Introduction
Laryngeal cancer remains one of the most frequently
detected head and neck cancer types (1); ~40% of affected patients are diagnosed
in the early stage, mostly due to seeking medical treatment for
hoarseness (2,3). According to the most recent National
Comprehensive Cancer Network guidelines (NCCN), early laryngeal
cancers of stage I (T1N0M0) and stage II (T2N0M0) should be treated
with definitive radiation therapy (RT) or surgery, such as
laryngeal laser surgery or partial laryngectomy (4). It has been reported that treatment
outcomes in terms of local control, laryngeal preservation and
survival rates are similar between those methods in patients with
T1N0M0 and T2N0M0 laryngeal cancer (3–6).
The local control rate after RT alone is reportedly
80–95% in patients with T1N0M0 laryngeal cancer (5–7);
however, the results change in T2N0M0 disease. Treatment outcomes
of T2N0M0 laryngeal cancer via RT alone and organ-preserving
surgery, with 50–85% local control rates, are insufficient compared
with those of T1N0M0 laryngeal cancer (7–11). Among
the various attempts to improve the treatment outcomes of patients
with T2N0M0 laryngeal cancer, previous studies have suggested
combining chemotherapy with RT (7,12,13).
Moreover, chemoradiation therapy (CRT) has been revealed to
increase the radiosensitivity of cancer cells, thus maximizing the
local control effect (14–16). In stage III and IV laryngeal cancer,
the comparative advantage of CRT has been reported in several
randomized trials (17,18). In T2N0M0 laryngeal cancer, however,
no randomized trials have compared treatment outcomes among RT
alone, CRT or surgery-based treatment (SBT), and retrospective
studies have been limited.
Therefore, the present study compared the long-term
treatment outcomes, including overall survival (OS) rates,
disease-specific survival (DSS) rates, local control rates and
disease-free survival (DFS) rates, of patients with T2N0M0
laryngeal cancer treated with RT, CRT or SBT. Furthermore, this
study aimed to provide an important basis for identifying the
impact of CRT on improving the prognosis of patients with T2N0M0
laryngeal cancer.
Patients and methods
Patient selection
Between March 2005 and July 2015, consecutive
patients with previously untreated T2N0M0 laryngeal squamous
carcinoma, who were treated in Chonnam National University Hwasun
Hospital (Hwasun, South Korea) were enrolled in the present study.
This study was conducted in a tertiary referral hospital with a
regional cancer center in South Korea. TNM staging, according to
the 8th American Joint Committee on Cancer staging system was used
(19). In all patients, flexible
laryngoscopic examination, diagnostic biopsy, computed tomography
(CT) and 18F-FDG positron emission tomography (PET)-CT
were performed for accurate staging before the treatment, and these
imaging examinations were reviewed to prevent inappropriate
enrollment in this study. Patients for whom a full record of their
staging, treatment method and follow-up results, including
radiologic studies and physical examinations, was unavailable were
excluded. After a retrospective review of the charts of 86
patients, three patients were excluded. In total, two patients did
not complete RT, due to financial problems or treatment refusal,
while one patient had double primary cancer (lung and larynx),
which could have confounded the interpretation of the treatment
outcomes. Thus, 83 patients (81 men and 2 women; age range, 47–85
years; mean age, 65.8 years), were ultimately enrolled in this
study.
Treatment methods
The enrolled patients were classified by treatment
method into the RT-only (n=27), CRT (n=46) and SBT (n=10) groups.
The treatment decision for patients was determined by considering
numerous factors, including lung and kidney function, the
profession of the patient (voice demand), lifestyle requirements
and psychological support, as well as the cancer extent (20,21).
All patients in the RT-only and CRT groups underwent
RT with curative intent. External beam RT was performed using
three-dimensional RT (3D-CRT) or intensity modulated RT (IMRT). The
patients received 3D-CRT with 6 MV photon beams produced by a
Clinac IX (Varian Medical Systems, Inc.) using the field-shrinking
technique (22). IMRT was conducted
using a Clinac IX or TomoTherapy (Accuray, Inc.). The gross target
volume (GTV) was the macroscopic extent of laryngeal disease on CT,
magnetic resonance imaging and PET-CT. The clinical target volume
(CTV) was delineated as a 1–2 cm margin from GTV, with
consideration of microscopic disease or larynx with a field of 6×6
cm. The radiation dose for CTV was administered at 2 Gy per dose,
66–70 Gy, five times a week. Elective nodal irradiation, including
bilateral level II, III and IV, was performed with a dose of 45 Gy.
Most patients, except three patients who underwent surgery only,
received bilateral elective nodal irradiation. Patients in the CRT
group underwent induction chemotherapy with either radiotherapy or
concurrent CRT (CCRT), or CCRT only. Then, two cycles of induction
chemotherapy were performed using cisplatin (75 mg/m2)
and 5-fluorouracil (5-FU) (1,000 mg/m2) with/without
docetaxel (70 mg/m2). Both followed CCRT, patients who
had induction chemotherapy with CCRT, and CCRT only, patients who
had CCRT only without induction chemotherapy, was performed with
cisplatin-based treatment with RT. Dosage of chemotherapy was
reduced to 75% of the original dose in subsequent cycles when
patients had toxicity >grade III hematologic or non-hematologic
toxicity. After induction chemotherapy, flexible laryngoscopic exam
and CT was performed to assess the response of chemotherapy. If
some patients had progressive or stable disease, surgery was
planned instead of RT or CCRT. However, none of the patients
underwent surgery on poor response of induction chemotherapy.
SBT-grouped patients had surgery only or surgery
with adjuvant RT. The adjuvant RT was performed as aforementioned.
The radiation dose of adjuvant RT was administered at 50–68 Gy.
Patient characteristics
The characteristics of the 83 enrolled patients (men
81; women 2; mean age, 65.8±8.0 years; age range, 47–85 years) are
shown in Table I. The mean patient
follow-up period was 70.8±37.9 months (follow-up range, 10.7–153.3
months). The distribution of the primary subsite was as follows:
Glottis, 43 cases; supraglottis, 36 cases; and subglottis, four
cases. In 43 patients with glottic cancer, 42 patients, except one
patient with impaired vocal cord mobility, had the tumor extent to
the supraglottis or subglottis. In 36 patients with supraglottic
cancer, all patients had the tumor invade the mucosa of the
adjacent subsite of supraglottis or glottis. In four patients with
subglottic cancer, all patients had tumor extend to the vocal
cords. With regards to the smoking history, 48 patients (57.8%)
were current smokers, 33 patients (39.8%) were ex-smokers and two
patients (2.4%) did not have a smoking history. For alcohol
history, 50 patients (60.2%) were social drinkers, ten patients
(12%) were heavy drinkers and 23 patients (27.7%) did not have an
alcohol history. For primary treatment, 27 patients had RT alone,
46 patients had CRT and ten underwent SBT. Given that the
distribution of primary sites was different for each treatment
group, subgroup analysis was performed, according to the primary
site, when analyzing the treatment outcome. In addition, analyses
concerning anterior commissure and subglottic extension, which can
effect glottic cancer treatment outcomes, were also performed.
 | Table I.Characteristics of patients. |
Table I.
Characteristics of patients.
| Parameters | Total (n=83) | RT alone (n=27) | CRT (n=46) | SBT (n=10) | P-value |
|---|
| Age, years | 65.8 (47–85) | 67.5 (47–85) | 64.5 (51–77) | 66.9 (53–76) | 0.292 |
| Sex |
|
|
|
| 0.214 |
| Male | 81 | 27 | 45 | 9 |
|
|
Female | 2 | 0 | 1 | 1 |
|
| Primary
subsite |
|
|
|
| 0.002 |
|
Glottis | 43 | 21 | 20 | 2 |
|
|
Supraglottis | 36 | 6 | 22 | 8 |
|
|
Subglottis | 4 | 0 | 4 | 0 |
|
| RT dose, Gy | 66.6
(50.4–71.0) | 67.2
(57.5–71.0) | 66.9
(57.6–71.0) | 62.5
(50.4–68.4) | 0.081 |
| Follow-up duration,
months | 70.8
(10.7–153.3) | 72.2
(14.1–149.9) | 72.6
(10.7–145.6) | 58.8
(17.1–153.3) | 0.573 |
Treatment response assessment and
toxicity evaluation
Treatment response for the primary lesion and
evaluation of lymph nodes and distant metastasis were assessed via
physical examination involving flexible laryngoscope exam, CT and
PET-CT in the fourth and twelfth weeks after the end of the first
treatment. Regular follow-up was performed every 1–6 months
thereafter.
Recurrence was defined as the first treatment
failing due to the primary lesion or other sites including nodal or
distant metastasis (7). Local
control was defined as cure of the primary lesion without
recurrence after the first treatment (7). Patients who experienced recurrence
underwent salvage treatments such as surgery, chemotherapy or RT.
Furthermore, treatment failure was defined as a disease with
inoperable locoregional progression or distant metastasis despite
the salvage treatment (7). The
recurrence rate, local control rate and treatment failure rate were
defined as an event occurring within the follow-up period without
regular periods as 3- or 5-year.
OS was calculated from the date of the first
diagnosis to mortality, or the last follow-up. DSS was calculated
from the date of the first diagnosis to laryngeal cancer-associated
mortality or the last follow-up. DFS was calculated from the date
of diagnosis to the date of the first recurrence or the last
follow-up.
Acute toxicity during RT and chemotherapy was graded
according to the National Cancer Institution (NCI) Common Toxicity
Criteria (CTC) version 5.0 (23).
Laboratory results regarding hematologic, hepatic, renal and
non-hematologic complications such as dermatitis, mucositis,
nausea, diarrhea and fever, occurring after and during treatment
were reviewed.
Statistical analysis
Survival curves were calculated by the Kaplan-Meier
method and compared using the log-rank test, and the Renyi-type
test from the survMisc Package in R. Multivariate analysis was
performed using by Cox's regression. Unpaired t-test and Fisher's
exact test were used to compare between two groups. ANOVA and
nonparametric Kruskal-Wallis test was used for multiple comparison
and the post hoc test was performed by Bonferroni. Analyses were
performed using SPSS version 23.0 (SPSS, Inc.) and R version 3.9.2
(R Foundation for Statistical Computing). P<0.05 was considered
to indicate a statistically significant difference.
Result
Local control, recurrence and
treatment failure
For primary treatment, 27 patients had RT alone, 46
patients had CRT and ten underwent SBT. The difference in the
distribution of the primary subsite was significant among the
treatment groups (Table I; P=0.002).
Accordingly, when analyzing treatment outcomes, subgroup analysis
by primary subsite was conducted. No significant differences were
observed in age, sex or RT dose (Table
I).
Recurrence was observed in 26/83 patients. Moreover,
the RT, CRT and SBT groups had significantly different recurrence
rates of 44.4% (12/27), 19.6% (9/46) and 50% (5/10) (Table II; P=0.03). In the subgroup
analysis, the CRT group had a significantly lower recurrence rate
compared with the RT group (P=0.03). Among the 26 patients, 12 were
treated successfully. After salvage treatment, treatment failure
was not associated with treatment group (Table II; P=0.24).
 | Table II.Pattern of the recurrence and
treatment failure according to the treatment method. |
Table II.
Pattern of the recurrence and
treatment failure according to the treatment method.
| Parameters | Total (n=83) | RT (n=27) | CRT (n=46) | SBT (n=10) | P-value |
|---|
| Recurrence |
|
|
|
| 0.03 |
| No | 57 (68.7%) | 15 (55.6%) | 37 (80.4%) | 5 (50%) |
|
|
Yes | 26 (31.3%) | 12 (44.4%) | 9 (19.6%) | 5 (50%) |
|
| Recur sites |
|
|
|
|
|
| Primary
recur | 20 | 12 | 6 | 2 |
|
| Nodal
recur | 5 | 0 | 4 | 1 |
|
| Distant
metastasis | 9 | 4 | 3 | 2 |
|
| Treatment
failure |
|
|
|
| 0.24 |
| No | 69 (93.1%) | 20 (74.1%) | 41 (89.1%) | 8 (80%) |
|
|
Yes | 14 (16.9%) | 7 (25.9%) | 5 (10.9%) | 2 (20%) |
|
Among the 27 patients in the RT group, 12
experienced recurrence in the primary site and four had distant
metastasis. Furthermore, the local control rate of the RT group was
55.6% (15/27). Among these 12 patients, five were successfully
salvaged by surgery and adjuvant chemotherapy. However, after
salvage treatment, seven patients (25.9%) experienced treatment
failure.
Among the 46 patients in the CRT group, six
experienced recurrence in the primary site, four had nodal
recurrence and three had distant metastasis. Moreover, the local
control rate of the CRT group was 87.0% (40/46). The CRT group also
had a significantly higher local control rate compared with the RT
group. Among these nine patients, four were successfully salvaged
by surgery, adjuvant chemotherapy and RT. After salvage treatment,
five (10.9%) experienced treatment failure.
Among the ten patients in the SBT group, two
experienced recurrence in the primary site, one had nodal
recurrence and two had distant metastasis. The local control rate
of the SBT group was 80.0% (8/10). Among these five patients, three
could be successfully salvaged by surgery, adjuvant chemotherapy
and RT, while two (20%) experienced treatment failure.
Survival rates
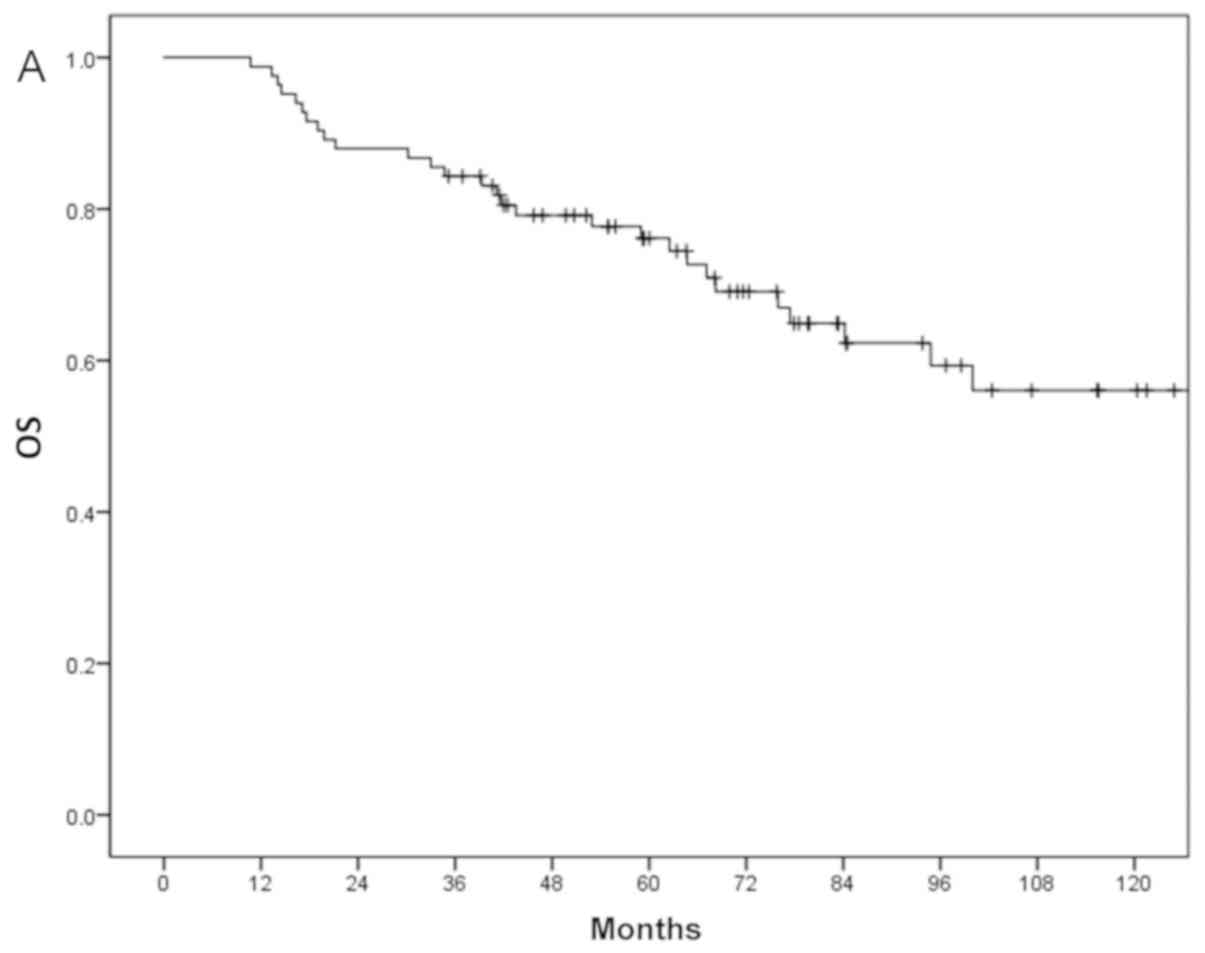
The 3- and 5-year OS of all patients were 79 and
69%, respectively (Fig. 1A).
Moreover, the 3- and 5-year DSS of all patients were 87 and 86%,
respectively (Fig. 1B). During a
mean long-term follow-up duration of 70.8 months, 12 mortalities
occurred due to disease progression and 16 due to causes unrelated
to the laryngeal cancer, such as second primary cancer (lung cancer
in two patients; esophageal cancer, hepatocellular carcinoma,
cholangiocarcinoma, pancreatic cancer and myelodysplastic syndrome
in one patient each); pneumonia in 5; and brain hemorrhage,
esophageal variceal bleeding, urinary tract infection and unknown
in one patient each. In addition, the 3- and 5-year DFS of all
patients were both 68% (Fig.
1C).
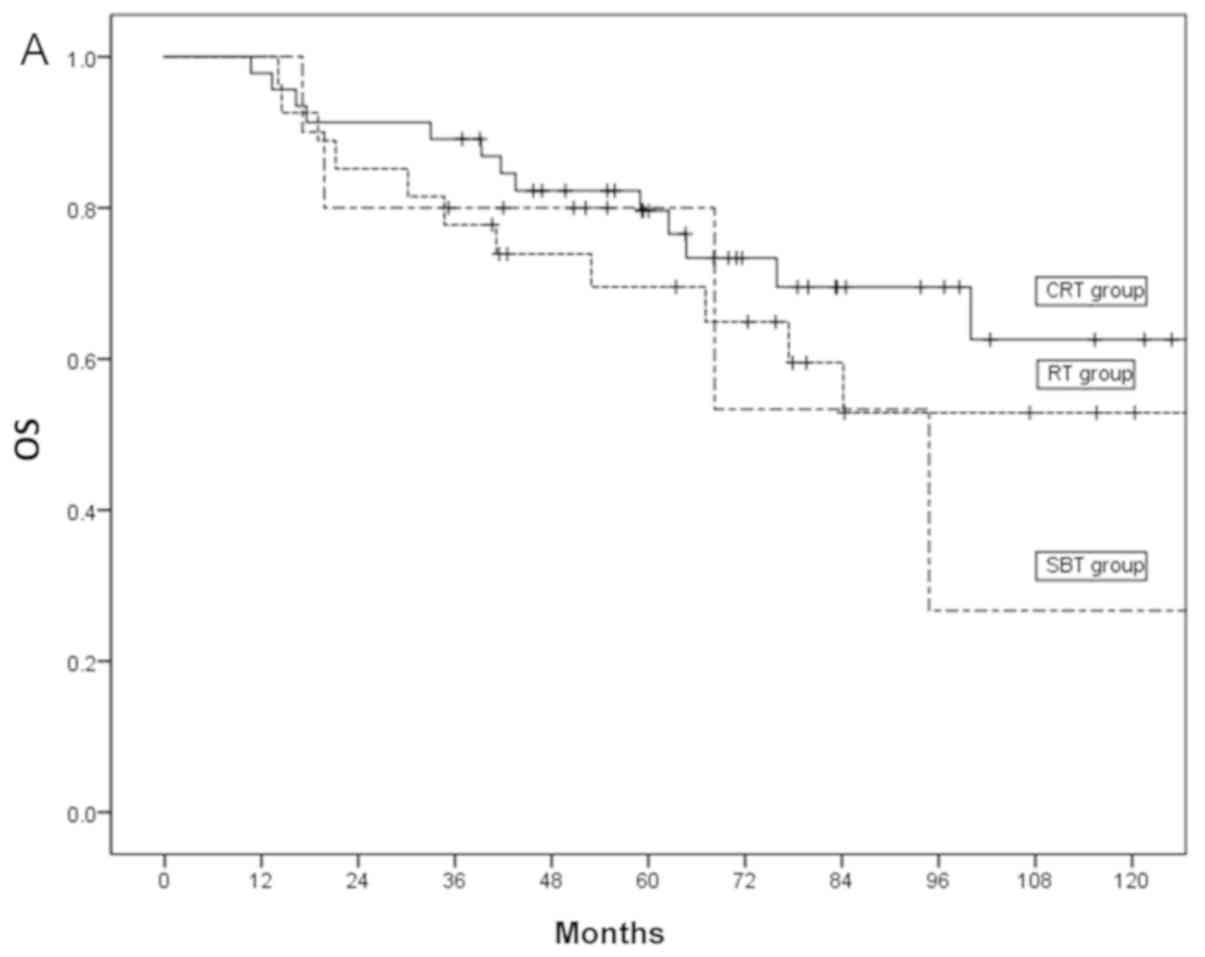
The 3- and 5-year OS of the RT group were 74 and
65%, those of the CRT group were 82 and 73%, and those of the SBT
group were 80 and 53%, respectively (P=0.23; Fig. 2A). The 3- and 5-year DSS of the RT
group were 85 and 80%, while those of the CRT and SBT groups were
both 89% (P=0.29; Fig. 2B).
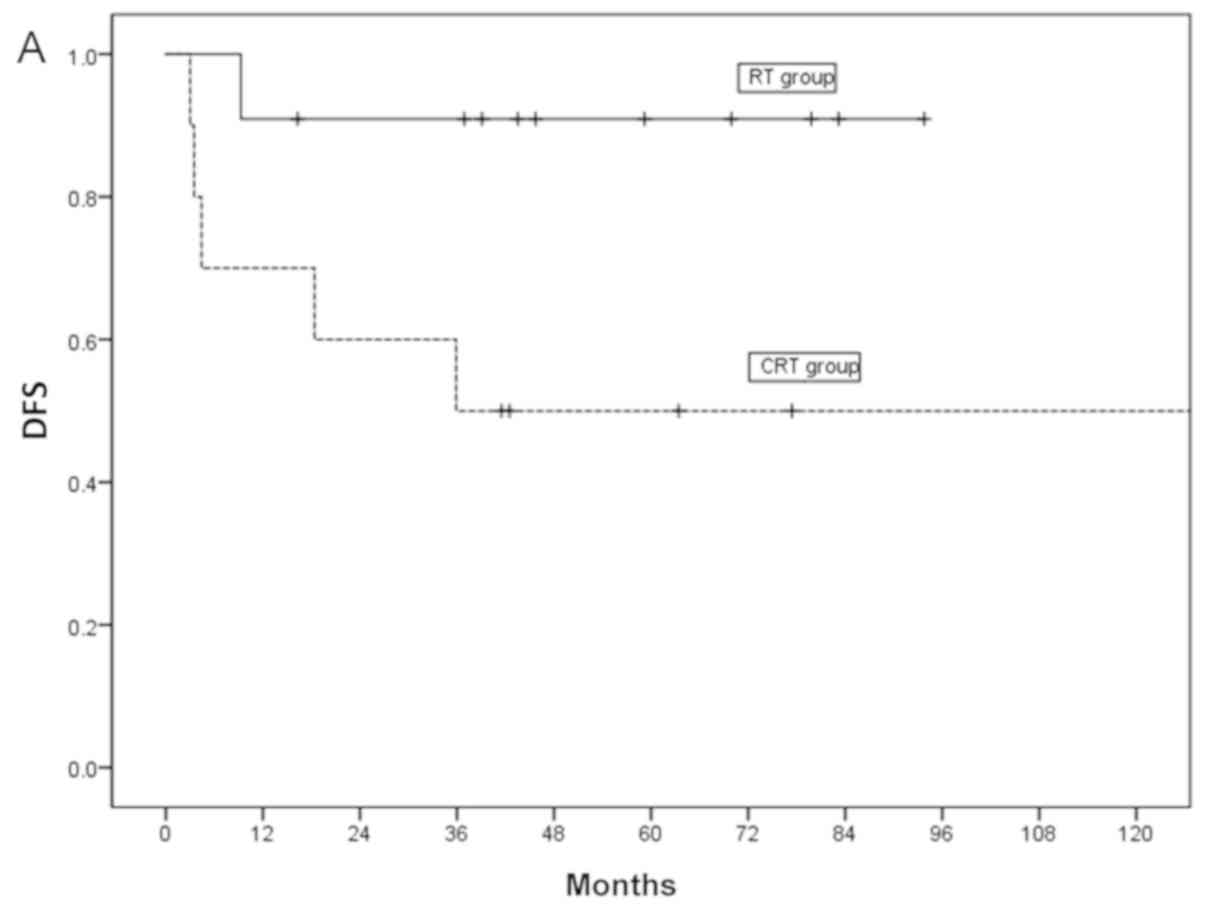
However, the 3- and 5-year DFS in the RT group was
both 55%, those of the SBT group were both 50% and those of the CRT
group were both 80%. Moreover, the DFS demonstrated significant
differences among treatment methods (P=0.02; Fig. 2C). In the subgroup analysis, the DFS
of the CRT group was significantly higher compared with those of
the RT and SBT groups (P=0.02). To assess the significant factors
for DFS, multivariate Cox regression analysis was performed of the
following variables: Age, sex, treatment method and RT dose. Among
the variables, the treatment method was the most important factor
that influenced DFS, and CRT had a superior prognosis compared with
RT and SBT (Table III).
 | Table III.Results of the multivariate analysis
of the disease-free survival rate. |
Table III.
Results of the multivariate analysis
of the disease-free survival rate.
| Parameters | Hazard ratio
[Exp(B)] | 95% CI | P-value |
|---|
| Age | 0.99 | 0.94–1.04 | 0.72 |
| Primary
subsite |
|
| 0.87 |
|
Glottis | 1.0 (ref) |
|
|
|
Supraglottis | 1.19 | 0.43–3.30 | 0.72 |
|
Subglottis | 1.65 | 0.18–15.2 | 0.66 |
| RT dose | 0.99 | 0.99–1.00 | 0.41 |
| Treatment
method |
|
| 0.01 |
|
CRT | 1.0 (ref) |
|
|
| RT | 3.8 | 1.35–10.75 | 0.01 |
|
SBT | 6.7 | 1.56–29.39 | 0.01 |
Treatment outcomes of glottic
cancer
In total, 43 patients with glottic cancer were
enrolled in this study: 21 had RT alone, 20 had CRT and two had
SBT. Of them, 35 (81.4%) had anterior commissure (AC) invasion,
while 23 patients (53.5%) had AC and subglottic invasion (data not
shown). The SBT group was small, and thus the treatment outcomes of
only the RT and CRT groups were compared. A total of 13 patients
(30.2%) experienced recurrence. Furthermore, the CRT group had a
tendency towards a lower recurrence rate compared with the RT group
(42.9% in RT vs. 15% in CRT); however, there was no statistical
significance (P=0.09; data not shown). It was indicated that the
DFS of the CRT group was higher compared with the RT group (56% 3-
and 5-year DFS in RT vs. 85% in CRT); however, this difference was
not statistically significant (P=0.053). In addition, no
significant differences were seen in OS and DSS according to RT and
CRT groups (P>0.05; data not shown).
To evaluate the impact of AC and subglottic invasion
on treatment outcomes of glottic cancer, a subgroup analysis was
performed according to AC and subglottic invasion status. In 21
patients with AC and subglottic invasion, the DFS of the CRT group
was significantly higher compared with the RT group (P=0.045;
Fig. 3A). However, in 20 patients
without subglottic invasion, DFS did not differ between the RT and
CRT groups (P=0.47; Fig. 3B).
Treatment outcomes of supraglottic
cancer
In total, 36 patients with supraglottic cancer were
enrolled in this study: Six had RT alone, 22 had CRT and eight had
SBT (Table I). Moreover, 12 patients
(33.3%) experienced recurrence (data not shown). The CRT group had
a lower recurrence rate compared with the RT and SBT groups (50.0%
in RT and SBT vs. 22.7% in CRT, data not shown); however, there was
no statistical significance (P=0.24; data not shown). Furthermore,
no significant differences were seen in OS, DSS and DFS according
to treatment methods (P>0.05; data not shown).
Toxicity
The RT and CRT groups were graded according to
NCI-CTC version 5.0 (Table IV). It
was found that more patients in the CRT group compared with the RT
group showed symptoms of toxicity. Furthermore, the RT group had
grade I dermatitis, followed by mucositis. Although the RT group
demonstrated more non-hematologic toxicity, neutropenia was the
most common finding in hematologic toxicity. However, neutropenia
occurred with grade I toxicity, which was not life threatening.
Moreover, there were no cases of grade IV toxicity among the RT
group, and only four grade III events were identified. In addition,
no treatment-associated mortalities occurred in the RT group.
 | Table IV.Toxicity occurring in RT and CRT
groups was graded according to National Cancer Institution Common
Toxicity Criteria version 5.0. |
Table IV.
Toxicity occurring in RT and CRT
groups was graded according to National Cancer Institution Common
Toxicity Criteria version 5.0.
| Grade | I | II | III | IV | Total |
|---|
| RT group (n) |
|
|
|
|
|
|
Hematologic |
|
|
|
|
|
|
Anemia | 5 | 2 | 1 | 0 | 8 |
|
Leukopenia | 1 | 1 | 0 | 0 | 2 |
|
Neutropenia | 19 | 1 | 0 | 0 | 20 |
|
Thrombocytopenia | 1 | 0 | 0 | 0 | 1 |
|
Hepatic |
|
|
|
|
|
|
LFT | 3 | 0 | 0 | 0 | 3 |
|
Renal |
|
|
|
|
|
|
Cr |
|
|
|
|
|
|
Non-Hematologic | 4 | 1 | 0 | 0 | 5 |
|
Nausea | 0 | 0 | 1 | 0 | 1 |
|
Diarrhea | 1 | 0 | 0 | 0 | 1 |
|
Fever | 0 | 0 | 0 | 0 | 0 |
|
Dermatitis | 23 | 2 | 1 | 0 | 26 |
|
Mucositis | 8 | 9 | 1 | 0 | 18 |
| CRT group (n) |
|
|
|
|
|
|
Hematologic |
|
|
|
|
|
|
Anemia | 23 | 15 | 1 | 0 | 39 |
|
Leukopenia | 15 | 15 | 6 | 2 | 38 |
|
Neutropenia | 17 | 15 | 6 | 4 | 42 |
|
Thrombocytopenia | 16 | 1 | 3 | 1 | 21 |
|
Hepatic |
|
|
|
|
|
|
LFT | 4 | 2 | 1 | 0 | 7 |
|
Renal |
|
|
|
|
|
|
Cr |
|
|
|
|
|
|
Non-Hematologic | 13 | 5 | 0 | 0 | 18 |
|
Nausea | 10 | 3 | 1 | 0 | 14 |
|
Diarrhea | 6 | 0 | 0 | 0 | 6 |
|
Fever | 3 | 0 | 0 | 0 | 3 |
|
Dermatitis | 34 | 9 | 0 | 0 | 43 |
|
Mucositis | 22 | 11 | 2 | 1 | 36 |
In the CRT group, neutropenia and dermatitis were
the most common findings according to the toxicity criteria. Unlike
the RT group, which had distinctive neutropenia, the CRT group
demonstrated various toxicities resulting in abnormal hematological
findings, mostly neutropenia, anemia and leukopenia. Moreover, the
CRT group had higher toxicity in non-hematologic categories.
Compared with the RT group, the CRT group also demonstrated
significantly more symptoms, such as nausea and diarrhea. However,
dermatitis and mucositis were commonly found in the CRT group as in
the RT group. In total, eight patients in the CRT group were
categorized as having grade IV toxicity. However, one patient
delayed the CRT for 2 months due to severe thrombocytopenia; after
recovering, the patient completed the remaining RT treatments. It
was also identified that no treatment-associated mortalities
occurred in the CRT group.
Discussion
The larynx is an essential organ for speech and
swallowing that greatly impacts the quality of life (20). Thus, the goal in the treatment of
laryngeal cancer is to treat the disease, while preserving
laryngeal function and quality of life (3). However, the requirement to preserve
laryngeal function further complicates the decision of treatment
methods for laryngeal cancer (21).
Moreover, the treatment decision process for patients with
laryngeal cancer has to consider numerous factors, such as cancer
extent, lung function, the occupation of the patients, lifestyle
requirements and psychological support (21). Early laryngeal cancer (T1N0M0 and
T2N0M0) is characterized by a low-volume tumor and a low incidence
of nodal metastasis; thus, the effective local control of primary
treatment is important for laryngeal preservation (5).
There are several guidelines for laryngeal cancer by
the NCCN, American Society of Clinical Oncology and United Kingdom
National Multidisciplinary Specialty Associations, which recommend
that a single treatment modality, including larynx-preserving
surgery or RT alone, for early laryngeal cancer are accepted
treatment options (4,21,24).
However, treatment outcomes of T2N0M0 laryngeal cancer are
distinctively worse compared with those of T1N0M0 laryngeal cancer,
despite both stages being considered the early stage (24,25).
Patients with T2N0M0 in particular have a 25–50% recurrence rate,
which is ≥2 times higher compared with that of patients with
T1N0M0, due to local control failure in laryngeal cancer (25). Previous studies have identified
consistent insufficient treatment outcomes in terms of survival
rate, such as laryngeal-preserving survival rate, OS and DSS, in
T2N0M0 laryngeal cancer (10,11).
Therefore, the present study focused on comparing long-term
treatment outcomes according to treatment method in patients with
T2N0M0 laryngeal cancer. Furthermore, patients in this study were
divided into RT, CRT and SBT groups by their initial treatment
method. However, there were only ten patients in the SBT group,
which is insufficient to enable assessment of the treatment
outcomes of the SBT group, and thus is a limitation of the study;
although the comparison between the RT and CRT groups remains
important.
In this study, the CRT group had a significantly
higher local control rate (87.0 vs. 55.6%), lower recurrence rate
(19.6 vs. 44.4%) and higher DFS (80 vs. 55% in 5 years) compared
with the RT group. The multivariate analysis results also suggested
that the treatment method was the most significant factor for DFS,
in that the CRT group had a superior prognosis compared with the RT
and SBT groups. However, there was no significant intergroup
difference in OS or DSS, as improved salvage treatment after
recurrence could dilute the adverse effects of recurrence on OS and
DSS. Akimoto et al (7)
reported that voice preservation and DFS in patients treated with
CRT are significantly superior compared with those of RT alone in
T2N0M0 laryngeal cancer. Previous studies by Furusaka et al
(12) and Nishimura et al
(13) also revealed that CRT
improves laryngeal preservation compared with RT alone in T2N0M0
laryngeal cancer. Thus, the results of these previous studies are
consistent with those of the present study. In laryngeal cancer,
local control is directly associated with laryngeal preservation
(14). With the development of the
larynx-preserving surgery technique, larynx-preserving surgery is
possible without total laryngectomy, even in patients with
recurrent laryngeal cancer after RT (14). However, in these cases, due to the
serious quality of life deterioration, such as chronic aspiration,
the improvement in local control is essential for improving
functional laryngeal preservation (17). Thus, in T2N0M0 laryngeal cancer, CRT
may be an alternative method that improves local control and, in
turn, improves laryngeal preservation and DFS.
It was hypothesized that the poor local control rate
of the RT group may be due to understaging. With regards to the
present study, in the clinical staging system, based on CT and
laryngoscopy findings, some patients classified as having T2N0M0
laryngeal cancer may actually have minor paraglottic and
pre-epiglottic space invasion (T3), or minor thyroid cartilage
invasion (T4a), which are not prominent on a CT scan. Moreover,
these patients with deep tissue invasion could experience a
difference in local control between the RT and CRT groups; however,
there is no method to filter out these patients in the clinical
setting. The present study reviewed the CT scans of all enrolled
patients, but no classifications were changed from T2N0M0 to T3 or
T4a. In particular, most patients with T2N0M0 glottic cancer had AC
invasion, which can cause minor invasion of the thyroid cartilage
as there is no perichondrium at the insertion of Broyles' ligament,
making it difficult to identify on a CT scan (26). The present results suggested that
81.4% of the patients had AC invasion, while 53.5% had AC invasion
and subglottic invasion, which could be assumed due to deep tissue
invasion of the cancer. The present results supported the above
hypothesis, as for patients with AC and subglottic invasion, the
CRT group had significantly improved DFS compared with the RT
group; while among patients without subglottic invasion, there was
no statistically significant intergroup difference.
Toxicity is an issue that is associated with
chemotherapy (27). In the present
study, more patients in the CRT group compared with the RT group
showed toxicity. In particular, the proportion of patients with a
toxicity higher than grade III was increased in the CRT group.
However, there were no cases of mortality due to toxicity, and all
cases of treatment-associated toxicity were manageable.
Furthermore, the improvement of various drugs and the ability of
infection control to manage acute toxicity of chemotherapy,
including the severe neutropenia and associated fever,
significantly contributed to the low mortality and morbidity of
chemotherapy in the present study.
The main limitation of the current study is that the
sample size of case series was not sufficient for an accurate
multivariate analysis. This study was conducted in a tertiary
referral hospital with a regional cancer center in South Korea, but
the number of patients with T2N0M0 laryngeal cancer did not
approach 100 cases during the 10 years. Therefore, further studies
with larger focused cohorts are required to assess the
applicability of the present results, and a multicenter randomized
trial may be an alternative. However, the present study provided a
basis for conducting a randomized trial to improve treatment
outcome for T2N0M0 laryngeal cancer.
In conclusion, while this retrospective study is
insufficient to draw definitive conclusions, the present results
indicated that CRT had a positive impact on local control and DFS
with manageable toxicity in T2N0M0 laryngeal cancer. Thus, a
randomized prospective trial should be performed to compare RT, CRT
and SBT for treating T2N0M0 laryngeal cancer.
Acknowledgements
The authors would like to thank Dr SS Kweon (Chonnam
National University) for the consultation of statistics.
Funding
This study was supported by the Chonnam National
University Hwasun Hospital Institute for Biomedical Science
(Hwasun, South Korea; grant no. HCRI 16922-22) and the National
Research Foundation of Korea (Daejeon, South Korea; grant no.
2017R1C1B1009843).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
TMY and SCL designed the present study EKJ, JJ and
TMY analyzed the data and drafted the initial manuscript. SJ, JK,
DHL and JKL acquired and analyzed the data. WC, HKK, JH, HS, WB, SC
and IC contributed to the interpretation of data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Institutional Review Board of Chonnam National University
Hwasun Hospital (Hwasun, South Korea; approval no. CNUHH-2019-178)
under the exemption of patients informed consent. The Ethics
Committee of the Institutional Review Board allows the exemption of
patients informed consent for retrospective studies, as a
retrospective study has a extremely low risk of influencing
subjects, and obtaining consent is very difficult in the
retrospective studies.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Groome PA, O'Sullivan B, Irish JC,
Rothwell DM, Schulze K, Warde PR, Schneider KM, Mackenzie RG,
Hodson DI, Hammond JA, et al: Management and outcome differences in
supraglottic cancer between Ontario, Canada, and the Surveillance,
Epidemiology, and End Results areas of the United States. J Clin
Oncol. 21:496–505. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mendenhall WM, Werning JW, Hinerman RW,
Amdur RJ and Villaret DB: Management of T1-T2 Glottic carcinomas.
Cancer. 100:1786–1792. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pfister DG, Spencer S, Adelstein D, Adkins
D, Brizel DM, Burtness B, Busse PM, Caudell JJ, Cmelak AJ, Colevas
AD, et al: National Comprehensive Cancer Network: NCCN Clinical
Practice Guidelines in Oncology (NCCN Guidelines) Head and Neck
Cancers (Version 1). 2020.https://www.nccn.orgApril 07–2020
|
|
5
|
Feng Y, Wang B and Wen S: Laser surgery
versus radiotherapy for T1-T2N0 glottic cancer: A meta-analysis.
ORL J Otorhinolaryngol Relat Spec. 73:336–342. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoo J, Lacchetti C, Hammond JA and Gilbert
RW; Head; Neck Cancer Disease Site Group, : Role of endolaryngeal
surgery (with or without laser) versus radiotherapy in the
management of early (T1) glottic cancer: A systematic review. Head
Neck. 36:1807–1819. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Akimoto T, Nonaka T, Kitamoto Y, Ishikawa
H, Ninomiya H, Chikamatsu K, Furuya N, Hayakawa K, Mitsuhashi N and
Nakano T: Radiation therapy for T2N0 laryngeal cancer: A
retrospective analysis for the impact of concurrent chemotherapy on
local control. Int J Radiat Oncol Biol Phys. 64:995–1001. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Laccourreye O, Weinstein G, Brasnu D,
Trotoux J and Laccourreye H: Vertical partial laryngectomy: A
critical analysis of local recurrence. Ann Otol Rhinol Laryngol.
100:68–71. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brumund KT, Gutierrez-Fonseca R, Garcia D,
Babin E, Hans S and Laccourreye O: Frontolateral vertical partial
laryngectomy without tracheotomy for invasive squamous cell
carcinoma of the true vocal cord: A 25-year experience. Ann Otol
Rhinol Laryngol. 114:314–322. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Furusaka T, Matuda H, Saito T, Katsura Y
and Ikeda M: Long-term observations and salvage operations on
patients with T2N0M0 squamous cell carcinoma of the glottic larynx
treated with radiation therapy alone. Acta Otolaryngol.
132:546–551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arshad H, Jayaprakash V, Gupta V, Cohan
DM, Ambujakshan D, Rigual NR, Singh AK and Hicks WL Jr: Survival
differences between organ preservation surgery and definitive
radiotherapy in early supraglottic squamous cell carcinoma.
Otolaryngol Head Neck Surg. 150:237–244. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Furusaka T, Matsuda A, Saito T, Katsura Y
and Ikeda M: Concurrent chemoradiation therapy with docetaxel (DOC)
for laryngeal preservation in T2N0M0 glottic squamous cell
carcinomas. Acta Otolaryngol. 133:99–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nishimura G, Tsukuda M, Mikami Y, Matsuda
H, Horiuchi C, Taguchi T, Takahashi M, Kawakami M, Watanabe M, Niho
T, et al: Efficacy of concurrent chemoradiotherapy for T1 and T2
laryngeal squamous cell carcinoma regarding organ preservation.
Anticancer Res. 29:661–666. 2009.PubMed/NCBI
|
|
14
|
American Society of Clinical Oncology, .
Pfister DG, Laurie SA, Weinstein GS, Mendenhall WM, Adelstein DJ,
Ang KK, Clayman GL, Fisher SG, Forastiere AA, et al: American
society of clinical oncology clinical practice guideline for the
use of larynx-preservation strategies in the treatment of laryngeal
cancer. J Clin Oncol. 24:3693–3704. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Urba S, Wolf G, Eisbruch A, Worden F, Lee
J, Bradford C, Teknos T, Chepeha D, Prince M, Hogikyan N and Taylor
J: Single-cycle induction chemotherapy selects patients with
advanced laryngeal cancer for combined chemoradiation: A new
treatment paradigm. J Clin Oncol. 24:593–598. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lefebvre JL: Laryngeal preservation in
head and neck cancer: Multidisciplinary approach. Lancet Oncol.
7:747–755. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Forastiere AA, Zhang Q, Weber RS, Maor MH,
Goepfert H, Pajak TF, Morrison W, Glisson B, Trotti A, Ridge JA, et
al: Long-term results of RTOG 91–11: A comparison of three
nonsurgical treatment strategies to preserve the larynx in patients
with locally advanced larynx cancer. J Clin Oncol. 31:845–852.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pignon JP, le Maître A, Maillard E and
Bourhis J; MACH-NC Collaborative Group, : Meta-analysis of
chemotherapy in head and neck cancer (MACH-NC): An update on 93
randomised trials and 17,346 patients. Radiother Oncol. 92:4–14.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC Cancer Staging Manual. 8th. Springer; New
York, NY: 2017, View Article : Google Scholar
|
|
20
|
Korean Society of Thyroid-Head and Neck
Surgery Guideline Task Force, . Ahn SH, Hong HJ, Kwon SY, Kwon KH,
Roh JL, Ryu J, Park JH, Baek SK, Lee GH, et al: Guidelines for the
surgical management of laryngeal cancer: Korean society of
thyroid-head and neck surgery. Clin Exp Otorhinolaryngol. 10:1–43.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Forastiere AA, Ismaila N, Lewin JS, Nathan
CA, Adelstein DJ, Eisbruch A, Fass G, Fisher SG, Laurie SA, Le QT,
et al: Use of Larynx-Preservation strategies in the treatment of
laryngeal cancer: American society of clinical oncology clinical
practice guideline update. J Clin Oncol. 36:1143–1169. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shanei A, Abedi I, Saadatmand P,
Amouheidari AR and Akbari-Zadeh H: Comparison of 3D conformal and
intensity modulated radiotherapy in early stage oral tongue cancer:
Dosimetric and radiobiological evaluation. Int J Radiat Res.
18:33–42. 2020.
|
|
23
|
US Department of Health and Human
Services; National Institutes of Health; National Cancer Institute,
. Common Terminology Criteria For Adverse Events (CTCAE), Version
5.0. November 27–2017.
|
|
24
|
Jones TM, De M, Foran B, Harrington K and
Mortimore S: Laryngeal cancer: United Kingdom National
Multidisciplinary guidelines. J Laryngol Otol. 130:S75–S82. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hirasawa N, Itoh Y, Ishihara S, Kubota S,
Itoh J, Fujimoto Y, Nakashima T and Naganawa S: Radiotherapy with
or without chemotherapy for patients with T1-T2 glottic carcinoma:
Retrospective analysis. Head Neck Oncol. 2:202010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mor N and Blitzer A: Functional anatomy
and oncologic barriers of the larynx. Otolaryngol Clin North Am.
48:533–545. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Culakova E, Thota R, Poniewierski MS,
Kuderer NM, Wogu AF, Dale DC, Crawford J and Lyman GH: Patterns of
chemotherapy-associated toxicity and supportive care in US oncology
practice: A nationwide prospective cohort study. Cancer Med.
3:434–444. 2014. View
Article : Google Scholar : PubMed/NCBI
|