Introduction
According to the World Health Organization, cervical
cancer is the fourth most frequent cancer in women representing
6.6% of all female cancers worldwide. In 2018, there have been an
estimated 570,000 new cases. Worldwide, approximately 266,000 women
died of cervical cancer in 2012 (1).
Approximately 75% of cervical carcinomas are squamous cell cancers.
It is well established that an infection with human papilloma virus
(HPV) is responsible for the formation of cervical cancer in more
than 90% of all cancers (2). Up to
date, there are more than 120 subtypes of HPV described and at
least 14 are classified ‘high risk’, i.e. oncogenic for cervical
cancer (1). HPV16 and HPV18 are most
prominent among the sexually transmitted ‘high risk’ viruses
causing more than 70% of cervical cancers (3). In high-income countries, a vaccination
of adolescents protects against the infection with most high-risk
subtypes of HPV and prevents the development of cervical cancer.
Furthermore, in these countries a widely performed screening by
PAP-testing provides an early diagnosis of pre-invasive cervical
lesions and, under these circumstances, the therapeutic success
increased during the last decades. However, the diagnosis of
early-stage pre-cancerous neoplasia might be further improved by
the identification of molecular markers for early diagnosis,
prediction and prognosis as well as for the establishment of novel
therapeutic targets in cervical carcinomas.
The treatment options for cervical cancer include
the radical hysterectomy with pelvic and paraaortal lymphonodectomy
or chemoradiotherapy according to the cancer stage. Following the
guidelines of the European society for Medical Oncology from 2017
and the German S3 guideline from 2014, chemoradiotherapy following
surgery should be restricted to cases of up-staging after surgery
(4,5). A fertility-preserving surgery is
possible for early detected cancers of small size or low risk. In
severe cases with metastases, the therapy can include actual
medications e.g., the anti-VEGF antibody Bevacizumab (6). Otherwise, the search for new molecular
structures as possible target options is ongoing.
26S proteasome non-ATPase regulatory subunit 9
(PSMD9) is the human homolog of the protein Bridge-1 from rat.
Thomas et al described the transcriptional co-activator
Bridge-1 as a PDZ-domain containing protein that binds to E12-box
DNA-binding protein and transcription factors PDX-1 and E47 and is
functioning as a transcriptional co-regulator in the glucose
homeostasis (7).
PSMD9 has already been investigated in tumors. In a
cohort of 157 patients with breast cancer, Langlands et al
used a PSMD9 antibody for an immunohistochemical analysis,
following the idea that a lack of proteasome function could be
linked to the sensitivity of breast cancer cells for radiotherapy
(8). Indeed, they found that low
expression of PSDM9 in the tumor was associated with less local
recurrences in patients treated with radiotherapy. Banz-Jansen
et al detected PSMD9 protein and mRNA in tumor tissues of
breast cancer patients (9).
Furthermore, they could show that PSMD9 expression is regulated by
activin A, an inhibitor of breast carcinoma cell proliferation.
Vice versa, the same group showed that downregulation of PSMD9 in
MCF-7 breast cancer cells resulted in a decrease of the activin A
signal transduction proteins Smad-2, −3 and −4 (10). This indicates that PSMD9 could be
involved in the signaling cascade of activin A and might be
critical for the growth regulation of breast cancer cells or cancer
cells in general.
This study evaluated the expression of PSMD9 on
tissue samples from patients with cervical cancer by
immunohistochemistry.
Materials and methods
Patients
A total of 102 patients with squamous cell cancer of
the cervix were included into the retrospective immunohistological
analysis of PSMD9 expression in formalin fixed, paraffin embedded
(FFPE)-tissue samples. All patients gave their written informed
consent for the use of their tissues and the publication of
results. The local ethics committee at the University of Lübeck
approved this study with the number 15–134 on June 9, 2015.
The patients had undergone hysterectomy including
lymph node excision in the Department of Gynecology and Obstetrics
at the University Medical Center Schleswig-Holstein, Campus Lübeck
between 2003 and 2012. Patients with carcinoma in situ
(preinvasive), adenocarcinoma and those having received neoadjuvant
radio- or chemotherapy were excluded from the study.
Formaldehyde fixation and paraffin embedding was
performed immediately after surgery. The patients' data and disease
specific information were taken from medical records and
pathologists' reports. The immunhistochemical data for the
expression of the proliferation marker MIB-1 were taken from a
previous study using the same tissue microarrays (TMAs) (11).
Tissue micro arrays
Tissue microarrays (TMA) were prepared from
FFPE-tissue samples using a semi-automated arrayer (TMArrayer;
Pathology Devices. Inc.). The arrays were made as described
(12,13). In brief, with a hollow
stainless-steel needle, one tumor containing tissue cylinder and
another one from peritumoral stroma were taken for each patient.
The tumor areas had previously been evaluated on hematoxylin
stained 4 µm sections of entire FFPE-samples. After assembly, the
TMA-blocks were hardened first at 42°C for 2 h and then at room
temperature over-night. Sections of 4 µm thickness were cut with a
microtome and spread onto glass slides.
For the immunohistochemistry of the TMAs a
monoclonal murine anti-Bridge-1 antibody (Clone 30, cat. no.
612458; BD Biosciences) was used in a 1:50 dilution in
Bond-Polymer-Antibody-Diluent (Leica Biosystems). Previously, the
slides were deparaffinized and pretreated for antigen retrieval in
Epitope Retrieval Solution (Leica Biosystems) for 20 min. The
tissues were submerged with the primary antibody for 15 min and
washed. Antibody reactions were detected with a horseradish
peroxidase linked secondary antibody using the
Bond-Polymer-Refine-Detection-Kit (Leica Biosystems) including
DAB-chromogen staining and a hematoxylin counterstain. The tissues
were dehydrated and mounted with Cytoseal-60 (Thermo Fischer
Scientific). The PSMD9 expression was evaluated using the immune
reactive score (IRS) by Remmele and Stegner (14). The IRS (0–12) multiplies scores for
the percent of positive cells (PP; 0–4) and for staining intensity
(SI; 0–3). An IRS ≥3 was classified as positive expression.
Statistics
To compare the PSMD9 expression between tumor
tissues and peritumoral stromata the Wilcoxon matched-pairs signed
rank test was used. The age of patients and data from the patients'
clinical reports with polytomous variables were correlated with the
IRS of PSMD9 expression by the Spearman correlation coefficient
(rs). Patients' data with dichotomous outcome were used
to calculate odds ratios with simple logistic regression.
Statistical significance was defined at P≤0.05. Multiple testing of
the same cohort might lead to a false discovery of significant
results. Therefore, the false discovery rate (FDR) was corrected
using the algorithm by Benjamini & Hochberg in a web-based
calculator (15).
Results
In 96 patients PSMD9 expression could be evaluated
by immunohistochemistry in both, tumor and peritumoral stroma
tissues (Fig. 1). An expression of
PSMD9, defined as positive with an IRS ≥3, could be detected in all
tumor tissues and in 90% of the peritumoral stromata. According to
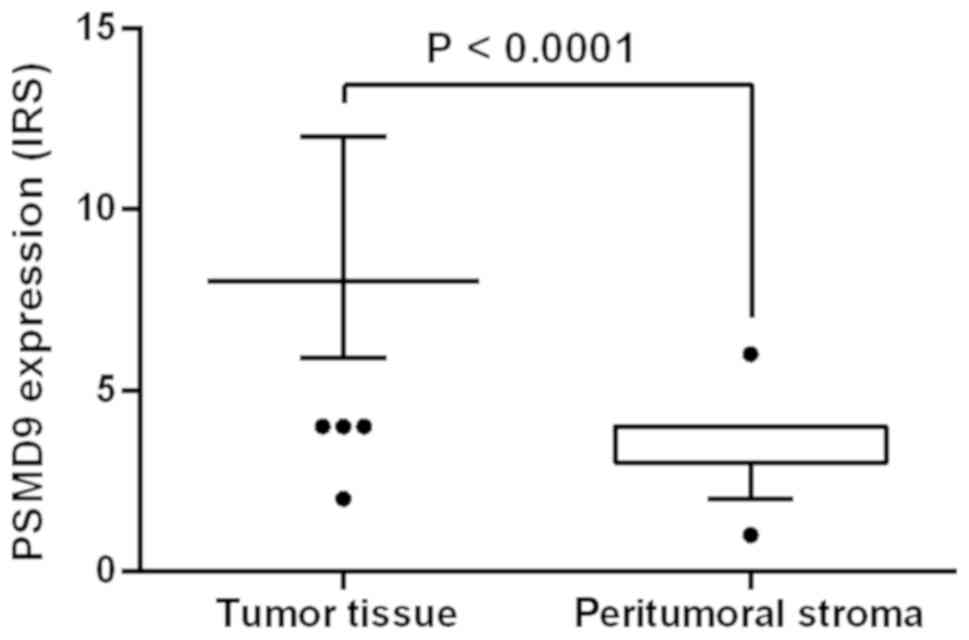
Table I and Fig. 2, PSMD9 was detected statistical
significantly higher (P≤0.0001) even after controlling the FDR
(P=0.0012) in tumor tissues (IRS=8.48±1.9) compared to the
surrounding peritumoral stroma (IRS=3.18±0.81).
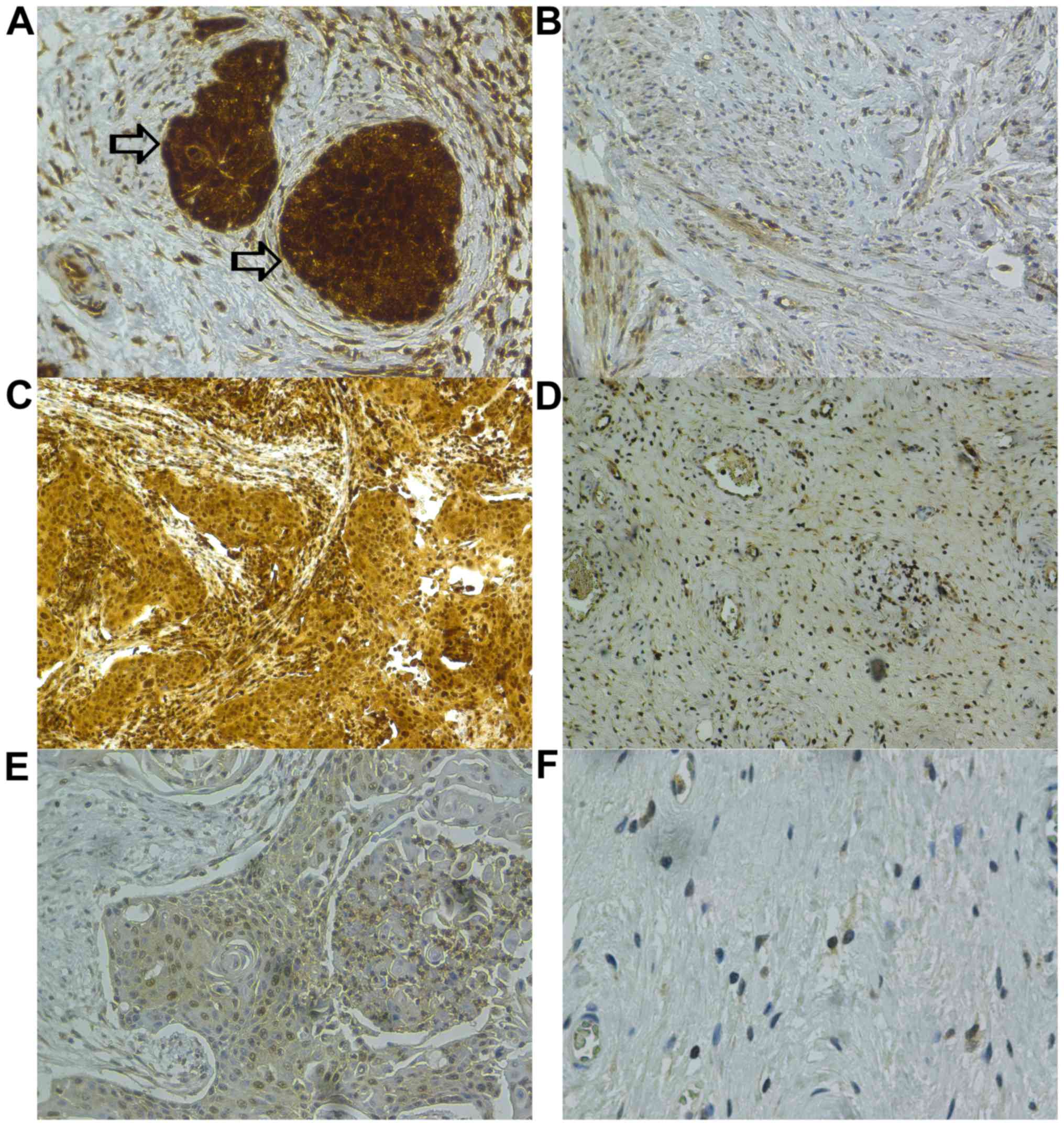 | Figure 1.Immunohistochemistry of PSMD9 in
cervical cancer and peritumoral stroma tissues with varying IRS.
Antibody reactions were visualized using DAB-chromogen staining and
a hematoxylin counterstain was used. (A) Tumor tissues indicated by
the arrows: IRS 12 (PP 4, SI 3); magnification, ×20. (B)
Peritumoral stroma: IRS 2 (PP 2, SI 1); magnification, ×20. (C)
Tumor tissue: IRS 8 (PP 4, SI 2); magnification, ×10. (D)
Peritumoral stroma: IRS 3 (PP 3, SI 1); magnification, ×10. (E)
Tumor tissue: IRS 4 (PP 4, SI 1); magnification, ×20. (F)
Peritumoral stroma: IRS 1 (PP 1, SI 1); magnification, ×40. PSMD9,
Proteasome 26S non-ATPase Subunit 9; IRS, immunoreactive score; PP,
percentage positive cells; SI, staining intensity. |
 | Table I.Patients' data and results of the
PSMD9 expression. |
Table I.
Patients' data and results of the
PSMD9 expression.
| Variable | Patients (n) | Value | P-value | FDR P-value |
|---|
| Age | 102 |
|
|
|
|
rs (95% confidence
interval) |
| 0.03926
(−0.1611–0.2365) | 0.3469 | 0.5204 |
| Tissuesd | 96 | IRS (mean ± SD) |
|
|
| cervical
carcinoma |
| 8.48±1.89 |
|
|
|
peritumoral stroma |
| 3.18±0.81 | ≤0.0001c | 0.0012b |
| MIB-1 expression | 102 |
|
|
|
|
rs (95% confidence
interval) |
| 0.2866
(0.0928–0.4595) | 0.0017b | 0.0102a |
| Tumor size | 101 | IRS (mean ± SD) |
|
|
| T1 | 62 | 8.71±2.01 |
|
|
| T2 | 34 | 8.18±1.42 |
|
|
| T3 | 3 | 9.33±2.31 |
|
|
| T4 | 2 | 6.00±2.83 |
|
|
|
rs (95% confidence
interval) |
| −0.1584
(−0.3483–0.0441) | 0.0568 | 0.1136 |
| Nodal status | 93 | IRS (mean ± SD) |
|
|
| N
=0 | 69 | 8.43±1.94 |
|
|
| N
≥1 | 24 | 8.17±1.44 |
|
|
| Odds
ratio (95% confidence interval) |
| 0.92
(0.6987–1.191) | 0.5293 | 0.5774 |
| Metastasis | 102 | IRS (mean ±
SD) |
|
|
| M
=0 | 97 | 8.56±1.95 |
|
|
| M
≥1 | 5 | 8.00 |
|
|
| Odds
ratio (95% confidence interval) |
| 0.8537
(0.5323–1.367) | 0.5186 | 0.5774 |
| Lymphangiosis
carcinomatosa | 58 | IRS (mean ±
SD) |
|
|
| L0 | 38 | 8.58±1.98 |
|
|
| L1 | 20 | 8.8±2.09 |
|
|
| Odds
ratio (95% confidence interval) |
| 1.065
(0.794–1.426) | 0.6697 | 0.6697 |
| Grading (UICC) | 99 |
|
|
|
| G1 | 2 | 8 |
|
|
| G2 | 47 | 8.6±1.86 |
|
|
| G3 | 50 | 8.48±1.96 |
|
|
|
rs (95% confidence
interval) |
| −0.0158
(−0.2182–0.1879) | 0.4384 | 0.5774 |
| FIGO staging | 102 | IRS (mean ±
SD) |
|
|
| Stage
I | 62 | 8.71±2.02 |
|
|
| Stage
II | 34 | 8.29±1.57 |
|
|
| Stage
III | 3 | 9.33±2.31 |
|
|
| Stage
IV | 3 | 6.67±2.31 |
|
|
|
rs (95% confidence
interval) |
| −0.1336
(−0.325–0.0683) | 0.0904 | 0.155 |
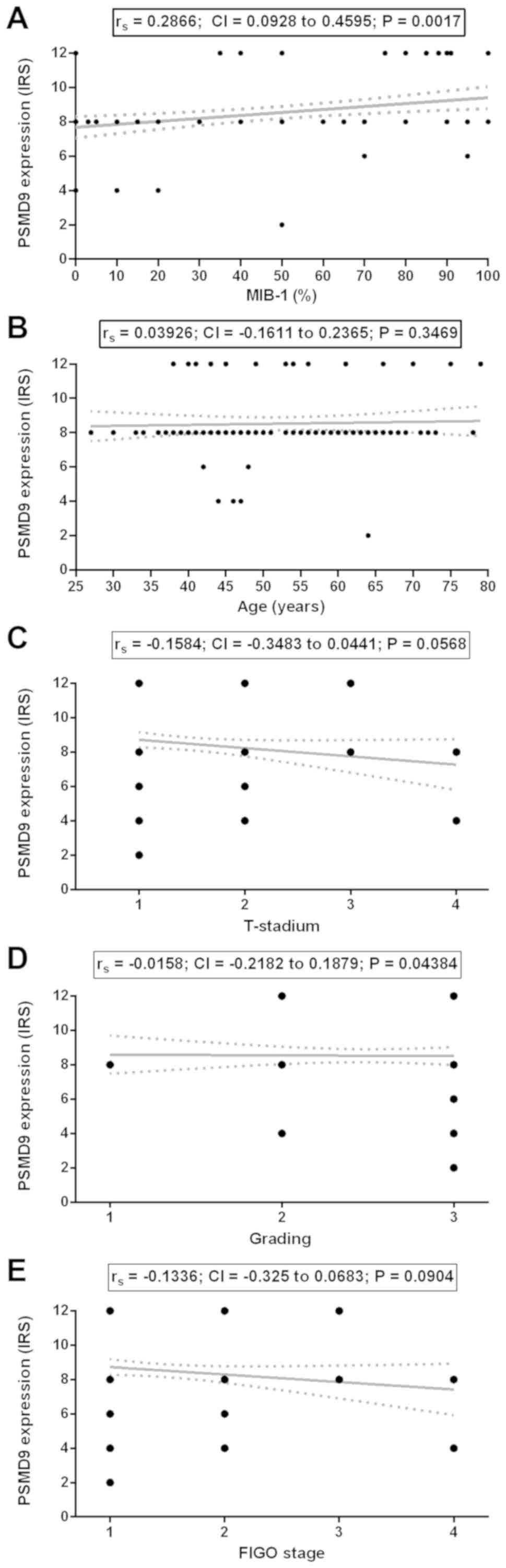
The IRS of PSMD9 significantly correlated with the
expression of the proliferation marker MIB-1 with a fair
correlation (rs: 0.2866; CI: 0.0928–0.4595; P=0.0017;
FDR: P=0.0102; Table I, Fig. 3A).
According to Table I,
all included patients (n=102) had a mean age ± standard deviation
(mean ± SD) of 53.28±12.36 years ranging from 27 to 79 years. The
patients' age did not correlate with the expression of PSMD9
(rs: 0.3926; CI: −0.1611–0.2365; P=0.3469; Fig. 3B). The patients' tumor
classifications concerning tumor size (rs: −0.1584; CI:
−0.3483–0.0441; P=0.0568; Fig. 3C),
tumor cell grading (rs: −0.0158; CI: −0.2182–0.1879;
P=0.4384; Fig. 3D) and FIGO
classifications (rs: −0.1336; CI: −0.325–0.0683;
P=0.0904; Fig. 3E) did not correlate
with the expression of PSMD9 in tumor tissues. The graphs in
Fig. 3C-E have a different
appearance to Fig. 3A and B, because
in categorical data most values shared the same points on the line
of each category. The odds ratios (OR) for lymph node evasion (OR:
0.92; CI: 0.6987–1.191; P=0.5293; Fig.
3), distant metastases (OR: 0.8537; CI: 0.5323–1.367; P=0.5186;
Fig. 3), and the appearance of
lymphangiosis carcinomatosa (OR: 1.065; CI: 0.794–1.426; P=0.6697;
Fig. 3) were not significantly
changed with the expression of PSMD9 in tumor tissues.
Follow-up data from 102 patients were collected
between 2 and 160 months after surgery with a mean ± SD of
65.14±39.4 months. According to Table
II, 18 patients experienced a recurrence of the disease and 84
were recurrence free. The mean ages (mean ± SD) of patients with
recurrence (52.89±13.89 years; range: 34–75 years) and recurrence
free patients (53.37±12.1 years; range: 27–79 years) were not
different (P=0.9013). PSMD9 expression (mean ± SD) was stronger in
patients with a recurrence (9.33 +/-1.94) compared to those without
a recurrence (8.36 +/-1.87). The odds for a recurrence were
estimated 1.304 fold (CI: 1.0–1.706; P=0.0497) when the IRS for
PSMD9 was 1 point higher. After controlling the FDR, the odds ratio
was not significant anymore (FDR: P=0.1136).
 | Table II.Cancer recurrences and results of the
PSMD9 expression. |
Table II.
Cancer recurrences and results of the
PSMD9 expression.
| Cancer
recurrence | patients (n) | IRS (mean ±
SD) | P-value | FDR P-value |
|---|
| All patients | 102 | IRS (mean ±
SD) |
|
|
|
Recurrence | 18 | 9.33 ± 1.94 |
|
|
|
Recurrence free | 84 | 8.36 ± 1.87 |
|
|
| Odds
ratio (95% confidence interval) |
| 1.304 (1.0 to
1.706) | 0.0497a | 0.1136 |
| Radiation following
surgery | 62 | IRS (mean ±
SD) |
|
|
|
Recurrence | 13 | 8.92 ± 1.75 |
|
|
|
Recurrence free | 49 | 7.84 ± 1.07 |
|
|
| Odds
ratio (95% confidence interval) |
| 1.974 (1.186 to
4.239) | 0.0076b | 0.0304a |
| Chemoradiotherapy
following surgery | 47 | IRS (mean ±
SD) |
|
|
|
Recurrence | 7 | 9.14 ± 1.95 |
|
|
|
Recurrence free | 40 | 7.95 ± 1.29 |
|
|
| Odds
ratio (95% confidence interval) |
| 1.983 (1.101 to
4.369) | 0.0222a | 0.0666 |
Two subgroups were established, one with patients
having received radiotherapy (n=62) and within this subgroup
another one including patients having received combined
chemoradiotherapy (n=47; Table II).
The IRS (mean ± SD) of PSMD9 expression was higher in patients
after receiving radiotherapy with a recurrence (8.92±1.75; n=13)
compared to recurrence-free patients (7.84±1.07; n=49). The
estimated odds of a recurrence for patients with a radiotherapy was
1.974 (CI: 1.186–4.239; P=0.0076) and the result was still
significant after controlling the FDR (P=0.0304). In the subgroup
of patients having received chemoradiotherapy, who are included in
the radiotherapy subgroup, PSMD9 had an odds ratio of 1.983 for a
recurrence (CI: 1.101–4.369; P=0.0222) which was not significant
anymore after FDR control (P=0.0666). These patients expressed
PSMD9 with higher IRS (mean ± SD; 9.14±1.95; n=7) compared to
recurrence-free patients (7.95±1.29; n=40). The mean ages (mean ±
SD) within the subgroup of patients who received radiotherapy after
surgery were not different (P=0.4382) between patients with a
recurrence (54.77+/-14.6 years; range: 34–75 years) and recurrence
free patients (51.39±11.24 years; range: 27–71 years). The same was
true for patients with chemoradiotherapy after surgery. Patients
with a recurrence (55.71±15.03 years; range: 36–73 years) were not
different in age (mean ± SD) from recurrence free patients
(50.86±10.91 years; range: 27–69 years; P=0.3976).
Discussion
In our collective of cervical cancer patients, PSMD9
was overexpressed in all cancer tissues compared to peritumoral
stroma. Thus, PSMD9 could play a role as a tumor marker in cervical
cancer. An upregulation of PSMD9 in tumor cells could be reasonable
due to its role in the proteasome. Rapidly dividing cells require
an increased protein turnover and therefore, the proteins
assembling the proteasome are upregulated (16). Moreover, PSMD9 expression correlated
with the expression of the proliferation marker MIB-1. The MIB-1
antibody recognizes the cell cycle progression marker Ki-67.
Together with another tumor marker, p16INK4a, Ki-67 is a
useful diagnostic tool for the classification of precancerous
cervical intraepithelial neoplasia (CIN) (17,18). One
could hypothesize that PSMD9 as a tumor and proliferation marker in
cervical tumor tissue might also be associated with higher TNM- or
FIGO-stages of cervical cancer. Here, the study that included only
patients who underwent hysterectomy was not able to point onto this
question. An explanation might be the fact that patients with
neoadjuvant chemoradiotherapy and thereby, most of the severe
cases, were excluded from this study, because patients with higher
FIGO stages who did not undergo surgery according to present
guidelines were excluded. According to the existing guidelines,
FIGO stages 3 and 4 were treated with neoadjuvant
chemoradiotherapy. In our patient collective only three patients
each were included with FIGO stages III and IV, respectively. These
patients underwent hysterectomy with clinical up-staging following
surgery.
In the subgroups of patients who received
radiotherapy or combined chemoradiotherapy following surgery,
stronger PSMD9 expression resulted in significantly higher odds for
the appearance of a recurrence. The patients with chemoradiotherapy
were included in the radiotherapy-subgroup. After controlling the
FDR, only the radiotherapy-subgroup was still significant. As a
conclusion from this result, PSMD9 might be a candidate protein for
the prediction of radiation sensitivity in cervical carcinoma
patients. However, the present patient collective with recurrence
following radiotherapy was small with only 14 patients. Overall,
within the patient collective, only 18 of 102 patients showed
recurrence. This might be caused by the exclusion of higher cancer
stages. With regard to the small number of patients, these
findings, though statistically significant, must be interpreted
with care and further studies would be advisable. Otherwise,
Langlands et al received a similar result in a study with
breast cancer patients. They found an association of PSMD9
expression with a shorter time to recurrence in a subgroup of 110
patients who had radiotherapy (8).
The same publication describes that breast cancer cell lines are
sensitized to radiotherapy in vitro after siRNA mediated
downregulation of PSMD9.
It is widely accepted that a HPV infection is a
prerequisite of cervical cancer. Furthermore, an HPV infection
might influence the sensitivity to radiation therapy. Sabeena and
colleagues collected follow-up data from ten published studies
containing quantitative data from cervical cancer patients with
radiation therapy and HPV infection status before or after
treatment. In single publications significant prognostic values for
testing HPV were described and the overall outcome showed 3 times
the odds to develop a recurrence for patients with positive HPV
after radiation, but statistical significance could not be reached
(19). There seems to be higher
prognostic value in HPV testings after therapy then before.
Badaracco and colleagues tested HPV subtypes in a follow-up of 18
cervical cancer patients and found more recurrences when the HPV
was still persistent after the chemoradiotherapy (20). Song and colleagues found the same in
a bigger cohort of cervical cancer patients of 156 patients
(21). Yu and colleagues advise to
perform HPV tests after cervical cancer treatment, since they found
a significant higher risk for recurrence in patients with high-risk
HPV infection after therapy (22).
Deng and colleagues tested the viral load in 246 patients before
cervical cancer therapy with radical hysterectomy. High viral load
was associated with the depth of lymphovascular invasion and with
the recurrence of the disease (23).
A major limitation of this study is the
unavailability of HPV data in our patient collective, because HPV
testing was only performed in approximately ten percent of the
patients. It would be a great value to integrate data of HPV
subtype infection and virus loads especially from periods following
the therapy. According to the herein before mentioned literature,
we thereby would have been able to show if the influence of HPV
biased the results regarding radiation response. A further
limitation of our study is the relatively small number of patients.
Especially, the low rate of recurrences in our cohort could have
diminished the statistical outcome. The low rate of recurrences was
caused by the including only patients with a surgery. Patients with
higher FIGO-stages have therefore been excluded, because, following
the guidelines, they must immediately be treated by
chemoradiotherapy. At least, the accuracy of the evaluation of the
PSMD9 expression by immunohistochemistry is limited. The staining
as well as the evaluation by humans cannot reach perfectness.
Otherwise, the IHC staining was performed in a routine lab and
highly experienced pathologists controlled the tumor tissue
selection as well as the visual IRS evaluation.
The question, whether the different PSMD9
expressions described here and elsewhere, relied on function of
PSMD9 as the transcriptional cofactor or as a part of the small
subunit of the proteasome, cannot be answered. Several groups have
already investigated an influence of the proteasome activity on
radiation sensitivity in cancer treatment. S. Kamer et al
observed in the cervical cancer cell line SiHa that the proteasome
function inhibitor bortezomid sensitized the cells for radiation
(24). By contrast, in the cervical
carcinoma cell line SiHa, Pajonk et al detected a resistance
to radiation under hypoxic conditions when they inhibited the
proteasome activity with MG-132 (25). The proteasome as a drug target is
further discussed in a review by Crawford et al (26). The function of the proteasome itself
is a probable player in tumor development. The proteasome is
responsible for the degradation of proteins with important
functions for the cell cycle regulation e.g., IκB, the inhibitor of
transcription factor NFκB, or the cyclin dependent phosphatase
p27KIP. Therefore, proteasome function and its proteins
might be a target option in cancer treatment. Recently, Harish and
colleagues have pointed out the importance of PDZ-binding proteins
e.g., PSMD9 for biological functions and their usefulness as
therapeutic targets. In a binding complex with hnRNPA1 PSMD9
regulates NFκB signaling. The PDZ-domain was found to be the
responsible region in PSMD9 that carries the functionality for
regulation (27). Han et al
described the role of TIP-1, another PDZ-domain containing protein,
for the resistance against radiotherapy in malignant glioma cells.
TIP-1 was overexpressed in malignant glioma patients with
radiotherapy resistance. Furthermore, TIP-1 was found as a binding
partner in a protein complex that enforced the ubiquinization of
p53 and its degradation. As a consequence, p53 was unable to direct
the malignant glioma cells into apoptosis that would normally be
induced by DNA damage after radiation (28).
To conclude, the overexpression of PSMD9 has been
found to be associated with unfavorable tumor outcome and treatment
resistance in this study and by others in various cancers. Further
investigations about the role of PSMD9 in cancer development and
treatment are advisable.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FK evaluated the data and wrote the manuscript. LS
evaluated the immunohistochemistry and prepared the data. JRI
performed the immunohistochemical stainings and evaluated the
histological sections. FH constructed the TMAs and revised the
manuscript. KB contributed to the evaluation of the data and
revised the manuscript. AR conducted the clinical evaluation of the
study. CBJ conceived of and conducted the study, and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The local ethics committee at the University of
Lübeck approved the study with the number 15–134 on June 9, 2015.
All patients gave their written informed consent for the use of
their tissues and data after pseudonymisation.
Patient consent for publication
All patients gave their written informed consent for
the publication of the results including their data after
pseudonymization.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization: Cervical
cancer. https://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer/en/2019.
|
|
2
|
zur Hausen H: Papillomaviruses in the
causation of human cancers-a brief historical account. Virology.
384:260–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Olusola P, Banerjee HN, Philley JV and
Dasgupta S: Human papilloma virus-associated cervical cancer and
health disparities. Cells. 8(pii): E6222019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marth C, Landoni F, Mahner S, McCormack M,
Gonzalez-Martin A and Colombo N; ESMO Guidelines Committee, :
Cervical cancer: ESMO clinical practice guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 28 (Suppl 4):iv72–iv83. 2017.
View Article : Google Scholar
|
|
5
|
Leitlinienprogramm Onkologie (Deutsche
Krebsgesellschaft DK, AWMF), . S3-Leitlinie Diagnostik, Therapie
und Nachsorge der Patientin mit Zervixkarzinom, Langversion, 1.0,
2014, AWMF-Registernummer: 032/033OL. http://leitlinienprogramm-onkologie.de/Leitlinien.7.0.html2014.
|
|
6
|
Cohen PA, Jhingran A, Oaknin A and Denny
L: Cervical cancer. Lancet. 393:169–182. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thomas MK, Tsang SW, Yeung ML, Leung PS
and Yao KM: The roles of the PDZ-containing proteins bridge-1 and
PDZD2 in the regulation of insulin production and pancreatic
beta-cell mass. Curr Protein Pept Sci. 10:30–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Langlands FE, Dodwell D, Hanby AM, Horgan
K, Millican-Slater RA, Speirs V, Verghese ET, Smith L and Hughes
TA: PSMD9 expression predicts radiotherapy response in breast
cancer. Mol Cancer. 13:732014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Banz-Jansen C, Munchow B, Diedrich K and
Finas D: Bridge-1 is expressed in human breast carcinomas:
Silencing of Bridge-1 decreases Smad2, Smad3 and Smad4 expression
in MCF-7 cells, a human breast cancer cell line. Arch Gynecol
Obstet. 284:1543–1549. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Banz C, Munchow B and Diedrich K: Bridge-1
is expressed in human granulosa cells and is involved in the
activin A signaling cascade. Fertil Steril. 93:1349–1352. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hoellen F, Waldmann A, Banz-Jansen C,
Holtrich U, Karn T, Oberländer M, Habermann JK, Hörmann M, Köster
F, Ribbat-Idel J, et al: Claudin-1 expression in cervical cancer.
Mol Clin Oncol. 7:880–884. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hoellen F, Waldmann A, Banz-Jansen C, Rody
A, Heide M, Köster F, Ribbat-Idel J, Thorns C, Gebhard M,
Oberländer M, et al: Expression of cyclooxygenase-2 in cervical
cancer is associated with lymphovascular invasion. Oncol Lett.
12:2351–2356. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oberländer M, Alkemade H, Bünger S, Ernst
F, Thorns C, Braunschweig T and Habermann JK: A ‘waterfall’
transfer-based workflow for improved quality of tissue microarray
construction and processing in breast cancer research. Pathol Oncol
Res. 20:719–726. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German). PubMed/NCBI
|
|
15
|
Hemmerich W: Rechner zur Adjustierung des
α-Niveaus: StatistikGuru. https://statistikguru.de/rechner/adjustierung-des-alphaniveaus.htmlJuly
21–2019
|
|
16
|
McBride WH, Iwamoto KS, Syljuasen R,
Pervan M and Pajonk F: The role of the ubiquitin/proteasome system
in cellular responses to radiation. Oncogene. 22:5755–5773. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kisser A and Zechmeister-Koss I: A
systematic review of p16/Ki-67 immuno-testing for triage of low
grade cervical cytology. BJOG. 122:64–70. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu L, Fei L, Liu X, Pi X, Wang L and Chen
S: Application of p16/Ki-67 dual-staining cytology in cervical
cancers. J Cancer. 10:2654–2660. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sabeena S, Kuriakose S, Damodaran B,
Ravishankar N and Arunkumar G: Human papillomavirus (HPV) DNA
detection in uterine cervix cancer after radiation indicating
recurrence: A systematic review and meta-analysis. J Gynecol Oncol.
31:e202020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Badaracco G, Savarese A, Micheli A, Rizzo
C, Paolini F, Carosi M, Cutillo G, Vizza E, Arcangeli G and Venuti
A: Persistence of HPV after radio-chemotherapy in locally advanced
cervical cancer. Oncol Rep. 23:1093–1099. 2010.PubMed/NCBI
|
|
21
|
Song YJ, Kim JY, Lee SK, Lim HS, Lim MC,
Seo SS, Kang S, Lee DO and Park SY: Persistent human papillomavirus
DNA is associated with local recurrence after radiotherapy of
uterine cervical cancer. Int J Cancer. 129:896–902. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu MC, Austin RM, Lin J, Beck T, Beriwal
S, Comerci JT, Edwards RP, Sukumvanich P, Kelley J and Olawaiye AB:
The role of high-risk human papilloma virus testing in the
surveillance of cervical cancer after treatment. Archives of
pathology & laboratory medicine. 139:1437–1440. 2015.
View Article : Google Scholar
|
|
23
|
Deng T, Feng Y, Zheng J, Huang Q and Liu
J: Low initial human papillomavirus viral load may indicate worse
prognosis in patients with cervical carcinoma treated with surgery.
J Gynecol Oncol. 26:111–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kamer S, Ren Q and Dicker AP: Differential
radiation sensitization of human cervical cancer cell lines by the
proteasome inhibitor velcade (bortezomib, PS-341). Arch Gynecol
Obstet. 279:41–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pajonk F, Grumann T and McBride WH: The
proteasome inhibitor MG-132 protects hypoxic SiHa cervical
carcinoma cells after cyclic hypoxia/reoxygenation from ionizing
radiation. Neoplasia. 8:1037–1041. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Crawford LJ, Walker B and Irvine AE:
Proteasome inhibitors in cancer therapy. J Cell Commun Signal.
5:101–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Harish M, Kannan S, Puttagunta S, Pradhan
MR, Verma CS and Venkatraman P: A novel determinant of PSMD9 PDZ
binding guides the evolution of the first generation of super
binding peptides. Biochemistry. 58:3422–3433. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Han M, Wang H, Zhang HT and Han Z:
Expression of TIP-1 confers radioresistance of malignant glioma
cells. PLoS One. 7:e454022012. View Article : Google Scholar : PubMed/NCBI
|