Introduction
Papillary thyroid carcinoma (PTC) is the most common
pathological type of thyroid carcinoma, accounting for
approximately 70–80% of all cases diagnosed (1,2). Thyroid
carcinomas occur mostly in women with an incidence of 1/100,000
individuals, and this number is still rising (3). A consensus has not yet been reached in
terms of the pathogenesis of PTC. Studies have shown that the
occurrence of PTC may be associated with life style, genetic
factors, ionizing radiation exposure, iodine intake, endocrine
imbalance and obesity (4–6). Some researchers believed that a variety
of oncogenes, as well as tumor-suppressor genes, are involved in
the progression of PTC (7). In
recent years, it was found that long non-coding RNAs (lncRNAs) are
abnormally expressed in cancers, suggesting that lncRNAs may be
involved in cancer onset and progression. HOX transcript antisense
RNA (HOTAIR) is the first example of an lncRNA that has
trans-regulatory function (8). According to the literature, HOTAIR is
closely associated with the onset of cancers such as breast cancer,
liver cancer, colorectal cancer, laryngeal cancer and
nasopharyngeal carcinoma (9–13). Zhu et al reported that HOTAIR
single nucleotide polymorphisms (SNPs) are associated with the risk
of PTC, and one of the SNPs was found to be a variant susceptible
to PTC, which was identified only in women (14). In another study conducted through
database analysis, Li et al reported that HOTAIR is
overexpressed in PTC tissues, and patients with higher HOTAIR
expression exhibit poorer prognosis in general (15).
PTC is a type of thyroid cancer with a relatively
good patient prognosis. Cervical lymph node metastases often occur
at the early stage of PTC, presenting in 20–50% of all PTC patients
(16). Diagnosis of lymph node
metastasis often depends upon postoperative pathological tests due
to the limitations of ultrasonography, resulting in a lack of
specificity (17,18). Lack of accurate preoperative
diagnosis of lymph node metastasis makes it difficult to decide
whether a lymph node dissection is needed and where the dissection
area is located. In the present study, PTC patients were divided
into a metastasis-negative group and a metastasis-positive group
based on ultrasound findings. The expression level of lncRNA-HOTAIR
in serum was determined, and its role in regulating the PTC cell
line TPC-1 was explored, to reveal the possible pathogenesis of PTC
with lymph node metastasis.
Materials and methods
Reagents
The following reagents were purchased from
commercial sources: RPMI-1640 medium and fetal bovine serum (FBS)
from Gibco; Thermo Fisher Scientific, Inc. TRIzol reagent,
Lipofectamine 2000 reagent and Opti-MEM medium were purchased from
Invitrogen; Thermo Fisher Scientific, Inc. Fluorescent dyes for
quantitative PCR were obtained from Bio-Rad Laboratories, Inc. A
reverse transcription kit was obtained from Toyobo Co., Ltd. and a
BCA kit (cat. no. P0009) was from Beyotime Biotechnology.
Monoclonal antibodies for E-cadherin (cat. no. 3195), vimentin
(cat. no. 5741), β-catenin (cat. no. 8480), and Wnt inhibitory
factor 1 (WIF1, cat. no. 2064) were purchased from Cell Signaling
Technology, Inc. Internal reference β-actin antibody (cat. no.
20536-1-AP) and horseradish peroxidase (HRP)-labeled goat
anti-rabbit secondary antibody (cat. no. SA00001-2) were from
Proteintech Group, Inc. and the siRNA was obtained from
RiboBio.
Subjects
A total of 89 patients with PTC who were admitted to
Beijing Geriatric Hospital from February 2018 to March 2019 were
recruited in this study. Patients who met the following criteria
were eligible for this study: i) patients who were diagnosed with
PTC regardless of cervical lymph node metastasis through
preoperative color Doppler ultrasound examination; ii) patients who
had surgical indications; iii) patients who were confirmed to have
PTC by postoperative pathological tests. Patients who presented
with the following criteria were excluded from this study: i)
patients who had other conditions such as cardiovascular and
cerebrovascular diseases, endocrine diseases, genetic diseases, and
other cancers; and ii) patients who had previous history of
chemotherapy, radiotherapy and surgery. All patients were informed
of the study and signed informed consent forms. This study was
approved by the Medical Ethics Committee of Beijing Geriatric
Hospital (Beijing, China). Peripheral blood was collected before
surgery, and thyroid cancer tissues were collected after surgery
for pathological examination to confirm the diagnosis of PTC.
Ultrasonography
Color Doppler ultrasonography was performed before
surgery on a GE Logiq P5 ultrasound machine equipped with a 7.5–10
MHz frequency probe (General Electric Co.). The patient was placed
in the supine position with the neck fully exposed. The
sonographers were doctors who had been engaged in color Doppler
ultrasound examination for more than 5 years and had extensive
experience in thyroid ultrasound examination. Lymph nodes in areas
covering the bilateral thyroid gland, isthmus and cervix were
carefully checked for size, morphology, location, internal echo,
blood flow and calcification. After evaluating the sonographic
findings, those lymph nodes presenting two or more of the following
ultrasound signs were regarded as metastasis-positive (18): i) longest diameter of the central
lymph node >5 mm and longest diameter of the lateral lymph node
>8 mm; ii) longitudinal nodal diameter to transverse diameter
ratio (L/T) <2; iii) eccentric or absent hilum; and iv)
hyperechoic mass with hypoechoic rim accompanied by cystic
formation and calcification. The subjects were divided into a
metastasis-positive group and a metastasis-negative group based on
the ultrasonographic findings and metastatic assessment. There were
36 patients allocated to the metastasis-positive group with a mean
age of 47.8±4.9 years. There were 53 patients allocated to the
metastasis-negative group with a mean age of 48.3±5.8 years.
Whole blood total RNA extraction and
quantitative fluorescent PCR
Whole blood total RNA was extracted using the
RNAprep Pure high-efficiency blood total RNA extraction kit (cat.
no. DP443, Tiangen Biotech) in accordance with the kit user manual.
The obtained RNA concentration was measured using a
spectrophotometer. Complementary DNA (cDNA) was synthesized from 1
µg of RNA via reverse transcription in accordance with the manual
provided in the reverse transcription kit. U6 was used as
the internal reference gene. The primer sequences were as follows:
U6 forward, GACAAGCCCTACCTACAG, and U6 reverse,
GATGATACAGTACAAGTCGC; HOTAIR forward, GGAGTGAGTCCCATCCATCT, and
HOTAIR reverse, TGCCCAATGGTACTACAGAAGAT. The PCR amplification
reaction was performed as follows: Pre-denaturation at 95°C for 5
min, 40 cycles of 95°C for 5 sec (denaturation), 60°C for 30 sec
(annealing), and 72°C for 30 sec (extension), followed by extension
at 72°C for 5 min at the end of the cycles. All samples were run in
triplicate. The relative expression level of the target gene was
calculated using the 2−ΔΔCt method (19), where ΔCt=Cttarget
gene-Ctinternal reference and
ΔΔCt=ΔCtexperimental group-ΔCtcontrol
group.
PTC cell culture
The PTC cell lines TPC-1, HTori-3 and FTC-133 were
purchased from the US ATCC Cell Bank. The cells were cultured in
RPMI-1640 medium supplemented with 10% FBS in a humidified
incubator supplied with 5% CO2 and maintained at 37°C.
The medium was changed every other day, and the cells were passaged
at about 80–90% confluency.
HOTAIR knockdown using siRNA
Cells were digested with 0.25% trypsin at about
80–90% confluency. The cells were seeded in 6-well plates at
3×105 cells/well, and randomly divided into a control
group, an siRNA group and an siRNA+LiCl group. Cell transfection
was performed when the cells were approximately 30–50% confluent.
Cells in the control group were transfected with a nonsense
sequence, and cells in the siRNA group were transfected with the
siHOTAIR sequence. The transfection protocol is briefly described
below. Ten microliters of siRNA (10 nM) was added to 250 µl of
Opti-MEM medium. Lipofectamine 2000 (5 µl) was added to another 250
µl of Opti-MEM medium. After incubation at room temperature for 5
min, the two solutions were combined, mixed and incubated for
another 5 min. This transfection mixture was added to the cells in
the 6-well plates. After 4–6 h of incubation, the medium was
replaced with complete medium. The group with LiCl was incubated
with 20 mM LiCl and siRNA for 24 h. LiCl can activate the
Wnt/β-catenin pathway and subsequent experiments were carried out
72 h after transfection. The forward and reverse sequences of the
nonsense sequence were 5′-UUCUCCGAACGUGUCACGUTT-3′ and
5′-ACGUGACACGUUCGGAGAATT-3′, respectively. The forward and reverse
sequences of the siHOTAIR sequence were: 5′-GCACAGAGAAAUGGCAAAUU-3′
and 5′-UUUGCCAUUAUCUCUGUGCUU-3′, respectively.
MTT assay
The transfected TPC-1 cells were digested and seeded
in a 96-well plate at 3×103 cells/200 µl/well. Six
replicate wells were set for each group. The cells were cultured
for 5 consecutive days, during which cell proliferation was
measured each day in accordance with a protocol described below.
Twenty microliters of MTT (5 mg/ml) was added to each well,
followed by incubation at 37°C for 4 h. The medium was aspirated
carefully and replaced with 150 µl of DMSO. The plate was shaken in
the dark for 10 min at room temperature, followed by measurement of
the absorbance in each well at a wavelength of 450 nm using a
microplate reader. A cell proliferation curve was plotted using
days as the abscissa and the absorbance at 450 nm as the
ordinate.
Cell scratch assay
TPC-1 cells were seeded in 6-well plates at
3×105 cells/well. When the cells were 100% confluent,
the medium was removed and replaced with serum-free medium. After
starvation for 24 h, at least three even scratches were created in
the bottom of each well using a pipette tip in a perpendicular way.
The plate was rinsed three times with PBS to remove detached cells,
followed by adding serum-free medium and incubation for 24 h.
Images were captured at 0 and 24 h under an inverted microscope at
10×10 times to measure the widths of the scratches. Cell migration
rate=(Scratch width at 0 h-Scratch width at 24 h)/Scratch width at
0 h.
Transwell assay
TPC-1 cells were seeded in Transwell chambers with
an 8.0-µm pore membrane at 1×104 cells/300 µl/well. When
the cells were attached, the medium was switched to serum-free
medium. After starvation for 24 h, the Transwell chambers were
inserted into a 24-well plate containing 600 µl serum-free medium
per well. After incubation for 24 h, a cotton swab was used to
gently rub the area inside the chamber to remove non-migrated
cells. The migrated cells were fixed by immersing the chambers in
4% paraformaldehyde for 10 min. The fixed cells were stained with
0.1% crystal violet, followed by washing under running water to
rinse off the excessive dye and drying at room temperature. Images
were captured and the migrated cells were counted under an inverted
microscope at 10×40 times.
Western blot assay
After digestion, the cells were centrifuged at 3,445
× g for 5 min, followed by removal of the supernatant. Two hundred
microliters of RIPA lysis buffer and 4 µl of protease inhibitor
were added to the cell pellet, followed by sonication on ice for 5
min. After complete lysis, the mixture was centrifuged at 12,000 ×
g for 15 min in a low temperature centrifuge. The supernatant was
collected, and 10 µl was used for total protein concentration
measurement using the BCA assay. Loading buffer 5X was added to the
remaining supernatant, followed by boiling at 100°C for 10 min.
Equal amounts of 30 µg total proteins from the different samples
were loaded on gels containing 5% stacking gel and 10% separation
gel for electrophoretic analysis. The gels were run at a constant
voltage of 80 V until bromophenol blue entered the stacking gel
with minimal distortion of the bands, when the voltage was changed
to 120 V until the target bands were separated. The protein bands
were transferred from gel to PVDF membranes by a wet transfer
method under a constant current of 275 mA for 80 min. After the
membrane was blocked with 5% milk at room temperature for 2 h, the
corresponding diluted primary antibody (dilution factor, 1:1,000)
was added, including antibodies for E-cadherin, vimentin, β-catenin
and Wnt inhibitory factor 1 (WIF1). After incubation at 4°C
overnight and subsequent washing, the HRP-labeled goat anti-rabbit
secondary antibody supplemented with 2% milk (dilution factor:
1:10,000) was added, followed by incubation at room temperature for
1 h. After development, the images were analyzed using Image J
software (1.52a) (National Institutes of Health, Bethesda, MD, USA)
for gray values of the bands. β-actin was used as an internal
reference. The ratio of the gray value of the target protein to the
gray value of β-actin was regarded as the expression level of that
protein.
Statistical analysis
SPSS 23 software (IBM Corp.) was used for
statistical analysis. Each test was repeated more than three times.
Data are expressed as mean ± standard deviation. The unpaired
t-test was used for PCR. Other data were firstly analyzed by
one-way ANOVA to determine the overall difference, and then the
Bonferroni pairwise comparison was performed. P<0.05 was
indicative of a statistically significant difference.
Results
Serum expression of lncRNA-HOTAIR in
lymph node metastasis-positive patients
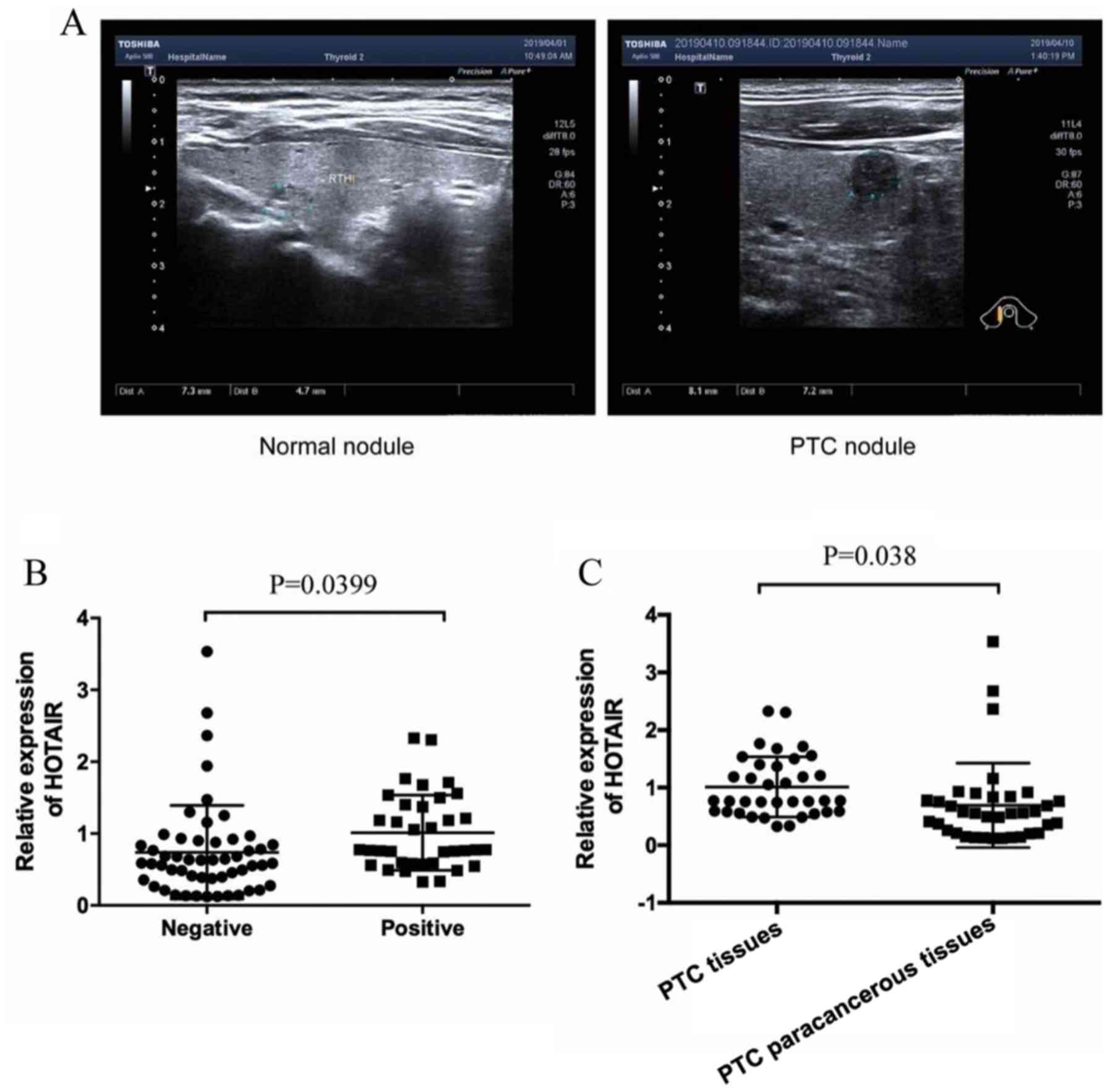
Ultrasonograms of PTC nodules and normal thyroid
nodules are shown in Fig. 1A.
Serum levels of lncRNA-HOTAIR in both
metastasis-positive and metastasis-negative PTC patients were
determined. As shown in Fig. 1B, the
relative expression level of lncRNA-HOTAIR in the lymph node
metastasis-positive group was 1.01±0.52 and 0.74±0.65 in the
metastasis-negative group, The difference was statistically
significant, P=0.0399. The relative expression of lncRNA-HOTAIR in
lymph node metastasis-positive PTC tumor tissues was significantly
higher than that in the PTC paracancerous tissues (0.69±0.73),
HOTAIR was significantly overexpressed in PTC tissues (P=0.038) and
the difference was statistically significant as shown in Fig. 1C.
Modulating effect of HOTAIR on PTC
cell proliferation
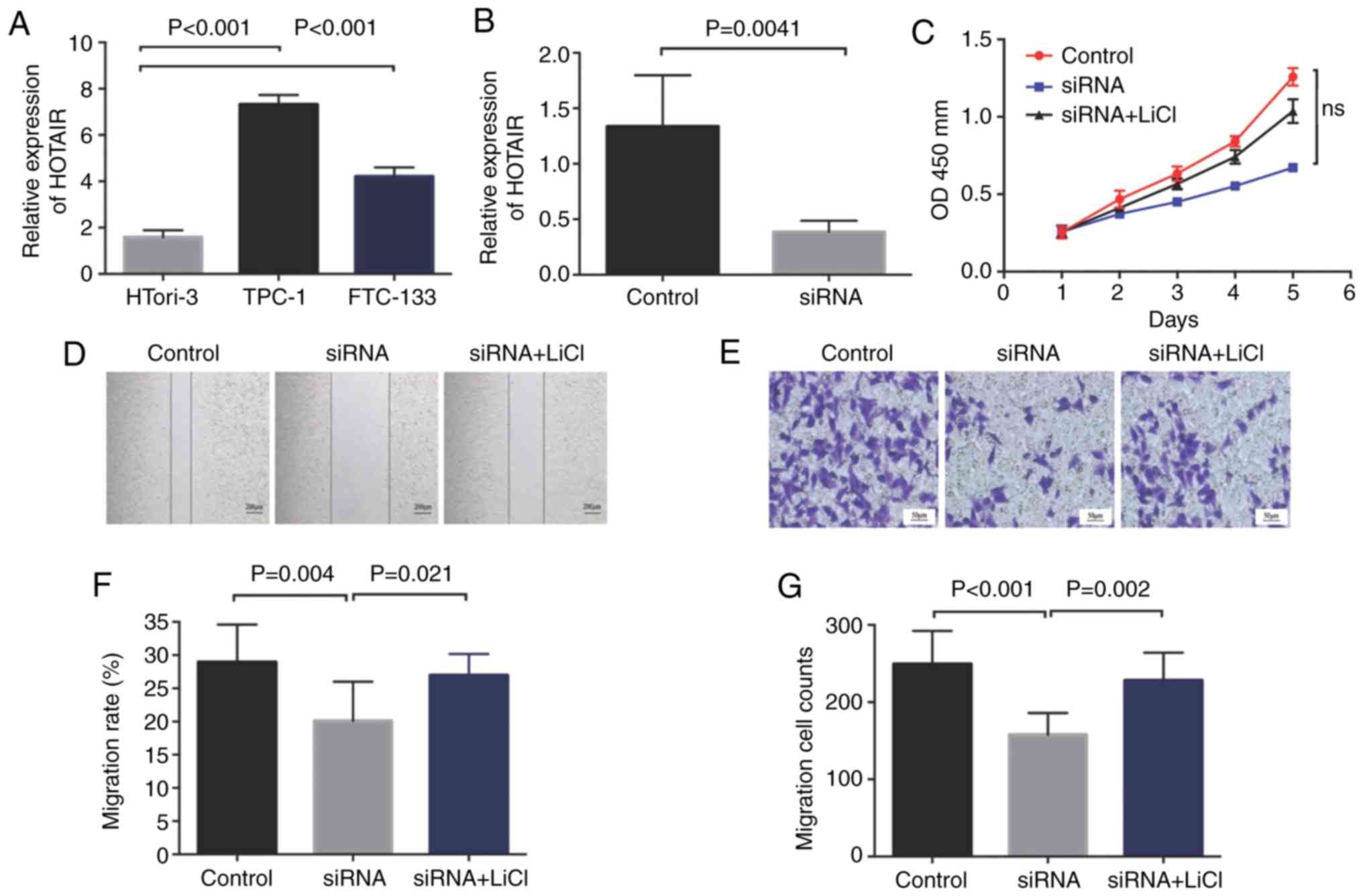
We assessed HOTAIR levels in the TPC-1, HTori-3 and
FTC-133 cell lines and found that the HOTAIR level in the TPC-1
cell line was the highest (Fig. 2A),
which was then selected for subsequent experiments. After knockdown
of lncRNA-HOTAIR expression with small interfering RNA, the
expression of HOTAIR in the transfected TPC-1 cells was decreased
by 3.45 times (Fig. 2B). The
difference was statistically significant (P=0.0041). The MTT assay
showed that after siRNA interference on TPC-1 cells, TPC-1 cell
proliferation was significantly slower than that of the control
group, but the difference did not reach a statistical difference
(Fig. 2C).
Effect of HOTAIR on PTC cell
migration
Cell migration rates in the siRNA group, the control
group and the siRNA+LiCl group were 20.11±5.46, 28.95±5.23 and
26.96±3.20%, respectively, as measured by a scratch assay
indicating that the migration rate in the siRNA group was
significantly lower than that in the control group (P=0.004). The
migration rate in the siRNA group was significantly lower than that
in the siRNA+LiCl group (P=0.021). Migrated cell numbers in the
siRNA group, the control group and siRNA+LiCl group were
157.86±25.99, 249.42±39.79 and 228.00±36.11, respectively, as
measured by the Transwell assay. The siRNA group was significantly
lower than that in the control group, P<0.001. The siRNA group
was significantly lower than that in the siRNA+LiCl group, P=0.002
(Fig. 2D-G).
Knockdown of HOTAIR and its impact on
the Wnt/β-catenin signaling pathway
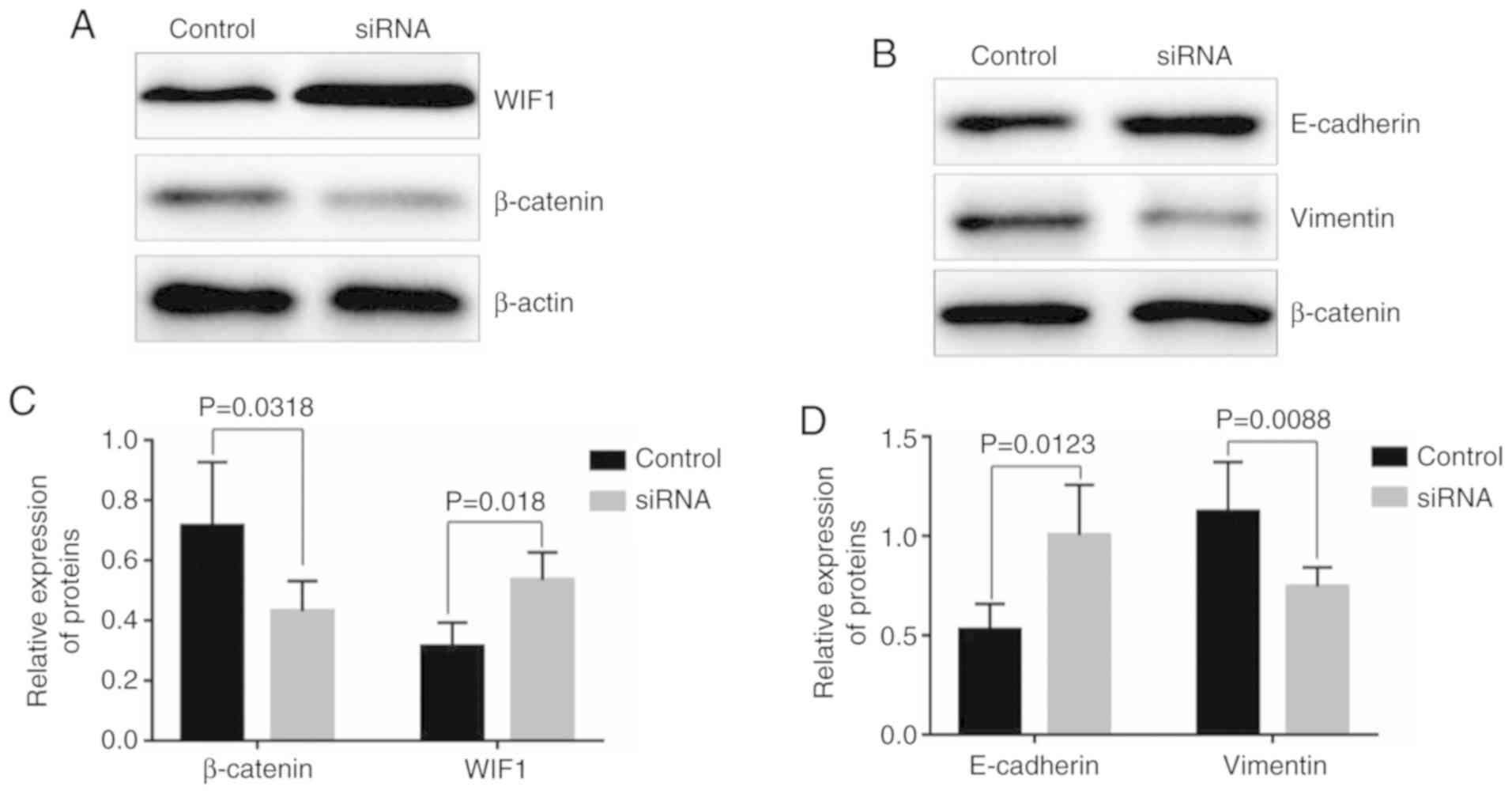
Compared with the control group, knockdown of HOTAIR
significantly increased the relative expression of WIF1 (0.54±0.08,
P=0.018), while knockdown of HOTAIR significantly decreased the
expression of β-catenin (0.43±0.09, P=0.0318) (Fig. 3A and C). The relative expression
levels of E-cadherin and vimentin were 0.53±0.12 and 1.12±0.23,
respectively, in the control groups, while the levels of the two
proteins were 1.00±0.23 and 0.75±0.09, respectively, in the siRNA
groups. Apparently, HOTAIR knockdown increased the expression of
E-cadherin (P=0.0123), but decreased the expression of vimentin
(P=0.0088) (Fig. 3B and D). In order
to further confirm that HOTAIR affects the proliferation and
migration of PTC cells through the Wnt/β-catenin pathway, we added
the agonist (LiCl) of Wnt/β-catenin after siRNA interference, and
found that the proliferation and migration of PTC cells were
increased compared with that of the siRNA group, almost similar to
that of the control cell group (Fig.
2C-G).
Discussion
Thyroid cancer is one of the most common endocrine
malignancies in the world, and papillary thyroid carcinoma (PTC)
has the highest incidence (1).
Despite multiple treatment strategies for PTC, 30% of patients with
PTC show a high tendency for lymph node metastasis and recurrence
within 10 years (19). Long
non-coding RNA HOX transcript antisense RNA (lncRNA-HOTAIR) has
been reported to promote PTC proliferation, invasion and migration,
while high levels of lncRNA-HOTAIR are potential biomarkers for PTC
patients and are associated with poor prognosis (15,20,21).
However, previous studies did not include the presence or absence
of cervical lymph node metastasis. In addition, we further explored
the specific mechanism of the involvement of lncRNA-HOTAIR in
cervical lymph node metastasis and focused on the role of
epithelial-mesenchymal transition (EMT). Therefore, the present
study has innovative and clinical application value. It was found
that the expression of lncRNA-HOTAIR was upregulated in the lymph
node metastasis-positive group compared with the
metastasis-negative group among the PTC patients. These findings
suggest that HOTAIR overexpression in PTC patients may be closely
associated with lymph node metastasis. To further investigate the
relationship between HOTAIR overexpression and PTC lymph node
metastasis, the HOTAIR gene in the PTC cell line TPC-1 was
knocked down using siRNA, followed by assessment of the
proliferation and migration of the transfected cells. Impacts on
the Wnt/β-catenin pathway and cell phenotype were also assessed
using western blot analysis to explore the mechanism underlying the
HOTAIR-mediated promotion of cell migration.
HOX transcript antisense RNA (HOTAIR), which was
first discovered in a study on homeotic genes in Drosophila
melanogaster (8), demonstrates
abnormal expression in cancer tissues. Researchers have reported
that HOTAIR is overexpressed in PTC and peripheral blood (15,20,21). The
results of this study were consistent with those of previous
studies. We also found that the proliferation of TPC-1 cells was
significantly inhibited after HOTAIR knockdown using siRNA. The
findings suggest that HOTAIR may be closely associated with the
growth and metastasis of PTC, and HOTAIR overexpression may promote
the occurrence and metastasis of PTC through a certain mechanism.
This result is consistent with the findings of Di et al
(20). HOTAIR overexpression can
also downregulate the expression of tumor-suppressor gene miR-1,
thereby promoting the onset of thyroid cancers through the HOTAIR/
miR-1/CCND2 axis (22,23). In addition, PTC susceptibility, onset
and progression were found to be closely associated with the three
SNP loci of HOTAIR (14). This
evidence indicates that lncRNA-HOTAIR plays an important role in
PTC onset and progression.
EMT is a biological process that plays an important
role in tumor metastasis. EMT is excessively activated in tumor
tissues, during which mesenchymal cell phenotypes increase, such as
vimentin and N-cadherin; while epithelial cell phenotypes decrease,
such as E-cadherin (24). Liu et
al (25) reported that cell
invasion was reduced after HOTAIR silencing in gastric cancer
cells, and the cell phenotypes were altered as well, indicating
that HOTAIR can promote tumor invasion by activating the process of
EMT. In addition, HOTAIR was found to be able to modulate cell
invasion and metastasis in breast cancer MCF-7 cells as well
through the p53/Akt/JNK pathway (26). The above-mentioned reports and our
findings indicate that HOTAIR may play an important role in tumor
metastasis.
The EMT process in tumor cells is modulated by the
Wnt/β-catenin signaling pathway. Activation of the Wnt/β-catenin
signaling pathway upregulates the expression of Snail and ZEB1
which are known to play a significant role in EMT, leading to tumor
metastasis (27,28). In the present study, the impact of
HOTAIR silencing on the Wnt/β-catenin signaling pathway was
evaluated. It was found that the expression of Wnt inhibitor 1
(WIF1) was significantly increased, while the expression of
β-catenin was significantly reduced after HOTAIR silencing. In
addition, the expression of the epithelial cell phenotype
E-cadherin was increased, while the expression of the mesenchymal
cell phenotype vimentin was reduced. The findings suggest that
HOTAIR can promote TPC-1 cell migration and invasion through the
Wnt/β-catenin pathway. The Wnt/β-catenin signaling pathway is a
type of canonical Wnt signaling pathway. When it is in an inactive
state, β-catenin is phosphorylated by the β-catenin destruction
complex assembled by APC/GSK-3β/CKIα/Axin, leading to its
degradation by ubiquitination. When the pathway is activated, the
β-catenin destruction complex is unstable, leading to accumulation
of β-catenin in the cytoplasm. Excessive β-catenin then
translocates into the nucleus where it causes transcription of
downstream target genes (29).
Therefore, activation of the Wnt/β-catenin signaling pathway plays
an important role in proliferation, apoptosis, cell cycle and
onset/progression of cancer cells. WIF1 is a protein that
antagonizes Wnt activity. It binds to Wnt in the extracellular
space, therefore blocking the Wnt signal transduction, leading to
inhibition of the Wnt/β-catenin pathway (30), and the weakening of WIF1 can result
in the overactivation of Wnt/β-catenin. In addition, to further
demonstrate that HOTAIR regulates the Wnt pathway and promotes
tumor growth and metastasis, LiCl was used in a rescue assay.
Studies have reported that LiCl can activate the Wnt/β-catenin
pathway. After adding LiCl to activate Wnt/β-catenin, we observed
that the migration, invasion and proliferation of the TPC-1 cells
was not inhibited by HOTAIR siRNA and was reversed similar to
levels of the control. Therefore, it was further confirmed that
HOTAIR regulated the proliferation and invasion of TPC-1 cells
through Wnt/β-catenin (31). These
results demonstrate that HOTAIR may cause activation of the
Wnt/β-catenin pathway by inhibiting WIF1, resulting in PTC cell
proliferation and metastasis, and EMT may also be involved.
In summary, in the present study it was found that
lncRNA-HOTAIR was overexpressed in the serum of patients with lymph
node metastasis of PTC. In in vitro experiments, HOTAIR was
found to promote cell growth and migration of PTC cells by
modulating the Wnt/β-catenin pathway. This evidence indicates the
correlation between HOTAIR expression and lymph node metastasis of
PTC. Moreover, the results of this study suggest that high levels
of lncRNA-HOTAIR in the blood of patients with clinical PTC can
predict the possibility of lymph node metastasis, which is of
referential significance for clinical diagnosis and treatment. It
is undeniable that there are shortcomings in this study. Firstly,
ultrasonography lacks specific indicators for lymph node
metastases, and sonographers may have subjective judgments or lack
experience. Although efforts were made to reduce the impact of
these factors, missed diagnosis or even misdiagnosis are still
possible. Secondly, the sample size of this study was small,
lacking tumor-related indicators and patient prognosis data, and
the relationship between HOTAIR and clinical and pathological
features were not further analyzed. It is necessary to further
expand the sample size to improve the study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LW and YS conceived and designed the study, and
drafted the manuscript. LW, BL and MZ collected, analyzed and
interpreted the experiment data, and revised the manuscript
critically for important intellectual content. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Beijing Geriatric Hospital (Beijing, China). Signed written
informed consents were obtained from all of the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Enewold L, Zhu K, Ron E, Marrogi AJ,
Stojadinovic A, Peoples GE and Devesa SS: Rising thyroid cancer
incidence in the United States by demographic and tumor
characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev.
18:784–791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fofi C, Festuccia F, Barberi S, Apponi F,
Chiacchiararelli L, Scopinaro F, Punzo G and Menè P: Hemodialysis
in patients requiring 131I treatment for thyroid carcinoma. Int J
Artif Organs. 36:439–443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davies L and Welch HG: Increasing
incidence of thyroid cancer in the United States, 1973–2002. JAMA.
295:2164–2167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu XH, Chen GG, Vlantis AC and van
Hasselt CA: Iodine mediated mechanisms and thyroid carcinoma. Crit
Rev Clin Lab Sci. 46:302–318. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Renehan AG, Soerjomataram I, Tyson M,
Egger M, Zwahlen M, Coebergh JW and Buchan I: Incident cancer
burden attributable to excess body mass index in 30 European
countries. Int J Cancer. 126:692–702. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nikiforov YE: Is ionizing radiation
responsible for the increasing incidence of thyroid cancer? Cancer.
116:1626–1628. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ushenkova LN, Koterov AN and Biryukov AP:
RET/PTC Gene rearrangements in the sporadic and radiogenic thyroid
tumors: Molecular genetics, radiobiology and molecular
epidemiology. Radiats Biol Radioecol. 55:229–249. 2015.(In
Russian). PubMed/NCBI
|
|
8
|
Rinn JL, Kertesz M, Wang JK, Squazzo SL,
Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E and
Chang HY: Functional demarcation of active and silent chromatin
domains in human HOX loci by noncoding RNAs. Cell. 129:1311–1323.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang Z, Zhou L, Wu LM, Lai MC, Xie HY,
Zhang F and Zheng SS: Overexpression of long non-coding RNA HOTAIR
predicts tumor recurrence in hepatocellular carcinoma patients
following liver transplantation. Ann Surg Oncol. 18:1243–1250.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kogo R, Shimamura T, Mimori K, Kawahara K,
Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S, et al:
Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin
modification and is associated with poor prognosis in colorectal
cancers. Cancer Res. 71:6320–6326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li D, Feng J, Wu T, Wang Y, Sun Y, Ren J
and Liu M: Long intergenic noncoding RNA HOTAIR is overexpressed
and regulates PTEN methylation in laryngeal squamous cell
carcinoma. Am J Pathol. 182:64–70. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nie Y, Liu X, Qu S, Song E, Zou H and Gong
C: Long non-coding RNA HOTAIR is an independent prognostic marker
for nasopharyngeal carcinoma progression and survival. Cancer Sci.
104:458–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu H, Lv Z, An C, Shi M, Pan W, Zhou L,
Yang W and Yang M: Onco-lncRNA HOTAIR and its functional genetic
variants in papillary thyroid carcinoma. Sci Rep. 6:319692016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li HM, Yang H, Wen DY, Luo YH, Liang CY,
Pan DH, Ma W, Chen G, He Y and Chen JQ: Overexpression of lncRNA
HOTAIR is associated with poor prognosis in thyroid carcinoma: A
study based on TCGA and GEO data. Horm Metab Res. 49:388–399. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Z, Zeng W, Liu C, Wang S, Xiong Y, Guo
Y, Li X, Sun S, Chen T, Maimaiti Y, et al: Diagnostic accuracy of
ultrasonographic features for lymph node metastasis in papillary
thyroid microcarcinoma: A single-center retrospective study. World
J Surg Oncol. 15:322017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cho SJ, Suh CH, Baek JH, Chung SR, Choi YJ
and Lee JH: Diagnostic performance of CT in detection of metastatic
cervical lymph nodes in patients with thyroid cancer: A systematic
review and meta-analysis. Eur Radiol. 29:4635–4647. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhan J, Diao X, Chen Y, Wang W and Ding H:
Predicting cervical lymph node metastasis in patients with
papillary thyroid cancer (PTC)-Why contrast-enhanced ultrasound
(CEUS) was performed before thyroidectomy. Clin Hemorheol
Microcirc. 72:61–73. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xing M: Molecular pathogenesis and
mechanisms of thyroid cancer. Nat Rev Cancer. 13:184–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Di W, Li Q, Shen W, Guo H and Zhao S: The
long non-coding RNA HOTAIR promotes thyroid cancer cell growth,
invasion and migration through the miR-1-CCND2 axis. Am J Cancer
Res. 7:1298–1309. 2017.PubMed/NCBI
|
|
21
|
Zhang Y, Yu S, Jiang L, Wang X and Song X:
HOTAIR is a promising novel biomarker in patients with thyroid
cancer. Exp Ther Med. 13:2274–2278. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nasser MW, Datta J, Nuovo G, Kutay H,
Motiwala T, Majumder S, Wang B, Suster S, Jacob ST and Ghoshal K:
Down-regulation of micro-RNA-1 (miR-1) in lung cancer. Suppression
of tumorigenic property of lung cancer cells and their
sensitization to doxorubicin-induced apoptosis by miR-1. J Biol
Chem. 283:33394–33405. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reid JF, Sokolova V, Zoni E, Lampis A,
Pizzamiglio S, Bertan C, Zanutto S, Perrone F, Camerini T, Gallino
G, et al: miRNA profiling in colorectal cancer highlights miR-1
involvement in MET-dependent proliferation. Mol Cancer Res.
10:504–515. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luo Y, Cui X, Zhao J, Han Y, Li M, Lin Y,
Jiang Y and Lan L: Cells susceptible to epithelial-mesenchymal
transition are enriched in stem-like side population cells from
prostate cancer. Oncol Rep. 31:874–884. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu YW, Sun M, Xia R, Zhang EB, Liu XH,
Zhang ZH, Xu TP, De W, Liu BR and Wang ZX: LincHOTAIR
epigenetically silences miR34a by binding to PRC2 to promote the
epithelial-to-mesenchymal transition in human gastric cancer. Cell
Death Dis. 6:e18022015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu Y, Lv F, Liang D, Yang Q, Zhang B, Lin
H, Wang X, Qian G, Xu J and You W: HOTAIR may regulate
proliferation, apoptosis, migration and invasion of MCF-7 cells
through regulating the P53/Akt/JNK signaling pathway. Biomed
Pharmacother. 90:555–561. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bao Z, Xu X, Liu Y, Chao H, Lin C, Li Z,
You Y, Liu N and Ji J: CBX7 negatively regulates migration and
invasion in glioma via Wnt/β-catenin pathway inactivation.
Oncotarget. 8:39048–39063. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang Y, Zhang N, Zhu J, Hong XT, Liu H, Ou
YR, Su F, Wang R, Li YM and Wu Q: Downregulated connexin32 promotes
EMT through the Wnt/β-catenin pathway by targeting Snail expression
in hepatocellular carcinoma. Int J Oncol. 50:1977–1988. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sawa M, Masuda M and Yamada T: Targeting
the Wnt signaling pathway in colorectal cancer. Expert Opin Ther
Targets. 20:419–429. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sánchez-Hernández D, Sierra J,
Ortigão-Farias J and Guerrero I: The WIF domain of the human and
Drosophila Wif-1 secreted factors confers specificity for Wnt or
Hedgehog. Development. 140:3849–3858. 2013. View Article : Google Scholar
|
|
31
|
Zhou Z, Liu Y, Ma M and Chang L: Knockdown
of TRIM44 inhibits the proliferation and invasion in papillary
thyroid cancer cells through suppressing the Wnt/β-catenin
signaling pathway. Biomed Pharmacother. 96:98–103. 2017. View Article : Google Scholar : PubMed/NCBI
|