Introduction
UV radiation, smoking and various other
environmental factors can cause DNA damage (1). In the human body, DNA damage can be
corrected by direct repair, base excision repair and nucleotide
excision repair (NER) to maintain genome stability. Among these,
the NER system is the primary repair method (1). If DNA damage is not removed or repaired
in time, it can lead to various diseases, such as cancer and
neurodegenerative diseases, Parkinson's disease, Alzheimer's
disease, Huntington's disease, aging (2). Xeroderma pigmentosum group D (XPD) is a
major protein involved in NER. As an important DNA repair gene, it
serves an essential role in transcription factor IIH (TFIIH)
protein complex-mediated NER and its transcription process.
Mutations in the human embryonic XPD gene can result in xeroderma
pigmentosum, Cockayne syndrome and fur dystrophy syndrome (3). The risk of skin cancer in individuals
with xeroderma pigmentosum is 1,000 times higher than that in
normal individuals. From a molecular basis, xeroderma pigmentosum
is a birth defect of NER in which UVB-associated DNA damage cannot
be repaired (4). Previous studies
have revealed associations between XPD gene polymorphisms and
prostate cancer, lung cancer, bladder cancer, basal cell carcinoma
and malignant melanoma (5–8). Investigations into the molecular
mechanism of the susceptibility to cutaneous melanoma has revealed
that mutated XPD is a biomarker of skin malignant melanoma
(2). However, to the best of our
knowledge, the role of the XPD gene and its protein product in
malignant melanoma cells has not yet been reported.
In the present study, the XPD gene was cloned and
the recombinant pEGFP-N1/XPD and pcDNA3.1(+)/XPD expression
plasmids were constructed and transfected into human malignant
melanoma cells. By observing XPD expression, its intracellular
localization and its effects on cellular proliferation, the
biological effects of XPD on malignant melanoma cells were
analyzed.
Materials and methods
Reagents and antibodies
Human cervical squamous cell carcinoma epithelial
HeLa cells and malignant melanoma A375 cells were purchased from
The Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences. pEGFP-N1 and pcDNA3.1(+) plasmids were obtained from
Clontech Laboratories, Inc. and Invitrogen (Thermo Fisher
Scientific, Inc.), respectively. Restriction enzymes, RNAiso
extraction reagent, reverse transcription reagent part of the
PrimeScript™ RT reagent and PrimeSTAR high fidelity enzyme were
purchased from Takara Bio, Inc. Lipofectamine® 2000 was
purchased from Invitrogen (Thermo Fisher Scientific, Inc.). T4 DNA
ligase and the DNA gel recovery and plasmid extraction kits were
obtained from Promega Corporation. The XPD antibody was ordered
from Abcam (cat. no. ab102682), while GM130 (cat. no. sc-55590) and
KDEL antibodies (cat. no. sc-58774) were obtained from Santa Cruz
Biotechnology, Inc. Goat anti-rabbit IgG-HRP-labeled (cat. no.
4030-05), goat anti-rabbit IgG-FITC-labeled (cat. no. sc-2012) and
goat anti-mouse IgG-TRITC-labeled (cat. no. 4030-03) antibodies
were provided by OriGene Technologies, Inc. DMEM and FBS were
purchased from Gibco (Thermo Fisher Scientific, Inc.). RIPA buffer
(cat. no. P0013C) was purchased from (Beyotime, Institute of
Biotechnology). Primers were synthesized by Shanghai Shenggong
Biology Engineering Technology Service, Ltd., and the enhanced
chemiluminescence (ECL) reagent was obtained from Merck KGaA.
Construction of the pEGFP-N1/XPD
recombinant plasmid
HeLa cells were cultured in DMEM supplemented with
10% FBS and antibiotics (100 U/ml penicillin and 100 g/ml
streptomycin) at 37°C (5% CO2) for 2 weeks. Total RNA
was extracted using the RNAiso reagent and reverse transcribed at
37°C for 15 min into cDNA. The primers were designed according to
the human XPD intact mRNA sequence published on GenBank
(NM_001130867) (9), and the
restriction endonuclease sites of HindIII and SalI
were added to the 5′-ends of the forward and reverse primers,
respectively. The primer sequences were as follows: External nest
primer XPD2-F1, 5′-TCAACGTGGACGGGCTCCTGGTCTA-3′ and XPD2-R1,
5′-TATTTGGCTGCATCTTTGCTACTGG-3′; nested primer XPD2-F2, 5′-CCC AAG
CTT ATG CGG GAG CTC AAA CGC ACG CTG-3′ (HindIII restriction
site underlined) and XPD2-R2, 5′-GCG TCG ACT GGG GAT GAG ATC TTT
TTT GGT TCC TG-3′ (SalI restriction site underlined). The
reaction system of the PrimeSTAR enzyme was configured according to
the manufacturer's protocol, and the target gene was amplified by
nested PCR using the thermocycling conditions of one cycle at 55°C
for 15 sec followed by 38 cycles at 72°C for 90 sec. The pEGFP-N1
empty vector and the purified PCR product were double-digested with
HindIII and SalI sequentially. After purification and
recovery of the digestion products by DNA gel recovery kit, the XPD
gene fragment and the pEGFP-N1 vector fragment were ligated at a
ratio of 5:1 in the presence of T4 DNA ligase, at 16°C for 12 h.
The ligation product was transformed into competent Escherichia
coli DH5α cells (cat. no. RR420A; Takara Bio Inc.) and cultured
overnight on LB solid medium containing a final concentration of 30
µg/ml kanamycin. The resulting colonies were individually selected,
added to Luria-Bertani medium (cat. no. CM0007; Beijing Leagene
Biotech Co., Ltd.) and shaken at 37°C for 16 h; the recombinant
plasmid was extracted using a plasmid extraction kit and identified
by BglII digestion. The positive plasmid was sequenced by
Shanghai Shenggong Biology Engineering Technology Service, Ltd.
Liposome-mediated transfection
The constructed recombinant pEGFP-N1/XPD expression
vector was validated by DNA sequencing and subsequently transfected
(1 µg/ml) into malignant melanoma A375 cells which were cultured in
DMEM supplemented with 10% FBS at 37°C in a 5% CO2
incubator) using Lipofectamine® 2000.
Western blot analysis
Total protein of the A375 cells was extracted using
the RIPA buffer. The protein content was measured using the
bicinchoninic acid assay (Beyotime Institute of Biotechnology).
Equal amounts of protein (40 µg) were separated via 12% SDS-PAGE,
and the separated proteins were subsequently transferred onto a
nitrocellulose membrane. Membranes were blocked with 5% milk in
TBST. Next, the membrane was incubated with the XPD (dilution,
1:500; cat. no. ab102682; Abcam) and GAPDH (dilution, 1:3,000; cat.
no. 60004-1-Ig; Proteintech Group, Inc.) primary antibodies and
shaken for 2 h at room temperature. After washing 3 times with TBST
(TBS containing 0.1% Tween-20), the membrane was incubated with a
horse radish peroxidase goat anti-rabbit (IgG) secondary antibody
(1:10,000; cat. no. ab6721; Abcam) and shaken for 2 h at room
temperature. After washing 3 times with TBST, protein bands were
visualized using an ECL reagent. GAPDH was used as the protein
loading control. The experiment was repeated three times.
Immunofluorescence staining
The XPD2-F2 and XPD2-stop-R2 primers (5′-GCG TCG ACT
TAG GGA TGA GAT CTT TTT TGGT TC-3′; underlined for SalI
restriction site) were used To observe the localization of the XPD
gene in A375 cells. The eukaryotic expression plasmid
pcDNA3.1(+)/XPD was constructed by amplification, digestion,
ligation and transformation, as aforementioned, and subsequently
transfected (1 µg/ml) into A375 cells for 48 h using
Lipofectamine® 2000. The Golgi membrane protein marker
GM130 and the endoplasmic reticulum membrane protein marker KDEL
were used for labeling, and the location of the gene was detected
by immunofluorescence staining. Briefly, cells were fixed with
freshly prepared 4% paraformaldehyde for 10 min at room
temperature. The cells were washed with PBS thrice for 5 min each
time and then permeabilized with 0.2-0.5% Triton-100 (prepared in
PBS) for 10 min at room temperature. Next, the cells were washed
again thrice in PBS and subsequently blocked with 2% BSA for 30 min
at room temperature. Following blocking the cells were incubated
with primary antibodies against GM130 (1:200) and KDEL (1:200) for
1 h at room temperature. The cells were again washed thrice with
PBS and then incubated with secondary antibody (1:500) for 1 h at
room temperature. Cells were visualized using a fluorescence
microscope (magnification, ×400).
MTT assay
Following pcDNA3.1(+) and pcDNA3.1(+)/XPD
transfection into A375 cells for 24, 48 and 72 h, 5×103
cells/well were seeded into 96-well plates. After 3 days of cell
culture, 20 µl MTT (5 mg/ml) was added to each well for 4 h. The
culture supernatant was subsequently discarded, and 150 µl triple
solution of dimethyl sulfoxide was added to each well and shaken
for 10 min to fully dissolve the purple formazan crystals. The
light absorption value of each well was measured at a wavelength of
490 nm.
Statistical analysis
Each experiment was repeated three times and the
data are presented as the mean ± SD. Statistical analyses were
conducted using SPSS v17.0 (SPSS, Inc.). A Student's unpaired
t-test was used to compare differences between two groups, such as
pcDNA3.1(+) 48 h and pcDNA3.1(+)/XPD 48 h groups, pcDNA3.1(+) 72 h
and pcDNA3.1(+)/XPD 72 h groups. One-way ANOVA was used to compare
differences among multiple groups, such as the DMEM, pcDNA3.1(+)
and pcDNA3.1(+)/XPD groups. ANOVA was followed by Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Cloning and identification of the XPD
gene
Following the reverse transcription of HeLa cell
total RNA, a nested PCR method was used to obtain the expected
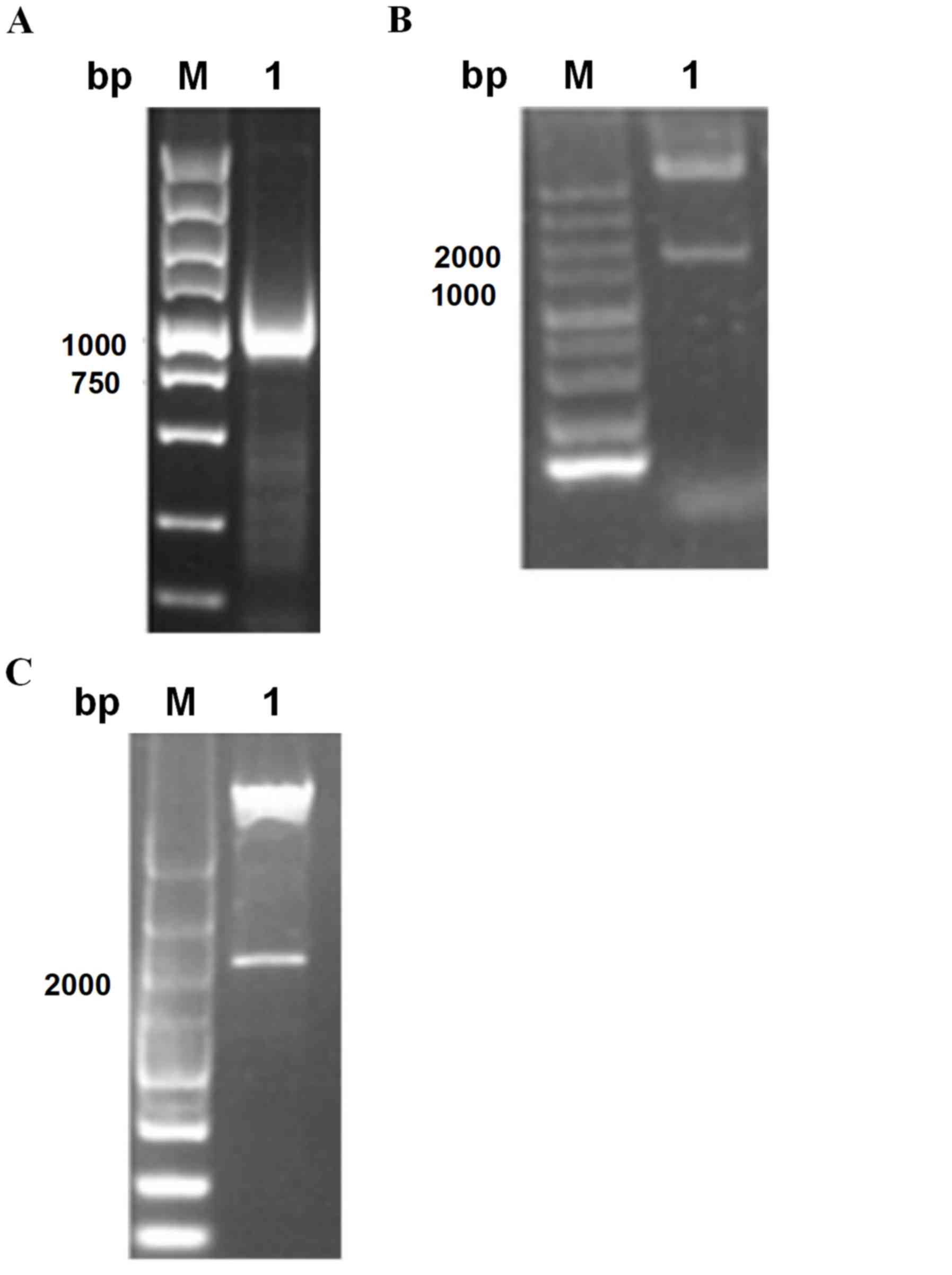
1,234 bp band (Fig. 1A). The PCR
amplification products of pEGFP-N1 and XPD cDNA were digested using
HindIII and SalI, respectively. After gel recovery,
ligation, transformation and plasmid extraction, enzymatic
digestion was performed. Due to the presence of the BglII
restriction site at the multiple cloning site of pEGFP-N1 and the
3′-end of XPD, BglII single digestion resulted in 4,737 and
1,200 bp products, as shown in Fig.
1B. The eukaryotic expression vector pcDNA3.1(+) was digested
with HindIII and XhoI, while the PCR products were
digested with HindIII and SalI. The digestion
products were subsequently ligated using SalI and
XhoI sites (per the principle of homologous enzymes) to
obtain the pcDNA3.1(+)/XPD plasmid. Following identification with
HindIII and XbaI, the present results were consistent
with the expectations (Fig. 1C).
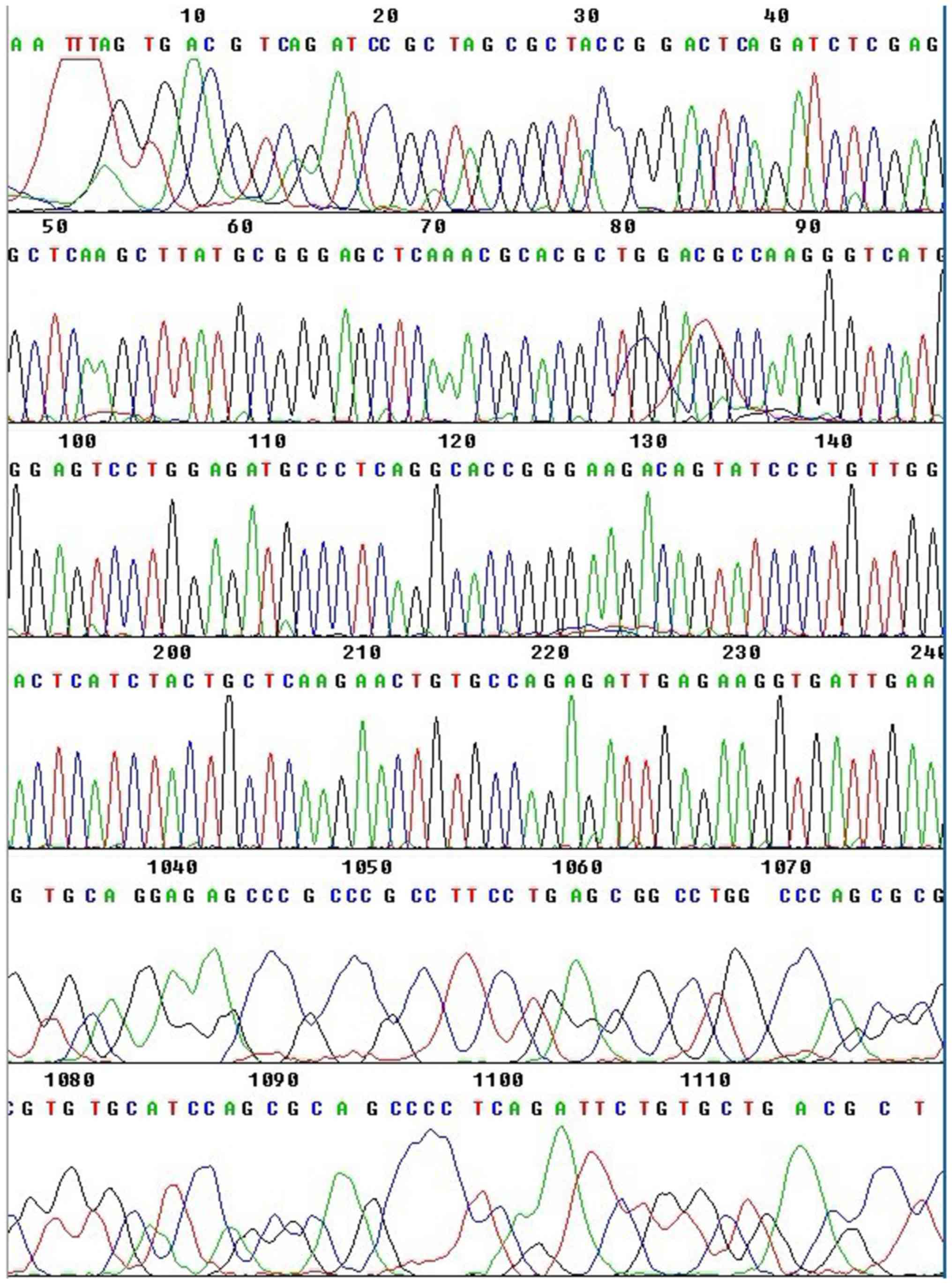
Additionally, the obtained sequencing results (Fig. 2) were consistent with the human XPD
sequence reported on GenBank.
Recombinant plasmid transfection into
A375 cells
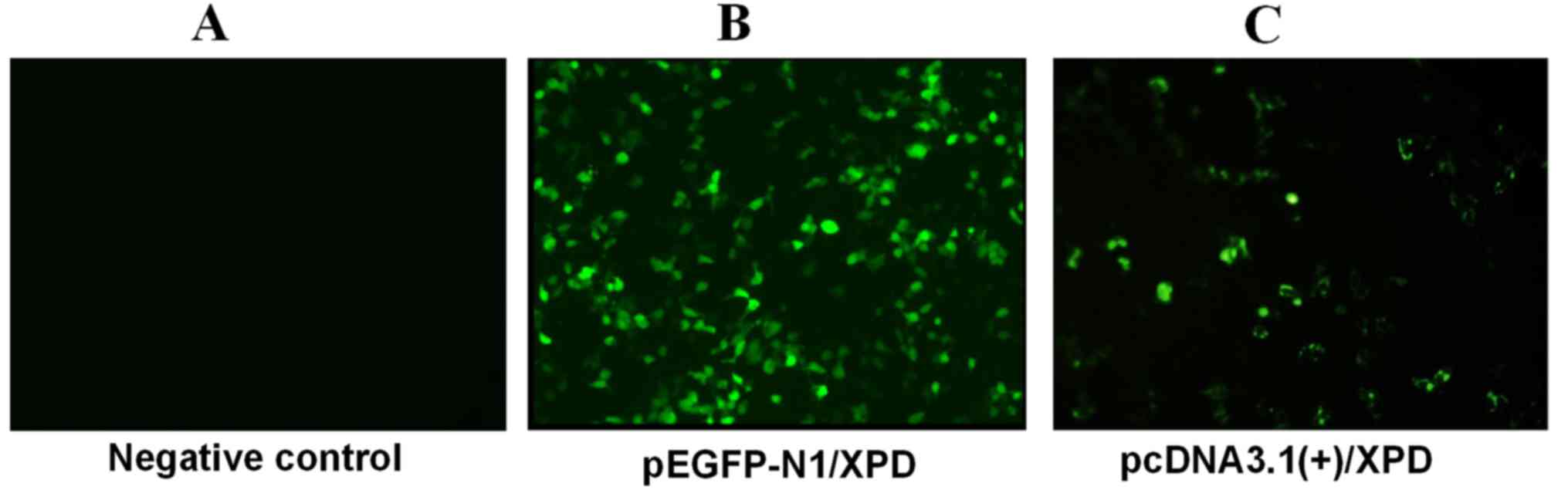
The negative control (PBS), pEGFP-N1 and
pEGFP-N1/XPD recombinant plasmids were transfected into malignant
melanoma A375 cells using Lipofectamine® 2000. GFP
expression was observed under a fluorescence microscope. The
results revealed fluorescence in both the recombinant pEGFP-N1/XPD-
and the empty pEGFP-N1 plasmid-transfected cells, while the
negative control group exhibited no fluorescence, which proved that
the recombinant plasmid was successfully transfected into A375
cells (Fig. 3).
Detection of XPD protein
expression
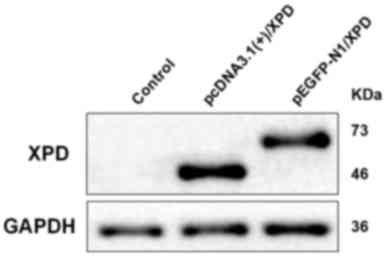
Following transfection of pEGFP-N1/XPD and
pcDNA3.1(+)/XPD into A375 cells, XPD expression was analyzed via
western blot analysis. The results revealed that XPD was expressed
in the transfection groups (Fig. 4).
The 46 kDa band corresponds to pcDNA3.1(+)/XPD, the 73 kDa band
corresponds to pEGFP-N1/XPD. As pEGFP-N1/XPD has green fluorescent
protein, it can present as a 73 kDa band which is larger than
pcDNA3.1(+)/XPD.
Localization of XPD to the endoplasmic
reticulum
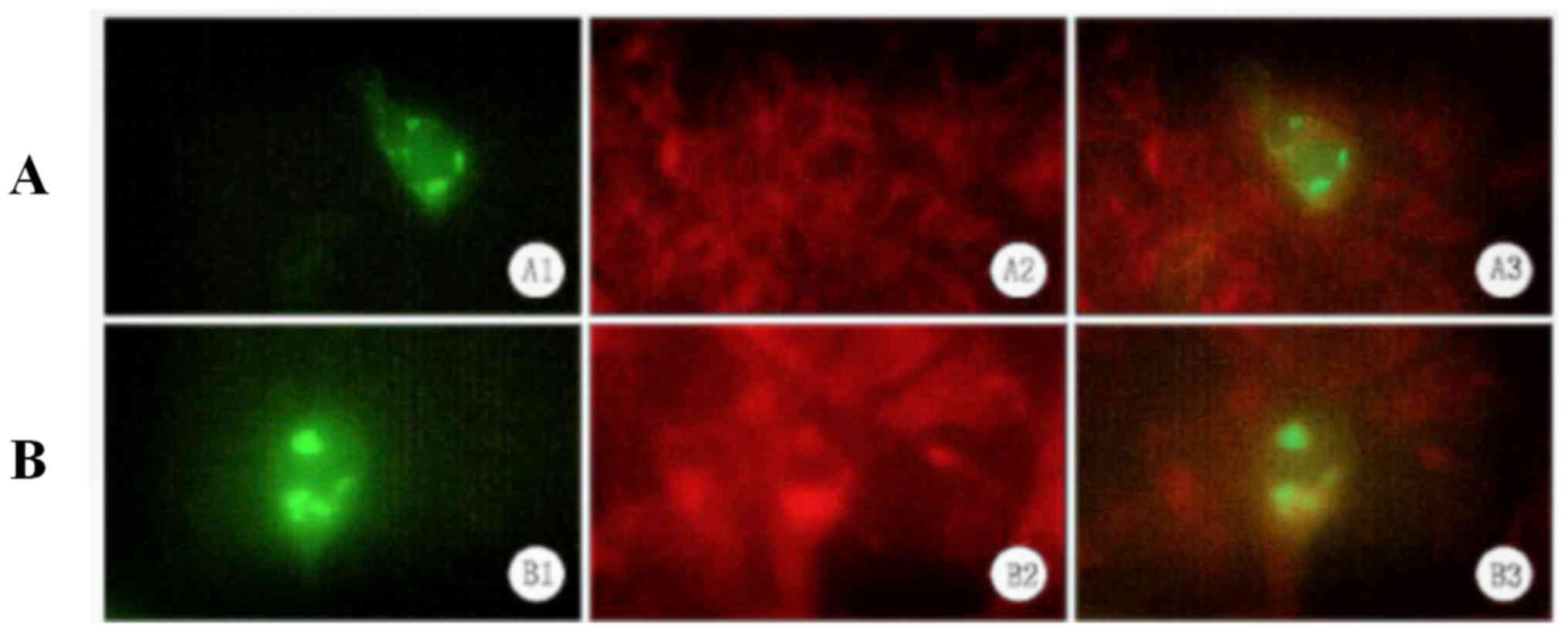
Since pEGFP-N1/XPD -transfected A375 cells exhibited
a clustered distribution of XPD-EGFP (as indicated by fluorescence
microscopy), the subcellular location of XPD was analyzed. Using
the Golgi membrane protein marker GM130 and the endoplasmic
reticulum membrane protein marker KDEL, immunofluorescence staining
revealed that XPD was localized to the endoplasmic reticulum
(Fig. 5).
Inhibition of A375 cell proliferation
by XPD
Following transfection of pcDNA3.1(+) and
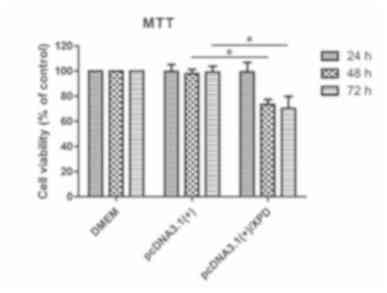
pcDNA3.1(+)/XPD into A375 cells for 24, 48 and 72 h, cell
proliferation was analyzed via MTT assay. The cell proliferation of
the pcDNA3.1(+)/XPD group was significantly lower than that of the
DMEM and pcDNA3.1(+) groups after 48 and 72 h of transfection
(P<0.05; Fig. 6), indicating that
XPD inhibits the proliferation of A375 cells.
Discussion
In recent years, the incidence of skin cancer has
been increasing worldwide, and this trend will continue to increase
as the global population ages (10).
This increase is primarily caused by the destruction of the ozone
layer, which leads to an increase in the number of ultraviolet rays
reaching the earth's surface (11).
Previous study has demonstrated that UVB light can cause numerous
types of DNA damage, leading to skin cancer (12). Therefore, DNA repair is important in
maintaining the genetic integrity of the skin.
Normal human keratinocytes have a well-established
DNA damage repair system to prevent gene mutations caused by
UVB-associated DNA damage. The NER pathway is the primary means of
repairing UVB-induced DNA damage, and the main line of defense
against carcinogenesis caused by UVB rays (13). To date, two NER pathways have been
discovered, including transcriptional coupling repair and whole
genome nucleic acid excision repair, which are complex processes
involving >30 gene products (14). XPD is the major protein of the
nucleic acid excision repair process; the XPD gene is located on
chromosome 19 and encodes a protein with ATP-dependent DNA helicase
activity (7). The XPD protein is the
second largest subunit of the TFIIH complex, which is composed of 9
subunits (namely XPB, XPD, p62, p52, p44, p34, cdk7, cyclinHT and
MAT1); XPB, p62, p52, p44 and p34 form a core subcomplex object,
while cdk7, cyclinHT and MAT1 form a subcomplex with the cdk active
kinase (CAK) (3). XPD acts primarily
as a scaffold that mediates CAK anchoring on the core subcomplex
(5). During NER, XPD is responsible
for opening the DNA duplex at the damaged position from the 5′ to
3′direction, allowing damage-specific nucleases to cut the damaged
DNA from both sides (5). During
transcription, the role of XPD is to maintain the structural
stability of the TFIIH complex and to promote transcription
amplification (6). Therefore, XPD
serves an important role in the TFIIH complex-mediated NER and its
transcription process (3). In
addition, XPD participates in various physiological and
pathological processes, such as cellular proliferation, apoptosis
and tumorigenesis (13,15–22). In
the present study, the XPD gene was cloned into a eukaryotic
expression vector to construct the pEGFP-N1/XPD recombinant
plasmid; this was confirmed by sequencing, which was consistent
with the XPD sequence published on GenBank.
Malignant melanoma is a type of malignant tumor
originating from neural crest melanocytes (22). It mainly occurs in the skin and is
the third most common skin malignant tumor (22). Additionally, it commonly results in
metastasis and relapse, leading to a poor prognosis (11). The etiology of malignant melanoma is
not fully understood, but an animal study has confirmed that UVB is
closely associated with malignant melanoma formation (23). Indeed, UVB can cause various types of
DNA damage, and can lead to the development of cutaneous malignant
melanoma (13). Studies on the
molecular mechanisms of the susceptibility to cutaneous malignant
melanoma genes have suggested that this susceptibility is
associated with sequence variation mutations in NER genes (2,24).
Mutated XPD may be a biomarker of skin malignant melanoma (1). Kertat et al (25) revealed that the Gln/Gln genotype of
the XPD 751 codon is a genetic marker of male malignant melanoma,
and that the Lys/Gln genotype is important for predicting tumor
progression.
To investigate the biological effects of XPD on
malignant melanoma cells, the XPD gene was cloned and the
recombinant pEGFP-N1/XPD plasmid was constructed and transfected
into malignant melanoma A375 cells. GFP expression was confirmed by
fluorescence expression of XPD-EGFP. Additionally, western blot
analysis revealed that XPD was successfully expressed in
transfected cells, indicating that an XPD eukaryotic expression
system was successfully constructed. Furthermore,
immunofluorescence analysis was used to detect the location of XPD
in the endoplasmic reticulum. An MTT assay revealed that the
proliferative capacity of malignant melanoma A375 cells transfected
with pcDNA3.1(+)/XPD was significantly lower than that of cells
transfected with pcDNA3.1(+) or DMEM, suggesting that XPD inhibits
the proliferation and metabolism of A375 cells. Therefore, the
present study provides a basis to further clarify the function of
XPD in malignant melanoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation (grant no. 81350023).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MG designed the study. YuW and YZ performed the
experiments and wrote the manuscript. YaW and CP analyzed and
interpreted data. All authors have read and approved the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee TH and Kang TH: DNA oxidation and
excision repair pathways. Int J Mol Sci. 20:60922019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li C, Hu Z, Liu Z, Wang LE, Strom SS,
Gershenwald JE, Lee JE, Ross MI, Mansfield PF, Cormier JN, et al:
Polymorphisms in the DNA repair genes XPC, XPD, and XPG and risk of
cutaneous melanoma: A case-control analysis. Cancer Epidemiol
Biomarkers Prev. 15:2526–2532. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lehmann AR: The xeroderma pigmentosum
group D (XPD) gene: One gene, two functions, three diseases. Genes
Dev. 15:15–23. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsao H: Update on familial cancer
syndromes and the skin. J Am Acad Dermatol. 42:939–969. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sobti RC, Berhane N, Melese D, Mahdi SA,
Gupta L, Thakur H and Singh N: Impact of XPD gene polymorphism on
risk of prostate cancer on North Indian population. Mol Cell
Biochem. 362:263–268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schabath MB, Delclos GL, Grossman HB, Wang
Y, Lerner SP, Chamberlain RM, Spitz MR and Wu X: Polymorphisms in
XPD exons 10 and 23 and bladder cancer risk. Cancer Epidemiol
Biomarkers Prev. 14:878–884. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lovatt T, Alldersea J, Lear JT, Hoban PR,
Ramachandran S, Fryer AA, Smith AG and Strange RC: Polymorphism in
the nuclear excision repair gene ERCC2/XPD: Association between an
exon 6-exon 10 haplotype and susceptibility to cutaneous basal cell
carcinoma. Hum Mutat. 25:353–359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yin J, Li J, Ma Y, Guo L, Wang H and Vogel
U: The DNA repair gene ERCC2/XPD polymorphism Arg 156Arg (A22541C)
and risk of lung cancer in a Chinese population. Cancer Lett.
223:219–226. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
"xrefwindow" href="http://www.ncbi.nlm.nih.gov/pubmed/NOT_FOUND" id="d7e616" name="d7e616" shape="rect">PubMed/NCBI
|
|
10
|
Armstrong BK, Kricker A and English DR:
Sun exposure and skin cancer. Australas J Dermatol. 38 (Suppl
1):S1–S6. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Swetter SM, Johnson TM, Miller DR, Layton
CJ, Brooks KR and Geller AC: Melanoma in middle aged older men: A
multi-institutional survey study of factors related to tumor
thickness. Arch Dermatol. 145:397–404. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Afaq F, Adhami VM and Mukhtar H:
Photochemoprevention of ultraviolet B signaling and
photocarcinogenesis. Mutat Res. 571:153–173. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alisha A, Perween N, Saijo M, Ghaskadbi SS
and Ghaskadbi S: Analysis of the conserved NER helicases (XPB and
XPD) and UV-induced DNA damage in Hydra. Biochim Biophys Acta Gen
Subj. 1862:2031–2042. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wood RD, Mitchell M and Lindahl T: Human
DNA repair genes, 2005. Mutat Res. 577:275–283. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuptsova-Clarkson N, Ambrosone CB, Weiss
J, Baer MR, Sucheston LE, Zirpoli G, Kopecky KJ, Ford L, Blanco J,
Wetzler M and Moysich KB: XPD DNA nucleotide excision repair gene
polymorphisms associated with DNA repair deficiency predict better
treatment outcomes in secondary acute myeloid leukemia. Int J Mol
Epidemiol Genet. 1:278–294. 2010.PubMed/NCBI
|
|
16
|
Wang HY, Xiong GF, Zhang JX, Xu H, Guo WH,
Xu JJ and Xiong XY: The role of XPD in cell apoptosis and viability
and its relationship with p53 and cdk2 in hepatoma cells. Med
Oncol. 29:161–167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yilmaz E, Celik O, Celik E, Turkcuoglu I,
Simsek Y, Karaer A, Otlu B, Gulbay G and Yesilada E: XPD and XRCC1
gene polymorphism in patients with normal and abnormal cervical
cytology by pap smear. Eur Rev Med Pharmacol Sci. 16:1713–1718.
2012.PubMed/NCBI
|
|
18
|
Szkandera J, Absenger G, Liegl-Atzwanger
B, Pichler M, Stotz M, Gerger S, Zacherl M, Renner W, Haijun M,
Leithner A and Gerger A: Common gene variants in RAD51, XRCC2 and
XPD are not associated with clinical outcome in soft-tissue sarcoma
patients. Cancer Epidemiol. 37:1003–1009. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Constantinescu-Aruxandei D,
Petrovic-Stojanovska B, Penedo JC, White MF and Naismith JH:
Mechanism of DNA loading by the DNA repair helicase XPD. Nucleic
Acids Res. 44:2806–2815. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shkarupa VM, Mishcheniuk OY,
Henyk-Berezovska SO, Palamarchuk VO and Klymenko SV: Polymorphism
of DNA repair gene XPD Lys751Gln and chromosome aberrations in
lymphocytes of thyroid cancer patients exposed to ionizing
radiation due to the chornobyl accident. Exp Oncol. 38:257–260.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Santovito A, Delsoglio M, Manitta E, Picco
G, Meschiati G, Chiarizio M, Gendusa C and Cervella P: Association
of GSTT1 null, XPD 751 CC and XPC 939 CC genotypes with increased
levels of genomic damage among hospital pathologists. Biomarkers.
22:557–565. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
de Carvalho Lima EN, Piqueira JRC and
Maria DA: Advances in carbon nanotubes for malignant melanoma: A
chance for treatment. Mol Diagn Ther. 22:703–715. 2018. View Article : Google Scholar
|
|
23
|
Pfeifer GP and Besaratinia A: UV
wavelength-dependent DNA damage and human non-melanoma and melanoma
skin cancer. Photochem Photobiol Sci. 11:90–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Goode EL, Ulrich CM and Potter JD:
Polymorphisms in DNA repair genes and associations with cancer
risk. Cancer Epidemiol Biomarkers Prev. 11:1513–1530.
2002.PubMed/NCBI
|
|
25
|
Kertat K, Rosdahl I, Sun XF, Synnerstad I
and Zhang H: The Gln/Gln genotype of XPD codon 751 as a genetic
marker for melanoma risk and Lys/Gln as an important predictor for
melanoma progression: A case control study in the Swedish
population. Oncol Rep. 20:179–183. 2008.PubMed/NCBI
|