Introduction
Meningiomas are the most commonly occurring primary
intracranial tumors, accounting for 20.0–33.8% of all intracranial
tumors (1,2). According to the WHO histological
classification, tumors may be classified as benign (grade 1),
atypical (grade 2) or malignant (grade 3) (3,4).
Atypical and malignant meningiomas account for ~5.0% of all
meningiomas (2). Although
meningiomas are mostly benign, the probability of an associated
hemorrhage is 1.3–2.4% (5–7). Hemorrhage can occur within the tumors,
surrounding the tumors, and within the brain parenchyma,
subarachnoid space and subdural space (8–10).
During such events, substantial bleeding may lead to severe
clinical consequences. Cheng and Lin (11) reported that the mortality of
hemorrhagic meningioma was as high as 38.5% in the computed
tomography (CT) era and 77.8% in the pre-CT era. Bosnjak et
al (12) reported that the
overall mortality and morbidity rates of hemorrhagic meningioma
were 21.1 and 32.6% in 2001, respectively.
The exact causes of hemorrhage in meningioma are
currently unknown. The most common hypotheses are infarction of the
tumor with secondary bleeding, increased density of blood vessels
inside the tumor, direct tumor invasion into one of the cerebral
arteries, mechanical stretching and distortion of the cortical
bridging veins, and histamine-related vasodilatation or venous
hypertension due to occlusion of the venous sinus (6,13).
In the present study, a case of sphenoidal ridge
meningioma with repeated bleeding episodes, manifested as
intratumoral parenchymal hemorrhages, is described. The progressive
process eventually resulted in the formation of a cerebral hernia,
which played an important role in the causal analysis of the
hemorrhage. In addition, a literature review on data between 2006
and 2019 was performed to provide supplemental information on the
causes of meningioma hemorrhage.
Case report
Medical history
A 35-year-old female patient was admitted to the
Sanbo Brain Hospital of the Capital Medical University (Beijing,
China) on November 19, 2018, with a complaint of an intermittent
week-long headache, aggravated nausea and vomiting. The patient had
experienced episodes of intermittent headache 1 week prior to the
aforementioned symptoms and had been admitted to a local hospital.
CT examination of the head showed hemorrhagic stroke, following
which, mannitol dehydration treatment (20%, once every 12 h; 125 ml
each time) was applied. After 4 days, the patient started showing
aggravated symptoms, accompanied by nausea and vomiting. A repeat
head CT demonstrated aggravated hemorrhage. The patient was then
transferred to Sanbo Brain Hospital. Upon admission, the nervous
system examination results were as follows: A Glasgow Coma Scale
score (14) of 13, lethargy, motor
aphasia, unequal pupil size (left, 5.0 mm; right, 2.0 mm),
disappearance of the left light reflex and a responsive right light
reflex. The muscular strength of the right limb was grade III
(Medical Research Council sum score) (15), tension was increased, deep reflex was
hyperactive and right pathological reflex was positive. Admission
blood tests revealed a normal platelet count, hemoglobin level,
international normalized ratio and activated partial thromboplastin
time.
The study was approved by the Ethics Committee of
Sanbo Brain Hospital. Written informed consent for publication was
obtained from the patient.
Imaging examination
On November 12, 2018, at the beginning of the onset
of hemorrhage, the CT examination showed bloody high-density
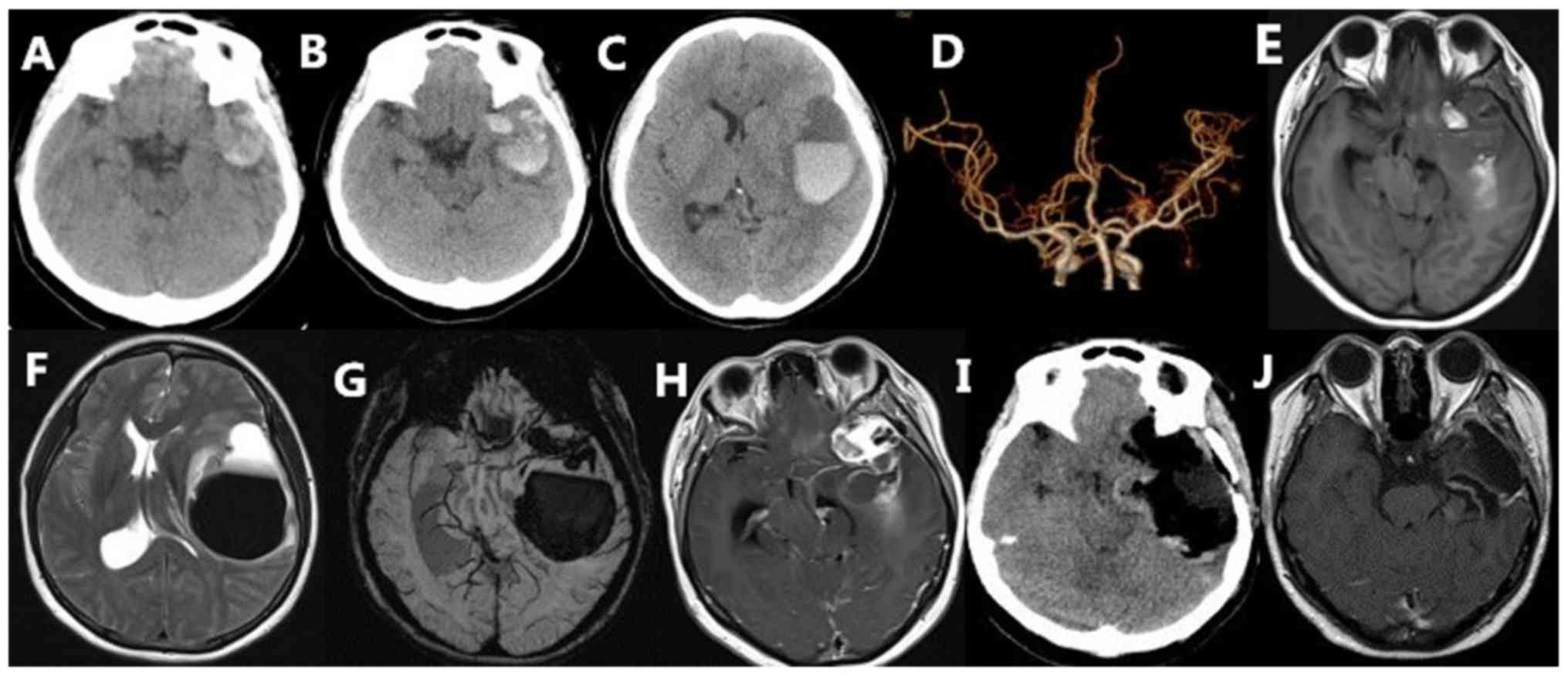
shadows in the left temporal region (Fig. 1A), whereas CT angiography did not
show any aneurysms or arteriovenous malformations (Fig. 1D). Another CT scan performed 3 days
later showed a significant increase in bleeding as compared with
the previous scan, and the blood was observed to have entered the
brain parenchyma and have formed a secondary temporal lobe gyrus
hernia (Fig. 1B and C). A magnetic
resonance imaging (MRI) scan of the head revealed that the left
temporal space-occupying lesions showed isointense and hyperintense
signals on T1-weighted imaging (Fig.
1E), hyperintense and hypointense signals on T2-weighted
imaging and a visible liquid level (Fig.
1F) on November 19, 2018. Susceptibility-weighted imaging (SWI)
showed that the hemorrhage site was located in the brain parenchyma
and within and around the tumor (Fig.
1G). Enhanced scanning showed that the lesions were uniformly
enhanced, whereas the surrounding hematoma was ring-enhanced and
displayed the meningeal tail sign (Fig.
1H). Post-surgical CT and MRI examinations showed that the
lesions had been completely removed (Fig. 1I and J).
Intraoperative and postoperative
conditions
Emergency resection was performed on admission. No
adherent veins were found around the tumor. The intratumoral
hemorrhage that was causing blood flow into the brain parenchyma
was evaluated. After the operation, the patient regained
consciousness and limb function gradually recovered. After 9 days,
the patient was discharged from hospital. At the time of discharge,
the patient could walk independently, but still experienced
incomplete aphasia. The left pupil was large (4.5 mm), but
sensitive to light reflection. The patient was then transferred to
a rehabilitation hospital for recovery treatment. At the 3-month
follow-up, the speech and pupil functions had recovered, and a
re-examination with MRI showed no recurrence of the tumor (Fig. 1J).
Histology
Gross morphology of the fresh tumor tissue excised
from surgery was fixed in 10% neutral buffered formalin overnight
for histological examinations at room temperature (24-26°C).
Furthermore, samples were specially dehydrated, paraffin embedded
and sectioned at 5-µm thickness following the procedures. After
that sections were stained with hematoxylin and eosin (H&E)
and/or immunohistochemical stains. The pathology result was
analyzed by 2 experienced specialists in the field of
neuropathology according to WHO classification of CNS tumors (2016)
(4).
Immunohistochemistry
Gross morphology of the fresh tumor tissue excised
from surgery was fixed in 10% neutral buffered formalin overnight
for histological examinations at room temperature. Formalin-fixed
sections were deparaffinized and stained with hematoxylin and eosin
(H&E) and immunohistochemistry according to the manufacturers'
instructions. In brief, 5-µm sections were deparaffinized. The
sections were then treated with 3% H2O2 for 5
min at room temperature to block endogenous peroxidase activity.
Antigen retrieval was performed by steaming the slides in target
retrieval solution, citrate pH 6 (Agilent Technologies Inc.) for 15
min and then cooling for 15 min. Then, the slides were blocked with
5% fetal bovine serum (Jackson ImmunoResearch Laboratories, Inc.)
diluted in wash buffer with 1% bovine serum albumin (Merk KGaA) at
room temperature for 15 min and incubated with primary antibodies
against epithelial membrane antigen (EMA; 1:100; cat. no. E29;
DAKO, Agilent Technologies, Inc.), Ki-67 (1:50 dilution; cat. no.
MIB-1; Labvision), progesterone receptor (PR; 1:150 dilution; cat.
no. ab8327; Abcam); CD34 (1:100; cat. no. ab8158; Abcam); vimentin
(1:150; cat. no. ab9254; Abcam) at 4°C overnight. After being
washed with phosphate-buffered saline 3 times, the sections were
stained with anti-mouse/rabbit polymer horse radish
peroxidase-labeled secondary antibody (cat. no. PV-6000D; Zhongshan
Bio-Tech Co., Ltd) for 30 min at 37°C. Then, 3,3′diaminobenzidine
(DAB) was applied for color development at room temperature for 5
min and sections were subsequently counterstained with hematoxylin.
Each slide was individually reviewed and scored using Faramount
mounting solution (Agilent Technologies Inc.) by 2 experienced
neuropathologists using light microscopy (×100-200).
Pathological findings
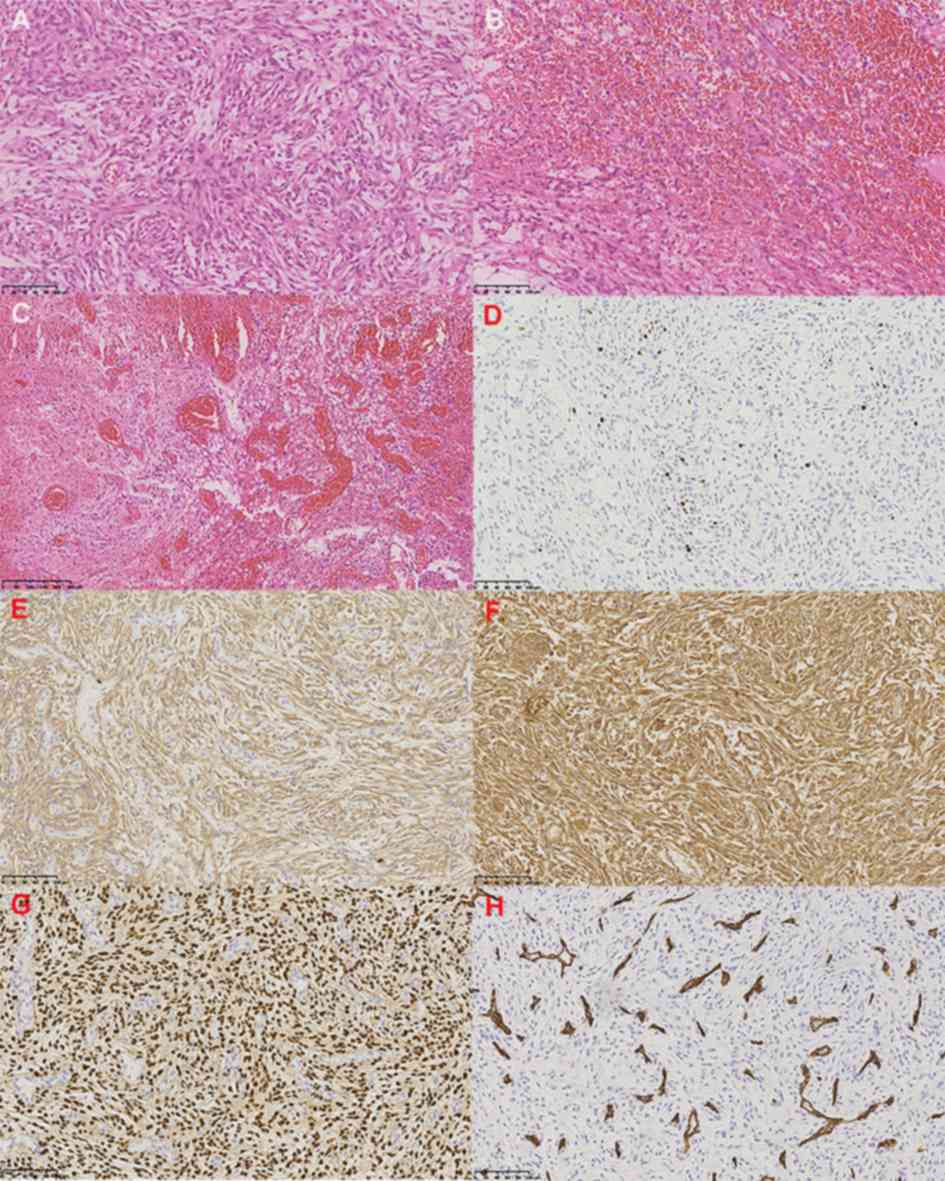
H&E-stained section demonstrated diffuse
proliferation of spindle shaped tumor cells arranged in bundles and
storiform (Fig. 2A), without
necrosis, mitosis and invasion of brain tissue. Hemorrhagic and
proliferative blood vessels were observed within the tumor
(Fig. 2B and C). Therefore, Grade 1
fibrous meningioma was diagnosed, according to the classification
criteria of 2016 WHO (4). Ki-67
expression was 5–10% (Fig. 2D).
Immunohistochemical staining revealed positive expression of
epithelial membrane antigens, vimentin and progesterone (Fig. 2E-G). The CD34 marker demonstrates
vascular dysplasia (Fig. 2H).
Literature review on hemorrhagic
meningiomas (2006–2019)
A total of 40 cases of hemorrhagic meningioma were
reviewed (Table I). The mean age of
onset was 56.23±12.91 years, and the male to female ratio was
1:1.35. Cystic changes were observed in 5.88% (2/34) and
recurrences were observed in 11.76% (4/34) of cases. The mortality
rate was 9.09% (3/33). Intratumor hemorrhage accounted for 80.00%
(32/40) of cases, and 37.50% (15/40) presented with solely
intratumoral hemorrhage. Multiple ventricular, peritumoral and
subdural hemorrhages also occurred. The proportion of solely
subdural hemorrhage cases was 17.50% (7/40), and only 1 case of
solely subarachnoid hemorrhage was reported (2.5%). In terms of
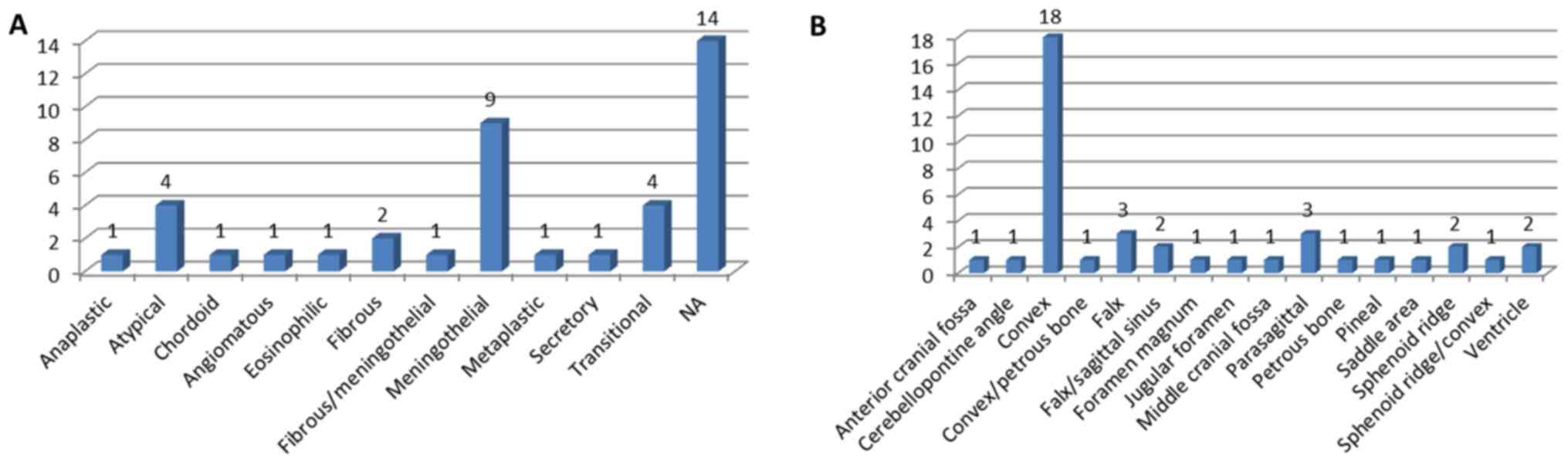
pathological type, the meningothelial tumor occurred in 34.62%
(9/26) of cases (Fig. 3A) and in
45.00% (18/40) of cases convex lesions were mostly observed
(Fig. 3B).
 | Table I.Clinical summary of 40 cases of
hemorrhagic intracranial meningioma. |
Table I.
Clinical summary of 40 cases of
hemorrhagic intracranial meningioma.
| First author/s,
year | Age, years | Sex | Hemorrhage
type | Pathological
type | Tumor location | Cystic | Incentive | Relapse | Outcome | (Refs.) |
|---|
| Ravindra VM, et
al, 2019 | 38 | M | ITH | Atypical | Saddle area | N | N | N | Normal | (56) |
| Mandour C, et
al, 2018 | 61 | M | ITH/IVH | Meningothelial | Sphenoid ridge | N | N | N | Hemiparesis | (13) |
| Hu S, et al,
2018 | 58 | M | ITH | NA | Falx/sagittal
sinus | N | N | N | Epilepsy | (57) |
| Basil G, et
al, 2018 | 54 | F | ITH | NA | Jugular
foramen | Y | N | N |
Hoarseness/hypoglossal weakness | (58) |
| Entezami P, et
al, 2018 | 49 | M | ITH | NA | Cerebellopontine
angle | N |
Radiotherapy/shunt | Y | Facial
palsy/hearing loss | (59) |
| Ravindran K, et
al, 2017 | 36 | F | ITH/SDH | NA | Sphenoid ridge | N | Postnatal | N | Normal | (60) |
| Byard RW, 2017 | 46 | M | ITH/ICH |
Fibrous/meningothelial | Parasagittal | N | N | N | Died | (61) |
| Broggi M, et
al, 2017 | 45 | F | ITH/PTH | Atypical | Convex | N | N | Y | NA | (62) |
| Diehl C, et
al, 2016 | 58 | M | ITH/IVH | NA | Ventricle | N | Thrombolytic | Y | Hemiparesis | (49) |
| Wang HC, et
al, 2016 | 39 | F | ITH/PTH | Fibrous | Falx | NA | NA | NA | Normal | (6) |
| Wang HC, et
al, 2016 | 45 | M | ITH | Atypical | Parasagittal | NA | NA | NA | Normal | (6) |
| Wang HC, et
al, 2016 | 51 | F | ITH/PTH | Transitional | Convex | NA | NA | NA | Normal | (6) |
| Wang HC, et
al, 2016 | 53 | F | ITH/PTH | Angiomatous | Convex | NA | NA | NA | Normal | (6) |
| Wang HC, et
al, 2016 | 59 | F | ITH | Atypical | Convex | NA | NA | NA | Hemiparesis | (6) |
| Wang HC, et
al, 2016 | 64 | M | ITH/PTH | Fibrous | Parasagittal | NA | NA | NA | Normal | (6) |
| Aoyama Y, et
al, 2015 | 59 | F | SAH | Transitional | Foramen magnum | N | N | N | NA | (63) |
| Ito Y, et
al, 2015 | 78 | F | ITH | Anaplastic | Convex | N |
Anticoagulation | N | Hemiparesis | (64) |
| Levine AB &
MacDougall KW, 2014 | 69 | M | ITH/SDH | NA | Convex | N | N | N | Confusion
improved | (65) |
| Eljebbouri B, et
al, 2014 | 51 | M | SDH | Meningothelial | Convex | N | N | N | Normal | (66) |
| Chonan M, et
al, 2013 | 67 | F | SDH | Meningothelial | Convex | N | N | N | Normal | (67) |
| Lee KH, et
al, 2013 | 23 | F | ITH | Chordoid | Pineal | Y | Pregnancy | N | Normal | (18) |
| Sasagawa Y, et
al, 2013 | 72 | F | ITH | Eosinophilic | Convex | N | N | N | Motor
aphasia/hemiparesis | (68) |
| Rocha AJ, et
al, 2013 | 52 | M | ITH/SDH | Meningothelial | Convex | N | N | N | NA | (69) |
| Kumar S, et
al, 2013 | 30 | F | ITH | Meningothelial | Convex | N | Pregnancy | N | NA | (20) |
| Krisht KM, et
al, 2012 | 50 | F | ITH | Metaplastic | Middle cranial
fossa | N | N | N | Normal | (70) |
| Eom KS & Kim
TY, 2012 | 75 | F | ITH | Meningothelial | Convex | N | Trauma | N | Consciousness not
improved | (71) |
| Yamaguchi S, et
al, 2011 | 34 | F | ITH | Meningothelial | Convex | N | Angiography | N | NA | (50) |
| Czyż M, et
al, 2011 | 69 | F | SDH | NA | Falx/sagittal
sinus | N | N | N | Normal | (72) |
| Bellut D, et
al, 2011 | 65 | F | ITH/IVH | Transitional | Convex | N | N | Y | Swallowing
disturbances | (73) |
| Miyajima Y, et
al, 2010 | 63 | F | ITH/PTH | Transitional | Convex/petrous
bone | N | Angiography | N | Hemiparesis | (51) |
| Lakshmi Prasad G,
et al, 2010 | 73 | M | ITH/SDH | NA | Sphenoid
ridge/convex | N | N | N | NA | (74) |
| Worm PV, et
al, 2009 | 64 | M | SDH | NA | Anterior cranial
fossa | N | Pulmonary function
tests | N | Normal | (52) |
| de Almeida JP,
et al, 2009 | 66 | F | ITH | NA | Convex | N | N | N | Normal | (53) |
| Kashimura H, et
al, 2008 | 55 | M | SDH | Meningothelial | Convex | N | N | N | Normal | (30) |
| Miyazawa T, et
al, 2008 | 66 | F | ITH | NA | Falx | N | Aspirin | N | Hemiparesis | (75) |
| Romeike BF, et
al, 2007 | 57 | F | ITH/IVH | NA | Ventricle | N | N | N | Died | (76) |
| Romero JR, et
al, 2007 | 66 | M | ITH/ICH | NA | Falx | N | Warfarin | N | Died | (77) |
| Mitsuhara T, et
al, 2006 | 60 | F | SDH | Meningothelial | Petrous bone | N | N | N | Normal | (78) |
| Ziyal IM, et
al, 2006 | 57 | M |
ITH/ICH/SDH/SAH | Secretory | Convex | N | N | N | Nerve
palsy/neurological status improved | (79) |
| Di Rocco F, et
al, 2006 | 72 | M | SDH | NA | Convex | N | N | N | NA | (80) |
Discussion
Meningiomas are usually benign tumors that arise
from arachnoidal cap cells and are detected by the symptoms caused
by increased intracranial pressure and seizures. Progressive
neurological deficits may also occur, depending on the tumor
location and growth (16).
Hemorrhagic meningioma is a rare disease with an incidence of
2.0–2.2% in Asia in the last two decades (6,7).
Repeated hemorrhages are rare, with only 2 cases having been
reported (17,18). From these cases, plus the present
case, meningioma patients with repeated hemorrhages were found to
be young, with the average age of onset being 28±6.24 years
(Table II). One of the previous
cases was in a pregnant woman, and a possible cause could be the
fluctuation of hormone levels during pregnancy, a hypothesis that
has also been supported by other studies (19,20). In
the present study, the patient also displayed a high level of
progesterone. It was also reported that the internal tumor
hemorrhage progressed gradually and eventually broke into the
peritumoral area and the brain parenchyma. As tumors have a higher
cellular density than the brain parenchyma, it is not surprising
that tumoral blood can encroach the brain parenchyma after breaking
through the tumor. This may also explain why there are more
peritumoral and parenchymal hemorrhages than intratumoral
hemorrhages. Another possible cause of hemorrhage was found to be
the high proliferation index of tumors. Niiro et al
(7) reported that the MIB-1 labeling
index of 5 grade I meningioma cases was 5.8±2.2. Ki-67 is known to
increase with the grade of meningioma and may be associated with
the biological behavior of the invasion (21,22).
Sunada et al (23) also
reported that a high cell proliferation index may activate certain
mechanisms leading to malignant tumor hemorrhage. In addition,
abnormal vascular proliferation, as in the case reported in the
present study, may also be one of the causes of hemorrhage
(Fig. 2C and H).
 | Table II.Clinical features of repeated
hemorrhage of meningioma. |
Table II.
Clinical features of repeated
hemorrhage of meningioma.
|
|
|
| Hemorrhage |
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| First author/s,
year | Age, years | Sex | n | Type | Intervals | Tumor location | Pathological
type | Possible cause | (Refs.) |
|---|
| Scotti G et
al, 1987 | 26 | F | 3 | SAH | 1.33 months | Foramen magnum,
C1 | Papillar,
epithelial, psammomatous bodies (mixed ingredients) | NA | (17) |
| Lee KH et
al, 2013 | 23 | F | NA | ITH | NA | Pineal region | Chordoid | Pregnancy | (18) |
| Present case,
2019 | 35 | F | 2 | ITH, ICH | 3 days | Sphenoid ridge | Fibrous | N |
|
A review of previous studies revealed that little
research has been conducted on the imaging features of meningioma
hemorrhage. In most cases, intensive MRI scans have been used;
however, T1- and T2-weighted imaging can offer reference support in
determining the timing of the hemorrhage. Based on the timeline,
intracranial hemorrhage can be classified into hyperacute stage
(the first few hours), acute stage (1–3 days), early subacute stage
(3–7 days), late subacute stage (4–7 days to 1 month), and chronic
stage (1 month to years) (24,25). The
bleeding time of meningioma can also be evaluated by MRI signal
information. The SWI MRI technique is sensitive to paramagnetic
blood products, such as methemoglobin, deoxyhemoglobin, ferritin
and hemosiderin. SWI is considered the most sensitive tool
available for the detection of cerebral microbleeds (26–28). MRI
revealed the subacute hemorrhage in the patient of the present
study. The tumor diameter seemed to be unrelated to meningioma
hemorrhage, and there are reports of secondary bleeding from small
tumors (29,30). In addition, the literature review
showed that calcification of hemorrhagic meningiomas is rare
(31), which may be due to the high
tumor density that makes bleeding difficult.
In 2005, Bosnjak et al (12) conducted a study on 134 cases of
hemorrhagic meningioma and showed that factors that may lead to
bleeding are a patient age of <30 or >70 years, convex and
intraventricular lesions, and a fibrous tumor type. The mortality
rate was reported to be 21.1%. In the present study, 40 cases of
hemorrhagic meningioma, reported in the past 14 years, were
reviewed and convex meningioma was shown to have a higher
probability of hemorrhage. Although numerous pathological types of
meningioma can produce hemorrhage, a meningoepithelial tumor is the
type most likely to do so. Niiro et al (7) investigated 57 cases of hemorrhagic
meningioma, and also showed that meningothelial is the most common
pathological type, followed by fibrotic type and hemangiomas.
Cystic changes are relatively rare, and were observed in 5.88% of
the hemorrhagic meningioma cases. The incidence of cystic
meningiomas was 1.7–10% (32). It is
questionable whether the same disease occurs simultaneously into
two separate processes of a rare disease progression and whether
there is a correlation between these two processes. However, some
reports argue that cystic meningiomas are prone to hemorrhage
(33,34). With the development of imaging
detection and diagnosis technologies, the mortality rate due to
meningiomas has decreased. In the present study, the mortality rate
was found to have decreased to 9.09% in the past 14 years (12).
The precise causes of hemorrhage in meningioma are
unknown. The most regularly expounded theories are of tumor
infarction with secondary bleeding, direct invasion of the tumor
into a cerebral artery, mechanical stretching and distortion of the
cortical bridging veins, and histamine-associated vasodilatation or
venous hypertension as a result of venous sinus occlusion (13). A recent study have also found that
the average number of undifferentiated vessels in hemorrhagic
meningioma patient groups is significantly higher than that in the
control groups, thereby confirming their existence as factors
involved in the mechanism of hemorrhage (6). In the case report of the present study,
the patient showed an onset of intratumor hemorrhage, followed by
aggravated bleeding that penetrated into the brain parenchyma.
Further analysis of relevant hemorrhage cases (mostly individual
cases) in the past 14 years indicated that a majority of
hemorrhages occur from inside the tumor, i.e., they are
precipitated by an internal factor. Intratumoral hemorrhage is
recorded to account for 80.00% (32/40) of cases. In such cases, it
is estimated that the hemorrhage may have started inside the tumor
and progressed, leading to the blood entering the peritumoral and
subdural areas, and even encroaching the brain parenchyma. Due to
the high density of the tumor, hemorrhage caused by an external
factor, such as a peritumoral blood vessel penetrating into the
brain parenchyma, is unlikely. Moreover, in the present study, the
literature review focused on evaluating traceable causative
factors, including embolization therapy (35–40),
pregnancy (19,20), radiotherapy (41,42), air
travel (43), surgery (44), trauma (8), long-term cough (45), anticoagulation therapy (46–48),
thrombolytic therapy (49), cerebral
angiography (50,51) and pulmonary function examination
(52). Prevention and intervention
targeted at these factors may reduce the probability of
hemorrhage.
Hemorrhage of the meninges is often a serious event
with a high mortality rate. However, several studies have reported
shrinkage in tumor size following a hemorrhagic event (53). Surgical intervention for hemorrhagic
meningiomas is the unanimously accepted treatment (11,54,55). In
the present case, the patient had a secondary cerebral hernia,
which was cured after proactive treatment. Early intervention is
necessary to eliminate the risk of secondary bleeding, which can
often lead to severe consequences.
In conclusion, repeated hemorrhages in meningiomas
are extremely rare and the causes have not been identified yet.
Increased Ki-67 and abnormally proliferating blood vessels may be
potential causes of hemorrhage. Early diagnosis and rapid surgical
intervention are essential to prevent further episodes of bleeding,
which may have fatal consequences for the patient. A limitation of
the present study is the insufficient number of studies reviewed;
however, the rare case reported will add to the scant literature on
such cases and will offer a basis for future research in this
field.
Acknowledgements
Not applicable.
Funding
The study was supported by the Beijing Municipal
Science and Technology Commission of China (grant no.
Z181100001718199).
Availability of data and materials
The datasets used or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CJY, CKY and SH designed the study; YKY and NL
collected data and revised the manuscript for important
intellectual content; XLQ performed the histological examination of
the samples; ZCY analyzed the data and SH wrote the article. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Sanbo Brain Hospital (Beijing, China).
Patient consent for publication
Written informed consent was obtained from the
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Claus EB, Bondy ML, Schildkraut JM,
Wiemels JL, Wrensch M and Black PM: Epidemiology of intracranial
meningioma. Neurosurgery. 57:1088–1095. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wiemels J, Wrensch M and Claus EB:
Epidemiology and etiology of meningioma. J Neurooncol. 99:307–314.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bromowicz J: Spontaneous hemorrhage in
brain tumors. Neurol Neurochir Pol. 17:471–475. 1983.(In Polish).
PubMed/NCBI
|
|
6
|
Wang HC, Wang BD, Chen MS, Li SW, Chen H,
Xu W and Zhang JM: An underlying pathological mechanism of
meningiomas with intratumoral hemorrhage: Undifferentiated
microvessels. World Neurosurg. 94:319–327. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Niiro M, Ishimaru K, Hirano H, Yunoue S
and Kuratsu J: Clinico-pathological study of meningiomas with
haemorrhagic onset. Acta Neurochir (Wien). 145:767–72. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bruno MC, Santangelo M, Panagiotopoulos K,
Piscopo GA, Narciso N, Del Basso De Caro MI, Briganti F and Cerillo
A: Bilateral chronic subdural hematoma associated with meningioma.
Case report and review of the literature. J Neurosurg Sci.
47:215–27. 2003.PubMed/NCBI
|
|
9
|
Martínez-Lage JF, Poza M, Martínez M,
Esteban JA, Antúnez MC and Sola J: Meningiomas with haemorrhagic
onset. Acta Neurochir (Wien). 110:129–32. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jones NR and Blumbergs PC: Intracranial
haemorrhage from meningiomas: A report of five cases. Br J
Neurosurg. 3:691–698. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng MH and Lin JW: Intracranial
meningioma with intratumoral hemorrhage. J Formos Med Assoc.
96:116–120. 1997.PubMed/NCBI
|
|
12
|
Bosnjak R, Derham C, Popović M and Ravnik
J: Spontaneous intracranial meningioma bleeding:
Clinicopathological features and outcome. J Neurosurg. 103:473–484.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mandour C, Laaguili J, Gazzaz M and
Mostarchid BE: Spontaneous intraventricular hemorrhage caused by
sphenoid meningioma. J Neurol Surg A Cent Eur Neurosurg.
79:434–435. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Teasdale G and Jennett B: Assessment of
coma and impaired consciousness. A practical scale. Lancet.
2:81–84. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kleyweg RP, van der Meché FG and Schmitz
PI: Interobserver agreement in the assessment of muscle strength
and functional abilities in Guillain-Barré syndrome. Muscle Nerve.
14:1103–1109. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kouyialis AT, Stranjalis G, Analyti R,
Boviatsis EJ, Korfias S and Sakas DE: Peritumoural haematoma and
meningioma: A common tumour with an uncommon presentation. J Clin
Neurosci. 11:906–909. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scotti G, Filizzolo F, Scialfa G, Tampieri
D and Versari P: Repeated subarachnoid hemorrhages from a cervical
meningioma. Case report. J Neurosurg. 66:779–781. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee KH, Lall RR, Chandler JP, Bigio EH and
Mao Q: Pineal chordoid meningioma complicated by repetitive
hemorrhage during pregnancy: Case report and literature review.
Neuropathology. 33:192–198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chow MS, Mercier PA, Omahen DA, Wood SL
and Johnson JA: Recurrent exophytic meningioma in pregnancy. Obstet
Gynecol. 121 (Suppl 1):S475–S478. 2013.
|
|
20
|
Kumar S, Gupta V and Khandelwal N:
Hemorrhage in meningioma: An unwanted outcome of pregnancy. Neurol
India. 61:329–330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bohra H, Rathi KR, Dudani S, Bohra A,
Vishwakarma S and Sahai K: The study of MIB-1 LI and CD 34 as a
marker of proliferative activity and angiogenesis in different
grades of meningioma. J Clin Diagn Res. 10:EC14–EC17.
2016.PubMed/NCBI
|
|
22
|
Olar A, Wani KM, Sulman EP, Mansouri A,
Zadeh G, Wilson CD, DeMonte F, Fuller GN and Aldape KD: Mitotic
index is an independent predictor of recurrence-free survival in
meningioma. Brain Pathol. 25:266–275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sunada I, Nakabayashi H, Matsusaka Y and
Yamamoto S: Meningioma associated with acute subdural hematoma-case
report. Radiat Med. 16:483–486. 1998.PubMed/NCBI
|
|
24
|
Smith EE, Rosand J and Greenberg SM:
Hemorrhagic stroke. Neuroimaging Clin N Am. 15259–272. (ix)2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Parizel PM, Makkat S, Van Miert E, Van
Goethem JW, van den Hauwe L and De Schepper AM: Intracranial
hemorrhage: Principles of CT and MRI interpretation. Eur Radiol.
11:1770–1783. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma X, Bai Y, Lin Y, Hong X, Liu T, Ma L,
Haacke EM, Zhou J, Wang J and Wang M: Amide proton transfer
magnetic resonance imaging in detecting intracranial hemorrhage at
different stages: A comparative study with susceptibility weighted
imaging. Sci Rep. 7:456962017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Haacke EM, Mittal S, Wu Z, Neelavalli J
and Cheng YC: Susceptibility-weighted imaging: Technical aspects
and clinical applications, part 1. AJNR Am J Neuroradiol. 30:19–30.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mittal S, Wu Z, Neelavalli J and Haacke
EM: Susceptibility-weighted imaging: Technical aspects and clinical
applications, part 2. AJNR Am J Neuroradiol. 30:232–252. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sato K, Sugawara T, Fujiwara S, Mizoi K
and Yoshimoto T: A case of small meningioma with acute subdural
hematoma. No Shinkei Geka. 17:687–690. 1989.(In Japanese).
PubMed/NCBI
|
|
30
|
Kashimura H, Arai H, Ogasawara K, Beppu T,
Kurose A and Ogawa A: Lipomatous meningioma with concomitant acute
subdural hematoma-case report-. Neurol Med Chir (Tokyo).
48:466–469. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Levy A and Mansuy L: Two cases of cerebral
tumors with atypical calcification; chronic subdural meningioma and
hematoma. J Radiol Electrol Arch Electr Medicale. 32:821–822.
1951.(In Undetermined Language). PubMed/NCBI
|
|
32
|
Wang P, Han S, Liu N, Yu C, Qi X, Zhu M,
Zhang X, Wang LI and Yan C: Peritumoral cystic meningioma: A report
of two cases and review of the literature. Exp Ther Med.
11:904–908. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wakamoto H, Miyazaki H, Hayashi T,
Shimamoto Y and Ishiyama N: Intratumoral hemorrhage associated with
cystic meningioma under observation: A case report. No Shinkei
Geka. 26:247–252. 1998.(In Japanese). PubMed/NCBI
|
|
34
|
Kuzeyli K, Cakir E, Usul H, Karaarslan G,
Yazar U, Baykal S, Reis A and Cobanoglu U: Intratumoral
haemorrhage: A clinical study. J Clin Neurosci. 11:490–192. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ishihara H, Ishihara S, Niimi J, Neki H,
Kakehi Y, Uemiya N, Kohyama S, Yamane F, Kato H, Suzuki T, et al:
The safety and efficacy of preoperative embolization of meningioma
with N-butyl cyanoacrylate. Interv Neuroradiol. 21:624–630. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu SC, Boet R, Wong GK, Lam WW and Poon
WS: Postembolization hemorrhage of a large and necrotic meningioma.
AJNR Am J Neuroradiol. 25:506–508. 2004.PubMed/NCBI
|
|
37
|
Kallmes DF, Evans AJ, Kaptain GJ, Mathis
JM, Jensen ME, Jane JA and Dion JE: Hemorrhagic complications in
embolization of a meningioma: Case report and review of the
literature. Neuroradiology. 39:877–880. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Motozaki T, Otuka S, Sato S, Nakao S, Ban
S, Fukumitsu T and Yamamoto T: Preoperative embolization with
gelfoam powder for intracranial meningioma causing unusual
peritumoral hemorrhage-with reference to the mechanism of
hemorrhage. No Shinkei Geka. 15:95–101. 1987.(In Japanese).
PubMed/NCBI
|
|
39
|
Suyama T, Tamaki N, Fujiwara K, Hamano S,
Kimura M and Matsumoto S: Peritumoral and intratumoral hemorrhage
after gelatin sponge embolization of malignant meningioma: Case
report. Neurosurgery. 21:944–946. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Watanabe K, Matsumura K, Matsuda M and
Handa J: Meningioma with intratumoral and subdural hemorrhage as an
immediate complication of therapeutic embolization. Case report.
Neurol Med Chir (Tokyo). 26:904–907. 1986.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim CH, Kim DG, Paek SH, Chung HT, Choi YL
and Chi JG: Delayed bleeding after gamma knife surgery for
meningioma. Acta Neurochir (Wien). 146:741–742. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kwon Y, Ahn JS, Jeon SR, Kim JH, Kim CJ,
Lee JK, Kwun BD, Lee DH and Kim SY: Intratumoral bleeding in
meningioma after gamma knife radiosurgery. J Neurosurg. 97 (5
Suppl):S657–S662. 2002. View Article : Google Scholar
|
|
43
|
Goldberg CR and Hirschfeld A: Hemorrhage
within brain tumors in association with long air travel. Acta
Neurochir (Wien). 144:289–293. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Maeda K, Gotoh H, Chikui E and Furusawa T:
Intratumoral hemorrhage from a posterior fossa tumor after cardiac
valve surgery-case report. Neurol Med Chir (Tokyo). 41:548–550.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bloomgarden GM, Byrne TN, Spencer DD and
Heafner MD: Meningioma associated with aneurysm and subarachnoid
hemorrhage: Case report and review of the literature. Neurosurgery.
20:24–26. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Spektor S, Ashkenazi E and Israel Z:
Intracranial haemorrhage from a meningioma in a patient receiving
aspirin prophylaxis: A case report. Acta Neurochir (Wien).
134:51–53. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Toledo E, Shalit MN and Segal R: Spinal
subdural hematoma associated with anticoagulant therapy in a
patient with spinal meningioma. Neurosurgery. 8:600–603. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Everett BA, Kusske JA and Pribram HW:
Anticoagulants and intracerebral hemorrhage from an unsuspected
meningioma. Surg Neurol. 11:233–235. 1979.PubMed/NCBI
|
|
49
|
Diehl C, Haux D, Sahm F, Unterberg AW and
Beynon C: Intracranial tumour haemorrhage following intravenous
thrombolysis. J Clin Neurosci. 26:145–146. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yamaguchi S, Suzuki SO, Matsuo Y, Uesaka
T, Matsukado K and Iwaki T: Exacerbation of radiation induced
meningioma due to hemorrhage after cerebral angiography: A case
report. No Shinkei Geka. 39:45–50. 2011.(In Japanese). PubMed/NCBI
|
|
51
|
Miyajima Y, Oka H, Utsuki S, Shimizu S,
Suzuki S and Fujii K: Intracranial hemorrhage associated with
convexity meningioma after cerebral angiography-case report. Neurol
Med Chir (Tokyo). 50:67–70. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Worm PV, Ferreira MP, Ferreira NP and
Cechetti F: Subdural haematoma in a patient with meningioma. Arq
Neuropsiquiatr. 67:308–310. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
de Almeida JP, Petteys RJ, Sciubba DM,
Gallia GL and Brem H: Regression of intracranial meningioma
following intratumoral hemorrhage. J Clin Neurosci. 16:1246–1249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Okuno S, Touho H, Ohnishi H and Karasawa
J: Falx meningioma presenting as acute subdural hematoma: Case
report. Surg Neurol. 52:180–184. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kohli CM and Crouch RL: Meningioma with
intracerebral hematoma. Neurosurgery. 15:237–240. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ravindra VM, Gozal YM, Palmer C and
Couldwell WT: Hemorrhagic atypical planum sphenoidale meningioma
with intermittent vision loss-rare presentation of apoplexy. World
Neurosurg. 121:71–76. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hu S, Zhang Y, Sun Y, Yu Y, Wang J, Dai H,
Sun F and Hu C: Lung metastases from intracranial bleeding
meningioma: A case report. Medicine (Baltimore). 97:e04572018.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Basil G, Urakov T, Knudsen MG and Morcos
J: Jugular tubercle meningioma with hemorrhagic conversion
mimicking a ruptured thrombosed giant vertebrobasilar aneurysm.
World Neurosurg. 119:108–112. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Entezami P, Riccio A and Kenning TJ:
Intratumoral hemorrhage within petrous meningioma. World Neurosurg.
117:246–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ravindran K, Gaillard F and Lasocki A:
Spontaneous subdural haemorrhage due to meningioma in the
post-partum setting. J Clin Neurosci. 39:77–79. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Byard RW: Parasagittal meningioma: A not
so benign entity. Med Sci Law. 57:175–178. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Broggi M, Restelli F and Acerbi F: Role of
tumor vessels' features in determining risk of bleeding in
Meningiomas: Which came first, the Chicken or the Egg. World
Neurosurg. 95:590–593. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Aoyama Y, Ohta S, Sakaki S and Fujita T:
Foramen magunum meningioma presented as subarachnoid haemorrhage.
No Shinkei Geka. 43:445–450. 2015.(In Japanese). PubMed/NCBI
|
|
64
|
Ito Y, Nakajima M, Watari M, Sakamoto T,
Hashimoto Y, Tajiri S, Takada A and Ando Y: Intratumoral hemorrhage
in a patient with malignant meningioma under anticoagulant therapy.
J Stroke Cerebrovasc Dis. 24:e91–e92. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Levine AB and MacDougall KW: Subdural
hematoma: A rare presentation of a convexity meningioma. Can J
Neurol Sci. 41:506–507. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Eljebbouri B, Mandour C and ElMostarchid
B: Acute headache originating from a bleeding convexity meningioma.
Headache. 54:1222–1223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chonan M, Niizuma K, Koyama S, Kon H,
Sannohe S, Kurotaki H, Midorikawa H, Sasaki T and Nishijima M: An
operated case of a meningioma causing acute subdural hematoma. No
Shinkei Geka. 41:235–239. 2013.(In Japanese). PubMed/NCBI
|
|
68
|
Sasagawa Y, Tachibana O, Iida T and Iizuka
H: Oncocytic meningioma presenting with intratumoral hemorrhage. J
Clin Neurosci. 20:1622–1624. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Rocha AJ, Saade N and Silva AZ: Meningioma
associated with non-traumatic subdural hematoma: An outstanding
appearance of this common intracranial tumor. Arq Neuropsiquiatr.
71:4172013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Krisht KM, Altay T and Couldwell WT:
Myxoid meningioma: A rare metaplastic meningioma variant in a
patient presenting with intratumoral hemorrhage. J Neurosurg.
116:861–865. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Eom KS and Kim TY: Isolated hemorrhagic
contusion of an incidental meningioma. Ulus Travma Acil Cerrahi
Derg. 18:277–279. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Czyż M, Jarmundowicz W, Szarek D, Tabakow
P and Markowska-Wojciechowska A: Bilateral chronic subdural
haematomas in a patient with meningioma of the superior sagittal
sinus-case report and pathophysiological study. Neurol Neurochir
Pol. 45:500–504. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Bellut D, Nern C, Burkhardt JK, Könü D,
Bertalanffy H and Krayenbühl N: Acute recurrent haemorrhage of an
intracranial meningioma. J Clin Neurosci. 18:992–993. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lakshmi Prasad G, Ramdurg SR, Suri A and
Mahapatra AK: A rare association of meningioma with intratumoral
bleed and acute subdural hematoma. Neurol India. 58:977–978. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Miyazawa T, Uozumi Y, Toyooka T and Shima
K: Hemorrhage from a falx meningioma after internal use of low-dose
aspirin. J Stroke Cerebrovasc Dis. 17:325–327. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Romeike BF, Joellenbeck B, Skalej M,
Scherlach C, Kirches E and Mawrin C: Intraventricular meningioma
with fatal haemorrhage: Clinical and autopsy findings. Clin Neurol
Neurosurg. 109:884–887. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Romero JR, Sakai O, Rice MB and Babikian
VL: Intracranial hemorrhage sparing meningioma in an anticoagulated
patient. J Neuroimaging. 17:246–250. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Mitsuhara T, Ikawa F, Ohbayashi N, Imada
Y, Abiko M and Inagawa T: A case of petrotentorial meningioma
presented as an acute subdural hemorrhage. No Shinkei Geka.
34:827–832. 2006.(In Japanese). PubMed/NCBI
|
|
79
|
Ziyal IM, Bilginer B, Celik O, Ozcan OE
and Ozgen T: Tentorial meningioma on follow-up presenting with
sudden deterioration due to intra- and peritumoral hemorrhage. Acta
Neurochir (Wien). 148:1315–1316. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Di Rocco F, Mannino S, Puca A, Lauriola L
and Pompucci A: Intracranial meningiomas associated with
non-traumatic chronic subdural hematoma. Acta Neurochir (Wien).
148:1097–1102. 2006. View Article : Google Scholar : PubMed/NCBI
|