Introduction
Colon cancer is the third most common cancer in the
world with a very high mortality rate (1). In China, the incidence of colon cancer
increased with an average rate of 2.34% per year between 1990 and
2016 (2). Age, diabetes, gene
mutation, abnormal expression of non-coding RNA and gastroenteric
bowel disease are all possible high-risk factors for colon cancer
(3–7). The pathogenesis of colon cancer
involves activation of multiple signal pathways. In colon cancer,
mitochondrial retrograde pathway mediated by TFAM promotes tumor
occurrence by inhibiting Wnt/β-catenin pathway (8). The intestinal insulin /IGF-1 pathway
promotes the formation of colon cancer tumors by promoting the
expression of FoxO1 (9).
Inhibitor of growth 4 (ING4) belongs to the growth
inhibitory factor family and is a type II tumor suppressor gene
(10). Ma et al (11) found that the inhibitory effect of
ING4 on melanoma can be realized by activating Fas/caspase-8
apoptosis pathway. Qian et al (12) believed that ING4 can inhibit the
growth and metastasis of liver cancer tumor by inhibiting NF-κB and
upregulating FoxO3. ING4 plays an important role in colon cancer.
In colon cancer, ING4 inhibits cell proliferation and reverses
epithelial-mesenchymal transition by regulating the expression of
target proteins such as p-Stat3, Ki-67, p21 and E-cadherin
(13–15).
GLI1 protein belongs to the zinc finger protein
Kruppel family, and GLI1 is closely related to cancer. GLI1 can
inhibit the expression of E-adherin protein in breast cancer cells
after being activated by SHH protein, and eventually weaken the
ability of cell migration and invasion (16). The high expression of GLI1 in glioma
cells can promote the proliferation and activity of glioma cells
and reduce the sensitivity of cells to vincristine (17). In colon cancer, GLI1 is activated by
PI3K/Akt/NF-κB pathway and can regulate cell biological processes
such as epithelial-mesenchymal transition and cell cycle (18). In addition, the co-expression of GLI1
and p-S6K protein is closely related to lymph node metastasis and
TNM staging (19).
Bevacizumab is a common anticancer drug. In order to
understand the effect of bevacizumab on colon cancer and its
relationship with GLI1 and ING4, the rat colon cancer model was
induced by azoxymethane (AOM) and treated with bevacizumab.
Materials and methods
AOM-induced colon cancer model in
rats
SD male rats were randomly divided into control
group, sham operation group, negative control group (NC group),
model group, low-dose bevacizumab group and high-dose bevacizumab
group with 10 rats in each group. Except control group and sham
operation group, rats in other groups received intraperitoneal
injection of AOM (1 ml, 15 mg/kg, dissolved in PBS buffer) once
every 2 weeks. The rats in the control group did not receive any
treatment, while the rats in the sham-operation group received
intraperitoneal injection of 1 ml of PBS buffer once every 2 weeks.
After modeling, the low-dose bevacizumab group was treated with 25
g/l of bevacizumab (dissolved in 0.9% NaCl solution), the high-dose
group was treated with 100 g/l bevacizumab (dissolved in 0.9% NaCl
solution), and the NC group was treated with 0.9% NaCl solution
injection. The length and width of the tumor were measured and
recorded every 1 week starting from 0 weeks after modeling, and the
tumor was weighed 3 weeks after modeling to record the tumor
quality. Tumor volume = tumor length, tumor width 2, tumor
inhibition rate = 100% (1 - tumor mass in bevacizumab treatment
group/tumor mass in negative control group).
The study was approved by the Ethics Committee of
General Hospital of Heilongjiang Province Land Reclamation Bureau
(Harbin, China).
Cell culture and transfection
Colon cancer cells SW480 and SW620 were purchased
from the Cell Bank of the Typical Culture Preservation Committee of
the Chinese Academy of Sciences (Shanghai). The above cells were
were cultured in animal cell incubator at 37°C with 5%
CO2 before transfection. The culture medium system was
RPMI-1640 medium + 10% fetal bovine serum solution + 1%
penicillin/streptomycin solution. Subsequent experiments would be
carried out after cell culture to logarithmic growth phase.
The day before transfection, the culture medium was
replaced with fetal bovine serum-free culture medium. On the day of
transfection, cells were inoculated into 6-well plates with
1×105 cells/well. GLI1, siRNA, ING4 siRNA and NC were
all purchased from Shanghai Sangon Biotech. Cell lines were
transfected with Lipofectamine 2000 transfection kit (Invitrogen;
Thermo Fisher Scientific, Inc.). The procedures referred to the kit
instructions. After 8 h of transfection, fresh culture was
replaced. Then cells were cultured in animal cell incubator at 37°C
with 5% CO2. Subsequent experiments can be carried out
after 48 h of continuous culture.
Flow cytometer
The sample to be tested was prepared into cell
suspension. The number of cells was controlled to 1×106.
Cells were immobilized in 70% ethanol ice-cold solution for 30 min
at 4°C. The ethanol solution was then removed and the cell
particles were incubated in Annexin V-FITC/7-AAD mixed solution.
FACScan flow cytometer (BD Biosciences) was used to analyze the
apoptosis.
MTT assay
Transfected cells with good growth status were
enzymatically hydrolyzed for 2 min, then the enzyme solution was
sucked and fresh culture medium was added to prepare cell
suspension. Four 96-well plates were taken and cells were
inoculated into the well plates according to the specification of
5×103 cells/100 µl/well, with 3 wells in each group. One
well plate was taken out every 24 h, 5 mg/ml MTT solution was added
10 µl/well, the cells were continued to culture for 1 h, then the
culture medium was sucked, and the OD value was measured at 570 nm
with an enzyme reader. The experiment was repeated 3 times to
visualize the cell activity-time curve.
Colon cancer cells were inoculated into 96-well
plates with 8×103 cells per well, cultured in animal
cell incubator at 37°C with 5% CO2 for 12 h, then added
with bevacizumab (1-1,000 g/l, with the increase of concentration
gradient rises) for further culture. After 48 h, 10 l MTT was added
to each well for 1 h in dark room. After that, the culture medium
was sucked. The OD value at 570 nm was measured by an
enzyme-labeled instrument, and the cell activity-concentration
curve was drawn. SPSS analysis curve was used to determine the
IC50 value of the semi-inhibitory concentration. The
experiment was repeated 3 times.
Western blot analysis
Part of rat colon cancer tissue was taken and cut to
pieces, and tissue samples were treated with cell protein extract
(cell lysate: protease inhibitor: phosphatase inhibitor = 98:1:1),
the extractive solution was centrifuged at 1.6×104 × g
at 4°C for 15 min, and the supernatant was collected. The
supernatant was subjected to SDS-PAGE electrophoresis to
distinguish proteins. The proteins were transferred to NC membrane
and placed for 1 h at room temperature (the blocking solution was
5% skim milk-PBS solution). GLI1, ING4, caspase-3, Bax, β-catenin,
Bcl2, PTEN, PI3K, Akt, NF-κB primary antibodies were then added and
placed overnight at 4°C. PBS solution was used for washing, the
operation was repeated three times, goat anti-rabbit secondary
antibody (HRP crosslinking) was added, and the mixture was placed
for 1 h at room temperature. Finally, the membrane was washed with
PBS solution and visualized using enhanced chemiluminescence
method. The internal reference protein was β-actin, and the
relative expression level of the protein to be detected = gray
value of the band to be detected/gray value of the β-actin band.
GLI1, ING4, caspase-3, Bax, β-catenin, Bcl2, PTEN, PI3K, Akt,
NF-κB, β-actin and goat anti-rabbit antibody were all purchased
from Shanghai Abcam Company.
Statisticsal analysis
The above index data were input into SPSS 20.0
software package (Asia Analytics Formerly SPSS China), and GraphPad
Prism 6.0 was used for statistical analysis. Each experiment was
repeated 3 times. The measurement data were expressed by mean ± SD.
Independent sample t-test was used for the data comparison method
between the two groups. One-way ANOVA was used for the comparison
among multi-groups, LSD-t test for pairwise comparison afterwards.
All data were tested by two-tailed test. The value of 95% was taken
as confidence interval, the difference was statistically
significant when P<0.05.
Results
Bevacizumab inhibits GLI1 and promotes
ING4
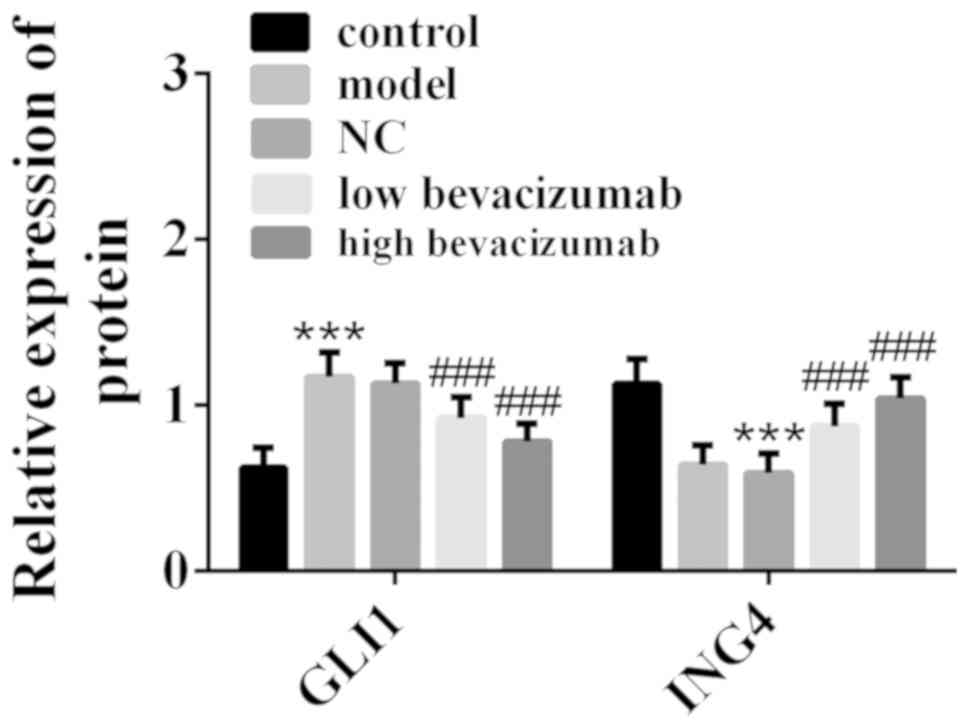
In this study, AOM was used to induce colon cancer
model in rats, and bevacizumab was given to colon cancer model
rats. The expression of GLI1 and ING4 in rat colon cancer tissues
was detected by western blot analysis, and the results are shown in
Fig. 1. Compared with the control
group, GLI1 expression was upregulated and ING4 expression was
downregulated in the model group. Compared with the model group,
GLI1 expression was downregulated in low-dose bevacizumab group and
high-dose bevacizumab group, while ING4 expression was upregulated.
In addition, the change degree of GLI1 and ING4 in low-dose
bevacizumab group was less than those in high-dose bevacizumab
group. The above results indicated that bevacizumab can inhibit
GLI1 and promote ING4 for colon cancer treatment.
Bevacizumab effectively inhibits tumor
growth
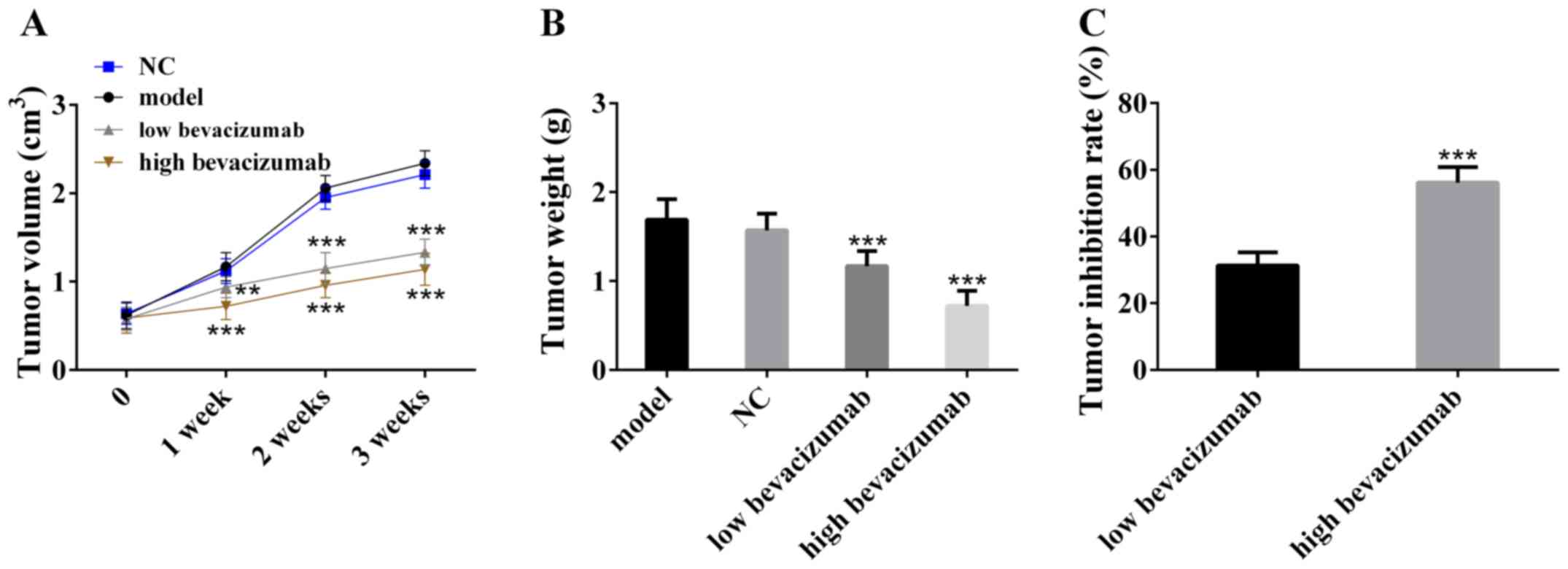
In order to verify the inhibitory effect of
bevacizumab on colon cancer tumors, the tumor weight and volume
were measured and the tumor inhibitory rate was calculated. The
results are shown in Fig. 2.
Compared with the model group, the tumor volume in the bevacizumab
treatment group decreased and increased slowly with the time. The
tumor weight of bevacizumab treatment group was also statistically
smaller than that of the model group. In addition, the tumor
inhibition rate of low-dose bevacizumab group was lower than that
of high-dose bevacizumab group. The above results indicated that
bevacizumab can inhibit tumor growth, and its inhibitory effect may
be dose-dependent.
Bevacizumab promotes apoptosis by promoting
caspase-3, Bax, PTEN and inhibiting β-catenin, Bcl2, PI3K, Akt,
NF-κB. In order to understand the molecular mechanism of
bevacizumab in the treatment of colon cancer, the apoptosis was
detected by flow cytometry and the expression of related proteins
was detected by western blot analysis. Fig. 3A showed that compared with the
control group, the apoptosis rate of the model group was reduced.
Compared with the model group, the apoptosis rate of bevacizumab
treatment group increased, and the apoptosis rate of low-dose group
was lower than that of high-dose group. Fig. 3B and C showed that compared with the
control group, the expression of PTEN, caspase-3 and Bax in the
model group was downregulated, and the expression of PI3K, Akt,
NF-κB, β-catenin and Bcl2 were upregulated. Compared with the model
group, the expression of PTEN, caspase-3 and Bax in bevacizumab
treatment group was upregulated, while PI3K, Akt, NF-κB, β-catenin
and Bcl2 were downregulated, and the change degree in high dose
group was greater than that in low dose group. The above results
showed that bevacizumab can promote cell apoptosis by promoting the
expression of caspase-3, Bax and PTEN and inhibiting β-catenin,
Bcl2, PI3K, Akt and NF-κB.
 | Figure 3.Bevacizumab promotes cell apoptosis by
promoting caspase-3, Bax, PTEN and inhibiting β-catenin, Bcl2,
PI3K, Akt, NF-κB. (A) Bevacizumab promotes cell apoptosis;
***P<0.001, compared with control group, and
###P<0.001, compared with model group. (B)
Bevacizumab upregulates PTEN and downregulates PI3K, Akt, NF-NF-κB;
***P<0.001, compared with control group, #P<0.05
and ##P<0.01, compared with model group. (C)
Bevacizumab upregulates caspase-3 and Bax and inhibits Bcl2 and
β-catenin; ***P<0.001, compared with model group, and
#P<0.05 and ##P<0.01, compared with
model group. |
GLI1 inhibits apoptosis, while ING4
promotes apoptosis
In order to understand the effect of GLI1 and ING4
on colon cancer, siRNA was used to silence GLI1 and ING4 of colon
cancer cells, MTT was used to detect cell activity, and flow
cytometry was used to detect cell apoptosis. Fig. 4 showed that the deletion of GLI1
inhibits cell activity and promotes cell apoptosis. The deletion of
ING4 increases cell activity and inhibits cell apoptosis. The above
results showed that GLI14 inhibits cell apoptosis and promotes cell
proliferation, while ING4 inhibits cell proliferation and promotes
cell apoptosis.
Effect of GLI1 and ING4 on apoptosis
protein and PTEN/PI3K/Akt/NF-κB pathway
Fig. 5A and B showed
that ING4 upregulates the expression levels of caspase-3 and Bax,
and downregulates β-catenin and Bcl2. GLI1 upregulates β-catenin
and Bcl2, and downregulates caspase-3 and Bax. Fig. 5C and D showed that ING4 promotes PTEN
expression and inhibits PI3K, Akt, NF-κB. GLI1 inhibits PTEN
expression and promotes PI3K, Akt and NF-κB expression.
GLI1 and ING4 affect the sensitivity
of colon cancer cells to bevacizumab
In order to discuss the significance of GLI1 and
ING4 in bevacizumab in the treatment of colon cancer, the
IC50 value was collected by MTT method, and the effects
of GLI1 and ING4 on the sensitivity of cell bevacizumab were
discussed. The results of Fig. 6
showed that, compared with NC group, the deletion of GLI1 increases
the IC50 value of cells with bevacizumab, while the
deletion of ING4 decreases the IC50 value of cells with
bevacizumab. The above results showed that GLI1 decreases the
sensitivity of cells to bevacizumab, while ING4 increases the
sensitivity of cells to bevacizumab.
Discussion
Bevacizumab is a humanized monoclonal antibody
against vascular endothelial growth factor, which can inhibit tumor
by inhibiting vascular endothelial growth factor (20). The results in this study verified
that the tumor volume and weight are significantly reduced after
treating with bevacizumab, and the curative effect of bevacizumab
may be dose-dependent. The results showed that bevacizumab could
upregulate ING4, promote cell apoptosis and inhibit cell activity.
The increase of ING4 can promote the downstream NF-κB and FoxO3
apoptosis pathways (11,12), so the increase of ING4 is the direct
cause of the increase of cell apoptosis rate. In this study, the
downregulation of caspase-3, Bax and β-catenin expression and the
increase of apoptosis rate in colon cancer cells after knockout of
ING4 also prove that ING4 can induce apoptosis.
GLI1 is a promoter of colon cancer (18,19). The
results showed that the loss of GLI1 leads to increased apoptosis,
decreased cell activity, and upregulated expression of caspase-3,
Bax and β-catenin. The above results indicated that GLI1 inhibits
cell apoptosis to promote colon cancer. It is worth mentioning that
bevacizumab also caused GLI1 downregulation after treating colon
cancer model rats. ING4 upregulation caused by bevacizumab may be
the reason for GLI1 downregulation. In colon cancer, PI3K/Akt/NF-κB
pathway can be used as upstream factor to activate GLI1, while
PI3K/Akt/NF-κB pathway is negatively regulated by PTEN (18,21).
Previous studies have shown that ING4 is co-expressed with PTEN
(22–24). In addition, ING5, a homologous
protein of ING4, inhibits Akt activity (25). In this study, after bevacizumab
treatment of colon cancer model rats, PTEN expression is
upregulated, while PI3K, Akt, NF-κB expression is downregulated.
Based on the results of this study and previous studies, it is
speculated that the upregulation of ING4 caused by bevacizumab can
promote PTEN expression, while PTEN and ING4 will jointly inhibit
the activity of PI3K/Akt/NF-κB pathway. Finally, this series of
cascade reactions lead to the decrease of GLI1 expression with the
decrease of upstream pathway activity.
The results also suggested that GLI1 can reduce the
sensitivity of colon cancer cells with bevacizumab, while ING4 can
improve the sensitivity of colon cancer cells with bevacizumab,
which indicates that regulating GLI1 and ING4 expression in
clinical application is helpful to improve the efficacy of
bevacizumab.
The results of this study showed that bevacizumab
inhibit colon cancer cells by regulating the expression of GLI1 and
ING4, and found that the dosage of bevacizumab affected its
therapeutic effect on colon cancer. In the future experimental
design, the expression of GLI1 and ING4 can be used as the standard
to further discuss the effects of different doses of bevacizumab on
colon cancer model rats, and to select the better dose. In
addition, it is also possible to find upstream and downstream
factors that change by regulating the expression of GLI1 and ING4
in colon cancer, thus supplementing the mechanism network of colon
cancer.
In conclusion, GLI1 downregulation and ING4
upregulation were found in the treatment of colon cancer model rats
with bevacizumab, and it is believed that the upregulation of ING4
caused by bevacizumab can inhibit GLI1 expression through
PTEN/PI3K/Akt/NF-κB pathway. In addition, GLI1 can reduce the
sensitivity of colon cancer to bevacizumab, while ING4 can enhance
the sensitivity of colon cancer to bevacizumab. Therefore, in
clinical treatment, targeted regulation of the expression levels of
GLI1 and ING4 will help to improve the effectiveness of bevacizumab
in the treatment of colon cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
BS wrote the manuscript, interpreted and analyzed
the data. CW performed flow cytometer, MTT assay and Western
blotting. FM contributed to observation indexes analysis. The final
version was read and adopted by all the authors. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
General Hospital of Heilongjiang Province Land Reclamation Bureau
(Harbin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vasaikar S, Huang C, Wang X, Petyuk VA,
Savage SR, Wen B, Dou Y, Zhang Y, Shi Z, Arshad OA, et al Clinical
proteomic tumor analysis consortium, : Proteogenomic analysis of
human colon cancer reveals new therapeutic opportunities. Cell.
177:1035–1049.e19. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang L, Cao F, Zhang G, Shi L, Chen S,
Zhang Z, Zhi W and Ma T: Trends in and predictions of colorectal
cancer incidence and mortality in China from 1990 to 2025. Front
Oncol. 9:982019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Laish I, Mizrahi J, Naftali T and Konikoff
FM: Diabetes mellitus and age are risk factors of Interval colon
cancer: A case-control Study. Dig Dis. 37:291–296. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haggar FA and Boushey RP: Colorectal
cancer epidemiology: Incidence, mortality, survival, and risk
factors. Clin Colon Rectal Surg. 22:191–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu RT, Gutkind JS, Johnson DE and Grandis
JR: PIK3CA mutations in colorectal and breast cancer: Impact on
oncogenesis and response to nonsteroidal anti-inflammatory drugs.
In: Targeting Cell Survival Pathways to Enhance Response to
Chemotherapy. Johnson DE (ed). Academic Press. (Cambridge, MA).
123–144. 2019.
|
|
6
|
Oh M, McBride A, Yun S, Bhattacharjee S,
Slack M, Martin JR, Jeter J and Abraham I: BRCA1 and BRCA2 gene
mutations and colorectal cancer risk: Systematic review and
meta-analysis. J Natl Cancer Inst. 110:1178–1189. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sabry D, El-Deek SEM, Maher M, El-Baz MAH,
El-Bader HM, Amer E, Hassan EA, Fathy W and El-Deek HEM: Role of
miRNA-210, miRNA-21 and miRNA-126 as diagnostic biomarkers in
colorectal carcinoma: Impact of HIF-1α-VEGF signaling pathway. Mol
Cell Biochem. 454:177–189. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wen YA, Xiong X, Scott T, Li AT, Wang C,
Weiss HL, Tan L, Bradford E, Fan TWM, Chandel NS, et al: The
mitochondrial retrograde signaling regulates Wnt signaling to
promote tumorigenesis in colon cancer. Cell Death Differ.
26:1955–1969. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ostermann AL, Wunderlich CM, Schneiders L,
Vogt MC, Woeste MA, Belgardt BF, Niessen CM, Martiny B, Schauss AC,
Frommolt P, et al: Intestinal insulin/IGF1 signalling through FoxO1
regulates epithelial integrity and susceptibility to colon cancer.
Nat Metab. 1:371–389. 2019. View Article : Google Scholar
|
|
10
|
Du Y, Cheng Y and Su G: The essential role
of tumor suppressor gene ING4 in various human cancers and
non-neoplastic disorders. Biosci Rep. 39:392019. View Article : Google Scholar
|
|
11
|
Ma Y, Cheng X, Wang F, Pan J, Liu J, Chen
H, Wang Y and Cai L: ING4 inhibits proliferation and induces
apoptosis in human melanoma A375 cells via the Fas/caspase-8
apoptosis pathway. Dermatology. 232:265–272. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qian F, Hu Q, Tian Y, Wu J, Li D, Tao M,
Qin L, Shen B and Xie Y: ING4 suppresses hepatocellular carcinoma
via a NF-κB/miR-155/FOXO3a signaling axis. Int J Biol Sci.
15:369–385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao L, Chen S, Zhang C, Chen C, Lu N,
Jiang Y, Cai Y, Yin Y and Xu J: ING4 enhances paclitaxel's effect
on colorectal cancer growth in vitro and in vivo. Int J Clin Exp
Pathol. 8:2919–2927. 2015.PubMed/NCBI
|
|
14
|
Chen Y, Huang Y, Hou P, Zhang Z, Zhang Y,
Wang W, Sun G, Xu L, Zhou J, Bai J, et al: ING4 suppresses tumor
angiogenesis and functions as a prognostic marker in human
colorectal cancer. Oncotarget. 7:79017–79031. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qu H, Yin H, Yan S, Tao M, Xie Y and Chen
W: Inhibitor of growth 4 suppresses colorectal cancer growth and
invasion by inducing G1 arrest, inhibiting tumor angiogenesis and
reversing epithelial-mesenchymal transition. Oncol Rep.
35:2927–2935. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Riaz SK, Ke Y, Wang F, Kayani MA and Malik
MFA: Influence of SHH/GLI1 axis on EMT mediated migration and
invasion of breast cancer cells. Sci Rep. 9:66202019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoon JW, Lamm M, Iannaccone P and
Walterhouse D: GLI1 contributes to proliferation, survival and
drug-resistance of Ewing sarcoma (EWS) cell. AACR. 36612019.
|
|
18
|
Yang Z, Zhang C, Qi W, Cui Y and Xuan Y:
GLI1 promotes cancer stemness through intracellular signaling
pathway PI3K/Akt/NFκB in colorectal adenocarcinoma. Exp Cell Res.
373:145–154. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang X, Yao Y and Zhu X: The influence of
aberrant expression of GLI1/p-S6K on colorectal cancer. Biochem
Biophys Res Commun. 503:3198–3204. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mulder K, Scarfe A, Chua N and Spratlin J:
The role of bevacizumab in colorectal cancer: Understanding its
benefits and limitations. Expert Opin Biol Ther. 11:405–413. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Georgescu MM: PTEN tumor suppressor
network in PI3K-Akt pathway control. Genes Cancer. 1:1170–1177.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Watson M, Berger P, Frank S, Winn M and
Miranti C: CREB1 and ATF1 differentially regulate terminal prostate
luminal differentiation by controlling the timing of ING4
expression, while CREB1 prevents ING4 expression upon PTEN loss in
prostate cancer. Cancer Res. 78 (16 Suppl):Abstract B031. 2018.
|
|
23
|
Rakshit N, Yang S, Zhou W, Xu Y, Deng C,
Yang J, Yu H and Wei W: Adenovirus-mediated co-expression of ING4
and PTEN cooperatively enhances their antitumor activity in human
hepatocellular carcinoma cells. Acta Biochim Biophys Sin
(Shanghai). 48:704–713. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Yang J, Sheng W, Xie Y and Liu J:
Adenovirus-mediated ING4/PTEN double tumor suppressor gene
co-transfer modified by RGD enhances antitumor activity in human
nasopharyngeal carcinoma cells. Int J Oncol. 46:1295–1303. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barlak N, Capik O, Sanli F, Kilic A,
Aytatli A, Yazici A, Ortucu S, Ittmann M and Karatas OF: ING5
inhibits cancer aggressiveness by inhibiting Akt and activating p53
in prostate cancer. Cell Biol Int cbin.11227. 2019.
|