Introduction
Colon cancer is a common gastrointestinal cancer.
More than 900,000 patients are diagnosed with colon cancer each
year, and its incidence is the third among all cancers (1). Colon cancer is ranked third of
cancer-related deaths (2). In most
colon cancer patients, the spread of tumor cells is the main cause
of death of patients. Most patients with stage I and II colon
cancer can be cured by surgical resection. Approximately 70% of
patients with colon cancer with stage III lymph node metastasis can
be cured by surgery combined with adjuvant chemotherapy. It is
difficult to cure advanced metastatic colon cancer (stage IV)
(3). Despite significant advances in
surgical techniques and adjuvant chemotherapy, the survival rate of
colon cancer patients has only modest improvement due to the high
recurrence rate of colon cancer and advanced colon cancer at the
time of diagnosis (2,4). Therefore, the pathogenesis of
colorectal cancer needs to be further explored.
Studies have shown that diabetes, especially type 2
diabetes, can increase the risk of cancer and death, especially in
gastrointestinal cancers (5). Type 2
diabetes is an independent risk factor for colon cancer, and colon
cancer patients with diabetes have a worse prognosis than those
without diabetes (6). In addition,
in diabetic patients, the incidence of colon cancer increased by
1.27-1.40 times (7). Moreover,
patients with colon cancer and type 2 diabetes had a 5-year
survival rate reduction of 42% and a 21% increase in colon cancer
recurrence (8). However, the reasons
for this increase in risk are unclear. Some scholars have suggested
that the increase in the level of free insulin growth factor 1
(IGF1) may promote the proliferation of colon cancer cells
(9,10). However, the specific regulatory
mechanisms are still unclear.
In recent years, most human genome transcripts have
been transcribed into non-coding RNAs, such as small non-coding
RNAs (miRNAs). miRNAs do not encode any protein, but can regulate
biological processes by inhibiting the expression level of target
gene mRNA, thereby regulating the occurrence and progression of the
disease (11–13). Studies have shown that miRNAs are
differentially expressed in a variety of cancers, and
differentially expressed miRNAs can be involved in the development
and progression of cancer (14–17),
including colon cancer (18–20). For example, the expression level of
miR-3653 is significantly downregulated in colon cancer tissues and
cells, and the increased expression level of miR-3653 can
significantly inhibit the migration and invasion of colon cancer
cells by inhibiting the expression level of target gene Zeb2. It
also inhibits the epithelial-mesenchymal transition (ERT) of colon
cancer cells (18). miR-223-3p,
which is significantly upregulated in colon cancer tissues, can
promote colon cancer cell proliferation, invasion and migration by
inhibiting the target gene PRDM1 expression level (19). Among miRNAs, miR-98 is differentially
expressed in a variety of cancers and can regulate a variety of
cancer cell phenotypes. For example, miR-98, which is downregulated
in breast cancer tissues and cell lines, can further regulate
proliferation, invasion and migration ability of breast cancer
cells by targeting the expression level of the target gene Gab2
(GRB2-associated-binding protein 2) (21). miR-98 is downregulated in the
expression of squamous cell carcinoma of the head and neck can also
regulate the proliferation and invasion of cancer cells (22). In addition, studies have found that
miR-98 expression levels are downregulated in colon cancer and can
be used as tumor suppressors to regulate the Warburg effect of
colon cancer cells (23). However,
the current regulatory role and mechanism of miR-98 in patients
with diabetes mellitus complicated with colon cancer is not clear.
Therefore, in this experiment, we mainly explored the differential
expression of miR-98 and its mechanism of regulation in colon
cancer combined with type 2 diabetes mellitus.
Materials and methods
Tissue sample collection and cell
culture
Tumor tissues of 40 patients with type 2 diabetes
mellitus complicated with colon cancer and 40 colon cancer patients
were collected between January 2017 and January 2018. Of the 40
patients with type 2 diabetes mellitus and colon cancer, 21 were
male and 21 were female, aged of 61.7±5.9 years. Of the 40 colon
cancer patients, 22 were male and 18 were female, aged 63.1±6.5
years. Diagnostic standard for type 2 diabetes was based on 1999
World Health Organization (WHO) criteria for the diagnosis of type
2 diabetes: i) patients with a history of significant diabetes; ii)
oral glucose tolerance test 2 h blood glucose ≥11.1 mmol/l.
Patients with other types of diabetes and those with a history of
hyperthyroidism and glucocorticoid use were excluded. Colon cancer
patients were diagnosed by histopathology and did not receive any
treatment. Patients with diabetes mellitus combined with colon
cancer and colon cancer patients were in stage T3-T4 of colon
cancer. Informed consent was obtained from all the patients. The
experimental protocol was approved by the Ethics committee of The
First Hospital of Lanzhou University.
The human colon cancer cell line SW480 from ATCC
(American Type Culture Collection) was purchased from Beijing
Zhongyuan Heju Economic and Trading Co., Ltd. SW480 was cultured in
DMEM medium supplemented with 10% fetal bovine serum, and 100 U/ml
penicillin and 100 µg/ml streptomycin were added to prevent cell
contamination. SW480 cells were cultured at 37°C, in 5%
CO2 cell culture incubator. SW480 cells were divided
into two groups, one group was cultured using conventional medium,
and the other group was cultured using medium supplemented with 25
mM glucose (24,25).
Cell transfection
The human colon cancer SW480 cells in logarithmic
growth phase were prepared into cell suspensions, and the cells
were inoculated in 6-well plates at about 6×105 cells
per well. The 6-well plates were placed at 37°C culture chamber
with 5% CO2 continued for 24 h. The control mimic,
miR-98 mimic (miR-98 mimic) or miR-98 mimic and IGF1R
overexpression plasmid (pCDNA-IGF1R) were then transfected into
cells alone using the lipofection reagent Lipofectamine 3000. After
72 h of transfection, the transfected SW480 cells were collected
for subsequent experiments.
Real-time QPCR
Total RNA in tumor tissues of diabetic colon cancer
patients, adjacent normal tissues, colon cancer SW480 cells, and
human normal colon epithelial cells NCM460 were extracted using
TRIzol reagent. Using the extracted RNA as a template, a reverse
transcription reaction was carried out using a reverse
transcription kit (Revert Aid RT Reverse Transcription kit, K1691,
Thermo Fisher Scientific, Inc.) to obtain cDNA. Then, cDNA was used
as a template, and the expression level of miR-98 was detected by
PCR reaction using a SYBR Green Master Mix kit (4385618, Thermo
Fisher Scientific, Inc.) in the Mx3000P Real-Time PCR system, and
U6 was used as an internal reference. The miR-98 primer sequence
was: upstream primer, 5′-GGACTGAGGTAGTAAGTTG-3′; downstream primer,
5′-CATCAGATGCGTTGCGTA-3′ (26). The
U6 primer sequence was: upstream primer, 5′-GCTTCGGCA
GCACATATACTAAAAT-3′; downstream primer, 5′-CGC
TTCACGAATTTGCGTGTCAT-3′. The PCR reaction procedure was: 1 cycle of
(9°C, 5 min), 42 cycles of (92°C, 15 sec, 60°C, 15 sec, 72°C, 30
sec). The relative expression levels of miR-98 were analyzed using
the 2−ΔΔCt method.
MTT experiment
SW480 cells in the logarithmic growth phase were
made into cell suspensions and seeded into 96-well plates at a
seeding density of 5×103 cells per well, and then placed
at 37°C, in 5% CO2 cell culture incubator and cultured
for 3 days. After the completion of the culture, 20 µl of a 5 mg/ml
MTT reagent was added to each well of a 96-well plate, and then the
96-well plate was further cultured in a cell culture incubator
containing 5% CO2 at 37°C for 4 h. After 4 h, 150 µl of
the reagent was added to each well of a 96-well plate to dissolve
the crystals. Finally, the absorbance was measured at a wavelength
of 570 nm.
Transwell cell invasion assay
The upper chamber of the Transwell chamber for cell
invasion experiments was first uniformly coated with 50 µl of
Matrigel, and DMEM containing 10% fetal calf serum was added to the
lower chamber of the Transwell chamber. Then, a well-grown SW480
cell suspension was inoculated into the upper chamber of the
Transwell chamber, and the inoculated cell density was
1×105. SW480 cells in the upper chamber were placed at
37°C, in 5% CO2 cell incubator and cultured with
serum-free medium for 24 h. After the completion of the culture,
SW480 cells on the surface of the membrane were washed, and the
remaining SW480 cells were fixed with 4% paraformaldehyde and
stained with 0.1% crystal violet for 10 min. Finally, five regions
were randomly selected from the microscope field to count the
invasive SW480 cells.
Bioinformatics methods to screen
miR-98 target genes
In order to find potential target genes for miR-98,
bioinformatics software miRWalk database (http://www.ma.uni-heidelberg.de/apps/zmf/mirwalk/)
miRanda (http://www.microrna.org/microrna/getExprForm.do),
miRDB (http://mirdb.org/), miRNAMAP (http://mirnamap.mbc.nctu.edu.tw/) and TargetScan
(http://www.targetscan.org) were used for analysis.
Screening principle: miR-98 was complementary binding to the target
gene locus; miR-98 target gene locus did not contain a complex
secondary structure, binding site was highly conserved, miR-98 and
target gene mRNA were highly thermostable. According to the
predicted results, the IGF1R gene may be a target gene of
miR-98.
Dual luciferase assay
The target gene of miR-98 was verified using a dual
luciferase assay. The IGF1R gene wild-type 3′-untranslated region
(3′-UTR) and mutant 3′-UTR were constructed into the pmiRGLO vector
(Promega). The vector carrying the wild-type 3′-UTR and mutant
3′-UTR of the IGF1R gene was then transfected into SW480 cells
using the lipofection reagent Lipofectamine 3000, respectively, and
then the wild-type 3′-UTR SW480 cells were divided into two
subgroups, one subgroup transfected the miR-98 mimetic, and the
other subgroup transfected the control mimic, and the mutant 3′-UTR
SW480 cells were also divided into two subgroups, one subgroup
transfected with miR-98 mimics, another subgroup was transfected
with control mimics. After 48 h of transfection, the relative
luciferase activity in colon cancer cells was measured using a
microplate reader.
Western blot analysis
Total protein in colon cancer SW480 cells was
extracted using RIPA lysate. The total protein concentration after
extraction was quantified using a BCA protein quantification kit.
Lysate (40 µg) was separated using 12% SDS-PAGE and transferred to
a PVDF membrane. The PVDF membrane was blocked with 4% skim milk
powder for 1 h, and the mouse anti-human IGF1R protein antibody
(ab16890, Abcam) (dilution, 1:500) or mouse anti-human GAPDH
protein antibody (ab8245, Abcam) (dilution, 1:500) was incubated
overnight at 4°C with PVDF membrane, then incubated with HRP-linked
rabbit anti-mouse IgG secondary antibody (ab6728, Abcam) (dilution,
1:2000) under 37°C condition. In this experiment, GAPDH protein was
used as an internal reference protein. Finally, color development
was performed using an ECL chemiluminescence chromogenic kit, and
imaging was performed using a ChemiDoc MP chemiluminescence imaging
system. Western blot bands were quantified using ImageJ
software.
Statistical analysis
Statistical analysis was performed using SPSS 22.0,
and the data was expressed using mean ± standard deviation.
Differences between the two groups were analyzed using an
independent sample t-test. P<0.05 was considered a statistically
significant difference.
Results
miR-98 is downregulated in tumor
tissues and colon cancer cell lines of patients with diabetes
mellitus complicated with colon cancer
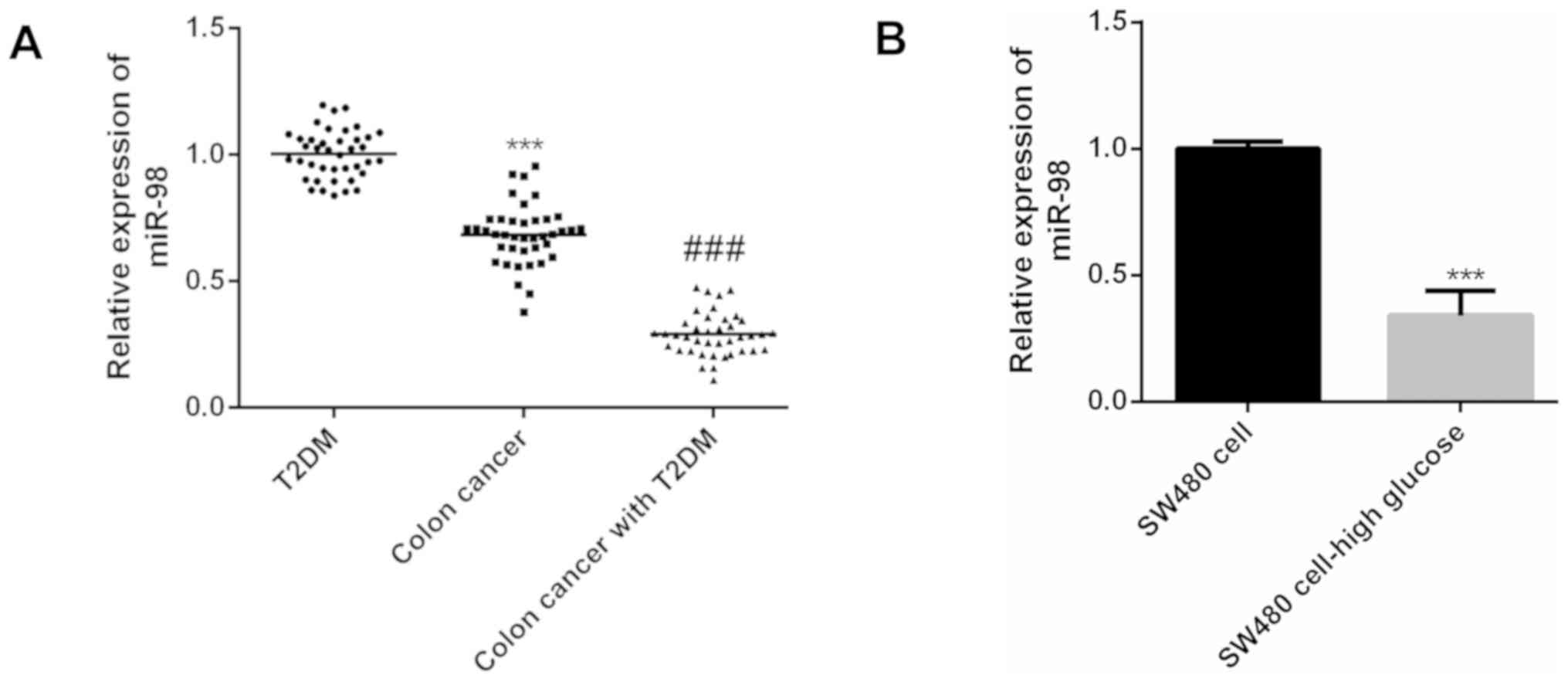
According to the results of real-time PCR, the
expression level of miR-98 in colon cancer patients was
significantly lower than that in diabetic patients (P<0.001),
compared with colon cancer patients, the expression level of miR-98
in patients with diabetes and colon cancer was significantly
decreased (P<0.001) (Fig. 1A).
Compared with human colon cancer SW480 cells cultured under normal
conditions, miR-98 was also significantly decreased in colon cancer
cell line SW480 cultured under high glucose conditions (P<0.01)
(Fig. 1B). Therefore, miR-98 has a
decreased expression level in tumor tissues of diabetic patients
with colon cancer and colon cancer cell lines under high glucose
conditions.
Upregulation of miR-98 expression
inhibits colon cancer cell proliferation and invasion
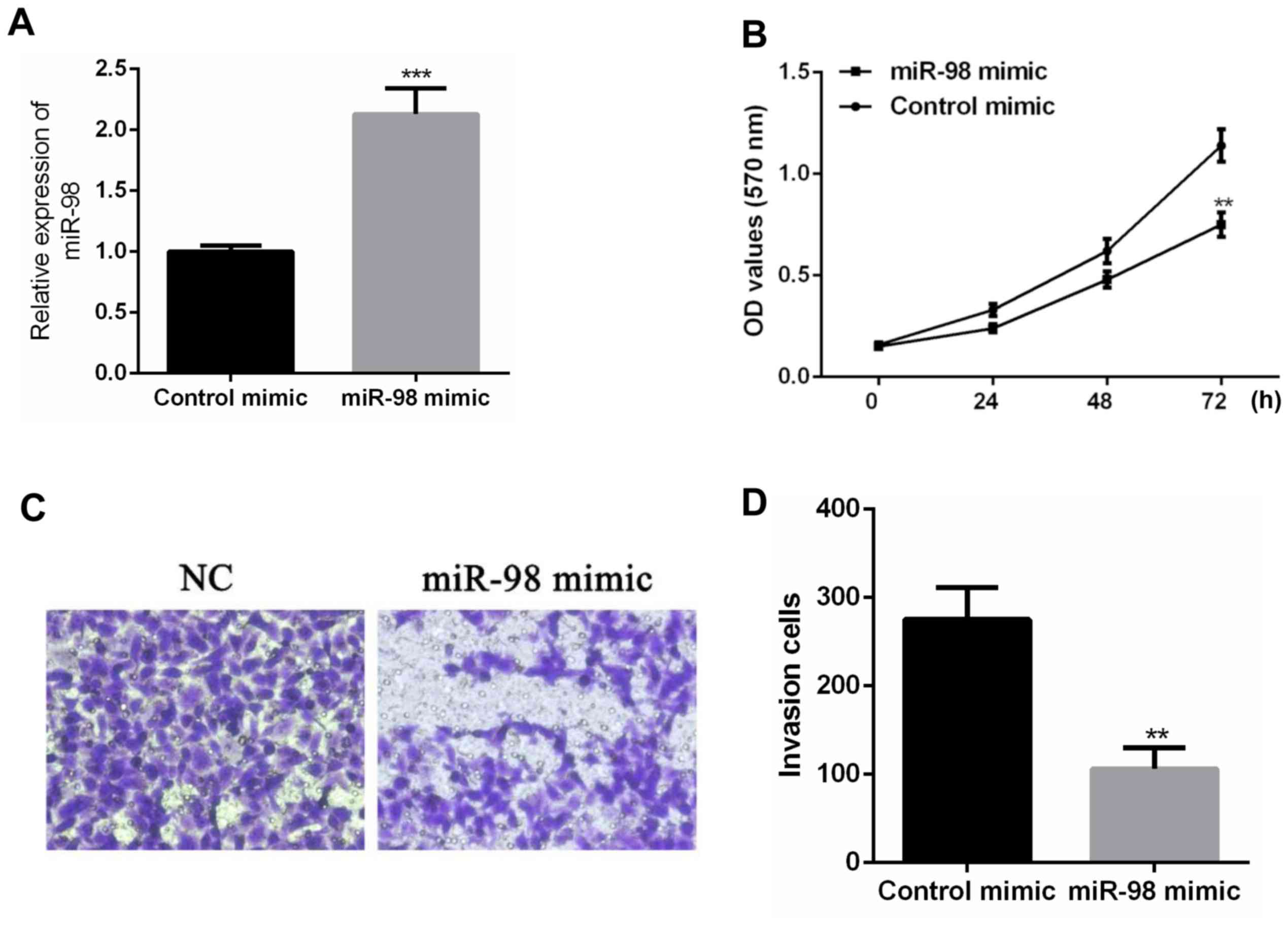
After transfecting control mimic and miR-98 mimic
into SW480 cells, real-time PCR was performed. According to the
results, miR-98 mimic can upregulate the expression level of miR-98
in SW48 cells compared with SW480 cells transfected with control
mimic (P<0.001) (Fig. 2A). After
the expression level of miR-98 was upregulated, the proliferation
of SW480 cells was detected. According to the results of MTT assay,
the upregulation of miR-98 significantly inhibited cell
proliferation compared with the control mimic group (P<0.01)
(Fig. 2B). Transwell cell invasion
assay showed that the invasive ability of SW480 cells was
significantly decreased (P<0.01) after upregulation of miR-98
expression compared with control mimic group (Fig. 2C). According to the above results,
the upregulation of miR-98 expression can inhibit the proliferation
and invasion of colon cancer cells.
miR-98 targets inhibition of its
target gene IGF1R expression level
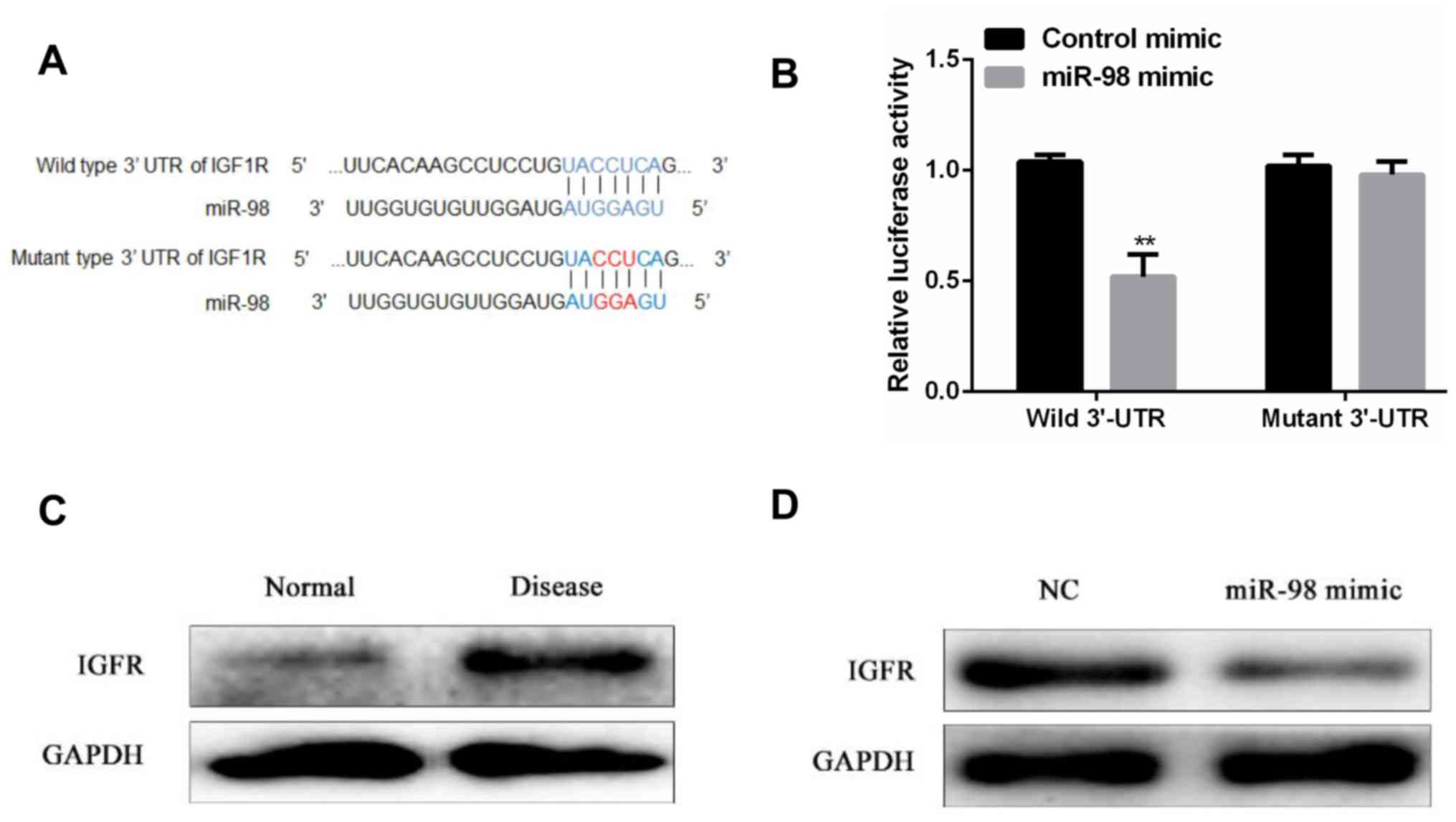
It is predicted by the bioinformatics database that
IGF1R may be a target gene of miR-98, and the potential binding
sequence of miR-98 and IGF1R 3′-UTR is shown in Fig. 3A. Dual luciferase assay was performed
to further verify whether there is a targeted binding relationship
between miR-98 and IGF1R. The results showed that there was no
significant difference in relative luciferase activity between the
miR-98 mimic group and the control group in the SW480 cells
transfected with the IGF1R mutant 3′-UTR, contrary to the SW480
cells transfected with the IGF1R wild-type 3′-UTR. Compared with
the control group, miR-98 mimic transfection significantly reduced
the relative luciferase activity in SW480 cells (P<0.01)
(Fig. 3B). Western blot analysis
showed that the protein expression level of IGF1R was significantly
higher in tumor tissues of patients with diabetes mellitus
complicated with colon cancer than in adjacent normal tissues
(Fig. 3C). In addition, IGF1R
protein expression levels were significantly downregulated after
upregulation of miR-98 expression levels compared to controls
(Fig. 3D). Based on the above
results, miR-98 can target and bind to the target gene IGF1R and
negatively regulates its expression level.
miR-98 regulates proliferation and
invasion of colon cancer cells by regulating target gene IGF1R
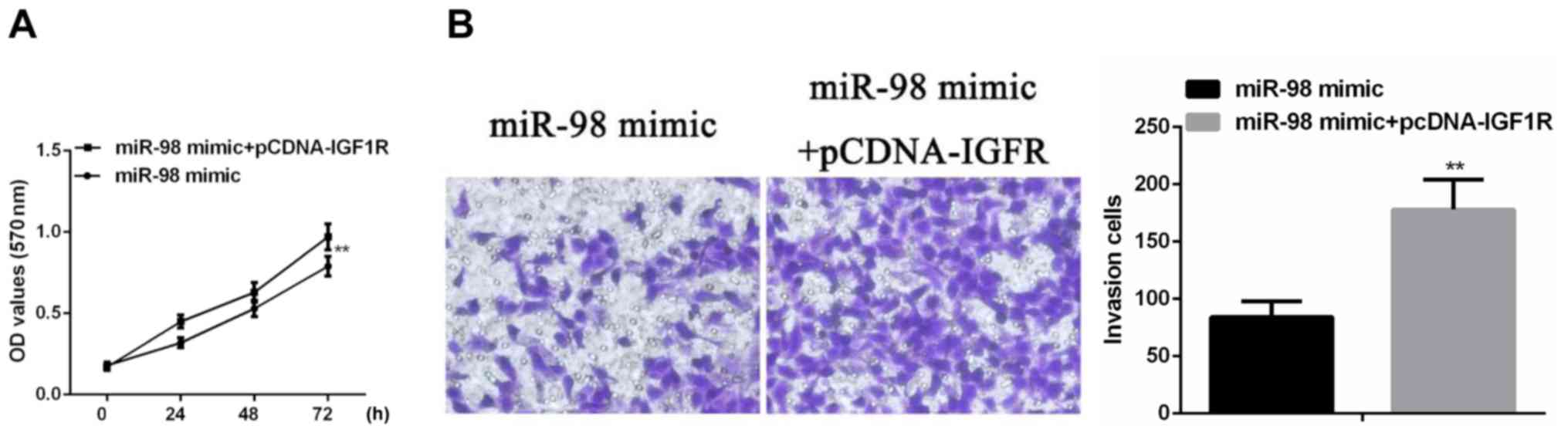
To further investigate the regulation of miR-98 on
the behavior of colon cancer cells, miR-98 mimic and pCDNA-IGF1R
were simultaneously transfected into SW480 cells, and cell
proliferation and invasion ability were examined. According to the
results of MTT assay, simultaneous transfection of miR-98 mimic and
pCDNA-IGF1R significantly increased the proliferation of colon
cancer cells compared with SW480 cells transfected with miR-98
mimic alone (P<0.01) (Fig. 4A).
Transwell cell invasion assay showed that transfection of miR-98
mimic and pCDNA-IGF1R significantly increased the invasiveness of
colon cancer cells compared with SW480 cells transfected with
miR-98 mimic alone (P<0.01) (Fig.
4B).
Discussion
In order to clarify the pathogenic mechanism of
patients with diabetes mellitus complicated with colon cancer,
miR-98 was used as the research object in this study. Firstly, the
expression level of miR-98 in patients with diabetes mellitus and
colon cancer was studied, and then the influence of the change of
miR-98 expression level on the proliferative capacity and invasive
ability of human colon cancer cells under high glucose conditions
was studied, followed by bioinformatics analysis and dual
luciferase assay to identify potential target genes of miR-98, and
the effects of the target genes on the proliferation and invasion
of human colon cancer cells under high glucose conditions were
explored, in order to clarify the regulatory mechanism of miR-98 on
diabetes and colon cancer, and to explore potential biological
targets for the treatment of diabetic colon cancer.
First, in order to study whether there is a
difference in the expression level of miR-98 in tumor tissues of
patients with diabetes mellitus complicated with colon cancer and
tumor tissues of colon cancer patients, 40 tumor patients with
diabetes mellitus combined with colon cancer and 40 colon cancer
patients after surgery, 40 colon cancer biopsies from patients with
diabetes mellitus were collected. and the expression of miR-98 in
colon cancer patients was lower than that in diabetic patients by
real-time fluorescent quantitative PCR. However, the expression
level of miR-98 in tumor tissues of diabetic patients combined with
colon cancer was also significant lower than that of colon cancer
patients, and the expression level of miR-98 in colon cancer cell
lines cultured under high glucose conditions was found to be
significantly lower than the colon cancer cell line cultured under
normal conditions. According to literature reports, the expression
level of miR-98 in tumor tissues was significantly reduced. For
example, the expression level of miR-98 was significantly decreased
in tumor tissues of patients with non-small cell lung cancer
(27). In laryngeal squamous cell
carcinoma tumor tissues and laryngeal squamous cell carcinoma cell
lines, the expression level of miR-98 was also significantly lower
than that of normal tissues and normal cell lines (28). In retinoblastoma, miR-98 expression
levels were also downregulated (29). In breast cancer patients' tumor
tissues and breast cancer cell lines, the expression level of
miR-98 was also found to be significantly lower than that of
adjacent normal tissues and normal cell lines (21). In addition, a team found that miR-98
was expressed at low levels in tumor tissues and colon cancer cells
of colon cancer patients (23). In
this experiment, the difference in the expression level of miR-98
between colon cancer cell lines and normal cells was not detected,
but miR-98 was found to have lower expression level under high
glucose conditions. The expression level of miR-98 in diabetic
colon cancer patients was lower than colon cancer patients,
suggesting that miR-98 may have a regulatory role in diabetes with
colon cancer.
The differential expression levels of miRNAs in
diseased and normal tissues usually indicate that miRNAs may play
important regulatory roles in disease. For example, miR-98, which
is significantly downregulated in tumor tissues of non-small cell
lung cancer patients and non-small cell lung cancer cell lines, can
regulate the migration and invasion ability of non-small cell lung
cancer cells (27). miR-98 is
downregulated in tumor tissues and laryngeal squamous cell
carcinoma cell lines of laryngeal squamous cell carcinoma, its
upregulation can inhibit the ERT of tumor cells and the invasive
behavior of laryngeal squamous cell carcinoma cell lines (28). The expression level of miR-98 is
downregulated in retinoblastoma, its upregulation can inhibit the
proliferation, invasion and migration ability of retinoblastoma
cells (29). In breast cancer,
upregulation of miR-98 expression by cell transfection can inhibit
breast cancer cell proliferation, invasion, migration, and
epithelial to mesenchymal transition (21). Therefore, we hypothesized that the
significant decrease in the expression level of miR-98 in diabetic
and colon cancer tissues in this experiment may indicate that
miR-98 can also regulate the behavior of cancer cells in this
disease. Therefore, we performed MTT assay and Transwell cell
invasion assay to investigate the changes in proliferation and
invasion ability of colon cancer cells after upregulation of miR-98
expression. According to the experimental results, it was found
that after the colon cancer cell line transfected with miR-98 mimic
significantly upregulated the expression level of miR-98, the cell
proliferation and invasion ability of colon cancer cell lines were
significantly inhibited. This result is similar to the regulation
of miR-98 on cancer cell behavior in other cancers. At the same
time, the results of this experiment are similar to those found by
other scholars in miR-98 in colon cancer. The researchers found
that miR-98, which is significantly downregulated in tumor tissues
and colon cancer cell lines of colon cancer patients, after
significantly increasing the expression level, it can significantly
inhibit the proliferation of colon cells. In addition, they also
found that upregulation in miR-98 expression level can inhibit
glucose absorption and lactate secretion, that is, inhibit the
glycolysis process of colon cancer cells. Glycolysis process is the
main functional metabolism of cancer cells. If the glycolysis
process is inhibited, it can inhibit the proliferation of cancer
cells (23). In this experiment, we
did not investigate the effect of miR-98 on the metabolic patterns
of colon cancer cells.
miRNAs are a class of non-coding RNAs that do not
encode any protein, but can be involved in the regulation of gene
expression levels at the post-translational level. The most
important way for miRNAs to regulate gene expression levels is
through targeting the non-translated region of mRNA to mediate the
post-translational silencing function of the gene, and the key
region of the sequence complementarity of the miRNA to its target
is located in the seed region of the 2-7 nucleotides at the 5′ end
of the miRNA (30). In cancer, the
main regulatory effect of miRNAs on cancer cell proliferation,
invasion and migration ability is through the regulation of the
expression level of its target genes. For example, in lung cancer
cell lines, miR-335 can target and negatively regulate the
expression level of transformer 2 beta homolog (Tra2β), and then
regulate the proliferation of lung cancer cells (31). In gastric cancer, miR-129-5p and
miR-129-3p can regulate the proliferation and migration ability of
gastric cancer cells by regulating the expression level of target
gene WWP1 (32). Moreover, miR-98
has also been found to regulate the cellular behavior of cancer
cells by regulating the expression levels of target genes. For
example, miR-98 can inhibit the cell proliferation, invasion and
migration ability of non-small cell lung cancer by targeting
inhibition of TWIST expression levels (27). Furthermore, miR-98e can also inhibit
the progression of retinoblastoma by targeting HMGA2 (29). In breast cancer, miR-98 can inhibit
the proliferation and metastasis of breast cancer cells by
targeting to inhibit the expression level of Gab2. In this
experiment, we found that miR-98 can inhibit the proliferation and
invasion of colon cancer cells, thus, we speculate that miR-98 may
inhibit the proliferation and invasive ability of colon cancer
cells by targeting the expression of target genes in this
experiment. Therefore, we first screened the potential target gene
of miR-98 through bioinformatics software, and then carried out the
dual luciferase assay to further verify the target gene of miR-98.
The experimental results showed that IGF1R is a target gene of
miR-98. It can be targeted to bind to the 3′-UTR region by miR-98.
Moreover, western blot analysis showed that the expression level of
IGF1R protein was significantly up-regulated in tumor tissues of
diabetic patients with colon cancer, which was contrary to the
expression of miR-98 in tumor tissues of patients with diabetes
mellitus with colon cancer. Furthermore, we found that IGF1R
protein expression levels were significantly inhibited when miR-98
expression levels were up-regulated. Based on the above results, it
is suggested that miR-98 can target to bind IGF1R, and miR-98 can
negatively regulate the expression level of IGF1R protein.
According to literature, we speculate that IGF1R protein may
mediate the regulation of miR-98 on the proliferation and invasion
of colon cancer cells. Therefore, we also changed the expression
levels of miR-98 and IGF1R by cell transfection, and found that
up-regulation of IGF1R expression can reverse the inhibition of
colon cancer cell proliferation and invasion ability caused by
up-regulation of miR-98 expression. Therefore, it is known that the
inhibitory effect of miR-98 on the proliferation and invasion
ability of colon cancer cells is achieved by targeted binding to
IGF1R.
The insulin-like growth factor receptor IGF1R is a
receptor protein located on the cell surface and binds to
insulin-like growth factor. This receptor binds to a ligand to
transmit cellular signals, causing cell growth and division. IGF1R
is found to be highly expressed in tumor cells, which promotes the
proliferation of cancer cells (33),
and IGF1R is considered to be a potential biological target for
cancer therapy, similar to the results of this experiment. In
addition, in the study of diabetes mellitus complicated with colon
cancer, some scholars have suggested that the increase in the level
of free insulin growth factor may promote the proliferation of
colon cancer cells (9,10). We hypothesized that IGF1R may have a
regulatory role in this process. In diabetic colon cancer cells,
decreased expression of miR-98 leads to increased expression of
IGF1R, elevated levels of IGF1R binds to more insulin growth
factors, transmitting cellular signals, leads to cancer cell
proliferation and malignant progression of the tumor. Therefore,
based on the results of the present study, we consider that miR-98
may be a potential biological target for the treatment of patients
with diabetes and colon cancer.
In conclusion, the expression level of miR-98 is
downregulated in diabetic colon cancer tumor tissues, and the
proliferation and invasion ability of colon cancer cells are
inhibited by targeted inhibition of the expression level of the
target gene IGF1R. miR-98 may be a potential biological target for
the treatment of patients with diabetes mellitus combined with
colon cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL put forward the proposition, designed the study
and wrote the manuscript. YuZ, performed PCR, MTT and Transwell
assay. YoZ was responsible for Dual luciferase assay and western
blot analysis. JW and RJ contributed to the analysis of the
observation indexes. The final version was read and adopted by all
the authors.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The First Hospital of Lanzhou University (Lanzhou, China). Informed
consent was obtained from all the patients.
Patient cnsent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Peng YC, Lin CL, Hsu WY, Chang CS, Yeh HZ,
Liao SC and Kao CH: The risk of colorectal cancer is related to
frequent hospitalization of IBD in an Asian population: results
from a nationwide study. QJM. 108:457–463. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Belov L, Zhou J and Christopherson RI:
Colorectal cancer thera-peutic antibodies. Encyclopedia of Cancer.
Schwab M: Springer. (Berlin, Heidelberg). 944–948. 2014.PubMed/NCBI
|
|
3
|
Ling H, Pickard K, Ivan C, Isella C, Ikuo
M, Mitter R, Spizzo R, Bullock M, Braicu C, Pileczki V, et al: The
clinical and biological significance of miR-224 expression in
colorectal cancer metastasis. Gut. 65:977–989. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dienstmann R, Vermeulen L, Guinney J,
Kopetz S, Tejpar S and Tabernero J: Consensus molecular subtypes
and the evolution of precision medicine in colorectal cancer. Nat
Rev Cancer. 17:79–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Al Omari A, Abdelkhaleq H, Al-Hussaini M,
Turfa R, Awad N, Hassan MM, Alfaqih MA and Garrett CR: Validation
of the survival benefits of metformin in middle Eastern patients
with type II diabetes mellitus and colorectal cancer. J Glob Oncol.
4:1–10. 2018. View Article : Google Scholar
|
|
6
|
Park JW, Lee JH, Park YH, Park SJ, Cheon
JH, Kim WH and Kim TI: Sex-dependent difference in the effect of
metformin on colorectal cancer-specific mortality of diabetic
colorectal cancer patients. World J Gastroenterol. 23:5196–5205.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakayama Y, Iijima T, Wakaume R, Takahashi
K, Matsumoto H, Nakano D, Miyaki M and Yamaguchi T: Microsatellite
instability is inversely associated with type 2 diabetes mellitus
in colorectal cancer. PLoS One. 14:e02155132019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meyerhardt JA, Catalano PJ, Haller DG,
Mayer RJ, Macdonald JS, Benson AB III and Fuchs CS: Impact of
diabetes mellitus on outcomes in patients with colon cancer. J Clin
Oncol. 21:433–440. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berster JM and Göke B: Type 2 diabetes
mellitus as risk factor for colorectal cancer. Arch Physiol
Biochem. 114:84–98. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin T: Why diabetes patients are more
prone to the development of colon cancer? Med Hypotheses.
71:241–244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu YZ, Chan KYY, Leung KT, Lam HS, Tam YH,
Lee KH, Li K and Ng PC: Dysregulation of miR-431 and target gene
FOXA1 in intestinal tissues of infants with necrotizing
enterocolitis. FASEB J. 33:5143–5152. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen B, Han S, Wang Y, Yang Z, Zou Z, Liu
J, Zhao Z, Wu R and Wang C: Bta-miR-152 affects intracellular
triglyceride content by targeting the UCP3 gene. J Anim Physiol
Anim Nutr (Berl). 103:1365–1373. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang X, Zhang M, Guo Q, Hu X, Zhao Z, Ni
L, Liu L, Wang X, Wang Z, Tong D, et al: MicroRNA-1297 inhibits
proliferation and promotes apoptosis in gastric cancer cells by
downregulating CDC6 expression. Anticancer Drugs. 30:803–811. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ghaemi Z, Soltani BM and Mowla SJ:
MicroRNA-326 functions as a tumor suppressor in breast cancer by
targeting ErbB/PI3K signaling pathway. Front Oncol. 9:6532019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yun Z, Meng F, Jiang P, Yue M and Li S:
microRNA-548b suppresses aggressive phenotypes of hepatocellular
carcinoma by directly targeting high-mobility group box 1 mRNA.
Cancer Manag Res. 11:5821–5834. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu J, Huang Y, Cheng Q, Wang J, Zuo J,
Liang Y and Yuan G: miR-1-3p suppresses the epithelial-mesenchymal
transition property in renal cell cancer by downregulating
Fibronectin 1. Cancer Manag Res. 11:5573–5587. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X, An D, Liu X, Wang X and Li B:
MicroRNA-27a downregulates the expression of Hsp90 and enhances the
radiosensitivity in esophageal squamous cell carcinoma. OncoTargets
Ther. 12:5967–5977. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu W, Luo X, Fu H, Liu L, Sun P and Wang
Z: miR-3653 inhibits the metastasis and epithelial-mesenchymal
transition of colon cancer by targeting Zeb2. Pathol Res Pract.
215:1525772019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chai B, Guo Y, Cui X, Liu J, Suo Y, Dou Z
and Li N: miR-223-3p promotes the proliferation, invasion and
migration of colon cancer cells by negative regulating PRDM1. Am J
Transl Res. 11:4516–4523. 2019.PubMed/NCBI
|
|
20
|
Jin Y, Cheng H, Cao J and Shen W: MicroRNA
32 promotes cell proliferation, migration, and suppresses apoptosis
in colon cancer cells by targeting OTU domain containing 3. J Cell
Biochem. 120:18629–18639. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi XY, Wang H, Wang W and Gu YH:
miR-98-5p regulates proliferation and metastasis of MCF-7 breast
cancer cells by targeting Gab2. Eur Rev Med Pharmacol Sci.
23:2847–2855. 2019.PubMed/NCBI
|
|
22
|
Tan H, Zhu G, She L, Wei M, Wang Y, Pi L,
Chen C, Zhang D, Tan P, Chen J, et al: miR-98 inhibits malignant
progression via targeting MTDH in squamous cell carcinoma of the
head and neck. Am J Cancer Res. 7:2554–2565. 2017.PubMed/NCBI
|
|
23
|
Zhu W, Huang Y, Pan Q, Xiang P, Xie N and
Yu H: MicroRNA-98 suppress Warburg effect by targeting HK2 in colon
cancer cells. Dig Dis Sci. 62:660–668. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Del Puerto-Nevado L, Minguez P, Corton M,
Solanes-Casado S, Prieto I, Mas S, Sanz AB, Gonzalez-Alonso P,
Villaverde C, Portal-Nuñez S, et al Diabetes Cancer Connect
Consortium, : Molecular evidence of field cancerization initiated
by diabetes in colon cancer patients. Mol Oncol. 13:857–872. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Bai YY, Yang Y, Hu F, Wang Y, Yu
Z, Cheng Z and Zhou J: Diabetes mellitus stimulates pancreatic
cancer growth and epithelial-mesenchymal transition-mediated
metastasis via a p38 MAPK pathway. Oncotarget. 7:38539–38550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang K, Dong L, Fang Q, Xia H and Hou X:
Low serum miR-98 as an unfavorable prognostic biomarker in patients
with non-small cell lung cancer. Cancer Biomark. 20:283–288. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou H, Huang Z, Chen X and Chen S: miR-98
inhibits expression of TWIST to prevent progression of non-small
cell lung cancers. Biomed Pharmacother. 89:1453–1461. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu M, Zhang C, Chen D, Chen S and Zheng
H: MicroRNA-98-HMGA2-POSTN signal pathway reverses
epithelial-to-mesenchymal transition in laryngeal squamous cell
carcinoma. Biomed Pharmacother. 117:1089982019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li W, Wang J, Zhang D, Zhang X, Xu J and
Zhao L: MicroRNA-98 targets HMGA2 to inhibit the development of
retinoblastoma through mediating Wnt/β-catenin pathway. Cancer
Biomark. 25:79–88. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Acunzo M, Romano G, Wernicke D and Croce
CM: MicroRNA and cancer - a brief overview. Adv Biol Regul. 57:1–9.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu J, Bian T, Feng J, Qian L, Zhang J,
Jiang D, Zhang Q, Li X, Liu Y and Shi J: miR-335 inhibited cell
proliferation of lung cancer cells by target Tra2β. Cancer Sci.
109:289–296. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ma L, Chen X, Li C, Cheng R, Gao Z, Meng
X, Sun C, Liang C and Liu Y: miR-129-5p and −3p co-target WWP1 to
suppress gastric cancer proliferation and migration. J Cell
Biochem. Nov 11–2018.(Epub ahead of print). doi:
10.1002/jcb.28027.
|
|
33
|
Kumar AS, Rayala SK and Venkatraman G:
Targeting IGF1R pathway in cancer with microRNAs: How close are we?
RNA Biol. 15:320–326. 2018. View Article : Google Scholar : PubMed/NCBI
|