Introduction
Breast cancer is the most common malignant tumor
among women worldwide and the second leading cause of tumor-related
death (1). The latest data show that
1,735,350 new cancer cases and 609,640 new cancer deaths are
expected in the United States by 2018 (2). In recent years, the incidence rate has
been increasing and the age of patients tends to be younger
(3). Without timely treatment,
breast cancer easily metastasize to heart, brain, lung and other
important organs, posing a serious threat to human life and health
(4). The survival rate of breast
cancer patients has improved due to the continuous progress of
surgical treatment, radiotherapy and chemotherapy and the wide
application of molecular targeting and immunotherapy, but there are
still approximately 20–30% of patients who relapse and develop
distant metastasis (5–7). The study of biomarkers can predict the
risk and metastasis of malignant tumors, which is helpful for the
treatment of breast cancer (8).
Therefore, finding molecular markers that affect the prognosis of
breast cancer is of important clinical significance.
In recent years, molecular markers of breast cancer
have become a hot topic in clinical research. It has been suggested
that KRT19 and CEACAM5 can be used as prognostic indicators for
breast cancer patients (9). MMP-2
was reported to help predict the occurrence of metastasis and death
in breast cancer patients (10).
Krüppel-like transcription factor family (KLFs), as transcription
factors with zinc finger domain, play an important role in growth
and progression of tumors (11,12).
Krüppel-like factor 9 (KLF9), one of the important members of KLFs
family (13), is highly-conserved
and widely expressed in higher mammals and human tissue (14,15).
Studies believe that KLFs are involved in the occurrence and
progression of various malignant tumors and regulate the
proliferation and differentiation of tumor cells. Their dynamic
expression is basically consistent with the change of malignant
degree of tumors (16,17). KLF9 inhibits multiple signal pathways
in glioblastoma stemness. For example, it inhibits tumor growth by
suppressing the ITGA6 in integrin pathway (18). The expression of KLF9 was decreased
in hepatocellular carcinoma, and its overexpression inhibits
migration and proliferation of hepatoma cells and accelerates
apoptosis (19). According to a
previous study (20), KLF9 may be an
inhibitor of invasive growth of breast cancer, and the expression
level of KLF9 in ‘early’ invasive cell populations, several
publicly expressed data sets and clinical breast cancer samples are
also significantly lower than that in normal tissue. However, there
are few studies on KLF9 expression in breast cancer tissue and its
correlation with prognosis.
Therefore, this study explored the relationship
between KLF9 and pathological features and prognosis of patients by
detecting expression of KLF9 in normal tissue and breast cancer
tissue.
Patients and methods
General data
Sixty-eight breast cancer patients admitted to
Ningde Hospital Affiliated to Fujian Medical University (Ningde,
China) from February 2014 to August 2015 were selected, aged
53.17±12.56 years, with height of 159.38±4.57 cm. Twenty cases were
poorly and moderately differentiated, and 48 cases were highly
differentiated. There were 35 cases at clinical stage I–II and 33
cases at stage III–IV. The specific data are shown in Table I. All patients were pathologically
diagnosed with breast cancer. Cancerous tissue (n=68) and normal
tissue (n=68) of patients were obtained as specimens. The normal
tissue was adjacent normal tissue and referred to the tissue 3 cm
away from the lesion, and there was no invasion of cancer
cells.
 | Table I.General data of breast cancer patients
(S)/[n (%)]. |
Table I.
General data of breast cancer patients
(S)/[n (%)].
| Factors | Breast cancer
patients (n=68) |
|---|
| Age (years) | 53.17±12.56 |
| Height (cm) | 159.38±4.57 |
| Weight (kg) | 50.26±10.54 |
| Menstrual state |
|
Premenopause | 29 (42.6) |
|
Postmenopause | 39 (57.4) |
| Tumor size |
| >5
cm | 15 (22.1) |
| ≤5
cm | 53 (77.9) |
| Lymph node
metastasis |
| Yes | 31 (45.6) |
| No | 37 (54.4) |
| Pathological
differentiation |
| Poor and
moderate | 20 (29.4) |
| High | 48 (70.6) |
| Clinical stage |
| I–II | 35 (51.5) |
|
III–IV | 33 (48.5) |
Inclusion criteria: no harmful habits, such as
smoking and drinking; complete clinical and pathological data;
Karnofsky score ≥60 points; clear consciousness and cooperative;
undergoing liver and kidney function, tumor markers, blood routine
and urine routine examinations within two weeks before surgery.
Exclusion criteria: liver dysfunction, severe organ
lesions; autoimmune diseases; pregnant or lactating women; mental
diseases, consciousness disorders and communication disorders.
This study was approved by the Ethics Committee of
Ningde Hospital Affiliated to Fujian Medical University, and a
detailed description of the experimental contents was explaned to
the patients. Complete informed consent forms were signed.
Detection of KLF9 mRNA expression by
quantitative real-time PCR (RT-qPCR)
The embedded tissue was cut into sections with a
thickness of 20 µm. Total RNAs were extracted with TRIzol kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.) according to
the operation instructions. The concentration and purity of RNAs
were determined by a spectrophotometer (Shanghai Mapada Instruments
Co., Ltd.) after dissolved with diethyl pyrocarbonate (DEPC)
treated water. When A260/A280 >1.8, the subsequent reactions
were carried out. cDNAs were prepared using cDNA reverse
transcription kit (Invitrogen; Thermo Fisher Scientific, Inc.).
Some were stored in a refrigerator at −80°C and the others were
treated with RT-qPCR (RT-qPCR instrument: ABI7500 Fast; and SYBR
Green RT-qPCR kit were from Applied Biosystems; Thermo Fisher
Scientific, Inc.) with a 20-µl reaction system in total.
Amplification conditions: predenaturation at 94°C for 30 sec,
denaturation at 95°C for 5 sec, then 60°C for 34 sec, for a total
of 40 cycles. Each sample was tested in 3 repeated wells, and the
experiment was repeated 3 times independently. The above operations
were strictly carried out in accordance with the instructions. In
this study, GAPDH was used as an internal reference, and the GAPDH
primer was synthesized by Shanghai Sangon Bioengineering Co., Ltd.
Forward primer: 5′-CTGGGCTACACTGACACC-3′, reverse primer:
5′-AAGTGGGTTGAGGCAATG-3′. The upstream primer and downstream primer
of KLF9 were produced by Guangzhou RiboBio Co., Ltd. Forward
primer: 5′-GGGAAACCTCCGAAAA-3′, reverse primer:
5′-CGTTCACCTGTATGCACTGTA-3′. The results were expressed with
relative quantitative method, and the relative expression of the
target gene in cancerous tissue and normal tissue was expressed by
2−∆Ct, where ∆Ct =
(CtKLF9-CtGAPDH).
Follow-up and outcome measures
Patients were followed up by hospital review and by
telephone. The survival time of patients was recorded 1 year, 2
years and 3 years after discharge. The difference of KLF9
expression level in cancerous tissue and normal tissue of breast
cancer patients was observed. According to the clinicopathological
features, the relationship between KLF9 and the pathological
features, prognosis was analyzed. The independent factors affecting
the survival of breast cancer was explored.
Statistical processing
SPSS 19.0 Software System (IBM, SPSS) was used to
perform statistical analysis on the experimental data. The counting
data were expressed as [n (%)], and Chi-square test was used to
carry out inter-group comparison. The measurement data were
expressed by mean ± SD, and the comparison between the two groups
was carried out by paired t-test. Survival analysis was carried out
with Kaplan-Meier and checked by Log-rank test. Cox regression was
used to analyze the factors affecting survival of breast cancer
patients, and P<0.05 was considered to indicate a statistically
significant difference.
Results
Comparison of KLF9 expression between
cancerous tissue and normal tissue
The expression level of KLF9 in cancerous tissue
(0.78±0.31) of breast cancer patients was significantly lower than
that in normal tissue (3.88±1.07), and the difference was
statistically significant (P<0.05) (Table II).
 | Table II.Comparison of KLF9 expression between
cancerous tissues and normal tissues (mean ± SD). |
Table II.
Comparison of KLF9 expression between
cancerous tissues and normal tissues (mean ± SD).
| Tissue | Cases | KLF9 |
|---|
| Cancerous tissue | 68 | 0.78±0.31 |
| Normal tissue | 68 | 3.88±1.07 |
| t value |
| 22.95 |
| P-value |
| <0.001 |
Relationship between pathological
features and KLF9 in cancer tissue
Expression of KLF9 in breast cancer tissue showed no
significant correlation with age, height, menstrual status, lymph
node metastasis or pathological differentiation (P>0.05).
However, it was significantly correlated with tumor size and
clinical stage (P<0.05) as shown Table III.
 | Table III.Relationship between KLF9 and
pathological features (mean ± SD). |
Table III.
Relationship between KLF9 and
pathological features (mean ± SD).
| Factors | Cases | KLF9 mRNA | t value | P-value |
|---|
| Age |
|
| 0.176 | 0.861 |
| >45
years | 42 | 0.77±0.44 |
|
|
| ≤45
years | 26 | 0.79±0.39 |
|
|
| Height |
|
| 0.088 | 0.930 |
| >160
cm | 36 | 0.76±0.48 |
|
|
| ≤160
cm | 32 | 0.77±0.45 |
|
|
| Menstrual
status |
|
| 0.569 | 0.571 |
|
Premenopause | 29 | 0.73±0.51 |
|
|
|
Postmenopause | 39 | 0.79±0.36 |
|
|
| Tumor size |
|
| 2.483 | 0.016 |
| >5
cm | 15 | 0.44±0.13 |
|
|
| ≤5
cm | 53 | 0.81±0.57 |
|
|
| Lymph node
metastasis |
|
| 0.402 | 0.689 |
|
Yes | 31 | 0.74±0.45 |
|
|
| No | 37 | 0.78±0.37 |
|
|
| Pathological
differentiation |
|
| 1.494 | 0.140 |
| Poor
and moderate | 20 | 0.68±0.38 |
|
|
|
High | 48 | 0.82±0.34 |
|
|
| Clinical stage |
|
| 5.158 | <0.001 |
|
I–II | 35 | 0.84±0.52 |
|
|
|
III–IV | 33 | 0.35±0.17 |
|
|
Relationship between KLF9 and survival
rate of patients
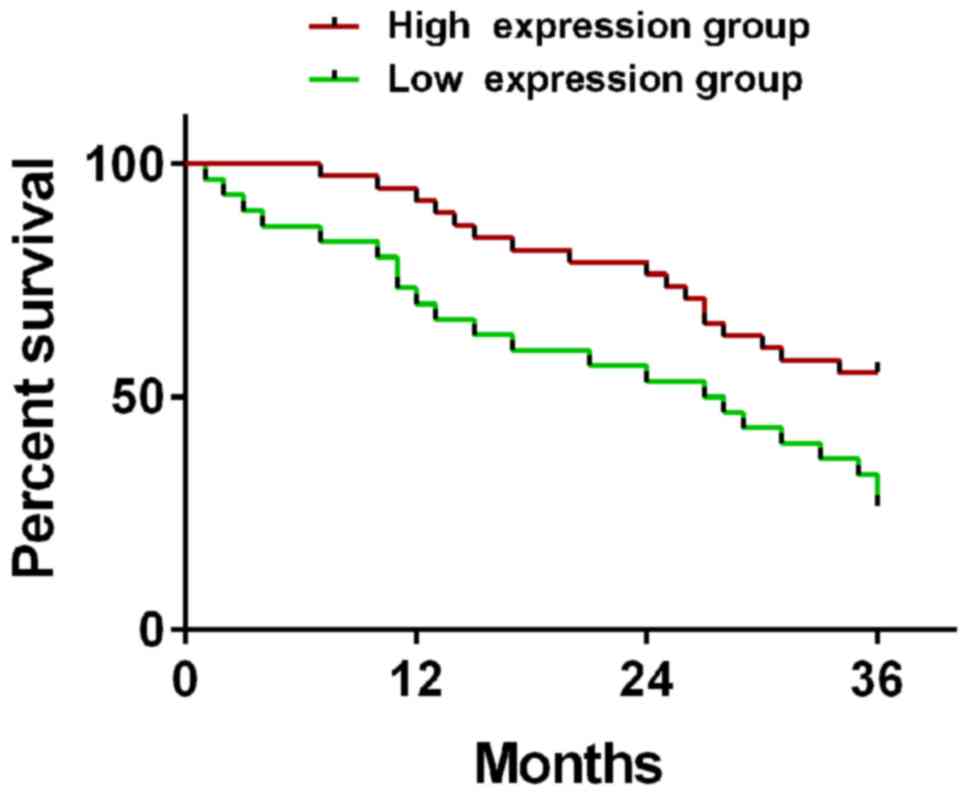
The patients were divided into high expression group
(≥0.78) and low expression group (<0.78) according to the
expression level of KLF9. The 1-, 2-, and 3-year survival rates of
patients in the high expression group (n=38) were 92.1, 76.3, and
55.3%, respectively, while those in the low expression group (n=30)
were 70.0, 53.3, and 26.7%, respectively. Therefore, the 1-, 2-,
and 3-year survival rates in the high expression group were
significantly higher than those in the low expression group
(t=15.72, 11.55, 16.21; P<0.001) (Fig. 1).
Cox regression analysis on survival
and death of breast cancer
Univariate Cox regression analysis on collected
3-year survival showed that tumor size (P=0.009), lymph node
metastasis (P=0.002), pathological differentiation (P=0.015),
clinical stage (P=0.013) and KLF9 (P=0.018) were the factors
affecting the survival of breast cancer patients. Multivariate Cox
regression analysis was carried out, and tumor size >5 cm, lymph
node metastasis, low pathological differentiation, high clinical
stage and low expression of KLF9 were important factors causing
death of patients (P<0.05), as shown in Tables IV, V
and VI.
 | Table IV.Univariate analysis of patient
survival [n (%)]. |
Table IV.
Univariate analysis of patient
survival [n (%)].
| Factors | Death group
(n=39) | Survival group
(n=29) | t value | P-value |
|---|
| Age |
|
| 0.212 | 0.646 |
| >45
years | 25 (64.1) | 17 (58.6) |
|
|
| ≤45
years | 14 (35.9) | 12 (41.4) |
|
|
| Height |
|
| 0.101 | 0.751 |
| >160
cm | 20 (51.3) | 16 (55.2) |
|
|
| ≤160
cm | 19 (48.7) | 13 (44.8) |
|
|
| Menstrual
status |
|
| 1.378 | 0.240 |
|
Premenopause | 19 (48.7) | 10 (34.5) |
|
|
|
Postmenopause | 20 (51.3) | 19 (65.5) |
|
|
| Tumor size |
|
| 6.761 | 0.009 |
| >5
cm | 13 (33.3) | 2 (6.9) |
|
|
| ≤5
cm | 26 (66.7) | 27 (93.1) |
|
|
| Lymph node
metastasis |
|
| 9.379 | 0.002 |
|
Yes | 24 (61.5) | 7
(24.1) |
|
|
| No | 15 (38.5) | 22 (75.9) |
|
|
| Pathological
differentiation |
|
| 5.941 | 0.015 |
| Poor
and moderate | 16 (41.0) | 4
(13.8) |
|
|
|
High | 23 (59.0) | 25 (86.2) |
|
|
| Clinical stage |
|
| 6.196 | 0.013 |
|
I–II | 15 (38.5) | 20 (69.0) |
|
|
|
III–IV | 24 (61.5) | 9
(31.0) |
|
|
| KLF9 |
|
| 5.605 | 0.018 |
| High
expression | 17 (43.6) | 21 (72.4) |
|
|
| Low
expression | 22 (56.4) | 8
(27.6) |
|
|
 | Table V.Assignments. |
Table V.
Assignments.
| Factors | Assignment |
|---|
| Tumor size | >5 cm: 1; ≤5 cm:
2 |
| Lymph node
metastasis | Yes: 1, No: 2 |
| Pathological
differentiation | Poor and moderate:
1, High: 2 |
| Clinical stage | I–II: 1; III–IV:
2 |
| KLF9 | High expression
(≥0.78): 1, low expression (<0.78): 2 |
 | Table VI.Multivariate analysis of factors
affecting survival. |
Table VI.
Multivariate analysis of factors
affecting survival.
| Factors | β | SD | χ2
value | P-value | HR (95% CI) |
|---|
| Tumor size | 1.849 | 0.933 | 3.927 | 0.048 | 6.354
(1.020–39.565) |
| Lymph node
metastasis | 2.062 | 0.727 | 8.048 | 0.005 | 7.862
(1.892–32.679) |
| Pathological
differentiation | 2.359 | 0.883 | 7.141 | 0.008 | 10.576
(1.875–59.647) |
| Clinical stage | −1.508 | 0.687 | 4.817 | 0.028 | 0.221
(0.058–0.851) |
| KLF9 | −1.727 | 0.748 | 5.325 | 0.021 | 0.178
(0.041–0.771) |
Discussion
Breast cancer is a malignant tumor type that is more
common in women (21). The main
cause is that breast epithelial cells proliferate abnormally under
the stimulation of various internal and external carcinogenic
factors, thus exceeding the limit of self-repair and leading to
carcinogenesis (22). Its main
manifestation is abnormal proliferation of cancer cells, which
destroys surrounding normal tissue and changes the normal structure
of the breast. At present, there is no effective method to cure
breast cancer clinically. In order to achieve early diagnosis and
treatment of breast cancer and improve the survival rate, it is of
great significance to find biomarkers for its prognosis (23).
KLF9 has been found to have a low expression in
endometrial cancer and colorectal cancer (24,25). It
regulates genes mainly by binding DNA with its 3 highly conserved
and classical Cys2/His2 zinc finger structures at carboxyl terminal
(26). KLF9 inhibits Notch1 promoter
directly indicated by chromatin immunoprecipitation (ChIP) assay
and luciferase reporter gene assay. Moreover, it also regulates
neurosphere cells in human glioblastomas by binding to Notch1
promoter and inhibiting Notch1 expression and downstream signal
transduction, indicating that KLF9 has differentiation and tumor
inhibition functions in tumor initiating cells (27). Moreover, a study has shown that KLF9
inhibits the migration and invasion of breast cancer cells. It is
negatively correlated with the expression of matrix
metalloproteinase-9 (MMP9), so it inhibits the invasion of cancer
cells by downregulating the expression and activity of MMP9
(28).
In this study, the results of RT-qPCR showed that
the KLF9 expression in cancerous tissue of breast cancer patients
was significantly lower than that in normal tissue. However, some
studies have shown that KLF9 has low expression in liver cancer
(29) and prostate cancer (30). Hosseini et al (31) found that KLF9 has low expression in
infiltrating cell lines and breast cancer tissue, which is
consistent with the results of this study. These results indicate
that KLF9 plays the role of tumor suppressor gene in the occurrence
and progression of tumors. The results of a previous study showed
that KLF9 inhibits cancer cell invasion, which was confirmed
through differences in expression between invasive and noninvasive
cell line, implicating the interaction between proliferation and
invasion; the results provide the first evidence that KLF9 is an
invasive growth inhibitor of breast cancer (20). In this study, the relationship
between KLF9 and pathological features of breast cancer patients
was studied, and it was found that the expression of KLF9 in
cancerous tissue was not significantly correlated with age, height,
menstrual status, lymph node metastasis and pathological
differentiation of patients (P>0.05), but was significantly
correlated with tumor size and clinical stage (P<0.05). It
indicates that the production of tumor inhibits the expression of
KLF9, and the expression of KLF9 decreases gradually with the
severity of the disease. The research of Chen et al
(32) showed that KLF9 is correlated
with lymph node metastasis, peritoneal effusion and clinical stage
of ovarian cancer. The lower the KLF9 level, the more serious the
disease is, lymph node metastasis is more likely, and the amount of
peritoneal effusion increases. The expression of KLF9 in pancreatic
cancer is closely related to the tumor differentiation and vascular
invasion, but has no correlation with gender, age, tumor location,
TNM stage, nerve invasion and lymph node metastasis (33), suggesting that KLF9 can reflect the
conditions of a variety of disease. Limame et al (20) demonstrated that KLF9 is significantly
downregulated in breast cancer compared with that in normal breast
epithelium, and its expression has no correlation with estrogen
receptor (ER), progesterone receptor (PR) or human epidermal growth
factor receptor-2 (HER-2). The results show that the downregulation
of the expression of this transcription factor is
receptor-independent in malignant transformation of breast
epithelial cells. The survival analysis in this study showed that
the 1-, 2-, and 3-year survival rates in KLF9 high expression group
were significantly higher than those in the low expression group.
The reason may be that KLF9 inhibits cell proliferation (31). Therefore, it is speculated that KLF9
expression is downregulated in breast cancer and may be closely
related to poor prognosis, suggesting that KLF9 can be used to
predict the survival of breast cancer patients. Multivariate Cox
regression analysis found that tumor size >5 cm, lymph node
metastasis, low pathological differentiation and high clinical
stage are all important factors that cause death of breast cancer
patients. Blanchette et al (34) found that HER-2 status, number at
first recurrence and metastasis to organs, number of lymph node
metastasis and treatment methods are independent prognostic factors
for patients with recurrent and metastatic breast cancer. Moreover,
a study showed that tumor size and lymph node metastasis number
were independent risk factors affecting the prognosis of breast
cancer patients (35). This study
found that low expression of KLF9 mRNA was one of the independent
prognostic indicators of breast cancer, which is similar to the
results of Chen et al (32)
on KLF9 in the prognosis of ovarian cancer. However, the mechanism
of KLF9 in the occurrence and progression of breast cancer needs to
be further studied.
This study comprehensively expounded the expression
of KLF9 in breast cancer and its relationship with prognosis. It
provides a good biomarker for the progression of breast cancer.
qPCR measurement has high sensitivity, wide linear range of
detection, good detection accuracy and good repeatability.
Moreover, the detection cost is low, and it is easy to operate.
Collectively, according to the results of RT-qPCR,
KLF9 expression in breast cancer tissue, and its expression level
is low related to tumor size and clinical stage. Moreover, tumor
size >5 cm, lymph node metastasis, low pathological
differentiation, high clinical stage and low expression of KLF9 are
all important factors that cause death of patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZJ conceived the study and wrote the manuscript. ZX
analyzed and interpreted the patient general data. ZX, ZJ, TH and
BS performed PCR. FL and KW were responsible for observation
indicators analysis. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Ningde Hospital Affiliated to Fujian Medical University (Ningde,
China). Patients who participated in this research had complete
clinical data. Signed informed consents were obtained from the
patients and/or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends - an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Taitt HE: Global Trends and prostate
cancer: A Review of incidence, detection, and mortality as
influenced by race, ethnicity, and geographic location. Am J Men
Health. 12:1807–1823. 2018. View Article : Google Scholar
|
|
3
|
Curado MP, Oliveira MM, Silva DRM and
Souza DLB: Epidemiology of multiple myeloma in 17 Latin American
countries: An update. Cancer Med. 7:2101–2108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boloker G, Wang C and Zhang J: Updated
statistics of lung and bronchus cancer in United States (2018). J
Thorac Dis. 10:1158–1161. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leithner D, Horvat JV, Ochoa-Albiztegui
RE, Thakur S, Wengert G, Morris EA, Helbich TH and Pinker K:
Imaging and the completion of the omics paradigm in breast cancer.
Radiologe. 58 (Suppl 1):7–13. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suppan C and Balic M: Early stage breast
cancer treatment and prognostic factors: Post San Antonio Breast
Cancer Symposium 2016. Memo. 10:82–85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), : Effects of chemotherapy and
hormonal therapy for early breast cancer on recurrence and 15-year
survival: An overview of the randomised trials. Lancet.
365:1687–1717. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feng Y, Spezia M, Huang S, Yuan C, Zeng Z,
Zhang L, Ji X, Liu W, Huang B, Luo W, et al: Breast cancer
development and progression: Risk factors, cancer stem cells,
signaling pathways, genomics, and molecular pathogenesis. Genes
Dis. 5:77–106. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang XM, Zhang Z, Pan LH, Cao XC and Xiao
C: KRT19 and CEACAM5 mRNA-marked circulated tumor cells indicate
unfavorable prognosis of breast cancer patients. Breast Cancer Res
Treat. 174:375–385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ramos EAS, Silva CT, Manica GC, Pereira
IT, Klassen LM, Ribeiro EM, Cavalli IJ, Braun-Prado K, Lima RS,
Urban CA, et al: Worse prognosis in breast cancer patients can be
predicted by immunohistochemical analysis of positive MMP-2 and
negative estrogen and progesterone receptors. Rev Assoc Med Bras
(1992). 62:774–781. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao Y, Cao Q, Lu L, Zhang X, Zhang Z, Dong
X, Jia W and Cao Y: Kruppel-like factor family genes are expressed
during Xenopus embryogenesis and involved in germ layer formation
and body axis patterning. Dev Dyn. 244:1328–1346. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kerr K, Qualmann K, Esquenazi Y, Hagan J
and Kim DH: Familial syndromes involving meningiomas provide
mechanistic insight into sporadic disease. Neurosurgery.
83:1107–1118. 2018.PubMed/NCBI
|
|
13
|
Cho N: Molecular subtypes and imaging
phenotypes of breast cancer. Ultrasonography. 35:281–288. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Apara A, Galvao J, Wang Y, Blackmore M,
Trillo A, Iwao K, Brown DP Jr, Fernandes KA, Huang A, Nguyen T, et
al: KLF9 and JNK3 interact to suppress axon regeneration in the
adult CNS. J Neurosci. 37:9632–9644. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yılmaz TU, Trabzonlu L, Güler SA, Baran
MA, Pösteki G, Erçin C and Utkan Z: Characteristics of special type
breast tumors in our center. Eur J Breast Health. 14:17–22. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song JL, Chen C, Yuan JP and Sun SR:
Progress in the clinical detection of heterogeneity in breast
cancer. Cancer Med. 5:3475–3488. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Noguchi K, Eguchi H, Konno M, Kawamoto K,
Nishida N, Koseki J, Wada H, Marubashi S, Nagano H, Doki Y, et al:
Susceptibility of pancreatic cancer stem cells to reprogramming.
Cancer Sci. 106:1182–1187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ying M, Tilghman J, Wei Y,
Guerrero-Cazares H, Quinones-Hinojosa A, Ji H and Laterra J:
Kruppel-like factor-9 (KLF9) inhibits glioblastoma stemness through
global transcription repression and integrin α6 inhibition. J Biol
Chem. 289:32742–32756. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fu DZ, Cheng Y, He H, Liu HY and Liu YF:
The fate of Krüppel-like factor 9-positive hepatic carcinoma cells
may be determined by the programmed cell death protein 5. Int J
Oncol. 44:153–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Limame R, de Beeck KO, Van Laere S, Croes
L, De Wilde A, Dirix L, Van Camp G, Peeters M, De Wever O, Lardon
F, et al: Expression profiling of migrated and invaded breast
cancer cells predicts early metastatic relapse and reveals
Krüppel-like factor 9 as a potential suppressor of invasive growth
in breast cancer. Oncoscience. 1:69–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Menyhárt O, Fekete JT and Győrffy B:
Demographic shift disproportionately increases cancer burden in an
aging nation: Current and expected incidence and mortality in
Hungary up to 2030. Clin Epidemiol. 10:1093–1108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vangangelt KMH, van Pelt GW, Engels CC,
Putter H, Liefers GJ, Smit VTHBM, Tollenaar RAEM, Kuppen PJK and
Mesker WE: Prognostic value of tumor-stroma ratio combined with the
immune status of tumors in invasive breast carcinoma. Breast Cancer
Res Treat. 168:601–612. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dieci MV, Vernaci G and Guarneri V:
Escalation and de-escalation in HER2 positive early breast cancer.
Curr Opin Oncol. 31:35–42. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kang L, Lü B, Xu J, Hu H and Lai M:
Downregulation of Krüppel-like factor 9 in human colorectal cancer.
Pathol Int. 58:334–338. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang J, Galvao J, Beach KM, Luo W, Urrutia
RA, Goldberg JL and Otteson DC: Novel roles and mechanism for
Krüppel-like factor 16 (KLF16) regulation of neurite outgrowth and
ephrin receptor A5 (EphA5) expression in retinal ganglion cells. J
Biol Chem. 291:18084–18095. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kang L and Lai MD: BTEB/KLF9 and its
transcriptional regulation. Yi Chuan. 29:515–522. 2007.(In
Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ying M, Sang Y, Li Y, Guerrero-Cazares H,
Quinones-Hinojosa A, Vescovi AL, Eberhart CG, Xia S and Laterra J:
Krüppel-like family of transcription factor 9, a
differentiation-associated transcription factor, suppresses Notch1
signaling and inhibits glioblastoma-initiating stem cells. Stem
Cells. 29:20–31. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bai XY, Li S, Wang M, Li X, Yang Y, Xu Z,
Li B, Li Y, Xia K, Chen H, et al: Krüppel-like factor 9
down-regulates matrix metalloproteinase 9 transcription and
suppresses human breast cancer invasion. Cancer Lett. 412:224–235.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun J, Wang B and Liu Y, Zhang L, Ma A,
Yang Z, Ji Y and Liu Y: Transcription factor KLF9 suppresses the
growth of hepatocellular carcinoma cells in vivo and positively
regulates p53 expression. Cancer Lett. 355:25–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shen P, Sun J, Xu G, Zhang L, Yang Z, Xia
S, Wang Y, Liu Y and Shi G: KLF9, a transcription factor induced in
flutamide-caused cell apoptosis, inhibits AKT activation and
suppresses tumor growth of prostate cancer cells. Prostate.
74:946–958. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hosseini MK, Gunel T, Gumusoglu E, Benian
A and Aydinli K: MicroRNA expression profiling in placenta and
maternal plasma in early pregnancy loss. Mol Med Rep. 17:4941–4952.
2018.PubMed/NCBI
|
|
32
|
Chen Y, Liu X, Li Y, Quan C, Zheng L and
Huang K: Lung cancer therapy targeting histone methylation:
opportunities and challenges. Comput Struct Biotechnol J.
16:211–223. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xie VK, Li Z, Yan Y, Jia Z, Zuo X, Ju Z,
Wang J, Du J, Xie D, Xie K, et al: DNA-methyltransferase 1 induces
dedifferentiation of pancreatic cancer cells through silencing of
Krüppel-Like factor 4 expression. Clin Cancer Res. 23:5585–5597.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Blanchette PS, Desautels DN, Pond GR,
Bartlett JMS, Nofech-Mozes S, Yaffe MJ and Pritchard KI: Factors
influencing survival among patients with HER2-positive metastatic
breast cancer treated with trastuzumab. Breast Cancer Res Treat.
170:169–177. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu T, Li D, He Y, Zhang F, Qiao M and Chen
Y: The expression level of CSDAP1 in lung cancer and its clinical
significance. Oncol Lett. 16:4361–4366. 2018.PubMed/NCBI
|