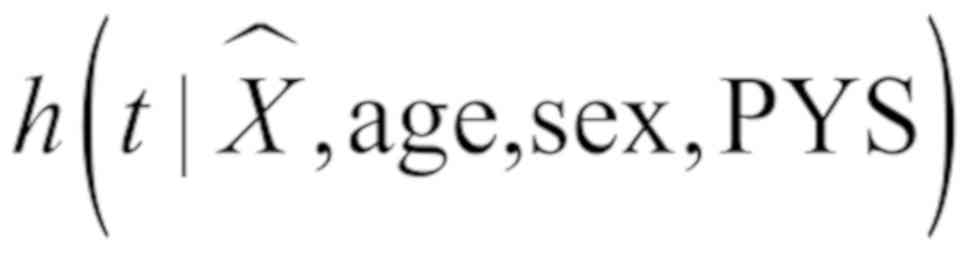Introduction
Lung cancer (LC) remains the most commonly diagnosed
cancer type worldwide, with 11.6% of total cancer cases, and is the
leading cause of cancer mortality, accounting for 18.4% of the
total cancer-associated mortalities (1). Non-small cell lung carcinoma (NSCLC)
accounts for ~80% of all LC types, with adenocarcinoma (LUAD) and
squamous cell carcinoma (LUSC) being the two major histological
types (2). LUAD and LUSC have
different cells of origin, location within the lung and growth
patterns, and can develop and progress via different molecular
mechanisms (3–6). Understanding the molecular mechanisms
underlying the progression and survival of LUAD and LUSC is
essential, and identifying the genetic difference between them may
facilitate development of suitable and precise treatment strategies
(6–8). Previous studies have demonstrated that
differentially expressed genes (DEGs) serve an important role in
the progression of both LUAD and LUSC (9–11).
Gantenbein et al (12) have
identified that upregulation of eukaryotic translation initiation
factor 6 in NSCLC is associated with poor overall survival in LUAD,
but not in LUSC. Qu et al (13) have demonstrated that interleukin-6
prevents the initiation, but enhances the progression of LC in a
mouse model. Immunohistochemical analysis by Huang et al
(14) has revealed that p16 protein
expression is associated with poor prognosis in LUSC.
However, previous studies have mainly focused on the
single level-omic analysis, such as differential gene expression
analysis, and primarily examined association rather than the causal
relationship between gene expression and LC survival (15). While the establishment of the
potential causal relationship is key for precise treatment of LC,
it is difficult to conduct causal inference in observational
studies due to bias, which results from reverse causation and
unobserved confounding factors (16). A powerful statistical tool to examine
the causal relationship between the modifiable exposure, such as
gene expression, and the outcome variable of interest (such as LC
survival) is instrumental variable analysis (IVA) (17–20). IVA
uses specific instrumental variables to estimate and test the
causal effect of the exposure variable of interest on the outcome
variable, under the assumptions that the instrumental variables are
strongly associated with the exposure (21). Furthermore, the instrumental variable
is independent of the confounders between the exposure and the
outcome, and the instrumental variable influences the outcome only
through the exposure (22).
Therefore, determining suitable instrumental variables is highly
important in IVA (23).
Generally, gene expression measured at the
transcript level affects clinical outcome or disease progression
more directly compared with gene methylation measured at the
DNA/epigenetics level (24–27). Biologically, for one specific gene,
methylation sites within the unique function of transcript start
site [e.g., within 1,500 bps ahead of a transcription start site
(TSS), but not including the 200 bps ahead of the TSS (TSS1500)]
can downregulate its expression, and deregulated expression can
further influence survival outcome (28,29). In
addition, deregulated methylation and gene expression level and
event are time sequential (29).
Previous studies have illustrated a correlation between DNA
methylation in the gene promoter region and gene expression
(30,31). However, in instrumental variable
analysis, more instruments can provide higher power than compared
with fewer instruments; TSS1500 regions include more CpG sites than
TSS200, and thus CpG sites in the TSS1500 region of one gene can be
selected as instrumental variables to explore the potential causal
relationship between gene expression and cancer survival outcome.
DNA methylation is a key epigenetic factor that regulates gene
expression, which has been described in several multi-omics
integrative analyses in cancer research (32–34).
In the present study, the aim to was to integrate
DNA methylation (level 3), RNA sequencing (RNA-seq; level 3),
clinical characteristics and survival outcome of patients with LUAD
and LUSC from The Cancer Genome Atlas (TCGA). Differentially
expressed genes (DEGs) and differentially expressed methylation
positions (DMPs) were identified using tumor and normal tissue from
patients with LUAD and LUAC. Furthermore, DMP CpG sites in the
TSS1500 and DEG were paired by gene, and the regulatory association
between them was assessed to identify candidate gene sets for
subsequent time-to-event IVA, which was used to establish the
potential causal effect of gene expression on LUAD and LUSC
survival, and to investigate the different genetic difference
between LUAD and LUSC. Various sensitivity analyses, including the
weak instrumental association test, the heterogeneity among
instrumental variables (IVs) and leave-one-out cross validation
(LOOCV) analysis, were conducted to ensure the robustness for
modeling misspecifications, and to improve the vailidity of the
results.
Materials and methods
Software
R (version 3.6.1; http://www.R-project.org/) was used to conduct data
processing and statistical analysis (35). edgeR (version 3.26.8) (36,37) and
ChAMP (version 2.14.0) (38) were
used with default settings for DEG and DMP analysis respectively.
An R package TwoSLSanalysis, which is available on GitHub
(https://github.com/LULIU1816/TwoSLSanalysis), was used
to implement the time-to-event IVA.
Data collection and processing
Gene expression, RNA-Seq and the corresponding
clinical data of patients with NSCLC were obtained from TCGA
(https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga).
Data were downloaded with published software TCGA-Assembler
(version 2.0; http://www.compgenome.org/TCGA-Assembler) (39) and TCGAbiolinks (version 3.9;
http://bioconductor.org/packages/release/bioc/html/TCGAbiolinks.html)
(40,41). DNA methylation was measured with
Infinium HumanMethylation450 BeadChip (Illumina, Inc.) with 485,577
CpG sites, among which 84,242 methylation sites were located on the
TSS1500. Gene expression was detected using the Illumina HiSeq2000
RNA Sequencing platform (Illumina, Inc.) with 20,502
transcripts.
To identify DEGs and DMPs, the methylation and gene
expression data were used from paired tumor and normal tissue. In
total, data from 50 pairs for LUSC and 57 pairs for LUAD were
matched for DEG analysis, and data from 40 pairs for LUSC and 29
pairs for LUAD were obtained for DMP or different methylation
region (DMR) analysis. For IVA, methylation, gene expression and
clinical information (demographic characteristics, survival and
treatment information) were downloaded from 504 patients with LUSC
and 522 patients with LUAD. Information included age, sex and
pack-years smoked (PYS) as covariates, as these have previously
been reported to be associated with the survival of patients with
LC (42,43). PYS was calculated by multiplying the
average number of packs of cigarettes smoked per day by the number
of years a person has smoked, which reflected smoking extent and
history. Overall survival (OS) was regarded as the survival outcome
and was defined as the time from diagnosis to death, and mortality
was the censoring variable. Patients with missing PYS, survival
time or methylation and gene expression information were excluded.
In addition, 287 patients with LUSC and 280 patients with LUAD were
included in the time-to-event IVA. The flow chart of all data
processing and analysis is presented in Fig. 1.
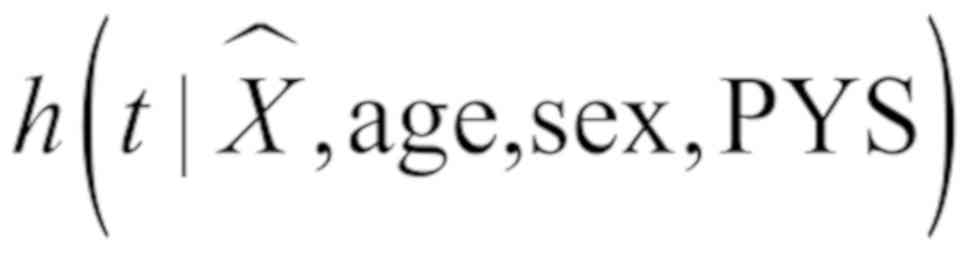 | Figure 1.Flow chart of data processing and
analysis. LUAD and LUSC followed the same process. First, the
candidate gene sets were selected from overlapping DEGs and DMPs in
TSS1500. Second, in stage I of IVA, the predicted expression value
for each gene X was obtained by regressing the gene
expression on the corresponding CpGs in TSS1500 with adjusted age,
sex and PYS. In stage II of IVA, the potential causal effect was
calculated by directly inputting the predicted gene expression
value X into the hazard model with adjustments for age, sex
and PYS. LUAD, lung adenocarcinoma; LUSC, lung squamous cell
carcinoma; DEGs, differentially expressed genes; DMPs,
differentially methylated probes; IVA, instrumental variable
analysis; PYS, pack-years smoked; TSS1500, 200-1,500 bp upstream of
a transcription start site. |
Identification of DEGs
The edgeR package was used to select the DEGs
(36,37). Read count and reads per kilobase per
million mapped reads matrix tables were extracted from classified
TCGA RNA-Seq data to assess the DEGs. The trimmed mean of M-values
method was used for normalization (44). In addition, the exact test, based on
the quantile-adjusted conditional maximum likelihood methods
(45), was used to define DEGs.
Using previously described methods (46), the present study identified DEGs
under the criteria that the absolute value of log2
fold-change (log-FC) of expression was >2 and the false
discovery rate (FDR) was <0.05.
Identification of DMPs
ChAMP package (https://www.bioconductor.org/packages/release/bioc/vignettes/ChAMP/inst/doc/ChAMP.html)
was used to identify the DMPs (41).
ChAMP is an integrated analysis pipeline that includes functions
for filtering low-quality probes based on detection P-values,
chromosomal location, presence of single nucleotide polymorphisms
in the probe sequence and cross-hybridization, adjustment for
Infinium I and II probe design, batch effect correction used
singular value decomposition, detecting DMPs, identifying DMRs and
detection of copy number aberrations (41,47).
LUAD- and LUSC-DMPs (P<0.05) were obtained from 485,577 CpGs
after quality control and normalization.
Time-to-event IVA
Traditional two-stage regression was used to perform
the time-to-event IVA. For one candidate gene, the instruments were
the corresponding CpGs in the region of TSS1500 obtained by DMPs,
and thus the number of IVs was gene-specific. Predicted gene
expression value in the first stage was obtained by treating the
differential methylation CpGs in the TSS1500 region as instrumental
variables. In the second stage, the Cox regression model was run
with the predicted gene expression used as the independent
variable. The model used was as follows:
Xˆ=α0+α1Z+α2age+α3sex+α4PYS
h(t|Xˆ,age,sex,PYS)h0(t)=exp(β1Xˆ+β2age+β3sex+β4PYS)
where Z is the methylation value of the
TSS1500 region of a specific gene, and X is the predicted
expression value of the specific gene. The present study defined
the linear regression of gene expression on CpGs in the TSS1500
region, age, sex and PYS in model I. α1 is a px1 vector
denoting the effect of CpGs on gene expression, p is the number of
the instrumental variables of one specific gene. In model II,
h(t|Xˆ,age,sex,PYS) is a
hazard function determined by the the predicted gene expression
value X and covariates age, gender and PYS.
h0(t) is the baseline hazard function. The
prediction gene expression value X was directly plugged into
the Cox model, and the parameter β1 represented
the potential causal effect of gene expression on LC survival. A
false discovery method was used to adjust multiple testing, and the
threshold of FDR-q value was set to 0.15 (48). In addition, proportional hazards
assumption was diagnosed by testing the correlation between the
Schoenfeld residuals and survival time, with zero correlation
indicating that the Cox model was valid (49).
Sensitivity analyses
Various sensitivity analyses were conducted to
ensure the robustness for modeling misspecifications and to ensure
the results were valid. Specifically, F statistic was used
to test the weak instrumental bias. In addition, the
I2-statisic was calculated to test the
heterogeneity among instrumental variables, and leave-one-out cross
validation (LOOCV) analysis was used to test whether one single
instrumental variable may have a strong causal effect on gene
expression. Weak association between instrumental variables and
gene expression is observed if the F-statistics is <10,
and heterogeneity among instrumental variables may exist when
I2-statisic is >50% (50–52).
Results
Descriptive statistics
The demographic characteristics of the 567 patients
with NSCLC are presented in Table I.
For the 280 patients with LUAD, the median age was 67 years, and
the proportion of female patients was 52.14%. The median PYS was
36.5 packs/year, and the median survival time was 216 months, with
a 24.29% censoring rate. For the 287 patients with LUSC, the median
age was 69 years, and the proportion of female patients was 26.13%.
The median PYS was 50 packs/year, and the median survival time was
224 months, with a 29.90% censoring rate. No significant
differences were observed in survival time (P=0.37), vital status
(P=0.13) or history of other malignancy distributions (P=0.08)
between LUAD and LUSC. However, age (P=0.007), sex
(P=3.77×10−10), race (P=0.01), PYS
(P=3.02×10−8), Kras gene analysis indicator
(P=9.27×10−7) and epidermal growth factor receptor
mutation status (P=1.38×10−7) were signifcantly
different between LUAD and LUSC.
 | Table I.Demographic and clinical
characteristics for study populations. |
Table I.
Demographic and clinical
characteristics for study populations.
| Variable | LUAD N=280 | LUSC N=287 | P-value |
|---|
| Age, median years
(interquartile range) | 67.00
(13.25) | 69.00
(11.00) | 0.01a |
| Sex, n (%) |
|
|
3.77×10−10 |
|
Female | 146 (52.14) | 75
(26.13) |
|
|
Male | 134 (47.86) | 212 (73.87) |
|
| Ethnicity, n
(%) |
|
| 0.01 |
|
Asian | 2
(0.71) | 3
(1.05) |
|
| Black
or African American | 29
(10.36) | 19
(6.62) |
|
|
White | 226 (80.71) | 218 (75.96) |
|
|
Unknown | 23
(8.21) | 47
(16.38) |
|
| Pack-years smoked,
median (interquartile range) | 36.50
(30.00) | 50.00
(33.87) |
3.02×10−8a |
| Survival time,
median months (interquartile range) | 216.00 (69.00) | 244.00
(975.00) | 0.37a |
| Dead, n (%) | 68
(24.29) | 87
(30.31) | 0.13 |
| History of other
malignancy, n (%) |
|
| 0.08 |
| No | 227 (81.07) | 249 (86.76) |
|
|
Yes | 53
(18.93) | 38
(13.24) |
|
| Kras gene analysis
indicator, n (%) |
|
|
9.27×10−7 |
| No | 149 (53.21) | 205 (71.43) |
|
|
Yes | 38
(13.57) | 10
(3.48) |
|
|
Unknown | 93
(33.21) | 72
(25.09) |
|
| EGFR mutation
status, n (%) |
|
|
1.38×10−7 |
| No | 124 (44.29) | 186 (64.81) |
|
|
Yes | 49
(17.5) | 16
(5.57) |
|
|
Unknown | 107 (38.21) | 85
(29.62) |
|
Time-to-event IVA for LUSC and
LUAD
The present study identified 1,543 DEGs in LUSC and
906 DEGs in LUAD (Tables SI and
SII). A total of 9,799
differentially methylated genes were located in genes in the
TSS1500 regions for LUAD (Table
SIII), among which 538 also differed in gene expression. In
addition, 12,283 differentially methylated CpGs were located in
genes in the TSS1500 regions for LUSC (Table SIV), among which 1,053 also differed
in gene expression. In total, 538 genes in LUAD and 1,053 genes for
LUSC were regarded as candidate genes after overlapping the DGEs
and DMPs in the TSS1500 region (Table
SV) for the downstream time-to-event IVA in order to identify
potential causal genes related to the survival of patients with LC
(Tables SVI and SVII). The present study only included 476
genes for LUAD and 922 genes for LUSC after removing genes with
missing methylation data. In addition, the proportional hazards
assumption in the second stage was confirmed to be valid for the
correlation between the Schoenfeld residuals and survival time.
The results of the present study identified eight
significant potential causal genes for LUAD survival and one
significant causal gene in LUSC using FDR-q<0.15 (Table II). The causal genes for LUAD were
aryl hydrocarbon receptor nuclear translocator like 2
(ARNTL2, HR=1.037; 95% CI: 1.017–1.056;
P=1.81×10−4; FDR-q=0.029), semaphorin 3G (SEMA3G,
HR=0.632; 95% CI: 0.504–0.792; P=6.79×10−5;
FDR-q=0.029), serum deprivation-response protein (SDPR,
HR=0.980; 95% CI: 0.969–0.990; P=1.38×10−4;
FDR-q=0.029), chloride intracellular channel protein 5
(CLIC5, HR=0.987; 95% CI: 0.980–0.995;
P=7.39×10−4; FDR-q=0.070), LIM zinc finger domain
containing 2 (LIMS2, HR=0.924; 95% CI: 0.884–0.967;
P=6.22×10−4; FDR-q=0.070), epithelial membrane protein 2
(EMP2, HR=0.997; 95% CI: 0.995–0.999;
P=2.52×10−3; FDR-q=0.150), carbonic anhydrase 7
(CA7, HR=1.34×10−9; 95% CI:
2.3×10−15−7.0×10−4; P=2.53×10−3;
FDR-q=0.150) and LOC116437 (HR=0.141; 95% CI: 0.040–0.496;
P=2.25×10−3; FDR-q=0.150). The causal gene for LUSC was
phosphatidylinositol-3,4,5-trisphosphate-dependent Rac exchange
factor 2 (PREX2, HR=1.958; 95% CI: 1.450–2.644;
P=1.16×10−5; FDR-q=0.011). All HR values were calculated
with 10-unit increment of gene expression.
 | Table II.Result of time-to-event instrument
variable analysis for causal genes. |
Table II.
Result of time-to-event instrument
variable analysis for causal genes.
| A, LUAD |
|---|
|
|---|
| Gene | Chr | Position | IVs | HR (95% CI) | P-value | FDR |
|---|
| ARNTL2 | 12 |
27,485,787-27,578,746 | cg26165146 | 1.037
(1.017–1.056) |
1.81×10−4 | 0.029 |
|
|
|
| cg17367616 |
|
|
|
|
|
|
| cg01986577 |
|
|
|
| SEMA3G | 3 |
52,467,268-52,479,112 | cg25134747 | 0.632
(0.504–0.792) |
6.79×10−5 | 0.029 |
| SDPR | 2 | 191,834,310-
191,847,088 | cg10082589 | 0.980
(0.969–0.990) |
1.38×10−4 | 0.029 |
|
|
|
| cg18843739 |
|
|
|
| CLIC5 | 6 | 45,866,188-
46,048,085 | cg23716866 | 0.987
(0.980–0.995) |
7.39×10−4 | 0.070 |
|
|
|
| cg14339765 |
|
|
|
|
|
|
| cg09347495 |
|
|
|
| LIMS2 | 2 |
128,395,996-128,439,360 | cg07262244 | 0.924
(0.884–0.967) |
6.22×10−4 | 0.070 |
|
|
|
| cg14282137 |
|
|
|
|
|
|
| cg08385249 |
|
|
|
|
|
|
| cg23966569 |
|
|
|
|
|
|
| cg22542731 |
|
|
|
| EMP2 | 16 | 10,622,279-
10,674,539 | cg04339790 | 0.997
(0.995–0.999) |
2.52×10−3 | 0.150 |
| CA7 | 16 |
66,878,282-66,888,052 | cg10352418 |
1.34×10−9
(2.33×10−15−7.0×10−4) |
2.53×10−3 | 0.150 |
|
|
|
| cg06438797 |
|
|
|
|
|
|
| cg11258532 |
|
|
|
|
|
|
| cg00182273 |
|
|
|
|
LOC116437 | 12 |
131,649,556-131,697,476 | cg20183756 | 0.141
(0.040–0.496) |
2.25×10−3 | 0.150 |
|
|
|
| cg03859668 |
|
|
|
|
| B, LUSC |
|
| Gene | Chr |
Position | IVs | HR (95%
CI) | P-value | FDR |
|
| PREX2 | 8 |
68,864,244-69,143,897 | cg13652336 | 1.958
(1.450–2.644) |
1.16×10−5 | 0.011 |
|
|
|
| cg16009633 |
|
|
|
|
|
|
| cg11549615 |
|
|
|
|
|
|
| cg05293738 |
|
|
|
|
|
|
| cg17747005 |
|
|
|
Sensitivity analyses
F-statistics of all causal genes used to
detect the weak instrumental bias were <10, which indicated a
weak association between instruments and gene expression due to the
small number of instrumental DMP sites within each gene (Table SVIII). Despite this, all the
significant genes were still to be identified powerfully.
The present study performed a heterogeneity test to
identify any instrumental outliers that may affect the results. The
results demonstrated that the I2-statistics were
75.9, 78.2 and 63.1% for ARNTL2, CLIC5 and PREX2,
respectively (Table SVIII). To
address the heterogeneity among the IVs, instrumental outliers were
removed; similar results were obtained when removing the
instrumental outliers. In addition, LOOCV analysis identified no
causal genes with outlier single instrument for both LUAD and LUSC
(Table SVIII).
Discussion
The present study integrated DNA methylation,
RNA-seq, clinical charancteristics and survival outcomes from TCGA
to investigate the potentical causal relationship between gene
expression and LUAD and LUSC survival, respectively.
The identified causal relationship between gene
expression and survival of disease was robust with respect to the
choice of statistical methods, and was assessed with various
sensitivity analyses. Non-overlapping causal genes between LUAD and
LUSC further highlighted the heterogeneity between these two
subtypes of LC. From the two-stage time-to-event IVA, the present
results indicated the potential causal role of ARNTL2, SEMA3G,
SDPR, CLIC5, LIMS2, EMP2, CA7 and LOC116437 in LUAD
survival, and PREX2 in LUSC survival. In addition, the
present study identified pivotal regulatory genes, the expression
levels of which were upregulated with poor survival, including
PREX2 in LUSC and ARNTL2 in LUAD. Furthermore,
several genes with downregulated expression levels associated with
poor survival were identified, including SEMA3G, SDPR, CLIC5,
LIMS2, EMP2, CA7 and LOC116437 in LUAD. The causal
effect of gene expression and NSCLC suggested that these genes may
be potential epigenetic therapeutic targets.
The majority of the potential causal genes
identified in the present study have also been detected by previous
studies, which have demonstrated a possible association with the
prognosis in NSCLC. ARNTL2 drives metastatic
self-sufficiency by orchestrating the expression of a complex
pro-metastatic secretome, and high ARNTL2 expression
predicts poor survival among patients with LUAD (53). In addition, SEMA3G is a
potential transcription gene associated with cancer susceptibility
candidate 9, and is significantly associated with the malignant
progression of LUSC (54). A
previous study using Oncomine and TCGA databases has demonstrated
that low expression of CLIC5 is associated with poor overall
survival after adjusting for age, sex and PYS (55). In addition, EPAS1, a
transcription factor that serves a vital role in tumor progression,
has been reported to directly regulate the LUAD-associated genes
EMP2 and LIMS2 (56).
It has been identified that upregulation of CA7 in tissues
from resectable NSCLC is a biomarker of good prognosis (57). As LUAD is a major subtype of NSCLC,
CA7 may have the same effect on LUAD. A xenograft study
demonstrated that SDPR may elicit a metastasis suppressor
function by directly interacting with ERK and have a limited
pro-survival role (58). A previous
study has reported that somatic alterations in PREX2
modulate the activity of immunomodulators, according to a
significant overlap between the Master Regulator- and
SYGNAL-PanImmune, which is associated with survival across all
cancer types (59). Thus,
upregulated PREX2 may lead to a short survival time, but
this has not been identified in previous studies. In addition,
there is no previous evidence that LOC116437 is the
potential causal gene in NSCLC.
The analysis pipeline used in the present study can
be considered as a gene-centered data integration method by
combining multi-omics data with clinical information. One single
level of genomic measurements can be insufficient to fully exploit
the knowledge underlying the etiology of cancer prognosis. Based on
the follow-up data from TCGA, gene expression was used as the
exposure variable, and survival time was the censored outcome
variable to avoid the reverse causation. For any one specific gene,
DMP sites within the promoter region TSS1500 were used as
instrumental variables, due to the biologically plausible
assumption that CpG sites in TSS1500 must first regulate gene
expression before affecting the survival. However, it may be
necessary to include additional instrumental variables to increase
the power of IVA. The present study only used DMPs within the
functional region of TSS1500, rather than including DMPs within the
gene body. Since DNA methylation in the gene body can be associated
with survival outcome through changes in gene expression and some
alternative mechanisms, these may possess the possibility of
violating the instrumental variable assumptions (60). The present study performed extensive
sensitivity analyses to ensure the robustness of the results and to
prevent any possible model assumption violation in the IVA.
However, the present study has certain limitations.
First, similar to other IVA studies, the present study assumed a
linear relationship between DMPs in the promoter region and the
corresponding gene expression. While a linear relationship can be
considered a first-order approximation to any non-linear
relationship, modeling a linear relationship can be suboptimal in
terms of power if the true relationship is non-linear. Second, the
censored rate of TCGA cohort was relatively high. Considering the
heterogeneity and various manifestations of NSCLC, the present
results should be verified in larger samples to evaluate the
findings among specific subgroups. Furthermore, the present results
should be interpreted with caution among other populations. The
analysis framework could be extended to other ethnicities to detect
the possible differences. In addition, several studies have
demonstrated that when the same dataset is used for the selection
of IVs and the estimation of instrument-exposure effect,
substantial selection bias occurs even if the selection threshold
is very stringent (61,62). Therefore, further studies are
required to investigate other independent samples to select
IVs.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by The National Natural
Science Foundation of China (grant nos. 81673272, 81703321 and
81872712), the Natural Science Foundation of Shandong Province
(grant no. ZR2019ZD02) and the Young Scholars Program of Shandong
University (grant no. 2016WLJH23).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZY and SY conceived the study. LL contributed to
data analysis, with assistance from SY and ZY. SY and PZ
contributed to the data interpretation. LL, SY and ZY wrote the
manuscript with participation from all other authors. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Travis WD, Brambilla E, Müller-Hermelink
HK and Harris CC: Pathology and genetics of tumours of the lung,
pleura, thymus and heart (WHO classification of tumours). IARC
Press Oxford University Press (distributor). (Lyon Oxford).
2004.
|
|
3
|
McKay JD, Hung RJ, Han Y, Zong X,
Carreras-Torres R, Christiani DC, Caporaso NE, Johansson M, Xiao X,
Li Y, et al: Large-scale association analysis identifies new lung
cancer susceptibility loci and heterogeneity in genetic
susceptibility across histological subtypes. Nat Genet.
49:1126–1132. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen M, Liu X, Du J, Wang XJ and Xia L:
Differentiated regulation of immune-response related genes between
LUAD and LUSC subtypes of lung cancers. Oncotarget. 8:133–144.
2017.PubMed/NCBI
|
|
5
|
Liu B, Chen Y and Yang J: LncRNAs are
altered in lung squamous cell carcinoma and lung adenocarcinoma.
Oncotarget. 8:24275–24291. 2017.PubMed/NCBI
|
|
6
|
Relli V, Trerotola M, Guerra E and Alberti
S: Distinct lung cancer subtypes associate to distinct drivers of
tumor progression. Oncotarget. 9:35528–35540. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shepherd FA, Rodrigues Pereira J, Ciuleanu
T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S,
Smylie M, Martins R, et al: Erlotinib in previously treated
non-small-cell lung cancer. N Engl J Med. 353:123–132. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang L, Zhu W, Streicher K, Morehouse C,
Brohawn P, Ge X, Dong Z, Yin X, Zhu G, Gu Y, et al: Increased
IR-A/IR-B ratio in non-small cell lung cancers associates with
lower epithelial-mesenchymal transition signature and longer
survival in squamous cell lung carcinoma. BMC Cancer. 14:1312014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bosse Y and Amos CI: A decade of GWAS
results in lung cancer. Cancer Epidemiol Biomarkers Prev.
27:363–379. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Välk K, Vooder T, Kolde R, Reintam MA,
Petzold C, Vilo J and Metspalu A: Gene expression profiles of
non-small cell lung cancer: Survival prediction and new biomarkers.
Oncology. 79:283–292. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gantenbein N, Bernhart E, Anders I,
Golob-Schwarzl N, Krassnig S, Wodlej C, Brcic L, Lindenmann J,
Fink-Neuboeck N, Gollowitsch F, et al: Influence of eukaryotic
translation initiation factor 6 on non-small cell lung cancer
development and progression. Eur J Cancer. 101:165–180. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qu Z, Sun F, Zhou J, Li L, Shapiro SD and
Xiao G: Interleukin-6 prevents the initiation but enhances the
progression of lung cancer. Cancer Res. 75:3209–3215. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang CI, Taki T, Higashiyama M, Kohno N
and Miyake M: p16 protein expression is associated with a poor
prognosis in squamous cell carcinoma of the lung. Br J Cancer.
82:374–380. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thomas ML and Marcato P: Epigenetic
modifications as biomarkers of tumor development, therapy response,
and recurrence across the cancer care continuum. Cancers (Basel).
10(pii): E1012018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Burgess S, Thompson DJ, Rees JMB, Day FR,
Perry JR and Ong KK: Dissecting causal pathways using mendelian
randomization with summarized genetic data: Application to age at
menarche and risk of breast cancer. Genetics. 207:481–487.
2017.PubMed/NCBI
|
|
17
|
Wright PG: The Tariff on Animal and
Vegetable Oils. Moulton HG: The Macmillan Company; New York, NY:
1928
|
|
18
|
Davies NM, Smith GD, Windmeijer F and
Martin RM: Issues in the reporting and conduct of instrumental
variable studies: A systematic review. Epidemiology. 24:363–369.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Palmer TM, Sterne JA, Harbord RM, Lawlor
DA, Sheehan NA, Meng S, Granell R, Smith GD and Didelez V:
Instrumental variable estimation of causal risk ratios and causal
odds ratios in Mendelian randomization analyses. Am J Epidemiol.
173:1392–1403. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen Y and Briesacher BA: Use of
instrumental variable in prescription drug research with
observational data: A systematic review. J Clin Epidemiol.
64:687–700. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Angrist JD, Imbens GW and Rubin DB:
Identification of causal effects using instrumental variables. J Am
Stat Assoc. 91:444–455. 1996. View Article : Google Scholar
|
|
22
|
Pearl J: Causality: Models, Reasoning, and
Inference. Harvey A: Cambridge University Press; Cambridge, New
York: 2000
|
|
23
|
Baser O: Too much ado about instrumental
variable approach: Is the cure worse than the disease? Value
Health. 12:1201–1209. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Glinsky GV: Integration of HapMap-based
SNP pattern analysis and gene expression profiling reveals common
SNP profiles for cancer therapy outcome predictor genes. Cell
Cycle. 5:2613–2625. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fabiani E, Leone G, Giachelia M, D'alo' F,
Greco M, Criscuolo M, Guidi F, Rutella S, Hohaus S and Voso MT:
Analysis of genome-wide methylation and gene expression induced by
5-aza-2′-deoxycytidine identifies BCL2L10 as a frequent methylation
target in acute myeloid leukemia. Leuk Lymphoma. 51:2275–2284.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang W, Baladandayuthapani V, Morris JS,
Broom BM, Manyam G and Do KA: iBAG: Integrative bayesian analysis
of high-dimensional multiplatform genomics data. Bioinformatics.
29:149–159. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de Tayrac M, Le S, Aubry M, Mosser J and
Husson F: Simultaneous analysis of distinct omics data sets with
integration of biological knowledge: Multiple Factor Analysis
approach. BMC Genomics. 10:322009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hou Y, Guo H, Cao C, Li X, Hu B, Zhu P, Wu
X, Wen L, Tang F, Huang Y and Peng J: Single-cell triple omics
sequencing reveals genetic, epigenetic, and transcriptomic
heterogeneity in hepatocellular carcinomas. Cell Res. 26:304–319.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Smith AA, Huang YT, Eliot M, Houseman EA,
Marsit CJ, Wiencke JK and Kelsey KT: A novel approach to the
discovery of survival biomarkers in glioblastoma using a joint
analysis of DNA methylation and gene expression. Epigenetics.
9:873–883. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lokk K, Modhukur V, Rajashekar B, Märtens
K, Mägi R, Kolde R, Koltšina M, Nilsson TK, Vilo J, Salumets A and
Tõnisson N: DNA methylome profiling of human tissues identifies
global and tissue-specific methylation patterns. Genome Biol.
15:r542014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Saif I, Kasmi Y, Allali K and Ennaji MM:
Prediction of DNA methylation in the promoter of gene suppressor
tumor. Gene. 651:166–173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Y, Baggerly KA, Orouji E, Manyam G,
Chen H, Lam M, Davis JS, Lee MS, Broom BM, Menter DG, et al:
Gene-specific methylation profiles for integrative
methylation-expression analysis in cancer research. bioRxiv.
2019.https://doi org/10.1101/618033April 24–2019
|
|
33
|
Denis M and Tadesse MG: Evaluation of
hierarchical models for integrative genomic analyses.
Bioinformatics. 32:738–746. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao Q, Shi X, Xie Y, Huang J, Shia B and
Ma S: Combining multidimensional genomic measurements for
predicting cancer prognosis: Observations from TCGA. Brief
Bioinform. 16:291–303. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Team RC: R: A language and environment for
statistical computing. R Foundation for Statistical Computing,
Vienna, Austria, 2019. https://www.R-project.org/
|
|
36
|
Robinson MD, McCarthy DJ and Smyth GK:
edgeR: A bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
McCarthy DJ, Chen Y and Smyth GK:
Differential expression analysis of multifactor RNA-Seq experiments
with respect to biological variation. Nucleic Acids Res.
40:4288–4297. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Morris TJ, Butcher LM, Feber A,
Teschendorff AE, Chakravarthy AR, Wojdacz TK and Beck S: ChAMP:
450k chip analysis methylation pipeline. Bioinformatics.
30:428–430. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu Y, Qiu P and Ji Y: TCGA-assembler:
Open-source software for retrieving and processing TCGA data. Nat
Methods. 11:599–600. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Colaprico A, Silva TC, Olsen C, Garofano
L, Cava C, Garolini D, Sabedot TS, Malta TM, Pagnotta SM,
Castiglioni I, et al: TCGAbiolinks: An R/bioconductor package for
integrative analysis of TCGA data. Nucleic Acids Res. 44:e712016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mounir M, Lucchetta M, Silva TC, Olsen C,
Bontempi G, Chen X, Noushmehr H, Colaprico A and Papaleo E: New
functionalities in the TCGAbiolinks package for the study and
integration of cancer data from GDC and GTEx. PLoS Comput Biol.
15:e10067012019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Janjigian YY, McDonnell K, Kris MG, Shen
R, Sima CS, Bach PB, Rizvi NA and Riely GJ: Pack-years of cigarette
smoking as a prognostic factor in patients with stage IIIB/IV
nonsmall cell lung cancer. Cancer. 116:670–675. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Peto J: That the effects of smoking should
be measured in pack-years: Misconceptions 4. Brit J Cancer.
107:406–407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Robinson MD and Oshlack A: A scaling
normalization method for differential expression analysis of
RNA-seq data. Genome Biol. 11:R252010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Robinson MD and Smyth GK: Small-sample
estimation of negative binomial dispersion, with applications to
SAGE data. Biostatistics. 9:321–332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Crow M, Lim N, Ballouz S, Pavlidis P and
Gillis J: Predictability of human differential gene expression.
Proc Natl Acad Sci USA. 116:6491–6500. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tian Y, Morris TJ, Webster AP, Yang Z,
Beck S, Feber A and Teschendorff AE: ChAMP: Updated methylation
analysis pipeline for Illumina BeadChips. Bioinformatics.
33:3982–3984. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li B, Severson E, Pignon JC, Zhao H, Li T,
Novak J, Jiang P, Shen H, Aster JC, Rodig S, et al: Comprehensive
analyses of tumor immunity: Implications for cancer immunotherapy.
Genome Biol. 17:1742016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Grambsch PM and Therneau TM: Proportional
Hazards Tests and Diagnostics Based on Weighted Residuals.
Biometrika. 81:515–526. 1994. View Article : Google Scholar
|
|
50
|
Staiger D and Stock JH: Instrumental
variables regression with weak instruments. Econometrica.
65:557–586. 1997. View Article : Google Scholar
|
|
51
|
Lawlor DA, Harbord RM, Sterne JA, Timpson
N and Davey Smith G: Mendelian randomization: Using genes as
instruments for making causal inferences in epidemiology. Stat Med.
27:1133–1163. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Brady JJ, Chuang CH, Greenside PG, Rogers
ZN, Murray CW, Caswell DR, Hartmann U, Connolly AJ, Sweet-Cordero
EA, Kundaje A and Winslow MM: An Arntl2-driven secretome enables
lung adenocarcinoma metastatic self-sufficiency. Cancer Cell.
29:697–710. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gao L, Guo YN, Zeng JH, Ma FC, Luo J, Zhu
HW, Xia S, Wei KL and Chen G: The expression, significance and
function of cancer susceptibility candidate 9 in lung squamous cell
carcinoma: A bioinformatics and in vitro investigation. Int J
Oncol. 54:1651–1664. 2019.PubMed/NCBI
|
|
55
|
Liu W, Ouyang S, Zhou Z, Wang M, Wang T,
Qi Y, Zhao C, Chen K and Dai L: Identification of genes associated
with cancer progression and prognosis in lung adenocarcinoma:
Analyses based on microarray from oncomine and the cancer genome
Atlas databases. Mol Genet Genomic Med. 7:e005282019.PubMed/NCBI
|
|
56
|
Liu Y, Xie D, He Z and Zheng L: Integrated
analysis reveals five potential ceRNA biomarkers in human lung
adenocarcinoma. PeerJ. 7:e66942019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ilie MI, Hofman V, Ortholan C, Ammadi RE,
Bonnetaud C, Havet K, Venissac N, Mouroux J, Mazure NM, Pouysségur
J and Hofman P: Overexpression of carbonic anhydrase XII in tissues
from resectable non-small cell lung cancers is a biomarker of good
prognosis. Int J Cancer. 128:1614–1623. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ozturk S, Papageorgis P, Wong CK, Lambert
AW, Abdolmaleky HM, Thiagalingam A, Cohen HT and Thiagalingam S:
SDPR functions as a metastasis suppressor in breast cancer by
promoting apoptosis. Proc Natl Acad Sci USA. 113:638–643. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Thorsson V, Gibbs DL, Brown SD, Wolf D,
Bortone DS, Ou Yang TH, Porta-Pardo E, Gao GF, Plaisier CL, Eddy
JA, et al: The immune landscape of cancer. Immunity.
48:812–830.e14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Koellinger PD and de Vlaming R: Mendelian
randomization: The challenge of unobserved environmental confounds.
Int J Epidemiol. 48:665–671. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hemani G, Zheng J, Elsworth B, Wade KH,
Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, et
al: The MR-Base platform supports systematic causal inference
across the human phenome. Elife. 7(pii): e344082018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Haycock PC, Burgess S, Wade KH, Bowden J,
Relton C and Davey Smith G: Best (but oft-forgotten) practices: The
design, analysis, and interpretation of Mendelian randomization
studies. Am J Clin Nutr. 103:965–978. 2016. View Article : Google Scholar : PubMed/NCBI
|