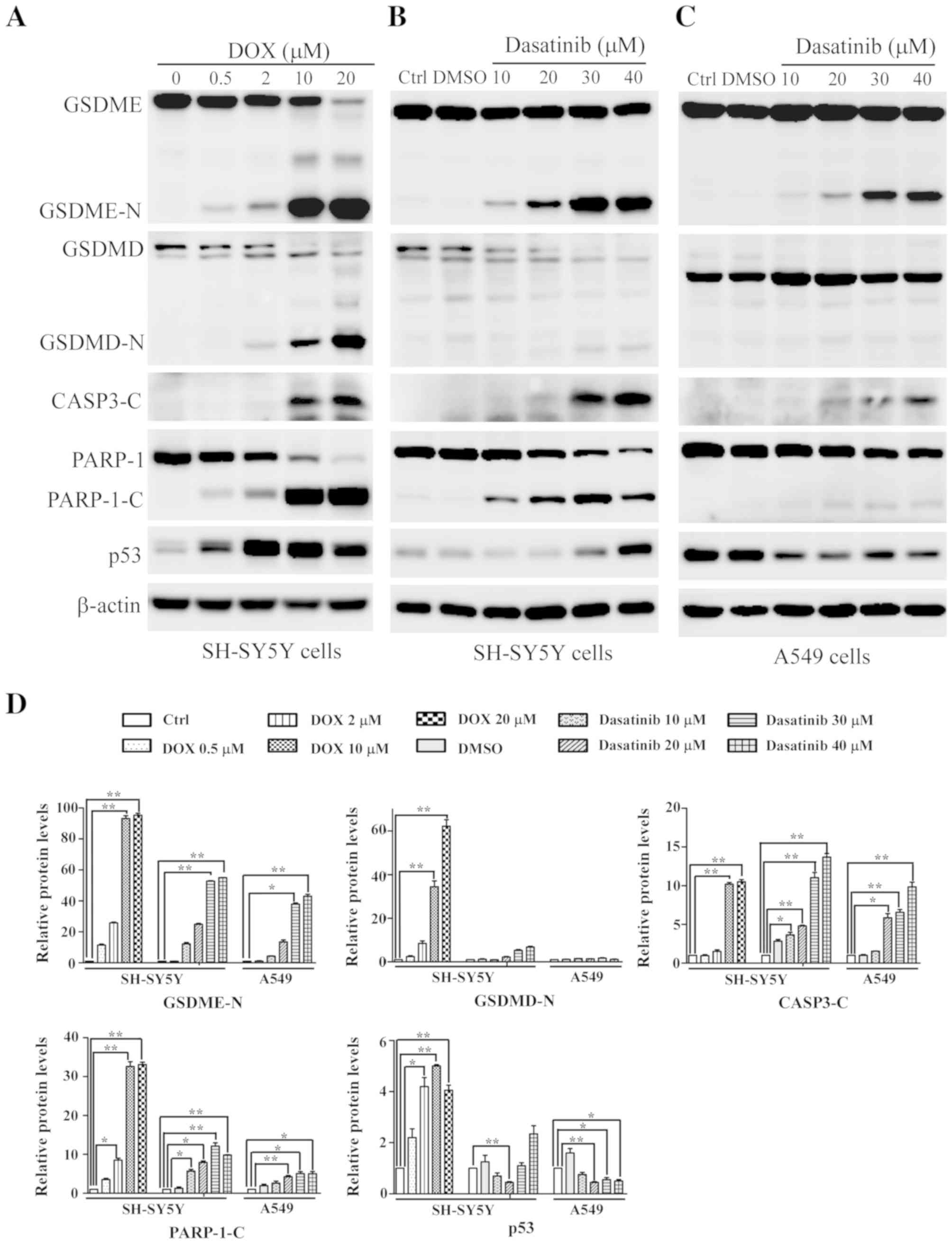 | Figure 3.Changes in pyroptosis-related
proteins detected with western blotting. SH-SY5Y cells were treated
with (A) DOX or (B) dasatinib for 24 h, and (C) A549 cells were
exposed to dasatinib for 48 h. One representative result from three
independent experiments is shown. (D) Relative amounts of protein
levels were quantified. *P<0.05, **P<0.01 represents the drug
treated groups vs. control group. DOX, doxorubicin; Ctrl, control;
GSDME, gasdermin E; GSDME-N N-terminal fragment of gasdemin E;
GSDMD, gasdermin D; GSDMD-N, N-terminal fragment of gasdemin D;
CASP3-C, cleaved caspase-3; PARP-1, poly (ADP-ribose) polymerase 1;
PARP-1-C, cleaved fragment of PARP-1. |
Effect of dasatinib on p53 expression
differs between SH-SY5Y and A549 cells
During apoptotic progression, the protein level of
tumor suppressor gene p53 gradually increases. Therefore,
the present study investigated whether p53 is associated with
dasatinib-induced pyroptosis. Increased p53 protein levels were
observed in SH-SY5Y cells after treatment with dasatinib or DOX,
especially in the DOX-treated group (Fig. 3A and B). By contrast, A549 cells
showed a reduction of p53 protein levels after exposure to
dasatinib (Fig. 3C), suggesting
differences in p53 expression between different cell lines in
response to dasatinib treatment.
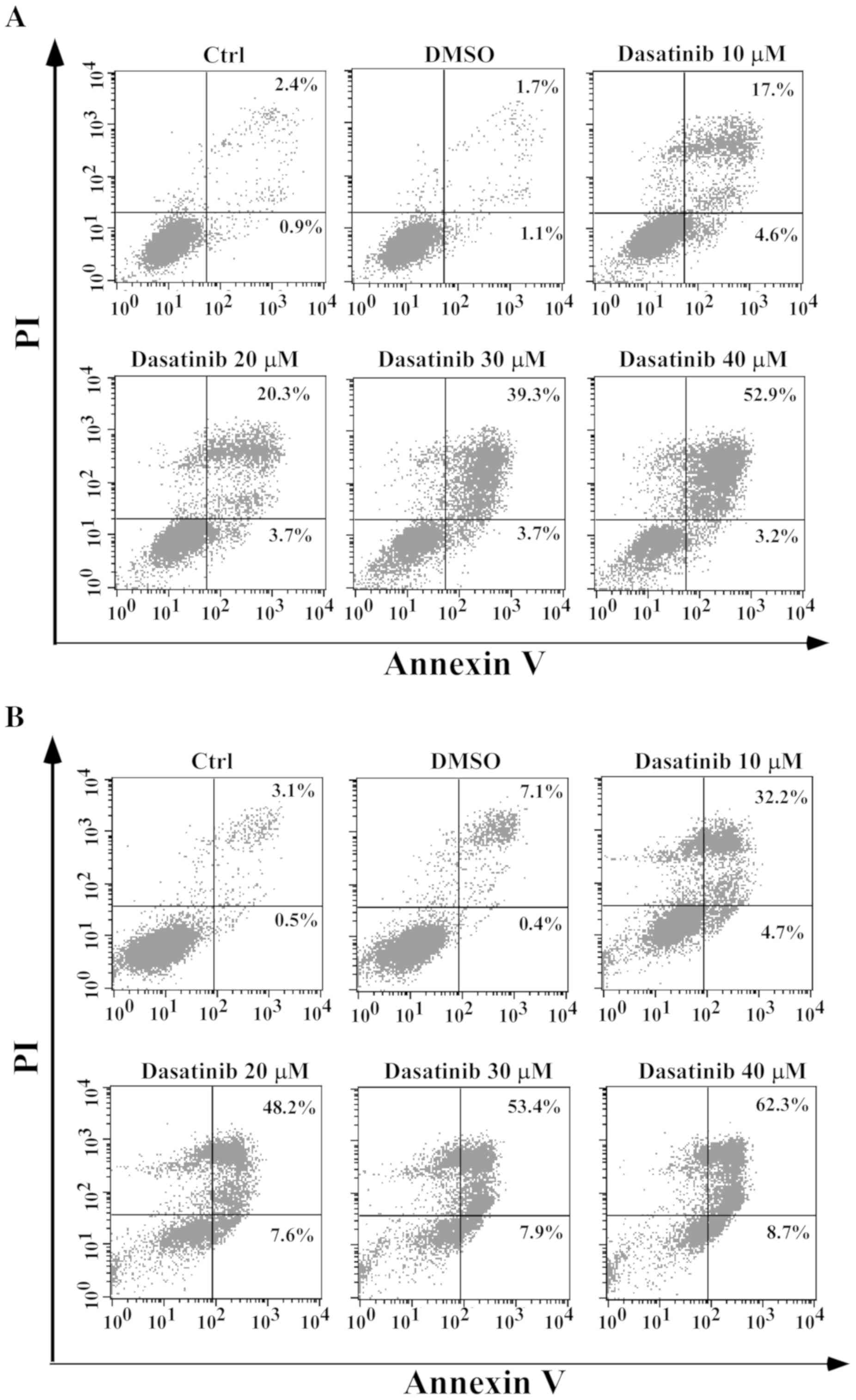
Dasatinib has distinct effects on the
apoptotic response in SH-SY5Y and A549 cells
As pyroptosis is secondary to apoptosis and the
cleavage of GSDME requires the activation of caspase-3 (13,14),
apoptotic characteristics in relation to pyroptosis were
investigated. In SH-SY5Y cells, apoptotic cells with Annexin V/PI
staining, activation of caspase-3 and PARP-1 cleavage were
associated with the occurrence of pyroptotic features after
exposure to dasatinib, in a concentration-dependent manner
(Figs. 3B and 4A). However, a notable apoptotic response
following dasatinib treatment was observed in the A549 cells. A
high percentage of Annexin V-stained cells and weak cleavages of
caspase-3 and PARP-1 were detected following treatment with 10 µM
dasatinib (Figs. 3C and 4B), inconsistent with the appearance of
pyroptotic features. This suggests that different pyroptotic events
occurred in the two cell lines after exposure to dasatinib.
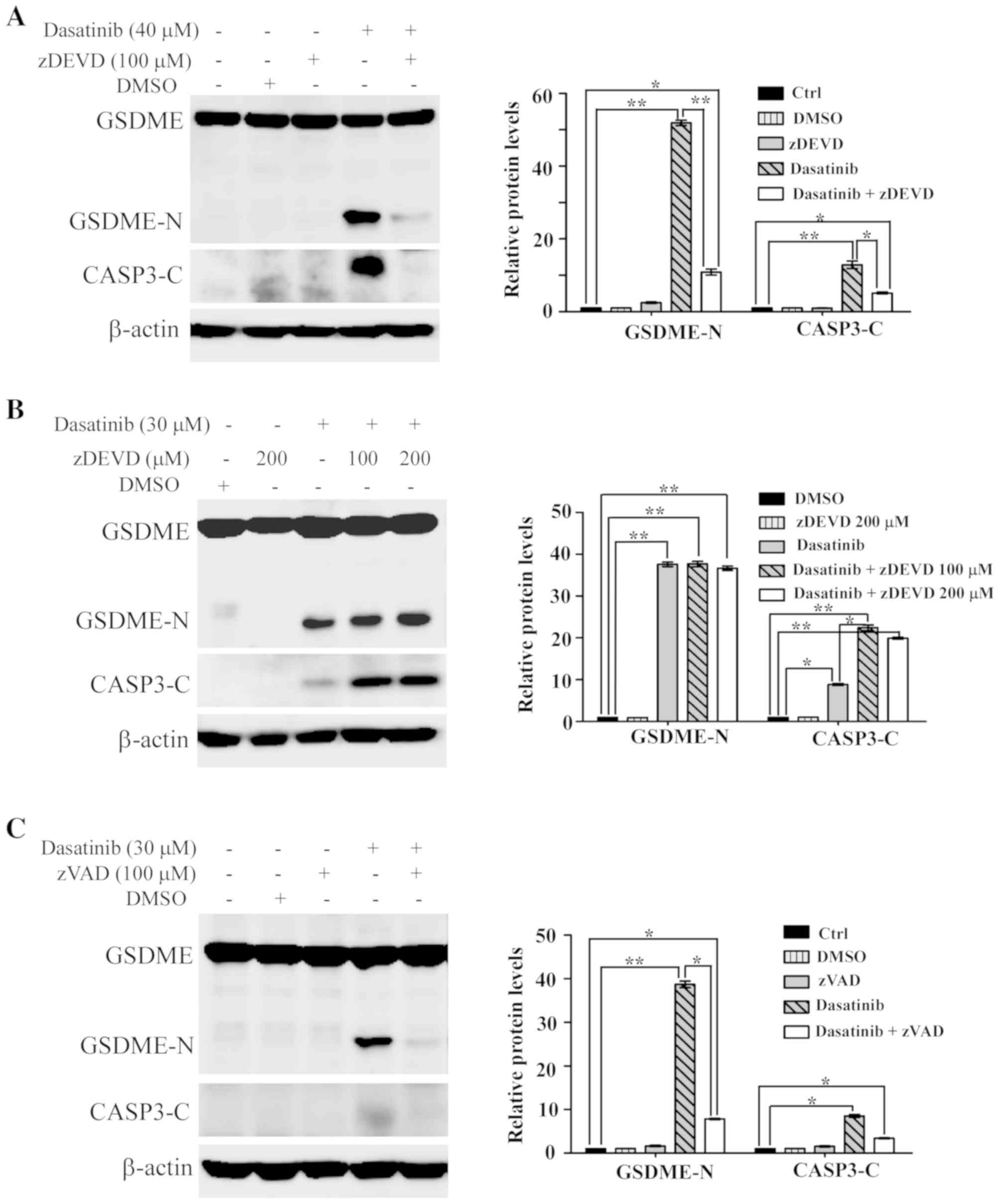
Activation of caspase is required for
dasatinib-induced pyroptosis
It has been reported that chemotherapy drug-induced
pyroptosis is mediated by caspase-3 (13,14). To
elucidate the role of caspase-3 in dasatinib-induced pyroptosis,
the specific caspase-3 inhibitor zDEVD was used to inhibit
activated caspase-3 in the cells. As shown in Fig. 5A, the cleavage of both caspase-3 and
GSDME was notably inhibited in SH-SY5Y cells pre-treated with
zDEVD. This suggests that the activation of caspase-3 was essential
to dasatinib-induced pyroptosis in SH-SY5Y cells.
Unexpectedly, the activation of caspase-3 and the
generation of GSDME-N fragments were not suppressed by
pre-treatment with zDEVD in A549 cells (Fig. 5B). However, the activation of
caspase-3 and the generation of GSDME-N fragments in A549 cells
were significantly suppressed by the pan-caspase inhibitor, zVAD
(Fig. 5C).
Number of cells affects A549 cell
sensitivity to dasatinib
As previously reported, the IC50 value of
dasatinib in A549 cells was >5 µM, as measured by the MTT method
(9). In the present study, the
IC50 value was 0.04 µM, as determined by the CCK-8
method. Therefore, the reason for this notable difference was
explored. A549 cells were seeded at various densities in a 96-well
plate. The IC50 value of dasatinib in A549 cells was 2.5
µM at a seeding density of 9×103 cells/well (Fig. 6A), suggesting that the number of
cells affects cell viability following dasatinib treatment.
After 24 h of exposure to dasatinib, the drug was
washed out and the cells were refreshed with a new culture medium.
After 6 h of refreshment, some of the cells had recovered and
adhered following 0.5 µM dasatinib treatment. The percentage of
non-adherent cells was only 13.2% 48 h after the medium was
replaced, suggesting that most of the A549 cells were still alive
(Fig. 6B and C). However, <10% of
the cells had adhered in the 2 or 5 µM dasatinib treatment groups
48 h after the medium was replaced. In order to differentiate the
live cells from the dead cells, a PI single staining experiment was
performed. These data showed high percentages of PI-stained cells
after exposure to 2 or 5 µM dasatinib for 24 h. Furthermore, the
proportion of PI positive cells remained stable after continuous
incubation with dasatinib for 72 h (Fig.
6D).
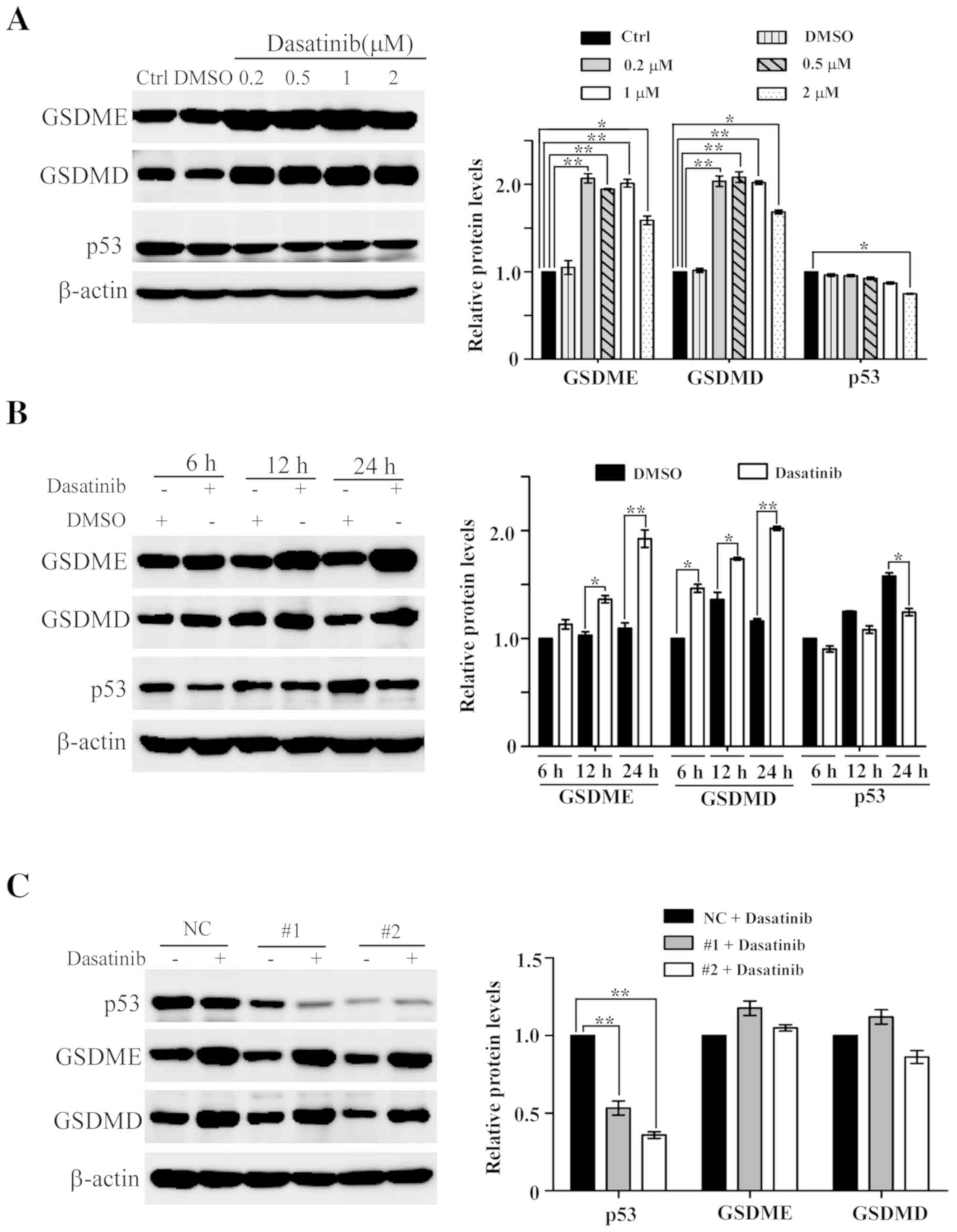
Low concentration of dasatinib results
in an increase of GSDME and GSDMD protein levels in A549 cells
During dasatinib-induced pyroptosis in A549 cells,
it was found that the protein levels of GSDME and GSDMD increased
slightly following treatment with 10 or 20 µM dasatinib (Fig. 3C). This led to further experiments to
clarify whether dasatinib treatment itself results in the increase
in protein levels. As shown in Fig.
7A, the protein levels of GSDME and GSDMD are significantly
elevated in A549 cells following treatment with lower
concentrations of dasatinib, and this increase was both
concentration-dependent and -independent of changes in p53 protein
levels. A time point experiment showed that the protein levels of
GSDME and GSDMD increased after A549 cells following exposure to
0.2 µM dasatinib for 6, 12 or 24 h (Fig.
7B). However, the p53 protein levels in the dasatinib-treated
groups were lower than those in the untreated groups.
To rule out the possibility that p53 participates in
the regulation of dasatinib-related GSDME and GSDMD expression, RNA
interference was used to knock down p53 in A549 cells. As shown in
Fig. 7C, p53 protein levels were
significantly reduced following siRNA transfection. The protein
levels of GSDME and GSDMD were still upregulated in the p53
knockdown cells treated with 0.5 µM dasatinib for 24 h,
demonstrating that the expression levels of GSDME and GSDMD are
upregulated in response to dasatinib in a p53-independent
manner.
Discussion
In the present study, the characteristics of
dasatinib-induced pyroptosis in gasdermin-expressing human SH-SY5Y
and A549 tumor cells, have been described. To the best of our
knowledge, this is the first report showing that dasatinib can
induce pyroptosis in tumor cells. Distinct pyroptotic features are
also shown in dasatinib-treated A549 and SH-SY5Y cells. In addition
to apoptosis, necroptosis and autophagy, pyroptosis is another type
of cell death induced by dasatinib action in both normal and tumor
cells. As dasatinib is used in the treatment of leukemia, it is
useful for the demonstration of dasatinib-induced pyroptosis in
leukemia cell lines. However, to the best of our knowledge, there
are few GSDME-expressing leukemia cell lines, including HL-60 cell
and K562 cell lines (13). The
significance of dasatinib-induced pyroptosis may be associated with
micro-environment conditions in tumor cells, such as inflammation
and therapy efficacy. It may be valuable to explain the mechanism
of dasatinib in overcoming drug resistance in lung tumors, such as
T790M resistance (18).
According to experiments in the present study, the
positive control drug DOX always induced typical pyroptosis in
several cell lines (data not shown), in contrast to dasatinib
treatment. There are no differences in the induction of pyroptosis
in both cell lines (Figs. 2 and
3). Apoptotic cells with Annexin
V/PI staining were not accurately detected in the DOX-treated
groups due to the overlapping of DOX auto-fluorescence and PI
fluorescence.
As previously reported, GSDME meditates the
induction of pyroptosis by chemotherapy agents (13,14).
Another protein linked to pyroptosis, GSDMD, may play a role in
tumor pathology. Treatment with dasatinib promoted the cleavage of
both GSDME and GSDMD in SH-SY5Y cells (Fig. 3B). In non-small cell lung cancer,
reduction of GSDMD can inhibit proliferation and is associated with
an improved prognosis (19).
Metformin can induce pyroptosis via the cleavage of GSDMD in human
esophageal carcinoma cells (20). It
is likely that more members of the gasdermin protein family will be
shown to have roles in tumor pathology and therapy efficacy.
The question remains as to the reason for different
GSDME-expressing tumor cells having distinct pyroptotic responses
upon treatment with chemotherapy agents. In the present study, it
was found that the caspase-3 specific inhibitor zDEVD did not
suppress the activation of caspase-3 and the apoptotic rate is
inconsistent with the cleavage of GSDME in A549 cells (Figs. 3C and 4B), therefore it is likely that other
caspases are involved. Caspase-1 can induce apoptosis in
GSDMD-deficient cells (21).
Caspase-8 is a switch molecule for apoptosis, pyroptosis and
necroptosis (22). It is commonly
known that the continuous expression of p53 leads to the initiation
of apoptosis after treatment with cytotoxic antitumor agents
(23). As pyroptosis is secondary to
apoptosis, high expression of p53 protein is proportional to the
amount of cleavage fragments of GSDME in SH-SY5Y cells treated with
dasatinib or DOX (Fig. 3). However,
pyroptotic characteristics were also observed upon weak activation
of caspase-3 and lower levels of p53 protein after exposure to
dasatinib in A549 cells (Figs. 3C
and 4B). The underlying mechanisms
of these effects are currently being studied in our lab.
In the present study it is reported that low
concentrations of dasatinib can result in an increase in protein
levels of GSDME and GSDMD in A549 cells in a p53-independent manner
(Fig. 7). To the best of our
knowledge, this is the first study to show that dasatinib can
induce to the upregulation of GSDME and GSDMD. Induction of GSDME
expression by p53 was previously elucidated as a response to
chemotherapy treatment, such as DOX or etoposide (15). The expression of GSDME is also
elevated by glucocorticoids and forskolin, an activator of protein
kinase A, however increased expression was not sufficient to induce
apoptosis (24). Transfection of
GSDME gene-carrying plasmids have been shown to increase apoptosis
in heptocellular carcinoma cells (25), suggesting that GSDME has cytotoxic
effects in tumor cells. It is unclear whether increased levels of
GSDME can inhibit cell proliferation in the present study.
Therefore, further investigation into the mechanism by which
dasatinib modulates the expression of GSDME and GSDMD proteins is
needed.
In the present study, it was discovered that higher
concentrations of dasatinib is required for induction of pyroptosis
in A549 cells compared with SH-SY5Y cells. A high percentage of
apoptotic cells was detected after exposure to 10 µM dasatinib
(Fig. 4), whereas it failed to
induce cleavage fragment of GSDME in A549 cells (Fig. 3C). It may be that dasatinib can
induce specific type of cell death in A594 cells. The cellular
context of A549 cells may be responsible for it. High endogenous
levels of nuclear factor erythroid 2-related factor 2 have been
reported in A549 cells (26). In
addition, high p53 expression was previously detected in A549 cells
(27). As CCK-8 in this study for
assessing cell survival, the sensitivity of A549 cells to dasatinib
was influenced by cell numbers (Fig.
6). The possibility that culture volume affects the action of
dasatinib on A549 cells has been ruled out (priliminary data not
shown). Notably, suppression of Src activity in A549 cells in
response to 150 nM dasatinib has been observed previously (9,28),
suggesting that lower concentrations of dasatinib can play an
inhibitory role. In a recent report, pyroptosis was shown to be
induced in A549 cells by the chemotherapy agent cisplatin, but not
by paclitaxel (29). These
differences may be due to the heterogeneity of A549 cell
populations as three sub-types were discovered according to cell
morphological and molecular features (30).
In conclusion, dasatinib can induce typical
pyroptosis in tumor cells and promote the expression of both GSDME
and GSDMD in some types of tumor cells. These findings will broaden
the understanding of the roles that pyroptosis plays in tumor
cells. In addition, it is helpful to explain the various actions of
dasatinib on normal and tumor cells.
Acknowledgements
Not applicable.
Funding
The study was supported by a grant from The Natural
Scientific Foundation of China (grant no. 31471150).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
QH conceived and designed the study. JZ and YC
performed the experiments and analyzed the data. QH and JZ drafted
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Institute Review Board.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DOX
|
doxorubicin
|
|
GSDMD
|
gasdermin D
|
|
GSDME
|
gasdermin E
|
|
LDH
|
lactate dehydrogenase
|
|
PARP-1
|
poly (ADP-ribose) polymerase 1
|
|
PI
|
propidium iodide
|
References
|
1
|
Rossari F, Minutolo F and Orciuolo E:
Past, present, and future of Bcr-Abl inhibitors: From chemical
development to clinical efficacy. J Hematol Oncol. 11:842018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shah NP, Tran C, Lee FY, Chen P, Norris D
and Sawyers CL: Overriding imatinib resistance with a novel ABL
kinase inhibitor. Science. 305:399–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tu MM, Lee FYF, Jones RT, Kimball AK,
Saravia E, Graziano RF, Coleman B, Menard K, Yan J, Michaud E, et
al: Targeting DDR2 enhances tumor response to anti-PD-1
immunotherapy. Sci Adv. 5:eaav24372019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mestermann K, Giavridis T, Weber J, Rydzek
J, Frenz S, Nerreter T, Mades A, Sadelain M, Einsele H and Hudecek
M: The tyrosine kinase inhibitor dasatinib acts as a pharmacologic
on/off switch for CAR T cells. Sci Transl Med. 11:eaau59072019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu Y, Tchkonia T, Pirtskhalava T, Gower
AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M,
et al: The Achilles' heel of senescent cells: From transcriptome to
senolytic drugs. Aging Cell. 14:644–658. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kirkland JL, Tchkonia T, Zhu Y,
Niedernhofer LJ and Robbins PD: The clinical potential of senolytic
drugs. J Am Geriatr Soc. 65:2297–2301. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Von Massenhausen A, Sanders C, Bragelmann
J, Konantz M, Queisser A, Vogel W, Kristiansen G, Duensing S,
Schrock A, Bootz F, et al: Targeting DDR2 in head and neck squamous
cell carcinoma with dasatinib. Int J Cancer. 139:2359–2369. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Das J, Chen P, Norris D, Padmanabha R, Lin
J, Moquin RV, Shen Z, Cook LS, Doweyko AM, Pitt S, et al:
2-aminothiazole as a novel kinase inhibitor template.
Structure-activity relationship studies toward the discovery of
N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)
−1-piperazinyl)]-2-methyl-4-pyrimidinyl]amino)]-1,3-thiazole-5-
carboxamide (dasatinib, BMS-354825) as a potent pan-Src kinase
inhibitor. J Med Chem. 49:6819–6832. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Johnson FM, Saigal B, Tran H and Donato
NJ: Abrogation of signal transducer and activator of transcription
3 reactivation after Src kinase inhibition results in synergistic
antitumor effects. Clin Cancer Res. 13:4233–4244. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang X, Wang J, Dai J, Shao J, Ma J, Chen
C, Ma S, He Q, Luo P and Yang B: Autophagy protects against
dasatinib-induced hepatotoxicity via p38 signaling. Oncotarget.
6:6203–6217. 2015.PubMed/NCBI
|
|
11
|
Xu Z, Jin Y, Yan H, Gao Z, Xu B, Yang B,
He Q, Shi Q and Luo P: High-mobility group box 1 protein-mediated
necroptosis contributes to dasatinib-induced cardiotoxicity.
Toxicol Lett. 296:39–47. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi J, Gao W and Shao F: Pyroptosis:
Gasdermin-mediated programmed necrotic cell death. Trends Biochem
Sci. 42:245–254. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Gao W, Shi X, Ding J, Liu W, He H,
Wang K and Shao F: Chemotherapy drugs induce pyroptosis through
caspase-3 cleavage of a gasdermin. Nature. 547:99–103. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rogers C, Fernandes-Alnemri T, Mayes L,
Alnemri D, Cingolani G and Alnemri ES: Cleavage of DFNA5 by
caspase-3 during apoptosis mediates progression to secondary
necrotic/pyroptotic cell death. Nat Commun. 8:141282017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Masuda Y, Futamura M, Kamino H, Nakamura
Y, Kitamura N, Ohnishi S, Miyamoto Y, Ichikawa H, Ohta T, Ohki M,
et al: The potential role of DFNA5, a hearing impairment gene, in
p53-mediated cellular response to DNA damage. J Hum Genet.
51:652–664. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu H, Zhang S, Wu J, Chen M, Cai MC, Fu Y,
Li W, Wang J, Zhao X, Yu Z, et al: Molecular targeted therapies
elicit concurrent apoptotic and GSDME-dependent pyroptotic tumor
cell death. Clin Cancer Res. 24:6066–6077. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Yin B, Li D, Wang G, Han X and Sun
X: GSDME mediates caspase-3-dependent pyroptosis in gastric cancer.
Biochem Biophys Res Commun. 495:1418–1425. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Watanabe S, Yoshida T, Kawakami H,
Takegawa N, Tanizaki J, Hayashi H, Takeda M, Yonesaka K, Tsurutani
J and Nakagawa K: T790M-selective EGFR-TKI combined with dasatinib
as an optimal strategy for overcoming EGFR-TKI resistance in
T790M-positive non-small cell lung cancer. Mol Cancer Ther.
16:2563–2571. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao J, Qiu X, Xi G, Liu H, Zhang F, Lv T
and Song Y: Downregulation of GSDMD attenuates tumor proliferation
via the intrinsic mitochondrial apoptotic pathway and inhibition of
EGFR/Akt signaling and predicts a good prognosis in non-small cell
lung cancer. Oncol Rep. 40:1971–1984. 2018.PubMed/NCBI
|
|
20
|
Wang L, Li K, Lin X, Yao Z, Wang S, Xiong
X, Ning Z, Wang J, Xu X, Jiang Y, et al: Metformin induces human
esophageal carcinoma cell pyroptosis by targeting the miR-497/PELP1
axis. Cancer Lett. 450:22–31. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsuchiya K, Nakajima S, Hosojima S, Thi
Nguyen D, Hattori T, Manh Le T, Hori O, Mahib MR, Yamaguchi Y,
Miura M, et al: Caspase-1 initiates apoptosis in the absence of
gasdermin D. Nat Commun. 10:20912019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fritsch M, Günther SD, Schwarzer R, Albert
MC, Schorn F, Werthenbach JP, Schiffmann LM, Stair N, Stocks H,
Seeger JM, et al: Caspase-8 is the molecular switch for apoptosis,
necroptosis and pyroptosis. Nature. 575:683–687. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
El-Deiry WS: The role of p53 in
chemosensitivity and radiosensitivity. Oncogene. 22:7486–7495.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Webb MS, Miller AL and Thompson EB: In CEM
cells the autosomal deafness gene dfna5 is regulated by
glucocorticoids and forskolin. J Steroid Biochem Mol Biol.
107:15–21. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang CJ, Tang L, Shen DW, Wang C, Yuan QY,
Gao W, Wang YK, Xu RH and Zhang H: The expression and regulation of
DFNA5 in human hepatocellular carcinoma DFNA5 in hepatocellular
carcinoma. Mol Biol Rep. 40:6525–6531. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Homma S, Ishii Y, Morishima Y, Yamadori T,
Matsuno Y, Haraguchi N, Kikuchi N, Satoh H, Sakamoto T, Hizawa N,
et al: Nrf2 enhances cell proliferation and resistance to
anticancer drugs in human lung cancer. Clin Cancer Res.
15:3423–3432. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang HX, Chen Y, Xu R and He QY: Nrf2
mediates the resistance of human A549 and HepG2 cancer cells to
boningmycin, a new antitumor antibiotic, in vitro through
regulation of glutathione levels. Acta Pharmacol Sin. 39:1661–1669.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sen B, Peng S, Tang X, Erickson HS,
Galindo H, Mazumdar T, Stewart DJ, Wistuba I and Johnson FM:
Kinase-impaired BRAF mutations in lung cancer confer sensitivity to
dasatinib. Sci Transl Med. 4:136ra702012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang CC, Li CG, Wang YF, Xu LH, He XH,
Zeng QZ, Zeng CY, Mai FY, Hu B and Ouyang DY: Chemotherapeutic
paclitaxel and cisplatin differentially induce pyroptosis in A549
lung cancer cells via caspase-3/GSDME activation. Apoptosis.
24:312–325. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tièche CC, Gao Y, Bührer ED, Hobi N,
Berezowska SA, Wyler K, Froment L, Weis S, Peng RW, Bruggmann R, et
al: Tumor initiation capacity and therapy resistance are
differential features of EMT-related subpopulations in the NSCLC
cell line A549. Neoplasia. 21:185–196. 2019. View Article : Google Scholar : PubMed/NCBI
|