Introduction
Gastric cancer (GC) is a malignant tumor of
epithelial origin and is the third most common cancer worldwide
(1). Currently, a combination of
surgery and perioperative adjuvant or neoadjuvant therapy are the
mainstay of treatment for GC, but depend on the disease stage and
pathological type (2,3). Although there has been a decline in the
incidence of GC in recent decades due to progress in clinical
interventions (4,5), the high morbidity and mortality
associated with the disease pose challenges to public health.
Studies suggest that multiple genetic and environmental factors
impact the risk and progression of GC (4,6). The
pathways underlying the pathogenesis of GC have not yet been fully
elucidated, and a deeper understanding of the dysregulation
involved in the intracellular signaling pathways may aid the
development of novel therapeutic strategies.
The activation of protein kinase B (AKT) by
phosphatidylinositol-3-kinase (PI3K) is required for cell
proliferation, invasion, apoptosis and angiogenesis, and is
associated with the progression of neoplasms (7–9). Several
components of the PI3K/AKT signaling pathway may serve as potential
therapeutic targets in a number of human tumors, including GC and
breast and lung cancer (8,10–12).
Cell cycle regulator D1 (cyclin D1) is activated by mammalian
target of rapamycin (mTOR) via phosphorylation of AKT at either
serine 473 or threonine 308 and is important in the G1/S transition
of tumor cells (13,14). Upregulation of cyclin D1 may lead to
shortening of the cell cycle and increased proliferation of tumor
cells, thereby expediting tumor progression. Matrix
metalloproteinases (MMPs) promote the degradation of the
extracellular matrix and therefore affect the invasion and
metastasis of cancer cells. The production of MMPs has been shown
to be regulated by the PI3K/AKT signaling pathway (15,16).
Activation of the PI3K/AKT signaling pathway inhibits apoptosis via
the expression of specific downstream proteins, including B-cell
lymphoma-2 (Bcl-2) and Bcl-2 associated X protein (BAX) (7,17).
Therefore, noveltherapeutic agents that specifically target the
PI3K/AKT signaling pathway may improve GC treatment.
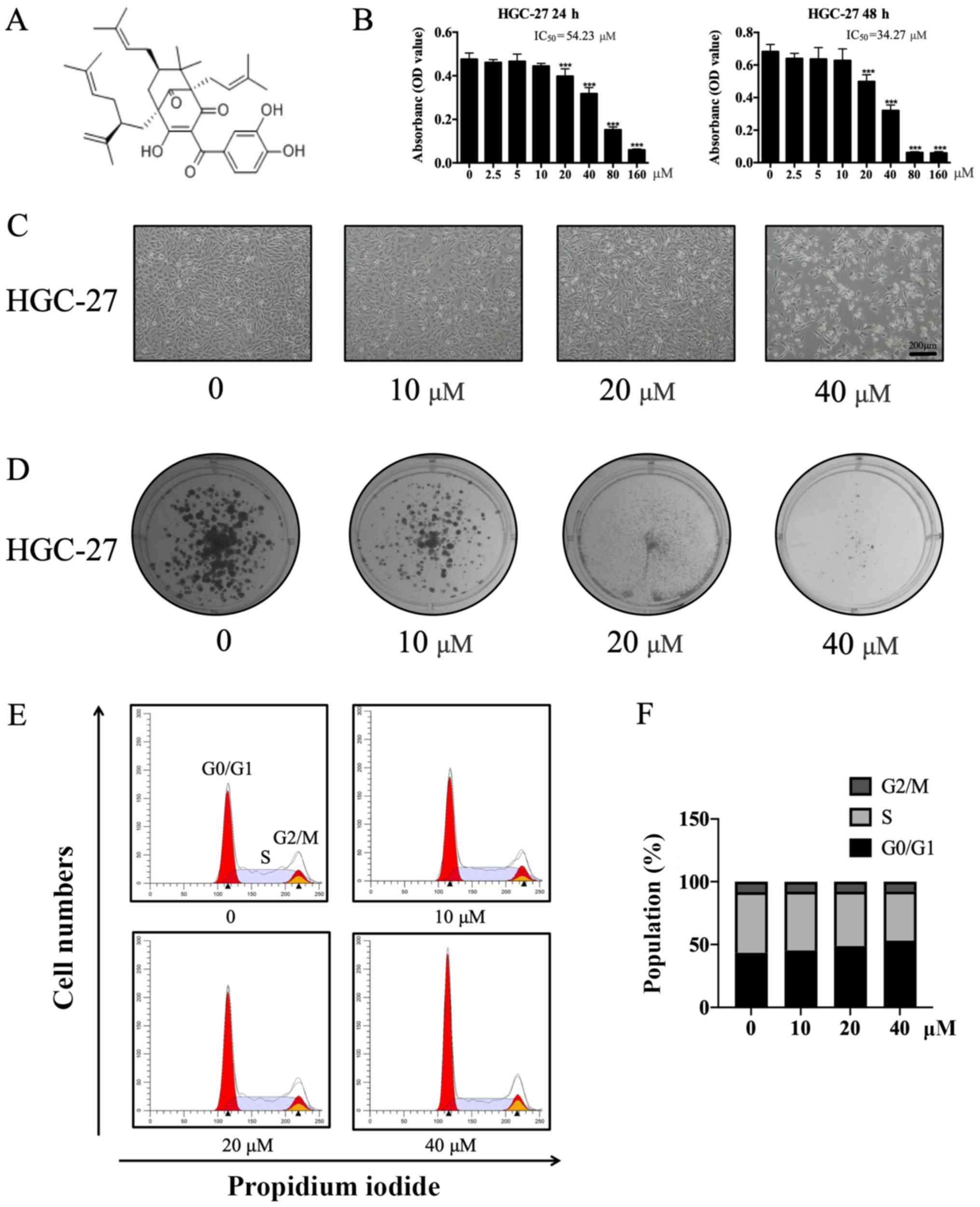
Garcinol (Fig. 1A), a
polyisoprenylated benzophenone derived from Garcinia indica
exhibits anti-inflammatory, acetyltransferase inhibitory,
antioxidant, and anticancer effects by regulating several signaling
pathways (18–20). Previous studies have shown the
therapeutic potential of garcinol for gastric ailments, such as
ulcers (18,21). Additionally, the anticancer effects
of garcinol have been demonstrated in a number of carcinomas in
vitro and in vivo. Specifically, garcinol exerted
inhibitory effects in colon and prostate cancer via the PI3K/AKT
signaling pathway (22,23). Additionally, garcinol decreased tumor
cell proliferation, angiogenesis and cell cycle progression, and
increased apoptosis in oral cancer (24). The results of the aforementioned
studies suggest that garcinol may serve as a potential
antineoplastic agent (20,24). However, the effects of garcinol in GC
and its underlying mechanism remain unclear.
The present study aimed to investigate the effects
of garcinol on the proliferation, invasion, and apoptosis of the GC
cell line HGC-27 and to further explore the associated mechanisms.
The results revealed that garcinol decreased colony formation
ability, viability, migration and invasion in a dose-dependent
manner. Moreover, garcinol promoted apoptosis. Further
investigation revealed that, garcinol exerted its inhibitory
effects on GC cells by regulating the PI3K/AKT signaling
pathway.
Materials and methods
Cell culture
The human GC cell line, HGC-27 (cat no. TCHu 22) was
obtained from The Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences and was tested for mycoplasma and
authenticated by STR profiling. The cells were cultured in
RPMI-1640 medium (Sigma-Aldrich; Merck KGaA) supplemented with 10%
fetal bovine serum (FBS; Sigma-Aldrich; Merck KGaA) and 1%
penicillin-streptomycin (PS; Beyotime Institute of Biotechnology)
at 37°C in a humidified atmosphere containing 5% CO2.
Garcinol was obtained from Sigma-Aldrich (Merck KGaA) and SC79 was
purchased from MedChemExpress.
Cell viability
HGC-27 (2×104 cells/ml) cells were seeded
in 100 µl medium per well in a 96-well plate. Cells were incubated
for 6 h and subsequently treated with increasing concentrations of
garcinol [0, 2.5, 5, 10, 20, 40, 80 and 160 µM in RPMI-1640 medium
supplemented with 10% FBS, 1% PS and 50 mM dimethyl sulfoxide
(DMSO)] for 48 h. A total of 10 µl MTT solution (5 mg/ml in PBS;
Beyotime Institute of Biotechnology) was added to each well and the
cells were incubated for an additional 4 h at 37°C. The medium was
then discarded and the purple formazan crystals were dissolved
using 150 µ1 of DMSO. After 10 min of oscillation in dark, the
absorbance was read at a wavelength of 570 nm using a SpectraMax
Plus 384 plate reader (Molecular Devices, -LLC). The median lethal
concentration (LC50) was calculated using SPSS software (version
24.0; IBM Corp.).
Clone formation assay
HGC-27 cells were seeded at a density of
1×103 cells/well in a 6-well plate. Cells were then
treated with 5 µM garcinol in serum-containing medium, or an equal
volume of DMSO dissolved in medium as a control, for 14 days. The
cells were subsequently fixed using 10% formalin for 20 min and
stained with 0.1% crystal violet for 5 min at room temperature.
Colonies containing >50 cells were counted under a light
microscope (Eclipse TS100; Nikon Corporation).
Wound healing assay
HGC-27 cells were cultured in 6-well plates to 80%
confluence and then serum starved for 24 h. A 100 µl sterile pipet
tip was used to create scratches in the confluent cell monolayers
and the cells were gently washed with PBS. The cells were incubated
with garcinol (0, 10, 20, or 40 µM in RPMI-1640 medium supplemented
with 10% FBS and 1% PS) and cells were cultured for an additional
48 h. An optical microscope (Eclipse TS100; Nikon Corporation) was
used to image the wound at 0 and 48 h and the width of the wound
was measured using Image-Pro Plus software (version 6.0; Media
Cybernetics, Inc.).
Matrigel invasion assay
Transwell chambers (8.0-µm pore size; Corning, Inc.)
coated with Matrigel (Corning, Inc.) were used to evaluate the
effect of garcinol on the invasiveness of HGC-27 cells. HGC-27
cells (1×105/ml) were seeded into the upper chambers of
the inserts in 200 µl serum-free RPMI-1640, while different
concentrations of garcinol (0, 10, 20 or 40 µM in RPMI-1640
supplemented with 10% FBS and 1% PS) was added to the lower
chambers. Cells were cultured for 48 h at 37°C in a humidified
atmosphere containing 5% CO2. The invading cells were
then fixed and stained using the same conditions described for the
clone formation assays. The stained HGC-27 cells were subsequently
imaged and counted.
Flow cytometry and cell cycle
analysis
HGC-27 cells were seeded in 6-well plates and
cultured to 60% confluence. The cells were subsequently treated
with garcinol (0, 10, 20 and 40 µM in RPMI-1640 medium supplemented
with 10% FBS and 1% PS) for an additional 48 h. The cells were
harvested, suspended in cold PBS and centrifuged at 114 × g. The
cells were then resuspended and centrifuged two more times. Annexin
V-FITC/PI (Beyotime Institute of Biotechnology) double staining was
performed according to the manufacturer's instructions. The cells
were incubated for 20 min at room temperature in the dark and
apoptosis was analyzed using a flow cytometer. For cell cycle
analysis, HGC-27 cells were treated as aforementioned, collected
and fixed with 70% ethanol at 4°C overnight. Cells were washed with
cold PBS and incubated with propidium iodide for 30 min at room
temperature in the dark. ModFit LT software (version 5.0;
www.vsh.com) was used to analysis the cell cycle
transition of HGC-27 cells treated with increasing concentrations
of garcinol.
Hoechst 33258 staining
HGC-27 cells were seeded in 96-well plates and
incubated with garcinol (0, 10, 20, and 40 µM) for 48 h. The cells
were subsequently fixed with 4% polyoxymethylene for 20 min at room
temperature and washed twice with PBS. The cells were stained with
10 µg/ml Hoechst 33258 for 5 min in the dark and washed twice with
PBS. The nuclear morphology of the HGC-27 cells was observed using
a fluorescence microscope (200× magnification).
Western blotting
HGC-27 cells were treated with garcinol (0, 10, 20
and 40 µM) for 48 h and subsequently harvested. The total cellular
protein was extracted using radio-immunoprecipitation assay lysis
buffer (Beyotime Institute of Biotechnology) at 4°C. The lysates
(10 µ (protein per lane, BCA assay) separated via SDS-PAGE on a 10%
gel and transferred onto nitrocellulose membranes. After blocking
with 5% non-fat powdered milk for 60 min at room temperature, the
membranes were incubated overnight at 4°C with the following
primary antibodies: Anti-PI3K (cat. no. 4249; 1:1,000; Cell
Signaling Technology, Inc.), anti-AKT (cat. no. ab32505; 1:1,000;
Abcam), anti-AKTp-Thr308 (cat. no. ab38449; 1:1,000,
Abcam), anti-AKTp-Ser473 (cat. no. ab81283; 1:1,000,
Abcam), anti-mTOR (cat. no. ab2732; 1:1,000; Abcam),
anti-mTORp-Ser2448 (cat. no. 5536; 1:1,000; Cell
Signaling Technology, Inc.), anti-cyclin D1 (cat. no. 60186-1-Ig;
1:1,000; ProteinTech, Inc.), anti-Bcl-2 (cat. no. 15071; 1:1,000;
Cell Signaling Technology, Inc.), anti-BAX (cat. no. 14796;
1:1,000; Cell Signaling Technology, Inc.), anti-MMP-2 (cat. no.
ab97779; 1:1,000; Abcam), anti-MMP-9 (cat. no. ab76003; 1:1,000;
Abcam) and anti-β-actin (cat. no. 3700; 1:1,000; Cell Signaling
Technology, Inc.). The membranes were then washed three times with
1% Tris-buffered saline/Tween-20 and incubated with the appropriate
horseradish peroxidase secondary antibodies (cat. no. A21020 and
A21010; 1:1,000; Abbkine Scientific Co., Ltd.) for 4 h at 4°C. The
protein bands were visualized using the BeyoECL kit (Beyotime
Institute of Biotechnology). β-actin was used as the loading
control.
Statistical analysis
Data are expressed as the mean ± SD of three
independent experiments. Statistical analyses were performed using
GraphPad Prism software (version 7.0; GraphPad Software, Inc.).
One-way analysis of variance with Tukey's post hoc test was used
for the statistical analysis of the groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Garcinol decreases the proliferation
and viability of GC cells
The proliferation and viability of HGC-27 cells were
significantly suppressed by garcinol. HGC-27 cells treated with a
low concentration of garcinol exhibited similar optical density
values to their respective negative controls (Fig. 1B and C). However, the optical density
values of HGC-27 cells treated with higher doses of garcinol were
significantly reduced (T-test: 0.50±0.042, 20 µM, P<0.001;
0.32±0.034, 40 µM, P<0.001; 0.06±0.005, 80 µM, P<0.001;
0.06±0.009, 160 µM, P<0.001). The median lethal concentration
(LC50) of garcinol treatment in HGC-27 cells was 34.27 µM. HGC-27
cells treated with garcinol (0, 10, 20 and 40 µM) for 48 h
displayed a marked decrease in cell growth (Fig. 1B and C). Furthermore, garcinol
significantly decreased the colony formation ability of HGC-27
cells in a dose-dependent manner (Fig.
1D). In order to further investigate the effects of garcinol on
the cell cycle transition of HGC-27 cells, cell cycle analysis was
performed. The percentage of cells in the G0/G1 phase was
significantly increased (ANOVA: 45.33±0.182, 10 µM, P<0.05;
48.86±1.148, 20 µM, P<0.001; 53.11±0.769, 40 µM,
P<0.001)while the percentage of cells in the S phase was
decreased (ANOVA: 46.69±0.201, 10 µM, P<0.05; 43.15±1.151, 20
µM, P<0.001; 38.91±0.757, 40 µM, P<0.001) in garcinol-treated
cells compared with controls (43.39±0.350, 0 µM, G0/G1 phase;
48.66±0.424, 0 µM, S phase; Fig. 1E and
F).
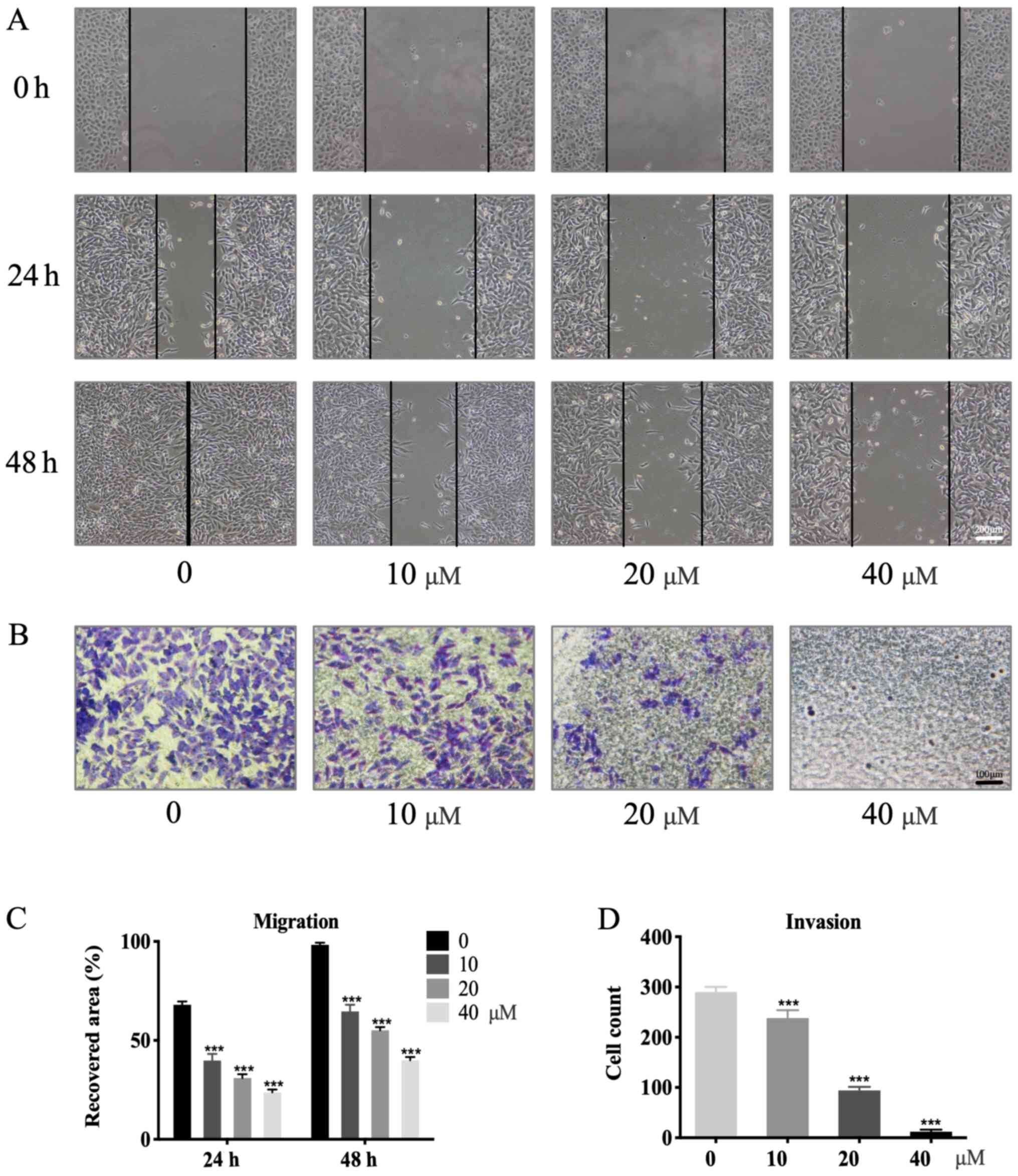
Garcinol inhibits the migration and
invasion of GC cells
Garcinol exerted a dose-dependent inhibitory effect
on migration and invasion in HGC-27 cells (Fig. 2A and B). Wound-healing assays were
performed to investigate the effects of garcinol on the migration
of HGC-27 cells. Compared with the control group (98.3±0.9%;
Fig. 2C) the percentage wound
closure exhibited a significant decrease with increasing garcinol
concentrations after 48 h of treatment: 64.6±2.75% (10 µM;
P<0.001), 55.1±1.3% (20 µM; P<0.001), 40.0±1.2% (40 µM;
P<0.001; Fig. 2C). A similar
trend was observed at the 24-h time point (P<0.001; Fig. 2C). Matrigel-coated Transwell chambers
were used to evaluate the inhibitory effects of garcinol on the
invasion of HGC-27 cells. The control group exhibited the highest
number of invading cells (255.3±13.0). This number significantly
decreased with increasing concentrations of garcinol at 48 h:
196.7±13.3 (10 µM; P<0.001), 83.3±6.3 (20 µM; P<0.001) and
12.0±3.2 (40 µM; P<0.001; Fig.
2D).
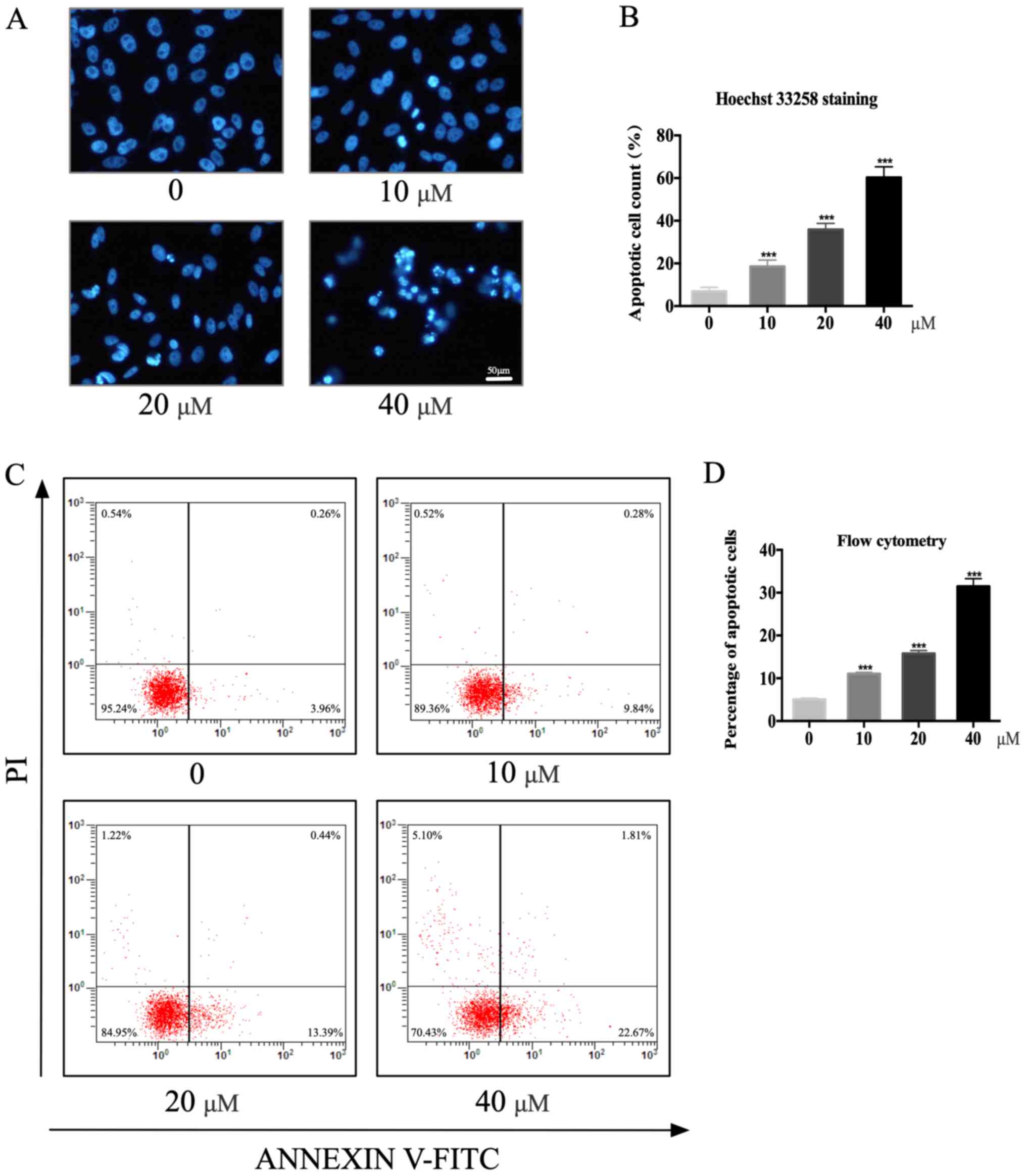
Garcinol induces apoptosis of GC
cells
Garcinol was found to induce the programmed cell
death of HGC-27 cells. Hoechst 33258 staining was used to observe
nuclear changes in HGC-27 cells. Cells with condensed and
fragmented nuclei were considered apoptotic (Fig. 3A). The number of apoptotic HGC-27
cells increased from 6.9±1.52 in the control group to 18.6±2.46
(P<0.001), 35.9±2.34 (P<0.001) and 60.3±4.10 (P<0.001) in
cells treated with 10, 20 and 40 µM garcinol for 48 h, respectively
(Fig. 3B). The annexin V-FITC/PI
staining assay revealed a similar trend. In particular, the number
of early apoptotic HGC-27 cells (annexin
V+/PI−) significantly increased with garcinol
concentrations as follows: 11.1±0.32% (10 µM; P<0.001),
15.8±0.67% (20 µM; P<0.001), 31.5±1.81% (40 µM; P<0.001;
Fig. 3C and D).
Garcinol down-regulates the activation
of the PI3K/AKT signaling pathway and its downstream effectors
Western blotting was used to determine the
concentration-dependent effects of garcinol on the expression of
several key proteins in the PI3K/AKT signaling pathway in HGC-27
cells. Garcinol significantly inhibited the levels of
AKTp-Thr308 and AKTp-ser473 in HGC-27 cells,
in a dose-dependent manner, while PI3K and total AKT levels were
not affected (Fig. 4A and B).
Additionally, garcinol significantly reduced the phosphorylation of
mTOR, while the expression of total mTOR remained stable
(P<0.05; Fig. 4A and B). Cyclin
D1 levels were then examined to further evaluate the impact of
garcinol on the G1/S transition of GC cells. Garcinol treatment was
found to decrease cyclin D1 levels in HGC-27 cells in a
dose-dependent manner (P<0.05; Fig.
4C and D). Garcinol also significantly reduced the expression
of the proteolytic enzymes MMP-2 and MMP-9 (P<0.05; Fig. 4E and F), which are considered to be
crucial for the malignant invasion and metastasis of carcinomas
(15,16). Furthermore, an increase in expression
of the pro-apoptotic BAX protein, together with a decrease in
expression of the anti-apoptotic Bcl-2 protein, was observed
(P<0.05; Fig. 4C and D).
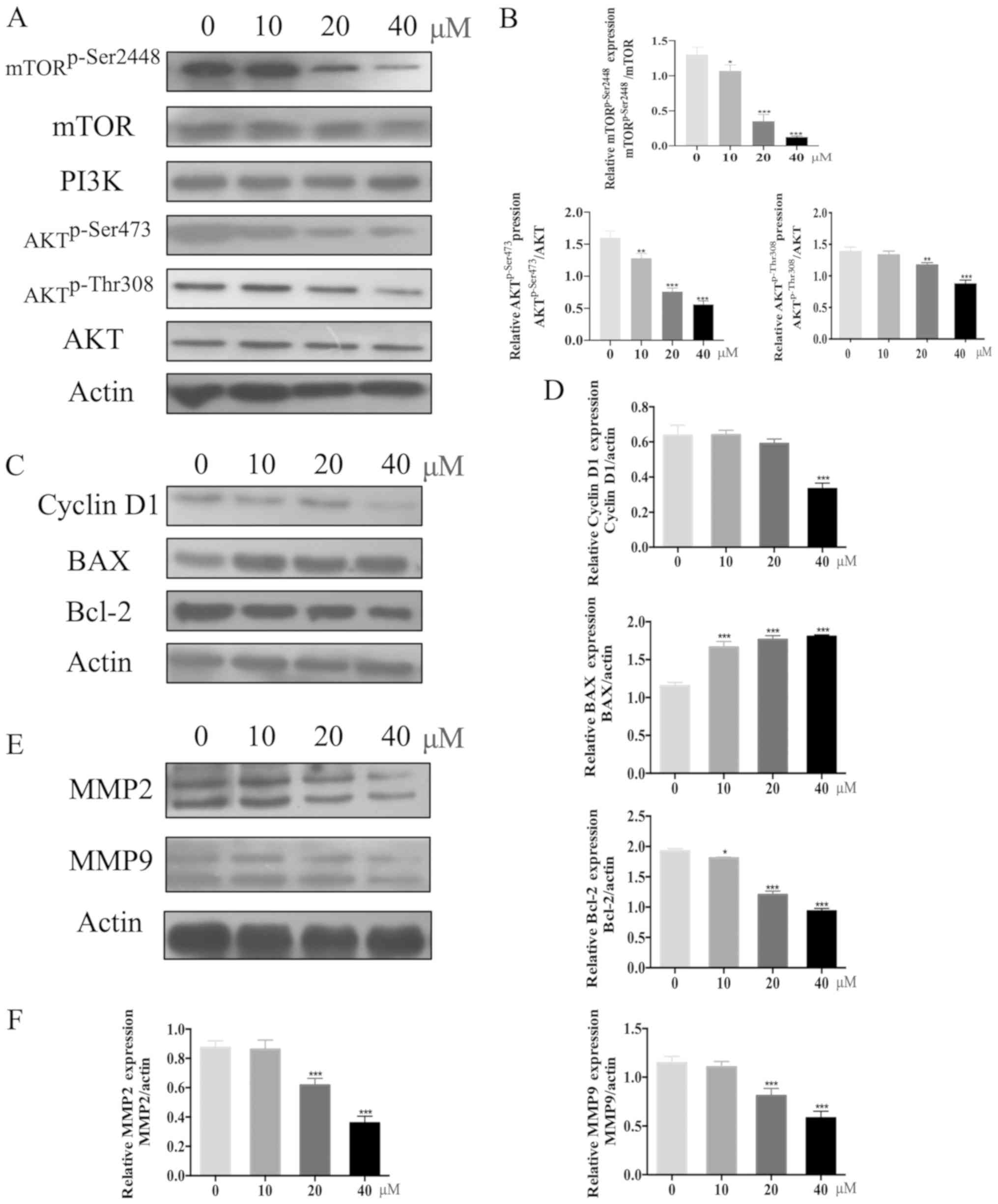 | Figure 4.Garcinol downregulateds PI3K/AKT and
its downstream signaling pathway. (A) HGC-27 cells were treated
with garcinol (0, 10, 20 and 40 µM) for 48 h and western blotting
was used to determine the protein expression levels of PI3K, AKT,
AKTp-Thr308, AKTSer473, mTOR,
mTORp-Ser2448 and β-actin. (B) Quantitative analysis for
the western blotting. The protein expression levels of PI3K, AKT,
AKTp-Thr308, AKTSer473, mTOR,
mTORp-Ser2448 and β-actin in each dose group (10, 20 and
40 µM) were compared with those in the no drug treatment group (0
µM). *P<0.05, **P<0.01 and ***P<0.005 vs. 0 µM. (C) HGC-27
cells were treated with garcinol (0, 10, 20 and 40 µM) for 48 h and
the protein expression levels of cyclin D1, BAX, Bcl-2 and β-actin
were determined by western blotting. (D) Semi-quantitative analysis
was performed to compare the protein expression levels of cyclin
D1, BAX, Bcl-2 and β-actin in each dose group (10, 20 and 40 µM)
with those in the no drug treatment group (0 µM). *P<0.05 and
***P<0.005 vs. 0 µM. (E) Western blotting was used to determine
the protein expression levels of MMP-2, MMP-9 and β-actin in HGC-27
cells under the treatment of garcinol (0, 10, 20 and 40 µM) for 48
h. (F) Protein expression levels of MMP-2, MMP-9 and β-actin in
each dose group (10, 20 and 40 µM) were quantitatively analyzed and
compared with those in the no drug treatment group (0 µM).
***P<0.005 vs. 0 µM. Each experiment was performed in
triplicate. MMP, matrix metalloprotease. |
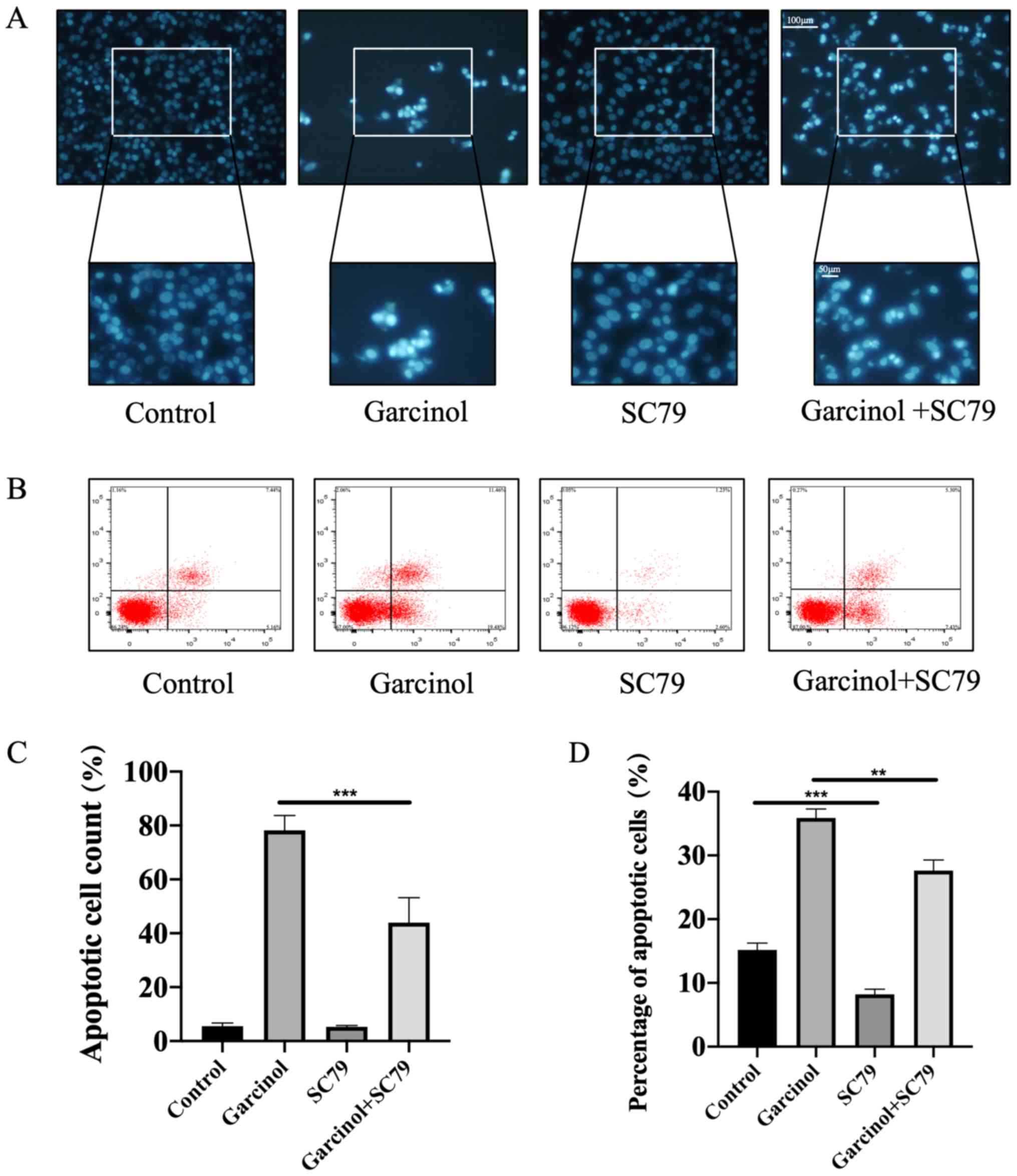
Specific AKT agonist SC79 rescues
garcinol-induced inhibitory effects in HGC-27 cells
The specific AKT agonist SC79 (25) was used to rescue the garcinol-induced
inhibitory effects on HGC-27 cell proliferation, invasion and
apoptosis. The MTT assay was performed to identify the most
effective dose of SC79 (Fig. 5A).
Western blotting was used to detect the expression levels of key
proteins in the PI3K/AKT signaling pathway. Garcinol significantly
decreased the expression level of AKTp-Thr308 in HGC-27 cells, an
effect that was abrogated with SC79 treatment (Fig. 5B). The inhibitory effect of garcinol
on cell migration (Fig. 5C and E)
and invasion (Fig. 5D and F) was
also abrogated following treatment with SC79. The effects of SC79
on garcinol-induced apoptosis of HGC-27 cells were investigated
using Hoechst 33258 staining and flow cytometry analysis. The
results revealed that SC79 significantly decreased the number of
apoptotic cells compared with the garcinol only-treated group
(Fig. 6A-D).
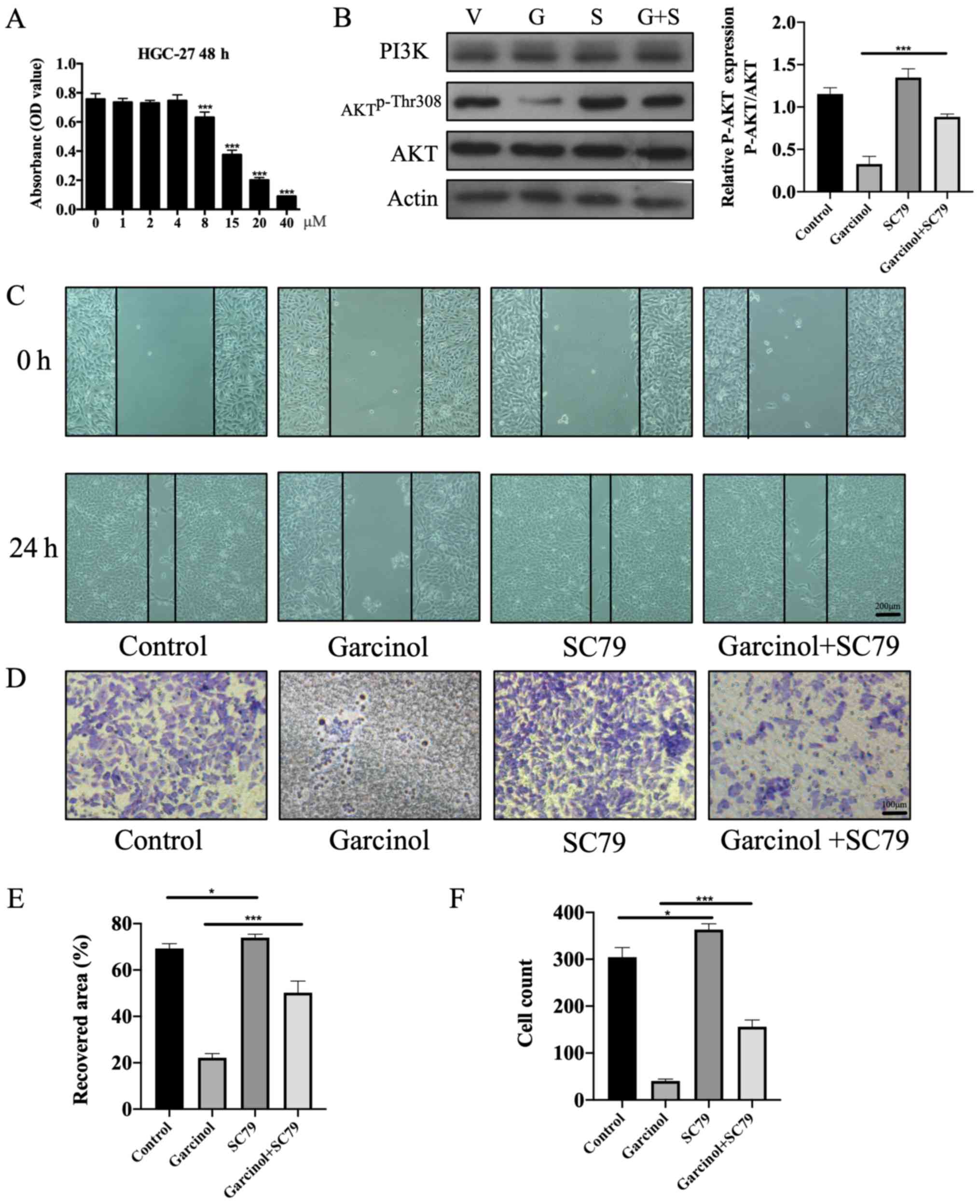 | Figure 5.SC79, a specific AKT agonist, rescues
the inhibitory effect of garcinol on the proliferation and invasion
of HGC-27 cells. (A) Safe effective dose of SC79 on HGC-27 cells at
48 h was measured by MTT assay. The absorbance of each dose group
(1, 2, 4, 8, 15, 20 and 40 µM) was compared with no SC79 treatment
group (0 µM). ***P<0.005 vs. 0 µM. (B) Western blotting was
performed to estimate the protein expression levels of PI3K, AKT,
AKTp-Thr308 and β-actin in HGC-27 cells at 48 h. ***P<0.005, as
indicated. (C) Width of the wound was measured at 0 and 24 h. Scale
bar, 200 µm. (D) A Transwell invasion assay was performed to
investigate the invasion of HGC-27 cells in the different groups at
48 h. Scale bar, 100 µm. (E) Recovered area of each group was
compared with vacant control group at 24 h. *P<0.05 and
***P<0.005, as indicated. (F) Invaded cell counts of each
intervention group were compared with the vacant control group at
48 h. *P<0.05 and ***P<0.005, as indicated. V, vacant
control; G, garcinol; S, SC79; G+S, garcinol+SC79. |
Discussion
GC is one of the most common malignant diseases
(1) and is responsible for the
second largest number of cancer-associated mortalities. There are
major challenges in developing effective therapeutic strategies for
advanced GC, and novel therapies are urgently required.
Garcinol is a bioactive phytochemical with
anti-carcinogenic properties. Previous studies have demonstrated
that garcinol decreases proliferation and induces apoptosis in
numerous tumor cells (18,19,26,27). The
anti-neoplastic activities of garcinol are reported to occur via a
variety of tumor-associated signaling pathways. Garcinol inhibited
autophagy and increased apoptosis of prostate cancer cells by
regulating the PI3K/AKT signaling pathway (23). Furthermore, garcinol suppressed the
progression of pancreatic (28) and
breast (29) carcinomas by mediating
NF-κB signaling and down-regulated the growth of hepatocellular
carcinoma cells by modulating STAT3 (30). However, to the best of our knowledge,
the effects and underlying mechanisms of garcinol in GC cells have
not been previously reported. The results obtained in the present
study revealed that garcinol significantly reduced the viability
and colony formation ability of GC cell line HGC-27 in a
dose-dependent manner. Garcinol resulted in a significant,
dose-dependent decrease in the migration and invasion of HGC-27
cells, which was accompanied by an increase in apoptosis.
Additionally, the percentage of HGC-27 cells in the G0/G1 phase was
significantly increased while the percentage in the S phase was
significantly decreased following treatment with garcinol, implying
significant cell cycle arrest. The G0/G1 phase is essential for DNA
replication and cell division, while a sustained G1 block may
result in apoptosis in HGC-27 cells (31–33).
Therefore, garcinol may serve as a potential therapeutic agent for
GC.
Tumor growth and metastasis are crucial steps in
tumorigenesis. Previous studies have indicated that mTOR is
expressed in 60–80% of gastric adenocarcinomas (34,35). The
regulation of cyclin D1 production by mTOR is the primary mechanism
by which mTOR mediates cell proliferation (36). Moreover, overexpression of cyclin D1
promotes the G1-S cell cycle transition and accelerates the GC
tumorigenesis (37). Therefore,
mTOR/cyclin D1 inhibitors may suppress tumor proliferation. The
present study revealed that garcinol decreased the protein
expression of mTOR, mTORp-Ser2448 and cyclin D1 in
HGC-27 cells in a dose-dependent manner, suggesting that it
suppresses the viability and proliferation of GC cells. Moreover,
garcinol significantly reduced the expression of MMP-2/9 proteins
in a dose-dependent manner. As proteolytic enzymes, the MMP family
participates in the degradation of the extracellular matrix and
MMP-2/9 proteins play important roles in invasion and angiogenesis
in malignant tumors (38). The
present study revealed that garcinol may inhibit the proliferation
and extravasation of gastric carcinoma cells, thereby exerting
anti-neoplastic effects and decreasing the rate of malignant
progression.
A dynamic balance between cell proliferation and
death is necessary to maintain homeostasis. Cancer cells are
characterized by their ability to evade tumor suppressors and cell
death and to sustain proliferative signaling and replication, thus
activating invasion and metastasis (39). Apoptosis appears to be attenuated in
numerous malignancies, thus enabling tumor cells to resist cell
death and to proliferate. Apoptosis is regulated by the
proapoptotic- and anti-apoptotic Bcl-2 family members (40). The upregulation of BAX and
downregulation of Bcl-2 increases apoptosis. In agreement with this
concept, the results obtained in the present study revealed that
garcinol increased HGC-27 cell apoptosis by down-regulating Bcl-2
and an up-regulating BAX in a dose-dependent manner. These results
indicated that garcinol may induce programed cell death in GC and
may serve as a promising therapeutic agent.
The mechanism underlying the anti-tumor effects of
garcinol may be attributed to the modulation of the PI3K/AKT
signaling pathway. The PI3K/AKT signaling pathway is closely
associated with neoplastic transformation, since the activation of
AKT is known to drive cellular growth, differentiation and survival
(41–43). Phosphorylated AKT enters the nucleus
and activates mTOR and downstream signaling, which subsequently
accelerates neoplasm progression (44). Previous studies suggested that the
PI3K/AKT/mTOR pathway is activated in gastric tumor tissues,
compared with non-tumor tissues (14,35,45),
indicating that targeted blocking of this pathway may be able to
suppress gastric tumorigenesis. Additionally, activated AKT
promotes the expression of MMPs, and reduces the binding of the
Bcl-2/XL-associated death (BAD) gene promoter to Bcl-2/XL (46,47), to
increase the invasion and decrease the apoptosis of gastric
carcinoma cells. The present study revealed that the protein levels
of AKTp-Thr308, AKTp-Ser473 and
mTORp-Ser2448 in HGC-27 cells decreased following
treatment with garcinol in a dose-dependent manner. These
aforementioned results suggested that garcinol exerts its antitumor
effects in HGC-27 cells by inhibiting the PI3K/AKT signaling
pathway. In order to further validate these findings, rescue
experiments using SC79, a specific agonist of AKT, were performed.
The results revealed that SC79 abrogated the garcinol-induced
inhibitory effects on HGC-27 cells, which further consolidated the
evidence garcinol inhibits the PI3K/AKT signaling pathway.
In conclusion, the present study demonstrated that
garcinol suppresses tumorigenesis in GC by decreasing cell
proliferation, inhibiting cell invasion and migration and promoting
cell apoptosis. Further investigation revealed that garcinol is
likely to exhibit these effects by inhibiting the PI3K/AKT
signaling pathway and downregulating the expression of Cylin D1,
MMP2, MMP9 in HGC-27 cells, which can be rescued by SC79, a
specific agonist of AKT, at the cellular level (Fig. S1). Therefore, garcinol may play
crucial roles in GC and may serve a novel therapeutic agent.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Foundation of China grant (grant nos. 81670472,
81700502 and 81800538), The Medical Health Scientific Project of
Zhejiang (grant no. 2019KY226) and The Technology Development
Research Plan of Shaoxing (grant no. 2018C30097).
Availability of data and materials
All data generated and/or analyzed during this study
are included in this published article.
Authors' contributions
AM, CG and XW conceived and designed the research.
YZ, CG, XZ performed the experiments and analyzed the data. YZ
wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Cutsem E, Sagaert X, Topal B,
Haustermans K and Prenen H: Gastric cancer. Lancet. 388:2654–2664.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Orditura M, Galizia G, Sforza V,
Gambardella V, Fabozzi A, Laterza MM, Andreozzi F, Ventriglia J,
Savastano B, Mabilia A, et al: Treatment of gastric cancer. World J
Gastroenterol. 20:1635–1649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Karimi P, Islami F, Anandasabapathy S,
Freedman ND and Kamangar F: Gastric cancer: Descriptive
epidemiology, risk factors, screening, and prevention. Cancer
Epidemiol Biomarkers Prev. 23:700–713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song Z, Wu Y, Yang J, Yang D and Fang X:
Progress in the treatment of advanced gastric cancer. Tumour Biol.
39:10104283177146262017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu WK, Cho CH, Lee CW, Fan D, Wu K, Yu J
and Sung J: Dysregulation of cellular signaling in gastric cancer.
Cancer Lett. 295:144–153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hennessy BT, Smith DL, Ram PT, Lu Y and
Mills GB: Exploiting the PI3K/AKT pathway for cancer drug
discovery. Nat Rev Drug Discov. 4:988–1004. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang J, Xu J, Dong Y and Huang B:
Down-regulation of HIF-1α inhibits the proliferation, migration,
and invasion of gastric cancer by inhibiting PI3K/AKT pathway and
VEGF expression. Biosci Rep. 38:BSR201807412018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gupta AK, Cerniglia GJ, Mick R, Ahmed MS,
Bakanauskas VJ, Muschel RJ and McKenna WG: Radiation sensitization
of human cancer cells in vivo by inhibiting the activity of PI3K
using LY294002. Int J Radiat Oncol Biol Phys. 56:846–853. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ao R, Guan L, Wang Y and Wang JN:
Silencing of COL1A2, COL6A3, and THBS2 inhibits gastric cancer cell
proliferation, migration, and invasion while promoting apoptosis
through the PI3k-Akt signaling pathway. J Cell Biochem.
119:4420–4434. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kumari S, Puneet, Prasad SB, Yadav SS,
Kumar M, Khanna A, Dixit VK, Nath G, Singh S and Narayan G: Cyclin
D1 and cyclin E2 are differentially expressed in gastric cancer.
Med Oncol. 33:402016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Riquelme I, Tapia O, Espinoza JA, Leal P,
Buchegger K, Sandoval A, Bizama C, Araya JC, Peek RM and Roa JC:
The Gene expression status of the PI3K/AKT/mTOR pathway in gastric
cancer tissues and cell lines. Pathol Oncol Res. 22:797–805. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou R, Xu L, Ye M, Liao M, Du H and Chen
H: Formononetin inhibits migration and invasion of MDA-MB-231 and
4T1 breast cancer cells by suppressing MMP-2 and MMP-9 through
PI3K/AKT signaling pathways. Horm Metab Res. 46:753–760. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou H, Wu J, Wang T, Zhang X and Liu D:
CXCL10/CXCR3 axis promotes the invasion of gastric cancer via
PI3K/AKT pathway-dependent MMPs production. Biomed Pharmacother.
82:479–488. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang F, Lee JT, Navolanic PM, Steelman
LS, Shelton JG, Blalock WL, Franklin RA and McCubrey JA:
Involvement of PI3K/Akt pathway in cell cycle progression,
apoptosis, and neoplastic transformation: A target for cancer
chemotherapy. Leukemia. 17:590–603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu C, Ho PC, Wong FC, Sethi G, Wang LZ
and Goh BC: Garcinol: Current status of its anti-oxidative,
anti-inflammatory and anti-cancer effects. Cancer Lett. 362:8–14.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saadat N and Gupta SV: Potential role of
garcinol as an anticancer agent. J Oncol. 2012:6472062012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oike T, Ogiwara H, Torikai K, Nakano T,
Yokota J and Kohno T: Garcinol, a histone acetyltransferase
inhibitor, radiosensitizes cancer cells by inhibiting
non-homologous end joining. Int J Radiat Oncol Biol Phys.
84:815–821. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamaguchi F, Saito M, Ariga T, Yoshimura Y
and Nakazawa H: Free radical scavenging activity and antiulcer
activity of garcinol from garcinia indica fruit rind. J Agric Food
Chem. 48:2320–2325. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liao CH, Sang S, Ho CT and Lin JK:
Garcinol modulates tyrosine phosphorylation of FAK and subsequently
induces apoptosis through down-regulation of Src, ERK, and Akt
survival signaling in human colon cancer cells. J Cell Biochem.
96:155–169. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Tsai ML, Chiou LY, Ho CT and Pan
MH: Antitumor activity of garcinol in human prostate cancer cells
and xenograft mice. J Agric Food Chem. 63:9047–9052. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aggarwal S and Das SN: Garcinol inhibits
tumour cell proliferation, angiogenesis, cell cycle progression and
induces apoptosis via NF-κB inhibition in oral cancer. Tumor
Biology. 37:7175–7184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jo H, Mondal S, Tan D, Nagata E, Takizawa
S, Sharma AK, Hou QM, Shanmugasundaram K, Prasad A, Tung JK, et al:
Small molecule-induced cytosolic activation of protein kinase Akt
rescues ischemia-elicited neuronal death. Proc Natl Acad Sci USA.
109:105812012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou XY, Cao J, Han CM, Li SW, Zhang C, Du
YD, Zhou QQ, Zhang XY and Chen X: The C8 side chain is one of the
key functional group of Garcinol for its anti-cancer effects.
Bioorg Chem. 71:74–80. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ahmad A, Sarkar SH, Aboukameel A, Ali S,
Biersack B, Seibt S, Li YW, Bao B, Kong D, Banerjee S, et al:
Anticancer action of garcinol in vitro and in vivo is in part
mediated through inhibition of STAT-3 signaling. Carcinogenesis.
33:2450–2456. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Parasramka MA and Gupta SV: Garcinol
inhibits cell proliferation and promotes apoptosis in pancreatic
adenocarcinoma cells. Nutr Cancer. 63:456–465. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ahmad A, Wang Z, Ali R, Maitah MY, Kong
DJ, Banerjee S, Padhye S and Sarkar FH: Apoptosis-inducing effect
of garcinol is mediated by NF-kappaB signaling in breast cancer
cells. J Cell Biochem. 109:1134–1141. 2010.PubMed/NCBI
|
|
30
|
Sethi G, Chatterjee S, Rajendran P, Li F,
Shanmugam MK, Wong KF, Kumar AP, Senapati P, Behera AK, Hui KM, et
al: Inhibition of STAT3 dimerization and acetylation by garcinol
suppresses the growth of human hepatocellular carcinoma in vitro
and in vivo. Molecular Cancer. 13:662014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hammarton TC, Engstler M and Mottram JC:
The Trypanosoma brucei Cyclin, CYC2, is required for cell
cycle progression through G1 phase and for maintenance of procyclic
form cell morphology. J Biol Chem. 279:24757–24764. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arand J and Sage J: G1 cyclins protect
pluripotency. Nat Cell Biol. 19:149–150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gao Z, Zhu M, Wu Y, Gao P, Qin Z and Wang
H: Interferon-lambda1 induces G1 phase cell cycle arrest and
apoptosis in gastric carcinoma cells in vitro. Oncol Rep.
32:199–204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lang SA, Gaumann A, Koehl GE, Seidel U,
Bataille F, Klein D, Ellis LM, Bolder U, Hofstaedter F, Schlitt HJ,
et al: Mammalian target of rapamycin is activated in human gastric
cancer and serves as a target for therapy in an experimental model.
Int J Cancer. 120:1803–1810. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Feng W, Brown RE, Trung CD, Li W, Wang LW,
Khoury T, Alrawi S, Yao J, Xia K and Tan D: Morphoproteomic profile
of mTOR, Ras/Raf Kinase/ERK, and NF-κB pathways in human gastric
adenocarcinoma. Ann Clin Lab Sci. 38:195–209. 2008.PubMed/NCBI
|
|
36
|
Advani SH: Targeting mTOR pathway: A new
concept in cancer therapy. Indian J Med Paediatr Oncol. 31:132–136.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Resnitzky D and Reed SI: Different roles
for cyclins D1 and E in regulation of the G1-to-S transition. Mol
Cell Biol. 15:34631995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou SY, Baltimore D, Cantley LC, Kaplan
DR and Franke TF: Interleukin 3-dependent survival by the Akt
protein kinase. Proc Natl Acad Sci USA. 94:11345–11350. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Geng W and Zhang HY: Research on the
mechanism of HP mediated PI3K/AKT/GSK3β pathways in gastric cancer.
Eur Rev Med Pharmacol Sci. 21 (Suppl 3):S33–S37. 2017.
|
|
43
|
Nagy TA, Frey MR, Yan F, Israel DA, Polk
DB and Peek RM Jr: Helicobacter pylori regulates cellular migration
and apoptosis by activation of phosphatidylinositol 3-kinase
signaling. J Infect Dis. 199:641–651. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Morita M, Gravel SP, Hulea L, Larsson O,
Pollak M, St-Pierre J and Topisirovic I: mTOR coordinates protein
synthesis, mitochondrial activity and proliferation. Cell Cycle.
14:473–480. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tapia O, Riquelme I, Leal P, Sandoval A,
Aedo S, Weber H, Letelier P, Bellolio E, Villaseca M, Garcia P and
Roa JC: The PI3K/AKT/mTOR pathway is activated in gastric cancer
with potential prognostic and predictive significance. Virchows
Archiv. 465:25–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mayer IA and Arteaga CL: The PI3K/AKT
pathway as a target for cancer treatment. Annu Rev Med. 67:11–28.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cheng TC, Din ZH, Su JH, Wu YJ and Liu CI:
Sinulariolide suppresses cell migration and invasion by inhibiting
matrix metalloproteinase-2/-9 and urokinase through the
PI3K/AKT/mTOR signaling pathway in human bladder cancer cells. Mar
Drugs. 15:E2382017. View Article : Google Scholar : PubMed/NCBI
|