Introduction
Anthracycline anticancer drugs, such as doxorubicin
(DOX), daunorubicin (DNR), and idarubicin (IDA), are broadly used
in the treatment of various cancers, including acute leukemia
(1–3). These drugs mediate cancer cell death
through intercalation between DNA base pairs and inhibition of
topoisomerase II (1–3). One of the limitations associated with
DOX and DNR therapy is the development of drug resistance through
overexpression of the drug transporter such as P-glycoprotein
(P-gp) (4–6). On the other hand, IDA, which is used in
acute myelogenous leukemia (AML) and acute lymphoblastic leukemia
(ALL) therapy, is highly lipophilic compared to DOX and DNR, and is
imported into cells faster than the aforementioned anthracyclines,
as well as less affected by P-gp-mediated drug efflux (7,8).
However, AML and ALL therapy with IDA also face the limitation of
drug resistance.
One of the characteristics of cancer cells is their
metabolic alteration, known as the Warburg effect (9,10). The
rapidly proliferating cancer cells predominantly use the less
efficient aerobic glycolytic pathway for ATP synthesis, resulting
in high glucose demand in cancer cells. Therefore, inhibition of
glycolysis is expected to have a stronger impact on cancer cells
than on normal cells. Much research has focused on glycolysis
inhibition as a strategy for cancer therapy (11,12).
The non-metabolic glucose analog 2-deoxy-D-glucose
(2-DG) is a glycolysis inhibitor (12). 2-DG is transported into the cells and
metabolized by hexokinase to 2-deoxy-D-glucose-6-phosphate, which
is not further metabolized and accumulates in the cells (12). The structure of 2-DG is similar to
that of mannose, which is important for N-glycosylation in proteins
and normal protein folding in the endoplasmic reticulum (ER). It
has been reported that inhibition of N-glycosylation by its
inhibitor Tunicamycin (Tm) induced ER stress (13). Inhibition of N-glycosylation by 2-DG
also increases misfolded proteins in the ER and results in ER
stress-induced cell death (14). It
has been thought that this dual effect of 2-DG provokes cell death
and suppresses cell proliferation in cancer cells. Therefore, it is
relevant to understand the potential of 2-DG in the mitigation of
IDA-resistance in the context of leukemia therapy. In this study,
the cytotoxic effect of 2-DG on in-house established IDA-resistant
P388 (P388/IDA) leukemia cells was evaluated.
Materials and methods
Materials
P388 mouse leukemia cells (P388 cells), which has
been used for model of leukemia cells (15–17),
were provided by Daiichi Pharmaceutical Co., Ltd. (Tokyo, Japan).
RPMI-1640 medium was purchased from Nissui Pharmaceutical Co., Ltd.
(Tokyo, Japan). 2-DG (D6134) and tunicamycin (Tm; T7765) were
purchased from Sigma-Aldrich (St. Louis, MO, USA). IDA was obtained
from Wako (Tokyo, Japan). The Cell Counting Kit-8 (CK04), Lactate
Assay Kit-WST (L256), and Glucose Assay Kit-WST (G264) were
purchased from Dojindo (Kumamoto, Japan). Anti-caspase-3
(19677-1-AP), anti-Poly (ADP-ribose) polymerase (PARP; 9542), and
anti-β-actin (A5441) antibodies were purchased from Proteintech
(Rosemont, IL, USA), Cell Signaling Technology (MA, USA), and
Sigma-Aldrich, respectively.
Cell culture and establishment of
IDA-resistant P388 cells
P388 leukemia cells were grown in RPMI-1640 medium
that contained 50 µM 2-mercaptoethanol and 10% fetal bovine serum,
under 5% CO2 at 37°C. To establish P388/IDA
cells, at first, P388 cells were cultured with 0.001 µM IDA for 1
week in 12-well plates. Cells underwent culture with increasingly
higher concentrations (1.5 to 2-fold) of IDA every 1–2 weeks.
Finally, P388/IDA cells were maintained in RPMI-1640 medium with
0.1 µM IDA. In total, the establishment of the drug-resistant cells
took more than 6 months. When the cells were used for analyses, the
cells were cultured in drug-free medium for more than 3 days.
Measurement of pH, lactic acid
production, and glucose consumption
P388 or P388/IDA cells (1.0×106 cells/ml)
were cultured in RPMI-1640 medium for 24 h in 6-well plates. The pH
of each medium was measured by LAQUAtwin (Horiba, Ltd., Kyoto,
Japan). The pH of RPMI-1640 medium before culturing was 7.9. Lactic
acid production and glucose consumption were measured by Lactate
Assay Kit-WST and Glucose Assay Kit-WST, respectively, according to
the manufacturers instructions. In brief, cultured media were
centrifuged at 10,000 × g for 3 min and the supernatants were used
for analysis. The respective reaction buffers were incubated with
the supernatants for 30 min at 37°C, and then the
absorbance was measured at 450 nm. Lactic acid concentrations were
calculated using a lactic acid standard and then normalized to cell
number. Glucose concentrations in culture media were calculated
using a glucose standard. To determine relative glucose consumption
per cell, glucose concentration after culturing was subtracted from
that before culturing and then normalized to cell number.
Cell viability
P388 or P388/IDA cells (1.5×105 cells/ml)
were cultured in RPMI-1640 with IDA (0–5 µM), 2-DG (0–500 µM), or
Tm (0–1 µg/ml) for 48 h in 96-well plates. Cell viability was
measured by Cell Counting Kit-8 according to the manufacturers
instructions.
Western blot analysis
P388 or P388/IDA cells (1.5×105 cells/ml)
were treated with IDA (0–5 µM) and/or 2-DG (250 µM) for 24 h in
12-well plates. Western blot analysis was performed as previously
described (18). In brief, proteins
were separated on 7.5% or 15% acrylamide gels for SDS-PAGE and
transferred to nitrocellulose membranes. Membranes were incubated
with anti-caspase-3 (1:1,000), anti-PARP (1:1,500), and
anti-β-actin (1:5,000) antibodies. Membranes were revealed with
horseradish peroxidase-conjugated secondary antibodies (anti-mouse
or anti-rabbit IgG).
Statistical analysis
Data are presented as the mean ± standard deviation
and were analyzed using a Students t-test or One-way ANOVA (post
hoc test, Tukey Kramer method). BellCurve for Excel version 3.20
was used for statistical analysis (Social Survey Research
Information Co., Ltd.). P<0.05 was considered statistically
significant.
Results
Characterization of IDA-resistant P388
leukemia cells
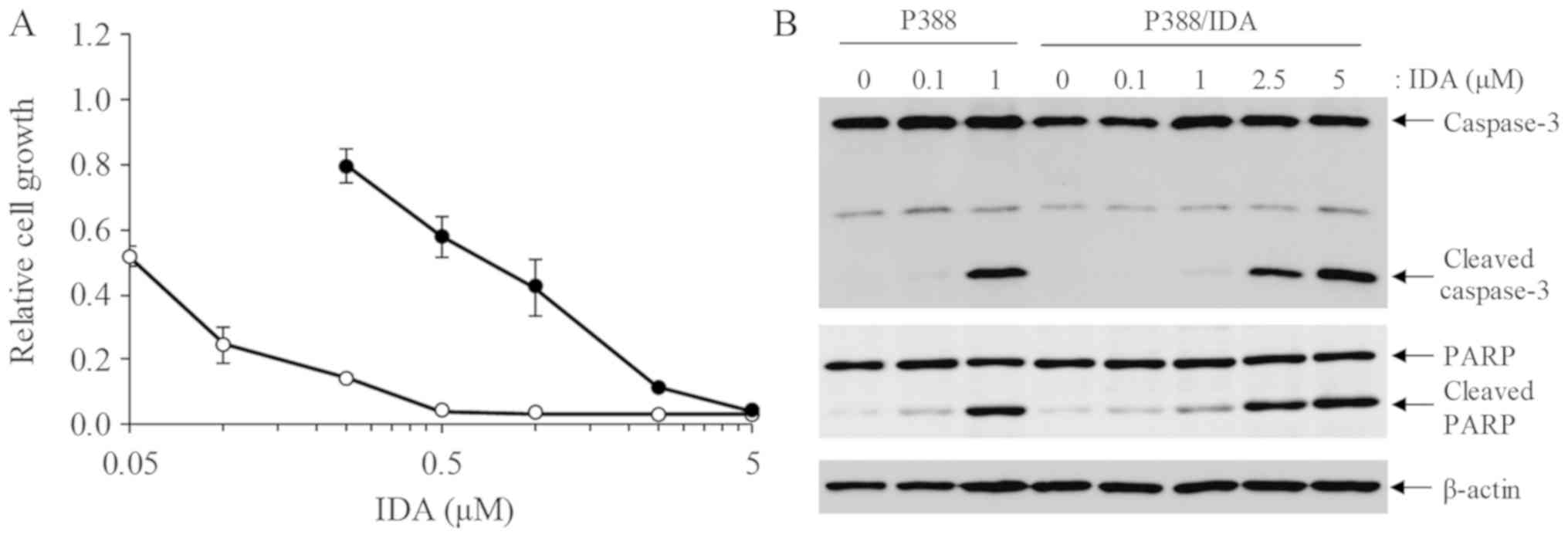
To compare the sensitivity of P388 and P388/IDA
cells to IDA, a cell viability assay was performed 48 h after IDA
treatment in both cell lines. The IC50 of IDA on P388
was 0.05±0.02 µM, whereas on P388/IDA cells was 0.66±0.18 µM
(P<0.05). IDA sensitivity of P388/IDA cells was approximately
10-fold lower than that of P388 cells (Fig. 1A). Western blot analysis showed that
the apoptosis markers cleaved caspase-3 and cleaved PARP were
detected in P388/IDA cells undergoing treatment with high
concentrations of IDA (Fig. 1B).
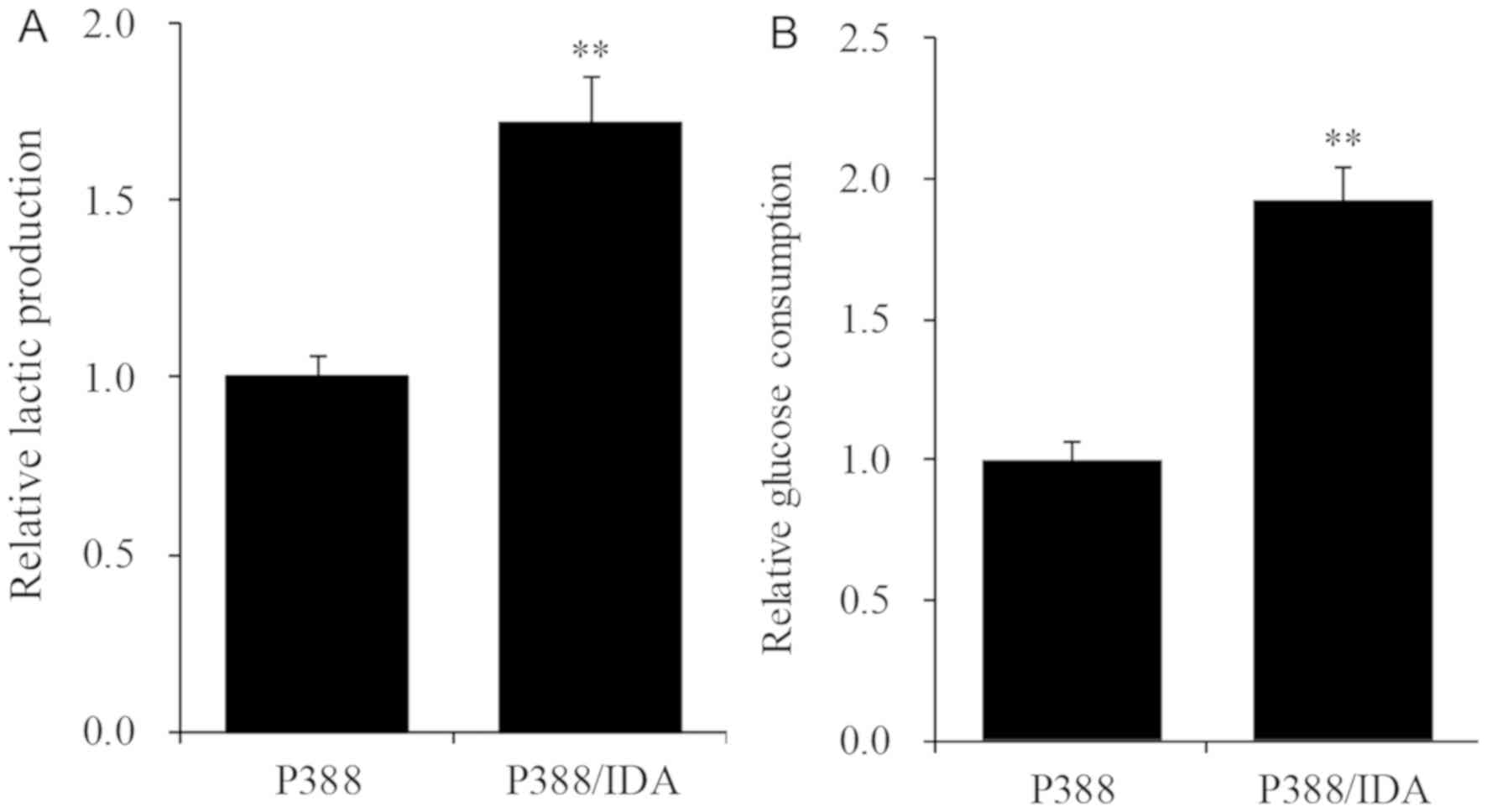
To examine the metabolism of IDA/P388 cells, we
measured the pH of culture media 24 h after incubation. The pH in
P388 and P388/IDA cells was 7.4±0.1 and 6.9±0.1, respectively.
Lactic acid production in IDA/P388 cells was approximately 1.7-fold
higher than that in P388 cells (Fig.
2A) and glucose consumption in the IDA-resistant cell line
increased about 1.9-fold relatively to the parent cell line
(Fig. 2B).
Effect of 2-DG and Tm on cell
proliferation
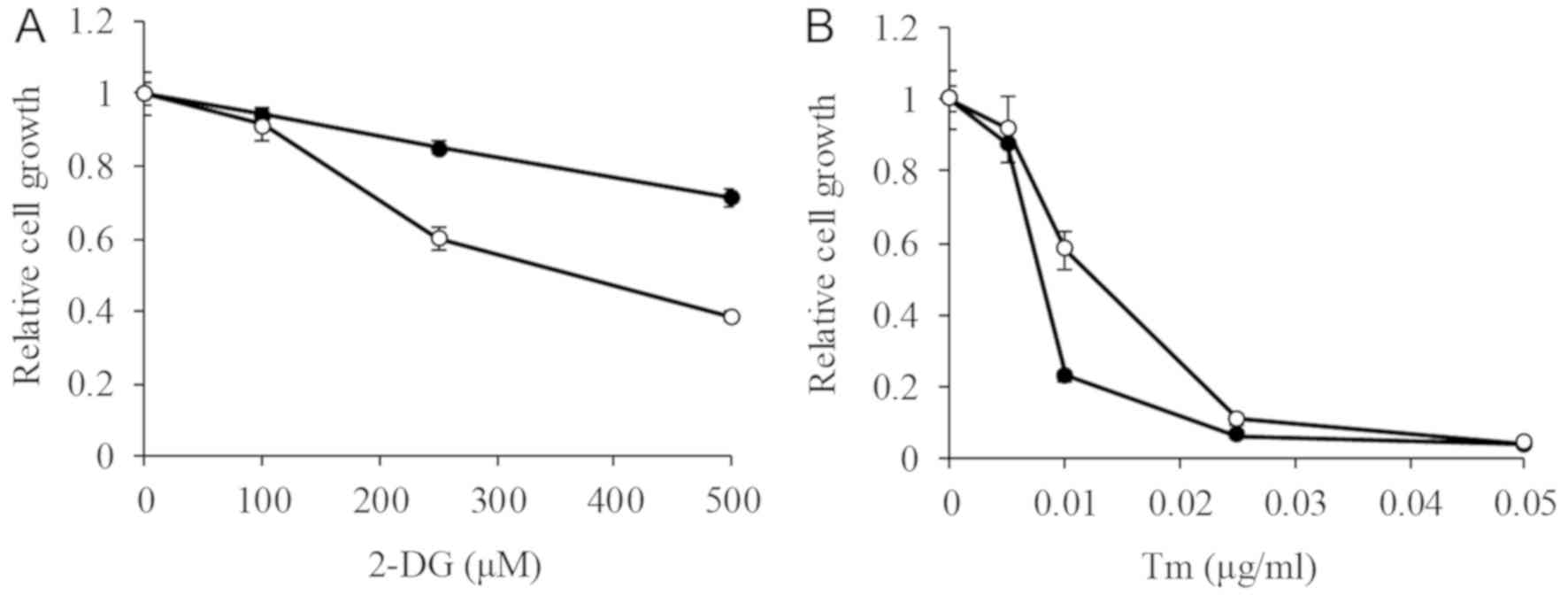
We next compared the cytotoxicity of the glycolysis
inhibitor 2-DG on both cell lines. The IC50 of 2-DG on
P388 was higher than the tested concentrations (>500 µM), while
on P388/IDA cells was 392.6±41.1 µM (Fig. 3A). 2-DG both suppressed glycolysis
and induced ER stress. To examine the impact of ER stress on the
cells, the effect of Tm was assessed. The IC50 of Tm on
P388 and P388/IDA cells was 9.2±1.6 and 16.8±2.4 ng/ml (P<0.05),
respectively (Fig. 3B). The effect
of Tm on P388 cells was about 1.8-fold higher than that in P388/IDA
cells.
2-DG enhanced the cytotoxic effect of
IDA on P388/IDA cells
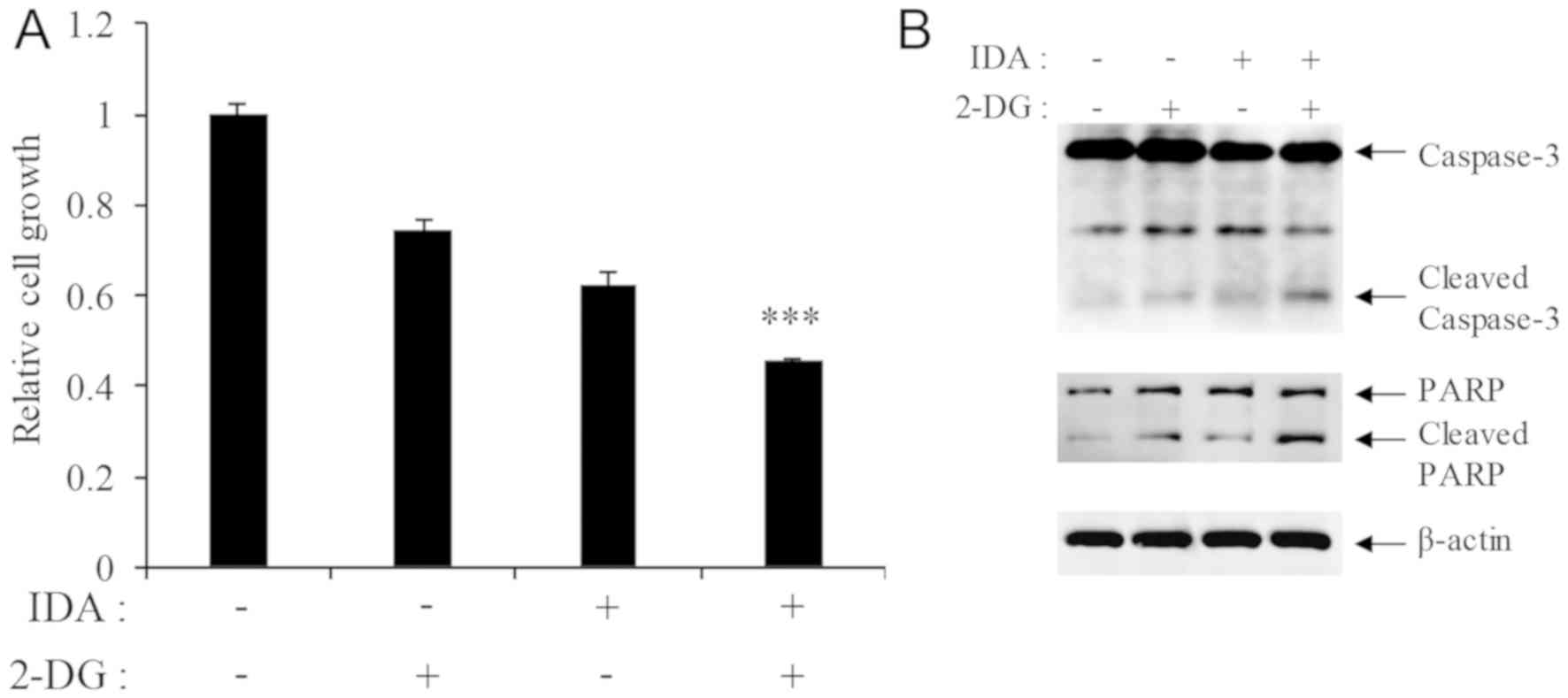
Simultaneous treatment of P388/IDA cells with 250 µM
2-DG and 0.5 µM IDA enhanced cell death in comparison with each
agent alone (Fig. 4A). The
expression of cleaved caspase-3 and cleaved PARP was also increased
in co-treated cells (Fig. 4B).
Discussion
IDA-resistance in acute leukemia poses a critical
problem for cancer chemotherapy. One of the reasons for the
development of resistance to anthracycline anticancer drugs is the
overexpression of P-gp in cancer cells (4,5). We
found that P-gp was overexpressed in P388/IDA cells compared to
P388 cells (data not shown). However, the highly lipophilic drug
IDA is less affected by P-gp expression than other anthracyclines
(7,8). Therefore, targeting P-gp to overcome
IDA-resistance in leukemia is not an efficient strategy. Metabolic
alteration, such as through activation of the glycolysis pathway,
in drug-resistant cancers has been the focus of potential cancer
therapies (19). While it is known
that the expression of genes related to glucose metabolism are
upregulated in drug-resistant cancer cells compared to parent cells
(19,20), the effect of metabolic alteration on
drug resistance is poorly understood. In this paper, IDA-resistant
P388 leukemia cells increased their dependence on glycolysis for
ATP production, as demonstrated by the increased lactic acid
production and glucose consumption. Therefore, we expected the
glycolysis inhibitor 2-DG to positively affect IDA-resistant P388
cells. It has been reported that 2-DG exerts synergistic cytotoxic
effects with other reagents (21).
In P388/IDA cells, the combination of IDA (0.1 µM) with 2-DG (100
µM), which are concentrations causing low levels of toxicity, could
not enhance cell death (data not shown). The cytotoxicity of this
combination is thought to be an additive effect, not a synergistic
effect.
Additionally, cytotoxicity of 500 µM 2-DG on P388
cells was low, but 2-DG elicited cytotoxic effects at lower
concentrations on P388/IDA cells. On the other hand, the ER stress
inducer Tm induced higher cytotoxicity in P388 cells than in
P388/IDA cells. These results suggest that P388/IDA cells are
resistant to ER stress, and the dominant effect of 2-DG on P388/IDA
cells is exerted through glycolysis inhibition. Therefore,
targeting the glycolytic pathway on IDA-resistant leukemia cells
could be a useful strategy to overcome drug-resistance. However,
clinical studies on the application 2-DG in cancers have reported
that 2-DG administration induces several side effects, such as
fatigue and dizziness, among others (22,23).
Additionally, the effective concentration of 2-DG on cancer cells
has been found to vary across different cancer cells (11,19,24,25). The
underlying mechanism of such differences is poorly understood and
the administration of high concentrations of 2-DG increases the
risk of side effects. To establish an effective cancer therapy
strategy using glycolysis inhibitors, further studies focusing on
reliable markers of effective cytotoxicity induced by glycolysis
inhibition are necessary.
In conclusion, this study demonstrated that the
glycolysis inhibitor 2-DG enhances IDA-induced cell death in
P388/IDA leukemia cells, in which glycolytic metabolism is
increased. A therapeutic combination of IDA and 2-DG might be a
potential strategy against IDA-resistant leukemia.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors contributions
TM conceived and designed the experiments. TM, YK
and EH performed the experiments and analyzed the data. TM and YS
wrote the manuscript. YS interpreted the data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
2-DG
|
2-deoxy-D-glucose
|
|
ALL
|
acute lymphoblastic leucemia
|
|
AML
|
acute myelogenous leucemia
|
|
DNR
|
daunorubicin
|
|
DOX
|
doxorubicin
|
|
ER
|
endoplasmic reticulum
|
|
IDA
|
idarubicin
|
|
P-gp
|
P-glycoprotein
|
|
Tm
|
tunicamycin
|
References
|
1
|
Gewirtz DA: A critical evaluation of the
mechanisms of action proposed for the antitumor effects of the
anthracycline antibiotics adriamycin and daunorubicin. Biochem
Pharmacol. 57:727–741. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Minotti G, Menna P, Salvatorelli E, Cairo
G and Gianni L: Anthracyclines: Molecular advances and
pharmacologic developments in antitumor activity and
cardiotoxicity. Pharmacol Rev. 56:185–229. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McGowan JV, Chung R, Maulik A, Piotrowska
I, Walker JM and Yellon DM: Anthracycline chemotherapy and
cardiotoxicity. Cardiovasc Drugs Ther. 31:63–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nooter K, Sonneveld P, Oostrum R,
Herweijer H, Hagenbeek T and Valerio D: Overexpression of the mdr1
gene in blast cells from patients with acute myelocytic leukemia is
associated with decreased anthracycline accumulation that can be
restored by cyclosporin-A. Int J Cancer. 45:263–268. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Berman E and McBride M: Comparative
cellular pharmacology of daunorubicin and idarubicin in human
multidrug-resistant leukemia cells. Blood. 79:3267–3273. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou XW, Xia YZ, Zhang YL, Luo JG, Han C,
Zhang H, Zhang C, Yang L and Kong LY: Tomentodione M sensitizes
multidrug resistant cancer cells by decreasing P-glycoprotein via
inhibition of p38 MAPK signaling. Oncotarget. 8:101965–101983.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mankhetkorn S, Dubru F, Hesschenbrouck J,
Fiallo M and Garnier-Suillerot A: Relation among the resistance
factor, kinetics of uptake, and kinetics of the
P-glycoprotein-mediated efflux of doxorubicin, daunorubicin,
8-(S)-fluoroidarubicin, and idarubicin in multidrug-resistant K562
cells. Mol Pharmacol. 49:532–539. 1996.PubMed/NCBI
|
|
8
|
Roovers DJ, van Vliet M, Bloem AC and
Lokhorst HM: Idarubicin overcomes P-glycoprotein-related multidrug
resistance: Comparison with doxorubicin and daunorubicin in human
multiple myeloma cell lines. Leuk Res. 23:539–548. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Orang AV, Petersen J, McKinnon RA and
Michael MZ: Micromanaging aerobic respiration and glycolysis in
cancer cells. Mol Metab. 23:98–126. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gu L, Yi Z, Zhang Y, Ma Z, Zhu Y and Gao
J: Low dose of 2-deoxy-D-glucose kills acute lymphoblastic leukemia
cells and reverses glucocorticoid resistance via N-linked
glycosylation inhibition under normoxia. Oncotarget. 8:30978–30991.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pelicano H, Martin DS, Xu RH and Huang P:
Glycolysis inhibition for anticancer treatment. Oncogene.
25:4633–4646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Garg AD, Maes H, van Vliet AR and
Agostinis P: Targeting the hallmarks of cancer with therapy-induced
endoplasmic reticulum (ER) stress. Mol Cell Oncol. 2:e9750892014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
DeSalvo J, Kuznetsov JN, Du J, Leclerc GM,
Leclerc GJ, Lampidis TJ and Barredo JC: Inhibition of Akt
potentiates 2-DG-induced apoptosis via downregulation of UPR in
acute lymphoblastic leukemia. Mol Cancer Res. 10:969–978. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Badiner GJ, Moy BC, Smith KS, Tarpley WG,
Groppi VE and Bhuyan BK: P388 leukaemia cells resistant to the
anthracycline menogaril lack multidrug resistant phenotype. Br J
Cancer. 62:378–384. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kiue A, Sano T, Naito A, Inada H, Suzuki
K, Okumura M, Kikuchi J, Sato S, Takano H, Kohno K, et al: Reversal
by two dihydropyridine compounds of resistance to multiple
anticancer agents in mouse P388 leukemia in vivo and in vitro. Jpn
J Cancer Res. 81:1057–1064. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aboudkhil S, Henry L, Zaid A and Bureau
JP: Effect of testosterone on growth of P388 leukemia cell line in
vivo and in vitro. Distribution of peripheral blood T lymphocytes
and cell cycle progression. Neoplasma. 52:260–266. 2005.PubMed/NCBI
|
|
18
|
Matsuo T and Sadzuka Y: Extracellular
acidification by lactic acid suppresses glucose deprivation-induced
cell death and autophagy in B16 melanoma cells. Biochem Biophys Res
Commun. 496:1357–1361. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song K, Li M, Xu X, Xuan LI, Huang G and
Liu Q: Resistance to chemotherapy is associated with altered
glucose metabolism in acute myeloid leukemia. Oncol Lett.
12:334–342. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Staubert C, Bhuiyan H, Lindahl A, Broom
OJ, Zhu Y, Islam S, Linnarsson S, Lehtiö J and Nordström A: Rewired
metabolism in drug-resistant leukemia cells: A metabolic switch
hallmarked by reduced dependence on exogenous glutamine. J Biol
Chem. 290:8348–8359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miwa H, Shikami M, Goto M, Mizuno S,
Takahashi M, Tsunekawa-Imai N, Ishikawa T, Mizutani M, Horio T,
Gotou M, et al: Leukemia cells demonstrate a different metabolic
perturbation provoked by 2-deoxyglucose. Oncol Rep. 29:2053–2057.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Raez LE, Papadopoulos K, Ricart AD,
Chiorean EG, Dipaola RS, Stein MN, Rocha Lima CM, Schlesselman JJ,
Tolba K, Langmuir VK, et al: A phase I dose-escalation trial of
2-deoxy-D-glucose alone or combined with docetaxel in patients with
advanced solid tumors. Cancer Chemother Pharmacol. 71:523–530.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stein M, Lin H, Jeyamohan C, Dvorzhinski
D, Gounder M, Bray K, Eddy S, Goodin S, White E and Dipaola RS:
Targeting tumor metabolism with 2-deoxyglucose in patients with
castrate-resistant prostate cancer and advanced malignancies.
Prostate. 70:1388–1394. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maximchik P, Abdrakhmanov A, Inozemtseva
E, Tyurin-Kuzmin PA, Zhivotovsky B and Gogvadze V:
2-Deoxy-D-glucose has distinct and cell line-specific effects on
the survival of different cancer cells upon antitumor drug
treatment. FEBS J. 285:4590–4601. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reyes R, Wani NA, Ghoshal K, Jacob ST and
Motiwala T: Sorafenib and 2-deoxyglucose synergistically inhibit
proliferation of both sorafenib-sensitive and -resistant HCC cells
by inhibiting ATP production. Gene Expr. 17:129–140. 2017.
View Article : Google Scholar : PubMed/NCBI
|