Introduction
Colorectal cancer (CRC) is the third most diagnosed
cancer worldwide with a high mortality rate, which reported 1.4
million new cases and 693,900 mortalities in 2012 (1). Based on the thorough understanding of
the CRC pathological process, immunotherapy and targeted therapy
have emerged and have greatly improved the prognosis of patients
with CRC (2). However, because the
occurrence and development of CRC is a complex process caused by
numerous factors, the prognosis of patients with advanced-stage CRC
remains unsatisfactory (3). It is
therefore crucial to identify the underlying mechanisms of CRC
tumorigenesis, in order to develop novel therapeutic
strategies.
The human genome consists of 2% genes coding for
proteins and several non-coding RNAs (ncRNAs) (4). Long non-coding (lnc)RNAs are a type of
ncRNAs that are defined as transcripts with lengths >200
nucleotides, and that are not translated into proteins (5,6).
Although lncRNAs were previously considered as transcriptional
noise, previous studies have reported that lncRNAs serve crucial
roles in the regulation of biological processes involved in
numerous diseases, including various types of cancer (6,7).
Previous studies have reported that the lncRNA KCNQ1OT1 is involved
in the progression of multiple tumors, including tongue cancer,
hepatocellular carcinoma, and breast cancer (8–10).
However, the underlying mechanisms of KCNQ1OT1 in CRC development
and metastasis remain unknown.
The present study investigated KCNQ1OT1 expression
in CRC tissues and its association with the prognosis of patients
with CRC. Furthermore, the role of KCNQ1OT1 in CRC cell
proliferation, migratory and invasive abilities, apoptosis and the
cell cycle was also analyzed. The underlying mechanisms of KCNQ1OT1
in CRC cells were subsequently investigated, which may help to
provide a new therapeutic and prognostic method for colorectal
cancer.
Materials and methods
Clinical samples
A total of 28 pairs of CRC and adjacent normal
tissues (>5 cm from the edge of tumor tissues) were collected
from patients with CRC between June 2018 and January 2019 at
Zhujiang Hospital, Southern Medical University, and preserved in
liquid nitrogen. All tissue samples were confirmed by
histopathology, and tumor staging was based on the NCCN Colon
Cancer Guidelines (11). The
clinicopathological information of all patients, including age,
sex, tumor size, pathological differentiation and
Tumor-Node-Metastasis TNM, were collected at the Department of
General Surgery of Zhujiang Hospital, Southern Medical University.
The patients recruited in the present study suffered from colon
adenocarcinoma (COAD) or rectum adenocarcinoma (READ), and all data
were analyzed together. The present study was approved by the
Ethics Committee of Zhujiang Hospital. All patients provided
written informed consent prior to the study.
Bioinformatics analysis
Gene Expression Profiling Interactive Analysis
(GEPIA http://gepia.cancer-pku.cn/) is an
online web server used for the analysis of RNA sequencing
expression data of 9,736 tumor samples and 8,587 normal samples
from The Cancer Genome Atlas (https://www.cancer.gov) and the Genotype-Tissue
Expression (https://commonfund.nih.gov/GTEx) databases, using a
standard processing pipeline (12).
Following submission of an analysis request, GEPIA can provide the
visual image results for users (12). The gene symbol KCNQ1OT1 was submitted
on GEPIA for differential expression analysis between data from CRC
and normal tissues, and provided results consisting of box plots,
stage plots and survival analysis.
Cell culture
The human CRC cell lines RKO, SW620, colo320, HCT116
and LoVo, and the normal colon epithelial cell line NCM460 were
obtained from the Southern Medical University Corporation. All
cells were maintained in RPMI-1640 (HyClone; GE Healthcare Life
Sciences) containing 10% FBS (Serana Europe GmbH) and placed at
37°C in a humidified incubator containing 5% CO2.
PI3K inhibitor was purchased from Selleck Chemicals.
The stably transfected SW620 and RKO cells were treated with 10 µM
PI3K inhibitor (LY294002) for 24 h (13) to suppress the PI3K/AKT signaling
pathway when the density of cells reached 50%.
RNA extraction and reverse
transcription-quantitative (RT-q) PCR
Total RNA was extracted from the primary tissues and
cell lines using TRIzol™ reagent (Takara Bio, Inc.), and reverse
transcribed into cDNA with the PrimeScript RT Kit (Takara Bio,
Inc.) according to the manufacturers' instructions. RT-qPCR
reactions were performed as follows: 37°C for 15 min, followed by
85°C for 5 sec. qPCR was performed using the TB Green premix
(Takara Bio, Inc.) in a CFX96 real-time PCR detection system
(Bio-Rad Laboratories, Inc.). The sequences of the primers (Sangon
Biotech, Co., Ltd.) were as follows: KCNQ1OT1 forward,
5′-CAAGCAGCCAGAAGGATGAGAAGG-3′ and reverse
5′-GGTCAGCACCAGAAGGCAGAATG-3′; and GAPDH forward,
5′-TGCACCACCAACTGCTTAGC-3′ and reverse 5′-GGCATGCACTGTGGTCATGAG-3′.
The relative expression level of KCNQ1OT1 normalized to endogenous
control and expressed as 2−ΔΔCq (14).
Lentivirus production and
transfection
Short hairpin RNAs (shRNAs) against human KCNQ1OT1
(sh-KCNQ1OT1-1 and sh-KCNQ1OT1-2) or scrambled oligonucleotides
(sh-nc) were ligated into a LV-5 (EF-1αF/GFP+Puro) vector (all from
Shanghai GenePharm Co., Ltd.). Lentivirus solution (108
TU/ml) was transfected into the SW620 and RKO lines using
Lipofectamine 3000 (Thermo Fisher Scientific, Inc,). The stably
transfected cells were selected by puromycin selection (5 µg/ml)
for ≥2 weeks. Subsequent experiments were performed after 2 weeks
of cell transfection.
Cell viability assay
The stably transfected SW620 and RKO cells were
seeded into 96-well plates at the density of 2×103 cells
per well. Cell viability was assessed every 24 h for 5 days using
the Cell Counting-Kit 8 reagent (CCK-8; Beijing Transgen Biotech
Co., Ltd.). Briefly, cells were incubated with 10 ul CCK-8 solution
for 2 h and absorbance was read at 450 nm on a microplate
reader.
Colony formation assay
The stably transfected cells were seeded in 6-well
plates at a density of 2×103 cells/well and cultured at
37°C for 12–14 days. Once the colonies were visible, cells were
washed twice with PBS, and colonies were fixed with 5%
paraformaldehyde for 20 min at room temperature and stained with
0.1% crystal violet (1 mg/ml) for 20 min. The stained colonies were
counted, and the colony formation rate was calculated using ImageJ
8.0 software (National Institutes of Health).
Wound healing assay
The stably transfected cells were seeded into 6-well
plates at a density of 5×105 cells/well and cultured
until they had reached 90% confluence. The complete medium was
replaced with serum-free medium and the cell monolayer was
scratched with a sterile 100-µl pipette tip. After further culture
for 48 h, the wound gap was observed under an inverted light
microscope (magnification ×100). The extent of wound healing was
calculated using the ImageJ 8.0 software. The wound healing
percentage was calculated using the following formula: (Scratch
healing area)/(original scratch area) ×100%.
Invasion assays
The stably transfected cells were suspended in
serum-free medium at a density of 2×105 cells/200 µl and
seeded into the Matrigel-coated upper chamber (8-µm pore size) of
Transwell inserts in 24-well plates at 37°C for 1 h. the lower
chamber was filled with 600 µl complete medium. Following 48 h of
culture, cells remaining on the upper surface of the filter were
swabbed off using cotton wool, and cells that had invaded the lower
surface were fixed with 5% paraformaldehyde for 20 min at room
temperature and stained with Giemsa for 10 min. Cells were observed
under an inverted light microscope (magnification ×200), and the
number of cells per field was calculated using ImageJ 8.0
software.
Cell cycle and apoptosis analysis
The stably transfected SW620 and RKO cells were
trypsinized (without EDTA) and washed with PBS. For cell cycle
analysis, 105 cells/100 µl cells were fixed with 70%
ethanol at 4°C for 12 h and resuspended in staining buffer
containing 450 µl propidium iodide (PI) and 50 µl RNaseA in the
dark for 30 min at room temperature (cat. no. KGA512; Nanjing
KeyGen Biotech Co., Ltd.) For apoptosis analysis, the Annexin V
APC/7AAD apoptosis kit (cat. no. KGF004; Nanjing KeyGen Biotech
Co., Ltd.) was used to stain for early and late apoptotic cells
according to the manufacturers' instructions. Subsequently, stained
cells were analyzed using a BD FACS-Verse flow cytometer (BD
Biosciences). Cell cycle distribution was obtained using ModFit 3.2
software (Verity Software House, Inc.), and the apoptosis rate was
calculated using FlowJo 7.6.1 software (FlowJo LLC).
Western blotting
Total proteins were extracted from cells and tissues
using the Protein Extraction Kit (cat. no. KGP2100; Nanjing KeyGen
Biotech Co., Ltd.) and quantified with a BCA assay (Nanjing KeyGen
Biotech Co., Ltd. KGPBCA). Proteins (20 ug) were separated by 12%
SDS-PAGE (Fude Biological Technology, Co. Ltd.) and transferred
onto polyvinylidene fluoride membranes (EMD Millipore). The
membranes were blocked with 5% skim milk (Fude Biological
Technology, Co., Ltd.) for 1.5 h at room temperature, and incubated
with primary antibodies against: PI3K (Wanleibio Co., Ltd.; cat.
no. WL03380; 1:1,000), phosphorylated (p-) PI3K (Wanleibio Co.,
Ltd.; cat. no. WL0226; 1:1,000), AKT (Wanleibio Co., Ltd.; cat. no.
WL003b; 1:1,000), p-AKT (Wanleibio Co., Ltd.; cat. no. WLP001a;
1:1,000), BAX (ProteinTech Group, Inc.; cat. no. 50599-2-lg;
1:5,000), BCL2 (ProteinTech Group, Inc.; cat. no. 12789-1-AP;
1:5,000) and GAPDH (Bioworld Technology, Inc.; cat. no. BS60630;
1:10,000) overnight at 4°C. The membranes were then incubated with
goat-anti-rabbit secondary antibody (Fude Biological Technology,
Co. Ltd.; cat. no. FDR007; 1:2,000) for 1 h at room temperature.
The immuno-positive bands were visualized using ECL developer
solution (Fude Biological Technology, Co., Ltd.) and an imaging
system (Bio-Rad Laboratories, Inc.).
Statistical analysis
All statistical analyses were performed using
GraphPad Prism 7.0 (GraphPad Software, Inc.). Data were presented
as the means ± standard deviation of at least three independent
experiments. Differences between two groups were assessed using the
t-test, whereas one-way ANOVA, followed by Newman-Keuls post-hoc
test were used to compare differences between multiple groups
(>2). P<0.05 was considered to indicate a statistically
significant difference.
Results
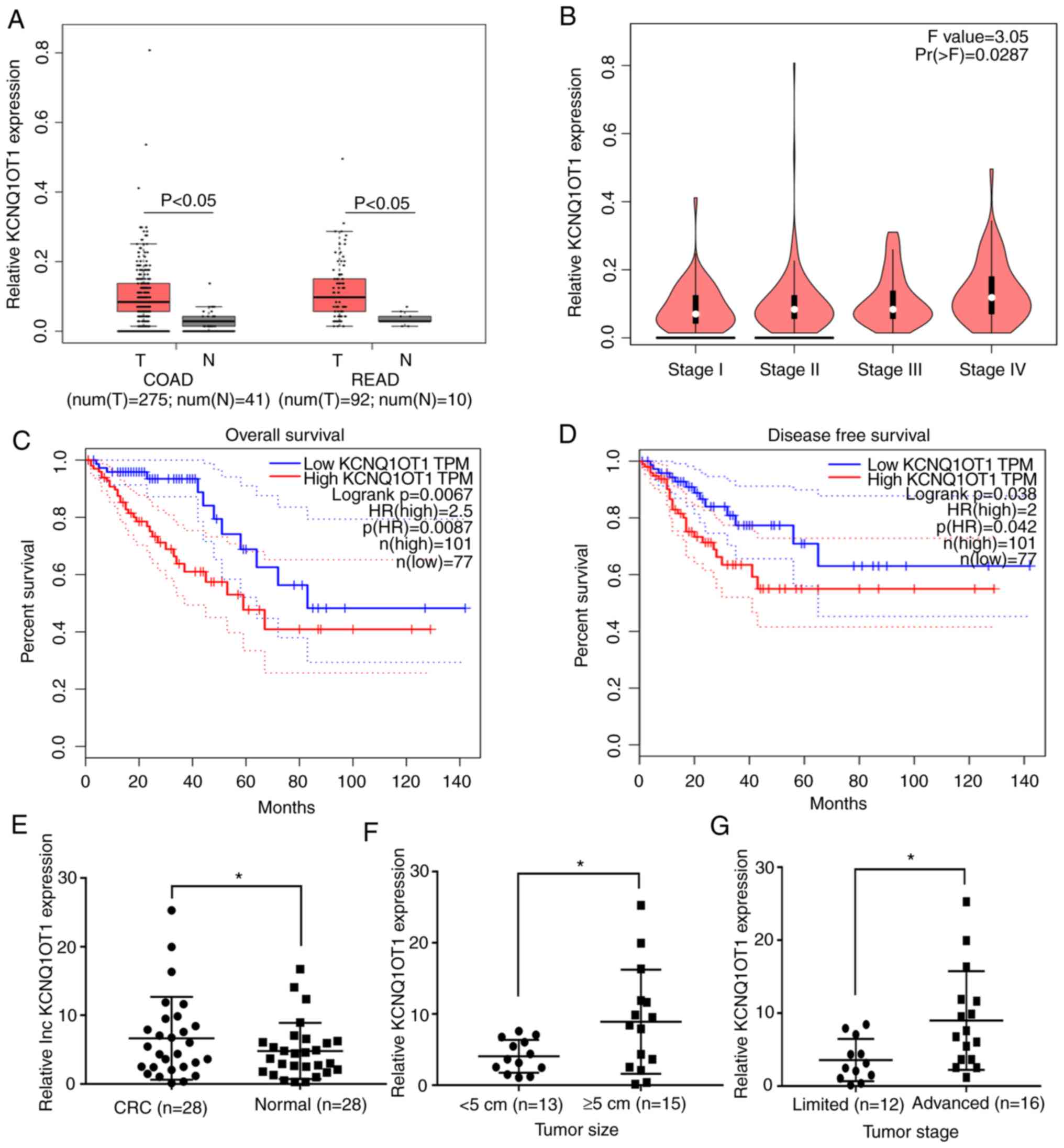
KCNQ1OT1 expression is upregulated in
CRC tissues and is associated with disease progression and poor
survival in patients with CRC
Previous studies have reported that KCNQ1OT1
expression is upregulated in various types of tumor, including
hepatocellular carcinoma, non-small cell lung cancer and tongue
cancer (8,9,15), and
that this is correlated with poor prognosis in patients. In the
present study, GEPIA was used to predict KCNQ1OT1 expression in
CRC. The results from box plot analysis in GEPIA demonstrated that
KCNQ1OT1 expression was significantly higher in tumor tissues
compared with normal tissues in patients with COAD and READ
(P<0.05; Fig. 1A). The results
from stage plot analysis in GEPIA demonstrated that KCNQ1OT1
expression increased with tumor stage progression in patients with
CRC (P=0.0287; Fig. 1B).
Furthermore, results from survival analysis in GEPIA demonstrated
that high KCNQ1OT1 expression in CRC tissues was associated with
lower overall and disease-free survival times (Fig. 1C and D). These predicted results
suggest that KCNQ1OT1 expression may be upregulated in CRC tissues
and may be associated with poor prognosis in patients with CRC.
Subsequently, 28 pairs of CRC and adjacent normal
tissues were collected from patients with CRC, and KCNQ1OT1
expression was examined by RT-qPCR. The results demonstrated that
the KCNQ1OT1 expression level was significantly higher in CRC
tissues compared with adjacent normal tissues (P=0.0142; Fig 1E). Furthermore, KCNQ1OT1 expression
level was significantly higher in CRC tissues obtained from large
tumors (≥5 cm) compared with those obtained from small tumors
(<5 cm; P=0.0302; Fig. 1F). In
addition, the KCNQ1OT1 expression level in CRC tissues obtained
from patients with advanced stage CRC (IIIB+IIIC+IV) was
significantly increased compared with those obtained from patients
with limited stage disease (I+II+IIIA; P=0.0372; Fig. 1G). These findings indicate that
KCNQ1OT1 may be associated with tumor growth and metastasis in
CRC.
KCNQ1OT1 knockdown inhibits CRC cell
proliferation in vitro
To investigate the role of KCNQ1OT1 in CRC growth,
in vitro experiments were performed. Firstly, KCNQ1OT1
expression was assessed by RT-qPCR in five CRC cell lines and the
normal NCM460 intestinal epithelial cell line. The results
demonstrate that KCNQ1OT1 expression level was upregulated in four
out of five CRC cell lines compared with the normal cell line
(Fig. 2A). SW620 and RKO cells were
subsequently selected to construct stably transfected cell lines
using two KCNQ1OT1-specifc shRNAs lentivirus or negative control
lentivirus. The results from RT-qPCR demonstrate that KCNQ1OT1
expression level was efficiently knocked down in the two cell lines
following transfection (Fig. 2B).
Subsequently, cell viability and colony formation assays were
performed to investigate the effect of KCNQ1OT1 on CRC cell
proliferation in vitro. The results demonstrate that
KCNQ1OT1 knockdown significantly decreased SW620 and RKO cell
viability (Fig. 2C and D).
Furthermore, the results of the colony formation assay demonstrated
that KCNQ1OT1 knockdown significantly inhibited clone formation
capacity in SW620 and RKO cells (Fig.
2E). These findings confirm that KCNQ1OT1 knockdown inhibits
CRC cell proliferation.
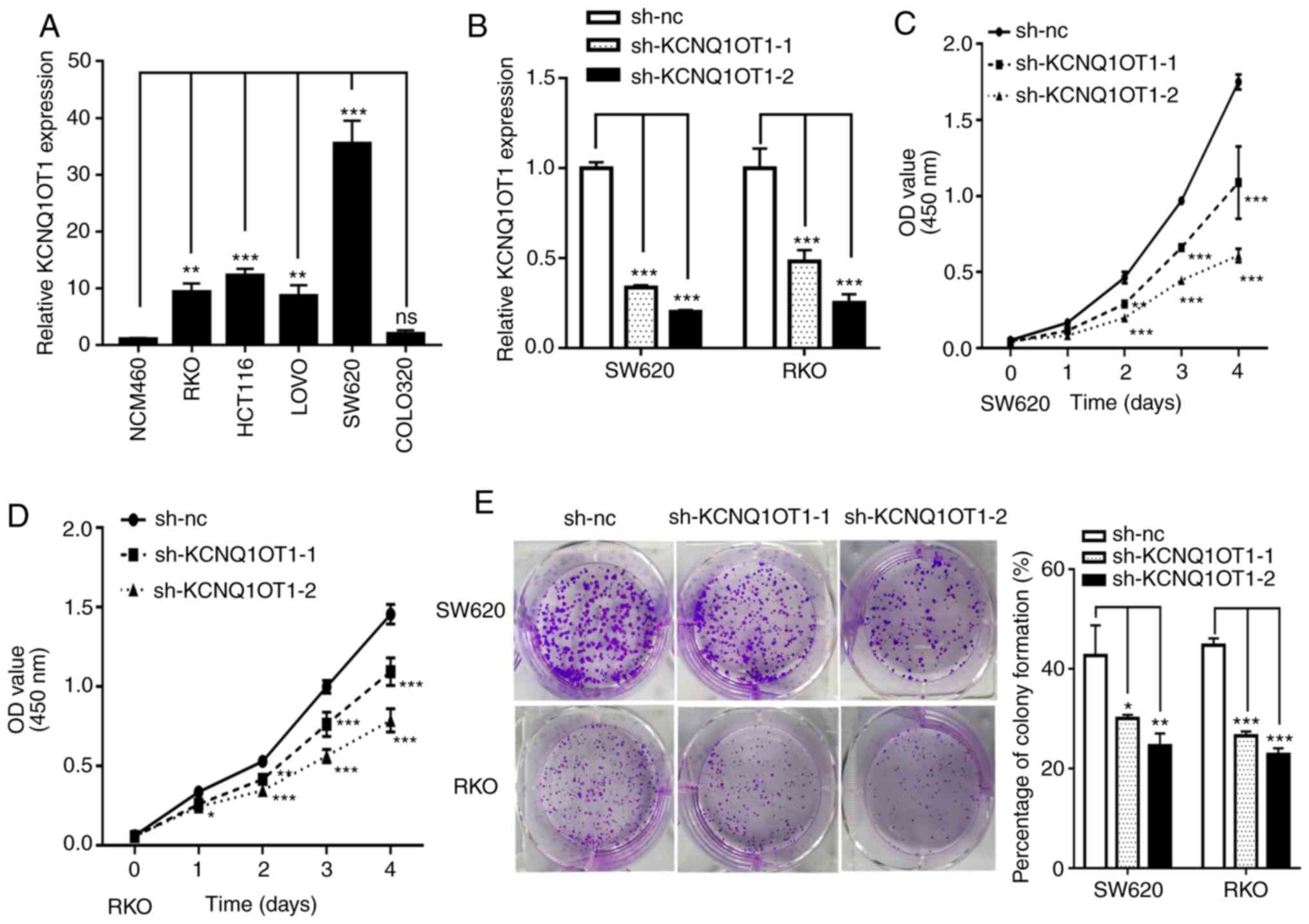 | Figure 2.KCNQ1OT1 knockdown inhibits CRC cell
proliferation in vitro. (A) Relative expression of KCNQ1OT1
in the CRC cell lines SW620, RKO, HCT116, LoVo and colo320, and the
normal intestinal epithelial cell line NCM460. (B) Relative
expression of KCNQ1OT1 in SW620 and RKO cells transfected with
sh-nc, sh-KCNQ1OT1-1 and sh-KCNQ1OT1-2 constructs. Viability of (C)
SW620 and (D) RKO cells following KCNQ1OT1 knockdown. (E) Colony
forming capacity of SW620 and RKO cells following KCNQ1OT1
knockdown. *P<0.05, **P<0.01 and ***P<0.001. CRC,
colorectal cancer; nc, negative control; OD, optical density; Sh,
short hairpin. |
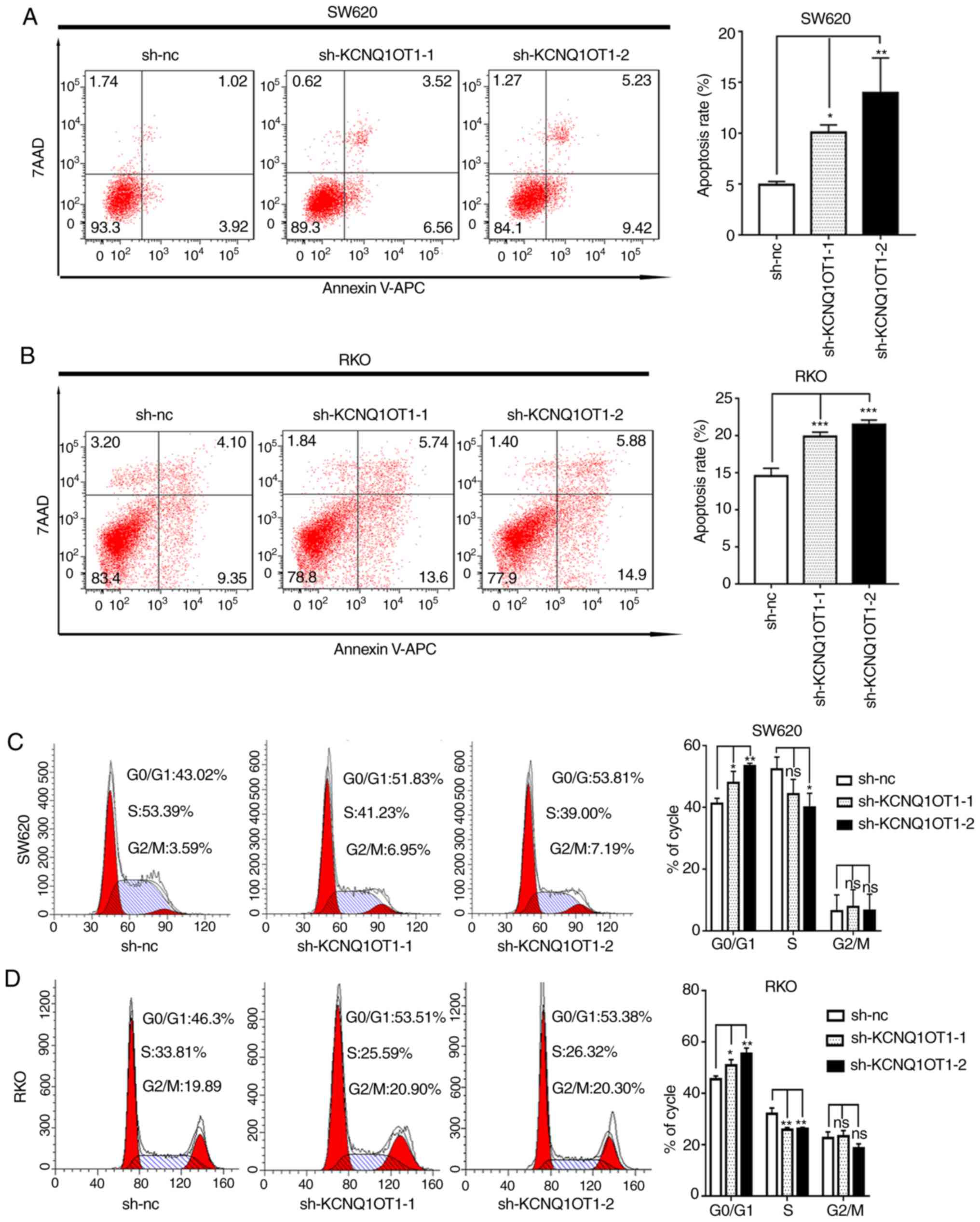
KCNQ1OT1 knockdown stimulates CRC cell
apoptosis and cell cycle arrest
A previous study reported that abnormal cell cycle
and apoptosis are associated with cancer growth (16). To better understand the underlying
mechanism of KCNQ1OT1 in CRC cell proliferation, apoptosis and cell
cycle distribution were evaluated in the present study. The results
demonstrated that the apoptotic rates of KCNQ1OT1 knockdown SW620
and RKO cells were significantly increased compared with the
control cells (Fig. 3A and B).
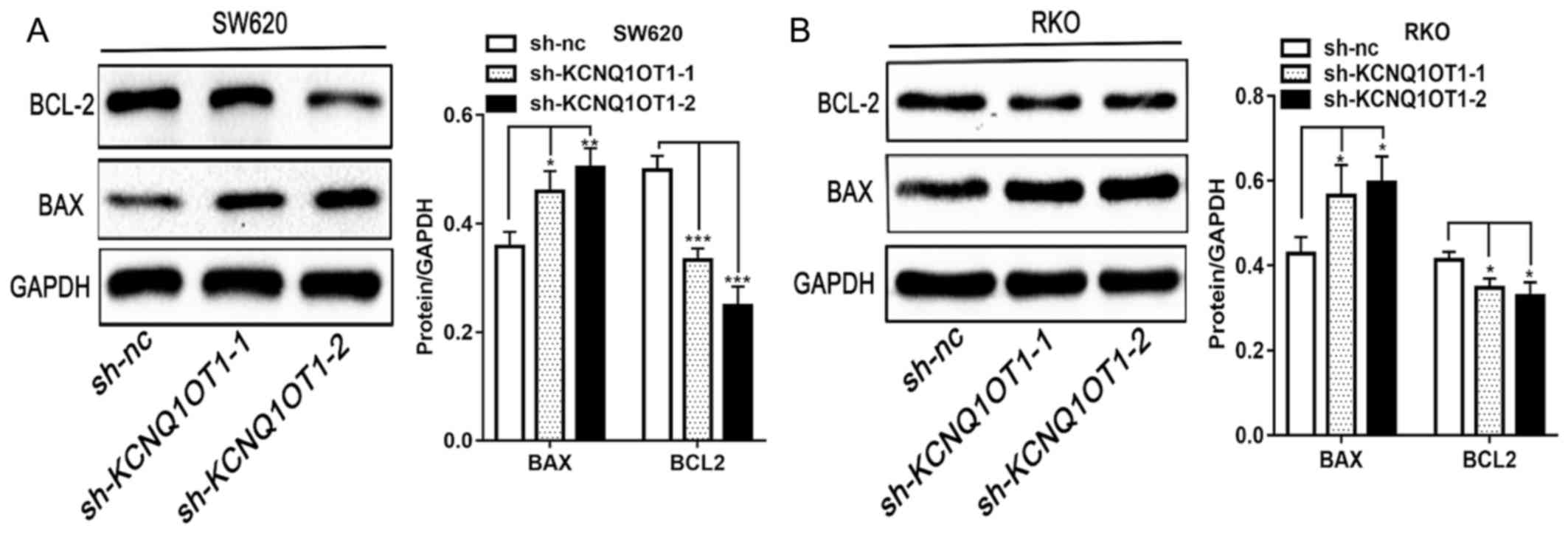
Furthermore, as presented in Fig. 4A and
B, the expression of the pro-apoptotic protein BAX and of the
anti-apoptotic protein BCL-2, in KCNQ1OT1 knockdown CRC cells was
significantly increased and decreased, respectively, compared with
control group. The results from western blotting are therefore
consistent with results from apoptosis detection by flow cytometry.
In addition, the results from cell cycle analysis demonstrated that
KCNQ1OT1 knockdown significantly increased the percentage of cells
in the G0/G1 phase and decreased the
percentage of cells in the S phase in SW620 and RKO cell lines,
compared with control cells (Fig. 3C and
D). These findings suggest that downregulation of KCNQ1OT1 in
CRC cells may induce apoptosis and arrest the cell cycle at the
G0/G1 phase.
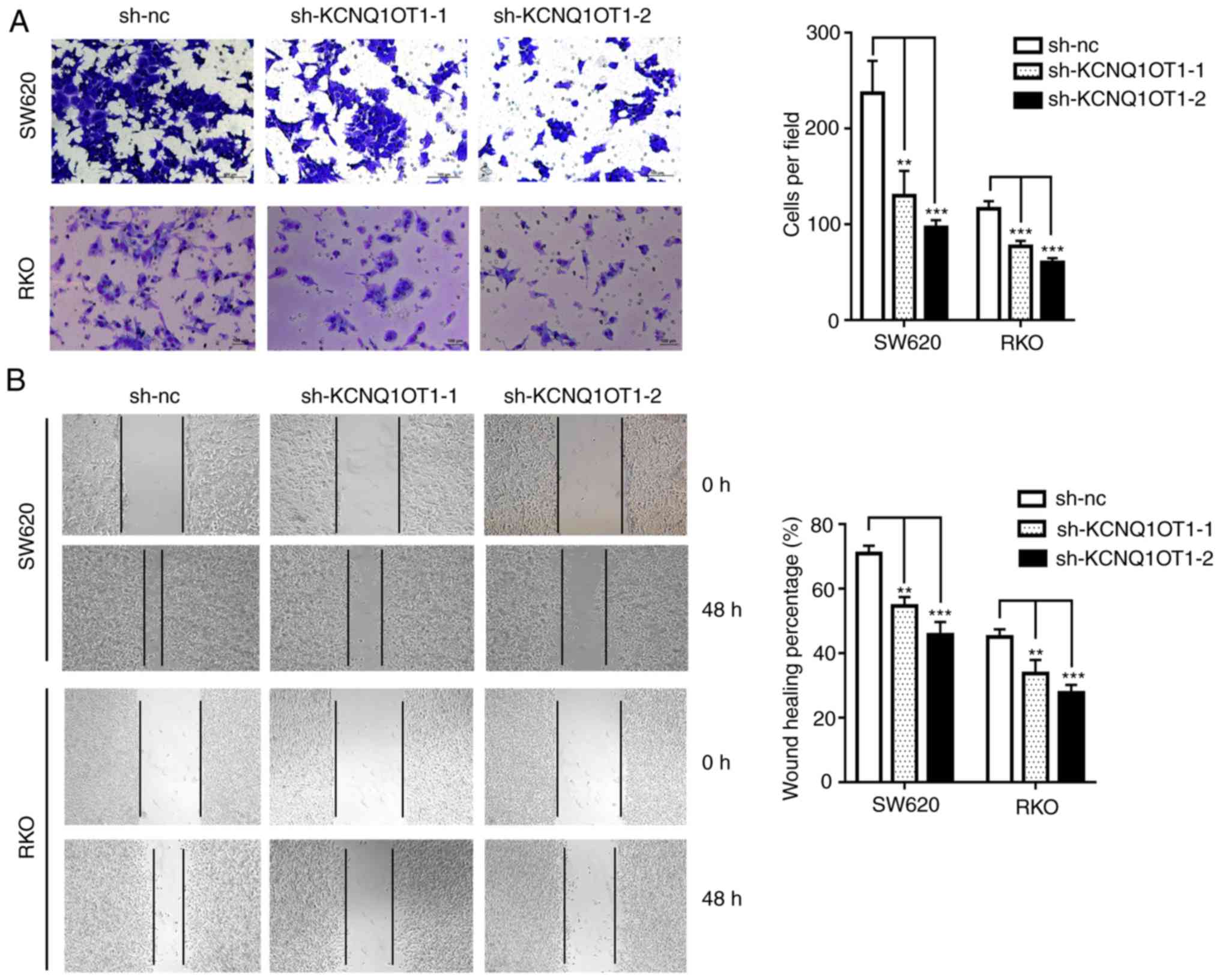
KCNQ1OT1 knockdown inhibits CRC cell
migratory and invasive abilities
In order to investigate the biological function of
KCNQ1OT1 in CRC cell invasiveness, a Transwell assay was performed.
The results demonstrate that the invasive ability of KCNQ1OT1
knockdown cells was significantly decreased compared with control
cells (Fig. 5A). Similarly, the
results from the wound healing assay demonstrated that silencing
KCNQ1OT1 in SW620 or RKO cells significantly decreased the scratch
healing ability (Fig. 5B). These
results indicate that KCNQ1OT1 knockdown may decrease CRC cell
invasion in vitro.
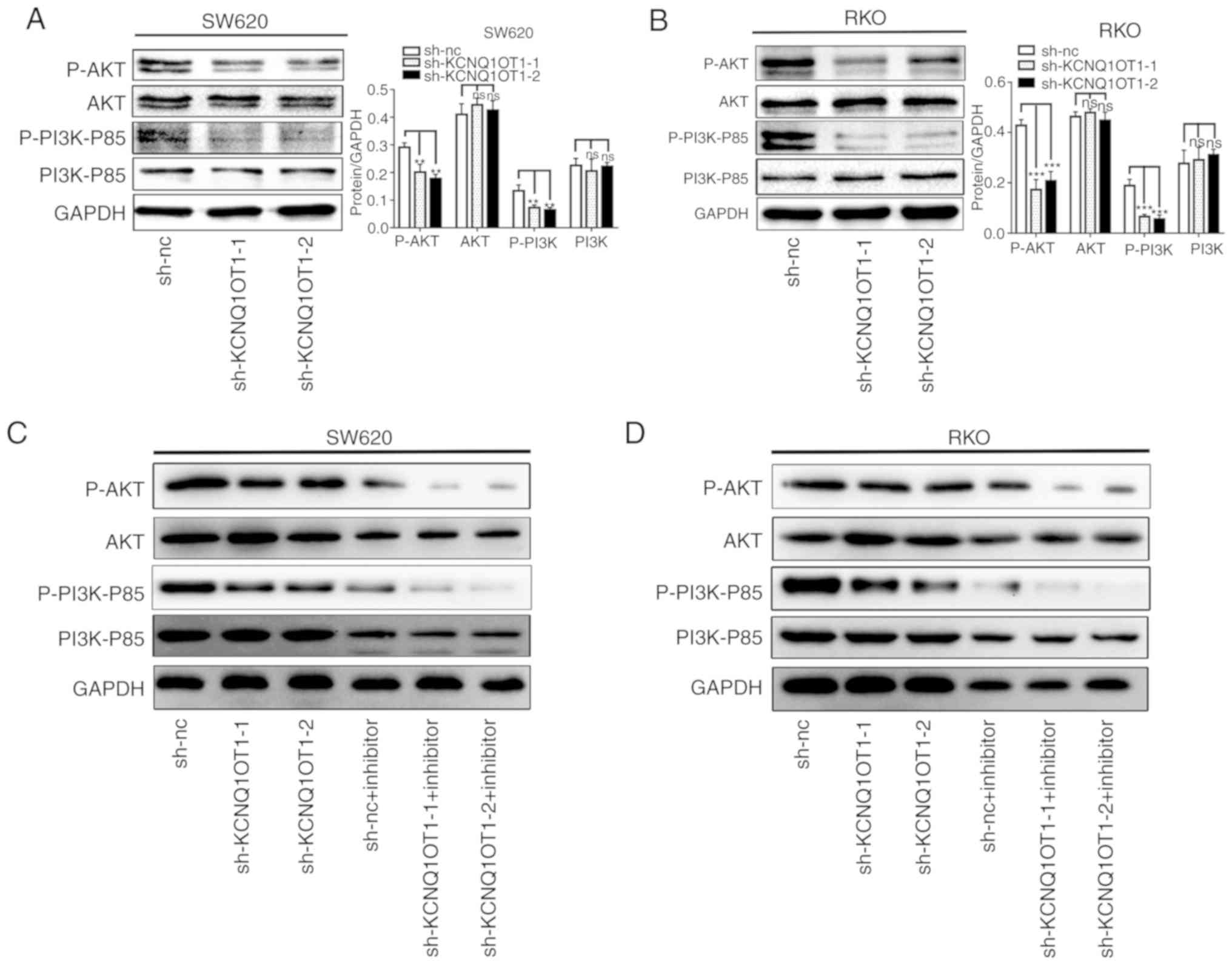
KCNQ1OT1 knockdown inhibits the
activation of the PI3K/AKT signaling pathway
Previous studies have reported that the PI3K/AKT
pathway is one of the most commonly altered pathways in human
cancer that is associated with the regulation of tumor growth and
metastasis (17,18). In order to identify the underlying
mechanism of KCNQ1OT1 in CRC progression, the PI3K/AKT pathway was
investigated. To do so, the expression of proteins from the
PI3K/AKT pathway was analyzed by western blotting in CRC cells
transfected with sh-KCNQ1OT1 or sh-nc. The results demonstrate that
KCNQ1OT1 knockdown inactivates the PI3K/AKT pathway, as evidenced
by the decreased expression of p-PI3K and p-AKT in SW620 and RKO
cell lines (Fig. 6A and B). In
addition, following CRC cell treatment with a PI3K inhibitor, the
level of p-PI3K and p-AKT was decreased compared with non-treated
cells (Fig. 6C and D). The
inhibition experiment was successful, confirming that KCNQ1OT1
inhibited the proliferation and invasion of colorectal cancer by
inhibiting the PI3K signaling pathway. These data suggest that
KCNQ1OT1 knockdown may inhibit PI3K/AKT signaling pathway
activation.
Discussion
Numerous studies have demonstrated that lncRNAs
serve crucial roles in malignant tumor progression, including CRC
(5,19). For example, Chen et al
(20) reported that lncRNA XIST is
significantly overexpressed in tumors from patients with CRC and is
associated with poor overall survival. Furthermore, it was
demonstrated that XIST can regulate CRC progression and metastasis
by competing with the micro-RNA miR-200b for the regulation of zinc
finger E-box binding homeobox 1 expression (20). In addition, Han et al
(21) reported that lncRNA CRNDE
could promote chemoresistance and CRC cell proliferation by
regulating the expression levels of miR-181a-5p and the activity of
the Wnt/β-catenin signaling pathway. A previous study reported that
β-catenin could directly target KCNQ1OT1 to promote CRC development
(22). However, the role and
underlying mechanism of KCNQ1OT1 in CRC remains unclear.
The present study demonstrated that KCNQ1OT1 is
highly expressed in CRC tissues compared with normal tissues, and
that high KCNQ1OT1 expression in CRC tissues is associated with
poor survival according to GEPIA predictions. These results were
similar to those observed in 28 CRC tissue samples, in which
KCNQ1OT1 was upregulated compared with adjacent normal tissues.
Furthermore, KCNQ1OT1 expression was significantly associated with
tumor size (<5 cm vs. ≥5 cm) and clinical stage (limited vs.
advanced). These results suggest that high KCNQ1OT1 expression may
be associated with the malignant progression of CRC. Previous
research demonstrates that KCNQ1OT1 overexpression serves a crucial
role in various types of tumor. For example, it was reported that
KCNQ1OT1 is upregulated in chemoresistant tongue squamous cell
carcinoma tissues compared with chemosensitive tissues, and that
high KNCQ1OT1 expression is positively correlated with poor
prognosis in patients (8). Another
study demonstrated that KCNQ1OT1 is highly expressed and promotes
tumor growth and metastasis in cholangiocarcinoma via
miR-140-5p/SRY-box transcription factor 4 axis (23). It was demonstrated that KCNQ1OT1 is
upregulated in patients with hepatocellular carcinoma (9), breast cancer (10), non-small-cell lung cancer (15) and glioma (24), and that it promotes malignant tumor
progression. The results from these studies indicate that KCNQ1OT1
may serve an oncogenic role in various types of cancer.
The present study demonstrated that KCNQ1OT1
knockdown inhibited the proliferation and migratory and invasive
abilities of CRC cells, which was consistent with previous studies
on cholangiocarcinoma and hepatocellular carcinoma (9,23).
Numerous studies have reported that the cell cycle and apoptosis
are associated with cancer cell proliferation and tumor growth
(16,25,26).
Subsequently, KCNQ1OT1 was knocked down in the present study and
the cell cycle and apoptosis were evaluated. The results
demonstrated that KCNQ1OT1 knockdown promoted apoptosis and cell
cycle arrest in CRC cells. These findings indicate that KCNQ1OT1
may regulate CRC cell proliferation, migratory and invasive
abilities, cell cycle and apoptosis in order to promote tumor
growth and metastasis.
The PI3K/AKT signaling pathway serves an essential
role in the growth and metastasis of multiple tumors. For example,
lncRNA ABHD11-AS1 promotes tumor progression by regulating PI3K/AKT
signaling in papillary thyroid carcinoma (27). In addition, lncRNA SNHG7 facilitates
CRC cell proliferation and metastasis via the PI3K/AKT/mTOR
signaling pathway, and by regulating GALNT7 expression via miR-34a
sponging (28). Following PI3K/AKT
pathway inactivation, G1/S transition, proliferation and
migratory and invasive abilities of cancer cells are inhibited,
whereas apoptosis is stimulated (17,28). The
results from the present study demonstrate that KCNQ1OT1 knockdown
decreases the protein expression of p-PI3K and p-AKT. These
findings suggest that CRC cell proliferation and metastasis may be
regulated by KCNQ1OT1 via the PI3K/AKT signaling pathway.
In conclusion, the present study demonstrated that
KCNQ1OT1 expression was significantly increased in CRC cells and
tissues compared with normal cells and tissues. Furthermore,
KCNQ1OT1 knockdown inhibited CRC cell proliferation and migratory
and invasive ability, promoted CRC cell apoptosis and arrested the
cell cycle via PI3K/AKT signal pathway inactivation. These findings
indicate that KCNQ1OT1 may be considered as a potential prognostic
marker and therapeutic target for CRC. However, the study had
several limitations. For example, the effect of KCNQ1OT1
overexpression in CRC cells in vitro or its function in
vivo, were not investigated. Future studies will further
explore the underlying mechanisms of KCNQ1OT1 in CRC, in
particular, its potential role as a competitive endogenous RNA.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Guangdong Province, China (grant no.
2017A030313533).
Availability of data and materials
The data generated during the study are included in
this article.
Authors' contributions
JY and CC designed the present study. QD and LC
conducted the main experiments and drafted the initial manuscript.
KZ conducted some minor experiments in this study. RH, CW and LX
analyzed the bioinformatics databases and assessed the expression
levels in clinical tissues. ZZ and XY analyzed the data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Zhujiang Hospital, Southern Medical University
(approval no. 2018-PTWK-005). All patients signed informed consent
prior to the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li Y, Liang L, Dai W, Cai G, Xu Y, Li X,
Li Q and Cai S: Prognostic impact of programed cell death-1 (PD-1)
and PD-ligand 1 (PD-L1) expression in cancer cells and tumor
infiltrating lymphocytes in colorectal cancer. Mol Cancer.
15:552016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Markowitz SD and Bertagnolli MM: Molecular
origins of cancer: Molecular basis of colorectal cancer. N Engl J
Med. 361:2449–2460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
ENCODE Project Consortium, . An integrated
encyclopedia of DNA elements in the human genome. Nature.
489:57–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang KC and Chang HY: Molecular mechanisms
of long noncoding RNAs. Mol Cell. 43:904–914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schmitz SU, Grote P and Herrmann BG:
Mechanisms of long noncoding RNA function in development and
disease. Cell Mol Life Sci. 73:2491–2509. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang S, Ma H, Zhang D, Xie S, Wang W, Li
Q, Lin Z and Wang Y: LncRNA KCNQ1OT1 regulates proliferation and
cisplatin resistance in tongue cancer via miR-211-5p mediated
Ezrin/Fak/Src signaling. Cell Death Dis. 9:7422018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li C, Miao R, Zhang J, Qu K and Liu C:
Long non-coding RNA KCNQ1OT1 mediates the growth of hepatocellular
carcinoma by functioning as a competing endogenous RNA of miR-504.
Int J Oncol. 56:857–858. 2020.PubMed/NCBI
|
|
10
|
Feng W, Wang C, Liang C, Yang H, Chen D,
Yu X, Zhao W, Geng D, Li S, Chen Z and Sun M: The dysregulated
expression of KCNQ1OT1 and Its interaction with downstream factors
miR-145/CCNE2 in breast cancer cells. Cell Physiol Biochem.
49:432–446. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
NCCN Clinical Practice Guidelines in
Oncology (NCCN Guidelines®)-Colon Cancer v1.2019. March
15–2019
|
|
12
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Kuang H, Xue J, Liao L, Yin F and
Zhou X: LncRNA AB073614 regulates proliferation and metastasis of
colorectal cancer cells via the PI3K/AKT signaling pathway. Biomed
Pharmacother. 93:1230–1237. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dong Z, Yang P, Qiu X, Liang S, Guan B,
Yang H, Li F, Sun L, Liu H, Zou G and Zhao K: KCNQ1OT1 facilitates
progression of non-small-cell lung carcinoma via modulating
miRNA-27b-3p/HSP90AA1 axis. J Cell Physiol. 234:11304–11314. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang JL, Cao SW, Ou QS, Yang B, Zheng SH,
Tang J, Chen J, Hu YW, Zheng L and Wang Q: The long non-coding RNA
PTTG3P promotes cell growth and metastasis via up-regulating PTTG1
and activating PI3K/AKT signaling in hepatocellular carcinoma. Mol
Cancer. 17:932018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lien EC, Dibble CC and Toker A: PI3K
signaling in cancer: Beyond AKT. Curr Opin Cell Biol. 45:62–71.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schmitt AM and Chang HY: Long noncoding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen DL, Chen LZ, Lu YX, Zhang DS, Zeng
ZL, Pan ZZ, Huang P, Wang FH, Li YH, Ju HQ and Xu RH: Long
noncoding RNA XIST expedites metastasis and modulates
epithelial-mesenchymal transition in colorectal cancer. Cell Death
Dis. 8:e30112017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han P, Li JW, Zhang BM, Lv JC, Li YM, Gu
XY, Yu ZW, Jia YH, Bai XF, Li L, et al: The lncRNA CRNDE promotes
colorectal cancer cell proliferation and chemoresistance via
miR-181a-5p-mediated regulation of Wnt/β-catenin signaling. Mol
Cancer. 16:92017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sunamura N, Ohira T, Kataoka M, Inaoka D,
Tanabe H, Nakayama Y, Oshimura M and Kugoh H: Regulation of
functional KCNQ1OT1 lncRNA by β-catenin. Sci Rep. 6:206902016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun H, Li Y, Kong H, Dai S and Qian H:
Dysregulation of KCNQ1OT1 promotes cholangiocarcinoma progression
via miR-140-5p/SOX4 axis. Arch Biochem Biophys. 658:7–15. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gong W, Zheng J, Liu X, Liu Y, Guo J, Gao
Y, Tao W, Chen J, Li Z, Ma J and Xue Y: Knockdown of long
non-coding RNA KCNQ1OT1 restrained glioma cells' malignancy by
activating miR-370/CCNE2 axis. Front Cell Neurosci. 11:842017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang M, Wang H, Hu X and Cao X: lncRNA
MALAT1 binds chromatin remodeling subunit BRG1 to epigenetically
promote inflammation-related hepatocellular carcinoma progression.
Oncoimmunology. 8:e15186282018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Z and Qin B: Prognostic and
clinicopathological significance of long noncoding RNA CTD-2510F5.4
in gastric cancer. Gastric Cancer. 22:692–704. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wen J, Wang H, Dong T, Gan P, Fang H, Wu
S, Li J, Zhang Y, Du R and Zhu Q: STAT3-induced upregulation of
lncRNA ABHD11-AS1 promotes tumour progression in papillary thyroid
carcinoma by regulating miR-1301-3p/STAT3 axis and PI3K/AKT
signalling pathway. Cell Prolif. 52:e125692019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Y, Zeng C, Hu J, Pan Y, Shan Y, Liu B
and Jia L: Long non-coding RNA-SNHG7 acts as a target of miR-34a to
increase GALNT7 level and regulate PI3K/Akt/mTOR pathway in
colorectal cancer progression. J Hematol Oncol. 11:892018.
View Article : Google Scholar : PubMed/NCBI
|