Introduction
Orthotopic liver transplantation (OLT) is an
effective treatment for various types of end-stage liver disease
and is the most appropriate alternative for treating HCC associated
with liver cirrhosis (1). However,
tumor recurrence following OLT is a common cause of poor prognosis
and poor long-term survival. Due to the high costs and limited
organ donors, the application of OLT requires accurate evaluation
of a patient's condition to guarantee the recipient benefits from
OLT without recurrence.
The Milan criteria (2) or the University of California San
Francisco (UCSF) criteria (3), which
are based on the tumor size and number, are the main criteria for
selecting recipients in numerous LT centers. The prognosis is
evaluated according to these criteria. However, it has been
observed that a number of patients with similar clinical features
display a different prognosis profile according to the Milan
criteria (4,5). Therefore, the biological characteristic
of tumors must be considered when evaluating patient prognosis.
Pathological differentiation is generally accepted as predictive of
recurrence and was internalized in the Hangzhou criteria for
transplantation (6). Microvascular
invasion (MVI), which represents tumor aggressiveness, is another
predictor of HCC recurrence (7).
However, the biological properties of tumors can only be evaluated
accurately by histological examination, which is limited by the
invasive nature of tumors and the high risk of sampling errors
caused by intra-tumoral heterogeneity (8).
In HCC, glucose metabolism is correlated with tumor
grade and aggressiveness, which is assessed by
18F-fluorodeoxyglucose (18F-FDG) positron
emission tomography/computed tomography (PET/CT). Glucose
metabolism was found to be a significant prognostic factor of
survival in patients with hepatic tumors undergoing curative
surgical resection (9). The maximum
standardized uptake value (SUVmax), which represents a single pixel
showing maximal metabolic uptake and reflects the degree of FDG
uptake, was shown to influence the survival of patients with
several different types of cancer, including HCC (10). In addition, metabolic tumor volume
(MTV) and total lesion glycolysis (TLG) reflected the metabolic
activity and volume of tumors, and were associated with the
recurrence risk of patients with HCC (11–13).
However, the incorporation of the metabolic burden indices and
candidate selection criteria for OLT remains controversial.
The aim of the present study was to analyze the
association between 18F-FDG PET/CT metabolic burden
indices or clinicopathological factors and the recurrence risk in
patients with HCC, following OLT. The Deauville-like scoring system
was used to classify the recurrence risk using the most important
metabolic indices as the input factors. Furthermore, univariate and
multivariate survival analyses were performed to determine the
influence of these metabolic indices on recurrence-free survival
(RFS) and to identify a priority candidate order based on metabolic
criteria.
Patients and methods
Patients
The present study included a total of 752 patients
who underwent OLT at Tianjin First Central Hospital (Tianjin,
China) between September 2013 and April 2017. Among these patients,
196 patients underwent a PET/CT examination. In total, 93 patients
(83 men and 10 women; mean age ± SD, 51.37±8.43 years; range, 29–70
years) were included in this retrospective analysis according to
the following criteria: i) No macrovascular invasion and no distant
metastasis, as confirmed by CT, magnetic resonance imaging (MRI) or
18F-FDG PET/CT; ii) patients who underwent PET/CT before
and after OLT; iii) patients who received OLT within 4 weeks of
PET/CT scan; iv) HCC confirmed by clinical diagnosis or
histopathological results; and v) patients who did not experience
recurrence following OLT were followed up for at least 12 months.
The following criteria were used to exclude patients: i) Patients
who received OLT only for liver cirrhosis or intracholangio
cellular carcinoma; ii) patients who did not undergo PET/CT
following OLT, whose recurrence could not be confirmed and iii)
patients whose follow-up data was missing.
Enhanced CT or MRI was performed to
confirm the site, size and number of tumors
In most patients, PET/CT was performed to assess the
presence or absence of metastasis. Candidates for LT were selected
mainly based on the Milan criteria (2). However, if there was neither major
vascular invasion nor extrahepatic metastasis, OLT was still
performed in some patients. Patients with different types of
hepatitis, along with clinical histopathological features,
including treatment history, serum α-fetoprotein (AFP) level, cell
differentiation, tumor number, tumor location, tumor size, liver
cirrhosis, lymph node metastasis, satellite nodules, microvascular
invasion (MVI) and interstitial vessel tumor embolus, were
recorded. Among the 93 patients, 91 had a clinical history of
hepatitis, including 84 cases with hepatitis B, 5 with hepatitis C,
1 with hepatitis B and D, and 1 with autoimmune hepatitis. The
other 2 patients were diagnosed with alcoholic liver cirrhosis
without a history of hepatitis.
Patients were routinely examined by ultrasound (US)
at days 7, 14 and 28 following OLT, in order to monitor the
progress of the transplanted liver. The AFP level was measured
every 2–3 months. CT or MRI scans were performed 3 months after
OLT. An 18F-FDG PET/CT scan was performed every 6 months
or complementarily used when serum AFP was increased, or other
suspicious symptoms or signs were observed, including pain, fever
and cough. The recurrence of lesions was confirmed by the
18F-FDG PET/CT examination or other imaging tools.
The study design was approved by the Institutional
Clinical Research Ethics Committee of Tianjin First Central
Hospital (approval no. 2018N103KY). No organs from executed
prisoners were used. Each organ donated or transplanted was
allocated by the China Organ Transplant Response System.
18F-FDG PET/CT
examination
All patients fasted for at least 6 h. Blood glucose
concentration was confirmed to be <7.1 mM in each patient prior
to administering 18F-FDG. 18F-FDG was
purchased from Tianjin Atom High Science Isotopes Medicine Co.,
Ltd. The radiochemical purity of 18F-FDG was 98%, which
was tested by the manufacturer. At 1 h post-intravenous injection
of 18F-FDG [5.55 MBq/kg (0.15 mCi/kg)], whole-body
PET/CT examination was performed using a Biograph mCT 64 system
(Siemens Healthineers) in the supine position. CT images were
acquired from the skull base to the upper thigh area for
attenuation map and lesion localization (94–140 mAs, 120 kVp, 5-mm
wide section). A 3.0-mm thick section was reconstructed for
attenuation correction followed by subsequent image fusion. PET
images of the same area were acquired following CT scans in
3-dimensional mode, with 6–7 bed positions. Images were
reconstructed using an iterative algorithm and were transported to
a dedicated workstation (syngoMMWP VE40A; Siemens Healthineers) and
analyzed using the syngo TrueD software (TRUED_SYSLATEST_VE10A40;
Siemens Healthineers).
PET/CT image analysis
All images were visually analyzed independently by
two experienced nuclear medicine specialists, and differences of
opinion were resolved by another PET/CT expert. For quantitative
analysis, the SUVmax and SUVmean based on
body weight, and SUV normalized to lean body mass (SUL) in the
primary tumor, were measured. For non-FDG-avid tumors, the enhanced
CT scans were used to determine the location and extent of the
primary tumor on PET/CT images. For FDG-avid tumors, the MTV,
referring to the volume of the tumor with the SUV in a given range
on the image (based on tumor volume), was manually measured by
3-dimensional globular region of interests (ROIs). The
SUVmax of 40% was used as the margin threshold for
volumetry, as previously described (14,15). The
SUVmean of the mediastinum (Msuv) was measured as from 1
cm3 volumetric globular ROIs in the descending aorta.
The SUVmean of the liver background (LBsuv) was measured
as from 20 cm3 volumetric globular ROIs, which were
drawn in locations where HCC was not detected in the liver. The
tumor-to-normal liver SUV ratio (TLR) and the tumor-to-mediastinum
SUV ratio (TMR) were calculated with the following equation: TLR or
TMR = SUVmax of the tumor/SUVmean of the
normal liver or mediastinum. TLG is a parameter that reflects the
average status of glycolysis in tumors, indicating the tumor burden
by incorporating MTV and SUVmean in the calculation
process. TLG was calculated as the product of MTV and
SUVmean. For each patient, if there were multiple tumors
in the liver with (<5 lesions) the MTV and SUVmean of
all tumors were calculated. If the number of lesions was >5, the
5 biggest tumors were calculated. MTV and SUVmean of the
whole liver were measured for diffuse tumors. For multiple tumors,
MTV or TLG was the sum of all lesions. The uptake-volume product
(UVP) (12), another metabolism
burden index, was reported to be a significant prognostic factor,
which exhibits a higher predictive power compared with other
indices. In the present study, UVP was calculated as the product of
MTV and mean TMR.
The tumors were semi-quantitatively scored based on
the Deauville 5-point scoring system (16). The scoring system is as follows: 1,
no uptake above the background; 2, uptake ≤mediastinum; 3, uptake
>mediastinum but ≤liver; 4, uptake moderately increased compared
with the liver at any site; and 5, uptake markedly increased
compared with the liver at any site. The SUVmax of the
lesions in the liver were measured and assigned a Deauville-like
score as follows: 1–3, PET-negative group (n=22/93; 23.66%); 4,
PET-weakly positive group (n=30/93; 32.26%); and 5, PET-markedly
positive group (n=41/93; 44.09%).
Statistical analysis
The IBM SPSS statistics software (version 20.0; IBM
Corp.) was used for statistical analysis. The χ2 test
was performed to compare clinicopathological factors and recurrence
states. Data are expressed as the mean ± SD. Msuv and LBsuv,
SUVmax and SUL, TMR and TLR were calculated and analyzed
using Pearson's correlation analysis. (P≤0.05 was considered to
indicate a statistically significant difference). An unpaired
Mann-Whitney U test was used to compare differences between the
metabolic indices, including SUVmax, TMR, TLR, SUL MTV,
TLG and UVP of the two groups with or without recurrence.
Recurrence-free survival (RFS) time was defined as the interval
between the date of OLT and the date when recurrence was detected
by follow-up imaging modalities. Receivers operating characteristic
(ROC) curve analyses were performed to predict recurrence based on
quantitative indices of PET/CT examination. Given the specificity
value, the cutoff values with the highest sensitivity for
quantitative factors were determined by an algorithm for optimal
cutoff determination (12). In the
survival analyses, the Kaplan-Meier method was used to assess the
cumulative RFS, and the log-rank test was performed to test the
statistical significance of the differences between groups. Cox
proportional hazards regression model was used to determine
predictors of RFS. Hazard ratios were calculated with corresponding
confidence intervals (CIs) set to 95%. P<0.05 was considered to
indicate a statistically significant difference.
Results
Follow-up information
The follow-up and other clinical information of the
patients are listed in Table I. The
mean follow-up time for all patients was 18.31±16.91 months (range,
1–60 months; 95% CI, 12.57–17.66). Among the 93 patients, 30 showed
no recurrence during the follow-up period of at least 12 months,
with a mean follow-up time of 37.13±14.69 months (range, 13–60
months; 95% CI, 20.00–29.14). A total of 63 patients had a
recurrence during the follow-up period, with a mean follow-up time
of 10.90±10.30 months (range, 1–55 months; 95% CI, 8.29–13.20). The
average recurrence period was 9.23±8.11 months (range, 1–35 months;
95% CI, 8.29–13.20).
 | Table I.Association of clinicopathological
features and recurrence. |
Table I.
Association of clinicopathological
features and recurrence.
| Variable | n | Non-recurrence,
n | Recurrence, n | χ2 | P-value |
|---|
| Gender |
|
|
| 0.307 | 0.579 |
|
Male | 83 | 26 | 57 |
|
|
|
Female | 10 | 4 | 6 |
|
|
| Age, years |
|
|
| 0.016 | 0.899 |
|
<60 | 76 | 25 | 51 |
|
|
|
≥60 | 17 | 5 | 12 |
|
|
| Treatment
history |
|
|
| 1.614 | 0.656 |
|
None | 36 | 11 | 25 |
|
|
|
Interventional therapy | 30 | 12 | 18 |
|
|
|
Excision | 14 | 3 | 11 |
|
|
|
Both | 12 | 4 | 8 |
|
|
| Drug
therapy | 1 | 0 | 1 |
|
|
| Hepatitis B/C DNA
copy |
|
|
| 2.483 | 0.115 |
|
Static | 67 | 25 | 42 |
|
|
|
Active | 25 | 5 | 20 |
|
|
| No
hepatitis | 1 | 0 | 1 |
|
|
| Hepatitis
types |
|
|
| 46.140 | 0.305 |
|
HBV | 84 | 28 | 53 |
|
|
|
HCV | 5 | 2 | 3 |
|
|
| NA | 4 | 0 | 4 |
|
|
| AFP, ng/ml |
|
|
| 5.450 | 0.020 |
|
<144 | 57 | 25 | 32 |
|
|
|
≥144 | 35 | 5 | 30 |
|
|
| NA | 1 | 0 | 1 |
|
|
| Milan criteria |
|
|
| 4.020 | 0.040 |
| In | 46 | 19 | 27 |
|
|
|
Out | 47 | 11 | 36 |
|
|
| Cell
differentiation |
|
|
| 6.172 | 0.046 |
|
Poorly | 23 | 3 | 20 |
|
|
|
Moderately | 61 | 23 | 38 |
|
|
|
Well | 3 | 0 | 3 |
|
|
| NA | 6 | 4 | 2 |
|
|
| Tumor number |
|
|
| 17.994 | <0.001 |
|
<3 | 54 | 27 | 27 |
|
|
| ≥3 | 39 | 3 | 36 |
|
|
| Tumor location |
|
|
| 17.936 | <0.001 |
| Right
lobe | 46 | 22 | 24 |
|
|
| Left
lobe | 10 | 5 | 5 |
|
|
|
Both | 35 | 2 | 33 |
|
|
| NA | 2 | 1 | 1 |
|
|
| Tumor size, cm |
|
|
| 17.350 | 0.001 |
|
<5 | 48 | 24 | 24 |
|
|
| ≥5 | 43 | 5 | 38 |
|
|
| NA | 2 | 1 | 1 |
|
|
| Liver
cirrhosis |
|
|
| 1.475 | 0.224 |
| No | 3 | 0 | 3 |
|
|
|
Yes | 87 | 29 | 58 |
|
|
| NA | 3 | 1 | 2 |
|
|
| Lymph nodes
metastasis |
|
|
| 2.558 | 0.110 |
| No | 88 | 31 | 57 |
|
|
|
Yes | 5 | 0 | 5 |
|
|
| Satellite
nodules |
|
|
| 12.740 | <0.001 |
| No | 63 | 28 | 35 |
|
|
|
Yes | 29 | 2 | 27 |
|
|
| NA | 1 | 0 | 1 |
|
|
| Stump or incisal
edge |
|
|
| 1.501 | 0.221 |
|
Negative | 89 | 30 | 59 |
|
|
|
Positive | 3 | 0 | 3 |
|
|
| NA | 1 | 0 | 1 |
|
|
| MVI |
|
|
| 10.874 | 0.001 |
| No | 52 | 25 | 27 |
|
|
|
Yes | 41 | 6 | 35 |
|
|
| Interstitial vessel
tumor embolus |
|
|
| 12.951 | <0.001 |
| No | 59 | 27 | 32 |
|
|
|
Yes | 33 | 3 | 30 |
|
|
| NA | 1 | 0 | 1 |
|
|
A total of 12 patients had intrahepatic recurrence,
16 patients had combined intrahepatic and extrahepatic recurrence
and 35 patients had extrahepatic recurrence. The sites of
recurrence were the liver (n=30), lung (n=24), lymph nodes (n=23),
adrenal gland (n=8), peritoneum (n=7), diaphragm (n=4), bone (n=4),
cancer embolus (n=4), spleen (n=1), face (n=1), pleura (n=1) or
widespread (n=1).
In total, 29 patients had early recurrence (≤6
months), with mean and median RFS times of 2.93±0.26 months (95%
CI, 2.42–3.44) and 3.00±0.217 months (95% CI, 2.57–3.43),
respectively. A total of 34 patients had a late recurrence (>6
months), with mean and median RFS times of 14.29±1.34 months (95%
CI, 11.67–16.92) and 12.00±0.472 months (95% CI, 11.07–12.93),
respectively (P<0.05).
During the follow-up, 7 patients died at 3, 4, 12,
15, 26 and 27.7 months, respectively. Since the number of patients
who died was low, the median overall survival (OS) was not
determined.
Association between
clinicopathological features and recurrence
Clinicopathological features were examined,
following the division of patients into different subgroups, to
assess their association with recurrence. ROC analyses were
performed, which indicated the cutoff values for age and AFP level
to be 60 years and 144 ng/ml, respectively. The subgroups of tumor
number and size were based on the Milan criteria.
Analysis by χ2 test revealed that
elevated AFP (≥144 ng/ml), Milan criteria, tumor number >3,
involvement of both right and left lobes, and tumor size >5 cm
were significant predictors of tumor recurrence. Patients with
poorly differentiated tumors, positive satellite nodules, MVI and
interstitial vessel tumor embolus had a higher recurrence rate
(P<0.05; Table I).
Prognostic value of metabolic indices
in preoperative PET/CT for tumor recurrence
Msuv and LBsuv were positively correlated
(P<0.05; r=0.867; data not shown). SUVmax and SUL, as
well as TMR and TLR, were positively correlated (both P<0.001;
r=0.995 and r=0.942, respectively; data not shown). The MTV, TLG
and UVP of the non-recurrence group were significantly lower
compared with those of the recurrence group, (P=0.018, P=0.009 and
P=0.028, respectively; data not shown). However, SUVmax,
TMR, TLR and SUL were not significantly different between the
recurrence group and the non-recurrence group (data not shown).
Association between Deauville-like
score and recurrence
The Deauville-like score in preoperative PET/CT was
summarized. Higher Deauville-like score was associated with higher
metabolic indices (P<0.05). The most important metabolic indices
including SUVmax, MTV, TLG and uptake-volume product
according the SUVmean of mediastinum (UVP-M) were
selected and are presented in Fig.
1.
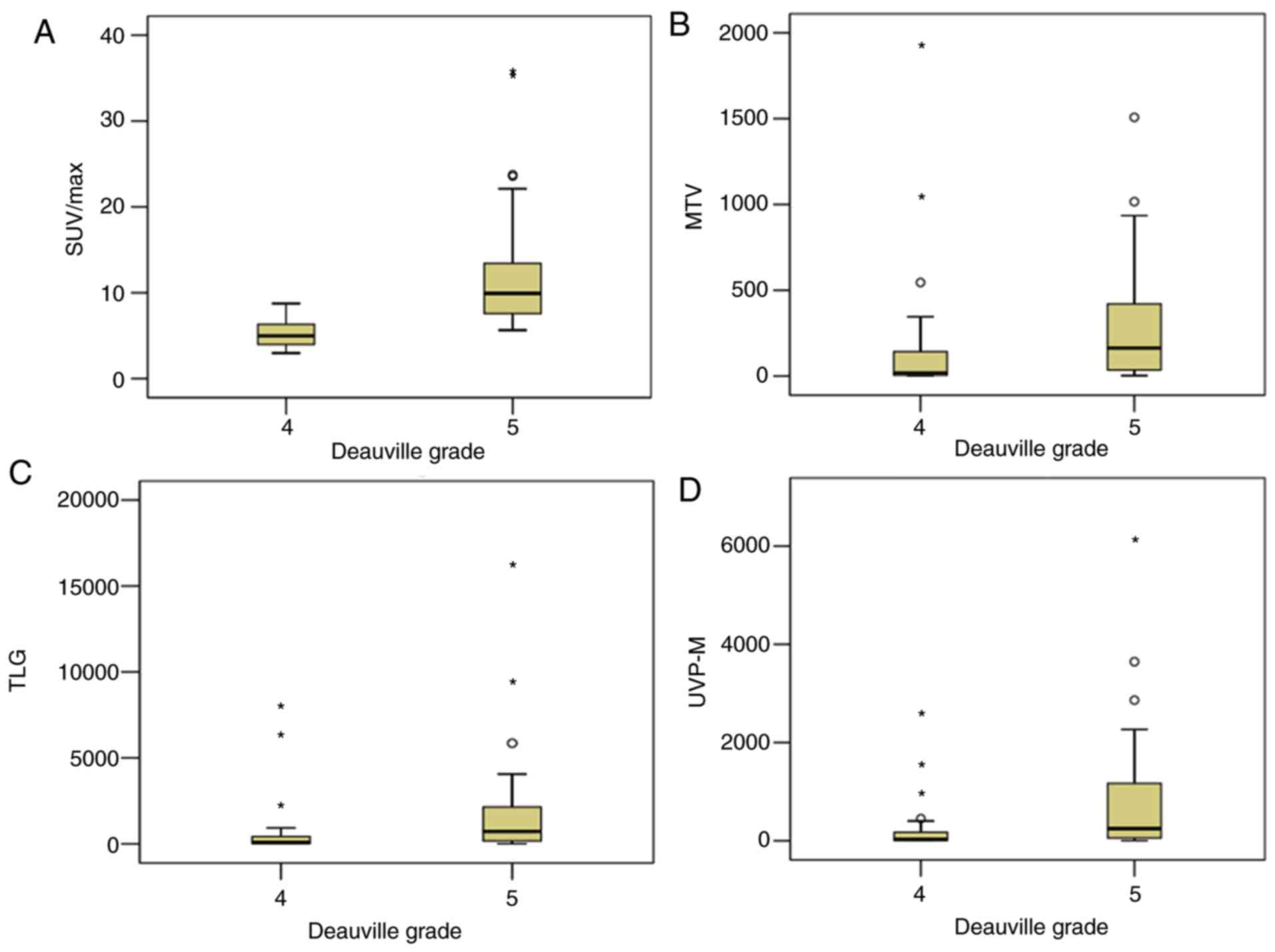 | Figure 1.Metabolic indices, including (A)
SUVmax, (B) MTV, (C) TLG and (D) UVP-M, were higher in PET-markedly
positive groups compared with PET-weakly positive groups, according
to Deauville-like criteria grades 5 and 4, respectively. The
asterisks and circles in the figure represent the extreme values
and outliers, respectively. SUVmax, maximum standardized uptake
value; MTV, metabolic tumor volume; TLG, total lesion glycolysis;
PET, positron emission tomography; UVP-M, uptake-volume product
according the SUVmean of mediastinum. |
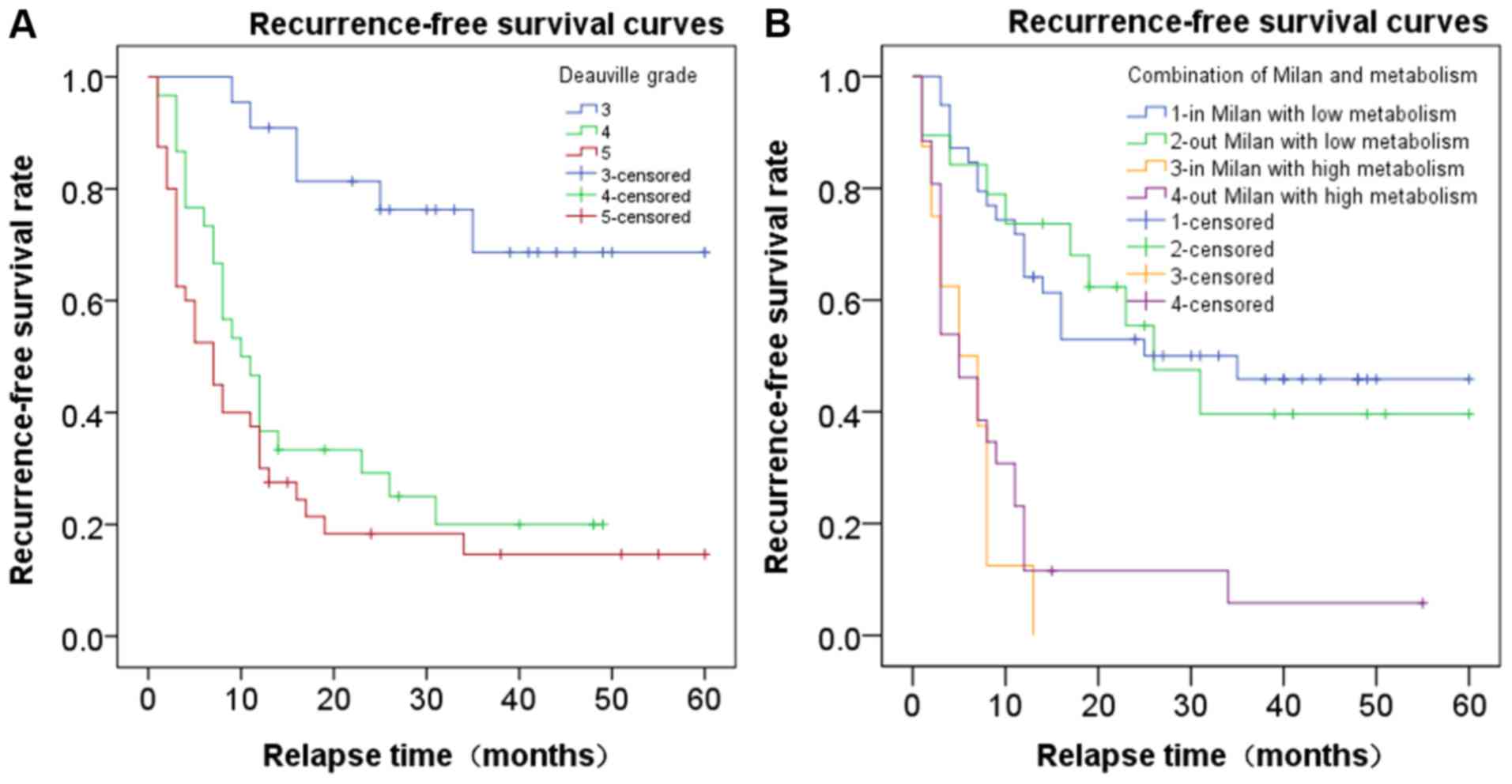
According to the Deauville-like score, 22 patients
had PET-negative lesions prior to OLT, with 6 patients (27.3%)
experiencing a recurrence at 9, 11, 16, 16, 25 and 35 months,
respectively, following OLT. The mean recurrence period was
18.67±9.73 months (range, 9–35 months; 95% CI, 8.46–28.88).
In the PET-weakly positive group, 23 of 30 (76.7%)
patients had a recurrence, with a mean recurrence period of
9.83±7.62 months (range, 1–31 months; 95% CI, 6.53–13.12). In the
PET-markedly positive group, 33 of 41 (80.5%) patients had a
recurrence, with a mean recurrence period of 6.97±7.04 months
(range, 1–34 months; 95% CI, 4.47–9.47 months) (P<0.001). The
recurrence period in the PET-markedly positive group was shorter
than that of the PET-weakly positive group, and the recurrence
periods of the two groups were shorter than that of the
PET-negative group. Therefore, the corresponding recurrence risks
for patients in the PET-negative (score 3), PET-weakly positive
(score 4) and PET-markedly positive groups (score 5) were low,
medium and high, respectively (P<0.05; Fig. 2A).
ROC curve analysis based on metabolic
indices determined by PET/CT
ROC analysis demonstrated the Deauville-like score
(PET-negative vs. PET-positive), MTV (cutoff value, 13.36), TLG
(cutoff value, 62.21) and UVP (cutoff value, 66.60) to be
significant predictors of recurrence risk (all P<0.05). Among
the metabolic indices, TLG had the highest AUC (0.725), with
sensitivity and specificity of 79.6 and 66.7% at the cutoff value
of 62.21 (Table II).
 | Table II.Receiver operating characteristic
analysis of metabolic burden indices for predicting recurrence. |
Table II.
Receiver operating characteristic
analysis of metabolic burden indices for predicting recurrence.
|
|
|
| 95% CI |
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Indices | AUC | P-value | Lower | Upper | Sensitivity | Specificity | Youden index | Cutoff value |
|---|
| MTV | 0.708 | 0.025 | 0.542 | 0.873 | 0.852 | 0.583 | 0.435 | 13.36 |
| TLG | 0.725 | 0.015 | 0.569 | 0.882 | 0.796 | 0.667 | 0.463 | 62.21 |
| UVP | 0.694 | 0.036 | 0.537 | 0.852 | 0.630 | 0.833 | 0.463 | 66.60 |
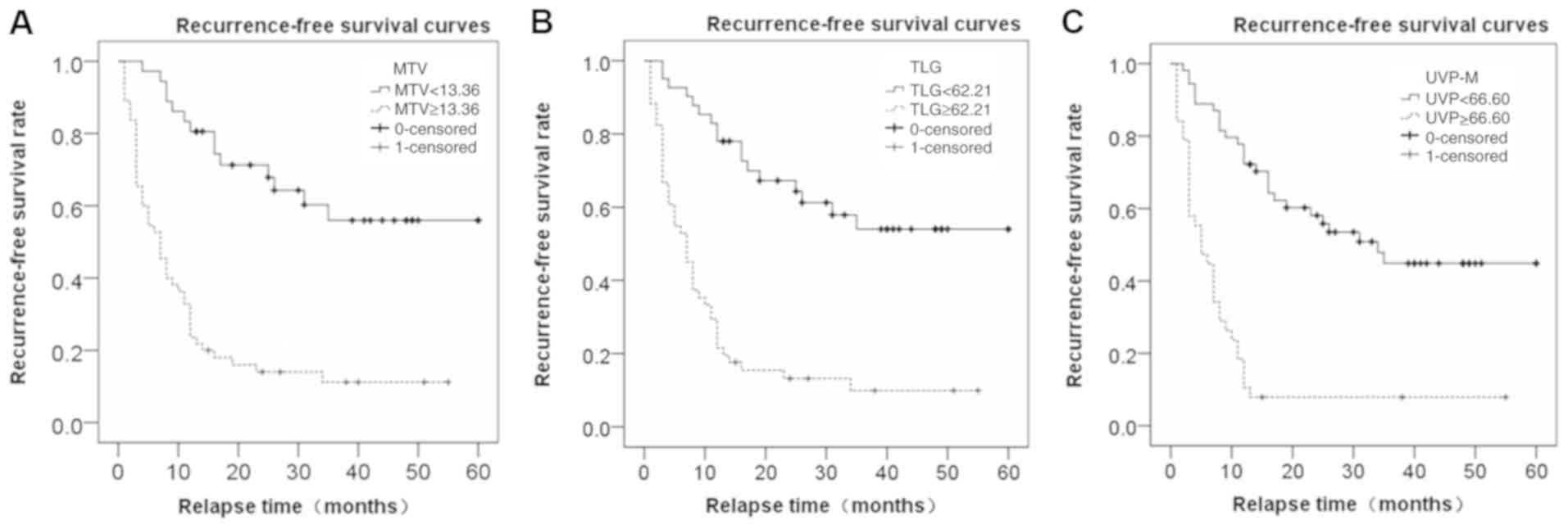
MTV=13.36 and TLG=62.21 were used as the cutoff
values, and the median RFS time of the lower MTV and TLG groups was
12 months (data not shown). The median RFS time of the higher MTV
and TLG groups was 5 months, and the χ2 values were
10.826 for the MTV group and 10.211 for the TLG group (P<0.05;
data not shown). With UVP-M=66.60 as the cutoff value, the median
RFS times in the lower and higher UVP-M groups were 12 and 4
months, respectively, and the χ2 value was 17.184
(P<0.05; data not shown). Fig. 3
displays the RFS curve based on the cutoff of MTV, TLG and UVP-M.
Patients with low MTV (<13.36) exhibited significantly improved
RFS than those with high MTV (≥13.36; P<0.05; Fig. 3A) and patients with low TLG
(<62.21) exhibited significantly improved RFS than those with
high TLG (≥62.21; P<0.05; Fig.
3B). Additionally, patients with low UVP-M (<66.60)
exhibited significantly improved RFS than those with high TLG
(≥66.60; P<0.05; Fig. 3C).
Combination of metabolic status and
Milan criteria to predict the recurrence risk
To further stratify the risk of HCC recurrence, the
TLG cutoff value of 62.21 was used to categorize FDG-positive
patients (n=56) into high-metabolic (n=45; TLG, ≥62.21) and
low-metabolic (n=11; TLG, <62.21) groups. TLG was found to be a
significant predictor of late recurrence (>6 months; P=0.04).
The RFS time in the low metabolic group (mean, 17.43 months;
median, 16 months; 95% CI, 8.74–23.26) was longer compared with the
high metabolic group (mean, 9.29 months; median, 12 months; 95% CI,
8.82–13.18) (P<0.001). However, TLG was not a significant
predictor of early recurrence (≤6 months; P=0.80).
The results revealed that patients with low
metabolic burden had longer RFS times compared with those with high
metabolic burden, regardless of the Milan criteria. Subsequently,
the patients were separated into four groups: 20 patients who met
the Milan criteria with low metabolism; 10 patients who did not
meet the Milan criteria with low metabolism; 8 patients who met the
Milan criteria with high metabolism; and 25 patients who did not
meet the Milan criteria and had high metabolism. The RFS curves of
these four groups are shown in Fig.
2B. Patients with low metabolism exhibited significantly
improved RFS than those with high metabolism, independently of
whether they met the Milan criteria or not (P<0.05).
Univariate and multivariate survival
analyses
In the univariate survival analysis, AFP, UVP-M,
Milan criteria, l node metastasis, number of tumor and MVI were
found to be significant prognostic factors of RFS (P<0.05). In
the multivariate survival analyses, AFP, UVP-M, Milan criteria,
lymph node metastasis, and number of tumors were identified as
significant prognostic factors (P=0.005, P<0.001, P=0.001,
P=0.001 and P=0.002, respectively; Table III).
 | Table III.Results of univariate analysis and
multivariate analysis. |
Table III.
Results of univariate analysis and
multivariate analysis.
|
| Univariate
analysis |
| Multivariate
analysis |
|
|---|
|
|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| AFP | 3.34 | 1.38–8.09 | 0.008 | 2.73 | 1.37–5.47 | 0.005 |
| UVP-M | 14.55 | 3.39–62.44 | <0.01 | 5.68 | 2.59–12.44 | <0.01 |
| Milan criteria | 0.030 | 0.01–0.20 | <0.01 | 0.26 | 0.12–0.57 | 0.001 |
| Lymph node
metastasis | 15.343 | 2.61–90.29 | 0.003 | 7.43 | 2.23–24.74 | 0.001 |
| Number of
tumor | 3.648 | 1.55–8.62 | 0.003 | 2.58 | 1.43–4.66 | 0.002 |
| MVI | 0.228 | 0.06–0.95 | 0.042 | 0.325 |
|
|
Discussion
Emerging evidence indicates that the Milan criteria
and radiographic up-to-seven (UTS) criteria (17), are too restrictive as selection tools
for liver transplantation candidates, and therefore, it is
necessary to expand these criteria (18,19).
Some expanded criteria, based on tumor number and size, such as the
UCSF criteria (20) or the Hangzhou
criteria (21), revealed that
selected patients had a similar prognosis profile compared with
those who met the Milan criteria. Kornberg et al (18) reported that the Milan criteria can
successfully select patients who are suitable for LT, and further
biological tumor evaluation is necessary beyond the Milan
boundaries. A number of studies have identified tumor size,
capsular invasion or positive resection margin, satellite nodules,
MVI, AFP, transaminase and cirrhosis as risk factors for HCC
recurrence (22–24). Cell differentiation and MVI, which
provide histological evidence of tumor penetration into small
vessels around the primary neoplasm, have become important markers
of tumor aggressiveness (25). The
findings of the present study are consistent with those reported in
previous studies (22,24). Additionally, both right and left lobe
involvement, as well as tumor number >3, which reflect higher
tumor burden, were found to be risk factors in the present
study.
A non-invasive method is required to provide
standardized patient selection to ensure their suitability for LT.
Previous studies demonstrated that 18FFDG PET/CT is a
powerful prognostic marker for patients with HCC following LT, and
it was found to be strongly correlated with pathological
characteristics of tumors, such as microvascular invasion and
differentiation (12,26,27). In
addition to FDG, other PET radioactive tracers, such as labeled
choline and acetate, are frequently used in clinical practice.
These factors are mainly designed to improve the specificity and
sensitivity of FDG PET-CT in detecting HCC and HCC metastasis.
11C-choline is a lipid tracer with strong avidity for HCC,
especially in well- and moderately-differentiated tumors (28). Several studies have described the
diagnostic potential of a dual tracer approach using radiolabeled
choline and 18F-FDG PET/CT in HCC (29,30).
11C-ACT-PET can be used to monitor abnormal uptake of local fatty
acids and is sensitive to the diagnosis of well-differentiated HCC.
However, the clinical application of 11C-ACT-PET is limited due to
the synthetic nature of the technology and the short half-life.
Accelerated glycolysis, determined by 18F-FDG PET/CT, is
highly correlated with glycolytic enzymatic activity and tumor
aggressiveness in malignant tumors. 18F-FDG is
transported into cells via glucose transporters (GLUTs). Previous
studies revealed that low 18F-FDG uptake was correlated
with high FDG-6-phosphatase activity, low expression of GLUT1 or
GLUT2, and high expression of P-glycoprotein (31,32).
High tumor uptake of FDG often indicates a poor clinical outcome
(30,33). FDG-PET has been combined with the
radiographic UTS criteria to select patients with HCC for LT
(18). Lee et al (34) evaluated the accuracy of the National
Cancer Center, Korea (NCCK) criteria using PET/CT (negative) and
total tumor size (<10 cm). The NCCK criteria produced comparable
results with preoperative PET/CT imaging and tumor pathological
characteristics relative to the Milan criteria. Therefore, the NCCK
criteria were proposed as new expanded criteria that can be used in
place of the traditional Milan criteria. The present study explored
the ability of metabolic indices, including MTV, TLG and UVP,
measured by FDG-PET/CT to predict tumor recurrence following
OLT.
SUVmax, TLR, MTV and TLG derived from
18F-FDG PET/CT are the most frequently used indices for
staging and risk stratification of patients prior to LT. SUL is
recommended for evaluating the metabolic state of SUV, based on
body weight, due to the increasing frequency of obesity cases. TLR
is superior to SUVmax in assessing tumor metabolism.
Studies have shown that high TLRs reflect a poor prognosis
(22,35). A study demonstrated that patients
with vascular invasion exhibited significantly higher AFP levels,
tumor (T)SUVmax, TSUVmax/LSUVmax ratio and
TSUVmax/LSUVmean ratio compared with those
without vascular invasion (36). In
another study, multivariate analysis revealed that peritumoral
enhancement and a TSUVmax/LSUVmean of ≥1.2
were significantly associated with MVI (37). TMR was compared with TLR in the
present study due to the heterogeneous metabolic activities in the
tissues of liver cirrhosis. The two indices showed similar
performances in predicting tumor recurrence. Thus, the selection of
patients by TMR rather than TLR is recommended, as the
SUVmax of the mediastinum is more stable than the
SUVmax of the liver with cirrhosis and hepatitis.
In a previous study, both TLR and UVP calculated by
the inferior vena cava (IVC) activity were identified as
significant prognostic factors of tumor recurrence in multivariate
analyses (12). The descending aorta
in the mediastinum was chosen as the background rather than the
IVC, which is often flattened in patients with cirrhosis. Patients
with low 18F-FDG uptake had significantly better
survival rates than those with higher 18F-FDG uptake
(38). In the present study, MTV,
TLG and UVP were strong predictors of recurrence in patients with
HCC following LT compared with other indices, such as
SUVmax, TLR and SUL. UVP and TLG in PET/CT were found to
be independent prognostic factors for RFS in patients with HCC
following LT, demonstrating a superior prognostic value for
HCC.
Lee et al (9)
retrospectively analyzed the data of 242 patients with HCC who
underwent staging by FDG PET and subsequent curative surgical
resection. The serum bilirubin level, MTV and TLG were found to be
independent prognostic factors of overall RFS and OS (P<0.05).
MTV and TLG were prognostic predictors for only extrahepatic RFS
(P<0.05). Furthermore, serum AFP, bilirubin levels, MTV and TLG
were prognostic factors of early intrahepatic RFS (P<0.05),
whereas hepatitis C virus positivity and serum albumin level were
independently prognostic predictors of late intrahepatic RFS
(P<0.05). Lee et al (39)
analyzed 191 patients who underwent FDG-PET scans and subsequent
living donor LT for HCC. Based on a multivariate analysis,
PET/CT-positive status was found to be an independent prognostic
predictor for disease-free survival, which influenced early
recurrence. HCC recurrence occurred early (≤6 months) in 20 (10.5%)
patients and late (>6 months) in 18 (9.4%) patients.
Studies have evaluated the prediction ability of FDG
uptake for tumor recurrence patterns. Previous studies revealed
that patients with high SUVmax had extrahepatic
recurrence and early recurrence, which reflected the aggressiveness
of HCC (13,40). Such patients had a poor prognosis due
to the limited therapeutic options (13,41). In
the present study, MTV, TLG and UVP in pre-transplantation PET
imaging were significantly higher in the recurrence group compared
with that in the non-recurrence group, indicating that higher MTV,
TLG or UVP were associated with shorter RFS time.
In PET-negative lesions, it is challenging to
measure MTV or TLG, due to poor contrast. These patients can
therefore be grouped into two types. In type-one patients, the
tumor itself is non-discernible from the background due to the
highly differentiated HCC in 18FDG PET/CT. In the second
category, the patients received therapies such as hepatectomy or
transarterial chemoembolization (TACE), which minimize the impact
of disease progression and are the most frequently used to control
HCC while awaiting OLT (42). These
PET-negative patients had a better prognosis; a total of 22
patients with negative FDG uptake in tumors showed better prognosis
or late recurrence following OLT. However, out of the PET-weakly
positive group, 23/30 (76.7%) patients had a recurrence, while of
the PET-markedly positive group, 33/41 (80.5%) patients had a
recurrence, with a shorter mean recurrence period compared with
that in the PET-negative and PET-weakly positive groups.
The Deauville-like score can be obtained from
PET-positive patients by visual examination, which reflects the
degree of tumor malignance. In the present study, Deauville-like
score reflected the metabolic indices to some extent; a higher
Deauville-like score was correlated with a higher MTV, TLG and UVP.
For most patients with HCC, the prognosis can be predicted by
performing visual assessment for the Deauville-like score. In
patients with Deauville-like scores of 4 and 5, the metabolic
indices should be evaluated during the decision-making process. TLG
reflects the average level of glycolysis in tumors and is closer to
the concept of tumor burden by taking into account the MTV and
SUVmean in the calculation process, and had the highest
AUC. Thus, patients were grouped into low and high metabolic groups
according to TLG in combination with the Milan criteria. Patients
in the low metabolic group had a better prognosis, regardless of
whether they met the Milan criteria or not.
Based on the findings of the presents study, a
PET/CT examination prior to LT in all patients is recommended in
order to preclude those with extrahepatic metastasis. The following
metabolic criteria provide an indication for the order of priority
of candidates for OLT: i) Patients with PET-negative imaging should
be indicated for OLT regardless of the size and number of tumors;
ii) patients meeting the Milan criteria with low metabolic indices
should undergo OLT; iii) patients not meeting the Milan criteria
with low metabolic indices should be considered for OLT; iv)
patients meeting the Milan criteria but with high metabolic burden
should undergo TACE to decrease metabolic burdens prior to LT; and
v) patients not meeting the Milan criteria with high metabolic
indices should be precluded or re-evaluated following treatment to
decrease metabolic burden (19).
Moreover, a PET/CT examination is strongly recommended within 3
months of OLT in patients with high metabolic indices who received
OLT, to detect early recurrence for timely therapy.
Apart from the intrinsic limitations of any
retrospective study, there are some limitations in the present
study. Firstly, the sample size was relatively small, as only
patients who had PET/CT before and after LT were considered.
Secondly, the recurrence rate of the patients was high due to
selection bias, since some non-recurrence patients who did not
undergo PET/CT examination following LT were excluded from the
study. Thus, the association of metabolic and volume indices with
RFS or OS following LT requires further investigation in a large
sample study.
In conclusion, Deauville-like score, MTV, TLG and
UVP, the metabolic indices assessed by PET/CT, are significant
prognostic factors of patients with HCC who undergo OLT. Therefore,
combining the metabolic indices of PET/CT examinations with the
existing criteria, such as the Milan criteria, may play an
important role in identifying patients who are eligible for
successful OLT.
Acknowledgements
The authors would like to thank Dr Tianpeng Hu and
Dr Momo Sun (Department of Nuclear Medicine, Tianjin First Central
Hospital, Tianjin, China) for their assistance in PET/CT image
analysis, and Dr Jinzhen Cai (Organ Transplantation Center, Tianjin
First Central Hospital, Tianjin, China) for providing pathological
and follow-up information of the patients.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ED, conception and design, acquisition and analysis
of data, manuscript preparation; DL, analysis and interpretation of
data; LW, acquisition of data; XF, analysis and interpretation of
data; JS, conception and design, supervision of data analysis; WX,
experimental design, supervision of data analysis and manuscript
revision. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study design was approved by the Institutional
Clinical Research Ethics Committee of Tianjin First Central
Hospital (approval no. 2018N103KY). No organs from executed
prisoners were used. Each organ donated or transplanted was
allocated by the China Organ Transplant Response System. All
participants provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
RFS
|
recurrence-free survival
|
|
CI
|
confidence interval
|
|
AFP
|
α-fetoprotein
|
|
18F-FDG
|
18F-fluorodeoxyglucose
|
|
PET
|
positron emission tomography
|
|
CT
|
computed tomography
|
|
SUV
|
standardized uptake value
|
|
SUL
|
SUV normalized to lean body mass
|
|
TLR
|
tumor-to-normal-liver SUV ratio
|
|
TMR
|
tumor-to-mediastinum SUV ratio
|
|
MTV
|
metabolic tumor volume
|
|
TLG
|
total lesion glycolysis
|
|
UVP
|
uptake-volume product
|
|
MVI
|
microvascular invasion
|
|
OLT
|
orthotopic liver transplantation
|
|
TACE
|
transarterial chemoembolization
|
References
|
1
|
Yao FY, Hirose R, LaBerge JM, Davern TJ
III, Bass NM, Kerlan RK Jr, Merriman R, Feng S, Freise CE, Ascher
NL, et al: A prospective study on downstaging of hepatocellular
carcinoma prior to liver transplantation. Liver Transpl.
11:1505–1514. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mazzaferro V, Regalia E, Doci R, Andreola
S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A and
Gennari L: Liver transplantation for the treatment of small
hepatocellular carcinomas in patients with cirrhosis. N Engl J Med.
334:693–699. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yao FY, Xiao L, Bass NM, Kerlan R, Ascher
NL and Roberts JP: Liver transplantation for hepatocellular
carcinoma: Validation of the UCSF-expanded criteria based on
preoperative imaging. Am J Transplant. 7:2587–2596. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shin WY, Suh KS, Lee HW, Kim J, Kim T, Yi
NJ and Lee KU: Prognostic factors affecting survival after
recurrence in adult living donor liver transplantation for
hepatocellular carcinoma. Liver Transpl. 16:678–684. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okuno T, Tsuruyama T, Haga H, Ueda M,
Takada Y, Maetani Y, Manabe T and Tamaki K: A comparative study of
pathological staging systems in predicting recurrent hepatocellular
carcinoma after liver transplantation. J Hepatobiliary Pancreat
Surg. 16:802–807. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen J, Xu X, Wu J, Ling Q, Wang K, Wang
W, Zhang M, Shen Y, Zhou L, Xie H, et al: The stratifying value of
Hangzhou criteria in liver transplantation for hepatocellular
carcinoma. PLoS One. 9:e931282014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rodríguez-Perálvarez M, Luong TV, Andreana
L, Meyer T, Dhillon AP and Burroughs AK: A systematic review of
microvascular invasion in hepatocellular carcinoma: Diagnostic and
prognostic variability. Ann Surg Oncol. 20:325–339. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pawlik TM, Gleisner AL, Anders RA,
Assumpcao L, Maley W and Choti MA: Preoperative assessment of
hepatocellular carcinoma tumor grade using needle biopsy:
Implications for transplant eligibility. Ann Surg. 245:435–442.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee JW, Hwang SH, Kim HJ, Kim D, Cho A and
Yun M: Volumetric parameters on FDG PET can predict early
intrahepatic recurrence-free survival in patients with
hepatocellular carcinoma after curative surgical resection. Eur J
Nucl Med Mol Imaging. 44:1984–1994. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin CY, Chen JH, Liang JA, Lin CC, Jeng LB
and Kao CH: 18F-FDG PET or PET/CT for detecting
extrahepatic metastases or recurrent hepatocellular carcinoma: A
systematic review and meta-analysis. Eur J Radiol. 81:2417–2422.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee JW, Yun M, Cho A, Han KH, Kim DY, Lee
SM and Lee JD: The predictive value of metabolic tumor volume on
FDG PET/CT for transarterial chemoembolization and transarterial
chemotherapy infusion in hepatocellular carcinoma patients without
extrahepatic metastasis. Ann Nucl Med. 29:400–408. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim YI, Paeng JC, Cheon GJ, Suh KS, Lee
DS, Chung JK and Kang KW: Prediction of Posttransplantation
Recurrence of Hepatocellular Carcinoma Using Metabolic and
Volumetric Indices of 18F-FDG PET/CT. J Nucl Med.
57:1045–1051. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hwang SH, Lee JW, Cho HJ, Kim KS, Choi GH
and Yun M: Prognostic Value of Metabolic Tumor Volume and Total
Lesion Glycolysis on Preoperative 18F-FDG PET/CT in Patients With
Very Early and Early Hepatocellular Carcinoma. Clin Nucl Med.
42:34–39. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moon SH, Hyun SH and Choi JY: Prognostic
significance of volume-based PET parameters in cancer patients.
Korean J Radiol. 14:1–12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Esfahani SA, Heidari P, Halpern EF,
Hochberg EP, Palmer EL and Mahmood U: Baseline total lesion
glycolysis measured with (18)F-FDG PET/CT as a predictor of
progression-free survival in diffuse large B-cell lymphoma: A pilot
study. Am J Nucl Med Mol Imaging. 3:272–281. 2013. View Article : Google Scholar : PubMed/NCBIPubMed/NCBI
|
|
16
|
Meignan M, Gallamini A, Meignan M,
Gallamini A and Haioun C: Report on the First International
Workshop on Interim-PET-Scan in Lymphoma. Leuk Lymphoma.
50:1257–1260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mazzaferro V, Llovet JM, Miceli R, Bhoori
S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi
GL, et al Metroticket Investigator Study Group, : Predicting
survival after liver transplantation in patients with
hepatocellular carcinoma beyond the Milan criteria: A
retrospective, exploratory analysis. Lancet Oncol. 10:35–43. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kornberg A, Witt U, Schernhammer M,
Kornberg J, Ceyhan GO, Mueller K, Friess H and Thrum K: Combining
18F-FDG positron emission tomography with Up-to-seven
criteria for selecting suitable liver transplant patients with
advanced hepatocellular carcinoma. Sci Rep. 7:141762017.
|
|
19
|
Kornberg A, Schernhammer M and Friess H:
18F-FDG-PET for Assessing Biological Viability and Prognosis in
Liver Transplant Patients with Hepatocellular Carcinoma. J Clin
Transl Hepatol. 5:224–234. 2017.PubMed/NCBI
|
|
20
|
Yao FY, Ferrell L, Bass NM, Watson JJ,
Bacchetti P, Venook A, Ascher NL and Roberts JP: Liver
transplantation for hepatocellular carcinoma: Expansion of the
tumor size limits does not adversely impact survival. Hepatology.
33:1394–1403. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng SS, Xu X, Wu J, Chen J, Wang WL,
Zhang M, Liang TB and Wu LM: Liver transplantation for
hepatocellular carcinoma: Hangzhou experiences. Transplantation.
85:1726–1732. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ahn SG, Kim SH, Jeon TJ, Cho HJ, Choi SB,
Yun MJ, Lee JD and Kim KS: The role of preoperative
[18F]fluorodeoxyglucose positron emission tomography in predicting
early recurrence after curative resection of hepatocellular
carcinomas. J Gastrointest Surg. 15:2044–2052. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Shi ZL, Yang X and Yin ZF:
Targeting of circulating hepatocellular carcinoma cells to prevent
postoperative recurrence and metastasis. World J Gastroenterol.
20:142–147. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Prasad KR, Young RS, Burra P, Zheng SS,
Mazzaferro V, Moon DB and Freeman RB: Summary of candidate
selection and expanded criteria for liver transplantation for
hepatocellular carcinoma: A review and consensus statement. Liver
Transpl. 17 (Suppl 2):S81–S89. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Figueras J, Ibañez L, Ramos E, Jaurrieta
E, Ortiz-de-Urbina J, Pardo F, Mir J, Loinaz C, Herrera L,
López-Cillero P, et al: Selection criteria for liver
transplantation in early-stage hepatocellular carcinoma with
cirrhosis: Results of a multicenter study. Liver Transpl.
7:877–883. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ye YF, Wang W, Wang T, Yu J, Geng L, Yu
SF, Yan S and Zheng SS: Role of 18F fludeoxyglucose
positron emission tomography in the selection of liver
transplantation candidates in patients with hepatocellular
carcinoma. Hepatobiliary Pancreat Dis Int. 16:257–263. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takeuchi S, Rohren EM, Abdel-Wahab R, Xiao
L, Morris JS, Macapinlac HA, Hassan MM and Kaseb AO: Refining
prognosis in patients with hepatocellular carcinoma through
incorporation of metabolic imaging biomarkers. Eur J Nucl Med Mol
Imaging Jun. 44:969–978. 2017. View Article : Google Scholar
|
|
28
|
Yamamoto Y, Nishiyama Y, Kameyama R, Okano
K, Kashiwagi H, Deguchi A, Kaji M and Ohkawa M: Detection of
hepatocellular carcinoma using 11C-choline PET: Comparison with
18F-FDG PET. J Nucl Med. 49:1245–1248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bertagna F, Bertoli M, Bosio G, Biasiotto
G, Sadeghi R, Giubbini R and Treglia G: Diagnostic role of
radiolabelled choline PET or PET/CT in hepatocellular carcinoma: A
systematic review and meta-analysis. Hepatol Int. 8:493–500. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Castilla-Lièvre MA, Franco D, Gervais P,
Kuhnast B, Agostini H, Marthey L, Désarnaud S and Helal BO:
Diagnostic value of combining 11C-choline and
18F-FDG PET/CT in hepatocellular carcinoma. Eur J Nucl
Med Mol Imaging. 43:852–859. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee JD, Yang WI, Park YN, Kim KS, Choi JS,
Yun M, Ko D, Kim TS, Cho AE, Kim HM, et al: Different glucose
uptake and glycolytic mechanisms between hepatocellular carcinoma
and intrahepatic mass-forming cholangiocarcinoma with increased
(18)F-FDG uptake. J Nucl Med. 46:1753–1759. 2005.PubMed/NCBI
|
|
32
|
Seo S, Hatano E, Higashi T, Nakajima A,
Nakamoto Y, Tada M, Tamaki N, Iwaisako K, Kitamura K, Ikai I, et
al: P-glycoprotein expression affects
18F-fluorodeoxyglucose accumulation in hepatocellular
carcinoma in vivo and in vitro. Int J Oncol. 34:1303–1312.
2009.PubMed/NCBI
|
|
33
|
Pant V, Sen IB and Soin AS: Role of
18F-FDG PET CT as an independent prognostic indicator in
patients with hepatocellular carcinoma. Nucl Med Commun.
34:749–757. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee SD, Kim SH, Lee EC, Park S-J, Kim YK,
Han SS and Park HM: Expanded selection criteria of living donor
liver transplantation for hepatocellular carcinoma using total
tumor size and 18 F-FDG-PET/CT. HPB (Oxford). 18:e192016.
View Article : Google Scholar
|
|
35
|
Hong CM, Ahn BC, Jang YJ, Jeong SY, Lee SW
and Lee J: Prognostic Value of Metabolic Parameters of
18F-FDG PET/CT and Apparent Diffusion Coefficient of MRI
in Hepatocellular Carcinoma. Clin Nucl Med. 42:95–99. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin CY, Liao CW, Chu LY, Yen KY, Jeng LB,
Hsu CN, Lin CL and Kao CH: Predictive Value of 18F-FDG
PET/CT for Vascular Invasion in Patients With Hepatocellular
Carcinoma Before Liver Transplantation. Clin Nucl Med.
42:e183–e187. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ahn SY, Lee JM, Joo I, Lee ES, Lee SJ,
Cheon GJ, Han JK and Choi BI: Prediction of microvascular invasion
of hepatocellular carcinoma using gadoxetic acid-enhanced MR and
(18)F-FDG PET/CT. Abdom Imaging. 40:843–851. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Asman Y, Evenson AR, Even-Sapir E and
Shibolet O: [18F]fludeoxyglucose positron emission tomography and
computed tomography as a prognostic tool before liver
transplantation, resection, and loco-ablative therapies for
hepatocellular carcinoma. Liver Transpl. 21:572–580. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee SD, Kim SH, Kim YK, Kim C, Kim SK, Han
SS and Park SJ: (18)F-FDG-PET/CT predicts early tumor recurrence in
living donor liver transplantation for hepatocellular carcinoma.
Transpl Int. 26:50–60. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ochi H, Hirooka M, Hiraoka A, Koizumi Y,
Abe M, Sogabe I, Ishimaru Y, Furuya K, Miyagawa M, Kawasaki H, et
al: 18F-FDG-PET/CT predicts the distribution of
microsatellite lesions in hepatocellular carcinoma. Mol Clin Oncol.
2:798–804. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kitamura K, Hatano E, Higashi T, Seo S,
Nakamoto Y, Yamanaka K, Iida T, Taura K, Yasuchika K and Uemoto S:
Preoperative FDG-PET predicts recurrence patterns in hepatocellular
carcinoma. Ann Surg Oncol. 19:156–162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cascales-Campos PA, Ramírez P, Lopez V,
Gonzalez R, Saenz-Mateos L, Llacer E, Sánchez Bueno F, Robles R,
Pons JA, Capel A, et al: Prognostic value of
18-Fluorodeoxyglucose-positron emission tomography after
transarterial chemoembolization in patients with hepatocellular
carcinoma undergoing orthotopic liver transplantation. Transplant
Proc. 47:2374–2376. 2015. View Article : Google Scholar : PubMed/NCBI
|