Introduction
Colorectal cancer is one of the common malignant
tumors of the digestive system in the gastrointestinal tract
(1), and its morbidity rate ranks
second only to gastric cancer (2).
With the development of social economy and the impact of people's
poor living and eating habits, the morbidity of colorectal cancer
shows lower average onset age. Early detection, early diagnosis and
early radical treatment are the key to the treatment of colorectal
cancer, but because the clinical features of early colorectal
cancer are easily overlooked by patients, most patients have
advanced colorectal cancer when they are diagnosed (3). Patients with advanced colorectal cancer
have a poor prognosis and a high mortality rate (4,5). At
present, the treatment of colorectal cancer commonly used in
clinical practice is mainly based on surgical excision,
irradiation, and combination of chemotherapy and radiotherapy
(6,7). Recent in-depth investigations of the
mechanism of colorectal cancer, is expected to provide new ideas
and targets for the treatment and prognosis of colorectal cancer by
regulating tumor suppressor genes or cancer-promoting genes to
affect the occurrence and development of cancer (8,9).
MicroRNAs (miRNAs) are a class of endogenous
non-coding single-stranded RNAs that have tumor promotion or tumor
inhibition function (10,11). Current research indicates that
miR-135a-5p plays a role in tumor suppressor miRNAs in gallbladder
carcinoma tissues (12). In recent
years, there have been reports that the inhibition of miR-214
expression significantly inhibits the proliferation of colorectal
cancer, process of the cell cycle, and development of migration
(13). Some researchers have
confirmed that miR-141 is abnormally expressed in cancerous
symptoms in cervical cancer, ovarian cancer and bladder cancer, and
the expression levels of miR-141 in tumor tissues were much lower
than those in normal tissues (14).
However, the biological characteristics of colorectal cancer SW620
cells, the correlation between miR-135a-5p and miR-141 and specific
effects of expression changes of miR-135a-5p and miR-141 on the
biological characteristics of colorectal cancer SW620 cells were
not clear. Therefore, this study investigated the characteristics
of expression levels of miR-135a-5p and miR-141 in colorectal
cancer and effects of the biological characteristics of colorectal
cancer SW620, in order to provide a new theoretical basis for the
diagnosis and treatment of colorectal cancer in molecular
biology.
Patients and methods
Data collection
Fifty-four specimens of cancer tissues and 54
specimens of corresponding adjacent tissues of colorectal cancer
patients who were treated in The Central Hospital of Wuhan (Wuhan,
China) from March 2014 to March 2015 were selected. The inclusion
and exclusion criteria were as follows: Patients had normal liver
and kidney function and no other malignant tumors; the tissue
sections were diagnosed as colorectal cancer tissues or adjacent
tissues by the pathology department of The Central Hospital of
Wuhan, and all specimens were placed in liquid nitrogen immediately
after excision. Patients who had undergone chemotherapy,
immunotherapy, and radiotherapy before surgery were excluded.
Patients and their families were informed in advance and informed
consent forms were signed before the study. The study was approved
by the Ethics Committee of The Central Hospital of Wuhan.
Main reagents, instruments and
detection methods
Main reagents and instruments
Human colorectal cancer cell line SW620 cells were
from Shanghai Enzyme Research Biotechnology Co., Ltd.; TRIzol
reagents were from Invitrogen; Thermo Fisher Scientific, Inc.;
RT-qPCR kit and miScript reverse transcription kit were from Dalian
Takara; HBS-1096A enzyme-mark analyzer was from Nanjing Detie
Experimental Equipment Co., Ltd.; real-time quantitative PCR
instrument was from Bio-Rad Laboratories, Inc.; DMEM medium was
from Gibco; Thermo Fisher Scientific, Inc.; Fetal bovine serum
(FBS) and trypsin were from Hyclone, GE Healthcare; Cell Counting
Kit-8 (CCK-8) kit was from Beijing Zhijie Fangyuan Technology Co.,
Ltd.; Transwell chamber was from BD Biosciences; CyFlow Cube 8 flow
cytometry was from Partec, Germany; primer sequences of miR-135a-5p
and miR-141 and internal reference U6, miRNA negative control and
other carriers were designed and synthesized by Shanghai Jima
Company (Table I).
 | Table I.Primer sequences of miR-135a-5p,
miR-141 and internal reference U6. |
Table I.
Primer sequences of miR-135a-5p,
miR-141 and internal reference U6.
| Name | Forward primers | Reverse primers |
|---|
| miR-141 |
5′-GAGCGGACCTTCAGATGAGA-3′ |
5′-GTGCAGGGTCCGAGGT-3′ |
| miR-135a-5p |
5′-ACACTCCAGCTGGGGTGCATTGTAGTTGCA-3′ |
5′-TGGTGTCGTGGAGTCG-3′ |
| U6 |
5′-CTCGCTTCGGCAGCACA-3′ |
5′-AACGCTTCACGAATTTGCGT-3′ |
| Blank group |
5′-UUCCGGUCCUUGCGUGGUTT3-3′ |
5′-ACGCAUGAGGUUCGTCCUGT-3′ |
| miRNA negative
control |
5′-UUCAAUCCCGCGACGUTT-3′ |
5′-ACGACGUGCGACUUGAGAATT-3′ |
Detection of miR-135a-5p and miR-141
RT-PCR was used to detect the expression levels of
miR-135a-5p and miR-141 in colorectal cancer tissues and adjacent
tissues. Total RNA in tissues was extracted and dissolved in 20 µl
of DEPC water according to the instructions of TRIzol reagent. The
total RNA was then reverse transcribed by a reverse transcription
kit, and the reaction system was as follows: M-MIV 1 µl, Olig(dT) 1
µl, RNA enzyme inhibitor 0.5 µl, d NTPs 1 µl, RNAse free water
supplemented to 15 µl. The incubation was for 60 min at 38°C. cDNA
(1 µl) was taken at 85°C for 5 sec; the synthesized cDNA was used
as a template for RT-qPCR amplification; preparation of PCR
reaction system was as follows: 10 × 2.5 µl PCR buffer, dNTPs 1 µl,
upstream primers 1 µl, downstream primers 1 µl, Taq DNA Polymerase
0.25 µl, ddH2O was made up to 25 µl. Reaction conditions
were as follows: pre-denaturation at 95°C for 15 min, denaturation
at 95°C for 15 sec, annealing at 60°C for 30 sec, with a total of
35 cycles; finnally, extended at 72°C for 15 min; three replicate
wells were set for each sample for 3 repeated experiments, and
miR-135a-5p and miR-141 were used with U6 as an internal reference.
After the end of the reaction, the amplification curve and the
melting curve of the Real-time PCR were confirmed, and the relative
amount of the target gene was calculated based on the resulting
parameters. The quantification of the target gene was calculated by
2−∆Ct.
Cell culture and transfection
The human colorectal cancer SW620 cells were placed
in a medium containing 10% FBS DMEM, placed in a CO2
incubator at 37°C, and when the fusion growth of cells reached 50%,
25% trypsin was added for digestion; after the digestion was
completed, it was placed in the medium to continue the culture and
complete the passage. Cells in logarithmic phase were transfected,
grouped before transfection, and cells that were not transfected
were set as blank group, negative RNA control (NC group),
miR-135a-5p inhibitor group and miR-141 mimic group; Lipofectamine
2000 and DNA were diluted and mixed according to the Lipofectamine
2000 manufacturer's kit instructions; NC, miR-135a-5p inhibitor and
miR-141 mimic were transfected into SW620 cells by Lipofectamine
2000, and incubated for 5 min at room temperature; finally, the
mixture was mixed with cells and transfected under the condition of
37°C and CO2; RT-qPCR was used to test the expression
levels of miR-135a-5p in SW620 cells transfected with miR-135a-5p
or miR-141 in miR-135a-5p inhibitor group, miR-141 mimic group, NC
group and blank group 48 h after transfection.
Cell proliferation detected by CCK8
SW620 cell lines in each group were inoculated in a
96-well plate at 100 µl per well 48 h after transfection; cells
were diluted into 4×103 cells/ml after pancreas
digestion, and then the culture plate was placed in a cell culture
incubator for 24 h; the old medium was discarded; finally these
samples were added to groups of miR-135a-5p inhibitor, miR-141
mimic, NC and blank. The culture plate was taken out and the old
culture medium was discarded 48 and 72 h after culture; 100 µl of
CCK8 solution was added to each well; the absorbance was measured
at 450 nm with a microplate reader to detect cell proliferation
after 4 h of incubation in a 37°C incubator, and the experiment was
repeated three times.
Invasive ability of cells in vitro detected by
Transwell chamber
Cells were digested by Trypsin, then centrifuged at
8,000 × g for 5 min at 4°C and the culture medium was discarded;
cells were washed twice with FBS, then the serum-free medium
containing BSA was resuspended, and the cell density was adjusted
to 5×104/ml. Medium containing FBS (1 ml) was added to
the lower chamber of a 6-well plate; 2 ml of cell suspension was
added to Transwell chamber; after 24 h of routine incubation, cells
in the Transwell chamber were wiped off with a cotton swab; after
the Transwell chamber was dried, the film was sealed and observed
by microscopic techniques.
Cell apoptosis in each group detected by flow
cytometry
After trypsin digestion, cells in miR-135a-5p
inhibitor group, miR-141 mimic group and 48 h after NC treatment
were collected; it was fixed for 24 h under ethanol with the
concentration of 75% and room temperature of 20°C, with a constant
temperature of 4°C, ethanol was discarded after centrifugation at
2,500 × g for 5 min; FBS was used again to rinse it, it was
centrifuged with a constant temperature of 4°C and at the speed of
2,500 × g for 5 min, then the supernatant was discarded; 500 µl DNA
staining solution was added to all samples and thoroughly mixed;
finally, the prepared solution was transferred to a flow tube and
incubated in the dark for 30 min and detected by CyFlow Cube 8 flow
cytometry.
Statistical analysis
SPSS 17.0 (Beijing Strong-Vinda Information
Technology Co., Ltd.) software system was applied in statistical
analysis; the counting data were expressed as [n (%)], and the
comparison between the two groups was performed by χ2
test; the measurement data were expressed by (mean ± SD), and the
comparison between groups was performed by t-test or F test;
P<0.05 was considered to indicate a statistically significant
difference.
Results
General data of patients (Table II)
Expression levels of miR-135a-5p and miR-141 in
colorectal cancer tissues and adjacent tissues
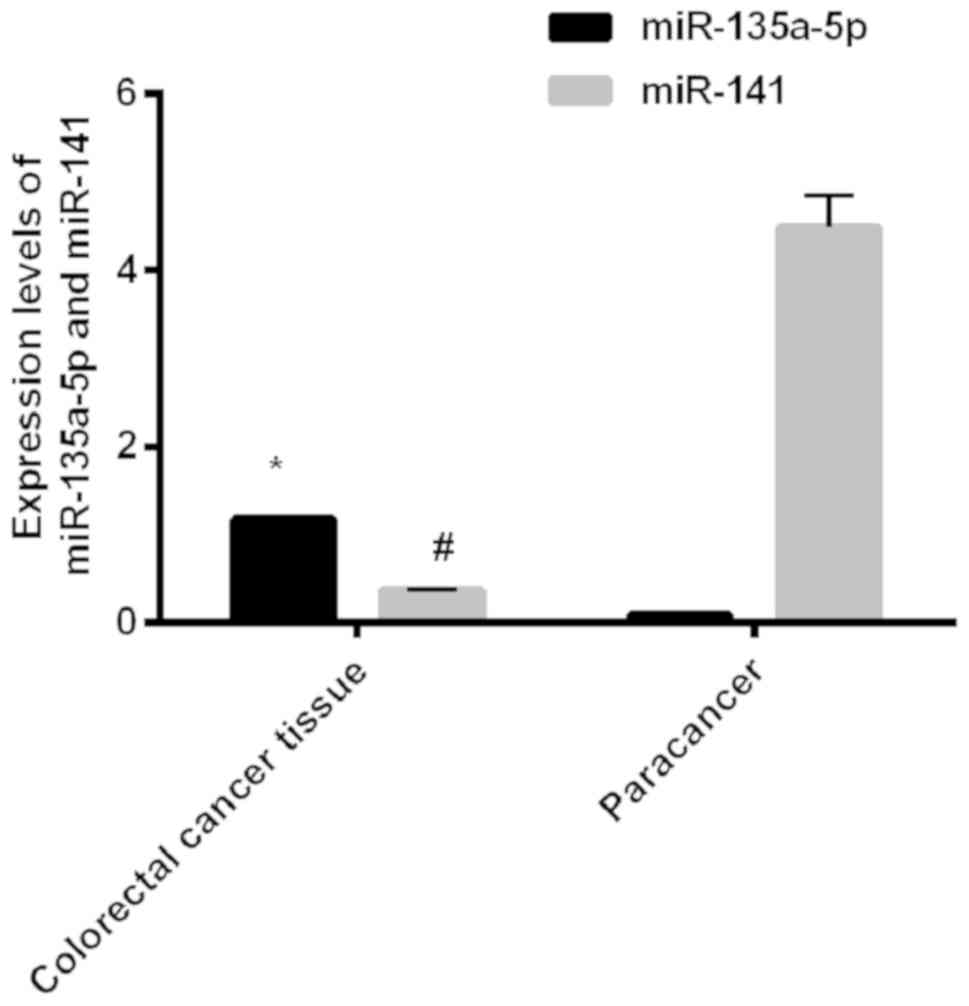
The expression levels of miR-135a-5p in colorectal
cancer tissues and adjacent tissues were, respectively, 1.18±0.02
and 0.10±0.02; the expression levels of miR-141 in colorectal
cancer tissues and adjacent tissues were, respectively, 0.37±0.01
and 4.48±0.36. The expression levels of miR-135a-5p in colorectal
cancer tissues were significantly higher than those in adjacent
tissues; the expression levels of miR-141 in colorectal cancer
tissues were significantly lower than those in adjacent tissues,
and differences between the two groups were statistically
significant (P<0.001) (Fig.
1).
Relative expression levels of miR-135a-5p and
miR-141 in each group after transfection
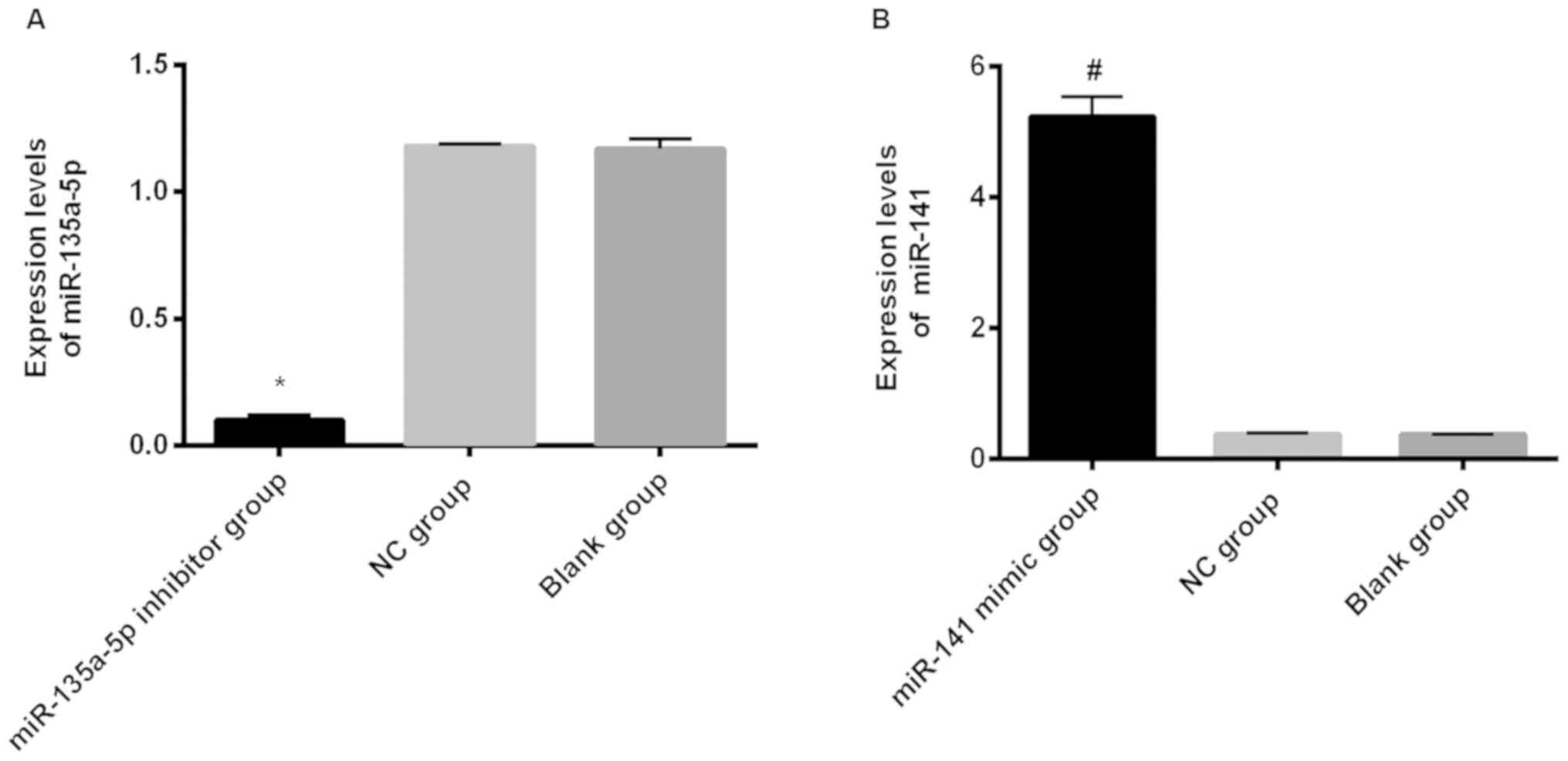
The expression levels of miR-135a-5p in the
miR-135a-5p inhibitor group, NC group and blank group were,
respectively, 0.10±0.01, 1.18±0.01 and 1.17±0.04; the expression
levels of miR-135a-5p in the miR-135a-5p inhibitor group were
significantly lower than those in the NC group and blank group, and
the difference was statistically significant (P<0.001). The
expression levels of miR-141 in the miR-141 mimic group, NC group
and blank group were, respectively, 5.23±0.31, 0.37±0.02 and
0.37±0.01; the expression levels of miR-141 in the miR-141 mimic
group were significantly higher than those in the NC group and the
blank group, and the difference was statistically significant
(P<0.001). There was no significant difference in the expression
levels of miR-141 and miR-135a-5p between the NC group and the
blank group (P>0.05) (Fig.
2).
Comparison of survival rate of colorectal cancer
SW620 cells after transfection
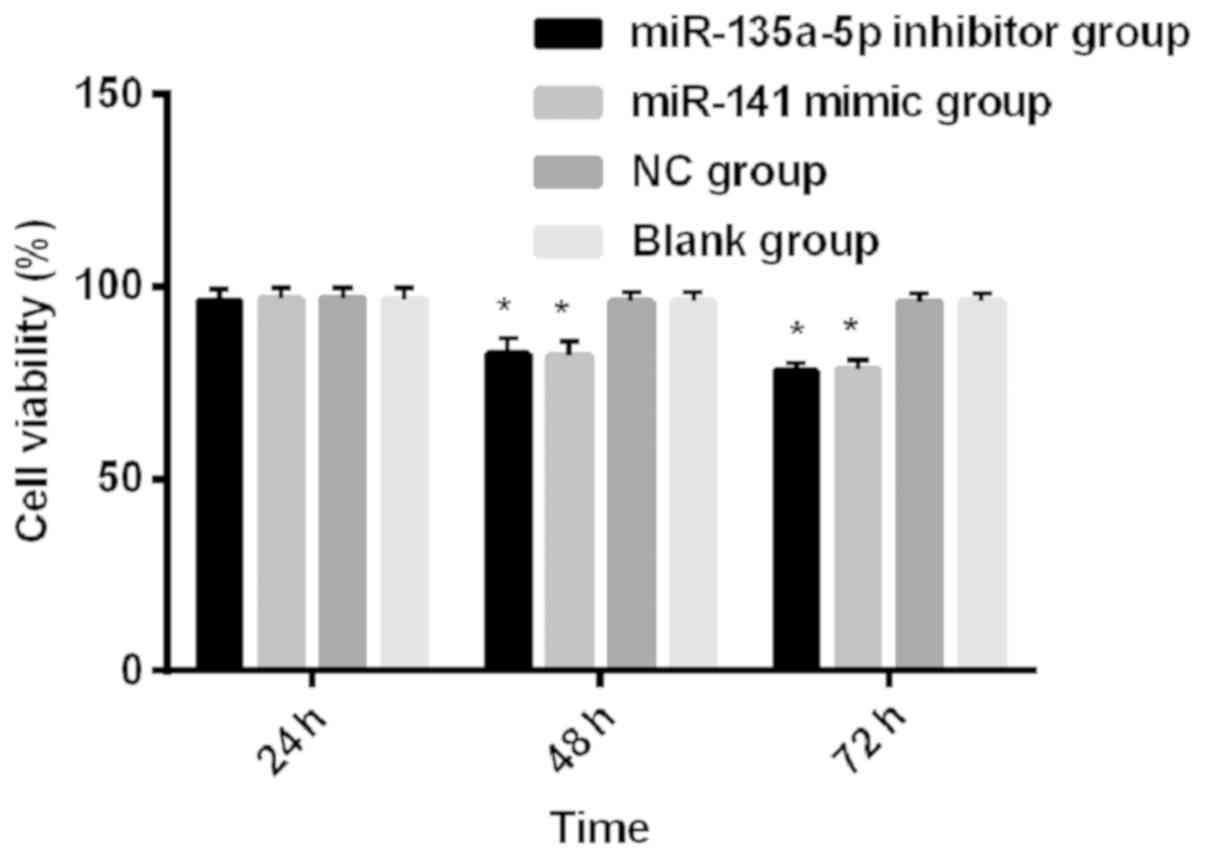
The results of intra-group comparison showed that
the cell survival rates of the miR-135a-5p inhibitor group and the
miR-141 mimic group showed a gradual downward trend from 24 to 72
h, and the differences were statistically significant at different
time points in the three groups (P<0.001); the cell survival
rates of the NC group and blank group at 72 and 48 h were
significantly lower than those of 24 h, and the differences were
statistically significant (P<0.05). The results of group
comparisons showed that the cell survival rates of the miR-135a-5p
inhibitor group, miR-141 mimic group, NC group and blank control
group were compared for 24 h, and differences were not
statistically significant (P>0.05). The cell survival rates of
the miR-135a-5p inhibitor group and miR-141 mimic group were
significantly lower than those of NC group and blank group at 48
and 72 h (P<0.001), and there was no significant difference
between the NC group and blank group at 48 and 72 h (P>0.05)
(Fig. 3).
Comparison of invasion of colorectal cancer SW620
cells in each group
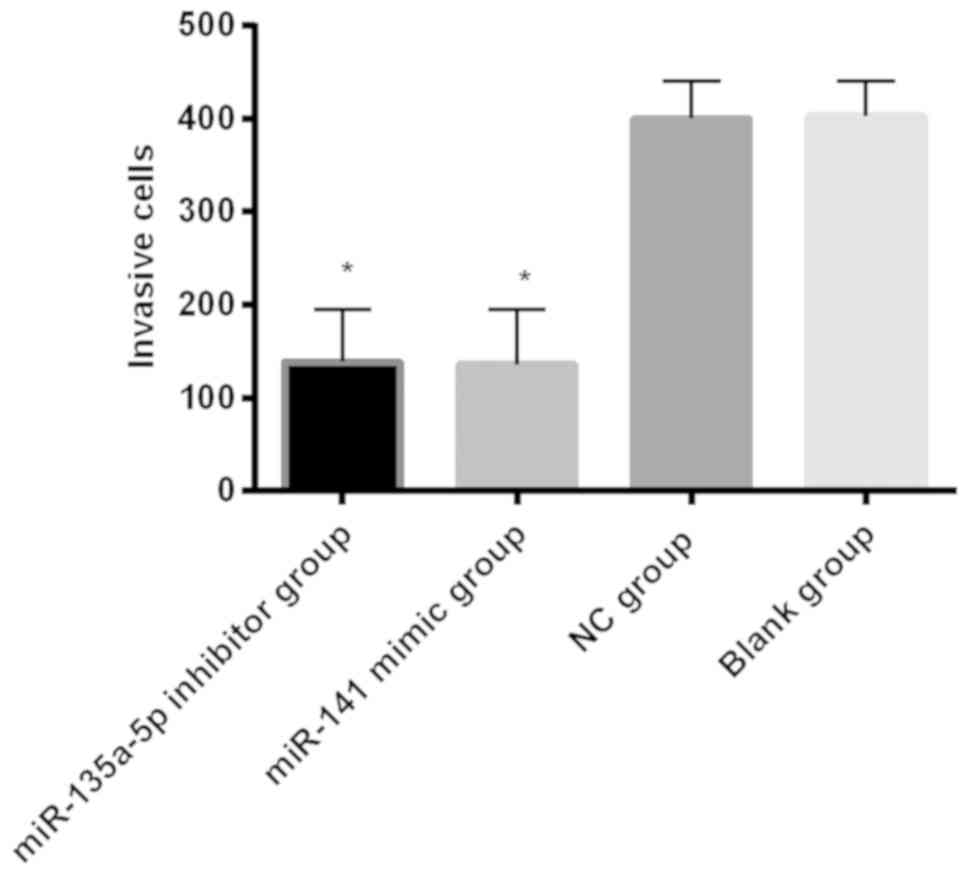
The number of invasion cells in the miR-135a-5p
inhibitor group, miR-141 mimic group, NC group and blank control
group was, respectively, 138.80±56.04, 136.24±58.44, 400.23±40.33
and 403.10±38.00; the number of invasion cells in miR-135a-5p
inhibitor group and miR-141 mimic group was significantly lower
than that in the blank group and NC group, and the differences were
statistically significant (P<0.001) (Fig. 4).
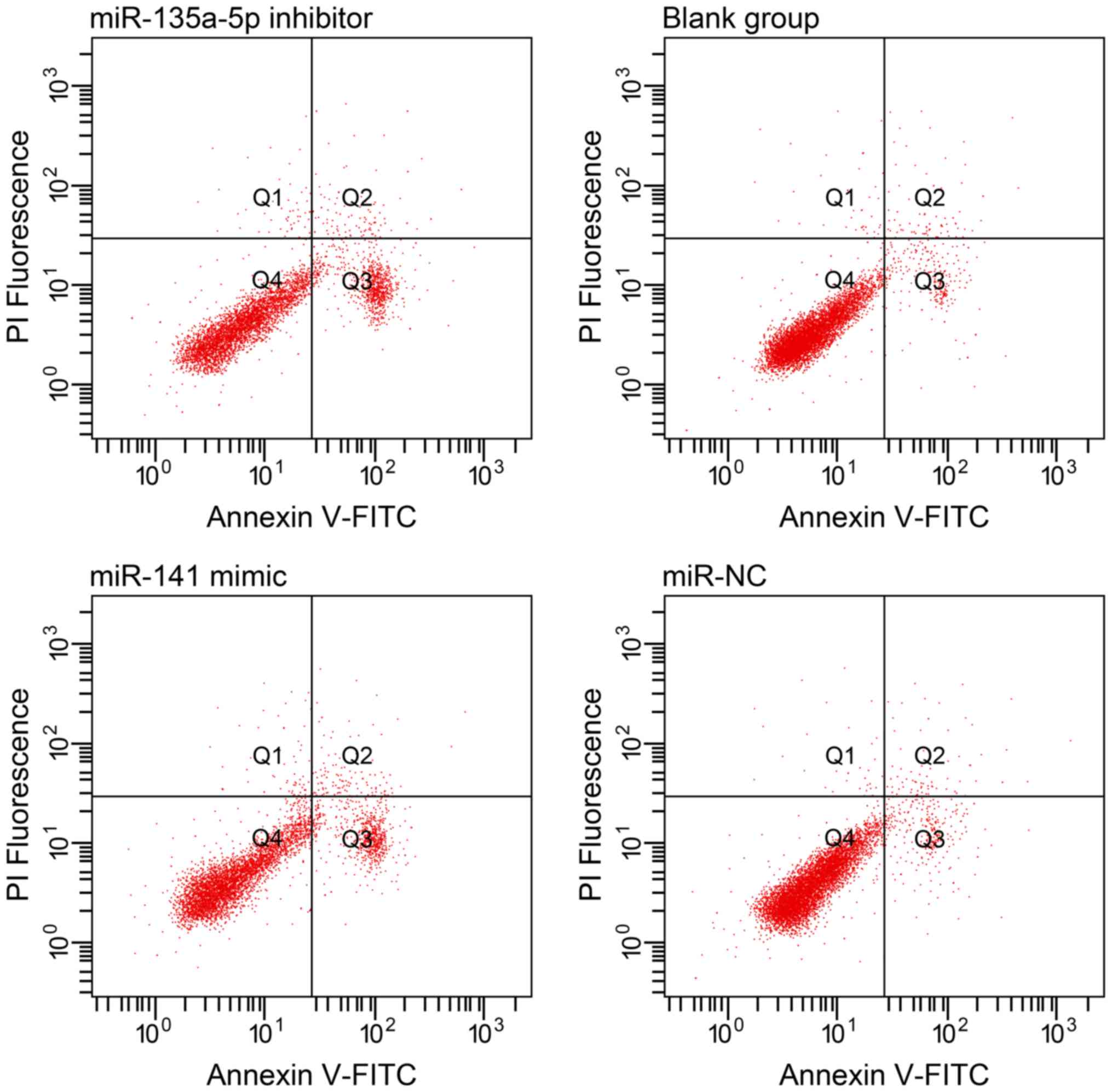
Comparison of apoptosis ability of colorectal
cancer SW620 cells in each group after transfection
The apoptosis rates of miR-135a-5p inhibitor group
and miR-141 mimic group were, respectively, 23.06±2.64% and
22.41±4.39%, but were significantly higher than those of the NC
group 4.10±0.20% and blank group 3.99±0.43% (P<0.001). There was
no significant difference in apoptosis rates between the NC group
and blank group (P>0.05) (Table
III and Fig. 5).
 | Table III.Comparison of apoptotic rate (%) of
each group. |
Table III.
Comparison of apoptotic rate (%) of
each group.
| Group | miR-135a-5p
inhibitor | miR-141 mimic
group | NC group | Blank group | F value | P-value |
|---|
| Apoptotic rate
(%) | 20.14±3.32 | 19.73±4.01 | 4.10±0.20 | 3.99±0.43 | 665.500 | <0.001 |
Discussion
The biological characteristics of colorectal cancer
cells affect the developmental effects of colorectal cancer
(15). Among them, proliferation and
apoptosis of tumor cells together determine the growth and decline
of tumor and control the growth rate of tumors (16). Numerous studies have shown that cell
proliferation is an important life feature of biological organisms
(17). Normal cell proliferation
will stop naturally to a certain extent, and cancer cells have
uncontrolled characteristics; the non-stop proliferation, invasion
and metastasis of cancer cells are the main causes of death in
patients with malignant tumors (18). miRNAs play an important role in
carcinogenesis and tumor progression, and expression of miRNAs
affects the invasiveness of tumor cells (19). Changes in the expression of miRNAs
have been shown to be associated with the development and
progression of malignant tumors (20). This study analyzed the expression
features of miR-135a-5p and miR-141 in colorectal cancer and the
effects of miR-135a-5p and miR-141 on the biological function of
colorectal cancer SW620 cells, in order to provide a new
theoretical basis for diagnosis and treatment of colorectal cancer
in molecular biology.
In this study, expression levels of miR-135a-5p and
miR-141 were first observed in colorectal cancer tissues and
adjacent normal tissues. The results showed that the expression
levels of miR-135a-5p in colorectal cancer tissues were
significantly higher than those in adjacent tissues. The expression
levels of miR-141 in colorectal cancer tissues were significantly
lower than those in adjacent tissues, with statistically
significant difference. Similar studies also confirmed that the
expression levels of miR-141 mRNA in renal cell carcinoma tissues
were significantly lower than those in adjacent tissues and normal
tissues. The results of Wang et al (21) showed that the expression levels of
miR-135a-5p in colorectal cancer tissues were significantly higher
than those in adjacent tissues, and differences were statistically
significant. In order to investigate the specific effects of
expression changes of miR-135a-5p and miR-141 in colorectal cancer,
we performed RT-qPCR to detect the expression levels of miR-135a-5p
and miR-141 in transfected human colorectal cancer SW620 cells in
groups of miR-135a-5p inhibitor, miR-141 mimic, and NC, and the
proliferation, invasion and apoptosis of SW620 cells were recorded
and regulated after miR-135a-5p and miR-141 were expressed. The
expression levels of miR-135a-5p in the miR-135a-5p inhibitor group
were significantly lower than those in the NC group and blank
group. The expression levels of miR-141 in the miR-141 mimic group
were significantly higher than those in the NC group and blank
group, with statistically significant difference. Moreover, the
cell survival rates of the miR-135a-5p inhibitor group and the
miR-141 mimic group were significantly lower than those of the NC
group and the blank group at 48 and 72 h.
The biological function of malignant tumor cells is
affected by the epithelial-mesenchymal transition process (22); Musavi Shenas et al (23) found that the miR-200 family was
closely related to the epithelial-mesenchymal transition process.
As a member of the miR-200 family, miR-141 promoted cell
transformation from benign to malignant and abnormal proliferation
of cells by inhibiting the epithelial-mesenchymal transition
process (24). Thus, we believe that
it is possible to indirectly inhibit the epithelial-mesenchymal
transition process of tumor cells and inhibit the action of tumors
by overexpressing miR-141. Invasion and apoptosis of colorectal
cancer SW620 cells were observed. The number of invasive cells in
the miR-135a-5p inhibitor group and miR-141 mimic group was
significantly lower than that in the NC group and blank group. The
apoptosis rate of the miR-135a-5p inhibitor group and miR-141 mimic
group was significantly higher than that of the NC group and blank
group. The death in patients with malignant tumors is often caused
by invasion and metastasis of cancer cells (25); when the invasion of cancer cells is
controlled and the apoptosis rate of cancer cells is accelerated,
the continued deterioration of the cancer has been relieved to some
extent (26). Similarly, studies on
miRNAs and cancer cells confirmed that the overexpression of
miR-141 effectively inhibited the number of invading cancer cells,
and the amplitude of apoptosis of cancer cells became significantly
larger (27). Therefore, we believe
that the number of invasive cells and apoptosis rate of human
colorectal cancer SW620 cells could be affected by appropriately
regulating miRNAs.
In summary, miR-135a-5p is highly expressed in
colorectal cancer tissues, and miR-141 expression is low in
colorectal cancer tissues; silent expression of miR-135a-5p and
overexpression of miR-141 inhibited the proliferation and invasion
of human colorectal cancer SW620 cells, and promoted apoptosis of
human colorectal cancer SW620 cells. It is concluded that
miR-135a-5p and miR-141 are involved in the biological process of
human colorectal cancer SW620 cells, and could be used as
diagnostic markers and therapeutics targets in colorectal
cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW wrote the manuscript. JW and JY performed PCR and
CCK8 test. HZ and YL were responsible for Transwell chamber test
and flow cytometry. DX and SM contributed to observation indexes
analysis. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Central Hospital of Wuhan (Wuhan, China). Patients who
participated in this research had complete clinical data. Signed
informed consents were obtained from the patients and/or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guinney J, Dienstmann R, Wang X, de
Reyniès A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda
G, Angelino P, et al: The consensus molecular subtypes of
colorectal cancer. Nat Med. 21:1350–1356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pietrantonio F, Petrelli F, Coinu A, Di
Bartolomeo M, Borgonovo K, Maggi C, Cabiddu M, Iacovelli R, Bossi
I, Lonati V, et al: Predictive role of BRAF mutations in patients
with advanced colorectal cancer receiving cetuximab and
panitumumab: A meta-analysis. Eur J Cancer. 51:587–594. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tauriello DVF and Batlle E: Targeting the
microenvironment in advanced colorectal cancer. Trends Cancer.
2:495–504. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moriarity A, O'Sullivan J, Kennedy J,
Mehigan B and McCormick P: Current targeted therapies in the
treatment of advanced colorectal cancer: A review. Ther Adv Med
Oncol. 8:276–293. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schmeel FC, Simon B, Sabet A, Luetkens JA,
Träber F, Schmeel LC, Ezziddin S, Schild HH and Hadizadeh DR:
Diffusion-weighted magnetic resonance imaging predicts survival in
patients with liver-predominant metastatic colorectal cancer
shortly after selective internal radiation therapy. Eur Radiol.
27:966–975. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van Hazel GA, Heinemann V, Sharma NK,
Findlay MP, Ricke J, Peeters M, Perez D, Robinson BA, Strickland
AH, Ferguson T, et al: SIRFLOX: Randomized phase III trial
comparing first-line mFOLFOX6 (plus or minus bevacizumab) versus
mFOLFOX6 (plus or minus bevacizumab) plus selective internal
radiation therapy in patients with metastatic colorectal cancer. J
Clin Oncol. 34:1723–1731. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mohammadi A, Mansoori B and Baradaran B:
The role of microRNAs in colorectal cancer. Biomed Pharmacother.
84:705–713. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsukamoto M, Iinuma H, Yagi T, Matsuda K
and Hashiguchi Y: Circulating exosomal microRNA-21 as a biomarker
in each tumor stage of colorectal cancer. Oncology. 92:360–370.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang W, Wan S, Yang Z, Teschendorff AE and
Zou Q: Tumor origin detection with tissue-specific miRNA and DNA
methylation markers. Bioinformatics. 34:398–406. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mansoori B, Mohammadi A, Ghasabi M,
Shirjang S, Dehghan R, Montazeri V, Holmskov U, Kazemi T, Duijf P,
Gjerstorff M, et al: miR-142-3p as tumor suppressor miRNA in the
regulation of tumorigenicity, invasion and migration of human
breast cancer by targeting Bach-1 expression. J Cell Physiol.
234:9816–9825. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Z, Yu X, Shen J, Law PT, Chan MT and Wu
WK: MicroRNA expression and its implications for diagnosis and
therapy of gallbladder cancer. Oncotarget. 6:13914–13921.
2015.PubMed/NCBI
|
|
13
|
Hu JL, He GY, Lan XL, Zeng ZC, Guan J,
Ding Y, Qian XL, Liao WT, Ding YQ and Liang L: Inhibition of
ATG12-mediated autophagy by miR-214 enhances radiosensitivity in
colorectal cancer. Oncogenesis. 7:162018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Penna E, Orso F and Taverna D: miR-214 as
a key hub that controls cancer networks: Small player, multiple
functions. J Invest Dermatol. 135:960–969. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ling H, Pickard K, Ivan C, Isella C, Ikuo
M, Mitter R, Spizzo R, Bullock M, Braicu C, Pileczki V, et al: The
clinical and biological significance of miR-224 expression in
colorectal cancer metastasis. Gut. 65:977–989. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fernandez S, Risolino M, Mandia N, Talotta
F, Soini Y, Incoronato M, Condorelli G, Banfi S and Verde P:
miR-340 inhibits tumor cell proliferation and induces apoptosis by
targeting multiple negative regulators of p27 in non-small cell
lung cancer. Oncogene. 34:3240–3250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fang F, Chang RM, Yu L, Lei X, Xiao S,
Yang H and Yang LY: MicroRNA-188-5p suppresses tumor cell
proliferation and metastasis by directly targeting FGF5 in
hepatocellular carcinoma. J Hepatol. 63:874–885. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lima CR, Geraldo MV, Fuziwara CS, Kimura
ET and Santos MF: MiRNA-146b-5p upregulates migration and invasion
of different papillary thyroid carcinoma cells. BMC Cancer.
16:1082016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liao J, Liu R, Shi YJ, Yin LH and Pu YP:
Exosome-shuttling microRNA-21 promotes cell migration and
invasion-targeting PDCD4 in esophageal cancer. Int J Oncol.
48:2567–2579. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo X, Han T, Hu P, Guo X, Zhu C, Wang Y
and Chang S: Five microRNAs in serum as potential biomarkers for
prostate cancer risk assessment and therapeutic intervention. Int
Urol Nephrol. 50:2193–2200. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Q, Zhang H, Shen X and Ju S: Serum
microRNA-135a-5p as an auxiliary diagnostic biomarker for
colorectal cancer. Ann Clin Biochem. 54:76–85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu X, Han S, Wu S, Bai Y, Zhang N and Wei
L: Dual role of twist1 in cancer-associated fibroblasts and tumor
cells promoted epithelial-mesenchymal transition of esophageal
cancer. Exp Cell Res. 375:41–50. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Musavi Shenas MH, Eghbal-Fard S,
Mehrisofiani V, Abd Yazdani N, Rahbar Farzam O, Marofi F and
Yousefi M: MicroRNAs and signaling networks involved in
epithelial-mesenchymal transition. J Cell Physiol. 234:5775–5785.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dong H, Weng C, Bai R, Sheng J, Gao X, Li
L and Xu Z: The regulatory network of miR-141 in the inhibition of
angiogenesis. Angiogenesis. 22:251–262. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bedke J, Heide J, Ribback S, Rausch S, de
Martino M, Scharpf M, Haitel A, Zimmermann U, Pechoel M, Alkhayyat
H, et al: Microvascular and lymphovascular tumour invasion are
associated with poor prognosis and metastatic spread in renal cell
carcinoma: A validation study in clinical practice. BJU Int.
121:84–92. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Casasent AK, Schalck A, Gao R, Sei E, Long
A, Pangburn W, Casasent T, Meric-Bernstam F, Edgerton ME and Navin
NE: Multiclonal invasion in breast tumors identified by topographic
single cell sequencing. Cell. 172:205–217.e12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng Y, Li S, Boohaker RJ, Liu X, Zhu Y,
Zhai L, Li H, Gu F, Fan Y, Lang R, et al: A microRNA expression
signature in taxane-anthracycline-based neoadjuvant chemotherapy
response. J Cancer. 6:671–677. 2015. View Article : Google Scholar : PubMed/NCBI
|