Introduction
Carcinoma of the digestive system, including
hepatocellular carcinoma (HCC), colorectal cancer (CRC), gastric
cancer (GC) and pancreatic cancer (PC), is one of the leading
causes of death worldwide (1).
Although the molecular mechanisms and genetic changes underlying
these cancer types have been gradually unveiled in recent years,
there remain obstacles in the treatment of these diseases, due to
complexity and heterogeneity in molecular pathogenesis. Therefore,
it is necessary to discover and develop more effective diagnostic
biomarkers and potential therapeutic targets for these
diseases.
Alanine-glyoxylate aminotransferase 2-like 1
(AGXT2L1) was initially discovered in brain tissues, and is
considered to be involved in the pathogenesis of schizophrenia and
bipolar disorder (2–4). Although AGXT2L1 belongs to a subfamily
of class II aminotransferases, its enzyme activity is much stronger
as a phosphorylase than as an aminotransferase (2). AGXT2L1 is identified to be a
phosphoethanolamine (PEtN) phosphorylase and can irreversibly
degrade PEtN to acetaldehyde, phosphate and ammonia. In a previous
study, it has been discovered that AGXT2L1 is significantly
decreased and potentially functions as a tumor suppressor in HCC,
and it may regulate the de novo lipogenesis of cancer cells
(5). That study was the first to
investigate the expression and function of AGXT2L1 in cancer.
However, its role remains obscure in other types of cancer. In the
present study, the aim was to detect the expression levels of
AGXT2L1 in carcinomas of the digestive system and determine its
biological functions in different types of digestive cancer.
Autophagy is a process in which cells degrade
organelles and proteins to accomplish both the self-maintenance of
cells and the recycling of intracellular substances (6,7). In the
processes of tumorigenesis and cancer progression, autophagy may be
activated to degrade unnecessary macromolecules to meet the
increased proliferation requirements (6,7). PEtN is
a precursor of phosphatidylethanolamine (PE) (8), which is the main target for GABA type A
receptor associated protein like 2 (ATG8) conjugation during
autophagosome formation (9).
Therefore, it was hypothesized that AGXT2L1 may be involved in a
regulatory mechanism of autophagy. Among the proteins involved in
autophagy, microtubule-associated protein 1 light chain 3 is the
most extensively used autophagy marker, which undergoes lipidation
with PE following autophagy induction, resulting in the formation
of the phagophore membrane-bound form, LC3-II (10). LC3 expression was detected via
western blotting and immunofluorescence, in order to determine
autophagy activity. Gene set enrichment analysis (GSEA), combined
with in vitro experiments were performed in the present
study, in order to determine the role AGXT2L1 plays in colon
cancer.
Together with a previous study on HCC, the present
study demonstrated that AGXT2L1 is downregulated in carcinomas of
the digestive system and may function as a promising diagnostic and
prognostic biomarker for these diseases.
Materials and methods
Clinical samples
Primary gastric cancer and CRC mucosal tissues and
corresponding adjacent normal mucosal tissues (5–10 cm away from
tumor) were obtained from 16 patients with GC (10 men and 6 women;
age range, 47–71 years; mean age, 58.8 years) and six patients with
CRC (3 men and 3 women; age range, 47–64 years; mean age, 56.2
years) undergoing surgical resection at Renmin Hospital of Wuhan
University (Wuhan, China) between October 2016 and January 2017.
All patients had never received chemotherapy or radiotherapy.
Written informed consent was obtained from each patient. All
tissues were snap-frozen in liquid nitrogen and stored at −80°C.
All samples were validated by two experienced histopathologists as
tumor or normal tissues.
Cell culture, culture conditions and
antibodies
The human colon cancer HCT116 and SW480 cell lines
were cultured in DMEM (Hyclone; GE Healthcare Life Science)
supplemented with 10% FBS, 100 U/ml penicillin and 100 mg/ml
streptomycin (Hyclone; GE Healthcare Life Science) at 37°C with 5%
CO2. A rabbit polyclonal anti-AGXT2L1 antibody was
obtained from Sigma-Aldrich (Merck KGaA; cat. no. HPA044546). A
mouse monoclonal anti-GAPDH antibody was obtained from Santa Cruz
Biotechnology, Inc. (cat. no. sc-47724). A rabbit anti-LC3B
antibody was obtained from Cell Signaling Technology, Inc. (cat.
no. 2775S). Horseradish peroxidase (HRP)-labeled goat anti-rabbit
immunoglobulin G (IgG) (H+L), HRP-labeled goat anti-mouse IgG (H+L)
and FITC-labeled goat anti-rabbit IgG (H+L) antibodies were
obtained from Beyotime Institute of Biotechnology (cat. no. A0208,
A0216 and A0562, respectively).
Tissue sampling and
immunohistochemistry staining
GC, CRC, PC and esophageal cancer tissues from
patients were retrieved from the Department of Pathology at Renmin
Hospital of Wuhan University, fixed in 10% neutral buffered
formalin fixation (Beijing Solarbio Science & Technology Co.,
Ltd.) for 24 h at room temperature and embedded in paraffin.
Paraffin-embedded tissue samples were cut into 5-µm-thick sections
and subsequently deparaffinized in xylene for 15 min at room
temperature and rehydrated in a descending ethanol series (100, 95,
70 and 50% for 5 min at room temperature, respectively).
Deparaffinized sections were incubated with in 0.1 M citric acid
buffer (pH 6.0; www.servicebio.cn), prior to incubation with 0.3%
hydrogen peroxide (in PBS) for 10 min at room temperature to
inhibit endogenous peroxidase activity. Sections were subsequently
blocked with 5% BSA (provided in the ready-to-use IHC kit; cat. no.
KIT-9710; Fuzhou Maxim Biotech. Co., Ltd..) at room temperature for
15 min. Tissue sections were incubated with primary antibody
directed against AGXT2L1 (1:200; cat. no. HPA044546; Merck KGaA)
overnight at 4°C. Following the primary incubation, sections were
incubated with a goat anti-rabbit IgG (H&L) biotin-conjugated
secondary antibody (provided in the ready-to-use IHC kit; cat. no.
KIT-9710; Fuzhou Maxim Biotech. Co., Ltd) for 15 min at room
temperature. An UltraSensitive™ SP (mouse/rabbit)
Immunohistochemistry kit (Fuzhou Maxim Biotech. Co., Ltd.) and a
3,3′-diaminobenzidine (DAB) kit (cat. no. DAB-0031; Fuzhou Maxim
Biotech Co., Ltd.) were used for immunohistochemistry. The slides
were incubated at room temperature in alkaline
phosphatase-conjugated streptavidin for 30 min followed by DAB
solution. The chromogenesis procedure was monitored under a light
microscope (BX43 Upright Microscope; magnification, ×100; Olympus
Corporation). The intensity and percentage of stained sections were
independently assessed by two certified pathologists from the
Department of Pathology, Renmin Hospital of Wuhan University
(Wuhan, China) and verified using ImageJ software (version 1.51j8,
National Institutes of Health).
Gene expression profile analysis
The gene expression profile GSE17536 (11) was downloaded from the National Center
for Biotechnology Information Gene Expression Omnibus database
(https://www.ncbi.nlm.nih.gov/geo). This
dataset contains general information regarding patients with CRC,
such as age, sex, tumor stage, tumor grade and survival data. The
patients were divided into two groups according to their AGXT2L1
expression level (top 50%, high; vs. bottom 50%, low) for the
receiver operating characteristic (ROC) curve analysis. ROC
analysis was performed to determine the optimal cut-off value for
the CRC survival group, as determined using Youden's index, in
order to screen AGXT2L1 expression. Based on this analysis,
patients were grouped into high and low expression subgroups.
Subsequently, the association between AGXT2L1 and the clinical
features of patients was analyzed. The GSE63089 (12) and GSE27342 (13,14)
datasets were downloaded from the National Center for Biotechnology
Information Gene Expression Omnibus database, in order to assess
AGXT2L1 expression in gastric cancer. For GSEA analysis, GSEA tools
(http://www.broadinstitute.org/gsea)
and the gene expression profile from the GSE17536 dataset were used
to analyze the potential genes influenced by AGXT2L1, as previously
described (5).
Small interfering (si)RNA
transfection
siRNAs were designed as previously described
(5), and were obtained from Shanghai
GenePharma Co., Ltd. At 24 h before transfection, HCT116 and SW480
cells were seeded into 6-well plates at a density of
1×106 cells/well. Once cells reached 30–50% confluence,
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to transfect siRNAs (100 pmol) into
cells. At 3 days following transfection, cells were collected and
protein was extracted. The most efficient siRNA was selected for
the subsequent experiments. The sequences used were as follows:
AGXT2L1 forward, 5′-CCGGAGAAACUCUCUGUUUTT-3′ and reverse,
5′-AAACAGAGAGUUUCUCCGGTT-3′; and non-targeting siRNA forward,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′.
Western blotting
Total protein was extracted from cells transfected
with siRNA and pre-frozen tissue samples using RIPA lysis buffer
supplemented with 1 mM PMSF (Beyotime Institute of Biotechnology).
Lysates were centrifuged at 13800 × g for 15 min at 4°C and the
supernatant was used for subsequent analysis. Total protein was
quantified using a bicinchoninic acid assay and 30 µg protein/lane
was separated via SDS-PAGE on a 10% polyacrylamide gel. The
separated proteins were subsequently transferred onto a
polyvinylidene difluoride membrane (Merck KGaA) and blocked with 5%
non-fat milk in TBS with 0.1% Tween-20 for 1 h at room temperature.
Membranes were washed three times in TBST buffer (5 min/wash) and
incubated with primary antibodies against AGXT2L1 (1:1,000; cat.
no. HPA044546; Merck KGaA), LC3B (1:1,000; cat. no. 2775S; Cell
Signaling Technology, Inc.) and GAPDH (1:400; cat. no. sc-47724;
Santa Cruz Biotechnology, Inc.) on a shaker, overnight at 4°C.
Following the primary incubation, membranes were incubated with
horseradish peroxidase (HRP)-labeled goat anti-rabbit
immunoglobulin G (IgG) (H+L) (cat. no. A0208) and HRP-labeled goat
anti-mouse IgG (H+L) (cat. no. A0216) for 2 h at room temperature
(both 1:1,000 and both from Beyotime Institute of Biotechnology).
Membranes were washed three times with TBS, with 0.1% Tween-20 and
protein bands were visualized using ECL reagents (Wuhan Servicebio
Technology Co., Ltd.). Signals were detected using the Gel Doc XR
system (Bio-Rad Laboratories, Inc.) and band intensity was measured
via densitometry using Image Lab software (version 5.1, Bio-Rad
Laboratories, Inc.).
RNA extraction and reverse
transcription-quantitative (RT-q)PCR
Total RNA was extracted from tissue samples using
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. cDNA synthesis was performed using a
PrimeScript™ RT reagent kit with gDNA Eraser (cat. no. RR047A;
Takara Bio, Inc.). The RT reaction conditions were as follows: 37°C
for 15 min, followed by 85°C for 5 sec. mRNA levels were quantified
with a Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) using SYBR Premix EX Taq II (RR820l; Takara Bio,
Inc.). The PCR conditions were as follows: Initial denaturation at
95°C for 30 sec; 38 cycles of denaturation at 95°C for 5 sec and
annealing/extension at 60°C for 34 sec. GAPDH was used as an
internal control. The primers were, AGXT2L1 forward,
5′-GCCGATGGACCTCATAGAAA-3′ and reverse, 5′-TGGGCTTCTTTCAGCATCTT-3′;
GAPDH forward, 5′-CAAGGCCAACCGCGAGAA-3′ and reverse,
5′-CCCTCGTAGATGGGCACAGT-3′. The expression of AGXT2L1 relative to
GAPDH was determined using the 2−ΔΔCq method (15). The reactions were repeated three
times.
Immunofluorescence detection
Cells were seeded onto coverslips in 24-well plates,
at a density of 1×104 and cultured at 37°C, in 5%
CO2 and 95% humidified air overnight. The next day,
cells were transfected with siRNA. After 24 h, the cells were
washed with PBS three times. Subsequently, 4% paraformaldehyde was
used to fix the cells for 15 min at room temperature. Next, cells
were permeabilized in 0.5% Triton X-100 for 10 min at room
temperature. Following that, cells were blocked in 5% BSA for 30
min at room temperature and incubated with the primary antibody
against LC3B (1:400; cat. no. 2775S; Cell Signaling Technology,
Inc.) overnight at 4°C, followed by incubation with secondary
antibodies at room temperature for 1 h. After the addition of the
FITC-labeled goat anti-rabbit IgG (H+L) secondary antibody (1:400;
cat. no. A0562; Beyotime Institute of Biotechnology), all steps
were performed in the dark. Images were captured under a
fluorescence microscope (Olympus Corporation).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 20.0; IBM Corp.) and GraphPad Prism software
(version 6.0.1; GraphPad Software, Inc.). The Kaplan-Meier method
and the log-rank test were used to analyze the associations between
AGXT2L1 expression and the survival of patients with CRC. The
associations between AGXT2L1 expression and clinicopathological
features were statistically analyzed by the χ2 test.
Other data were statistically analyzed using paired Student's
t-test. All data are presented as the mean ± standard deviation.
All experiments were performed in triplicate. P<0.05 was
considered to indicate a statistically significant difference.
Results
AGXT2L1 expression is decreased in
CRC
To explore the expression levels of AGXT2L1 in CRC,
normal, intraepithelial neoplasia and CRC tissues were collected.
Subsequently, AGXT2L1 expression was detected by
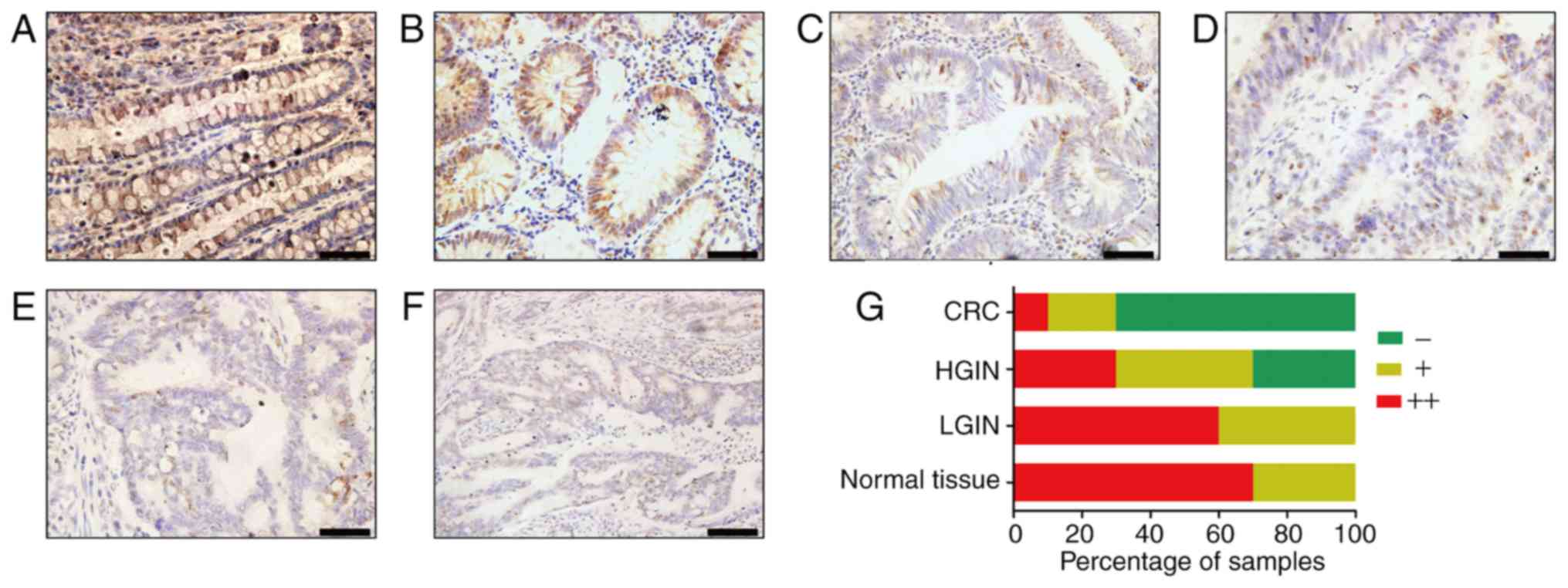
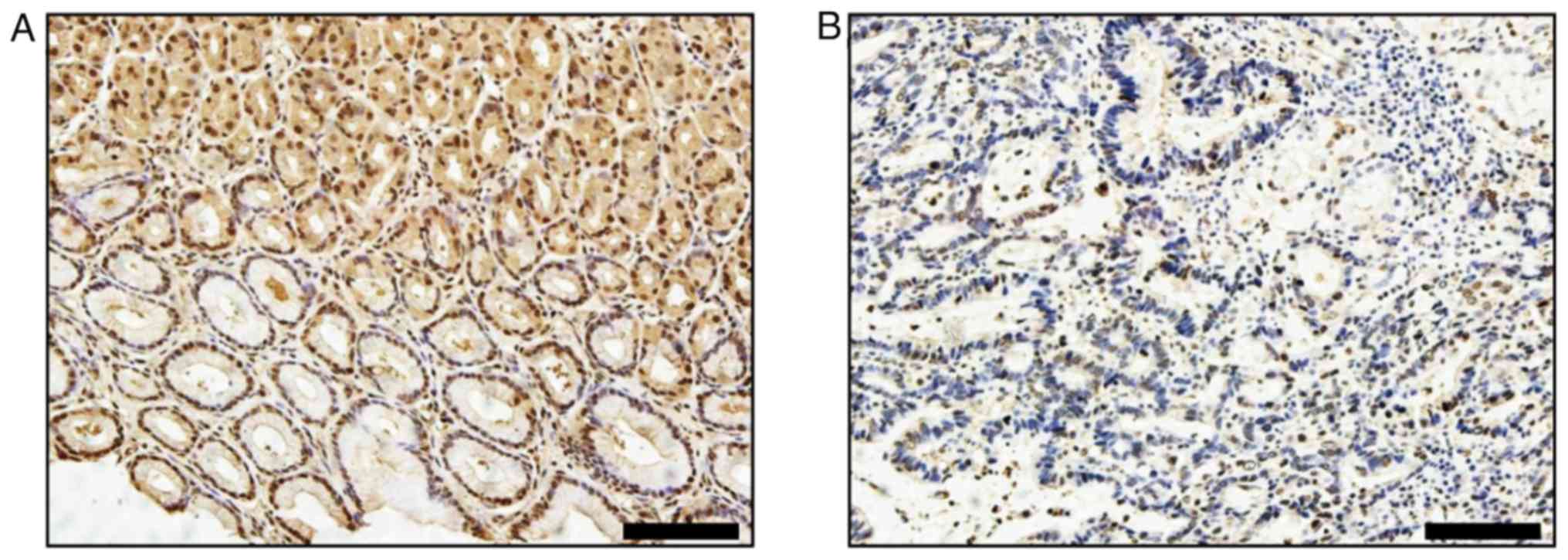
immunohistochemistry. Most normal tissues (Fig. 1A) and colonic low-grade
intraepithelial neoplasia (LGIN) tissues (Fig. 1B) were strongly AGXT2L1-positive
(70%, 7/10; 60%, 6/10, respectively), and a small number of tissues
were weakly positive (30%, 3/10; 40%, 4/10, respectively). In
colonic high-grade intraepithelial neoplasia (HGIN) tissues and CRC
tissues, most samples were AGXT2L1-negative or weakly positive. In
colonic HGIN (Fig. 1C), 30% (3/10)
of the tissues were AGXT2L1 negative, 40% (4/10) were weakly
positive and 30% (3/10) were positive; in CRC tissues (Fig. 1D-F), 70% (7/10) of the tissues were
AGXT2L1 negative, 20% (2/10) were weakly positive and only 10%
(1/10) were strongly positive. Notably, it was observed that
AGXT2L1 expression decreased gradually from normal tissue to
intraepithelial neoplasia to colon cancer (Fig. 1G), suggesting that AGXT2L1 serves an
important role in the malignant transformation of colonic tissue.
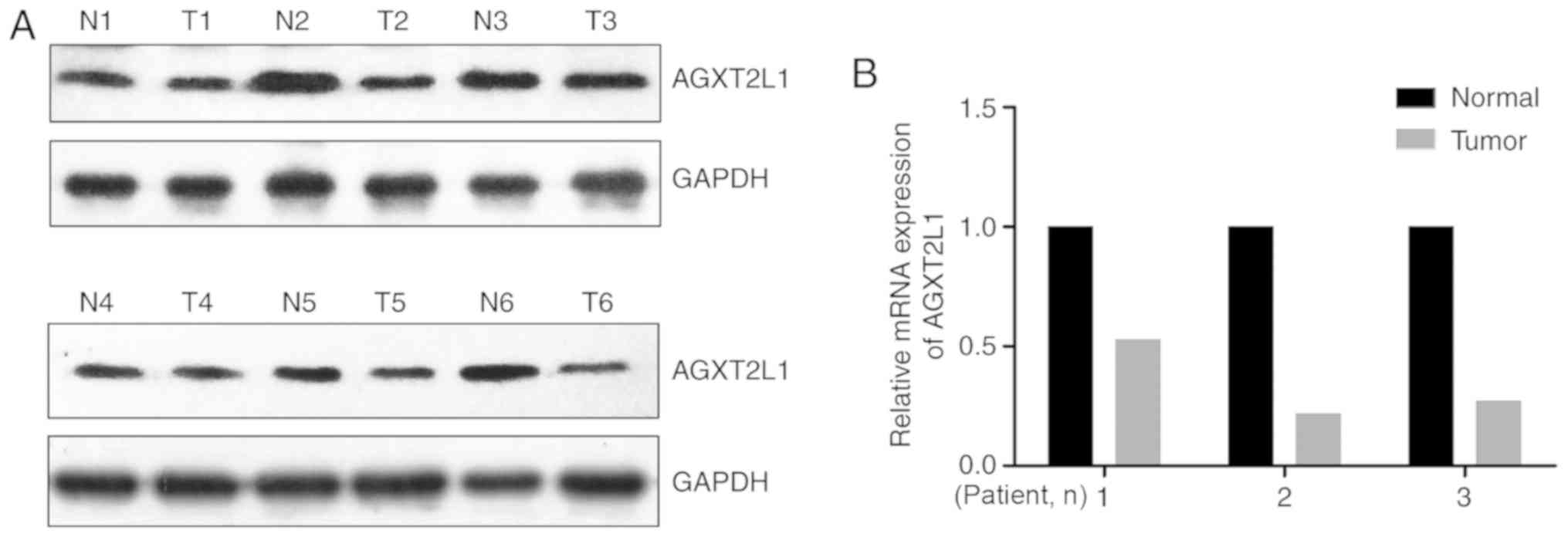
The expression levels of AGXT2L1 were also detected in six pairs of
CRC tissues and corresponding adjacent tissues by western blotting.
It was demonstrated that the expression levels of AGXT2L1 in CRC
tissues were lower compared with those in the adjacent tissues
(Fig. 2A). Additionally, it was
identified that the mRNA expression levels of AGXT2L1 were
decreased in three CRC tissues compared with in paired adjacent
tissues (Fig. 2B). Collectively,
these results indicated that AGXT2L1 was downregulated in CRC.
Clinicopathological features and the survival of
patients with CRC are associated with AGXT2L1 expression. A total
of 177 patients with CRC in the GES17536 dataset were divided into
two groups (high expression: ≥4.8355, n=32; low expression:
<4.8355, n=145). The association between the clinicopathological
features and AGXT2L1 expression was analyzed statistically using
the χ2 test. AGXT2L1 expression was closely associated
with age (P=0.045) and American Joint Committee on Cancer T stage
(P=0.027; Table I). Survival
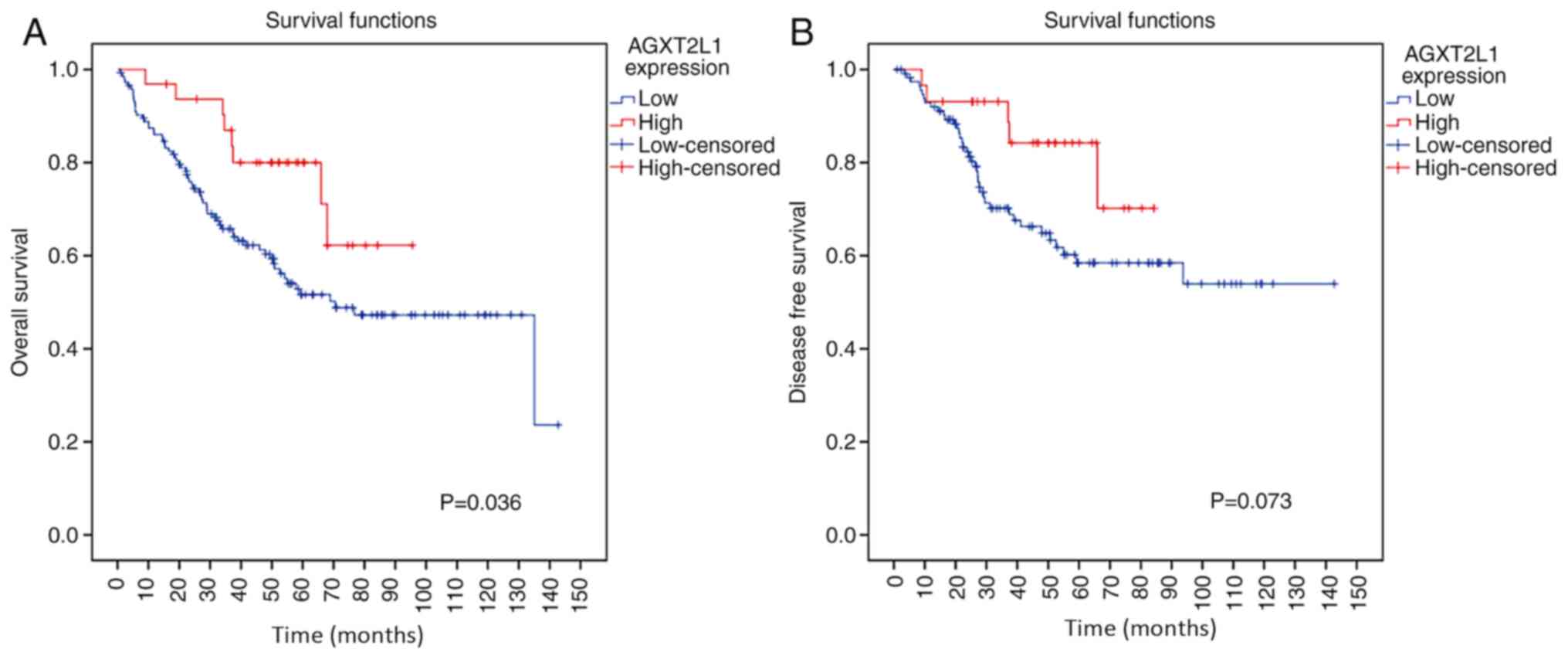
analysis was subsequently performed using the Kaplan-Meier method
to estimate the association between AGXT2L1 expression and the
survival of patients with CRC. It was demonstrated that AGXT2L1
expression was significantly associated with the prognosis of
patients with CRC (Fig. 3A);
patients with high expression levels of AGXT2L1 displayed longer
overall survival (P=0.036) and possibly suffered less incidence of
recurrence of the disease (P=0.073) compared with patients with low
expression levels of AGXT2L1 (Fig.
3B).
 | Table I.Association between AGXT2L1 expression
and clinicopathological features in patients with colorectal cancer
(GSE17536). |
Table I.
Association between AGXT2L1 expression
and clinicopathological features in patients with colorectal cancer
(GSE17536).
|
| AGXT2L1
expression |
|---|
|
|
|
|---|
| Characteristics | Patients, n | Low, n | High, n | P-value |
|---|
| Age, years |
|
|
| 0.045 |
|
≤65 | 78 | 69 | 9 |
|
|
>65 | 99 | 76 | 23 |
|
| Sex |
|
|
| 0.801 |
|
Male | 96 | 78 | 18 |
|
|
Female | 81 | 67 | 14 |
|
| AJCC stage |
|
|
| 0.027 |
| T1 | 24 | 16 | 8 |
|
| T2 | 57 | 43 | 14 |
|
| T3 | 57 | 51 | 6 |
|
| T4 | 39 | 35 | 4 |
|
| Grade |
|
|
| 0.592 |
| WD | 16 | 13 | 3 |
|
| MD | 134 | 108 | 26 |
|
| PD | 27 | 24 | 3 |
|
AGXT2L1 expression is also decreased
in GC and PC
Furthermore, the expression levels of AGXT2L1 were
examined in gastric tissues. Similar to the expression in colonic
tissues, AGXT2L1 expression also decreased gradually from normal
gastric tissue to intraepithelial neoplasia to GC (Fig. 4). In normal gastric tissues (Fig. 4A), 90% (9/10) of the samples were
strongly positive and 10% (1/10) of the samples were weakly
positive, whereas in LGIN tissues (Fig.
4B), 50% (5/10) of the samples were strongly positive and 50%
(5/10) of the samples were weakly positive. In HGIN tissues
(Fig. 4C), 20% (2/10) of the samples
were strongly positive and 80% (8/10) of the samples were weakly
positive. The absence of AGXT2L1 was detected only in GC tissues
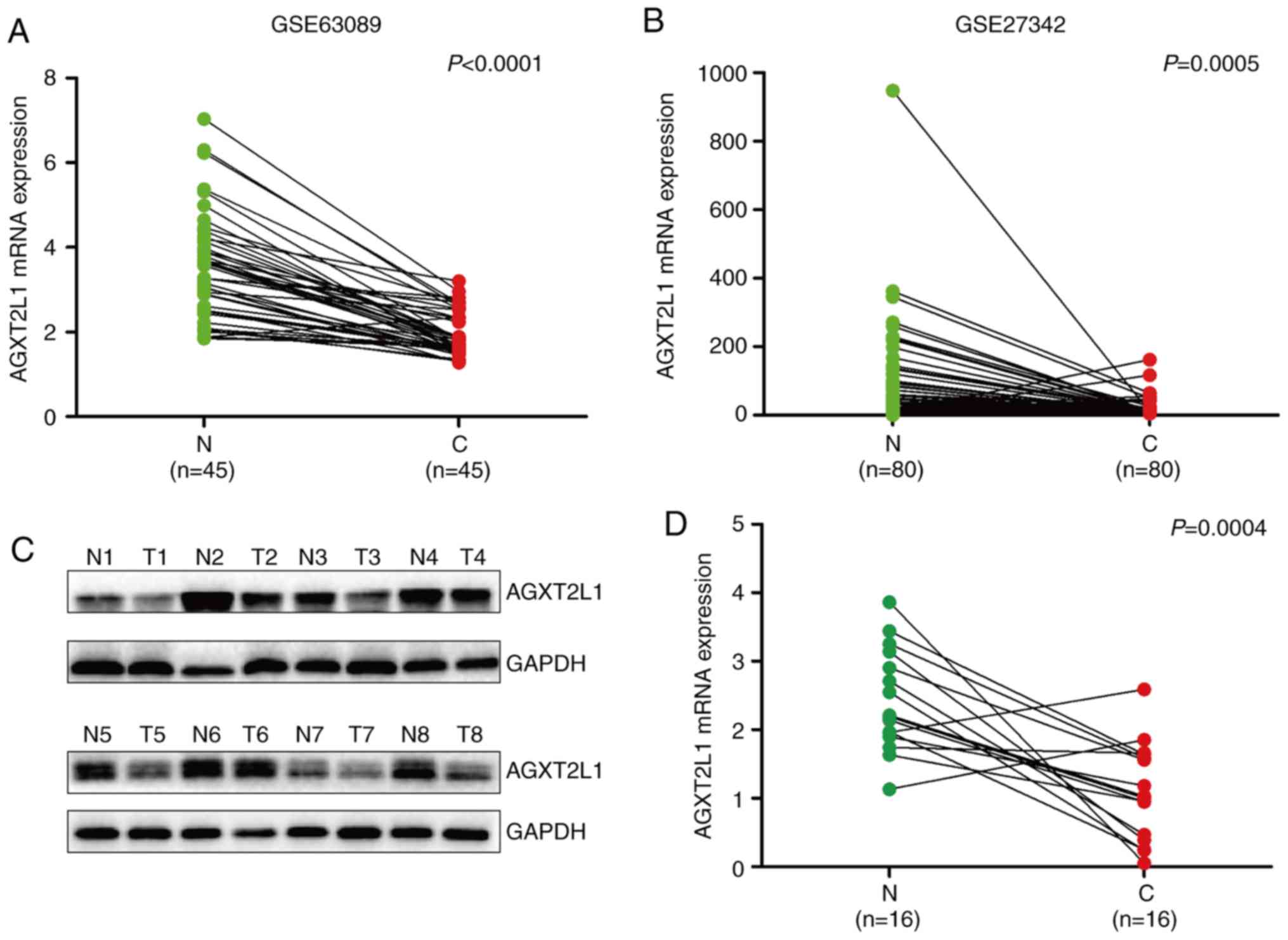
(80%; 16/20; Fig. 4D-F). Analysis of
the public datasets GSE63089 (Fig.
5A) and GSE27342 (Fig. 5B), and
the detection of AGXT2L1 by western blotting (Fig. 5C) and RT-qPCR (Fig. 5D) also demonstrated that AGXT2L1 was
downregulated in GC. Subsequently, AGXT2L1 was detected in four
pairs of PC tissues and their corresponding adjacent tissues. All
normal tissues (Fig. 6A) exhibited
high expression levels of AGXT2L1, whereas all cancerous tissues
(Fig. 6B) displayed low expression
or absence of AGXT2L1. AGXT2L1 expression was examined in
esophageal cancer tissues by immunohistochemistry, however, AGXT2L1
intensity was weak or absent in esophagus tissues (data not shown),
indicating that AGXT2L1 expression may be cell-type specific.
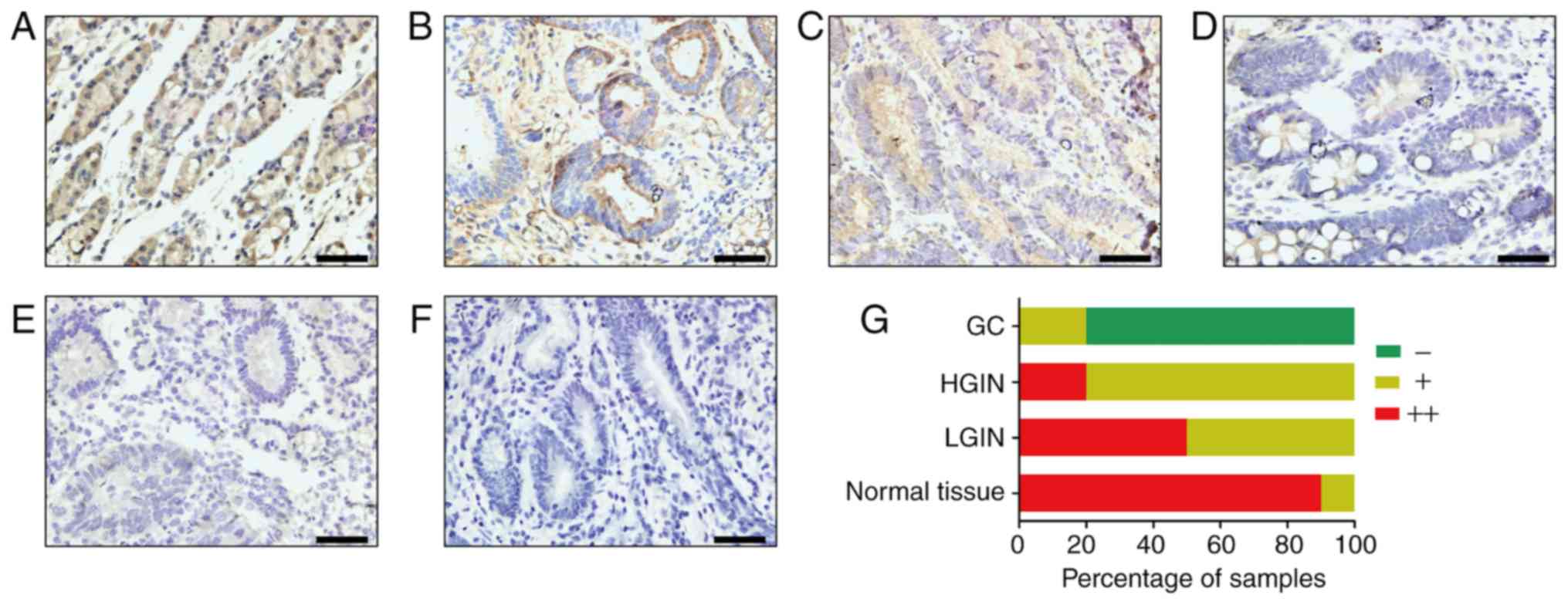 | Figure 4.AGXT2L1 expression is decreased in GC
tissues. AGXT2L1 expression in gastric tissues was determined by
immunohistochemistry. Representative images of (A) normal gastric
tissue, (B) LGIN and (C) HGIN, and (D) well-differentiated, (E)
poorly differentiated and (F) undifferentiated GC tissues are
shown. (G) Percentage of samples at each expression level in
normal, LGIN, HGIN and GC tissues. Scale bars, 50 µm. LGIN,
low-grade intraepithelial neoplasia; HGIN, high-grade
intraepithelial neoplasia; GC, gastric cancer; AGXT2L1,
alanine-glyoxylate aminotransferase 2-like 1. |
AGXT2L1 is associated with PI
metabolism and is involved in autophagy
Gene Set Enrichment Analysis using the CRC
expression profile data (GSE17536) was performed to predict the
function of AGXT2L1 in carcinomas of the digestive system. There
were significant differences in PI metabolism and the synthesis of
PIPs at the plasma membrane (Fig.
7A), between the two groups (AGXT2L1 low expression vs. high
expression). To investigate the role of AGXT2L1 in autophagy,
HCT116 and SW480 cells were transfected with AGXT2L1-siRNA and
NC-siRNA. Expression levels of LC3, specifically LC3II, were
increased in AGXT2L1-knockdown cells (Fig. 7B). In the immunofluorescence assay
(Fig. 7C), LC3 was consistently
expressed in a diffuse pattern in the control group, whereas in the
AGXT2L1-knockdown group, the pattern of LC3 was changed from
diffuse to punctate. These results provided preliminarily
validation that AGXT2L1 is involved in autophagy and it may
modulate autophagy via phospholipid metabolism, thus contributing
to cancer development.
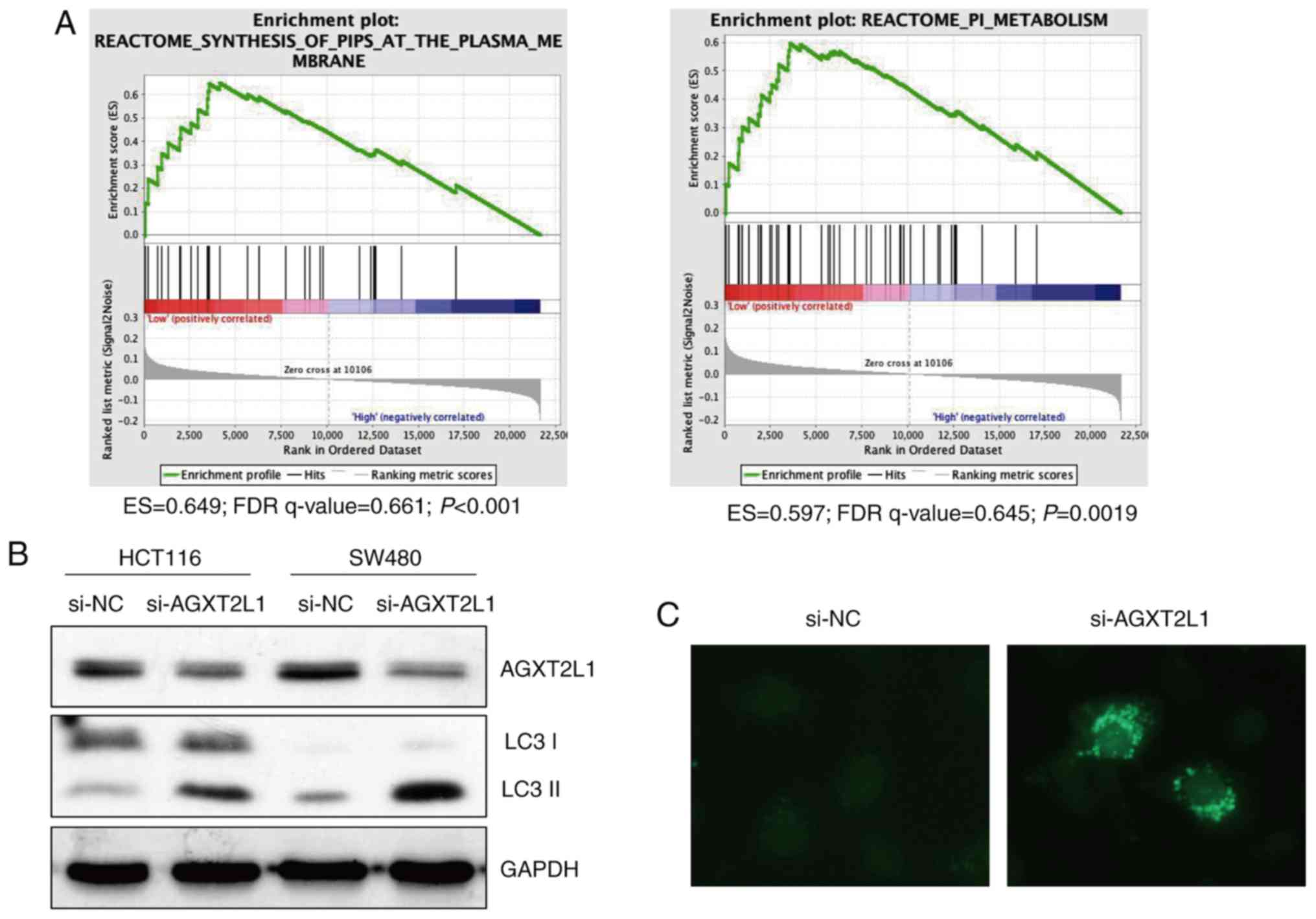 | Figure 7.AGXT2L1 is associated with
phosphatidylinositol metabolism and is involved in autophagy. (A)
Gene set enrichment analysis of GSE17536 indicated that AGXT2L1 may
regulate PI metabolism and PIPs biosynthesis in colorectal cancer.
(B) AGXT2L1 expression was decreased by siRNA transfection in
HCT116 and SW480 cells, whereas the expression of the autophagy
marker LC3II was increased. (C) LC3 was detected by
immunofluorescence (magnification, ×400). In AGXT2L1 knockdown
cells, the pattern of LC3 was altered from diffuse to punctate.
AGXT2L1, alanine-glyoxylate aminotransferase 2-like 1; ES,
enrichment score; FDR, false discovery rate; NC, negative control;
PI, phosphatidylinositol; PIP, phosphatidylinositol phosphates;
si-, small interfering RNA. |
Discussion
To the best of our knowledge, the present study is
the first to examine the expression levels of AGXT2L1 in CRC, GC,
PC and esophageal cancer. The limited sample size is a limitation
of the present study, and it would be beneficial to include more
samples in future studies to confirm the current findings. The
findings of the present study were consistent with those of a
previous study (5) on liver cancer,
demonstrating that AGXT2L1 is significantly downregulated in
cancerous tissues and may be a tumor suppressor; at least in
carcinomas of the digestive system of colorectum, stomach and
pancreas. However, the underlying mechanism by which AGXT2L1
regulates tumorigenesis and cancer progression remains unclear, and
several possible explanations are proposed here.
The present study demonstrated an association
between AGXT2L1 and autophagy. The role of autophagy in the process
of tumor development has always been considered as a double-edged
sword (16). However, it is certain
that autophagy can be activated to convert unnecessary molecules to
essential substances to promote tumor growth in established cancer
(17–19). For numerous years, the origin of the
autophagosomal membranes and the regulation underlying
autophagosome biogenesis aroused researchers' interests. It has
first been suggested that the phagophore is derived from the
endoplasmic reticulum (20).
Subsequently, there have been various assumptions that the membrane
might come from the Golgi, mitochondria, endosomes or the plasma
membrane (20). Currently, it is
widely accepted that autophagosomes are derived from endoplasmic
reticulum membranes, which are strongly enriched in PI(3)P
(21,22). The present study demonstrated that
AGXT2L1 may regulate PI metabolism and the synthesis of PIPs at the
plasma membrane. PI is a membrane phospholipid that can be
reversibly phosphorylated to generate several phosphoinositides,
including phosphatidylinositol 3-phosphate [PI(3)P] (21,22). It
has been reported that autophagosomes are derived from the
endoplasmic reticulum membrane, which is strongly enriched in
PI(3)P (22); thus, it was
hypothesized that AGXT2L1 may regulate the process of autophagy.
Therefore, it was speculated that AGXT2L1 may serve a role in the
formation of the omegasome. On the other hand, PE is an
indispensable component in the final step of autophagosome
formation (23). It has been
reported that a high level of PE can stimulate the conjugation of
the ATG8/LC3-PE complex, which is necessary during the elongation
and closure of the autophagic membrane (9,24). The
present study suggested that the downregulation of AGXT2L1 may
result in excessive storage of PEtN and an increase in PE
synthesis. In contrast to the mainstream view that PE may derive
from multiple membrane sources, the present study suggested that
the increased level of PE in autophagosomes may be due to the
abnormal AGXT2L1 expression.
Compared with normal cells, cancer cells are
relatively undifferentiated (25).
Aberrant metabolism of phospholipids is a crucial characteristic
during tumorigenesis and cancer progression (26). A number of studies have previously
linked PE to cell differentiation (26–28). For
example, lysophosphatidylethanolamine (LPE)-acyltransferase, which
regulates the synthesis of PE from LPE, has been reported to
modulate cardiac muscle differentiation (27). Lactamase β is significantly
upregulated in differentiated postmitotic muscle cells relative to
its expression in undifferentiated, actively cycling cells and
functions as a tumor suppressor by inhibiting phosphatidylserine
decarboxylase, in turn blocking the synthesis of mitochondrial PE
(26). As PEtN is the main precursor
of PE, and AGXT2L1 irreversibly and strictly degrades PEtN, the
downregulation of PEtN levels by AGXT2L1 may lead to inactivation
of the synthesis of PE and subsequent differentiation.
Furthermore, the imbalance of the membrane
PE/phosphatidylcholine ratio has a great impact on multiple
cellular processes, such as lipogenesis, endoplasmic reticulum
stress, mitochondrial respiration and oxidative capacity (29). PE and PC are the most abundant
phospholipids in the cell membrane, and a decreased PE/PC ratio
leads to increased cell membrane permeability, resulting in the
leakage of intracellular material that may trigger a series of
inflammatory responses (30), which
are stimulating factors in the process of tumorigenesis. All
aforementioned factors are involved in cancer biology, and it is
worth studying whether the downregulation of AGXT2L1 can take part
in cancer progression via these mechanisms in the future.
Acknowledgements
The authors would like to thank Mr. Xia Hong from
Hubei Key Laboratory of Digestive System, Renmin Hospital of Wuhan
University (Wuhan, China) for his administrative support and
excellent technical assistance in this work.
Funding
The present study was supported by National Natural
Science Foundation of China (grant nos. 81672387 and 81703030).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QD and HY designed the experiments. YD and LW
performed the experiments. YD and LW collected the tissues samples.
YD and LW performed the statistical analyses. QD, LW and YD wrote
the manuscript. HY and QD provided the funds and materials.
Ethics approval and consent to
participate
The Ethics Committee of Renmin Hospital of Wuhan
University approved the present study (approval no. WDRY2016-K033).
Written informed consent was obtained from each patient prior to
the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Veiga-da-Cunha M, Hadi F, Balligand T,
Stroobant V and Van Schaftingen E: Molecular identification of
hydroxylysine kinase and of ammoniophospholyases acting on
5-phosphohydroxy-L-lysine and phosphoethanolamine. J Biol Chem.
287:7246–7255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schiroli D, Cirrincione S, Donini S and
Peracchi A: Strict reaction and substrate specificity of AGXT2L1,
the human O-phosphoethanolamine phospho-lyase. IUBMB Life.
65:645–650. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shao L and Vawter MP: Shared gene
expression alterations in schizophrenia and bipolar disorder. Biol
Psychiatry. 64:89–97. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ding Q, Kang J, Dai J, Tang M, Wang Q,
Zhang H, Guo W, Sun R and Yu H: AGXT2L1 is down-regulated in
heptocellular carcinoma and associated with abnormal lipogenesis. J
Clin Pathol. 69:215–220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lai K, Killingsworth MC and Lee CS: The
significance of autophagy in colorectal cancer pathogenesis and
implications for therapy. J Clin Pathol. 67:854–858. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saha S, Panigrahi DP, Patil S and Bhutia
SK: Autophagy in health and disease: A comprehensive review. Biomed
Pharmacother. 104:485–495. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pavlovic Z and Bakovic M: Regulation of
Phosphatidylethanolamine Homeostasis - The Critical Role of CTP:
Phosphoethanolamine Cytidylyltransferase (Pcyt2). Int J Mol Sci.
14:2529–2550. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martens S, Nakamura S and Yoshimori T:
Phospholipids in Autophagosome Formation and Fusion. J Mol Biol.
Oct 27–2016.(Epub ahead of print). doi: 10.1016/j.jmb.2016.10.029.
View Article : Google Scholar
|
|
10
|
Ravikumar B, Sarkar S, Davies JE, Futter
M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M,
Korolchuk VI, Lichtenberg M, Luo S, et al: Regulation of mammalian
autophagy in physiology and pathophysiology. Physiol Rev.
90:1383–1435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smith JJ, Deane NG, Wu F, Merchant NB,
Zhang B, Jiang A, Lu P, Johnson JC, Schmidt C, Bailey CE, et al:
Experimentally derived metastasis gene expression profile predicts
recurrence and death in patients with colon cancer.
Gastroenterology. 138:958–968. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang X, Ni Z, Duan Z, Xin Z, Wang H, Tan
J, Wang G and Li F: Overexpression of E2F mRNAs associated with
gastric cancer progression identified by the transcription factor
and miRNA co-regulatory network analysis. PLoS One.
10:e01169792015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cui J, Chen Y, Chou WC, Sun L, Chen L, Suo
J, Ni Z, Zhang M, Kong X, Hoffman LL, et al: An integrated
transcriptomic and computational analysis for biomarker
identification in gastric cancer. Nucleic Acids Res. 39:1197–1207.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cui J, Li F, Wang G, Fang X, Puett JD and
Xu Y: Gene-expression signatures can distinguish gastric cancer
grades and stages. PLoS One. 6:e178192011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
16. Gual P, Gilgenkrantz H and Lotersztajn
S: Autophagy in chronic liver diseases: The two faces of Janus. Am
J Physiol Cell Physiol. 312:C263–C273. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kowalik MA, Perra A, Ledda-Columbano GM,
Ippolito G, Piacentini M, Columbano A and Falasca L: Induction of
autophagy promotes the growth of early preneoplastic rat liver
nodules. Oncotarget. 7:5788–5799. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sui X, Jin L, Huang X, Geng S, He C and Hu
X: p53 signaling and autophagy in cancer: A revolutionary strategy
could be developed for cancer treatment. Autophagy. 7:565–571.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Giatromanolaki A, Koukourakis MI, Harris
AL, Polychronidis A, Gatter KC and Sivridis E: Prognostic relevance
of light chain 3 (LC3A) autophagy patterns in colorectal
adenocarcinomas. Clin Pathol. 63:867–872. 2010. View Article : Google Scholar
|
|
20
|
Tooze SA: Current views on the source of
the autophagosome membrane. Essays Biochem. 55:29–38. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hurley JH and Young LN: Mechanisms of
Autophagy Initiation. Annu Rev Biochem. 86:225–244. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lamb CA, Yoshimori T and Tooze SA: The
autophagosome: Origins unknown, biogenesis complex. Nat Rev Mol
Cell Biol. 14:759–774. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qiu Y, Zheng Y, Wu KP and Schulman BA:
Insights into links between autophagy and the ubiquitin system from
the structure of LC3B bound to the LIR motif from the E3 ligase
NEDD4. Protein Sci. 26:1674–1680. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rockenfeller P, Koska M, Pietrocola F,
Minois N, Knittelfelder O, Sica V, Franz J, Carmona-Gutierrez D,
Kroemer G and Madeo F: Phosphatidylethanolamine positively
regulates autophagy and longevity. Cell Death Differ. 22:499–508.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Garcia H, Fleyshman D, Kolesnikova K,
Safina A, Commane M, Paszkiewicz G, Omelian A, Morrison C and
Gurova K: Expression of FACT in mammalian tissues suggests its role
in maintaining of undifferentiated state of cells. Oncotarget.
2:783–796. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Keckesova Z, Donaher JL, De Cock J,
Freinkman E, Lingrell S, Bachovchin DA, Bierie B, Tischler V, Noske
A, Okondo MC, et al: LACTB is a tumour suppressor that modulates
lipid metabolism and cell state. Nature. 543:681–686. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fotheringham J, Xu FY, Nemer M, Kardami E,
Choy PC and Hatch GM: Lysophosphatidylethanolamine acyltransferase
activity is elevated during cardiac cell differentiation. Biochim
Biophys Acta. 1485:1–10. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu Y, Chen K, Xing G, Li L, Ma B, Hu Z,
Duan L and Liu X: Phospholipid remodeling is critical for stem cell
pluripotency by facilitating mesenchymal-to-epithelial transition.
Sci Adv. 5:eaax75252019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
van der Veen JN, Kennelly JP, Wan S, Vance
JE, Vance DE and Jacobs RL: The critical role of
phosphatidylcholine and phosphatidylethanolamine metabolism in
health and disease. Biochim Biophys Acta Biomembr 1859B.
B1558–B1572. 2017. View Article : Google Scholar
|
|
30
|
Li Z, Agellon LB, Allen TM, Umeda M,
Jewell L, Mason A and Vance DE: The ratio of phosphatidylcholine to
phosphatidylethanolamine influences membrane integrity and
steatohepatitis. Cell Metab. 3:321–331. 2006. View Article : Google Scholar : PubMed/NCBI
|