Introduction
Traditional treatment methods of human cancer types
primarily include resection, chemotherapy or radiotherapy (1). Single or combination therapy can
control local tumors and prolong the lifespan of the patient
(2). However, these therapies have
limitations, such as causing greater trauma and toxic side effects
on patients, which impact their long-lasting anti-cancer effects
(3). Moreover, the recrudescence and
metastasis of tumors cannot be fully controlled by traditional
treatments (4).
Previous studies have reported that tumor occurrence
is associated with the immune system in the human body (5,6).
Furthermore, immunotherapy of tumors has been promising, and
multiple methods have been tested over the past few decades
(7,8). Previous studies on tumor immunotherapy
have revealed the characteristics of immune system response related
to cancer occurrence (9,10), and some of these studies have
involved the use of dendritic cells (DCs) (11). DCs connect the innate and adaptive
immune systems by functioning as antigen-presenting cells, and have
been used as tumor vaccines in several clinical experiments
(12,13). Furthermore, immunotherapy based on
DCs has been reported to be safe and capable of inducing anti-tumor
immunity (14).
The application of cancer vaccines has become an
important procedure, and immunotherapy has been used for the
standard treatment and continuous control of tumors (15). However, the immunoreactions triggered
by these vaccines are inefficient due to a lacking number of
lymphocytes (16). Therefore, it is
important to develop novel types of vaccines that can activate the
immune system to generate long-lasting immunity. It has been
suggested that vaccines may induce a strong immune response and
elicit immunological memory (15).
As for the preparation of vaccine, it has been
proved that the immune response is induced by autologous cancer
cells in the host, leading to a large number of tumor antigens
produced by the immune system (17).
Regardless of the final result, this type of vaccine includes the
main principles of cell-based vaccines: i) Complete inactivation of
tumor cells; ii) maintaining the immunogenicity of cells; and iii)
adherence to the laws and ethical guidelines. Physical methods,
such as freeze-thawing or ultrasonication, and chemotherapeutics
for the inactivation of the tumor cells have certain restrictions,
such as inadequate inactivation of cells and the addition of toxic
impurities (18). Moreover, certain
laws have forbidden the use of X-ray irradiation and lethal factors
of toxic drugs in humans (19).
Treatment with high hydrostatic pressure (HHP) has
helped overcome the aforementioned limitations (20,21).
Previous studies have reported the use of HHP as a disinfectant in
the pharmaceutical and foodstuff industries (22–24), and
thus microorganisms are killed without the use of radiation, heat
or chemicals (21,25). With regards to the applications of
HHP in medicine, it has been utilized to disinfected the bone,
while maintaining biomechanical stability (26), and has also been researched in
Alzheimer's disease (27). Previous
studies have investigated the application of HHP for the
development of the bacterial vaccines (28) and its effect on lipoprotein particles
(29). The effect of HHP on
biological particles has been previously examined (21). It has been revealed that proteins
cannot maintain their primary structures, and the tertiary and
quaternary structures of proteins could be transformed by HHP
(19). Moreover, biological enzymes
may lose their abilities after HHP treatment (30). Furthermore, the conformation of DNA
and lipid bilayers may undergo significant changes during HHP
(31).
To study the effective pressure treatment durations
(1, 5, 10, 30, 60 and 120 min) and the effective pressures (50,
100, 200, 300 and 500 MPa) of HHP to inactivate melanoma cells, B16
melanoma cells were subjected to different treatment conditions. In
addition, cell death was examined over a culture period of 48 h. A
clonogenic assay was also performed to assess the proliferative
abilities of the cells. Furthermore, HHP-treated cells were used in
the mouse models to determine immunogenicity.
Materials and methods
Cell line and culture
The cell line used was luciferase-labeled B16
melanoma cells (B16-F10; Xenogen Corporation), and mycoplasma
testing was performed for this cell line. Cells were cultivated
with DMEM (HyClone; GE Healthcare Life Sciences), 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.), 1% penicillin-streptomycin
(Beijing Transgen Biotech Co., Ltd.) and 1% L-glutamine (AMRESCO,
LLC). Melanoma cells were cultivated under conditions of 100%
relative humidity and 37°C in a 5% CO2 atmosphere.
HHP treatment of cells
Cells were separated from the petri dish with 0.25%
trypsin (HyClone; GE Healthcare Life Sciences). Then, cells were
centrifuged (1,500 × g at 4°C for 5 min), rinsed (sterile PBS) and
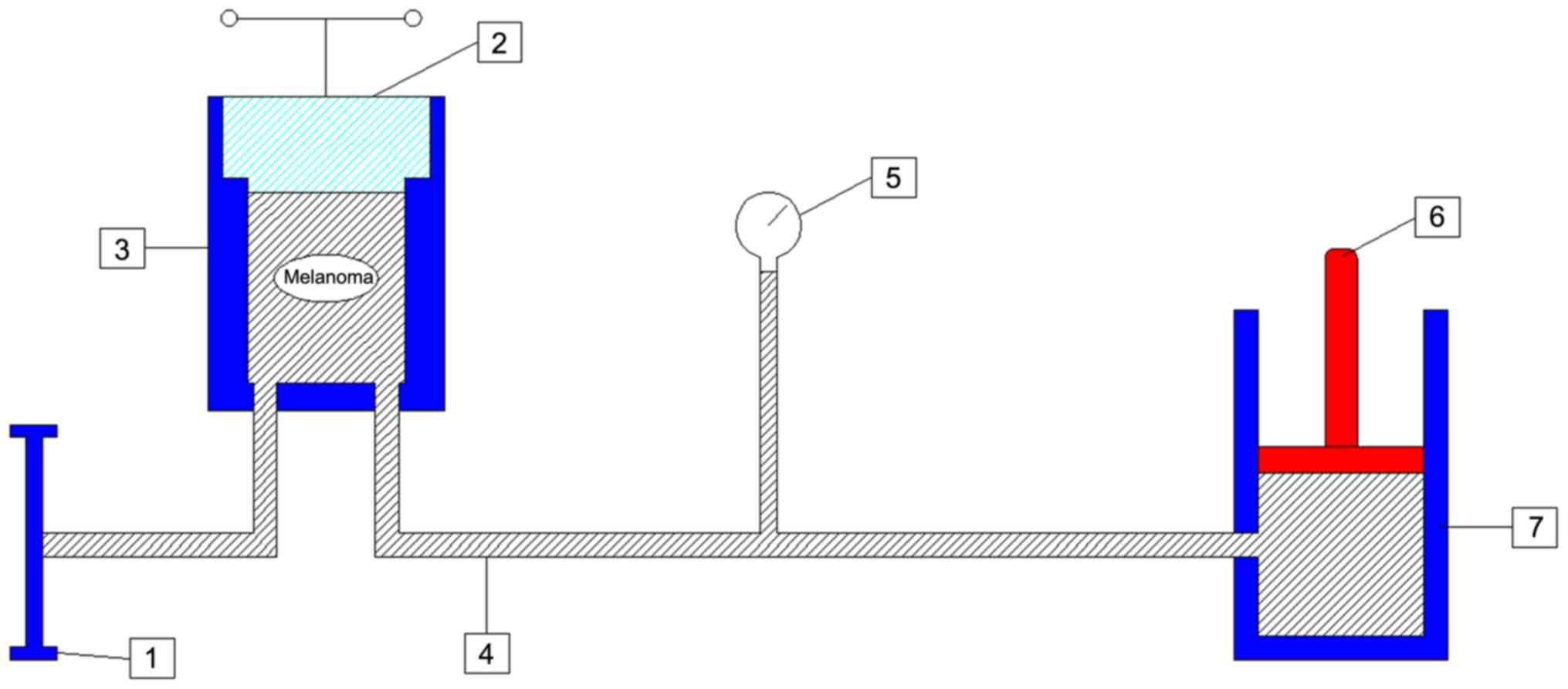
re-suspended in vitro. Soft-seal vacuum sterile plastic bags
(Alibaba Group) were used to avert the leakage of cells during the
treating processes (Fig. 1). The
plastic bag was filled with a cell suspension of 3.2×107
melanoma cells and securely closed. Then, the plastic bag was
placed into another soft plastic bag and a vacuum seal was created
to avoid the air bubbles reducing the pressure during the HHP
treatment. The plastic bags were placed in the pressure-autoclave
of the equipment [cat. no. DH600-0.8X2 (9242); Dalong Goepe.
(http://www.dalongyeya.com/)]. The
pressure chamber was locked using the screwing flanges and the
pressure was elevated with an electric pump. In the present study,
the pressure of HHP used was ≥50 MPa (1 MPa=10 bar=9.86923
atm=145.0377 psi). When the digital pressure sensor displayed the
desired numerical value (50, 100, 200, 300 and 500 MPa) the pump
was suspended. The equipment held the pressure for the different
required durations (1, 5, 10, 30, 60 and 120 min). The pressure was
detected continually using a digital pressure sensor. Then, the
valve was opened slowly, and the pressure was gradually
decompressed to atmospheric pressure.
Clonogenic assay
Following HHP treatment, 1×104 cells were
cultured in the cell culture dishes for 14 days. Then, 0.25%
methylene blue solution was used to stain the colonies in the
dishes (25°C for 10 min) and colonies were counted manually. The
colony-forming units (CFU) results were compared with the numbers
of colonies related to the untreated cells.
Immunization of mouse models
A total of 105 mice (C57BL/6 mice; female; age, 4–6
weeks; weight, 15.00±0.33 g; Beijing Vital River) were raised in
well-ventilated cages in these studies. Three mice were raised per
cage. The mice were given a sterile special diet (white noodle 37%,
corn noodle 22%, bran 17%, soybean meal 13%, fish meal 4%, egg 1%,
yeast powder 1%, bone meal 3%, salt 1% and cod liver oil 1%) and
water. The animals could access to food and water ad libitum. The
cages were kept under normal conditions for temperature (23±3°C),
humidity (55±15%) and light (12/12 h light-dark cycles). All animal
experiments were conducted according to the National Standard of
the Care and Use of Laboratory Animals (32), and the study was approved by the
Animal Ethics Committee of The First Hospital of Jilin University
(Changchun, China; Approval no. 2019-0456).
Then, emulsification was performed using two 5 ml
glass syringes and a medical tee joint to mix the treated cell
suspension with 1.5 ml complete Freund's adjuvant (Sigma-Aldrich;
Merck KGaA) for 30 min. Subsequently, 100 µl emulsified liquid was
injected under the skin of the posterior neck of the mice. The
injection was carried out at the same location three times, every 2
weeks. During the second and third injections, the same dosage
Freund's incomplete adjuvant (Sigma-Aldrich; Merck KGaA) was used.
The control group was injected with the same dose of emulsion of
saline and the Freund's adjuvant (the first injection) or the
Freund's incomplete adjuvant (the second and third injections). The
health and behavior of the mice were monitored every day after the
injection.
Detection of the immune effect
Thirty treatment groups and one control group (n=3
mice for each group) were prepared for this experiment. There were
five treatment groups (50, 100, 200, 300 and 500 MPa) at six
treatment durations (1, 5, 10, 30, 60 and 120 min). All experiments
were performed in triplicate. Seven days following the final
injection, the delayed-type hypersensitivity (DTH) test was
performed by subcutaneously injecting 5×106 melanoma
cells, which were suspended in 70 µl sterile PBS, into the palm of
the hind paw of the mice. The same volume of PBS was injected into
the other palm of the hind paw (the control group only received
injections of saline). The swelling degree of the paws was measured
after 24 h using a digital caliper (Iron Bridge Tools, Inc.).
The B16-F10 cell suspension was subcutaneously
injected into the flank of the mice; the concentration of the
suspension was 1×106 cells/ml and the volume was 0.1 ml.
An electric razor was used to remove the hair of the flank to
observe the apophysis of tumors. After the injection, the flank was
observed every day to identify the tumors. After the tumors
appeared, a digital caliper (Iron Bridge Tools, Inc.) was used to
measure the length (L) and the width (W) of these subcutaneous
tumors, which were measured every 2 days until day 14 following the
subcutaneous injection. The maximum diameter exhibited by a single
subcutaneous tumor was 9 mm. The volume of the tumor (V) was
calculated using a previously described formula (1): V=0.5 × 2L × W2.
Then, 0.4 ml the concentration of 1×106
cells/ml cell suspension was injected into the caudal vein. Five
treatment groups and one control group (n=2 mice for each group)
were prepared for this experiment. The number of mice used in
repeated experiments were the same. After 7 days of the injection,
ketamine was used for anesthesia via intraperitoneal injection, at
a concentration of 100 mg/kg (33).
When the anesthetic took effect (the heart rate and breath of the
mice were even, the muscles were relaxed, the limbs were not
active), 15 mg/ml D-luciferin potassium salt (Shanghai Sciencelight
Biology Science & Technology Co., Ltd.) was injected via
intraperitoneal injection, at a concentration of 10 mg/kg. Then,
mice were placed in the biofluorescence imager (IVIS Lumina XR; EMD
Millipore) for fluorescence imaging, following the manufacturer's
instructions, with the time of exposure at 1 min.
Mice were euthanized after the fluorescence imaging
test via cervical dislocation under the same anesthesia procedure
as aforementioned. The heart rate and respiration of mice stopped
completely, and the nerve reflex disappeared. The following
criteria for humane endpoints in oncological laboratory animals
were implemented: The weight of the subcutaneous tumor was <10%
of the original weight of the animal; the average diameter of the
tumor was <20 mm in the adult mice; no ulcer, necrosis or
infection emerged on the skin at the site of a tumor; no abdominal
cavities of the mice were abnormally dilated and the mice did not
have difficulty breathing or exhibit neuropsychiatric symptoms
(34). No mice had any of these
symptoms. The purpose of selecting and determining the humane
endpoint was to accurately predict the end point of the experiment
before the animals experienced unnecessary pain due to the
experiment, to shorten the experiment time to the greatest extent
and to avoid or reduce the pain and suffering caused to the animals
in the later period of the experiment (34). A small number of the experimental
animals in the present study exhibited slight abdominal
distention.
Statistical analysis
All experiments were performed ≥3 times. Data are
presented as the mean ± SD. Data were analyzed for statistical
significance using SPSS version 21.0 software (IBM Corp.). The
clonogenic assay, the DTH test and tumor growth curves were
analyzed via one-way ANOVA, followed by Bonferroni's correction.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clonogenic assay of HHP-treated
cells
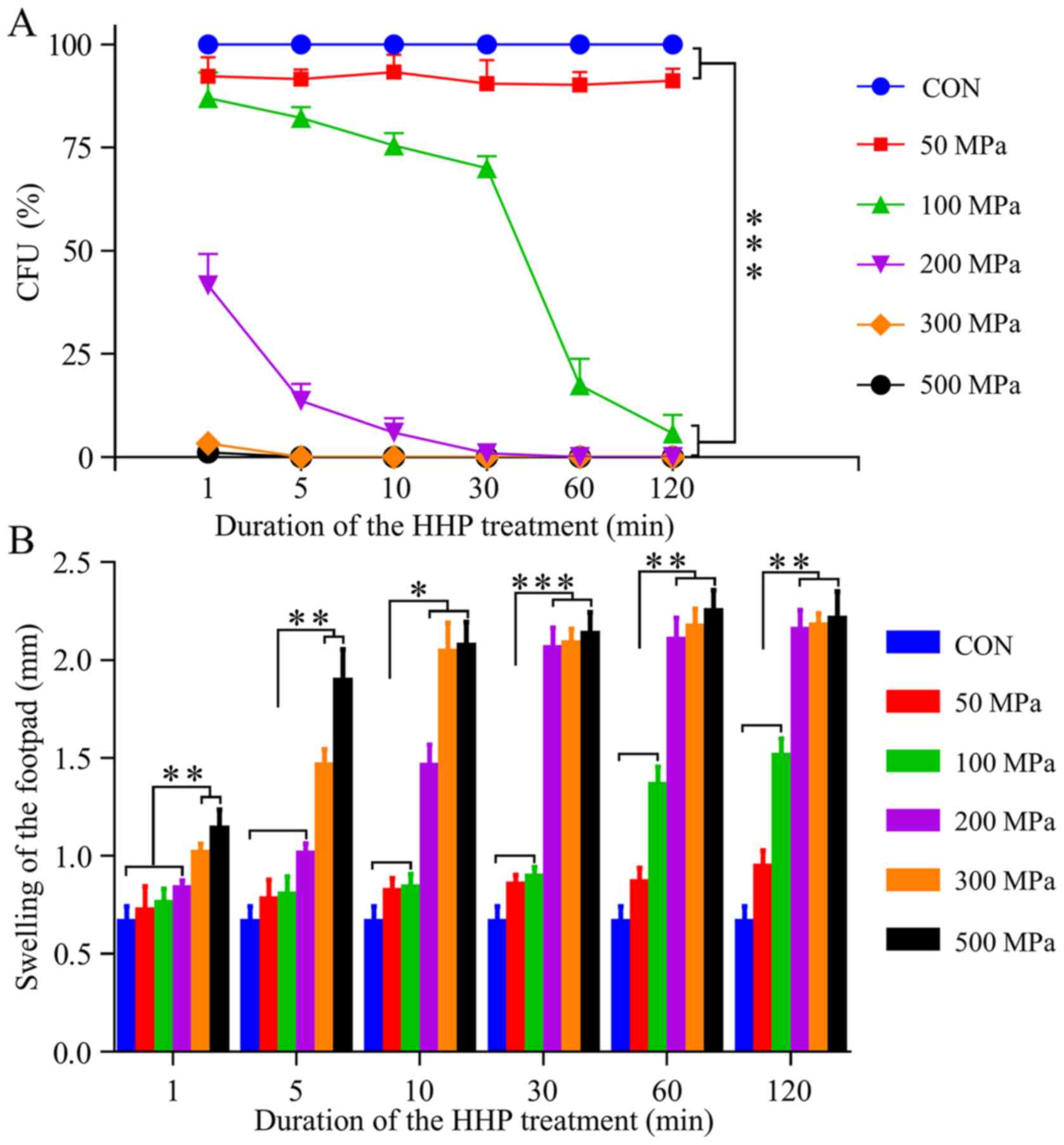
A clonogenic assay was performed to detect the CFU
of the treated cells in vitro. Regardless of the treatment
duration, the pressure of 50 MPa did not significantly affect the
proliferation of malignant cells. However, after treatment with 100
MPa for 1 min, the ability of colony-forming slightly declined. As
the duration increased, the CFU began to decrease, and when the
duration was >30 min, the percentage of CFU was notably
decreased. Moreover, the CFU (%) of cancer cells that were treated
for 120 min under the pressure of 100 MPa approached zero. In
addition, it was demonstrated that all tumor cells treated with
≥200 MPa for ≥30 min significantly lost their in vitro
ability to form colonies (P<0.001) (Fig. 2A).
Immunogenicity of HHP-treated
cells
The results of the DTH test indicated that groups
with 300 and 500 MPa had significant immune effects when the
duration was ≥5 min (Fig. 2B).
Furthermore, group with 200 MPa achieved the same significant
result when the duration was ≥30 min. It was revealed that almost
all HHP-inactivated cells held the immunogenicity response, except
the groups with 50 and 100 MPa for <60 min.
Mouse models were also used to test the
immunogenicity of HHP-treated cells. After the sixth day of the
subcutaneous injection in the flank, subcutaneous tumors were
observed in the majority of mice.
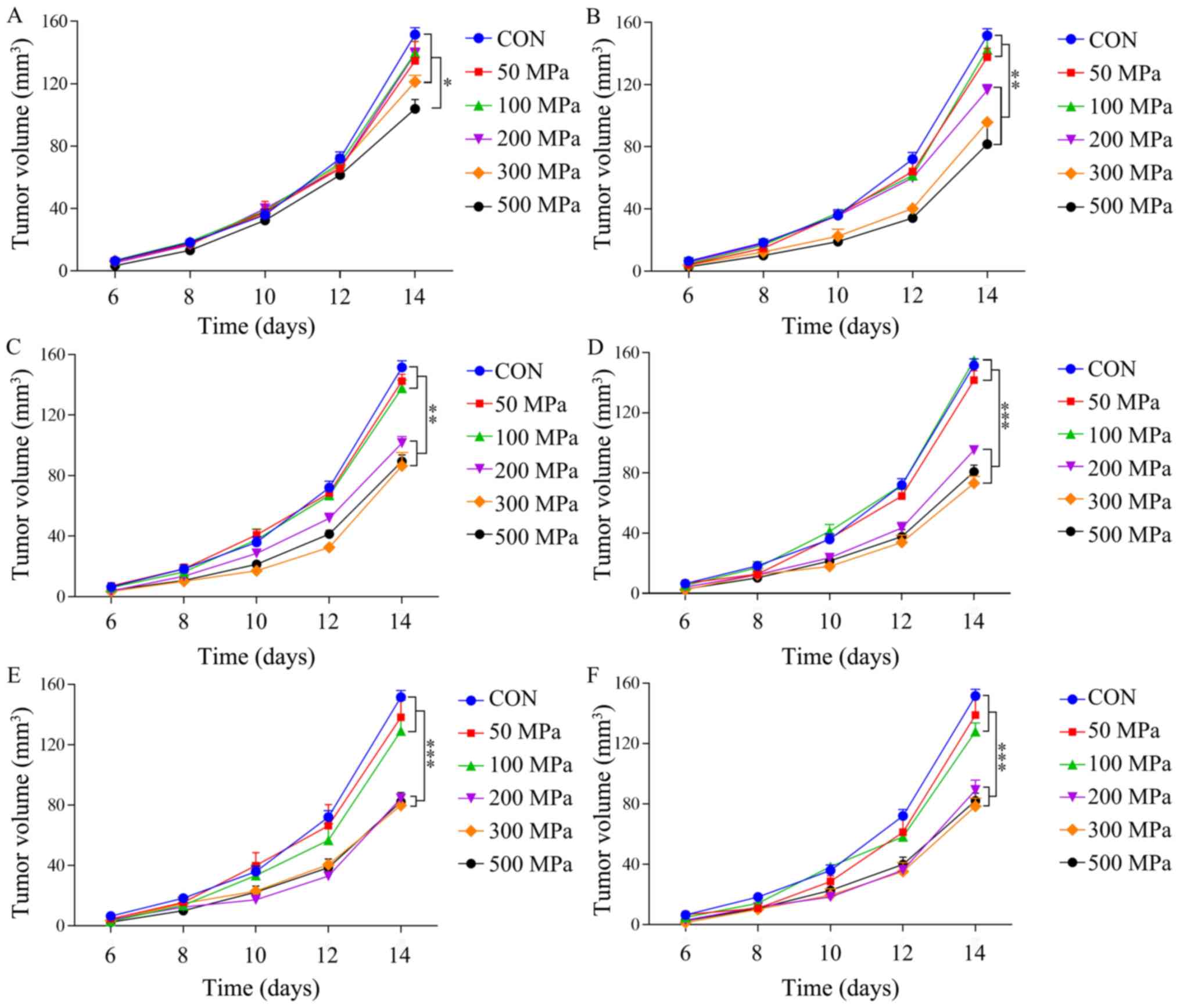
When the treatment duration was 1 min, the group
treated with 500 MPa had a significantly different V compared with
other groups at day 14 (Fig. 3A;
P<0.05). When the duration of HHP was 5 min, the groups treated
with 200, 300 and 500 MPa had a significantly decreased V compared
with other groups (Fig. 3B;
P<0.01). Furthermore, when the treatment duration was 10 min,
the groups treated with 200, 300 and 500 MPa also had a
significantly decreased V compared with other groups at day 14
(Fig. 3C; P<0.01). When the
treatment duration was ≥30 min, 200, 300 and 500 MPa groups had a
significantly decreased immune effect compared with the other
groups (Fig. 3D-F; P<0.001);
however, there were no significant differences between these three
groups (P>0.05).
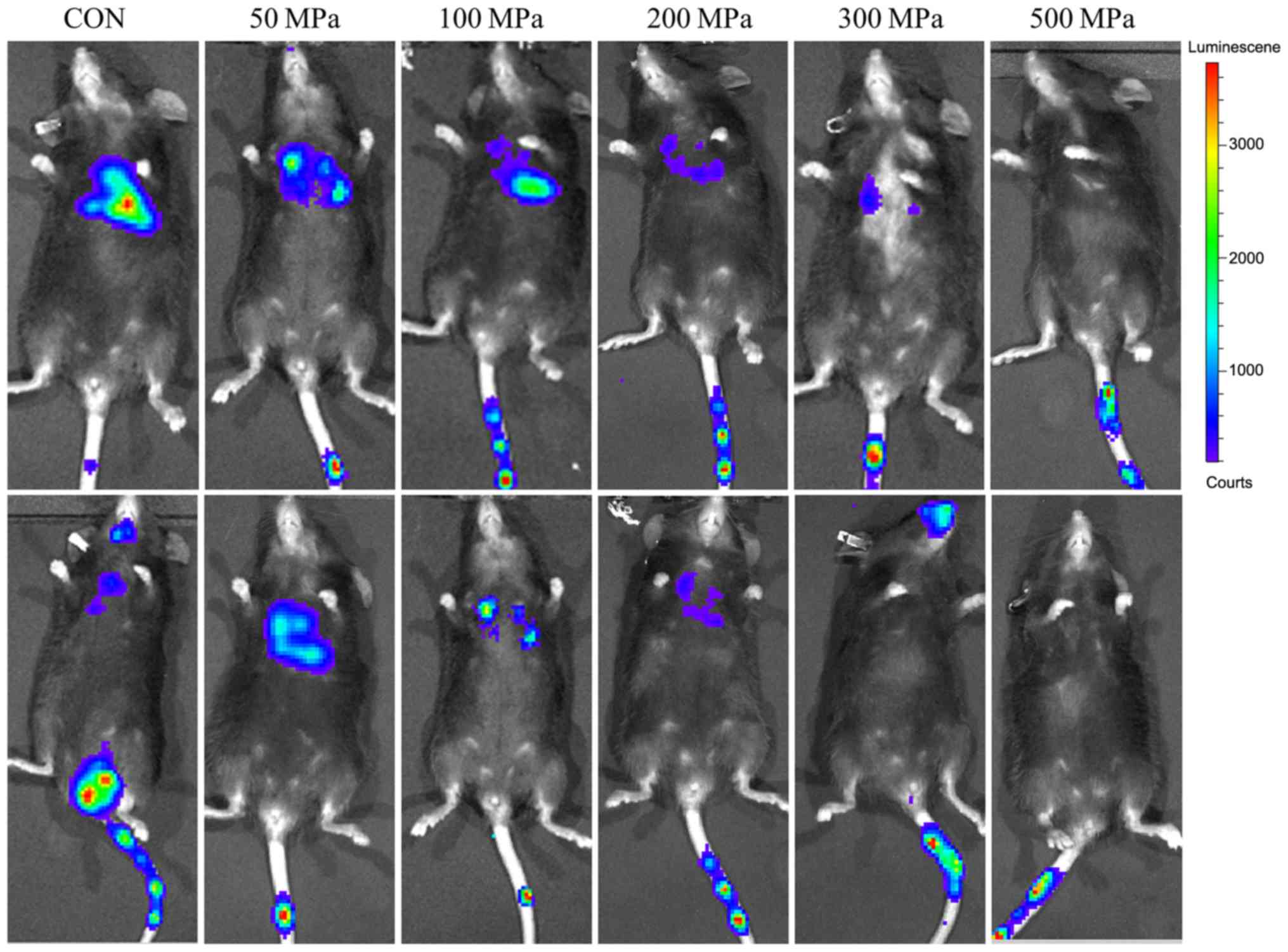
After 2 weeks of the injection, bioluminescence was
investigated (Fig. 4). The
immunofluorescence results suggested that a pressure of ≥200 MPa
could be used to develop a vaccine to suppress tumor growth, when
the duration of pressure was ≥30 min.
Discussion
A previous study reported the importance of the
immune system for treating melanoma (35). Moreover, as a promising therapeutic
method, an autologous malignant cell vaccine has been used to treat
correlative tumors (36). A previous
study suggested that inactivated cancer cells may be used as
vaccines for malignant diseases (37). However, for their potential use as an
anti-tumor treatment, it is important to inactivate the cancer
cells (38). Moreover, cell
inactivation should be in line with the three basic principles: i)
Complete inactivation of tumor cells; ii) maintaining the
immunogenicity of cells; and iii) adherence to the laws and ethical
guidelines, and the immunogenicity of the inactivation cells and
method of inactivation are directly associated with the treatment
effect. Retaining immunogenicity is the most important process and
the most significant challenge facing the development of autologous
whole tumor cell-based vaccines (36). Furthermore, these vaccines possess
qualities that targeted antigens do not, which can be identified
prospectively, and they can deliver numerous tumor-associated
antigens, which are highly expressed self-antigens. In contrast to
neo-antigens, autologous whole tumor cell-based vaccines should
only be able to activate the remaining low-affinity T cells and
would have to break self-tolerance (36). Moreover, several additional methods
have been developed to overcome these limitations, such as the
addition of adjuvants, repeated vaccinations or co-stimulators
(39). It has been reported that
whether the inactivated cells are immunogenic or not depends on the
inactivation methods of the cells, as well as the cell
death-initiating stimulus (40).
Furthermore, certain cell death inducers result in the exposure of
immunogenic factors on the surface or the release of immunogenic
signals into the extracellular fluid (41). In addition, the same anticancer agent
can cause the release of signals from certain tumor types, which is
due to the fact that this release requires the intervention of
specific signal transduction pathways (40).
Although HHP is known to denature proteins, it does
not affect the covalent bonds, meaning the primary and secondary
structures of the proteins are maintained, while their tertiary and
quaternary structures are changed (42). A previous study showed that
HHP-treated tumor cells affect the antigenic pool and DCs and
HHP-killed tumor cells may induce CD8+ T cell-mediated
responses (43). Moreover,
HHP-treatment induces immunogenic tumor cell death, and the
interaction between tumor cells killed with HHP and DC results in
the phagocytosis of cancer cells and activation of DC (44). Thus, previous studies have suggested
that the treatment of HHP fulfils the main requirements for a
clinical vaccine, in that it inactivates tumor cells effectively,
retains the immunogenicity of the cancer cells, had no intrinsic
toxicity and complied with the requirements of the Good
Manufacturing Practice (19).
Furthermore, HHP-treatment is a simple and reproducible method
(45). Therefore, this vaccination
has advantages over other preparation methods such as radiation,
freeze-thaw or heat treatment. Moreover, the primary aim of the
present study was to investigate whether different durations and
pressures affect the results of the vaccination.
When tumor cells were treated with different
pressures for the different durations, the effects on tumor cell
death and the structures of cellular elements were different
(19). Necrotic cells induce
inflammatory processes; however, apoptotic cells primarily cause
anti-inflammatory reactions (46).
In the present study, it was demonstrated that the treatment
durations did not affect the viability of the tumor cells when the
pressure was 50 or 100 MPa; the immune reactions induced by these
cells were too low to affect the development of tumors. However,
when the pressure was ≥200 MPa, it elicited a strong effect on cell
viability. Moreover, when the pressure of HHP treatment was 100–300
MPa, the degeneration of protein was reversible; however, when the
pressure was ≥300 MPa, the denaturation of protein was irreversible
(47). It was also indicated that
HHP treatment with ≥200 MPa resulted in the death of tumor cells in
the first 24 h of the culture, and none of these treated tumor
cells could form new colonies.
The vaccination of whole cancer cells supplies a
number of antigens to the immune system, and this could result in
long-lasting anti-tumor immunity (19). A previous study has reported that
serum antibodies could be released by vaccines of the treated cells
(48). In the present study, the
number of the specific IgG antibodies treated by the HHP treatment
(pressure ≥200 MPa, duration ≥30 min) was not decreased. Moreover,
the repellent T-lymphocyte reaction, which was measured by the DTH
response, was significantly increased. Thus, these results
suggested that as the pressure increased and the duration
prolonged, the immune effect of the melanoma cells could not
increase significantly, when the pressure ≥200 MPa and the duration
>30 min.
It has been reported that HHP-treated cancer cells
maintain their morphology, even for a number of weeks, and have
increased viscosity of the cytoplasm; this feature may be important
for an effective vaccine. Furthermore, it was speculated that
increased viscosity of the cytoplasm results in a sustained
released of relevant cell-derived antigens (49), and these characteristics are
important for the immunological effect of malignant cells. Thus,
these previous studies support the utility of HHP treatment in the
preparation of tumor cells vaccines.
The present results suggest that different pressures
and treatment durations may change the final immune efficacy of the
vaccine. During HHP treatment, the pressure was distributed
throughout the media and the pressure was distributed evenly
throughout the experimental samples; it has been reported that the
intensity of pressure propagates through all flexible packing
materials (50). As every compressed
material was given the same pressure quantity, any gradients were
mitigated, and thus the effects of HHP could not be attained under
the lower pressure (<200 MPa) and the shorter time (<30 min).
A previous study demonstrated that vaccination with HHP-killed
cancer cells combined with local RTx significantly inhibits tumor
growth and improves survival in tumor-bearing mice (51), using a compression time of 300 sec.
To the best of our knowledge, the present study was the first to
test the HHP-treated tumor vaccine efficacy at different
compression durations (1, 5, 10, 30, 60 and 120 min).
In the present study, a sensitive camera with a
sufficient exposure time was used to assess the emission of
fluorescence from fluorophores in the whole-body living animals,
which is known as fluorescence imaging in vivo (52). However, the thick tissue of the mice
can absorb and shelter autofluorescence, and thus the equipment may
not always receive and collect the fluorescent signals (52); therefore, sufficient exposure time
and luciferase are required to complete the test. In the present
study fluorescence intensity results suggested that a pressure of
≥200 MPa could be used to prepare a vaccine to suppress tumor
growth, when the treatment duration was ≥30 min.
It was speculated that the technique of HHP may
represent an alternative to efficiently develop whole-cell tumor
vaccines, when the pressure is ≥200 MPa and the treatment duration
is ≥30 min. The present study not only assessed the effect of
different pressures on vaccine efficacy, but also the effect of
different pressure durations. Moreover, future work will focus on
the immunity mechanism of the vaccination, as well as the optimum
pressure and treatment duration in order to optimize immune
stimulation. However, if the pressure is too high or the duration
is too long, this may decrease the immunogenicity of the cancer
cells; however, this requires further investigation. Moreover,
vaccines are injected multiple times to break the self-tolerance
along with the appropriate adjuvants (53). A limitation to the present study was
the lack of other assays to assess cell death, the cell cycle and
the immunophenotype of the tumor. In addition, there are numerous
difficulties that have to be overcome to develop beneficial tumor
vaccines. The development of tumor vaccines also has to follow the
same standard as radiotherapy and chemotherapy. Collectively, the
present study demonstrated a potential production method for
tumor-cell vaccines generated via HHP, and this provides a basis
for further investigation into the optimization of tumor
vaccines.
Acknowledgements
Not applicable.
Funding
The present study was supported by Fund of Jilin
Provincial Finance Department (grant no. 2018SCZWSZX-010).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
This study was designed and conceived by KL and BL.
The experimental procedures and data analysis were carried out by
KL, SY, ZM and BL. The manuscript was prepared by KL and BL. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were conducted according to
the National Standard of the Care and Use of Laboratory Animals,
and the study was approved by the Animal Ethics Committee of The
First Hospital of Jilin University (Approval no. 2019-0456).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DC
|
dendritic cells
|
|
HHP
|
high hydrostatic pressure
|
|
CFU
|
colony-forming units
|
|
DTH
|
delayed-type hypersensitivity
|
References
|
1
|
Jaklitsch MT, Grondin SC and Sugarbaker
DJ: Treatment of malignant mesothelioma. World J Surg. 25:210–217.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spagnolo F, Boutros A, Tanda E and
Queirolo P: The adjuvant treatment revolution for high-risk
melanoma patients. Semin Cancer Biol. 59:283–289. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lacy A and O'Kennedy R: Studies on
coumarins and coumarin- related compounds to determine their
therapeutic role in the treatment of cancer. Curr Pharm Des.
10:3797–3811. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Spiegel D and Classen C: Group therapy for
cancer patients: A research-based handbook of psychosocial care.
1st edition Basic Books; New York, NY: 2000
|
|
5
|
Hutchison S, Sahay B, de Mello SC, Sayour
EJ, Lejeune A, Szivek A, Livaccari AM, Fox-Alvarez S, Salute M,
Powers L and Milner RJ: Characterization of myeloid-derived
suppressor cells and cytokines GM-CSF, IL-10 and MCP-1 in dogs with
malignant melanoma receiving a GD3-based immunotherapy. Vet Immunol
Immunopathol. 216:1099122019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Indini A, Di Guardo L, Cimminiello C,
Prisciandaro M, Randon G, De Braud F and Del Vecchio M: Developing
a score system to predict therapeutic outcomes to anti-PD-1
immunotherapy in metastatic melanoma. Tumori. 105:465–473. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dolan DE and Gupta S: PD-1 pathway
inhibitors: Changing the landscape of cancer immunotherapy. Cancer
Control. 21:231–237. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khalil DN, Smith EL, Brentjens RJ and
Wolchok JD: The future of cancer treatment: Immunomodulation, CARs
and combination immunotherapy. Nat Rev Clin Oncol. 13:273–290.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li SY, Liu Y, Xu CF, Shen S, Sun R, Du XJ,
Xia JX, Zhu YH and Wang J: Restoring anti-tumor functions of T
cells via nanoparticle-mediated immune checkpoint modulation. J
Control Release. 231:17–28. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Montagne K, Onuma Y, Ito Y, Aiki Y,
Furukawa KS and Ushida T: High hydrostatic pressure induces
pro-osteoarthritic changes in cartilage precursor cells: A
transcriptome analysis. PLoS One. 12:e01832262017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bol KF, Aarntzen EH, Pots JM, Olde
Nordkamp MA, van de Rakt MW, Scharenborg NM, de Boer AJ, van
Oorschot TG, Croockewit SA, Blokx WA, et al: Prophylactic vaccines
are potent activators of monocyte-derived dendritic cells and drive
effective anti-tumor responses in melanoma patients at the cost of
toxicity. Cancer Immunol Immunother. 65:327–339. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Klippstein R and Pozo D:
Nanotechnology-based manipulation of dendritic cells for enhanced
immunotherapy strategies. Nanomedicine. 6:523–529. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schummer V, Flindt S and Hinz T: Tumor
vaccines and peptide-loaded dendritic cells (DCs).
Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz.
58:1254–1258. 2015.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gross S, Erdmann M, Haendle I, Voland S,
Berger T, Schultz E, Strasser E, Dankerl P, Janka R, Schliep S, et
al: Twelve-year survival and immune correlates in dendritic
cell-vaccinated melanoma patients. JCI Insight. 2:914382017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu Z, Ma Y, Shang Z, Hu S, Liang K, Liang
W, Xing X, Wang Y and Du X: Improving immunotherapy for colorectal
cancer using dendritic cells combined with anti-programmed
death-ligand in vitro. Oncol Lett. 15:5345–5351.
2018.PubMed/NCBI
|
|
16
|
Rosenblatt J and Avigan D: Cellular
immunotherapy for multiple myeloma. Best Pract Res Clin Haematol.
21:559–577. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Copier J, Ward S and Dalgleish A: Cell
based cancer vaccines: Regulatory and commercial development.
Vaccine. 25 (Suppl 2):B35S–B46S. 2007. View Article : Google Scholar
|
|
18
|
Bliznyuk A, Grossman Y and Moskovitz Y:
The effect of high pressure on the NMDA receptor: Molecular
dynamics simulations. Sci Rep. 9:108142019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weiss EM, Meister S, Janko C, Ebel N,
Schlücker E, Meyer-Pittroff R, Fietkau R, Herrmann M, Gaipl U and
Frey B: High hydrostatic pressure treatment generates inactivated
mammalian tumor cells with immunogeneic features. J Immunotoxicol.
7:194–204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Franco I, Castillo E, Perez MD, Calvo M
and Sanchez L: Effects of hydrostatic high pressure on the
structure and antibacterial activity of recombinant human
lactoferrin from transgenic rice. Biosci Biotechnol Biochem.
76:53–59. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Dai B, Deng Y and Zhao Y: In
vitro anti-inflammatory and antioxidant activities and protein
quality of high hydrostatic pressure treated squids (Todarodes
pacificus). Food Chem. 203:258–266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen H, Xiao G, Xu Y, Yu Y, Wu J and Zou
B: High hydrostatic pressure and Co-fermentation by lactobacillus
rhamnosus and gluconacetobacter xylinus improve flavor of
yacon-litchi-longan juice. Foods. 8:E3082019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Woo HJ, Park JB, Kang JH, Chun HH and Song
KB: Combined treatment of high hydrostatic pressure and cationic
surfactant washing to inactivate listeria monocytogenes on
fresh-cut broccoli. J Microbiol Biotechnol. 29:1240–1247. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamamoto K: Food processing by high
hydrostatic pressure. Biosci Biotechnol Biochem. 81:672–679. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang R, Ye M, Li X, Ji L, Karwe M and
Chen H: Evaluation of high hydrostatic pressure inactivation of
human norovirus on strawberries, blueberries, raspberries and in
their purees. Int J Food Microbiol. 223:17–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Diehl P, Schauwecker J, Mittelmeier W and
Schmitt M: High hydrostatic pressure, a novel approach in
orthopedic surgical oncology to disinfect bone, tendons and
cartilage. Anticancer Res. 28:3877–3883. 2008.PubMed/NCBI
|
|
27
|
Barnes CA, Robertson AJ, Louis JM,
Anfinrud P and Bax A: Observation of β-amyloid peptide
oligomerization by pressure-jump NMR spectroscopy. J Am Chem Soc.
141:13762–13766. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shearer AE and Kniel KE: High hydrostatic
pressure for development of vaccines. J Food Prot. 72:1500–1508.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Golub M, Lehofer B, Martinez N, Ollivier
J, Kohlbrecher J, Prassl R and Peters J: High hydrostatic pressure
specifically affects molecular dynamics and shape of low-density
lipoprotein particles. Sci Rep. 7:460342017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Winter R and Dzwolak W:
Temperature-pressure configurational landscape of lipid bilayers
and proteins. Cell Mol Biol (Noisy-le-grand). 50:397–417.
2004.PubMed/NCBI
|
|
31
|
Winter R: Synchrotron X-ray and neutron
small-angle scattering of lyotropic lipid mesophases, model
biomembranes and proteins in solution at high pressure. Biochim
Biophys Acta. 1595:160–184. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. National
Academies Press (US); Washington, DC: 2011
|
|
33
|
Varela A, Salagianni M, Galani I,
Andreakos E and Davos CH: The role of TLR7 receptors on cardiac and
vascular function of ApoE-/-mice. Eur J Echocardiography. 12 (Suppl
2):P7572011.
|
|
34
|
Leary SL, Underwood W, Anthony R, Cartner
S, Corey D, Grandin T, Greenacre C, Gwaltney-Bran S, McCrackin M
and Meyer R: AVMA guidelines for the euthanasia of animals: 2013
edition. American Veterinary Medical Association Schaumburg; IL:
2013
|
|
35
|
Zhuang X, Wu T, Zhao Y, Hu X, Bao Y, Guo
Y, Song Q, Li G, Tan S and Zhang Z: Lipid-enveloped zinc phosphate
hybrid nanoparticles for codelivery of H-2K b and H-2D b-restricted
antigenic peptides and monophosphoryl lipid A to induce antitumor
immunity against melanoma. J Control Release. 228:26–37. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
De Gruijl TD, van den Eertwegh AJ, Pinedo
HM and Scheper RJ: Whole-cell cancer vaccination: From autologous
to allogeneic tumor-and dendritic cell-based vaccines. Cancer
Immunol Immunother. 57:1569–1577. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Frey B, Schildkopf P, Rödel F, Weiss EM,
Munoz LE, Herrmann M, Fietkau R and Gaipl US: AnnexinA5 renders
dead tumor cells immunogenic-implications for multimodal cancer
therapies. J Immunotoxicol. 6:209–216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Estrela-Llopis VR, Chevichalova AV,
Tregubova NA, Shishko ED and Litvin PM: Platinum nanoparticles with
adsorptive layer of Chlorella vulgaris polysaccharides
inactivate tumor cells of ascitic ehrlich carcinoma, ovarian cancer
and leukemia. Nanocomposites, nanophotonics, nanobiotechnology, and
applications. Fesenko O and Yatsenko L: 156. Springer Proceedings
in Physics; Springer, Cham: 2015
|
|
39
|
Hollingsworth RE and Jansen K: Turning the
corner on therapeutic cancer vaccines. NPJ Vaccines. 4:72019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kepp O, Tesniere A, Schlemmer F, Michaud
M, Senovilla L, Zitvogel L and Kroemer G: Immunogenic cell death
modalities and their impact on cancer treatment. Apoptosis.
14:364–375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Melief CJM, van Hall T, Arens R, Ossendorp
F and van der Burg SH: Therapeutic cancer vaccines. J Clin Invest.
125:3401–3412. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gross M and Jaenicke R: Proteins under
pressure: The influence of high hydrostatic pressure on structure,
function and assembly of proteins and protein complexes. Eur J
Biochem. 221:617–630. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Urbanova L, Hradilova N, Moserova I,
Vosahlikova S, Sadilkova L, Hensler M, Spisek R and Adkins I: High
hydrostatic pressure affects antigenic pool in tumor cells:
Implication for dendritic cell-based cancer immunotherapy. Immunol
Lett. 187:27–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fucikova J, Moserova I, Truxova I,
Hermanova I, Vancurova I, Partlova S, Fialova A, Sojka L, Cartron
PF, Houska M, et al: High hydrostatic pressure induces immunogenic
cell death in human tumor cells. Int J Cancer. 135:1165–1177. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Weiss EM, Wunderlich R, Ebel N, Rubner Y,
Schlücker E, Meyer-Pittroff R, Ott OJ, Fietkau R, Gaipl US and Frey
B: Selected anti-tumor vaccines merit a place in multimodal tumor
therapies. Front Oncol. 2:1322012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gaipl US, Munoz LE, Rödel F, Pausch F,
Frey B, Brachvogel B, von der Mark K and Pöschl E: Modulation of
the immune system by dying cells and the phosphatidylserine-ligand
annexin A5. Autoimmunity. 40:254–259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Boonyaratanakornkit BB, Park CB and Clark
DS: Pressure effects on intra-and intermolecular interactions
within proteins. Biochim Biophys Acta. 1595:235–249. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Korn A, Frey B, Sheriff A, Gaipl U, Franz
S, Meyer-Pittroff R, Bluemelhuber G and Herrmann M: High
hydrostatic pressure inactivated human tumour cells preserve their
immunogenicity. Cell Mol Biol (Noisy-le-grand). 50:469–477.
2004.PubMed/NCBI
|
|
49
|
Frey B, Franz S, Sheriff A, Korn A,
Bluemelhuber G, Gaipl U, Voll R, Meyer-Pittroff R and Herrmann M:
Hydrostatic pressure induced death of mammalian cells engages
pathways related to apoptosis or necrosis. Cell Mol Biol
(Noisy-le-grand). 50:459–467. 2004.PubMed/NCBI
|
|
50
|
Patterson MF: Microbiology of
pressure-treated foods. J Appl Microbiol. 98:1400–1409. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Seitz C, Rückert M, Deloch L, Weiss EM,
Utz S, Izydor M, Ebel N, Schlücker E, Fietkau R, Gaipl US and Frey
B: Tumor cell-based vaccine generated with high hydrostatic
pressure synergizes with radiotherapy by generating a favorable
anti-tumor immune microenvironment. Front Oncol. 9:8052019.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Rao J, Dragulescu-Andrasi A and Yao H:
Fluorescence imaging in vivo: Recent advances. Curr Opin
Biotechnol. 18:17–25. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chang EY, Chen CH, Ji H, Wang TL, Hung K,
Lee BP, Huang AY, Kurman RJ, Pardoll DM and Wu T: Antigen-specific
cancer immunotherapy using a GM-CSF secreting allogeneic tumor
cell-based vaccine. Int J Cancer. 86:725–730. 2000. View Article : Google Scholar : PubMed/NCBI
|