Introduction
Liver cancer is a common malignant tumor, which was
reported to account for ~8.2% of cancer-associated mortality
globally in 2018 (1,2). Escaping immunological surveillance has
been extensively investigated in liver cancer in the past decade
(3); however, the precise mechanisms
remain unknown. Therefore, determining the molecular mechanisms
contributing to immune escape in liver cancer cells, and
identifying specific therapeutic targets, require further
examination; which will improve the efficacy of immunotherapy and
the development of novel immunotherapeutics.
Endoplasmic reticulum (ER) homeostasis is a basic
condition for cell survival; however, in a hostile tumor
microenvironment (TME), tumor cells frequently experience glucose
and oxygen deprivation, oxidative stress and loss of
Ca2+ homeostasis, which collectively contribute to the
production and accumulation of incompletely or incorrectly folded
proteins in the lumen of the ER (4).
Moreover, the accumulation of misfolded proteins results in ER
dysfunction, and thus, the cell enters a cellular state termed ‘ER
stress’ in order to maintain cell survival (5,6). Mild
and chronic upregulation of ER-stress activity enables malignant
cells to exhibit several aggressive characteristics, such as
invasion, metastasis and apoptotic-resistance (7). Furthermore, previous studies have shown
that ER stress may disrupt anti-tumor immunity by modulating the
role of tumor-associated macrophages (TAMs) residing in the TME
(8,9). In addition, the intercellular
interaction between ER-stressed tumor cells and resident immune
cells in the TME has gained attention in several different types of
cancer, for example liver cancer (10,11).
However, the mediators linking these two types of cells are not yet
fully understood.
Exosomes are a class of extracellular vesicles,
possessing a double-layer membrane structure, that are secreted by
almost all cell types and play a vital role in cell-to-cell
communication by acting as carriers of cell soluble proteins,
lipids and RNA (12,13). Exosomes released by tumor cells are
enriched in immunosuppressive molecules, as well as
biologically-active soluble factors, which may interact with
immunological effector cells in the TME, leading to the dysfunction
of anti-tumor immunity by delivering immunosuppressive signal
(14,15). By suppressing the functions of
immunological effector cells, tumor-derived exosomes promote tumor
progression and facilitate tumor cell escape from immunological
surveillance (16,17). Moreover, Chen et al (16) reported that exosomes released from
hypoxic epithelial ovarian cancer cells deliver a range of
microRNAs (miRNAs) to macrophages, and can remodel macrophages into
an oncogenic phenotype to promote tumor cell proliferation and
migration. However, whether exosome releasing from ER stressed
liver cancer cells is capable of immunosuppression remains to be
determined.
Therefore, the present study investigated the role
of exosomes, released from ER-stressed liver cancer cells, on
macrophage function. Furthermore, it was demonstrated that ER
stress-associated exosomes increased cytokine production via the
STAT3 pathway in macrophages.
Materials and methods
Cell culture
The human liver cancer cell line HepG2
(authenticated using short tandem repeat profiling) and the murine
macrophage cell line RAW264.7 were purchased from The Cell Bank of
Type Culture Collection of the Chinese Academy of Sciences. Cells
were cultured in DMEM (Thermo Fisher Scientific, Inc.) containing
10% heat-inactivated FBS (Thermo Fisher Scientific, Inc.) at 37°C
in a humidified 5% CO2 atmosphere.
Exosomes isolation
HepG2 cells were cultured in DMEM media containing
10% exosome-free FBS (System Biosciences, LLC) up to a confluence
of 80%. Exosomes from the supernatants of normal cultured HepG2
cells (Exo-con) and HepG2 cells treated with 2.5 µM tunicamycin
(Sigma-Aldrich; Merck KGaA) (Exo-TM) at 37°C for 24 h were purified
using ExoQuick Precipitation Solution (System Biosciences, LLC) at
the volume ratio of 1:5, according to the manufacturer's protocol.
Supernatants of the Exo-con group and the Exo-TM group were
collected and centrifuged at 3,000 × g for 15 min at room
temperature to remove cell debris. ExoQuick precipitation solution
was added to the centrifuged supernatant at a ratio of 1:5 (v/v),
agitated and incubated at 4°C for 12 h. After incubation, the
mixture was centrifuged at 3,000 × g for 30 min at 4°C, and the
supernatants were removed and discarded. The pellet was centrifuged
under the same conditions to remove excess fluid. Then, the plates
(collected exosome mass) were washed twice with sterile PBS.
Protein quantification of exosome preparations was measured using a
bicinchoninic acid assay kit (Beyotime Institute of
Biotechnology).
Transmission electron microscopy
(TEM)
The morphology and size of Exo-con and Exo-TM was
measured using TEM (JEM-1230; Jeol Ltd.). Exosomes were stored at
−80°C and thawed on ice when required. Then, 10 µl suspension
liquid (sterile PBS) was added onto the formvar carbon-coated
copper grids and the excess liquid was absorbed using a filter
paper. Subsequently, 30 µl 2% phosphotungstic acid was added to the
copper net to negatively stain exosomes at room temperature for 5
min, and the excess liquid was removed using filter paper. The
grids were washed with PBS three times and dried under an
incandescent lamp. Representative exosome images were captured
using TEM.
Construction of tissue microarray
(TMA)
TMA was constructed as previously reported (18). Formalin-fixed (using 4%
paraformaldehyde at room temperature for 24 h) and
paraffin-embedded liver cancer tumor tissues and paired healthy
liver tissues were obtained from the Department of Pathology of The
First Affiliated Hospital of Anhui Medical University between March
2004 and July 2010 as previously. A total of 89 patients were
included in this study (21 women and 68 men; age range, 28–76
years; mean ± standard deviation, 51.0±12.3 years). To identify the
target area for construction of TMAs, the 4-µm-thick specimen
sections were analyzed using 0.5% hematoxylin and eosin-staining at
room temperature for 1 min. Then, five representative 1-mm cores
(three tumor tissues and two paired healthy tissues) were obtained
from each patient and marked tissues were embedded into a new blank
paraffin block according to the design grid using a manual tissue
arrayer (Nantong Hengtai Graphite Equipment Systems Co., Ltd.,
http://www.smlnq.com/en/index.asp).
Immunohistochemical analysis
The primary hepatocellular carcinoma (HCC) tumor
tissues used were the same as used in a previous study (18). The protocol of the current study
conforms to the Ethical Guidelines of the 1975 Declaration of
Helsinki and was approved by the Ethics Committee of The First
Affiliated Hospital of Anhui Medical University (approval no.
20040158). Written consent was provided by all the enrolled
patients. Sections (thickness, 4 µm) were deparaffinized and 3%
hydrogen peroxide in methanol was used to block endogenous
peroxidase activity at room temperature for 10 min. The slides were
placed into heated (96-98°C) sodium citrate buffer (0.01 M; pH=6.0)
for 15 min for antigen retrieval and allowed to cool at room
temperature. Incubation with 5% BSA blocking solution (Beyotime
Institute of Biotechnology) was performed for 20 min to block
non-specific binding at room temperature. The sections were
subsequently incubated with primary antibodies, including
glucose-regulated protein 78 (GRP78; cat. no. ab108615; Abcam),
CD68 (cat. no. ab955; Abcam), IL-6 (cat. no. ab9324; Abcam) and
IL-10 (cat. no. ab34843; Abcam) in a moist chamber at 4°C
overnight; all primary antibodies were diluted at 1:200. After
incubation, PBS was used to wash the sections, which were then
incubated with a biotinylated secondary antibody (1:5,000; cat. no.
BA1004; Wuhan Boster Biological Technology, Ltd.) and
peroxidase-conjugated streptavidin (cat. no. BA1088; Wuhan Boster
Biological Technology, Ltd.) at room temperature for 15–20 min. TMA
sections were stained with 1% diaminobenzidine solution for 1 min
and counterstained using 0.5% hematoxylin for 30 sec at room
temperature, respectively. The binding of target antigen was
observed under an optical light Olympus microscope (magnification,
×100 and ×400).
The expression of GRP78 was scored by multiplying
the staining intensity (0 for negative staining; 1 for light
yellow; 2 for orange-brown and 3 for brown) with the percentage of
stained cells (0 for negative; 1 for ≤10%; 2 for 11–50%; 3 for
51–75%; and 4 for >75%). Moreover, the product <5 and ≥5
indicated low and high expression levels of GRP78 and the product
between 3–5 was grouped as medium expression (18). CD68, interleukin (IL)-6 and IL-10
distribution was scored by counting the mean percentage of positive
cells in five random fields of each sample.
Western blotting
Total cellular and exosomal proteins were lysed
using RIPA buffer (Beyotime Institute of Biotechnology) with 1 nM
PMSF, quantified with a bicinchoninic acid assay, and then ~20 µg
of total proteins were loaded per lane and resolved on 10% gels
using SDS-PAGE. Proteins were transferred onto PVDF membranes (EMD
Millipore) and blocked with 5% non-fat milk at room temperature for
2 h. The membranes were washed three times with Tris Buffered
saline Tween solution and then incubated with the following primary
antibodies: Mouse anti-β-actin (cat. no. 3700; Cell Signaling
Technology, Inc.), rabbit anti-CD63 (cat. no. ab217345; Abcam),
rabbit anti-CD81 (cat. no. ab109201; Abcam), rabbit anti-tumor
susceptibility gene 101 (TSG101; cat. no. ab125011; Abcam), rabbit
anti-Calnexin (cat. no. 2679; Cell Signaling Technology, Inc.),
rabbit anti-Janus kinase 2 (JAK2; cat. no. ab108596; Abcam), rabbit
anti-phosphorylated (p)-JAK2 (cat. no. ab32101; Abcam), rabbit
anti-STAT3 (cat. no. ab119352; Abcam), rabbit anti-p-STAT3 (cat.
no. ab76315; Abcam) and rabbit anti-GRP78 (cat. no. BS1154; Biogot
Technology Co., Ltd.) at 4°C overnight; all primary antibodies were
diluted at 1:1,000. Horseradish peroxidase-labeled anti-mouse (cat.
no. BS12478) or anti-rabbit (cat. no. BS13278) immunoglobulin G
(both Biogot Technology Co., Ltd.) were used as the secondary
antibodies at a dilution of 1:10,000 for 2 h at 37°C. The bands
were visualized using SuperSignal West Dura (Thermo Fisher
Scientific, Inc.) and the intensity of the bands were
semi-quantitative analyzed using Scion Image software (version
4.0.3.2; http://softwaretopic.informer.com/search-scion-image).
Immunofluorescence
To detect macrophages that had incorporated
exosomes, RAW264.7 cells were co-cultured with PKH67-
(Sigma-Aldrich; Merck-KGaA) labeled exosomes for 12 h at 37°C, and
subsequently fixed with 4% formaldehyde for 30 min at 37°C and
permeabilized with 0.1% Triton-X. Cells were imaged using a
confocal microscope (Leica Microsystems GmbH; magnification, ×100)
after counter-staining cells with DAPI (1 mg/ml) at room
temperature for 5 min.
To detect the expression levels of IL-6 and IL-10 in
CD68-positive cells, the sections were prepared as described for
immunohistochemistry and incubated at 4°C overnight with mouse
anti-CD68 (1:200; cat. no. ab955; Abcam), rabbit anti-IL-10 (1:200;
cat. no. ab34843; Abcam;) or rabbit anti-IL-6 (1:200; cat. no.
ab9324; Abcam) and goat anti-mouse IgG (H+L) unconjugated or goat
anti-rabbit IgG (H+L) unconjugated secondary antibodies (1:5,000;
cat. nos. BS13271 and BS12471; Biogot Technology Co., Ltd.) for 2 h
at 37°C. Cells were counter-stained with DAPI (100 ng/ml) for 5 min
at room temperature. Co-localization of CD68 with IL-6 or IL-10 was
determined using an Olympus fluorescence microscope (magnification,
×100).
Cytokine bead array (CBA) analyses of
inflammatory factors
RAW264.7 cells were co-cultured with Exo-con and
Exo-TM for 24 h at 37°C, then the culture supernatants were
collected to detect the expression levels of IL-10, IL-6, monocyte
chemo-attractant protein-1 (MCP-1) and tumor necrosis factor-α
(TNF-α). The aforementioned inflammatory factors were measured
using a mouse CBA kit (cat. no. 552364; BD Biosciences), according
to the manufacturer's protocol.
Statistical analysis
SPSS version 16.0 software (IBM Corp.) was used for
statistical analysis. Data are presented as the mean ± SD of ≥3
independent experiments. Kaplan-Meier analysis was performed to
determine the association between GRP78 expression and overall
survival (OS) time of patients with liver cancer and log-rank test
was used to determine statistical significance, while Spearman's
rank correlation analysis was performed to assess the association
between IL-10 and IL-6. The association between GRP78 expression
levels and clinicopathological characteristics was determined using
the χ2 test or Mann-Whitney test. A paired t-test was
used for comparison between two groups, and a one-way ANOVA
followed by Tukey's test post hoc test was used for comparing ≥3
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Activation of ER stress is associated
with poor prognosis in patients with liver cancer
To determine whether ER stress was increased in
liver cancer tissues, the expression of GRP78, an ER stress
biomarker, was measured in 89 paraffin-embedded liver cancer
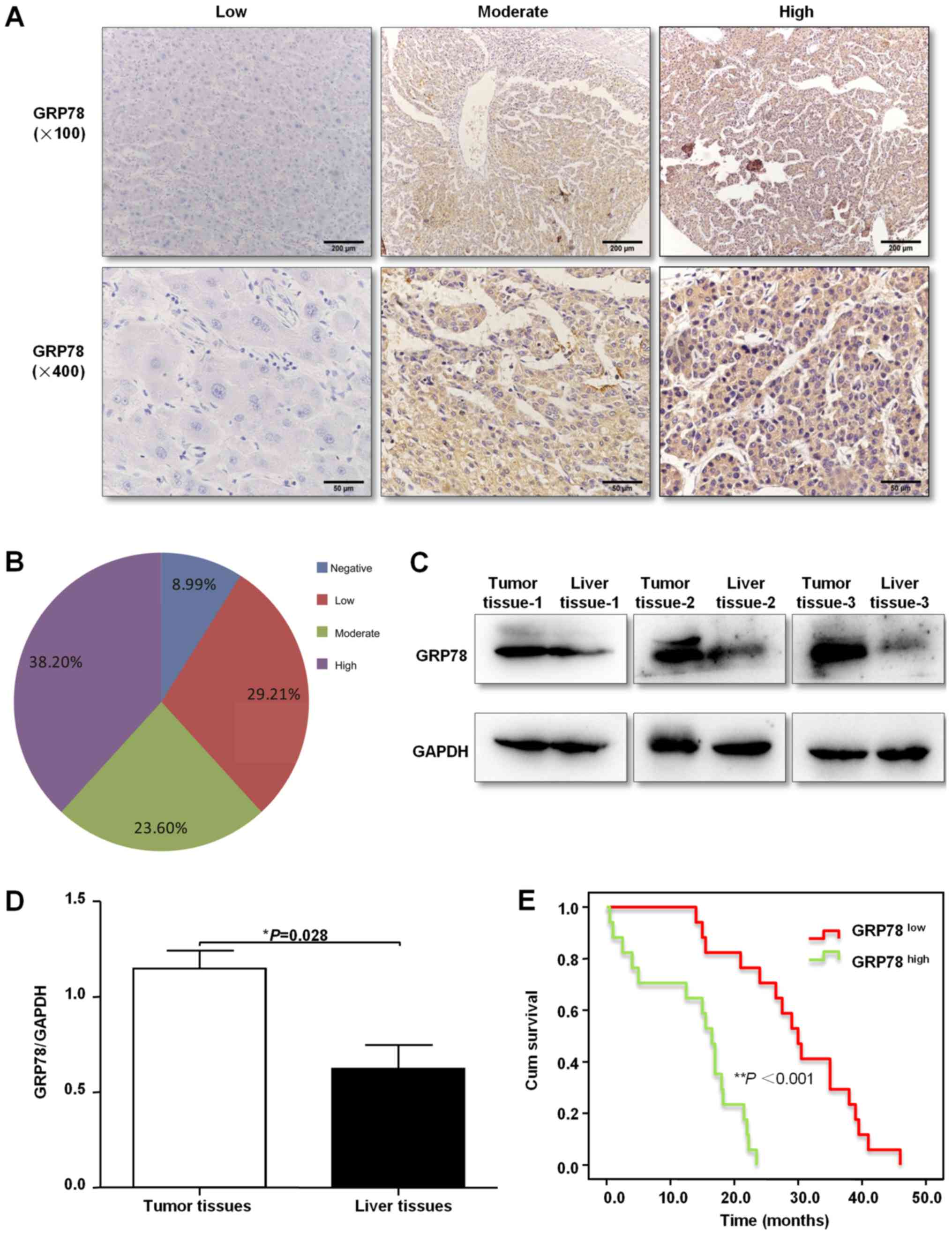
specimens using immunohistochemical staining. It was found that
GRP78 protein expression was primarily located in the cytoplasm in
a diffuse pattern (Fig. 1A). Of the
89 liver cancer specimens, 61.80% (55/89) of tissues stained
positive for GRP78 (Fig. 1B).
Moreover, GRP78 protein expression was also measured by western
blotting in three freshly resected liver cancer tissues, and it was
demonstrated that GRP78 expression was higher in tumor tissues
compared with the paired healthy liver tissues (Fig. 1C and D). Furthermore, the association
between clinicopathological characteristics and GRP78 expression in
patients with liver cancer is shown in Table I. The results indicated that GRP78
expression was associated with hepatitis, cirrhosis, larger tumor
size and poor differentiation (Table
I). In addition, Kaplan-Meier analysis was used to analyze the
association of GRP78 expression with overall survival (OS) time of
35 patients with liver cancer. It was identified that patients with
lower expression levels of GPR78 had a significantly longer OS time
compared with patients with higher expression levels of GPR78
(Fig. 1E).
 | Table I.Association between
clinicopathological features and GRP78 expression in patients with
hepatocellular carcinoma. |
Table I.
Association between
clinicopathological features and GRP78 expression in patients with
hepatocellular carcinoma.
|
| GRP78
expression |
|---|
|
|
|
|---|
| Clinicopathological
features | Case (n) | Low (n) | High (n) |
χ2/Z | P-value |
|---|
| Sex |
|
|
| 6.541 | 0.019a |
|
Female | 21 | 13 | 8 |
|
|
|
Male | 68 | 21 | 47 | 0.234 | 0.665 |
| Age, years |
|
<60 | 50 | 18 | 32 |
|
|
|
≥60 | 39 | 16 | 23 |
|
|
| Hepatitis |
|
|
| 6.75 | 0.013a |
| No | 23 | 14 | 9 |
|
|
|
Yes | 66 | 20 | 46 |
|
|
| Cirrhosis |
|
|
| 6.773 | 0.016a |
| No | 42 | 22 | 20 |
|
|
|
Yes | 47 | 12 | 35 |
|
|
| AFP value |
|
|
| −0.317 | 0.751 |
|
<20 | 39 | 16 | 23 |
|
|
| ≥20,
<400 | 21 | 7 | 14 |
|
|
|
≥400 | 29 | 11 | 18 |
|
|
| Clinical
stages |
|
|
| 1.136 | 0.333 |
|
I/II | 65 | 27 | 38 |
|
|
|
III/IV | 24 | 7 | 17 |
|
|
| Tumor size |
|
|
| −2.024 | 0.043a |
|
<5 | 29 | 15 | 14 |
|
|
| ≥5,
<10 | 46 | 16 | 30 |
|
|
|
≥10 | 14 | 3 | 11 |
|
|
| Differentiated
degree |
|
|
| −2.664 | 0.008a |
|
High | 18 | 17 | 15 |
|
|
|
Middle | 50 | 16 | 29 |
|
|
|
Low | 18 | 1 | 11 |
|
|
Activation of ER stress is associated
with macrophage recruitment and cytokines secretion
TAMs serve a vital role in liver cancer progression
(19). Therefore, to investigate
whether upregulation of ER stress activity is associated with
macrophage recruitment, the expression of the macrophage surface
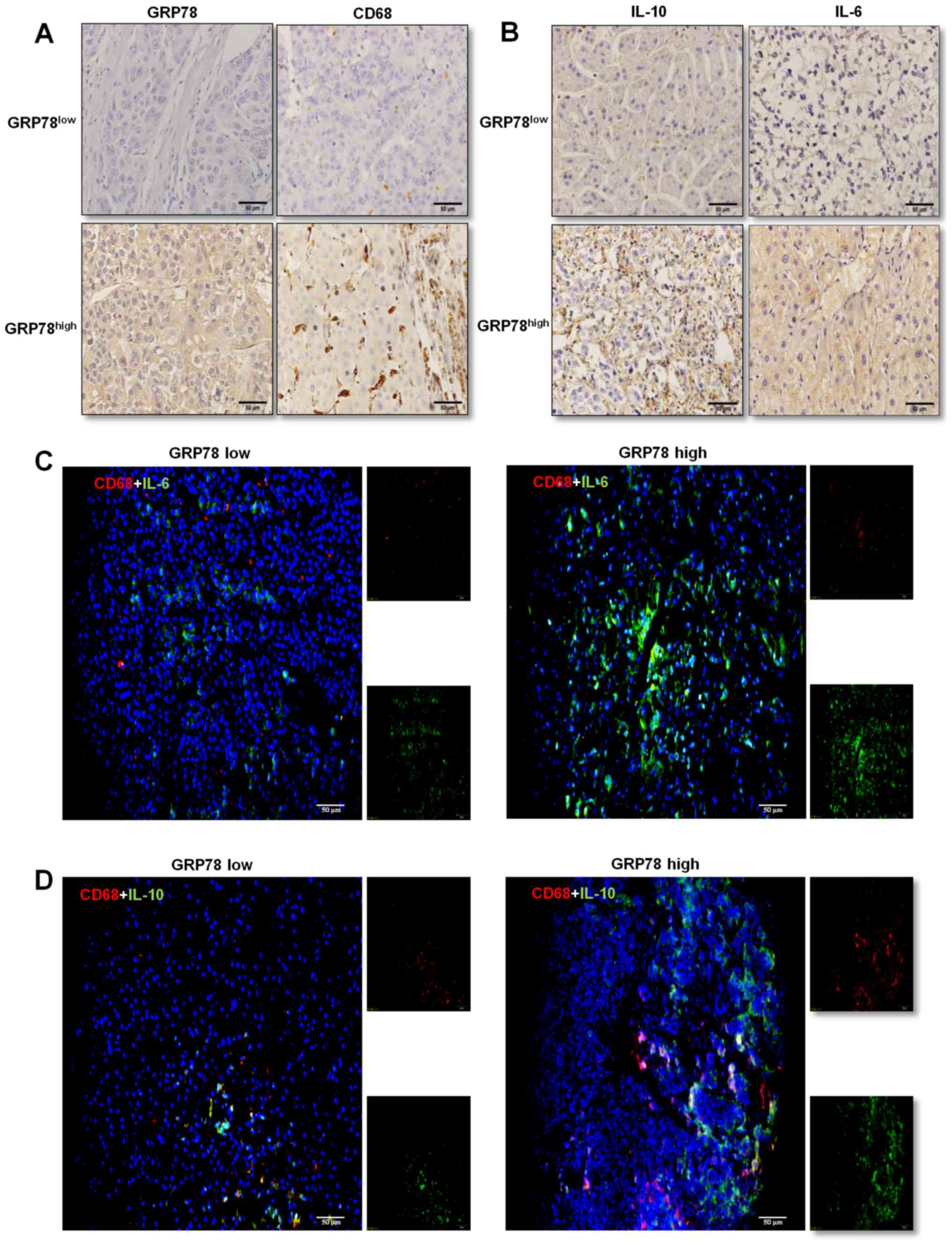
marker CD68 was determined in liver cancer tissues. The results
suggested that CD68-positive cells were distributed sporadically in
tissues with low GRP78 expression, whereas the number of
CD68-positive cells was higher and cells were present in clusters
in tissues with GRP78-high expression (Fig. 2A).
Furthermore, the association between the expression
levels of GRP78 and IL-10 and IL-6 in infiltrating macrophages was
assessed using immunohistochemical staining. Both low and high
GRP78 expressing liver cancer tissues stained positively for IL-10
and IL-6, and higher GRP78 expressing tissues secreted increased
quantities of IL-10 and IL-6 (Fig.
2B). As macrophages produce various cytokines including IL-10
and IL-6 (18,19), the expression levels of these
cytokines were determined in CD68+ macrophages in liver
cancer tissues using double immunofluorescence staining of
CD68/IL-6 (Fig. 2C) and CD68/IL-10
(Fig. 2D). The results demonstrated
that tissues overexpressed GRP78 protein frequently infiltrating
large number of macrophages, and these macrophages expressed higher
levels of IL-10 and lower levels of IL-6 than those macrophages
resided in HCC tissues that expressed lower levels of GRP78
protein. Thus, these results suggest that the activation of ER
stress may be correlated with macrophages infiltration and
cytokines secretion in liver cancer, and macrophages were
identified as an important group of immunosuppressive cells in
liver cancer.
Exosomes released from ER-stressed
liver cancer cells increase cytokine expression
To investigate whether ER stress-associated exosomes
affected cytokine expression in macrophages, HepG2 cells were
co-cultured using different concentrations of TM for 24 h, and the
GRP78 protein expression was detected using rabbit anti-GRP78. It
was found that the expression of GRP78 was increased in a
concentration-dependent manner and peaked when cells were incubated
with 2.5 µM TM (Fig. 3A and B).
HepG2 cells were also treated with 2.5 µM TM for 12, 24 and 48 h,
and GRP78 expression was increased in a time-dependent manner,
although it appears that 48 h has a slightly higher expression
level of GRP78 proteins than 24 h, there was no significant
difference between 24 and 48 h (Fig. 3C
and D). Therefore, treating HepG2 cells with 2.5 µM TM for 24 h
was considered the optical conditions to induce ER stress in
subsequent experiments.
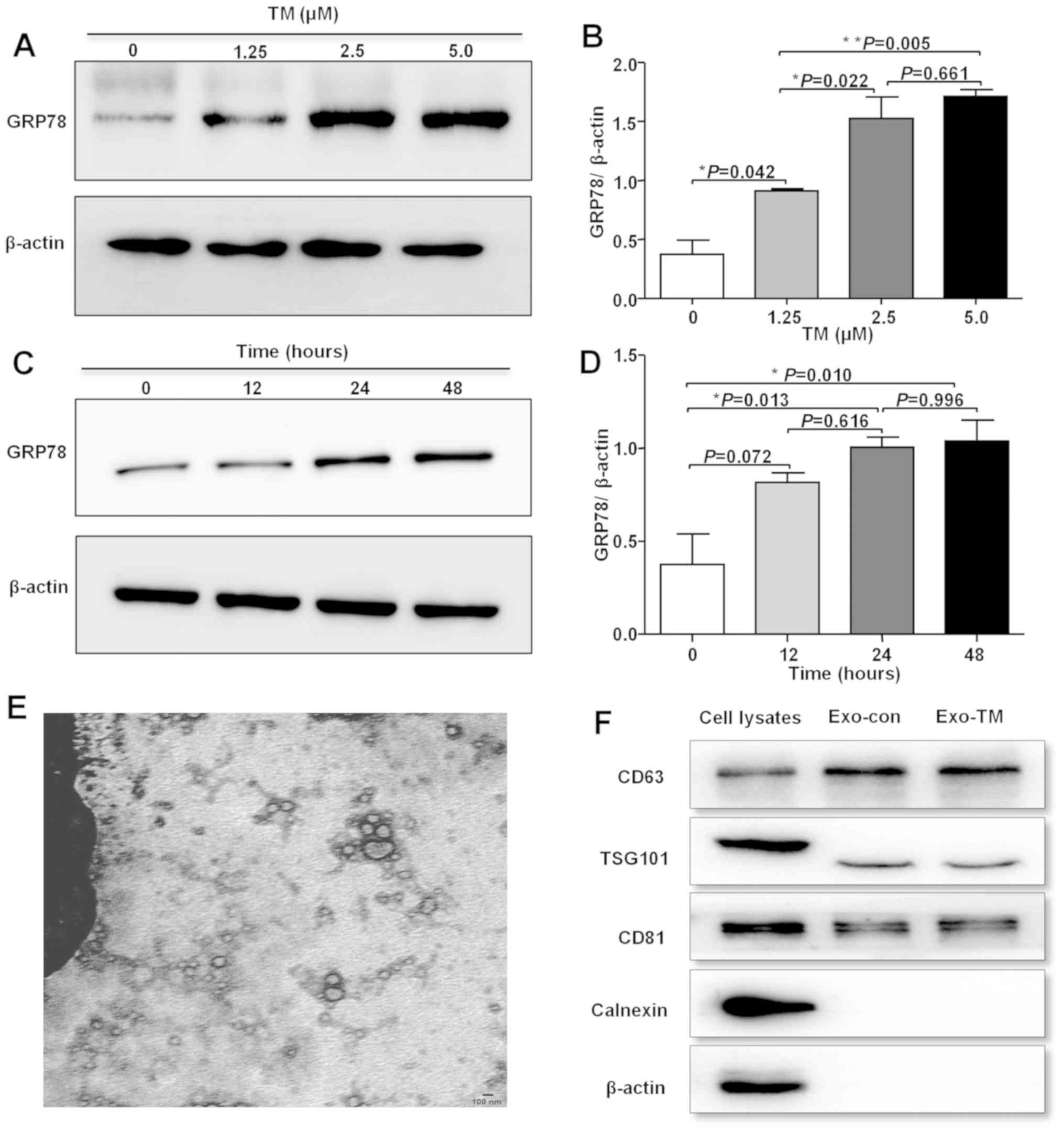 | Figure 3.Characteristics of ER
stress-associated exosomes. (A) HepG2 cells were treated with 0,
1.25, 2.5 and 5 µM TM for 24 h, and (B) GRP78 protein expression
was measured and semi-quantitatively analyzed, relative to β-actin
intensity. (C) HepG2 cells were treated with 2.5 µM TM for 12, 24
and 48 h, and (D) GRP78 protein expression was measured and
semi-quantitatively analyzed, relative to β-actin intensity. (E)
Representative transmission electron microscope images of Exo-TM.
Scale bar, 100 nm. (F) Expression levels of CD63, TSG101, CD81,
β-actin and Calnexin were measured using cell lysates or exosomes
by western blotting. *P< 0.05, **P< 0.01. ER, endoplasmic
reticulum; TM, tuniamycin; GRP78, glucose-regulated protein 78;
Exo-TM, exosomes from the supernatants of HepG2 cells treated with
2.5 µM tunicamycin; TSG101, tumor susceptibility gene 101; Exo-con,
exosomes from the supernatants of control HepG2 cells. |
To physically characterize exosomes secreted from
HepG2 cells, exosomes were isolated from the conditioned media of
TM-treated cells. The purified pellets were imaged using TEM, and
double-membrane structure vesicles that were within the expected
diameter range of exosomes (30–100 nm) were observed (Fig. 3E). Furthermore, the presence of CD63
and TSG101, two specific exosomal markers, was observed, and the
negative control, calnexin, was not identified in exosomes
(Fig. 3F). Thus, the results suggest
that the purified isolated pellets were exosomes.
Tumor cells can modulate the function of neighboring
or distant cells via exosome-mediated delivery of molecules to
recipient cells (20). To assess
whether ER-stressed liver cancer cells modulated the role of
macrophages via exosomes, RAW264.7 cells were cultured with
PKH67-labeled exosomes, and the labeled exosomes were observed to
be taken up by RAW264.7 macrophages (Fig. 4A). To determine the effect of ER
stress-associated exosomes on RAW264.7 cell immune function, cells
were incubated with Exo-con and Exo-TM, and the levels of IL-6,
MCP-1, IL-10 and TNF-α were detected using a CBA inflammatory
cytokine kit. The results indicated that IL-6 (Fig. 4B; P<0.05), IL-10 (Fig. 4C; P<0.05) and MCP-1 (Fig. 4D; P<0.01) levels were
significantly increased in Exo-TM treated cells, while the levels
of TNF-α (Fig. 4E; P>0.05) were
slightly decreased in Exo-TM treated RAW264.7 cells compared with
Exo-con treated cells. Collectively, the results suggest that
exosomes released from ER-stressed HepG2 cells influence the
expression profile of cytokines secreted by macrophages.
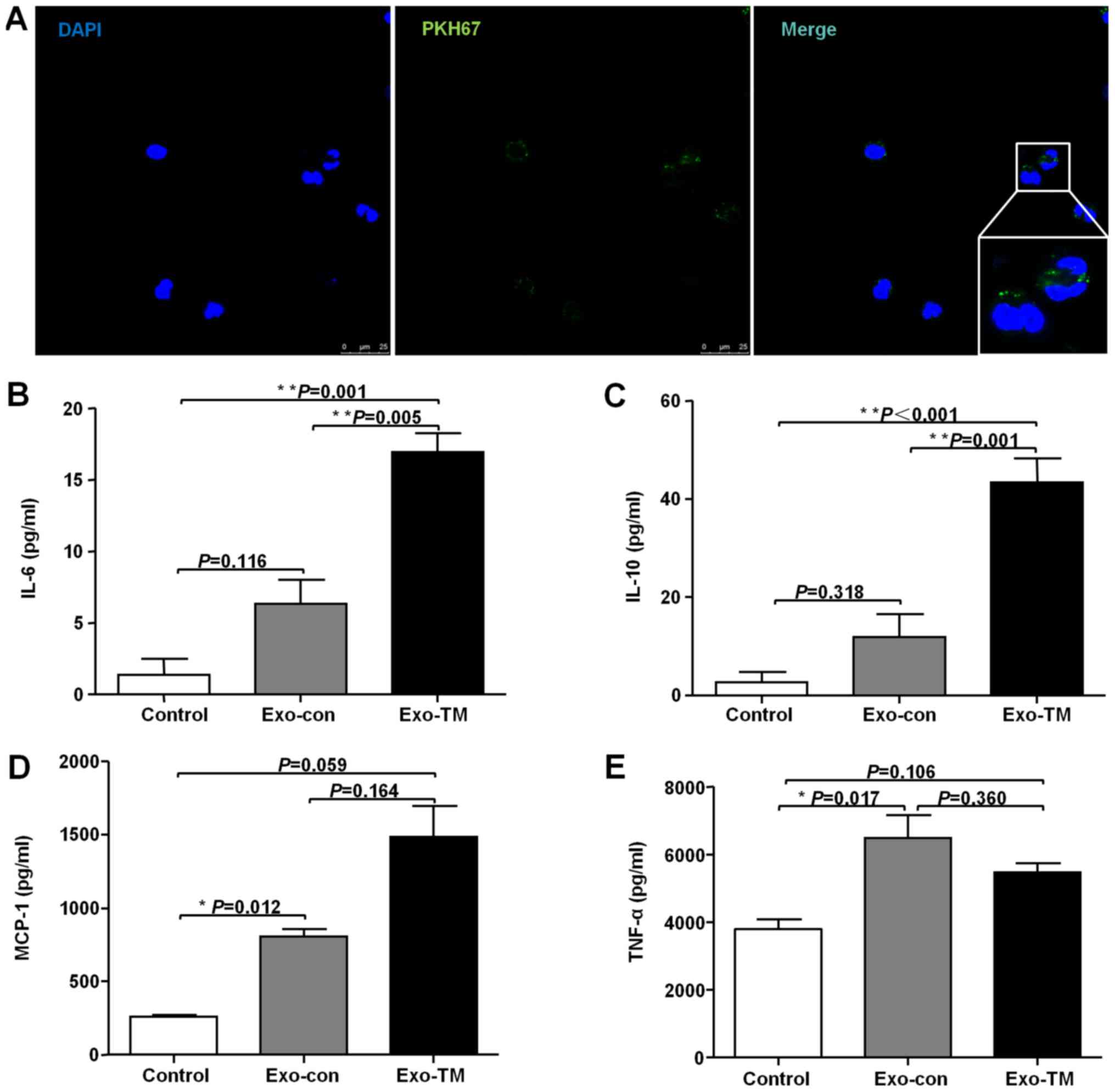 | Figure 4.Incubation with Exo-TM increases
expression of cytokines in macrophages in vitro. (A)
Confocal microscopy was used to measure the incorporation of
PKH67-labeled exosomes into RAW264.7 cells. Cells were incubated
with Exo-con and Exo-TM for 24 h, and (B) IL-6, (C) IL-10, (D)
MCP-1 and (E) TNF-α levels were measured using a CBA inflammatory
factor kit. *P< 0.05, **P< 0.01 with indicated groups.
Exo-Con, exosomes from the supernatants of control HepG2 cells;
Exo-TM, exosomes from the supernatants of HepG2 cells treated with
2.5 µM tunicamycin; IL, interleukin; MCP-1, monocyte chemotactic
protein-1; TNF-α, tumor necrosis factor-α; CBA, cytokine bead
array. |
ER stress-associated exosomes enhance
cytokines expression by activating the STAT3 pathway
The underlying mechanism via which ER
stress-associated exosomes affects cytokine expression in
macrophages was subsequently examined. The JAK2/STAT3 signaling
pathway plays a vital role in the macrophage inflammatory response
(21); therefore, whether the
Exo-TM-mediated inflammatory response is JAK2/STAT3-dependent was
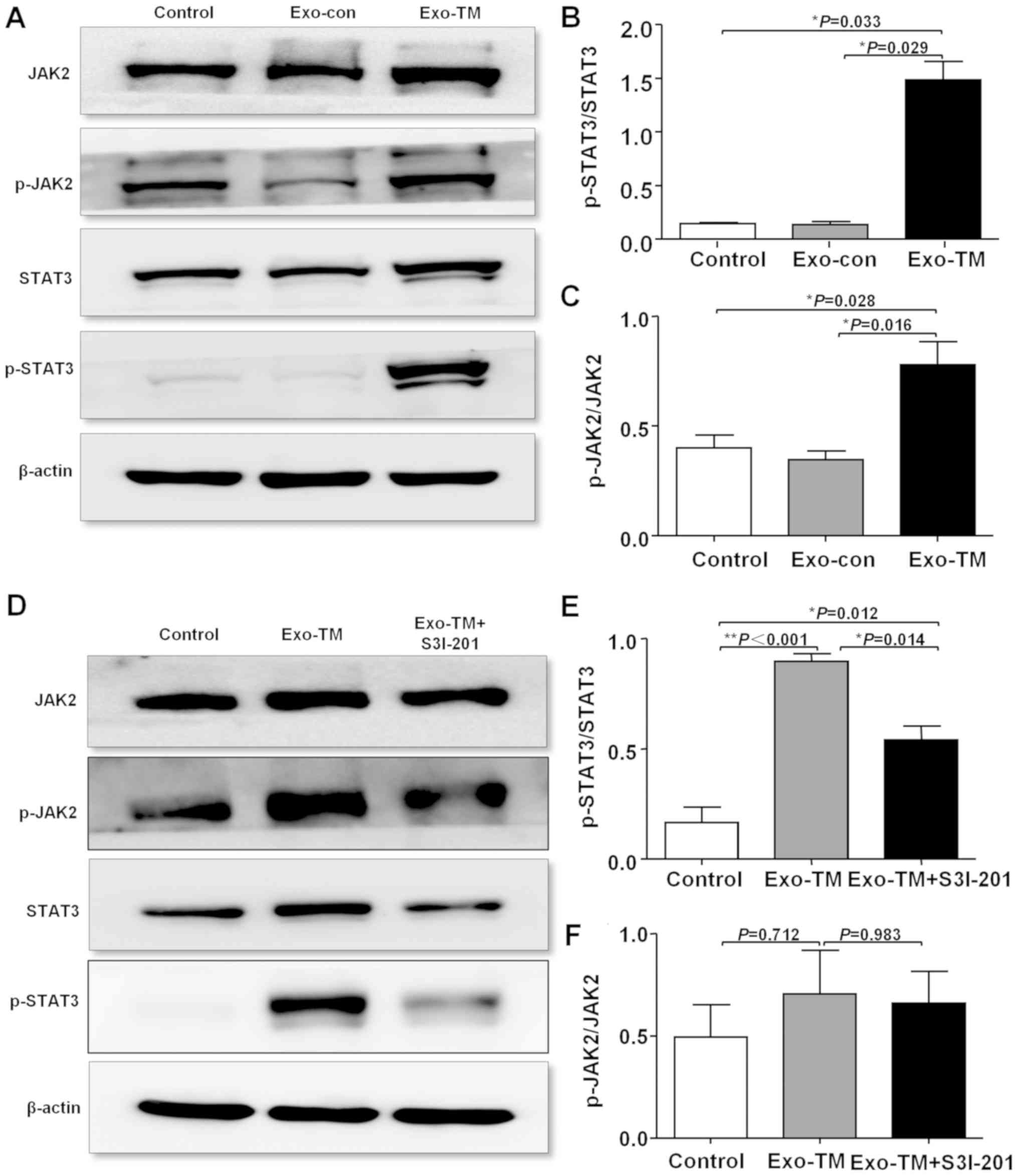
investigated. It was found that Exo-TM significantly increased the
protein expression levels of both p-JAK2 (P<0.05) and p-STAT3
(P<0.05; Fig. 5A-C). Furthermore,
inhibition of STAT3 activation, using a selective STAT3 inhibitor
(100 µM S3I-201; MedChemExpres) at 37°C for 24 h, significantly
decreased the Exo-TM-induced increase in p-STAT3 expression
(Fig. 5D and E; P<0.05), but only
slightly decreased p-JAK2 expression (Fig. 5D and F; P>0.05).
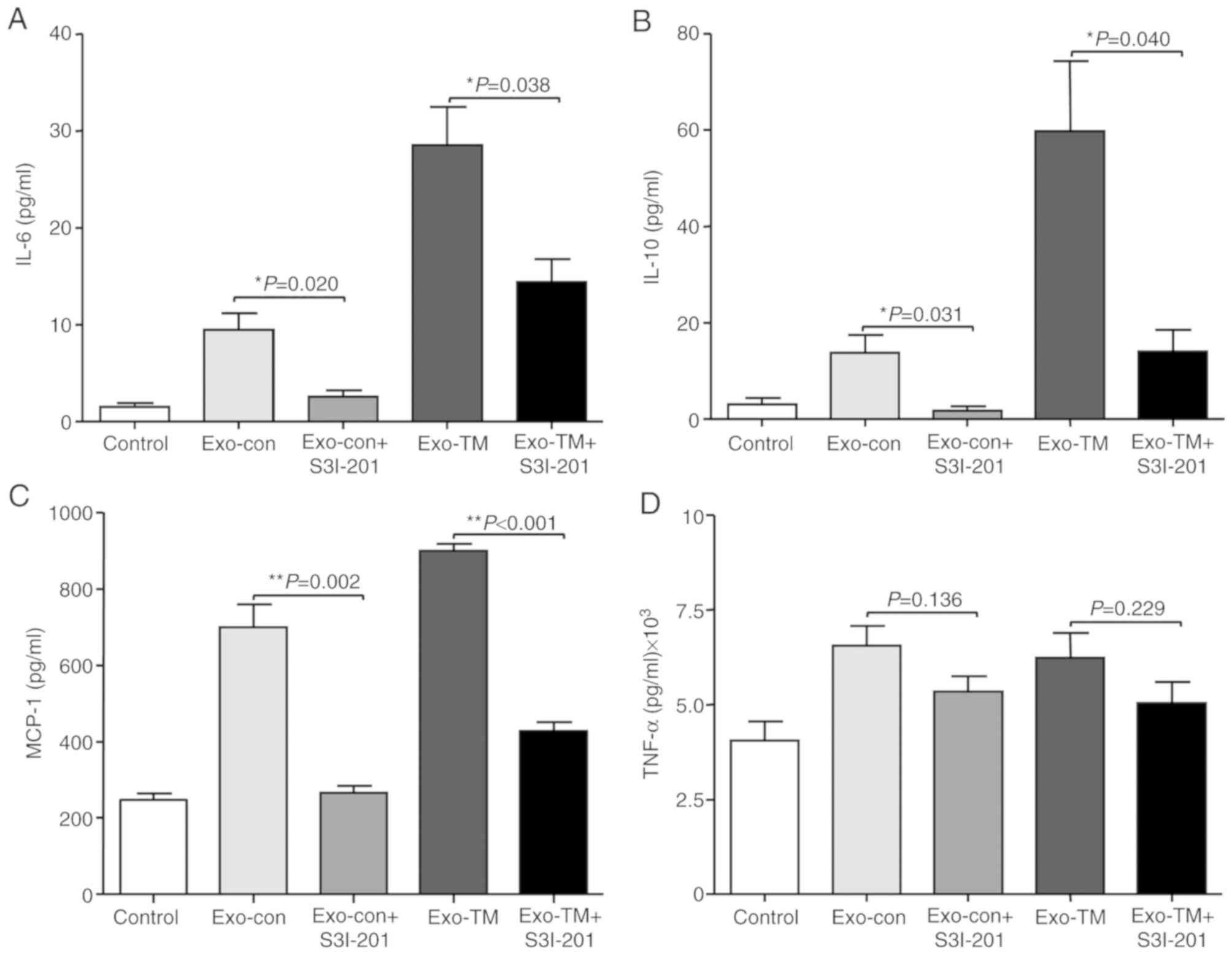
The results also demonstrated that inhibition of
STAT3 abrogated Exo-con- and Exo-TM-induced elevation of IL-6
(Fig. 6A; P<0.05), IL-10
(Fig. 6B; P<0.05) and MCP-1
(Fig. 6C; P<0.01) levels secreted
by RAW264.7 cells; however, inhibition of STAT3 did not affect
TNF-α levels (Fig. 6D; P>0.05).
Therefore, the results suggested that ER stress-associated exosomes
increased the levels of IL-6, MCP-1 and IL-10 in macrophages via
the JAK2/STAT3 signaling pathway.
Discussion
Primary liver cancer is one of the most common types
of malignancies (1,2). Moreover, surgical resection is the
primary treatment method for patients with early stage of liver
cancer (2). However, due to the
asymptomatic nature of the early stage of liver cancer, it is
frequently diagnosed at the later stages, at which point surgery
may not be suitable (2). Therefore,
understanding the causes and mechanisms of drug resistance in liver
cancer may improve the effects of chemotherapy on liver cancer, and
thus, increase survival.
Systemic chemotherapy and target therapy are the two
most frequently used treatment strategies for unresectable and
advanced liver cancer; however, these only improve OS modestly
compared with best supportive care, due to drug resistance
(22,23). Thus, novel treatment regimens are
required to prolong survival. Escaping immune surveillance is one
of the hallmarks of cancer (24,25), and
investigating the specific molecular mechanisms involved in the
modulation of immune escape in liver cancer cells may identify
potential therapeutic target for clinical treatment of liver
cancer.
Alterations in the TME, such as Ca2+
balance disorders, hypoxia and protein glycosylation inhibition,
result in aberrant accumulation of misfolded or unfolded proteins
in the ER, which cause ER stress to maintain homeostasis (4). Continuous activation of ER stress is a
symbolic trait in a number of cancer types, including liver cancer
(26,27), and serves a vital role in maintaining
homeostasis via the activation of the three unfolded protein
response (UPR) branches (28). In
the present study, it was identified that 61.8% (55/89) of liver
cancer tissues stained positively for GRP78, and activation of ER
stress was correlated with inflammation and aggressive disease
characteristics in patients with liver cancer. Moreover, a previous
study showed that several ER stress-associated genes, such as
PKR-like ER kinase, activating transcription factor 6 and inositol
essential enzyme 1α were upregulated in HCC tissues (18). However, the precise function of ER
stress in shaping the TME and the anti-tumor immune response has
not been previously examined. ER stress has been reported to induce
inflammatory responses, and numerous ER stress-associated diseases
also exhibit inflammatory phenotypes (29). Furthermore, ER stress-associated
inflammation is necessary for tissue remodeling, which contributes
to tissue injury and serves an important role in promoting the
development of a variety of inflammation-associated diseases
(30). It has also been shown that
ER-stressed tumor cells can either directly induce inflammation,
via the UPR pathway, or indirectly interact with innate immune
cells.
Cytokines released by ER-stressed tumor cells may
act as warning signals for non-tumor cells (31). Chronic inflammation is involved at
various stages of promoting tumor progression, via a number of
different mechanisms (32,33). For example, chronic inflammation may
promote the production of tumor-promoting cytokines from tumor
cells or tumor-associated immune cells, via the NF-κB and STAT3
signaling pathways (32,33). TAMs have also been reported to
express additional cytokines, such as TNF-α, IL-6 and IL-10
(34). IL-6 is a potent driver of
tumor growth and metastasis in several tumor models and can protect
against apoptosis via the activation of STAT3 in a hypoxic
microenvironment (35). Furthermore,
IL-10 is the primary anti-inflammatory factor secreted by TAMs,
which promotes tumor progression by enhancing tumor cell
proliferation, invasion, stimulating tumor angiogenesis and
inhibiting the anti-tumor immune response (36). MCP-1 is a small cytokine belonging to
the CC chemokine family, which has specific chemotactic activation
effect on monocytes and macrophages (37). Previous studies have shown that MCP-1
is an important pro-inflammatory cytokine that helps to recruit
TAMs in TME (38). TAMs also
secreted TNF-α to promote epithelial-mesenchymal transition and
cancer stemness (39). Moreover,
inflammation promotes tumor progression by inhibiting cell
apoptosis and activating angiogenesis (40,41). In
the present study, it was found that GRP78 expression was
positively associated with CD68, IL-10 and IL-6 levels. In
addition, protein expression levels of IL-10 and IL-6 in
CD68+ TAMs were increased in the GRP78-positive liver
cancer tissues, which was consistent with a previous study
(18). Therefore, the present
results suggested that ER stress plays an important role in
hampering anti-tumor immunity in liver cancer cells. However, the
mechanism via which ER stress exerts modulation of the TME, and
thus remodeling the anti-tumor effect of immune cells to promote
tumor progression, remains unknown.
Exosomes play a critical role in intercellular
communication by delivering their contents, such as proteins, DNA
and miRNA, to cells in local microenvironments or distant target
cells (14,42). In the present study, it was
hypothesized that exosomes may transmit ER stress-associated
signals to macrophages and modulate their function. In the present
study, purified exosomes were labeled with PKH67 and it was found
that RAW264.7 cells effectively engulfed the labeled exosomes
released by HepG2 cells. Moreover, ER-stressed liver cancer cells
released exosomes, which significantly increased the levels of
IL-6, IL-10 and MCP-1, and slightly increased TNF-α levels. It was
also demonstrated that treatment of cells with Exo-TM for 24 h
activated the JAK2/STAT3 signaling pathway, which has an important
role in inflammation response and liver cancer progression
(43). The contents of exosomes are
very complex; currently, >10,000 proteins, 200 lipids, 2,000
mRNAs and ~1,000 micro(mi)RNAs have been reported to be present in
exosomes secreted by different cells (44). In a previous study, exosomes derived
from ER-stressed liver cancer cells transmitted miRNA-23a-3p to
macrophages resulting in the upregulation of programmed
death-ligand 1 expression in macrophages (18). However, transfection of macrophages
with either miRNA-23a-3p mimics or inhibitor did not affect the
secretion of cytokines, such as IL-10 and IL-6 (data not shown).
Thus, the precise mechanism contributing to the upregulation of
cytokine secretion by ER stress-associated exosomes requires
further investigation.
STAT3 not only plays an indispensable role in early
embryonic development and differentiation of bone marrow cells, but
also participates in the regulation of physiological functions such
as tumor growth, differentiation, angiogenesis, invasion,
metastasis and immune escape (45).
Furthermore, the activation of STAT3 is an established pathway that
mediates the inflammatory immune response in response to cytokine
signals (46,47). To investigate whether STAT3 was
involved in Exo-TM-induced inflammation, the expression of p-STAT3
in RAW264.7 treated with Exo-TM was determined, and it was found
that p-STAT3 expression was increased in cells treated with Exo-TM
for 24 h. Furthermore, inhibition of STAT3 using S3I-201
significantly reduced p-STAT3 expression in cells and decreased the
levels of MCP-1, IL-6 and IL-10. Collectively, the present results
suggest that activation of the JAK2/STAT3 pathway may be a
potential mechanism, via which exosomes secreted by ER-stressed
liver cancer cells mediate the inflammatory response. S3I-201 not
only inhibits STAT3-STAT3 complex formation, STAT3-DNA binding and
transcriptional activities, but also inhibits STAT1 and STAT5
(48). Thus, STAT1 and STAT5 may be
also involved in S3I-201-induced cytokine expression reduction.
In conclusion, the present result suggested that
ER-stressed liver cancer cells promoted cytokine expression via
exosome-mediated activation of the JAK2/STAT3 pathway in
macrophages, which resulted in immunosuppression of macrophages,
thus facilitating tumor progression. Therefore, the present study
identified the potential of targeting the exosomal-STAT3 signaling
pathway to abrogate ER stress-associated immune suppression in
liver cancer.
Acknowledgements
The authors would like to thank Professor Wei Wei
and Professor Yujing Wu from the Institute of Clinical
Pharmacology, Anhui Medical University (Hefei, China) for their
technical assistance during the experimental stages of the present
study.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant nos. 81572430 and 81872047).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
GS and HW designed the present study and drafted the
initial manuscript. CH and LF performed the biological experiments.
JL and WH analyzed the data, while JL critically revised the
manuscript for important intellectual content. All authors have red
and approved the manuscript.
Ethics approval and consent to
participate
The protocol of the current study conforms to the
Ethical Guidelines of the 1975 Declaration of Helsinki and was
approved by the Ethics Committee of The First Affiliated Hospital
of Anhui Medical University (approval no. 20040158). Written
consent was provided by all the enrolled patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forner A, Reig M and Bruix J:
Hepatocellular carcinoma. Lancet. 391:1301–1314. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ringelhan M, Pfister D, O'Connor T,
Pikarsky E and Heikenwalder M: The immunology of hepatocellular
carcinoma. Nat Immunol. 19:222–232. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cubillos-Ruiz JR, Bettigole SE and
Glimcher LH: Tumorigenic and immunosuppressive effects of
endoplasmic reticulum stress in cancer. Cell. 168:692–706. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang M, Law ME, Castellano RK and Law BK:
The unfolded protein response as a target for anticancer
therapeutics. Crit Rev Oncol Hematol. 127:66–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hetz C and Papa FR: The unfolded protein
response and cell fate control. Mol Cell. 69:169–181. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen K, Johnson DW, Vesey DA, McGuckin MA
and Gobe GC: Role of the unfolded protein response in determining
the fate of tumor cells and the promise of multi-targeted
therapies. Cell Stress Chaperones. 23:317–334. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rubio-Patiño C, Bossowski JP, Chevet E and
Ricci JE: Reshaping the immune tumor microenvironment through IRE1
signaling. Trends Mol Med. 24:607–614. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoo YS, Han HG and Jeon YJ: Unfolded
protein response of the endoplasmic reticulum in tumor progression
and immunogenicity. Oxid Med Cell Longev. 2017:29692712017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rufo N, Garg AD and Agostinis P: The
unfolded protein response in immunogenic cell death and cancer
immunotherapy. Trends Cancer. 3:643–658. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seelige R, Searles S and Bui JD:
Mechanisms regulating immune surveillance of cellular stress in
cancer. Cell Mol Life Sci. 75:225–240. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ye L, Zhang Q, Cheng Y, Chen X, Wang G,
Shi M, Zhang T, Cao Y, Pan H, Zhang L, et al: Tumor-derived
exosomal HMGB1 fosters hepatocellular carcinoma immune evasion by
promoting TIM-1+ regulatory B cell expansion. J Immunother Cancer.
6:1452018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jella KK, Nasti TH, Li Z, Malla SR,
Buchwald ZS and Khan MK: Exosomes, their biogenesis and role in
inter-cellular communication, tumor microenvironment and cancer
immunotherapy. Vaccines (Basel). 6(pii): E692018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu C, Chen M, Jiang R, Guo Y, Wu M and
Zhang X: Exosome-related tumor microenvironment. J Cancer.
9:3084–3092. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li X, Wang Y, Wang Q, Liu Y, Bao W and Wu
S: Exosomes in cancer: Small transporters with big functions.
Cancer Lett. 435:55–65. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen X, Zhou J, Li X and Wang X, Lin Y and
Wang X: Exosomes derived from hypoxic epithelial ovarian cancer
cells deliver microRNAs to macrophages and elicit a tumor-promoted
phenotype. Cancer Lett. 435:80–91. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seo N, Akiyoshi K and Shiku H:
Exosome-mediated regulation of tumor immunology. Cancer Sci.
109:2998–3004. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu J, Fan L, Yu H, Zhang J, He Y, Feng D,
Wang F, Li X, Liu Q, Li Y, et al: Endoplasmic reticulum stress
causes liver cancer cells to release exosomal miR-23a-3p and
up-regulate programmed death ligand 1 expression in macrophages.
Hepatology. 70:241–258. 2019.PubMed/NCBI
|
|
19
|
Noy R and Pollard JW: Tumor-associated
macrophages: From mechanisms to therapy. Immunity. 41:49–61. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang Y, Liu K, Li Q, Yao Y and Wang Y:
Exosomes function in tumor immune microenvironment. Adv Exp Med
Biol. 1056:109–122. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cui J, Zhang F, Cao W, Wang Y, Liu J, Liu
X, Chen T, Li L, Tian J and Yu B: Erythropoietin alleviates
hyperglycaemia-associated inflammation by regulating macrophage
polarization via the JAK2/STAT3 signalling pathway. Mol Immunol.
101:221–228. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kudo M: Systemic therapy for
hepatocellular carcinoma: Latest advances. Cancers (Basel).
10(pii): E4122018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kulik L and El-Serag HB: Epidemiology and
management of hepatocellular carcinoma. Gastroenterology.
156:477–491.e1. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lapitz A, Arbelaiz A, Olaizola P, Aranburu
A, Bujanda L, Perugorria MJ and Banales JM: Extracellular vesicles
in hepatobiliary malignancies. Front Immunol. 9:22702018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
da Motta Girardi D, Correa TS, Crosara
Teixeira M and Dos Santos Fernandes G: Hepatocellular carcinoma:
Review of targeted and immune therapies. J Gastrointest Cancer.
49:227–236. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roth GS and Decaens T: Liver
immunotolerance and hepatocellular carcinoma: Patho-physiological
mechanisms and therapeutic perspectives. Eur J Cancer. 87:101–112.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Banerjee S and Zhang W: Endoplasmic
reticulum: Target for next-generation cancer therapy. Chembiochem.
19:2341–2343. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Corazzari M, Gagliardi M, Fimia GM and
Piacentini M: Endoplasmic reticulum stress, unfolded protein
response, and cancer cell fate. Front Oncol. 7:782017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Osorio F, Tavernier SJ, Hoffmann E, Saeys
Y, Martens L, Vetters J, Delrue I, De Rycke R, Parthoens E, Pouliot
P, et al: The unfolded-protein-response sensor IRE-1α regulates the
function of CD8α+ dendritic cells. Nat Immunol. 15:248–257. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Garg AD, Kaczmarek A, Krysko O,
Vandenabeele P, Krysko DV and Agostinis P: ER stress-induced
inflammation: Does it aid or impede disease progression? Trends Mol
Med. 18:589–598. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grootjans J, Kaser A, Kaufman RJ and
Blumberg RS: The unfolded protein response in immunity and
inflammation. Nat Rev Immunol. 16:469–484. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Räihä MR and Puolakkainen PA:
Tumor-associated macrophages (TAMs) as biomarkers for gastric
cancer: A review. Chronic Dis Transl Med. 4:156–163.
2018.PubMed/NCBI
|
|
33
|
Chen W, Jiang J, Xia W and Huang J:
Tumor-related exosomes contribute to tumor-promoting
microenvironment: An immunological perspective. J Immunol Res.
2017:10739472017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang X, Zeng Y, Qu Q, Zhu J, Liu Z, Ning
W, Zeng H, Zhang N, Du W, Chen C and sHuang JA: PD-L1 induced by
IFN-γ from tumor-associated macrophages via the JAK/STAT3 and
PI3K/AKT signaling pathways promoted progression of lung cancer.
Int J Clin Oncol. 22:1026–1033. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jeong SK, Kim JS, Lee CG, Park YS, Kim SD,
Yoon SO, Han DH, Lee KY, Jeong MH and Jo WS: Tumor associated
macrophages provide the survival resistance of tumor cells to
hypoxic microenvironmental condition through IL-6 receptor-mediated
signals. Immunobiology. 222:55–65. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ruffell B, Chang-Strachan D, Chan V,
Rosenbusch A, Ho CM, Pryer N, Daniel D, Hwang ES, Rugo HS and
Coussens LM: Macrophage IL-10 blocks CD8+ T cell-dependent
responses to chemotherapy by suppressing IL-12 expression in
intratumoral dendritic cells. Cancer Cell. 26:623–637. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ibi M, Horie S, Kyakumoto S, Chosa N,
Yoshida M, Kamo M, Ohtsuka M and Ishisaki A: Cell-cell interactions
between monocytes/macrophages and synoviocyte-like cells promote
inflammatory cell infiltration mediated by augmentation of MCP-1
production in temporomandibular joint. Biosci Rep. 38(pii):
BSR201712172018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hultgren EM, Patrick ME, Evans RL, Stoos
CT and Egland KA: SUSD2 promotes tumor-associated macrophage
recruitment by increasing levels of MCP-1 in breast cancer. PLoS
One. 12:e01770892017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen Y, Wen H, Zhou C, Su Q, Lin Y, Xie Y,
Huang Y, Qiu Q, Lin J, Huang X, et al: TNF-α derived from M2
tumor-associated macrophages promotes epithelial-mesenchymal
transition and cancer stemness through the Wnt/β-catenin pathway in
SMMC-7721 hepatocellular carcinoma cells. Exp Cell Res. 378:41–50.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu X, Tao P, Zhou Q, Li J, Yu Z, Wang X,
Li J, Li C, Yan M, Zhu Z, et al: IL-6 secreted by cancer-associated
fibroblasts promotes epithelial-mesenchymal transition and
metastasis of gastric cancer via JAK2/STAT3 signaling pathway.
Oncotarget. 8:20741–20750. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao X, Fan W, Xu Z, Chen H, He Y, Yang G,
Yang G, Hu H, Tang S, Wang P, et al: Inhibiting tumor necrosis
factor-alpha diminishes desmoplasia and inflammation to overcome
chemoresistance in pancreatic ductal adenocarcinoma. Oncotarget.
7:81110–81122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wan Z, Gao X, Dong Y, Zhao Y, Chen X, Yang
G and Liu L: Exosome-mediated cell-cell communication in tumor
progression. Am J Cancer Res. 8:1661–1673. 2018.PubMed/NCBI
|
|
43
|
Ding YF, Wu ZH, Wei YJ, Shu L and Peng YR:
Hepatic inflammation-fibrosis-cancer axis in the rat hepatocellular
carcinoma induced by diethylnitrosamine. J Cancer Res Clin Oncol.
143:821–834. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Théry C, Witwer KW, Aikawa E, Alcaraz MJ,
Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F,
Atkin-Smith GK, et al: Minimal information for studies of
extracellular vesicles 2018 (MISEV2018): A position statement of
the international society for extracellular vesicles and update of
the MISEV2014 guidelines. J Extracell Vesicles. 7:15357502018.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Belmokhtar K, Bourguignon T, Worou ME,
Khamis G, Bonnet P, Domenech J and Eder V: Regeneration of three
layers vascular wall by using BMP2-treated MSC involving HIF-1α and
Id1 expressions through JAK/STAT pathways. Stem Cell Rev Rep.
7:847–859. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Laudisi F, Cherubini F, Monteleone G and
Stolfi C: STAT3 Interactors as potential therapeutic targets for
cancer treatment. Int J Mol Sci. 19(pii): E17872018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang Y, Shen Y, Wang S, Shen Q and Zhou X:
The role of STAT3 in leading the crosstalk between human cancers
and the immune system. Cancer Lett. 415:117–128. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Siddiquee K, Zhang S, Guida WC, Blaskovich
MA, Greedy B, Lawrence HR, Yip ML, Jove R, McLaughlin MM, Lawrence
NJ, et al: Selective chemical probe inhibitor of Stat3, identified
through structure-based virtual screening, induces antitumor
activity. Proc Natl Acad Sci USA. 104:7391–7396. 2007. View Article : Google Scholar : PubMed/NCBI
|