Introduction
Cancer represents a major public health issue
worldwide, and is a leading cause of mortality (1). Globally, colorectal cancer (CRC) is the
third most frequent cancer type, with >1.4 million new cases and
>690,000 mortalities annually (2). For patients with CRC, survival time is
significantly dependent on the stage of cancer upon diagnosis, with
5 year survival rates of ~90, 70 and 13% for localized, regional
and distantly metastatic stages, respectively (3). CRC is slow to progress and noticeable
symptoms, such as bloody stools (hematochezia), abdominal pain,
fatigue and appetite loss (4).
Therefore, improving the prognosis for patients with CRC largely
depends upon early and accurate diagnosis.
Currently, the most accurate method of diagnosis of
CRC is colonoscopy combined with pathological biopsy (5); however, these tests are invasive and
expensive, potentially life-threatening complications, and patient
compliance is poor (6). Inexpensive
and non-invasive methods have been proposed, such as fecal occult
blood-based screening, but both the sensitivity and specificity are
lower than colonoscopy (7,8). Recently, certain researchers have
proposed that an exosome vesicle containing numerous specific
antigen proteins, DNA, long non-coding RNA (lncRNA) and microRNA
(miRNA) may have diagnostic value in CRC (9–11).
However, such a test would be clinically impractical as the
implementation may be complex, time-consuming, expensive, and the
sensitivity and specificity may also be low.
Serum tumor biomarkers may serve not only for
auxiliary diagnosis of CRC, but also as tools for estimating
survival and prognosis. Notably, commonly used tumor markers for
the diagnosis and assessment of patients with CRC are
carcinoembryonic antigen (CEA), cancer antigen (CA)19-9, CA125 and
CA242 (12,13). CEA may be the best biomarker for
differential diagnosis of malignant tumors, disease monitoring and
evaluation of efficacy, relative to the other markers as serum CEA
levels are an important prognostic factor and indicator of
therapeutic effect and recurrence in patients with CRC (14,15).
However, CA19-9, CA125 and CA242 have also been used as indicators
for CRC diagnosis, postoperative surveillance and the monitoring of
treatment effects (16–18).
Due to the highly heterogeneous nature of CRC, a
single tumor marker is unlikely to represent an accurate diagnostic
standard with sufficient sensitivity or specificity for all cases.
Recent studies have indicated that combining multiple tumor markers
may improve the accuracy of diagnostic and prognostic evaluations.
For example, CEA combined with CA242 achieved significantly higher
sensitivity compared with either alone (13). Furthermore, Wang et al
(18) demonstrated that CEA, CA19-9
and CA242, when analyzed together, improved the accuracy of
prognostic prediction in patients with CRC who underwent
surgery.
Serum neuron-specific enolase (NSE) is a
cell-specific isoenzyme of the glycolytic enzyme enolase, first
discovered in brain tissue extracts (19). NSE has been widely used as a clinical
biomarker for diagnosis and prognosis of various benign or
malignant diseases. For example, human serum NSE concentration is
directly proportional to the extent of brain damage caused by
conditions such as cerebral ischemia, and is therefore an important
biological marker for severe brain injury (20,21).
Additionally, NSE is a highly specific marker for neuronal and
peripheral neuroendocrine cells, it was the first marker used to
identify neuroendocrine cells, and as such is particularly used for
the diagnosis of malignant tumors (22). Serum NSE level is associated with
melanoma, seminoma, renal cell carcinoma, Merkel cell tumor,
carcinoid tumor, dysgerminoma, immature teratoma and malignant
pheochromocytoma, particularly in small cell lung cancer (SCLC),
but also in other cancer types (23). However, the potential value of NSE in
CRC is yet to be elucidated.
It has been hypothesized that that there is a high
positive rate for NSE in patients with CRC, and that NSE level may
be associated with tumor staging. Therefore, the present study
investigated the value of NSE for the diagnosis of CRC, and
determined its association with tumor staging and potential value
for prognostic evaluation. The present study also evaluated the
value of combining the detection of NSE, CEA, CA19-9, CA125 and
CA242 for the diagnosis of CRC.
Materials and methods
The Ethics Standards Committee of China-Japan Union
Hospital of Jilin University (Jilin, China) approved the protocol
of the present study. All patients provided written informed
consent and the experiments were performed in accordance with the
relevant guidelines and regulations of the aforementioned
hospital.
Patients and samples
Tumors were staged based on the
Tumor-Node-Metastasis (TNM) classification of the American Joint
Committee on Cancer Staging (eighth edition) (24).
The patient CRC group comprised 358 patients who
were hospitalized between July 2017 and March 2019 at The
China-Japan Union Hospital of Jilin University (Jilin, China), with
CRC of the rectum (n=193), left colon (n=87) or right colon (n=78).
The CRC group comprised 218 men and 140 women, aged between 27 and
85 years (median ± SD, 61.1±11.9 years). None of the patients had
received preoperative neoadjuvant chemoradiotherapy or any other
treatment for their tumor. For analyses, the CRC group was
stratified according to tumor site (rectum, left or right colon) or
clinical stage (I+II or III+IV).
All the included patients underwent surgery. Serum
samples were collected preoperatively. Patients with stage I–III
cancer were treated with radical surgery, while patients at stage
IV received either radical or palliative surgery. Postoperative
pathological examinations were performed to confirm a diagnosis of
CRC in all patients. All included patients had complete clinical
and pathological data. In addition, the control group consisted of
298 healthy volunteers (age range, 20–75 years; median ± SD,
55.8±8.9 years, 158 men and 140 women), who were free of any vital
infections or gastrointestinal disease.
Detection of serum tumor markers
Fasting venous blood samples (2 ml) were collected
from the elbow of all patients between 06:00 and 07:00 a.m. on the
second day following admission and submitted to the Central
Research Office of The China-Japan Union Hospital of Jilin
University for quantitative analysis of the relevant biomarkers.
Blood samples were centrifuged at room temperature, at 2,000 × g
for 5 min, and the supernatant was added to the corresponding tumor
kit for detection (Luminex Ltd.).
All lab tests were performed in accordance with the
standard operating procedures. The experiments were performed on
the day of sample collection, and the reports were used to guide
the clinical decisions of the physicians. The cut-off values for
the serum markers were in accordance with the recommendations of
the manufacturer (Tellgen Corporation); specifically, 25.00 and
5.00 ng/ml for NSE and CEA, respectively, and 37.00, 35.00 and
20.00 U/ml for CA19-9, CA125 and CA242, respectively. An overview
of the 5 markers and their association with CRC is displayed in
Table I.
 | Table I.An overview of the association
between biomarkers investigated in the present study and CRC. |
Table I.
An overview of the association
between biomarkers investigated in the present study and CRC.
| Marker | Rationale | (Refs.) |
|---|
| NSE | NSE is a tumor
marker widely used in the diagnosis of SCLC, but the association
between serum NSE levels and CRC are out of date and limited | (25,27,28) |
| CEA | CEA is an important
prognostic factor and an indicator of the therapeutic effect and
recurrence in patients with CRC | (12,13) |
| CA199 | Indications are
that elevated levels may be informative for some CRC cancers | (10,11) |
| CA125 | CA125 is more
sensitive for mucin-type ovarian cancer, and its combination with
CA125 can significantly improve the sensitivity of CRC detection in
clinic | (15) |
| CA242 | CA242 combined with
other markers may improve early diagnosis of CRC, and the accuracy
of prognostic prediction in surgically treated patients with
CRC | (16) |
Statistical analysis
Associations between the preoperative serum levels
of the 5 tumor biomarkers and clinicopathological characteristics
were analyzed using Pearson's chi-squared (χ2) test or
Fisher's exact probability test. Comparisons between the serum
levels of the 5 tumor biomarkers in the CRC and control groups were
evaluated using the Mann-Whitney U test. The areas under the
receiver operating characteristic (ROC) curve (AUC), 95% confidence
interval (CI), and Youden's index (sensitivity + specificity −1)
were calculated for each tumor biomarker, and the combination of
all 5 markers. Logistic regression was used to analyze the
diagnostic power of each of the 5 markers, and the combination of
the markers, for the CRC group and its various subgroups; the
Hosmer-Lemeshow goodness-of-fit test was used to assess the model.
The AUCs of the combination test and the individual biomarkers were
compared via the Z test using the MedCalc V15.2 software. P<0.05
was considered to indicate a statistically significant difference.
All of the aforementioned statistical analyses were performed using
SPSS v18.0 (SPSS, Inc.).
Results
Serum levels of the 5 tumor markers in
the control and CRC groups
The preoperative serum levels of the 5 markers in
the patients with CRC were significantly higher compared with the
control group. Specifically, the median levels of serum NSE and CEA
in the CRC (and median of control) group were 21.15 (15.63) and
3.01 (1.77) ng/ml, respectively; the median levels of serum CA19-9,
CA125 and CA242 were 11.08 (9.54), 12.49 (11.07), and 5.79 (3.74)
U/ml (Table II).
 | Table II.Median serum concentrations of the 5
tumor markers among the different groups. |
Table II.
Median serum concentrations of the 5
tumor markers among the different groups.
| Tumor type | Cases, n | Median NSE (IQR),
ng/ml | Median CEA (IQR),
ng/ml | Median CA19-9
(IQR), U/ml | Median CA125,
(IQR), U/ml | Median CA242 (IQR),
U/ml |
|---|
| Healthy
controls | 298 | 15.63
(12.88–18.41) | 1.77 (1.15,
2.94) | 9.54
(5.39,15.75) | 11.07
(8.14–15.14) | 3.74
(2.50–6.19) |
| Colorectal
cancer | 358 | 21.15
(16.53–26.92)a | 3.01
(1.61–6.58)a | 11.08
(5.68–21.77)a | 12.49
(9.46–18.63)a | 5.79
(3.45–10.26)a |
| Rectal cancer | 193 | 21.59
(17.67–27.62)a | 2.98
(1.71–6.72)a | 11.51
(6.00–20.00)a | 12.26
(9.46–17.07)a | 6.22
(3.46–10.81)a |
| Left colon
cancer | 87 | 22.04
(16.96–27.40)a | 2.69
(1.53–5.91)a | 12.94
(5.49–23.51)a | 11.67
(8.86–9.13)a | 5.33
(3.31–10.17)a |
| Right colon
cancer | 78 | 19.42
(15.19–25.87)a | 3.23
(1.53–7.58)a | 10.15
(5.03–22.21) | 14.86
(9.46–18.84)a | 5.40
(3.41–8.68)a |
The CRC group was further stratified according to
tumor site and for each subgroup (rectum, left colon and right
colon), the serum levels of NSE, CEA, CA12 and CA242 were
significantly higher compared with the control (Table II). Notably, the serum concentration
of CA19-9 was higher in the rectum and left colon cancer subgroups
compared with the control, but the levels of the right colon cancer
subgroup and control were not significantly different.
Associations between biomarkers and
clinicopathological parameters
The 5 markers were investigated for associations
with pathological parameters of CRC (Table III). Results of the χ2
tests indicated that the serum NSE level was significantly
associated with hematochezia and the N, M and pathological (p) TNM
stage, but not on any of the following: Vascular invasion, nerve
infiltration, tumor differentiation, tumor location, tumor size,
sex or age.
 | Table III.Association between the level of 5
biomarkers and clinicopathological parameters of patients with
colorectal cancer. |
Table III.
Association between the level of 5
biomarkers and clinicopathological parameters of patients with
colorectal cancer.
|
|
| NSE |
|
| CEA |
|
| CA19-9 |
|
| CA125 |
|
| CA242 |
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Clinicopathological
parameters | Cases | Neg | Pos | P-value |
χ2-value | Neg | Pos | P-value |
χ2-value | Neg | Pos | P-value |
χ2-value | Neg | Pos | P-value |
χ2-value | Neg | Pos | P-value |
χ2-value |
|---|
| Sex |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Male | 218 | 147 | 71 | 0.217 | 1.526 | 145 | 73 | 0.329 | 0.953 | 197 | 21 | 0.084 | 2.983 | 204 | 14 | 0.317 | 1.003 | 205 | 13 | 0.004 | 8.100 |
|
Female | 140 | 103 | 37 |
|
| 100 | 40 |
|
| 118 | 22 |
|
| 127 | 13 |
|
| 119 | 21 |
|
|
| Age, years |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
≤60 | 164 | 109 | 55 | 0.202 | 1.631 | 119 | 45 | 0.123 | 2.384 | 150 | 14 | 0.063a | 3.457 | 152 | 12 | 0.882 | 0.022 | 148 | 16 | 0.878 | 0.024 |
|
>60 | 194 | 141 | 53 |
|
| 126 | 68 |
|
| 165 | 29 |
|
| 179 | 15 |
|
| 176 | 18 |
|
|
| Tumor location |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Right
colon | 78 | 58 | 20 | 0.613 | 0.979 | 52 | 26 | 0.793 | 0.463 | 69 | 9 | 0.210 | 3.126 | 70 | 8 | 0.511 | 1.341 | 69 | 9 | 0.285 | 2.510 |
| Left
colon | 87 | 60 | 27 |
|
| 62 | 25 |
|
| 72 | 15 |
|
| 80 | 7 |
|
| 76 | 11 |
|
|
|
Rectum | 193 | 132 | 61 |
|
| 131 | 62 |
|
| 174 | 19 |
|
| 181 | 12 |
|
| 179 | 14 |
|
|
| Tumor size, cm |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ≤3 | 112 | 82 | 30 | 0.598 | 1.029 | 89 | 23 | 0.002a | 12.589 | 101 | 11 | 0.611 | 0.984 | 101 | 11 | 0.469 | 1.512 | 103 | 9 | 0.409 | 1.788 |
|
3–5 | 125 | 84 | 41 |
|
| 86 | 39 |
|
| 110 | 15 |
|
| 118 | 7 |
|
| 115 | 10 |
|
|
|
≥5 | 121 | 84 | 37 |
|
| 70 | 51 |
|
| 104 | 17 |
|
| 112 | 9 |
|
| 106 | 15 |
|
|
| T stage |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
T1+T2 | 67 | 47 | 20 | 0.950 | 0.004a | 62 | 5 |
<0.001a | 22.165 | 64 | 3 | 0.035a | 4.426 | 64 | 3 | 0.292 | 1.110 | 65 | 2 | 0.044a | 4.067 |
|
T3+T4 | 291 | 203 | 88 |
|
| 183 | 108 |
|
| 251 | 40 |
|
| 267 | 24 |
|
| 259 | 32 |
|
|
| N stage |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| N0 | 198 | 155 | 43 |
<0.001a | 15.017 | 155 | 43 |
<0.001a | 19.887 | 183 | 15 | 0.004a | 8.247 | 188 | 10 | 0.047a | 3.944 | 187 | 11 | 0.005a | 8.008 |
|
N1+N2 | 160 | 95 | 65 |
|
| 90 | 70 |
|
| 132 | 28 |
|
| 143 | 17 |
|
| 137 | 23 |
|
|
| M stage |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| M0 | 338 | 243 | 95 |
<0.001a | 12.200 | 239 | 99 |
<0.001a | 1.005 | 299 | 39 | 0.437 | 0.604 | 314 | 24 | 0.387 | 0.747 | 307 | 31 | 0.637 | 0.222 |
| M1 | 20 | 7 | 13 |
|
| 6 | 14 |
|
| 16 | 4 |
|
| 17 | 3 |
|
| 17 | 3 |
|
|
| Vascular
invasion |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| No | 260 | 187 | 73 | 0.160 | 1.971 | 190 | 70 | 0.002a | 9.471 | 232 | 28 | 0.239 | 1.386 | 240 | 20 | 0.861 | 0.031 | 239 | 21 | 0.135 | 2.229 |
|
Yes | 98 | 63 | 35 |
|
| 55 | 43 |
|
| 83 | 15 |
|
| 91 | 7 |
|
| 85 | 13 |
|
|
| Nerve
infiltration |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| No | 266 | 188 | 78 | 0.554 | 0.350 | 196 | 70 |
<0.001a | 13.200 | 240 | 26 | 0.027a | 4.900 | 250 | 16 | 0.063 | 3.461 | 248 | 18 | 0.003a | 8.977 |
|
Yes | 92 | 62 | 30 |
|
| 49 | 43 |
|
| 75 | 17 |
|
| 81 | 11 |
|
| 76 | 16 |
|
|
|
Differentiation |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Well | 8 | 7 | 1 | 0.429 | 1.691 | 8 | 0 |
<0.001a | 22.991 | 7 | 1 | 0.320 |
| 8 | 0 | 0.241 |
| 8 | 0 | 0.116 |
|
|
Moderate | 254 | 179 | 75 |
|
| 189 | 65 |
|
| 227 | 27 |
|
| 238 | 16 |
|
| 234 | 20 |
|
|
|
Poor | 96 | 64 | 32 |
|
| 48 | 48 |
|
| 81 | 15 |
|
| 85 | 11 |
|
| 82 | 14 |
|
|
| Stage |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
I+II | 193 | 152 | 41 |
<0.001a | 15.830 | 152 | 41 |
<0.001a | 20.649 | 179 | 14 | 0.003a | 8.967 | 184 | 9 | 0.026a | 4.976 | 182 | 11 | 0.008a | 7.027 |
|
III+IV | 165 | 98 | 67 |
|
| 93 | 72 |
|
| 136 | 29 |
|
| 147 | 18 |
|
| 142 | 23 |
|
|
| Hematochezia |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| No | 166 | 132 | 34 |
<0.001a | 13.783 | 127 | 65 | 0.316 | 1.005 | 142 | 24 | 0.185 | 1.753 | 150 | 16 | 0.162 | 1.951 | 146 | 20 | 0.126 | 2.343 |
|
Yes | 192 | 118 | 74 |
|
| 118 | 48 |
|
| 173 | 19 |
|
| 181 | 11 |
|
| 178 | 14 |
|
|
The serum CEA level was significantly associated
with vascular invasion, nerve infiltration, tumor differentiation,
and tumor size and stage; however, there was no association with
age, sex or hematochezia. Serum CA19-9 was significantly associated
with nerve infiltration, N stage and pTNM staging (P<0.05).
However, there was no significant association between positive
serum CA19-9 levels and M stage, vascular infiltration, tumor
differentiation, tumor location and sex (P>0.05). Similarly,
there were no significant statistical differences for preoperative,
or sex. The serum CA125 level was associated with the N stage and
pTNM staging, but not vascular invasion, nerve infiltration, tumor
differentiation or hematochezia. Furthermore, serum CA242 was
significantly associated with lymph node metastasis, nerve invasion
and T, N and pTNM staging, but not with M stage, tumor
differentiation, vascular invasion, hematochezia or sex.
Associations between biomarkers and
clinical stage of disease
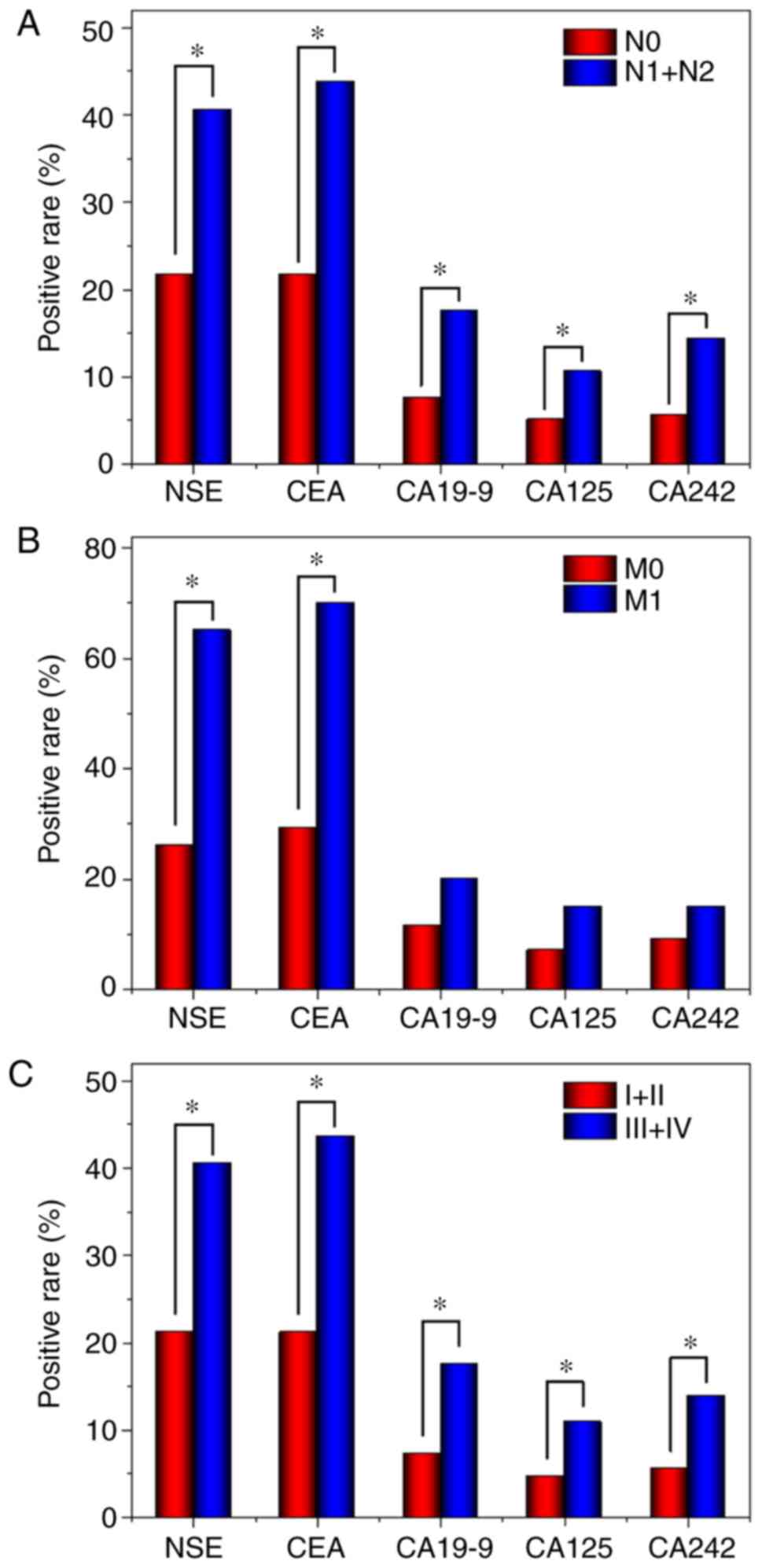
The CRC group was stratified by the clinical stages
(I+II or III+IV), and the positive rates of the 5 tumor markers
were calculated (Fig. 1) and were
revealed to increase with the clinical stage, with significant
differences in serum levels of each marker between the two
subgroups. In addition, the rate of positivity for the five tumor
markers were significantly higher in patients with lymph node
metastasis compared with those without. However, only the rate of
positivity for NSE and CEA were significantly higher in patients
with distant metastasis compared with those without distant
metastasis.
Logistic regression and ROC curve
analyses
For the CRC group, ROC curves were constructed for
each of the 5 tumor markers, and their combination (Fig. 2). Overall, for the 358 patients the
areas under the ROC curves (AUCs) for each of the markers were as
follows: NSE, 0.766; CEA, 0.682; CA19-9, 0.560; CA125, 0.590; and
CA242, 0.651. Notably, the AUC for the combination of all 5 markers
was 0.827.
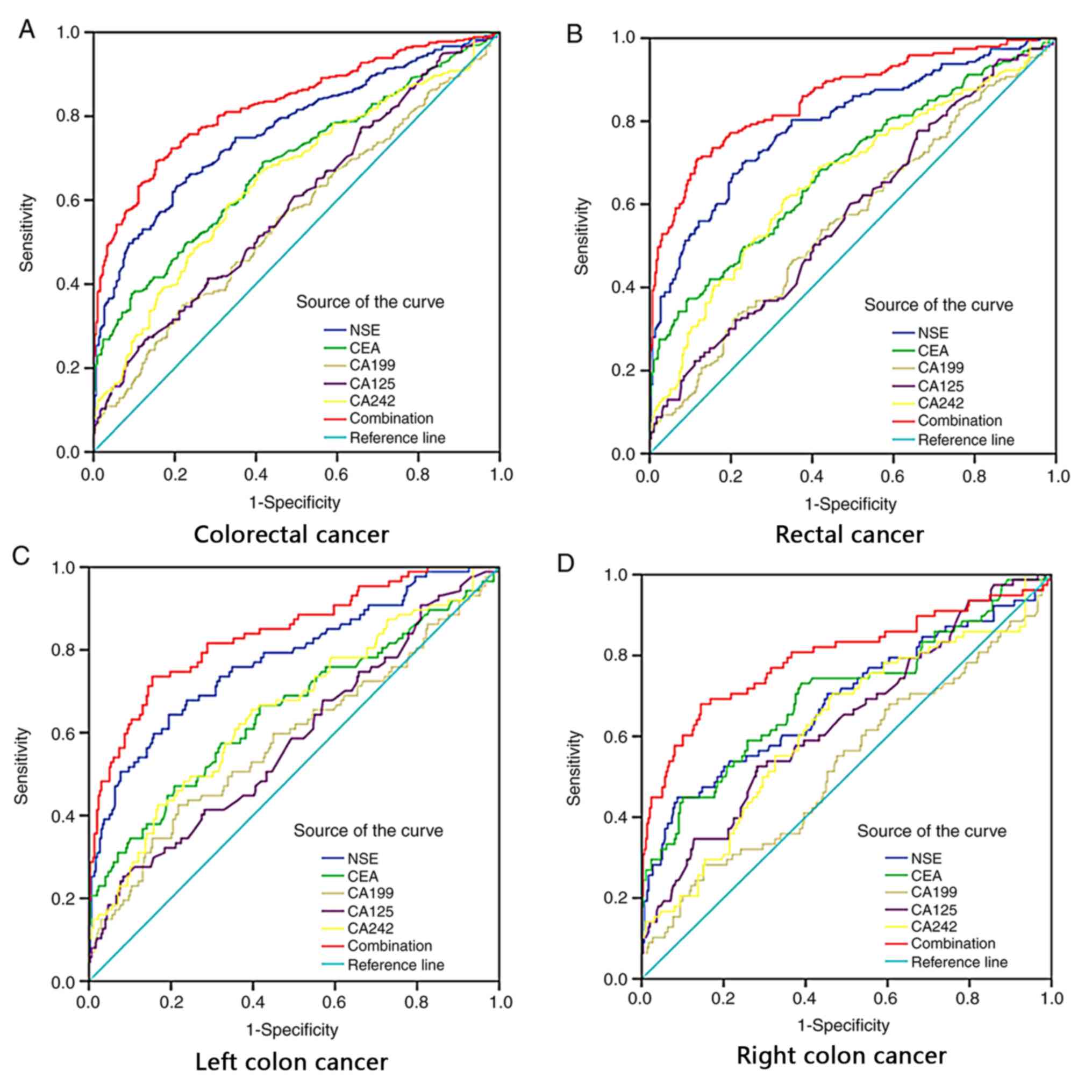 | Figure 2.Diagnostic value of combined
detection of the tumor markers NSE, CEA, CA19-9, CA125 and CA242
for CRC is superior to each independent tumor marker. ROC curves of
single NSE, CEA, CA19-9, CA125, CA242 and the combination in
predicting (A) colorectal cancer; (B) rectal cancer; (C) left colon
cancer; and (D) right colon cancer. NSE, neuron-specific enolase;
CEA, carcinoembryonic antigen; CA199, cancer antigen 19-9; CA125,
cancer antigen 125; CA242, cancer antigen 242; CRC, colorectal
cancer. |
ROC curves were also analyzed for each of the CRC
subgroups according to tumor site (rectum, left colon and right
colon). For the rectal subgroup, the AUCs of the NSE, CEA, CA19-9,
CA125, CA242 were: 0.794, 0.686, 0.562, 0.576 and 0.662,
respectively, and the AUC for the combination of all 5 markers was
0.858. In patients with left colon cancer, the AUCs of the
respective 5 markers and their combinations were: 0.777, 0.653,
0.584, 0.582 and 0.653. In patients with right colon cancer the
respective AUCs were: 0.708, 0.702, 0.529, 0.637 and 0.622.
The Hosmer-Lemeshow test was conducted to verify the
appropriateness of the logistic regression model for analyzing CRC,
and the various subtypes of tumor site in CRC. The results were as
follows: CRC, χ2=6.326, P=0.611; rectal CRC,
χ2=4.874, P=0.771; left colon CRC, χ2=9.771,
P=0.281; and right colon CRC, χ2=9.082, P=0.335.
The AUCs for predicted probability of CRC associated
with each tumor marker and logistic regression curves were
constructed. Among the 5 tumor markers analyzed individually, the
AUC of NSE was the highest (Table
IV). Yet, the AUC of the combination of all markers exceeded
that of any individual marker. For the diagnosis of CRC, both NSE
and CA242 had high sensitivity (63.41 and 66.2, respectively), but
the specificity of NSE was higher (79.53 vs. 59.73). For each of
the tumor site subgroups, the sensitivity of the combined tumor
markers was highest, but the specificity was lower.
 | Table IV.Area under the receiver operating
curve (AUC) and the corresponding 95% CI of the combination of NSE,
CEA, CA19-9, CA125 and CA242 (four different cancers versus healthy
controls). |
Table IV.
Area under the receiver operating
curve (AUC) and the corresponding 95% CI of the combination of NSE,
CEA, CA19-9, CA125 and CA242 (four different cancers versus healthy
controls).
| Tumor markers | Sensitivity | Specificity | Youden index | AUC | SE | 95% CI | P-value |
|---|
| Colorectal
cancer |
|
NSE | 63.41 | 79.53 | 0.43 | 0.766 | 0.018 | 0.732, 0.798 | <0.000 |
|
CEA | 37.71 | 90.60 | 0.28 | 0.682 | 0.021 | 0.644, 0.717 | <0.0001 |
|
CA199 | 34.92 | 78.19 | 0.13 | 0.560 | 0.022 | 0.521, 0.599 | 0.007 |
|
CA125 | 25.14 | 88.93 | 0.14 | 0.590 | 0.022 | 0.552, 0.688 | <0.0001 |
|
CA242 | 66.20 | 59.73 | 0.30 | 0.651 | 0.021 | 0.613, 0.688 | <0.0001 |
|
Combination | 69.30 | 84.60 | 0.54 | 0.827 | 0.016 | 0.796, 0.855 | <0.0001 |
| Rectum cancer |
|
NSE | 69.95 | 77.18 | 0.47 | 0.794 | 0.021 | 0.755, 0.829 | <0.0001 |
|
CEA | 37.71 | 90.60 | 0.30 | 0.686 | 0.025 | 0.643, 0.727 | <0.0001 |
|
CA199 | 33.68 | 78.19 | 0.12 | 0.562 | 0.027 | 0.517, 0.606 | 0.0202 |
|
CA125 | 77.72 | 33.89 | 0.12 | 0.576 | 0.026 | 0.531, 0.620 | 0.0041 |
The AUCs of the individual biomarkers and their
combination were compared using a Z test for the CRC group, and the
tumor location subgroups. For the CRC group overall, in comparison
with the combination of all markers the Z values were as
follows (all P<0.01): NSE, 5.47; CEA, 7.359; CA19-9, 11.159;
CA125, 10.476; and CA242, 7.865. In patients with rectal cancer,
the Z values for NSE, CEA, CA19-9, CA125 and CA242 compared with
the combination test were 4.997, 7.007, 10.123, 9.785 and 7.451,
respectively (all P<0.01). In patients with left colon cancer,
the respective Z values were 2.772, 4.864, 5.976, 6.191 and
4.887 (all P<0.01), compared with the combination test.
Discussion
Serum tumor markers are considered as biological
indicators detected from the serum or plasma of suspected tumor
patients. The increase of such indicators indicates tumor
existence, facilitating pathological analysis and evaluation of
tumor development (25). Serum tumor
biomarkers are useful for choosing treatment strategies,
particularly when the markers are convenient and economically
efficient to detect (26). CEA is
often secreted by tumors located in the digestive tract and is the
most widely used CRC marker (27).
CEA has good specificity and sensitivity for screening CRC and is a
valuable tool for evaluating the prognosis of patients with CRC
(28). The understanding of
malignant tumors and their associated serum markers has increased
in previous years, and this has indicated the potential of
biomarkers in facilitating improvements to the diagnosis and
evaluation of treatment effect and prognosis (29). However, the sensitivity and
specificity of a single serum tumor marker for CRC is not precise,
making it necessary to select and combine a variety of markers to
improve accuracy. Notably, Gao et al (30) identified a combination of serum
markers for the diagnosis of CRC, and also to appraise tumor status
to guide treatment, evaluate curative effect and predict
prognosis.
NSE is a tumor marker widely used in the diagnosis
of SCLC. In patients with SCLC, serum NSE levels are significantly
elevated (31), but they are also
higher in patients with non-small cell lung cancer (NSCLC) and
other types of tumor (32). The
association between serum NSE levels and CRC also has been
previously reported (33,34). In the present study, 30.16% of
patients with CRC tested positive for NSE, which was lower than the
rate for CEA (31.56%), but significantly higher compared with
CA19-9 (12.0%), CA125 (7.5%) and CA242 (9.5%). The positive rate
for NSE in patients with CRC is similar to that of CEA and
consequently, it was hypothesized that NSE may represent a
biomarker associated with colorectal tumor growth that may be used
for the diagnosis and evaluation of CRC progression.
In the present study, the association between serum
NSE values and clinical stage was investigated with the intention
of determining whether serum NSE may be used for the auxiliary
diagnosis and evaluation of tumor progression in patients with CRC.
Initially, the serum NSE levels of the 358 patients (CRC group)
were compared with that of 298 healthy people (control group). It
was revealed that the serum NSE levels in the CRC group were
significantly higher compared with the control. Subsequently, the
358 patients comprising the CRC group were stratified according to
the site of the tumor (specifically 193 rectum, 87 left colon and
78 right colon), and each was compared with the healthy control
group. Notably, for each of these subgroups, the serum NSE level
was significantly higher compared with the control group. This is
in accordance with previous studies, which reported that serum NSE
levels in patients with lung and non-colorectal tumors were
markedly elevated compared with healthy participants (23). Further research is required to
establish whether serum NSE levels may be used for CRC screening.
In the present study, the serum levels of CEA, CA19-9, CA125 and
CA242 were compared between the CRC and control groups, and it was
determined that all serum marker levels were significantly higher
in the CRC group, consistent with a previous study (35).
In addition, associations between each of the 5
markers in serum and certain clinicopathological parameters of CRC
were investigated. It was demonstrated that the serum NSE level was
significantly associated with pTNM staging, local lymphatic or
distant metastasis. This indicates that serum NSE level may be as
useful as CEA and other markers for the staging of patients with
CRC, and NSE level may represent a precise indicator of local
lymphatic or distant metastasis in CRC.
The cause of the difference in NSE levels between
early and late clinical stages in CRC is yet to be elucidated.
Perhaps, the NSE level is closely associated with (and may reflect
the rate of) tumor growth. Enolase is a multifunctional protein,
which is expressed abundantly in the cytosol. Upon stimulatory
signals, enolase can locate to the cell surface and contribute to
different pathologies, including injury, autoimmunity, infection,
inflammation, and cancer (36). The
present results warrant further experiments and follow-up
investigation to confirm whether NSE is associated with tumor
activity.
The sensitivity of CEA in the present study was
37.71%, which is lower than has been previously reported (37). Previously, it has been revealed that
patients who tested positive for serum CEA exhibited significantly
different T, N and M stages, tumor differentiation and pTNM staging
(38). In the present study,
preoperative positive CEA status was significantly associated with
vascular invasion, nerve infiltration and tumor size. This
indicated that CEA may be associated with CRC metastasis and tumor
progression. Hence, preoperative serum CEA levels may predict CRC
disease status (For example, tumor staging and lymph node
metastasis) and provide a guide for a more informed clinical
treatment strategy and prognosis. Furthermore, serum CEA level in
patients with CRC has previously been identified as an independent
predictor of both overall and disease-free survival time (39–41).
In the present study, serum CA242 status was
significantly associated with the presence of T and N stages, nerve
invasion and pTNM staging. This is consistent with previous studies
that revealed that CA242 was a prognostic factor in patients with
CRC (42). Similarly, in the present
study serum CA19-9 and CA125 levels were significantly associated
with lymph node metastasis and pTNM staging. The percentage of
patients at stage III+IV who were positive for CA19-9 or CA125 was
significantly higher compared with patients at stage I+II. These
results are consistent with previous reports (35,43).
Taken together, these results suggest that CA19-9 and CA125 exhibit
potential for the differential diagnosis, disease monitoring and
therapeutic evaluation of numerous malignant tumors.
The majority of patients with CRC first present with
blood in the stool as the primary symptom. Therefore, the
association between tumor markers and hematochezia was analyzed in
the present study. Of the 5 tumor markers tested, only NSE was
associated with preoperative hematochezia. To the best of our
knowledge, this finding has rarely been mentioned in other
literature. It was hypothesized that the tumor growth and tissue
characteristics of CRC result in symptoms of bloody stools
accompanied by an elevated serum NSE level. Thus, examining the
serum NSE of patients presenting with bloody stools may improve the
diagnostic rate of CRC.
The accuracies of the AUC values ≥0.97, 0.93–0.97,
0.75–0.93 and <0.75 are considered excellent, very good, good
and deficient or close to random, respectively (44). In the present study, in order to
assess the diagnostic potential of NSE and other single-tumor
biomarkers both individually and in combination, ROC curves were
constructed and the corresponding AUCs were calculated. The AUC of
NSE in patients with CRC was 0.766, and the AUCs stratified by
tumor site were 0.794, 0.777 and 0.708 for rectal, left and right
colon cancer, respectively. Thus, according to the AUC standard,
serum NSE may represent an independent tumor biomarker (AUC≥0.75).
However, the accuracy of NSE alone for the diagnosis for CRC was
not satisfactory. Therefore, other potential tumor markers (CEA,
CA19-9, CA125 and CA242) were investigated in a similar manner.
The sensitivity and specificity of NSE in the
diagnosis of CRC were 63.41 and 79.53%, respectively, in the
present study. Compared with the other four tumor markers commonly
used, NSE is relatively reliable for the diagnosis of CRC. For the
diagnosis of rectal colon cancer, the sensitivity and specificity
of NSE were 69.95 and 77.18%, respectively, and for left colon
cancer they were 64.37 and 80.54%, respectively. Compared with CEA,
CA19-9 and CA125, NSE exhibits good sensitivity for the diagnosis
of rectal cancer and CRC. However, for right colon cancer, although
the sensitivity of NSE was only 44.87%, the specificity was 91.28%.
Similarly, the sensitivity of CEA to CRC was only 37.71%, and the
sensitivity was 90.6%. The aforementioned results are similar to
those reported by McKeown et al (27), in which the reported specificity and
sensitivity of CEA for CRC screening were 36.00 and 87.00%,
respectively. In the present study, it was calculated that CA242
indicated a good sensitivity for rectal, and left and right colon
cancer (61.14–70.51%), while the sensitivities of CA19-9 and CA125
fluctuated from 25.14 to 77.72%, and the specificity was not
precise.
In the present study, the AUCs of single tumor
markers were significantly different between the patients overall
and the subgroups according to tumor site. To improve diagnostic
accuracy, 5 tumor biomarkers were combined using a logistic
regression model. The combined markers in the logistic regression
model exhibited a better diagnostic performance for CRC and the
sensitivity of diagnosis was also better.
In conclusion, the present study revealed that serum
NSE may represent an independent tumor biomarker for CRC, and that
it may be used for CRC auxiliary diagnosis. Furthermore, the
combined detection of the tumor markers NSE, CEA, CA19-9, CA125 and
CA242 was revealed as a significant combination of biomarkers that
may be used in the diagnosis of CRC. The present study is limited
in that the patients were all from a single center, the population
size was small and follow-up information was lacking (since it is
still ongoing). Therefore, whether NSE is a significant prognostic
indicator for patients with CRC is yet to be elucidated.
Nevertheless, the results of previous studies and the present study
indicate that the association between NSE and CRC may be of great
value in monitoring the recurrence of colorectal cancer and
selecting adjuvant therapy in the foreseeable future.
Acknowledgements
The authors would like to thank Dr. Sun from Jilin
University (Jilin, China) for providing statistical data
analysis.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
HL participated in the design of the experiment and
the analysis of all clinical data as well as the revision of the
paper. KS participated in the experimental design and paper
modification. BL participated in the collection and analysis of
pathological data. RL participated in the collection and analysis
of serum markers. ZW participated in the collection and analysis of
imaging data. ZX participated in data analysis and thesis revision.
All authors read and approved the final manuscript.
Ethics approval and consent to participate
and
The Ethics Standards Committee of China-Japan Union
Hospital of Jilin University approved this study. All patients
provided written informed consent form and the experiments were
performed in accordance with the relevant guidelines and
regulations of this hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors have declared that they have no
competing interests.
References
|
1
|
Stewart BW and Wild CP: World Cancer
Report 2014. IARC Press; Lyon: 2014
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marventano S, Forjaz M, Grosso G,
Mistretta A, Giorgianni G, Platania A, Gangi S, Basile F and Biondi
A: Health related quality of life in colorectal cancer patients:
State of the art. BMC Surg. 13 (Suppl 2):S152013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Helsingen LM, Vandvik PO, Jodal HC,
Agoritsas T, Lytvyn L, Anderson JC, Auer R, Murphy SB, Almadi MA,
Corley DA, et al: Colorectal cancer screening with faecal
immunochemical testing, sigmoidoscopy or colonoscopy: A clinical
practice guideline. BMJ. 367:l55152019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weiss JB, Cetel NS and Weiss DE: To the
Editor: Colorectal cancer screening: Colonoscopy has disadvantages.
Cleve Clin J Med. 86:774–776. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wong CK, Fedorak RN, Prosser CI, Stewart
ME, van Zanten SV and Sadowski DC: The sensitivity and specificity
of guaiac and immunochemical fecal occult blood tests for the
detection of advanced colonic adenomas and cancer. Int J Colorectal
Dis. 27:1657–1664. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Van Rossum LG, van Rijn AF, Verbeek AL,
van Oijen MG, Laheij RJ, Fockens P, Jansen JB, Adang EM and Dekker
E: Colorectal cancer screening comparing no screening,
immunochemical and guaiac fecal occult blood tests: A
cost-effectiveness analysis. Int J Cancer. 128:1908–1917. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan S, Han B, Gao S, Wang X, Wang Z, Wang
F, Zhang J, Xu D and Sun B: Exosome-Encapsulated microRNAs as
circulating biomarkers for colorectal cancer. Oncotarget.
8:60149–60158. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai J, Zuo X, Chen Z, Zhang Y, Wang J,
Wang J, Ye X and Zhao W: Long noncoding RNAs serve as potential
diagnostic biomarkers for colorectal cancer. J Cancer. 10:611–619.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang H, Zhu M, Shan X, Zhou X, Wang T,
Zhang J, Tao J, Cheng W, Chen G, Li J, et al: A panel of
seven-miRNA signature in plasma as potential biomarker for
colorectal cancer diagnosis. Gene. 687:246–254. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu H: Reference intervals for
gastrointestinal tumor markers (AFP, CEA, CA199 and CA724) in
healthy adults of han nationality in chongqing by roche ECLIA
system. Scand J Clin Lab Invest. 79:484–490. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong D, Zhang L, Jia L, Ji W, Wang Z, Ren
L, Niu R and Zhou Y: Identification of serum periostin as a
potential diagnostic and prognostic marker for colorectal cancer.
Clin Lab. 64:973–981. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song S, Hong JC, McDonnell SE, Koong AC,
Minsky BD, Chang DT and Liauw SL: Combined modality therapy for
rectal cancer: The relative value of posttreatment versus
pretreatment CEA as a prognostic marker for disease recurrence. Ann
Surg Oncol. 19:2471–2476. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tarantino I, Warschkow R, Schmied BM,
Güller U, Mieth M, Cerny T, Büchler MW and Ulrich A: Predictive
value of CEA for survival in stage I rectal cancer: A
population-based propensity score-matched analysis. J Gastrointest
Surg. 20:1213–1222. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Holdenrieder S, Dharuman Y, Standop J,
Trimpop N, Herzog M, Hettwer K, Simon K, Uhlig S and Micallef J:
Novel serum nucleosomics biomarkers for the detection of colorectal
cancer. Anticancer Res. 34:2357–2362. 2014.PubMed/NCBI
|
|
17
|
Webb A, Scott-Mackie P, Cunningham D,
Norman A, Andreyev J, O'Brien M and Bensted J: The prognostic value
of CEA, beta HCG, AFP, CA125, CA19-9 and C-erb B-2, beta HCG
immunohistochemistry in advanced colorectal cancer. Ann Oncol.
6:581–587. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Wang X, Yu F, Chen J, Zhao S,
Zhang D, Yu Y, Liu X, Tang H and Peng Z: Combined detection of
preoperative serum CEA, CA19-9 and CA242 improve prognostic
prediction of surgically treated colorectal cancer patients. Int J
Clin Exp Pathol. 8:14853–14863. 2015.PubMed/NCBI
|
|
19
|
Isgrò MA, Bottoni P and Scatena R:
Neuron-Specific enolase as a biomarker: Biochemical and clinical
aspects. Adv Exp Med Biol. 867:125–143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barone FC, Clark RK, Price WJ, White RF,
Feuerstein GZ, Storer BL and Ohlstein EH: Neuron-specific enolase
increases in cerebral and systemic circulation following focal
ischemia. Brain Res. 623:77–82. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Muller E, Shock JP, Bender A, Kleeberger
J, Högen T, Rosenfelder M, Bah B and Lopez-Rolon A: Outcome
prediction with serial neuron-specific enolase and machine learning
in anoxic-ischaemic disorders of consciousness. Comput Biol Med.
107:145–152. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schmechel D, Marangos PJ and Brightman M:
Neurone-Specific enolase is a molecular marker for peripheral and
central neuroendocrine cells. Nature. 276:834–836. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu L, Lina W and Xuejun Y: The diagnostic
value of serum CEA, NSE and MMP-9 for on-small cell lung cancer.
Open Med (Wars). 11:59–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The Eighth Edition AJCC Cancer Staging Manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qi W, Li X and Kang J: Advances in the
study of serum tumor markers of lung cancer. J Cancer Res Ther.
10:C95–C101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu L, Xu HX, Wang WQ, Wu CT, Xiang JF,
Liu C, Long J, Xu J, Fu de L, Ni QX, et al: Serum CA125 is a novel
predictive marker for pancreatic cancer metastasis and correlates
with the metastasis-associated burden. Oncotarget. 7:5943–5956.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McKeown E, Nelson DW, Johnson EK, Maykel
JA, Stojadinovic A, Nissan A, Avital I, Brücher BL and Steele SR:
Current approaches and challenges for monitoring treatment response
in colon and rectal cancer. J Cancer. 5:31–43. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Campos-da-Paz M, Dórea JG, Galdino AS,
Lacava ZGM and de Fatima Menezes Almeida Santos M: Carcinoembryonic
Antigen (CEA) and hepatic metastasis in colorectal cancer: Update
on biomarker for clinical and biotechnological approache. Recent
Pat Biotechnol. 12:269–279. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sung HJ and Cho JY: Biomarkers for the
lung cancer diagnosis and their advances in proteomics. BMB Rep.
30:615–625. 2008. View Article : Google Scholar
|
|
30
|
Gao Y, Wang J, Zhou Y, Sheng S, Qian SY
and Huo X: Evaluation of serum CEA, CA19-9, CA72-4, CA125 and
ferritin as diagnostic markers and factors of clinical parameters
for colorectal cancer. Sci Rep. 8:27322018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang P, Piao Y, Zhang X, Li W and Hao X:
The concentration of CYFRA 21-1, NSE and CEA in cerebro-spinal
fluid can be useful indicators for diagnosis of meningeal
carcinomatosis of lung cancer. Cancer Biomark. 13:123–130. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang JL, Wu DW, Cheng ZZ, Han WZ, Xu SW
and Sun NN: Expression of high mobility group box-B1 (HMGB-1) and
matrix metalloproteinase-9 (MMP-9) in non-small cell lung cancer
(NSCLC). Asian Pac J Cancer Prev. 15:4865–4869. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kaiser E, Kuzmits R, Pregant P, Burghuber
O and Worofka W: Clinical biochemistry of neuron specific enolase.
Clin Chim Acta. 183:13–31. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang WY, Shi G, Wu LP, Wei ST, Huang YN,
Tan LX, Yang RZ, Yan CX, Guo ET, Wang HY, et al: Analysis of
specific serum markers of colon carcinoma using a
bhattacharyya-based support vector machine. Genet Mol Res.
23:162017.
|
|
35
|
Ning S, Wei W, Li J, Hou B, Zhong J, Xie
Y, Liu H, Mo X, Chen J and Zhang L: Clinical significance and
diagnostic capacity of serum TK1, CEA, CA 19-9 and CA 72-4 levels
in gastric and colorectal cancer patients. J Cancer. 9:494–501.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Haque A, Ray SK, Cox A and Banik NL:
Neuron specific enolase: A promising therapeutic target in acute
spinal cord injury. Metab Brain Dis. 31:487–495. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fakih MG and Padmanabhan A: CEA monitoring
in colorectal cancer. What you should know. Oncology (Williston
Park). 20:579–587. 2006.PubMed/NCBI
|
|
38
|
Baqar AR, Wilkins S, Staples M, Angus Lee
CH, Oliva K and McMurrick P: The role of preoperative CEA in the
management of colorectal cancer: A cohort study from two cancer
centres. Int J Surg. 64:10–15. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takagawa R, Fujii S, Ohta M, Nagano Y,
Kunisaki C, Yamagishi S, Osada S, Ichikawa Y and Shimada H:
Preoperative serum carcinoembryonic antigen level as a predictive
factor of recurrence after curative resection of colorectal cancer.
Ann Surg Oncol. 15:3433–3439. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang WS, Lin JK, Chiou TJ, Liu JH, Fan FS,
Yen CC, Lin TC, Jiang JK, Yang SH, Wang HS and Chen PM:
Preoperative carcinoembryonic antigen level as an independent
prognostic factor in colorectal cancer: Taiwan experience. Jpn J
Clin Oncol. 30:12–16. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wiratkapun S, Kraemer M, Seow-Choen F, Ho
YH and Eu KW: High preoperative serum carcinoembryonic antigen
predicts metastatic recurrence in potentially curative colonic
cancer: Results of a five-year study. Dis Colon Rectum. 44:231–235.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang XQ, Chen C, Hou JX, Peng CW, Huang CQ
and Li Y: Preoperative serum carbohydrate antigen 242 is a useful
predictive and prognostic marker in colorectal cancer.
Hepatogastroenterology. 58:377–382. 2011.PubMed/NCBI
|
|
43
|
Okamura R, Hasegawa S, Hida K, Hoshino N,
Kawada K, Sugihara K and Sakai Y; Japanese Study Group for
Postoperative Follow-up of Colorectal Cancer, : The role of
periodic serum CA19-9 test in surveillance after colorectal cancer
surgery. Int J Clin Oncol. 22:96–101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li H, Zhang B, Hu X, Dong Y, Fan Q, Guo F,
Ren X, Zhou H, Tian W and Zhao Y: Serum helicobacter pylori FliD
antibody and the risk of gastric cancer. Oncotarget. 7:22397–22408.
2016. View Article : Google Scholar : PubMed/NCBI
|