Introduction
Gastric cancer is one of the most common types of
cancer worldwide, and a leading cause of cancer-related deaths
(1). Peritoneal metastasis is a
frequent recurrent pattern in gastric cancer, and is related to the
poor prognosis. Although various treatments including systemic or
intraperitoneal chemotherapy for peritoneal metastasis improved,
satisfactory outcomes have not been achieved (2,3). There
is thus a need for novel treatments in addition to conventional
surgery and chemotherapy.
Interactions between cancer cells and tumor stromal
cells have a key role in tumor progression, invasion and
metastasis. The tumor microenvironment consists of several kinds of
cells, such as endothelial cells, cancer-associated fibroblasts
(CAFs), and immune cells. We previously reported that activated
peritoneal mesothelial cells by transforming growth factor beta 1
(TGF-β1) caused to tumor invasion and progression (4). Moreover, the intraperitoneal cavity
contains a lot of M2 anti-inflammatory phenotype of macrophages,
which caused to the development of peritoneal metastasis in gastric
cancer (5).
Platelets are the discoid anucleate hematopoietic
cells that are responsible for maintaining hemostasis. On the other
hand, they have been recognized as key regulators for tumor
development and metastasis in several tumors (6–8).
Platelet aggregation in the blood vessels protects cancer cells
from several stress and immunocompetent cells through the platelet
coating around tumor cells. Platelets further promote cancer cell
attachment to intravascular endothelial cells, leading to
extravasation and the colonization of secondary tumors in new
microenvironments (9). However, few
studies have examined the role of platelets in primary tumors. We
previously found a correlation between extravasated platelet
aggregation (EPA) and epithelial-mesenchymal transition (EMT) in
breast cancer, and showed that patients with EPA were less
responsive to neo-adjuvant chemotherapy (10). Furthermore, EPA in primary gastric
cancer biopsy specimens was inversely correlated with pathological
response to preoperative chemotherapy, and was identified as an
independent prognostic factor (11).
Platelets contain a lot of TGF-β, which they secrete
following platelet activation (12,13).
TGF-β promotes the invasion ability and chemoresistance of tumor
cells via the induction of EMT, and also facilitates the induction
of immunosuppression by regulatory T (Treg) cells accumulation into
the tumor microenvironment (14).
TGF-β-induced forkhead box (FOX)P3-positive Treg cells have been
shown to participate in the maintenance of immunosuppression, and
to play critical roles in chemoresistance (15,16). In
addition to Treg cells, several studies have demonstrated the
importance of myeloid-derived suppressor cells (MDSCs) in
tumor-associated immune suppression (17,18).
MDSCs may promote the Treg cells infiltration into tumor stroma
through the secretion of TGF-β. Collectively, these findings
suggest that EMT, MDSCs, and Treg cell infiltration induced by EPA
are key regulators of cancer progression.
In the present study, we investigated the
relationship between EPA and prognosis in patients with gastric
cancer with peritoneal metastasis by analyzing the expression of
CD42b, SNAIL, FOXP3, and CD33 using immunohistochemistry.
Materials and methods
Patient samples
Sixty-two patients diagnosed with advanced gastric
cancer with peritoneal metastasis between 2001 and 2016 were
evaluated retrospectively. As inclusion criteria, all patients had
collected the peritoneal nodule by surgery included the staging
laparoscopy and diagnosed pathologically. Specimens from peritoneal
metastatic lesions were collected before chemo/radiotherapy.
Patients were excluded for the following reason: Poor general
condition or refuse treatment and are unable to treat the gastric
cancer.
All procedures were carried out in accordance with
the ethical standards of the responsible committees on human
experimentation and with the Helsinki Declaration of 1964 and later
versions. This study was approved by the Institutional Review Board
of Kanazawa University Graduate School of Medical Sciences (study
no. 2789). Written informed consent was obtained from all
patients.
Evaluation for clinical status
Primary and metastatic lesions were evaluated by
gastrointestinal endoscopy and contrast-enhanced computed
tomography scan. Peritoneal metastasis was diagnosed by laparoscopy
examination or open surgery before chemotherapy, and classified
into three categories according to the 15th edition of the General
Rules for Gastric Cancer Study of the Japanese Research Society for
Gastric Cancer: P1a (greater omentum, lesser omentum, anterior lobe
of the transverse colonic membrane, or membrane of the pancreatic
surface or spleen), P1b (a few scattered metastases to upper
abdominal peritoneum, namely, the parietal peritoneum close to the
umbilical side and the visceral peritoneum close to the cranial
transverse colon), and P1c (many metastases to middle or lower
peritoneum). The ascites level was evaluated by CT and classified
into four groups: None, mild (limited the pelvic cavity), moderate
(over the pelvic cavity), and severe (all over the abdominal
cavity). Univariate analyses of prognostic factors for overall
survival (OS) were performed. Patient-related factors included age,
sex, and European Cooperative Oncology Group (ECOG) performance
status was retrospectively examined. Tumor-related factors were
categorized according to the WHO Classification of tumours, 5th
edition (19).
Immunohistochemistry
EPA was investigated by immunostaining for CD42b.
CD42b (GPIbα) is platelet activation specific marker involved in
the process of coagulation (20).
All specimens were fixed in 10% formalin, embedded in paraffin, and
cut into the 3-µm tissue sections. The specimens were
deparaffinized through a graded series of xylene and ethanol. For
antigen retrieval, sections were pretreated in 1 mM citrate buffer
(pH 6.0), and autoclaved for 10 min at 120°C. Endogenous peroxidase
block was used by peroxidase block solution provided with the
EnVision kit for 20 min. After blocking endogenous peroxidase,
sections were incubated with 5% normal goat serum for 20 min to
block nonspecific staining. Sections were subsequently incubated
for 2 h at room temperature with anti-platelet antibody (1:100
dilution, anti-CD42b rabbit monoclonal; Abcam), anti-SNAIL antibody
(1:50 dilution, anti-SNAIL rabbit polyclonal antibody, ab180714;
Abcam), anti-FOXP3 antibody (1:50 dilution, anti-FOXP3 mouse
monoclonal, 236A/E7; Abcam), or anti-CD33 antibody (1:100 dilution,
anti-CD33 mouse monoclonal antibody, NCL-L-CD33; Leica Biosystems).
After the sections were washed in Phosphate-buffered saline: PBS,
immunoreactivity was visualized by EnVision reagent (Dako Co.), and
the slides were developed with diaminobenzidine and counterstained
with hematoxylin.
Evaluation of immunostaining
Immunostaining sections were evaluated in tumor
sites containing cancer cells. To evaluate CD42b expression,
immunostained cells were observed in five non-overlapping
intratumoral fields at 400× magnification. Cancer cells and
immunostained cells in the field were counted, and ≥10% of cancer
cells were stained were defined as positive and <10% were as
negative. For SNAIL evaluation, an immunoreactive score was used by
multiplying the staining intensity (0–3) and the stained cell ratio
(0–4). Specimens with a score 0 were classified as negative and
samples with a score 1–12 were classified as positive (21). FOXP3 cells were evaluated by counting
intratumoral fields under high power (×400) and the number of FOXP3
positive cells was defined as the mean number per field. The
average number of FOXP3 positive cells was calculated, and ≥5.5 was
defined as high infiltration and <5.5 as low infiltration
(22). CD33-positive cell
infiltration was evaluated by counting intratumoral fields under
high power (×400). The average number of CD33-positive cells was
evaluated: ≥11 was defined as high infiltration and <11 as low
infiltration (23).
Statistical analysis
Differences in CD42b expression and categorical
variables were analyzed using a χ2 test. Overall
survival rates were calculated by the Kaplan-Meier method, and the
log-rank test was used to compare results between survival times
and between subgroups. P<0.05 was taken to indicate statistical
significance. All statistical analysis was performed using SPSS v23
(SPSS).
Results
Patient and clinicopathological
characteristics
The clinicopathological characteristics of the 62
patients at the time of diagnosis of peritoneal metastasis are
shown in Table I. The median age was
63 (range, 28–83) years, and 26 patients were men and 36 patients
were women. 12 patients had a performance status (PS) ≥1, and the
remaining 50 patients had a PS of 0. 49 patients had initial and 13
patients had recurrent peritoneal metastasis. Primary gastric
cancer was intestinal-type adenocarcinoma in 13 patients and
diffuse-type adenocarcinoma in the remaining 49. 27 patients had a
macroscopic classification of Borrmann type 4. The P statuses
according to the Japanese Classification of Gastric Carcinoma 15th
edition were P1a in 8 cases, P1b in 5 cases, and P1c in 49 cases.
The levels of ascites were none in 23, mild in 17, moderate in 8,
and severe in 14. Ten patients had other distant metastases,
including liver, lung, or lymph node metastasis.
 | Table I.Clinical and pathological data of 62
patients with gastric cancer with peritoneal metastasis. |
Table I.
Clinical and pathological data of 62
patients with gastric cancer with peritoneal metastasis.
|
Characteristics | Value |
|---|
| Age, years (median,
range) | 63 (28–83) |
| Sex, n |
|
|
Male | 26 |
|
Female | 36 |
| Initial or
recurrence, n |
|
|
Initial | 49 |
|
Recurrence | 13 |
| ECOG performance
status, n |
|
| ≥1 | 12 |
| 0 | 50 |
| Borrmann
macroscopic type, n |
|
| 1 | 1 |
| 2 | 3 |
| 3 | 26 |
| 4 | 27 |
| 5 | 5 |
| Differentiation
(Lauren classification), n |
|
|
Intestinal | 13 |
|
Diffuse | 49 |
| Clinical T stage,
n |
|
| T1 | 0 |
| T2 | 0 |
| T3 | 11 |
| T4 | 51 |
| Clinical N stage,
n |
|
| N0 | 15 |
| N1 | 12 |
| N2 | 7 |
| N3 | 28 |
| P status, n |
|
|
P1a | 8 |
|
P1b | 5 |
|
P1c | 49 |
| Ascites, n |
|
|
None | 23 |
|
Mild | 17 |
|
Moderate | 8 |
|
Severe | 14 |
| Other distant
metastasis, n |
|
|
Negative | 52 |
|
Positive | 10 |
CD42b expression in peritoneal
metastasis
We investigated CD42b expression as a marker of EPA
in 62 patients with peritoneal metastasis. All peritoneal
metastasis specimens were collected before chemotherapy. CD42b
expression was observed in 56.5% (35/62) of peritoneal metastatic
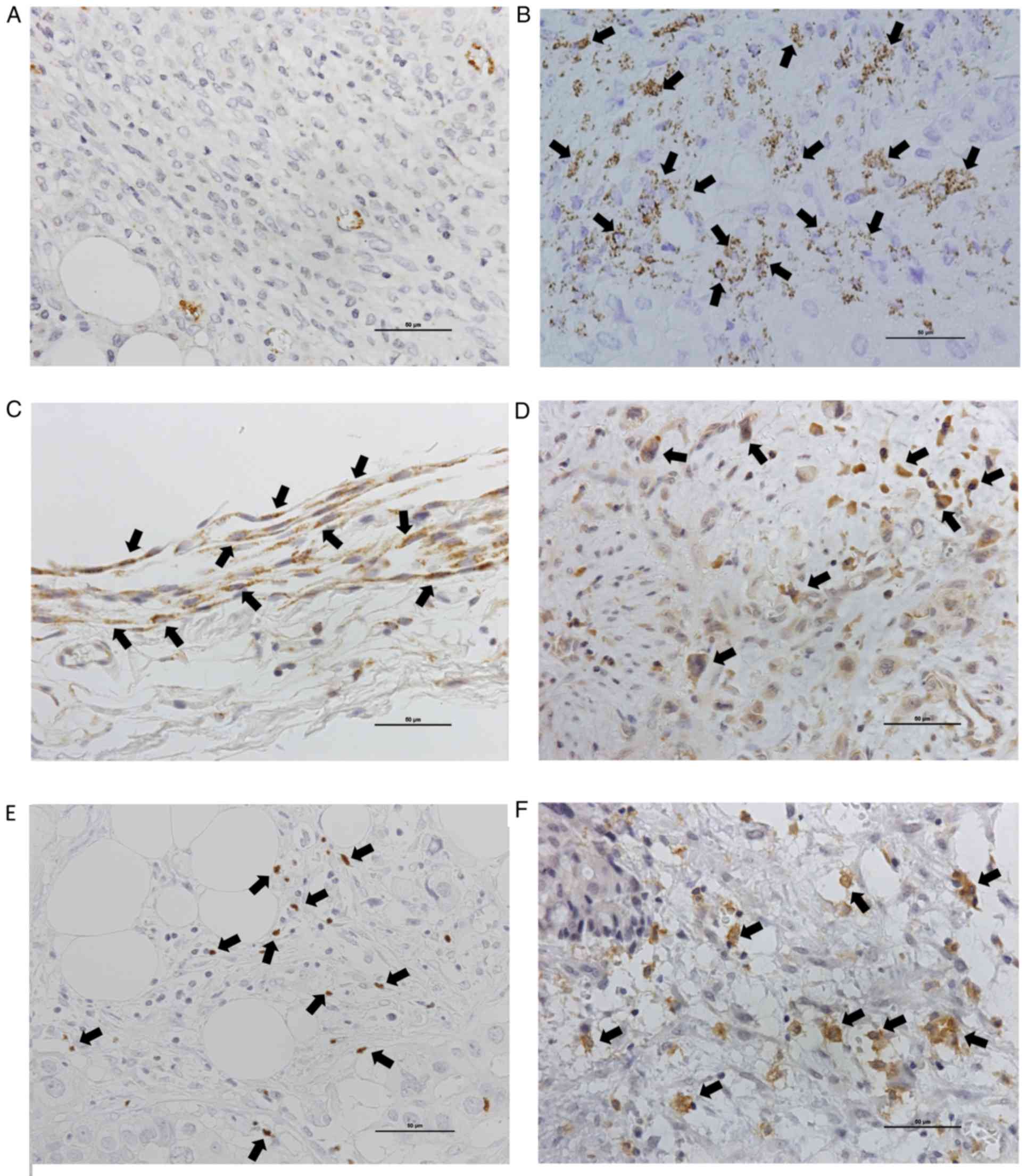
lesions (Fig. 1A and B). EPA was
observed around tumor cells and around CAFs (Fig. 1C).
Relationship between CD42b expression
and clinicopathological features
The relationships between CD42b expression and
clinicopathological features, including age, sex, PS, Borrmann
type, microscopic type, T stage, N stage, P status, ascites volume,
and other distant metastasis are shown in Table II. CD42b expression was clearly
related to sex (P<0.025) and microscopic type (P<0.038), but
not to age, performance status, T stage, N stage, P status, ascites
volume, or other distant metastasis.
 | Table II.Association between CD42b expression
and the clinicopathological characteristics of patients with
gastric cancer with peritoneal metastasis. |
Table II.
Association between CD42b expression
and the clinicopathological characteristics of patients with
gastric cancer with peritoneal metastasis.
| Variables | CD42(−), n
(n=27) | CD42(+), n
(n=35) | P-value |
|---|
| Age, years |
|
| 0.639 |
|
≥70 | 7 | 10 |
|
|
<70 | 20 | 25 |
|
| Sex |
|
| 0.025 |
|
Male | 7 | 19 |
|
|
Female | 20 | 16 |
|
| Initial or
recurrence |
|
| 0.697 |
|
Initial | 21 | 28 |
|
|
Recurrence | 6 | 7 |
|
| ECOG performance
status |
|
| 0.425 |
| 0 | 24 | 26 |
|
| ≥1 | 3 | 9 |
|
| Borrmann
macroscopic type |
|
| 0.373 |
| Type
4 | 10 | 17 |
|
|
Not | 17 | 18 |
|
| Microscopic |
|
| 0.038 |
|
Intestinal | 9 | 4 |
|
|
Diffuse | 18 | 31 |
|
| Clinical T
stage |
|
| 0.678 |
|
2-3 | 5 | 6 |
|
| 4 | 22 | 29 |
|
| Clinical N
stage |
|
| 0.405 |
|
0-2 | 16 | 18 |
|
| 3 | 11 | 17 |
|
| P status |
|
| 0.228 |
|
1a,1b | 8 | 5 |
|
| 1c | 19 | 30 |
|
| Ascites |
|
| 0.084 |
|
None-Moderate | 23 | 25 |
|
|
Severe | 4 | 10 |
|
| Other distant
metastasis |
|
| 0.296 |
|
Negative | 24 | 28 |
|
|
Positive | 3 | 7 |
|
| SNAIL
expression |
|
| 0.271 |
|
Negative | 11 | 9 |
|
|
Positive | 16 | 26 |
|
| FOXP3
infiltration |
|
| 0.564 |
|
Low | 18 | 21 |
|
|
High | 9 | 14 |
|
| CD33
infiltration |
|
| 0.022 |
|
Low | 17 | 8 |
|
|
High | 10 | 27 |
|
SNAIL, FOXP3, and CD33 expression
SNAIL expression was mainly confirmed in the nuclei
of cancer cells. Positive SNAIL expression was observed in 67.7%
(42/62) of cases (Fig. 1D). There
was no relationship between SNAIL and CD42b expression (P=0.271,
Table II). Furthermore, there was
no association between SNAIL expression and OS (P=0.601, Table III). The Treg cell marker FOXP3 was
also confirmed in the nuclei of T cells. High infiltration of
FOXP3-positive cells was detected in 16.1% (23/62) of cases
(Fig. 1E). There was no relationship
between FOXP3 and CD42b expression (P=0.564, Table II) or OS (P=0.823, Table III). High infiltration of
CD33-positive cells was detected in 59.6% (37/62) of cases
(Fig. 1F), and was clearly
correlated with CD42b expression (P=0.022, Table II), but not correlated with OS
(P=0.111).
 | Table III.Univariate analyses of
clinicopathological parameters associated with overall survival in
patients with gastric cancer with peritoneal metastasis. |
Table III.
Univariate analyses of
clinicopathological parameters associated with overall survival in
patients with gastric cancer with peritoneal metastasis.
| Variables | Odds ratio | 95% CI | No. | P-value |
|---|
| Age, years |
|
|
| 0.287 |
|
≥70 | 1.519 | 0.700–3.293 | 17 |
|
|
<70 |
|
| 45 |
|
| Sex |
|
|
| 0.522 |
|
Male | 1.211 | 0.673–2.180 | 26 |
|
|
Female |
|
| 36 |
|
| Initial or
recurrence |
|
|
| 0.286 |
|
Initial | 1.466 | 0.723–2.972 | 49 |
|
|
Recurrence |
|
| 13 |
|
| ECOG performance
status |
|
|
| 0.331 |
| 0 | 1.556 | 0.838–2.888 | 50 |
|
| ≥1 |
|
| 12 |
|
| Borrmann
macroscopic type |
|
|
| 0.736 |
|
Type4 | 0.905 | 0.506–1.619 | 27 |
|
|
Not |
|
| 35 |
|
| Microscopic |
|
|
| 0.535 |
|
Intestinal | 0.811 | 0.418–1.575 | 13 |
|
|
Diffuse |
|
| 49 |
|
| P status |
|
|
| 0.022 |
|
1a,1b | 2.242 | 1.070–4.698 | 13 |
|
| 1c |
|
| 49 |
|
| Ascites |
|
|
| 0.009 |
|
None-Moderate | 2.555 | 1.325–4.928 | 48 |
|
|
Severe |
|
| 14 |
|
| Other distant
metastasis |
|
|
| 0.043 |
|
Negative | 2.231 | 1.006–4.948 | 10 |
|
|
Positive |
|
| 52 |
|
| CD42b
expression |
|
|
| 0.018 |
|
Negative | 2.029 | 1.115–3.690 | 27 |
|
|
Positive |
|
| 35 |
|
| SNAIL
expression |
|
|
| 0.606 |
|
Negative | 0.85 | 0.459–1.576 | 20 |
|
|
Positive |
|
| 42 |
|
| FOXP3
infiltration |
|
|
| 0.823 |
|
Low | 1.073 | 0.580–1.983 | 39 |
|
|
High |
|
| 23 |
|
| CD33
infiltration |
|
|
| 0.111 |
|
Low | 1.712 | 0.878–3.341 | 25 |
|
|
High |
|
| 37 |
|
Relationship between patient
characteristics and overall survival
The relationships between clinicopathological
features and OS were evaluated by log-rank tests (Table III). OS was clearly lower in
patients with P1c (compared to P1a/P1b status), with severe ascites
(compared with no or moderate ascites), and in patients with other
distant metastases (Table III). OS
was not significantly related to age, sex, initial or recurrent
peritoneal metastasis, ECOG PS, Borrmann type, or microscopic
type.
Survival curves according to CD42b,
SNAIL, FOXP3, and CD33 expression
OS curves for gastric cancer with peritoneal
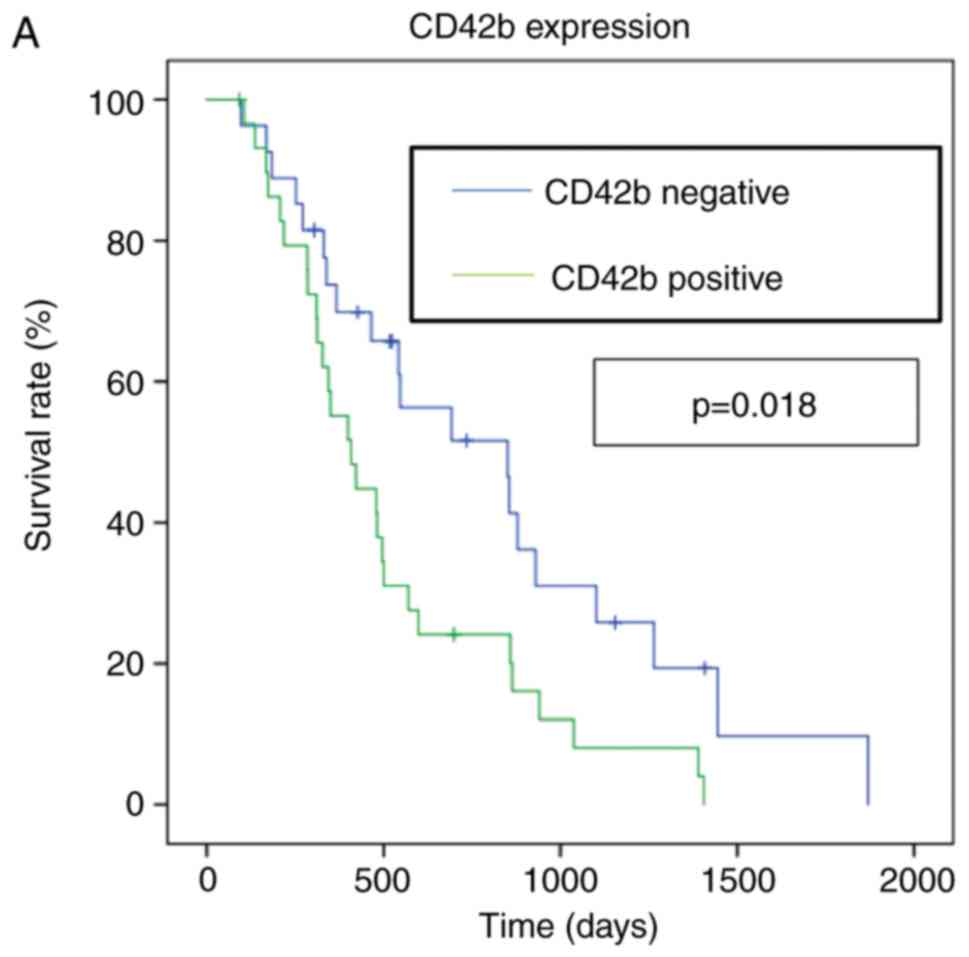
metastasis are shown in Fig. 2.
Median OS for CD42b-positive patients were 13.6 months compared
with 28.4 for CD42b-negative patients (hazard ratio 2.03, 95%
confidence interval 1.12–3.69, P=0.018). In contrast, SNAIL, FOXP3,
and CD33 expression in peritoneal metastatic lesions were not
significantly related to OS (Fig.
2B-D).
Discussion
We detected CD42b expression as a marker of EPA in
56.4% of peritoneal metastases for patients with gastric cancer in
the current study, and clarified that it was a poor prognosis
factor. All patients were diagnosed as Stage IV gastric cancer with
peritoneal metastasis. In our previous study, there were no
significant association between CD42b expression and clinical stage
(11). Generally, tumor stroma
contains fibroblasts which express the p-selectin, and tend to
aggregate the platelets (Fig. 1C).
In this study, we evaluated the gastric cancer cells and platelet
aggregation (Fig. 1B), and did not
evaluate the platelets around the fibroblasts. Platelets play an
important role in the tumor microenvironment during cancer
development, and have been shown to interact with tumor cells.
Mikami et al (24)
investigated that platelets facilitated the gastric cancer cells
growth and that this growth was disturbed by antiplatelet drugs
in vitro and in vivo. Platelets contain a large
amount of growth factors, such as TGF-β, platelet-derived growth
factor, epidermal growth factor, vascular endothelial growth factor
(VEGF), sphingosine 1-phospate, and basic fibroblastic growth
factor within the α-granules that are secreted following platelet
activation (25). Han et al
(26) reported that platelet pellet
(106 platelets) from breast cancer patients contained
higher TGF-β1 level (median 15.3 ng/ml) than control group (median
4.3 ng/ml). These growth factors affect the tumor progression,
angiogenesis, invasion, EMT, and metastasis, not only in the blood
vessels but also in the tumor stroma. Previous reports have
suggested a correlation between EPA and EMT (10); however our data found no association
between expression of the EMT marker SNAIL and EPA. This apparent
discrepancy could be explained by differences in the tumor
microenvironment between primary lesions and metastatic lesions,
given that various stromal cells, including CAFs, human peritoneal
mesothelial cells, mesenchymal stem cells, and M2 macrophages are
present in the peritoneal metastasis environment and affect the EMT
in tumor cells (5,27–29).
CD33 and CD11b are considered as basic markers of
MDSCs. Yu et al (30), also
reported that most CD33-positive cells in primary solid tumors were
MDSCs. The current results found a close relationship between CD42b
expression and CD33 infiltration in peritoneal metastasis. MDSCs
play a pivotal role in tumor-related immunosuppression, and are
recruited by several factors, including TGF-β, VEGF, and matrix
metalloproteinase 9, which are also secreted by platelets (31–33).
MDSCs promote tumor growth by shaping
immunosuppressive responses towards tumor tolerance, and also by
supporting several processes necessary for neoplastic progression,
such as tumor angiogenesis, cancer stemness, and metastasis
dissemination. Our findings thus showed that the presence of EPA in
the tumor microenvironment may induce the recruitment of MDSCs,
resulting in tumor progression.
A large volume of ascites fluid was associated with
a worse prognosis in this current study. However there was no
significant correlation between platelet aggregation and ascitic
fluid volume, there was a tendency for the volume of ascitic fluid
to be higher in CD42b-positive cases. We previously reported a
close relationship between ascites volume and VEGF levels in the
peritoneal cavity, with high levels of VEGF being correlated with a
poor prognosis (34). Expression for
VEGF was also detected in >70% of peritoneal metastases. These
results indicate that VEGF secretion by cancer cells and platelets
promote tumor development by inducing the angiogenesis in the
peritoneal cavity.
VEGF is important for inducing an immunosuppressive
microenvironment in several tumors via MDSCs (35). Horikawa et al (36) reported that VEGF in ovarian cancer
with peritoneal metastatic lesions inhibited immune functions
through MDSCs. Intratumoral MDSCs have also been shown to express
VEGF receptor 2, and VEGF/VEGF receptor 2 signaling directly
promoted MDSC differentiation and tumor infiltration (37,38).
Collectively, these data suggest that MDSCs induced by tumor- and
platelet-derived VEGF signaling play important roles in tumor
immune evasion.
The present study had several limitations. First,
regarding possible heterogeneity of tumor characteristics; the
pathology of gastric cancer with peritoneal metastasis is
complicated, and it is difficult to prove if a small biopsy sample
is characteristic of metastatic lesion. Second, it is not
sufficient to identify the MDSC by the CD33 staining, though the
MDSC marker is lack of defined it. Furthermore, the accumulation of
MDSCs by platelets is consideration in this study, it is necessary
to study in vitro and in vivo experiments. However,
there were few reports on the immune environment in gastric cancer
with peritoneal metastasis, our results are important. Third, this
investigation was conducted at a single institution, retrospective
study, with a relatively small sample size. However, it is
difficult to collect peritoneal metastatic tissue because gastric
cancer patients with peritoneal metastasis generally do not undergo
surgery. These factors should therefore be taken into account, and
further, prospective, multi-center studies are needed to confirm
the results before they can be generalized to daily clinical
work.
In conclusion, the results of the present study
suggest that EPA is associated with a poor prognosis in gastric
cancer patients with peritoneal metastasis. EPA may not only
increase tumor malignancy by secreting soluble factors such as
platelet-derived growth factor, basic fibroblastic growth factor,
and VEGF, but may also affect immunosuppression through the
infiltration of MDSCs into the tumor microenvironment. These data
indicate that EPA may represent a novel therapeutic target in
gastric cancer with peritoneal metastasis.
Acknowledgements
The authors would like to thank Dr Susan Furness for
editing a draft of this manuscript.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TY and SF conceived the study. TY performed the
experiments, analyzed data and wrote the manuscript. SF analyzed
and interpreted the data. JK, MO, SI, YO, ST, KO, SN and IM were
involved in conception and design. KN, TM, HTaj, HTak, IN and TO
were involved in analysis and interpretation of data. SF
contributed to manuscript writing and revision. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All procedures were carried out in accordance with
the ethical standards of the responsible committees on human
experimentation and with the Helsinki Declaration of 1964 and later
versions. This study was approved by the Institutional Review Board
of Kanazawa University Graduate School of Medical Sciences (study
number 2789). Written informed consent was obtained from all
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fushida S, Kinoshita J, Yagi Y, Funaki H,
Kinami S, Ninomiya I, Fujimura T, Nishimura G, Kayahara M and Ohta
T: Dual anti-cancer effects of weekly intraperitoneal docetaxel in
treatment of advanced gastric cancer patients with peritoneal
carcinomatosis: A feasibility and pharmacokinetic study. Oncol Rep.
19:1305–1310. 2008.PubMed/NCBI
|
|
3
|
Fushida S, Kinoshita J, Kaji M, Hirono Y,
Goda F, Yagi Y, Oyama K, Sudo Y, Watanabe Y and Fujimura T; Society
for Study of Peritoneal Carcinomatosis in Gastric Cancer, : Phase
I/II study of intraperitoneal docetaxel plus S-1 for the gastric
cancer patients with peritoneal carcinomatosis. Cancer Chemother
Pharmacol. 71:1265–1272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsukada T, Fushida S, Harada S, Yagi Y,
Kinoshita J, Oyama K, Tajima H, Fujita H, Ninomiya I, Fujimura T
and Ohta T: The role of human peritoneal mesothelial cells in the
fibrosis and progression of gastric cancer. Int J Oncol.
41:476–482. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamaguchi T, Fushida S, Yamamoto Y,
Tsukada T, Kinoshita J, Oyama K, Miyashita T, Tajima H, Ninomiya I,
Munesue S, et al: Tumor-associated macrophages of the M2 phenotype
contribute to progression in gastric cancer with peritoneal
dissemination. Gastric Cancer. 19:1052–1065. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lal I, Dittus K and Holmes CE: Platelets,
coagulation and fibrinolysis in breast cancer progression. Breast
Cancer Res. 15:2072013. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takagi S, Takemoto A, Takami M, Oh-Hara T
and Fujita N: Platelets promote osteosarcoma cell growth through
activation of the platelet-derived growth factor receptor-Akt
signaling axis. Cancer Sci. 105:983–988. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bambace NM and Holmes CE: The platelet
contribution to cancer progression. J Thromb Haemost. 9:237–249.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ludwig RJ, Boehme B, Podda M, Henschler R,
jager E, Tandi C, Boehncke WH, Zollner TM, Kaufmann R and Gille J:
Endothelial P-selectin as a target of heparin action in
experimental melanoma lung metastasis. Cancer Res. 64:2743–2750.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ihsikawa S, Miyashita T, Inokuchi M,
Hayashi H, Oyama K, Tajima H, Takamura H, Ninomiya I, Ahmed AK,
Harman JW, et al: Platelets surrounding primary tumor cells are
related to chemoresistance. Oncol Rep. 36:787–794. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saito H, Fushida S, Miyashita T, Oyama K,
Yamaguchi T, Tsukada T, Kinoshita J, Tajima H, Ninomiya I and Ohta
T: Potential of extravasated platelet aggregation as a surrogate
marker for overall survival in patients with advanced gastric
cancer treated with preoperative docetaxel, cisplatin and S-1: A
retrospective observational study. BMC Cancer. 17:2942017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Assoian RK, Komoriya A, Meyers CA, Miller
DM and Sporn MB: Transforming growth factor-beta in human
platelets. Identification of a major storage site, purification,
and characterization. J Biol Chem. 258:7155–7160. 1983.PubMed/NCBI
|
|
13
|
Labelle M, Begum S and Hynes RO: Direct
signaling between platelets and cancer cells induces an
epithelial-mesenchymal-like transition and promotes metastasis.
Cancer Cell. 20:576–590. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu H, Zhang T, Ye J, Li H, Huang J, Li X,
Wu B, Huang X and Hou J: Tumor-infiltrating lymphocytes predict
response to chemotherapy in patients with advance non-small cell
lung cancer. Cancer Immunol Immunother. 61:1849–1856. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nishikawa H and Sakaguchi S: Regulatory T
cells in tumor immunity. Int J Cancer. 127:759–767. 2010.PubMed/NCBI
|
|
16
|
Winkler I, Wilczynska B, Bojarska-Junak A,
Gogacz M, Adamiak A, Postawski K, Darmochwal-Kolarz D, Rechberger T
and Tabarkiewicz J: Regulatory T lymphocytes and transforming
growth factor beta in epithelial ovarian tumors-prognostic
significance. J Ovarian Res. 8:392015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kusmartsev S and Gabrilovich DI: Role of
immature myeloid cells in mechanisms of immune evasion in cancer.
Cancer Immunol Immunother. 55:237–245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Talmadge JE: Pathways mediating the
expansion and immunosuppressive activity of myeloid-derived
suppressor cells and their relevance to cancer therapy. Clin Cancer
Res. 13(18 Pt 1):5243–5248. 2007. View Article : Google Scholar
|
|
19
|
World Health Organization, .
Classification of tumours. 1:(5). World Health Organization.
(Geneva). 54–64. 2019.
|
|
20
|
Xu XR, Carrim N, Neves MA, McKeown T,
Stratton TW, Coelho RM, Lei X, Chen P, Xu J, Dai X, et al:
Platelets and platelet adhesion molecules: Novel mechanisms of
thrombosis and anti-thrombotic therapies. Thromb J. 14 (Suppl
1):S292016. View Article : Google Scholar
|
|
21
|
Keck B, Wach S, Goebell PJ, Kunath F,
Bertz S, Lehmann J, Stöckle M, Taubert H, Wullich B and Hartmann A:
SNAI1 protein expression is an independent negative prognosticator
in muscle-invasive bladder cancer. Ann Surg Oncol. 20:3669–3674.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oda N, Shimazu K, Naoi Y, Morimoto K,
Shimomura A, Shimoda M, Kagara N, Maruyama N, Kim SJ and Noguchi S:
Intratumoral regulatory T cells as an independent predictive factor
for pathological complete response to neoadjuvant paclitaxel
followed by 5-FU/epirubicin/cyclophosphamide in breast cancer
patients. Breast Cancer Res Treat. 136:107–116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong J, Li J, Liu SM, Feng XY, Chen S,
Chen YB and Zhang XS: CD33+/p-STAT1+ double-positive cell as a
prognostic factor for stage IIIa gastric cancer. Med Oncol.
30:4422013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mikami J, Kurokawa Y, Takahashi T,
Miyazaki Y, Yamasaki M, Miyata H, Nakajima K, Takiguchi S, Mori M
and Doki Y: Antitumor effect of antiplatelet agents in gastric
cancer cells: An in vivo and in vitro study. Gastric Cancer.
19:817–826. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stegner D, Dutting S and Nieswandt M:
Mechanistic explanation for platelet contribution to cancer
metastasis. Thromb Res. 133 (Suppl 2):S149–S157. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Han H, Cao FL, Wang BZ, Mu XR, Li GY and
Wang XW: Expression of angiogenesis regulatory proteins and
epithelial-mesenchymal transition factors in platelets of the
breast cancer patients. ScientificWorldJournal. 2014:8782092014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hong HN, Won YJ, Shim JH, Kim HJ, Han SH,
Kim BS and Kim HS: Cancer-associated fibroblasts promote gastric
tumorigenesis through EphA2 activation in a ligand-independent
manner. J Cancer Res Clin Oncol. 144:1649–1663. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun L, Wang Q, Chen B, Zhao Y, Shen B,
Wang X, Zhu M, Li Z, Zhao X, Xu C, et al: Human gastric cancer
mesenchymal stem cell-derived IL15 contributes to tumor cell
epithelial-mesenchymal transition via upregulation tregs ratio and
PD-1 expression in CD4+T cell. Stem Cells Dev.
27:1203–1214. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yan Y, Zhang J, Li JH, Liu X, Wang JZ, Qu
HY, Wang JS and Duan XY: High tumor-associated macrophages
infiltration is associated with poor prognosis and may contribute
to the phenomenon of epithelial-mesenchymal transition in gastric
cancer. Onco Targets Ther. 9:3975–3983. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu J, Wang Y, Yan F, Zhang P, Li H, Zhao
H, Yan C, Yan F and Ren X: Noncanonical NF-kB activation mediates
stat3-stimulated IDO upregulation in myeloid-derived suppressor
cells in breast cancer. J immunol. 193:2574–2586. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gabrilovich D, Ishida T, Oyama T, Ran S,
Kravtsov V, Nadaf S and Carbone DP: Vascular endothelial growth
factor inhibits the development of dendritic cells and dramatically
affects the differentiation of multiple hematopoietic lineages in
vivo. Blood. 92:4150–4166. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang L and Moses HL: Transforming growth
factor beta: Tumor suppressor or promoter? Are host immune cells
the answer? Cancer Res. 68:9107–9111. 2008.PubMed/NCBI
|
|
33
|
Melani C, Sangaletti S, Barazzetta FM,
Werb Z and Colombo MP: Amino-bisphosphonate-mediated MMP-9
inhibition breaks the tumor-bone marrow axis responsible for
myeloid-derived suppressor cell expansion and macrophage
infiltration in tumor stroma. Cancer Res. 67:11438–11446. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fushida S, Oyama K, Kinoshita J, Yagi Y,
Okamoto K, Tajima H, Ninomiya I, Fujimura T and Ohta T: VEGF is a
target molecule for peritoneal metastasis and malignant ascites in
gastric cancer: Prognostic significance of VEGF in ascites and
efficacy of anti-VEGF monoclonal antibody. Onco Targets Ther.
6:1445–1451. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Voron T, Marcheteau E, Pernot S, Colussi
O, Tartour E, Taieb J and Terme M: Control of the immune response
by pro-angiogenic factors. Front Oncol. 4:702014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Horikawa N, Abiko K, Matsumura N,
Hamanishi J, Baba T, Yamaguchi K, Yoshioka Y, Koshiyama M and
Konishi I: Expression of vascular endothelial growth factor in
ovarian cancer inhibits tumor immunity through the accumulation of
myeloid-derived suppressor cells. Clin Cancer Res. 23:587–599.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang J, Yan J and Liu B: Targeting
VEGF/VEGFR to modulate antitumor immunity. Front Immunol.
9:9782018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Secondini C, Coquoz O, Spagnuolo L,
Spinetti T, Peyvandi S, Ciarloni L, Botta F, Bourquin C and Rüegg
C: Arginase inhibition suppresses lung metastasis in the 4T1 breast
cancer model independently of the immunomodulatory and
anti-metastatic effects of VEGFR-2 blockade. Oncoimmunology.
6:e13164372017. View Article : Google Scholar : PubMed/NCBI
|