Introduction
Gastric cancer is the third leading cause of
cancer-associated mortality worldwide (1). Locally advanced gastric cancer (LAGC)
is a major treatment challenge and accounts for 80% of total
gastric cancer cases in China (2,3). The
current therapeutic strategy for LAGC is multidisciplinary with a
surgical procedure as the core. Accumulating evidence has revealed
that neoadjuvant therapy improves the efficacy of LAGC compared
with surgery alone (4–7).
Since the 2014 version of the guidelines of the
Japan Society for Endoscopic Surgery, distal gastrectomy by the
laparoscopic approach was recommended for stage I gastric cancer
(8). For advanced gastric cancer
following neoadjuvant therapy, however, the safety and efficacy of
laparoscopic approach following were unclear, as oncologic outcomes
of currently ongoing randomized trials are unknown (9,10). A
number of surgeons are now actively applying laparoscopic
gastrectomy (LG) to patients with LAGC receiving neoadjuvant
therapy. To the best of our knowledge, a limited number of studies
have reported the safety and efficacy of LG following neoadjuvant
therapy, particularly in terms of long-time survival.
Therefore, the aim of the present study was to
evaluate the postoperative safety and efficacy and the long-time
survival of patients who had undergone LG compared with patients
who had undergone open gastrectomy (OG) following neoadjuvant
therapy.
Patients and methods
Patient selection
This study retrospectively reviewed the medical
records of 270 patients with LAGC who underwent LG (n=49) or
conventional OG (n=221) surgery following neoadjuvant therapy
between January 2007 and December 2016 at the China National Cancer
Center (Beijing, China). LAGC was defined as clinical stage II–III
according to the eighth edition American Joint Committee on Cancer
(AJCC)/Union for International Cancer Control (UICC)
Tumor-Node-Metastasis (TNM) gastric cancer classification (11). There were 188 male (69.6%) and 82
female (30.4%) patients (male-to-female ratio, 2.29:1; median age,
54.8 years; range, 28–84 years). The database included data on
patient demographics, clinical history, past medical history,
family history, comorbidities, diagnostic tests, tumor
characteristics, therapeutic interventions, pathological data,
postoperative parameters and survival outcomes. All data were
backed up by source documents and the accuracy of the data was
periodically reviewed. The study procedures were approved by the
Institutional Review Board at the China National Cancer Center and
the patients provided informed consent at the time of sample
collection.
Procedures
Patients received a fluoropyrimidine-based
chemoradiotherapy regimen preoperatively. Surgery was performed 2–8
weeks after completion of the neoadjuvant therapy.
Histopathological examination was evaluated according to the
Mandard Tumor Regression Grading evaluation system (12).
Follow-up
Patients were followed-up every 3 months for the
first 2 years, every 6 months for the next 3 years, and every 6
months or yearly thereafter. For the postoperative follow-up, a
physical examination, complete blood-cell count, liver function
tests, serum carcinoembryonic antigen tests and chest radiography
were performed every 3 months or 6 months; abdominal and pelvic
computed tomography (CT) were performed every 6 months.
Gastroscopic examinations were done 1 year postoperatively and once
every 2 years thereafter. When a patient missed two consecutive
scheduled visits or voluntarily withdrew consent to participate
during the follow-up period, the patient was defined as lost to
follow-up and their data were censored. The last follow-up was
completed in May 2017 and included 251 patients.
Statistical analysis
Data of continuous variables are presented as the
mean ± standard deviation, whereas categorical variable data were
presented as percentages. Patient demographic and clinical
characteristics between the two groups were compared with Student's
t-test for continuous variables with normal distribution and
χ2 test for categorical variables. The Kaplan-Meier
method was used to estimate disease-free survival (DFS) and overall
survival (OS) rates, and the log-rank test was used to compare
survival distribution. Multivariate Cox regression analysis was
used to adjust for confounding factors that were significant in
univariate analysis and for non-balanced between-group variables.
Mean survival time (months) and 95% confidence interval (CI) were
calculated using the Kaplan-Meier method. A two-sided P<0.05 was
considered to indicate a statistically significant difference. All
analyses were performed using SPSS statistical software version
24.0 (SPSS, Inc.).
Results
Between January 2007 and December 2016, 270 patients
that underwent neoadjuvant therapy received LG (n=49) or OG
(n=221). None of the patients had a metastatic lesion detected
before or during the surgery. There were no deaths during the first
3 months after surgery. The OG and LG groups were balanced in terms
of their baseline characteristics, combined comorbidities, clinical
staging before neoadjuvant therapy, and chemotherapy regimens. The
number of neoadjuvant therapy cycles was statistically different
between the two groups (P=0.016). According to RECIST criteria
(version 1.1) (13) and tumor
regression grade, response on neoadjuvant therapy was not
significantly different between the two groups (Table I).
 | Table I.Demographic and clinicopathological
characteristics in laparoscopy and open gastrectomy group. |
Table I.
Demographic and clinicopathological
characteristics in laparoscopy and open gastrectomy group.
| Variable | Laparoscopy group
(n=49)a | Open gastrectomy
group (n=221)a | P-value |
|---|
| Age, years | 54.4 (10.9) | 54.9 (11.3) | 0.80 |
| Body mass index,
kg/m2 |
|
| 0.89 |
|
<18.5 | 3 (6.1) | 11 (5.0) |
|
|
18.5–22.9 | 22 (44.9) | 91 (41.2) |
|
|
23.0–27.4 | 20 (40.8) | 94 (42.5) |
|
|
≥27.5 | 4 (8.2) | 25 (11.3) |
|
| Sex, n (%) |
|
| 0.97 |
| Male | 34 (69.4) | 154 (69.7) |
|
|
Female | 15 (30.6) | 67 (30.3) |
|
| Comorbidities, n
(%) |
|
| 0.58 |
|
Diabetes | 3 (6.1) | 16 (7.2) |
|
| Coronary
artery disease | 0 (0.0) | 11 (5.0) |
|
|
Hypertension | 10 (20.4) | 42 (19.0) |
|
| Cerebral
infraction | 0 (0.0) | 5 (2.3) |
|
|
Hypothyroidism | 0 (0.0) | 2 (0.9) |
|
| Chronic
obstructive pulmonary disease | 0 (0.0) | 3 (1.4) |
|
| Hepatitis
B | 0 (0.0) | 4 (1.8) |
|
| Hepatitis
C | 0 (0.0) | 1 (0.5) |
|
| Previous abdominal
surgery, n (%) |
|
| 0.78 |
| Yes | 5 (10.2) | 19 (8.6) |
|
| No | 44 (89.8) | 202 (91.4) |
|
| Tumor location, n
(%) |
|
| 0.74 |
| Upper
1/3 | 13 (26.5) | 54 (24.4) |
|
| Middle
1/3 | 11 (22.5) | 44 (19.9) |
|
| Low
1/3 | 9 (18.4) | 61 (27.6) |
|
|
Upper-middle | 3 (6.1) | 16 (7.2) |
|
|
Middle-low | 13 (26.5) | 45 (20.4) |
|
|
Body-antrum | 0 (0.0) | 1 (0.5) |
|
| Family history of
cancer, n (%) |
|
| 0.47 |
| Yes | 13 (26.5) | 48 (21.7) |
|
| No | 36 (73.5) | 173 (78.3) |
|
| Clinical TNM stage, n
(%) |
|
| 0.08 |
| II | 4 (8.2) | 5 (2.3) |
|
| III | 45 (91.8) | 216 (95.5) |
|
| Neoadjuvant therapy
regime, n (%) |
|
| 0.10 |
|
XELOX | 3 (6.1) | 11 (5.0) |
|
|
FOLFOX | 1 (2.0) | 19 (8.6) |
|
| SOX | 13 (26.5) | 53 (24.0) |
|
| SP | 2 (4.1) | 19 (8.6) |
|
|
TXT+XELOX | 6 (12.2) | 17 (7.7) |
|
| TCF | 5 (10.2) | 28 (12.7) |
|
|
DOS | 7 (14.3) | 20 (9.1) |
|
|
TXT+SP | 0 (0.0) | 14 (6.3) |
|
|
Others | 5 (10.2) | 28 (12.7) |
|
| Cycle of
neoadjuvant therapy, n (%) |
|
| 0.02b |
|
1-3 | 23 (46.9) | 87 (39.4) |
|
|
4-6 | 21 (42.9) | 117 (52.9) |
|
|
>6 | 0 (0.0) | 12 (5.4) |
|
| Neoadjuvant therapy
toxicity, n (%) |
|
| 0.47 |
| No
toxicity | 13 (26.5) | 74 (33.5) |
|
| Grade
I/II | 33 (67.4) | 128 (57.9) |
|
| Grade
III/IV | 3 (6.1) | 19 (8.6) |
|
| Time between
neoadjuvant therapy and surgery, days | 36.4 (15.4) | 36.9 (18.0) | 0.86 |
| RECIST criteria
(version 1.1), n (%) |
|
| 0.93 |
| Partial
response | 34 (69.4) | 150 (67.9) |
|
| Stable
disease | 13 (26.5) | 64 (29.0) |
|
|
Progressive disease | 2 (4.1) | 7 (3.1) |
|
| Gastrectomy, n
(%) |
|
| 0.63 |
|
Distal | 29 (59.2) | 117 (52.9) |
|
|
Proximal | 4 (8.2) | 27 (12.2) |
|
|
Total | 16 (32.7) | 77 (34.8) |
|
| Borrmann type
(22), n (%) |
|
| 0.37 |
| I | 4 (8.2) | 8 (3.6) |
|
| II | 18 (36.7) | 62 (28.1) |
|
|
III | 23 (46.9) | 123 (55.7) |
|
| IV | 4 (8.2) | 25 (11.3) |
|
|
Unknown | 0 (0.0) | 3 (1.4) |
|
| Lauren type
(23), n (%) |
|
| 0.35 |
|
Intestinal | 13 (26.5) | 43 (19.5) |
|
|
Diffuse | 17 (34.7) | 66 (29.9) |
|
|
Mixed | 8 (16.3) | 34 (15.4) |
|
|
Unknown | 11 (22.5) | 78 (35.3) |
|
| Primary pathology,
n (%) |
|
| 0.65 |
| Poorly
differentiated adenocarcinoma | 32 (65.3) | 146 (66.1) |
|
|
Moderately differentiated
adenocarcinoma | 10 (20.4) | 31 (14.0) |
|
| Well
differentiated adenocarcinoma | 1 (2.0) | 3 (1.4) |
|
| Signet
ring cell carcinoma | 1 (2.0) | 13 (5.9) |
|
| Minor
adenocarcinoma remains | 2 (4.1) | 6 (2.7) |
|
| No
adenocarcinoma remains (complete response) | 2 (4.1) | 18 (8.1) |
|
|
Other | 1 (2.0) | 4 (1.8) |
|
| Resected lymph
nodes, n (%) | 32.9 (13.6) | 30.0 (14.0) | 0.19 |
| Metastatic lymph
nodes, n (%) | 2.4 (3.4) | 6.0 (9.1) |
<0.0001b |
| Mandard Tumor
Regression Grading (12), n (%) |
|
| 0.06 |
| 1 | 2 (4.1) | 17 (7.7) |
|
| 2 | 10 (20.4) | 32 (14.5) |
|
| 3 | 22 (44.9) | 62 (28.1) |
|
| 4 | 2 (4.1) | 8 (3.6) |
|
| 5 | 13 (26.5) | 102 (46.2) |
|
Postoperative stay was shorter and the number of
metastatic lymph nodes harvested was lower in the LG group compared
with that in the OG group (Tables I
and II). The number of resected
lymph nodes was similar between the two groups (Table I). The incidence of complications,
surgery time, blood loss and postoperative mortality were not
significantly different between the two groups (Table II).
 | Table II.Comparison of perioperative
parameters between the laparoscopic and open gastrectomy
groups. |
Table II.
Comparison of perioperative
parameters between the laparoscopic and open gastrectomy
groups.
| Variable | Laparoscopy group
(n=49)a | Open gastrectomy
group (n=221)a | P-value |
|---|
| Complication, n
(%) | 6 (12.2) | 26 (11.8) | 0.752 |
| Central
line infection | 0 (0.0) | 1 (0.5) |
|
| Wound
infection | 0 (0.0) | 3 (1.4) |
|
| Renal
failure | 0 (0.0) | 1 (0.5) |
|
|
Multiple organ failure | 0 (0.0) | 1 (0.5) |
|
| Delayed
gastric emptying | 1 (2.0) | 1 (0.5) |
|
|
Gastrointestinal
hemorrhage | 0 (0.0) | 5 (2.3) |
|
| Pleural
effusion | 1 (2.0) | 2 (0.9) |
|
|
Pneumonia | 0 (0.0) | 3 (1.4) |
|
| Fat
liquefaction | 1 (2.0) | 2 (0.9) |
|
|
Postoperative ileus | 0 (0.0) | 3 (1.4) |
|
|
Intra-abdominal infection | 1 (2.0) | 3 (1.4) |
|
|
Duodenal stump fistula | 1 (2.0) | 1 (0.5) |
|
|
Anastomotic leak | 1 (2.0) | 4 (1.8) |
|
|
Reoperation | 1 (2.0) | 1 (0.5) |
|
|
Postoperative mortality | 0 (0.0) | 0 (0.0) |
|
| Surgery time,
min | 221.5 (69.9) | 201.1 (56.7) | 0.060 |
| Estimated blood
loss, ml | 260.2 (232.1) | 241.1 (186.3) | 0.590 |
| Time to pull
gastric tube, days | 5.5 (2.0) | 6.6 (3.3) | 0.002b |
| Postoperative stay,
days | 11.1 (4.4) | 13.0 (7.3) | 0.020b |
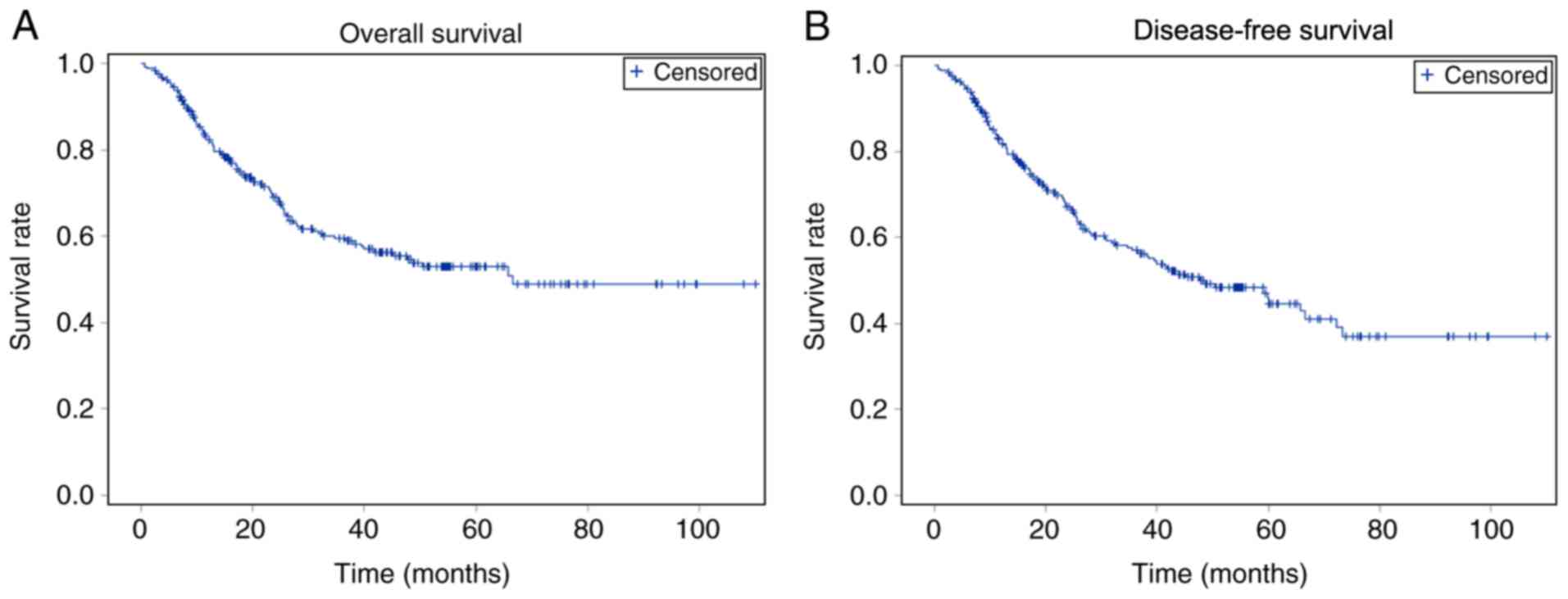
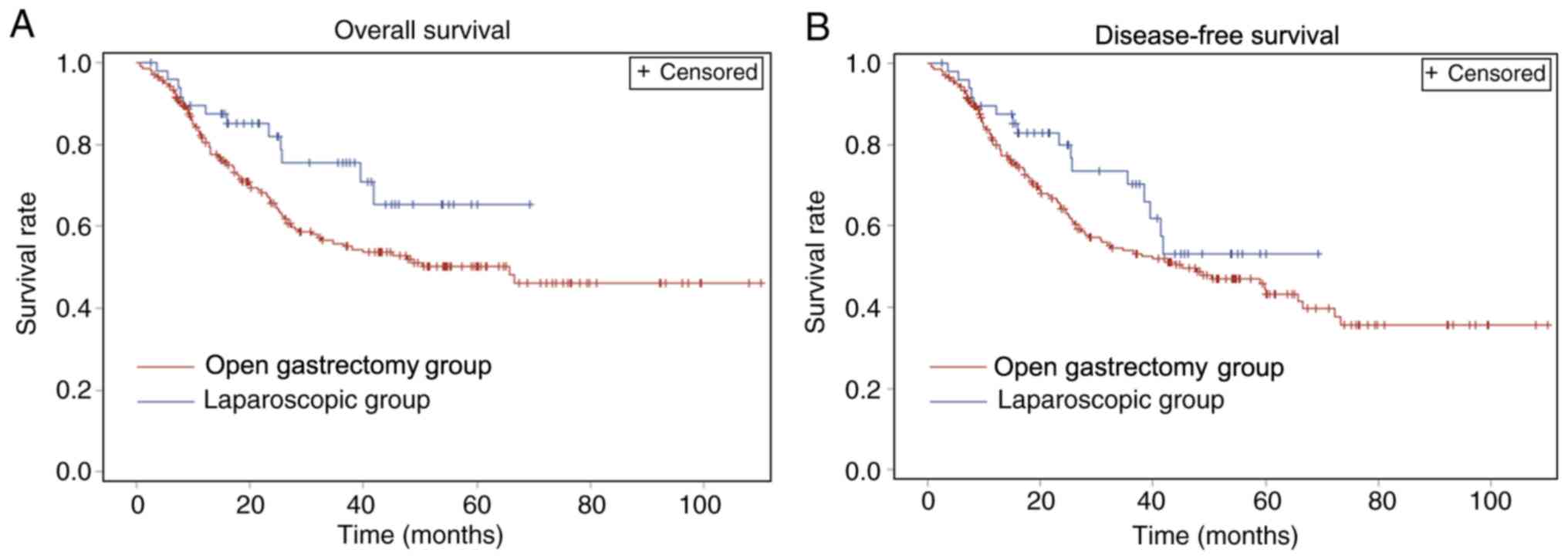
Fig. 1 shows the
rates of DFS and OS of all patients, whereas Table III and Fig. 2 demonstrate the rates of DFS and OS
in the LG and OG groups. The 75% DFS time was 15.6 (11.5–20.0)
months for the OG group and 25.7 (12.3–41.3) months for the LG
group. The 1-, 2-, 3- and 5-year OS rates for the LG group were
89.6, 82.1, 75.6 and 65.8%, respectively, and for the OG group were
81.6, 65.9, 55.9 and 49.7%, respectively. The 1-, 2-, 3- and 5-year
DFS rates for LG group were 89.6, 79.8, 70.4 and 53.3%,
respectively, and for OG group were 81.1, 64.4, 54.1 and 43.7%,
respectively. No significant difference was observed in OS and DFS
between the two groups. In addition, no significant difference was
observed in 1-, 2-, 3- and 5-year DFS and OS.
 | Table III.Comparison of survival status between
laparoscopic and gastrectomy group. |
Table III.
Comparison of survival status between
laparoscopic and gastrectomy group.
| Variable | Laparoscopy group
(n=49) | Open gastrectomy
group (n=221) | P-value |
|---|
| DFS,
monthsa | 25.7
(12.3–41.3) | 15.6
(11.5–20.0) | 0.27 |
| 1-year
rate | 0.896 | 0.811 |
|
| 2-year
rate | 0.798 | 0.644 |
|
| 3-year
rate | 0.704 | 0.541 |
|
| 5-year
rate | 0.533 | 0.437 |
|
| OS,
monthsa | 39.5
(12.3-)b | 16.1
(12.1–22.1) | 0.12 |
| 1-year
rate | 0.896 | 0.816 |
|
| 2-year
rate | 0.821 | 0.659 |
|
| 3-year
rate | 0.756 | 0.559 |
|
| 5-year
rate | 0.658 | 0.497 |
|
Discussion
According to the Clinical Practice Guidelines in
Oncology Gastric Cancer (version 2.2018), patients with potentially
resectable cT2 or higher, any N, and cM0 tumors are recommended to
receive perioperative chemotherapy (category 1) or perioperative
chemoradiaton (category 2B) (14).
Previous randomized control trials and retrospective studies have
demonstrated the safety and efficacy of LG for LAGC (15–18).
However, the evidence of safety and long-term results of
laparoscopic surgery for the treatment of LAGC after neoadjuvant
therapy were scarce.
A higher number of patients underwent conventional
OG following neoadjuvant therapy in the China National Cancer
Center compared with those that underwent LG, which may be due to
the following reasons. First, OG was selected for patients who were
diagnosed with bulky lymph nodes or lymph nodes fused together by
CT or MRI following neoadjuvant therapy. Second, a number of
surgeons in the China National Cancer Center only perform OG.
Third, several patients with severe coronary artery disease or
pulmonary disease were assigned to the OG group for surgical
safety.
The present study revealed that patients with LAGC
that underwent LG after neoadjuvant therapy had significantly
shorter postoperative stay compared to OG (11.1 vs. 13.0; P=0.020).
A randomized controlled trial has confirmed that the benefits of
the laparoscopic approach measured by early postoperative recovery
can safely be offered to select patients with LAGC (19). The results from a prospective study
also showed that laparoscopic distal gastrectomy after neoadjuvant
therapy has comparable results with open distal gastrectomy in
safety and efficacy in the short term (20).
The number of metastatic lymph nodes harvested in
the LG group was less than that in the OG group (2.4 vs. 6.0;
P<0.0001). Laparoscopic procedures have certain limitations,
such as difficult management of tumors with bulky
metastasis-positive nodes or large primary tumors, and unusual
tissue fibrosis or edema may present following neoadjuvant therapy,
which further increases surgical difficulty (21). Therefore, the majority of doctors
select conventional OG.
Resected lymph nodes, incidence of complications,
surgery time, blood loss and postoperative mortality were not
significantly different between the two groups. The results were
consistent with previous reports, which confirm the benefit of the
laparoscopic approach, measured by early postoperative recovery,
and that it can be safely offered to select patients with LAGC
after neoadjuvant therapy (14,15).
The OS and DFS of the laparoscopic group were
indicated to be longer than the open gastrectomy group; however,
there were no statistically significant differences between the two
groups. The 75% DFS time was 15.6 (11.5–20.0) months for the open
surgery group and 25.7 (12.3–41.3) months for the laparoscopic
surgery group (P=0.12). The upper confidence limit for the 75% OS
time among the laparoscopic group could not be calculated due to
the right-censoring of the data. The right-censoring may cause an
underestimate of the mean survival time and its standard error. The
major reason may be the small sample size; in addition, the
follow-up time was short. The cycle of neoadjuvant therapy was
statistically different between the two groups; however, it did not
affect the long-term survival result of the study (data not
shown).
Strengths and limitations should be considered when
interpreting the study results. To the best of our knowledge, this
cohort is the largest to date to compare the short-term and
long-term survival outcomes between OG and LG for LAGC after
neoadjuvant therapy. There were several limitations in this study.
First, it is a retrospective study with a limited sample size in a
single center. Second, patients were divided into different
surgical approach groups, based on their personal choice, because
either surgical type can be used according to doctors' clinical
judgments. Furthermore, the follow-up time was short, and follow-up
of these patients is ongoing.
The results of this study demonstrated that LG for
LAGC following neoadjuvant therapy may provide non-inferior
short-term and long-term survival outcomes compared with open
surgery, suggesting a laparoscopic approach may be justified for
patients with LAGC after neoadjuvant therapy. Multicenter
randomized controlled trials are required to investigate the
positive effects of LG for LAGC following neoadjuvant therapy.
Acknowledgements
Not applicable.
Funding
This research was supported by The National Key
R&D Program of China (grant. no. 2017YFC0908300).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC, DZ, AZ and JJ conceived and designed the study.
NW and HH collected the data. YC and YZ analyzed the data. NW wrote
the manuscript. YC and DZ reviewed and revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All study procedures were approved by the
Institutional Review Board at the China National Cancer Center and
the patients provided written informed consent at the time of
sample collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nashimoto A, Akazawa K, Isobe Y, Miyashiro
I, Katai H, Kodera Y, Tsujitani S, Seto Y, Furukawa H, Oda I, et
al: Gastric cancer treated in 2002 in Japan: 2009 annual report of
the JGCA nationwide registry. Gastric Cancer. 16:1–27. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cunningham D, Allum WH, Stenning SP,
Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ,
Falk SJ, Iveson TJ, et al: Perioperative chemotherapy versus
surgery alone for resectable gastroesophageal cancer. N Engl J Med.
355:11–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoshikawa T, Sasako M, Yamamoto S, Sano T,
Imamura H, Fujitani K, Oshita H, Ito S, Kawashima Y and Fukushima
N: Phase II study of neoadjuvant chemotherapy and extended surgery
for locally advanced gastric cancer. Br J Surg. 96:1015–1022. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ychou M, Boige V, Pignon JP, Conroy T,
Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM,
Saint-Aubert B, et al: Perioperative chemotherapy compared with
surgery alone for resectable gastroesophageal adenocarcinoma: An
FNCLCC and FFCD multicenter phase III trial. J ClinOncol.
29:1715–1721. 2011. View Article : Google Scholar
|
|
7
|
Tsuburaya A, Mizusawa J, Tanaka Y,
Fukushima N, Nashimoto A and Sasako M; Stomach Cancer Study Group
of the Japan Clinical Oncology Group, : Neoadjuvant chemotherapy
with S-1 and cisplatin followed by D2 gastrectomy with para-aortic
lymph node dissection for gastric cancer with extensive lymph node
metastasis. Br J Surg. 101:653–660. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Japanese Gastric Cancer Association, .
Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric
Cancer. 20:1–19. 2017. View Article : Google Scholar
|
|
9
|
Inaki N, Etoh T, Ohyama T, Uchiyama K,
Katada N, Koeda K, Yoshida K, Takagane A, Kojima K, Sakuramoto S,
et al: A Multi-institutional, prospective, phase II feasibility
study of laparoscopy-assisted distal gastrectomy with D2 lymph node
dissection for locally advanced gastric cancer (JLSSG0901). World J
Surg. 39:2734–2741. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hur H, Lee HY, Lee HJ, Kim MC, Hyung WJ,
Park YK, Kim W and Han SU: Efficacy of laparoscopic subtotal
gastrectomy with D2 lymphadenectomy for locally advanced gastric
cancer: The protocol of the KLASS-02 multicenter randomized
controlled clinical trial. BMC Cancer. 15:3552015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
In H, Solsky I, Palis B, Langdon-Embry M,
Ajani J and Sano T: Validation of the 8th Edition of the AJCC TNM
staging system for gastric cancer using the national cancer
database. Ann Surg Oncol. 24:3683–3691. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mandard AM, Dalibard F, Mandard JC, Marnay
J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P and Samama
G: Pathologic assessment of tumor regression after preoperative
chemoradiotherapy of esophageal carcinoma. Clinicopathologic
correlations. Cancer. 73:2680–2686. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
National Comprehensive Cancer Network, .
NCCN Clinical Practice Guidelines in Oncology Gastric Cancer
(version 2.2018). National Comprehensive Cancer Network. (Fort
Washington, DC). 2018.
|
|
15
|
Lee J and Kim W: Long-term outcomes after
laparoscopy-assisted gastrectomy for advanced gastric cancer:
Analysis of consecutive 106 experiences. J Surg Oncol. 100:693–698.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Du XH, Li R, Chen L, Shen D, Li SY and Guo
Q: Laparoscopy-assisted D2 radical distal gastrectomy for advanced
gastric cancer: Initial experience. Chin Med J (Engl).
122:1404–1407. 2009.PubMed/NCBI
|
|
17
|
Yu J, Hu J, Huang C, Ying M, Peng X, Wei
H, Jiang Z, Du X, Liu Z, Liu H, et al: The impact of age and
comorbidity on postoperative complications in patients with
advanced gastric cancer after laparoscopic D2 gastrectomy: Results
from the Chinese laparoscropic gastrointestinal surgery study
(CLASS) group. Eur J Surg Oncol. 39:1144–1149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu Y, Ying M, Huang C, Wei H, Jiang Z,
Peng X, Hu J, Du X, Wang B, Lin F, et al: Oncologic outcomes of
laparoscopy-assisted gastrectomy for advanced gastric cancer: A
large-scale multicenter retrospective cohort study from China. Surg
Endosc. 28:2048–2056. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J,
Xue Y, Suo J, Tao K, He X, et al: Morbidity and mortality of
laparoscopic versus open D2 distal gastrectomy for advanced gastric
cancer: A randomized controlled trial. J Clin Oncol. 34:1350–1357.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Z, Shan F, Wang Y, Li S, Jia Y, Zhang
L, Yin D and Ji J: Laparoscopic versus open distal gastrectomy for
locally advanced gastric cancer after neoadjuvant chemotherapy:
Safety and short-term oncologic results. Surg Endosc. 30:4265–4271.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kinoshita T and Kaito A: Current status
and future perspectives of laparoscopic radical surgery for
advanced gastric cancer. Transl Gastroenterol Hepatol. 2:432017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li C, Oh SJ, Kim S, Hyung WJ, Yan M, Zhu
ZG and Noh SH: Macroscopic Borrmann type as a simple prognostic
indicator in patients with advanced gastric cancer. Oncology.
77:197–204. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lauren P: The two histologic main types of
gastric carcinoma: Diffuse and so-called intestinal type carcinoma,
an attempt at a histo-clinical classification. Acta Parhol Microb
Scan. 64:31–49. 1965. View Article : Google Scholar
|