Introduction
Gastric carcinoma was reported to be the fifth most
common type of malignant cancer and the third most common cause of
tumour-associated mortality due to late diagnosis in the world in
2018 (1). There are contradictory
reports regarding the relationship between human epidermal growth
factor receptor (HER)2-positivity and the prognosis of patients
with gastric carcinoma (2–4). Previously, good prognosis has been
observed in patients with metastatic HER2-positive gastric
carcinoma who were treated with trastuzumab, a human epidermal
growth factor inhibitor (5,6). HER2 gene amplification or protein
overexpression has been detected in 7–30% of primary gastric
adenocarcinomas (2,4,7–9). It has been reported that HER2
amplification and overexpression are influenced by the histological
type and localisation of tumours (5–10). Due
to technological advances, HER2 can be easily identified; for
example immunohistochemistry and fluorescence, chromogenic or
silver in situ hybridisation have been used to detect HER2
protein overexpression and gene amplification (11,12).
Several studies have compared immunohistochemistry and in
situ hybridisation as methods for detecting HER2; a high degree
of agreement between the results of these methods was observed for
determining HER2 overexpression and amplification (7–9,13).
Grabsch et al (14) reported HER2 immunoreactivity in
<10% of gastric carcinoma cases and >5% of tumour cells, and
demonstrated that HER2 overexpression in gastric carcinoma was
heterogeneous (14). Furthermore,
Hofmann et al (9) reported
that heterogeneity of tumour cells was common when investigating
HER2 overexpression in gastric carcinoma. In breast carcinoma,
microarray methods have been useful for detecting HER2
amplification and overexpression (15). As heterogeneity is common in gastric
carcinoma, evaluating HER2 overexpression and amplification using
the microarray method may be insufficient. In breast cancer, HER2
amplification in the primary tumour may differ from that in any
metastasised lymph node tumours (16). Similarly, HER2 amplification
differences in the primary tumours and metastatic lymph nodes of
gastric carcinoma have also been investigated. Studies have
reported a high degree of agreement between HER2 results for
primary gastric tumours and synchronised lymph node metastases,
although discordant cases were also observed (10,17–20).
The aims of the present study were to investigate
and compare the efficacy of immunohistochemistry and silver in
situ hybridisation (SISH) for detecting HER2 amplification and
overexpression in the primary and metastatic lymph node tumours of
gastric adenocarcinoma. In addition, HER2 expression differences
were compared to assess the potential contribution of these
microarray methods to treatment planning.
Materials and methods
Patients
A total of 86 patients were diagnosed with gastric
adenocarcinoma using surgical resection material at the Pathology
Department of Pamukkale University (Denizli, Turkey) between
January 2008 and January 2014. The inclusion criteria for the
present study were a diagnosis of gastric carcinoma using surgical
resection material and the presence of paraffin embedded blocks for
this specimen. The exclusion criteria were: i) Absence of
metastases in the lymph nodes (n=20); ii) spilling of tumour
tissues from microarray sections of primary tumours or metastatic
lymph nodes (n=4); and iii) visualisation of tumour tissue in
restricted areas of a limited number of metastatic lymph nodes in
Node (N)1 (21) cases (n=2). Hence,
60 patients were included in the present study overall. Of these,
83% (n=50) were male and 17% (n=10) were female (male/female
ratio=5:1). The mean ± standard deviation and median age of
patients were 61±13 and 63 years, respectively (range, 33–86
years). The present study was approved by The Ethics Committee for
Non-Interventional Clinical Research at Pamukkale University (dated
06/12/2018; approval no. 60116787-020/47233).
Haematoxylin-eosin-stained preparations for all
selected cases were prepared from formalin-fixed (using 10%
buffered formalin overnight at room temperature) paraffin-embedded
tissue blocks from the archives of Pamukkale University. The blocks
that best reflected the relevant tumour morphology were selected
for each case. From primary tumours, 3–4 blocks, which best
represented the tumour across the widest area, were selected. From
metastatic lymph nodes of N2 and N3 cases, 3–4 blocks from the
largest metastatic lymph nodes were selected, where tumour tissue
predominated and covered most of the lymph node. In the five N1
cases included in the present study, the metastatic lymph nodes
were large enough to represent the tumour in a large area.
All medical and pathologic records were reviewed to
obtain patient data, including age at diagnosis, sex, tumour size,
histopathological type, tumour depth, number of lymph nodes and
metastatic lymph nodes. The Word Health Organisation 2010
classification (21) was used for
histological classification and pathological staging.
Microarray
The tissue microarray technique (22) was used to evaluate multiple cases in
a single section. The areas that best represented the tumour were
selected. In N2 and N3 cases, 3–4 distinct tissue samples were
obtained from either the primary tumour or metastatic lymph nodes
to represent the tumour. In N1 cases, 3–4 tissue samples were
obtained from distinct fields of a single block or two blocks to
represent the tumour. These tissue samples, which had a 2-mm
diameter, were embedded into recipient paraffin blocks. Then,
3–4-µm thick sections were collected from the recipient paraffin
blocks for immunohistochemical staining and SISH.
Immunohistochemistry
Immunohistochemical staining with HER2 antibody was
re-performed using the primary tumours and metastatic lymph nodes
of 60 cases. The 3–4-µm-thick isolated sections were dried in an
oven at 60°C for ≥2 h. The entire staining process, including
deparaffinisation and antigen retrieval, was performed on a
BenchMark XT fully automated immunohistochemistry-staining
equipment (Ventana Medical Systems, Inc.). The tissue sections were
deparaffinised using EZ Prep (Ventana Medical Systems, Inc.) at
75°C for 4 min and heat pre-treated in Cell Conditioning 1 solution
(Ventana Medical Systems, Inc.) for antigen retrieval at 95°C.
Tissue sections were incubated with the anti-HER2 rabbit monoclonal
primary antibody (clone SP3; 1:100; cat. no. 237R-16; Cell Marque;
Sigma-Aldrich; Merck KGaA) for 1 h at 37°C after inactivation of
endogenous peroxidase activity with 3% hydrogen peroxide (Ventana
Medical Systems, Inc.) for 4 min at 37°C. Non-specific antibody
binding was blocked with 3% bovine serum albumin (Ventana Medical
Systems, Inc.) for 15 min at 37°C. The sections were incubated with
a secondary antibody followed by the application of HRP Universal
Multimer for 8 min at 37°C using the ultraVIEW DAB Detection kit
(Ventana Medical Systems, Inc.). The slides were counterstained
with Hematoxylin II for 8 min at room temperature and bluing
reagent for 4 min at room temperature. All sections were scored
under a multi-head light microscope with ×40 magnification by three
pathologists (GG, YB and ÇDN) blinded to any of the
clinicopathological parameters, including patient outcome. The
scoring system proposed by Hofmann et al (9) was used for HER2 scoring as follows: 0,
0 or <10% staining in tumour cells; 1, noticeable, weak or
incomplete membranous staining in >10% of tumour cells; 2,
weak-moderate, complete or basolateral staining in >10% of
tumour cells; and 3, moderate-strong, complete or basolateral
staining in >10% of tumour cells. Scores 0 and 1 were grouped as
no or low HER2 expression, while scores 2 and 3 were grouped as
HER2 positive (+). Cases with scores of 2 and 3 were considered
positive for HER2 amplification. The highest HER2 score from 3–4
tissue samples, which were all prepared using the microarray block
method from the primary tumours or metastatic lymph nodes of the
same case, was accepted as the final score.
SISH
The SISH method was used to assess the primary
tumours and metastatic lymph nodes of 60 cases. For
deparaffinisation, 3-µm thick sections from the microarray blocks
were incubated in an oven at 60°C for 2 h. Slices were processed in
an automated BenchMark XT for SISH. After deparaffinisation with EZ
Prep (Ventana Medical Systems, Inc.) at 75°C for 4 min, slices were
incubated in citrate buffer for 12 min at 90°C and then in ISH
Protease 3 (Ventana Medical Systems, Inc.) for 16 min at 37°C,
denatured for 20 min at 80°C and finally hybridised for 6 h at
37°C. Samples were then subjected to the SISH multimer for 16 min,
silver chromogen for 4 min, red ISH multimer for 24 min and red
chromogen for 8 min, all at room temperature (all Ventana Medical
Systems, Inc.). Finally, the samples were incubated with Mayer's
haematoxylin for 8 min at room temperature and with bluing reagent
for 4 min at room temperature to stain the background. All sections
were analysed under a multi-head light microscope with ×40
magnification by three pathologists (GG, YB and ÇDN). The HER2 gene
was detected by a dinitrophenyl (DNP) labelled probe and visualized
utilizing VENTANA ultraView Silver ISH DNP Detection kit (cat. no.
760-098; Ventana Medical Systems, Inc.). The chromosome-17
centromere was targeted with a digoxigenin (DIG) labelled probe and
detected using VENTANA ultraView Red ISH DIG Detection kit (cat.
no. 760-505; Ventana Medical Systems, Inc.). The signals for the
HER2 gene and the chromosome-17 centromere were visualised in black
and red, respectively; amplification was analysed by manually
counting 20 consecutive cells under a light microscope with ×40
magnification. In cases with a HER2:centromeric probe for
chromosome 17 (CEP17) ratio of 1.8–2.2, 20 consecutive cells were
counted again. If HER2:CEP17 ratios were ≥2, these cells were
considered to indicate amplification (+). An absence of
amplification in 3–4 blocks prepared from the primary tumour or
metastatic lymph node of the same case using the microarray block
method was considered to indicate ‘no amplification’. If
amplification was detected in only one of the samples and not in
the others, amplification was still considered to be present.
Statistical analysis
All statistical analyses were performed using SPSS
version 17.0 (SPPS Inc.). The number of experimental repeats was 1.
The normal distribution of variables was examined using histograms
and the Kolmogorov-Smirnov test. Mean, standard deviation, median
and minimum-maximum values were used as statistical descriptors.
The κ test was used to compare HER2 results between the primary
tumours and metastatic lymph nodes, the single block method and the
microarray method, and the immunohistochemical method and the SISH
method. The concordance rate was calculated as the ratio of
concordant cases to total cases. The predictive values of the
microarray method, SISH method and metastatic lymph node HER2
results according to actual diagnoses were compared in binary
groups and assessed according to sensitivity, specificity, positive
predictive, negative predictive and false-negative rate values.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinicopathological features
The adenocarcinoma cases analysed in the present
study were as follows: 47% (n=28) Tubular, 5% (n=3) papillary, 10%
(n=6) mucinous, 35% (n=21) poorly cohesive and 3% (n=2) mixed
adenocarcinomas. Tumour (T) depth was classified as follows: 2%
(n=1) T1, 2% (n=1) T2, 18% (n=11) T3 and 78% (n=47) T4. The number
of metastatic lymph nodes in cases was assessed as follows: 8%
(n=5) N1, 28% (n=17) N2 and 63% (n=38) were N3. Distant metastasis
was present in 12% (n=7) of cases. The distribution of
clinicopathological features is shown in Table I.
 | Table I.Clinicopathological features of
patients with gastric carcinoma. |
Table I.
Clinicopathological features of
patients with gastric carcinoma.
| Clinicopathological
feature | No. (%) |
|---|
| Sex |
|
Male | 50 (83) |
|
Female | 10 (17) |
| Histological
type |
| Tubular
adenocarcinoma | 28 (47) |
|
Papillary adenocarcinoma | 3 (5) |
|
Mucinous adenocarcinoma | 6 (10) |
| Poorly
cohesive adenocarcinoma | 21 (35) |
| Mixed
adenocarcinoma | 2 (3) |
| T grade |
| 1 | 1 (2) |
| 2 | 1 (2) |
| 3 | 11 (18) |
| 4 | 47 (78) |
| N grade |
| 0 | 0 (0) |
| 1 | 5 (8) |
| 2 | 17 (28) |
| 3 | 38 (63) |
| M grade |
| 0 | 53 (88) |
| 1 | 7 (12) |
Comparison of immunohistochemical HER2
detection results in primary tumour sections prepared after single
or microarray blocking
All 42 cases with a HER2 score of 0 in a single
block also had a score of 0 in sections prepared after microarray
blocking. In five cases with a single block HER2 score of 1, 40%
(n=2) of these cases had a HER2 score 0, while 60% (n=3) had score
of 1 in sections prepared after microarray blocking. Moreover, five
cases had a single block HER2 score of 2; however, after microarray
blocking, 40% (n=2) of these cases had score 0, 40% (n=2) score of
2 and 20% (n=1) score of 3. In total, eight patients had a HER2
score of 3 in a single block; after microarray blocking, 25% (n=2)
of these cases had a score of 0, 13% (n=1) had a score of 2 and 63%
(n=5) had a score of 3 (Table II).
For primary tumours, there was a high degree of concordance (87%
concordance rate, κ=0.681, P<0.001) between the
immunohistochemical HER2 results from single blocks and sections
prepared after microarray blocking. When HER2 scores of 0 and 1
were grouped as no or low HER2 expression and scores of 2 and 3
were grouped as HER2+, the sensitivity of the microarray
method in comparison to the single block method was 69%, whereas
specificity was 100%, positive predictive value was 100%, negative
predictive value was 92% and the false-negative rate was 30% (data
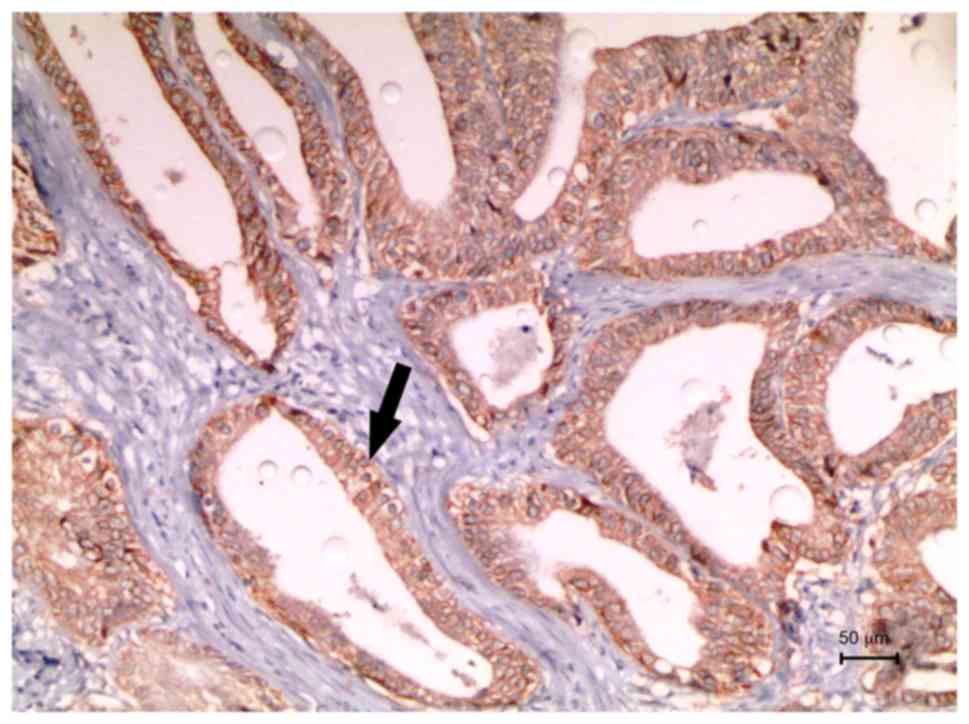
not shown). A case with a HER2 score of 3 after microarray blocking
is shown in Fig. 1.
 | Table II.Comparison of immunohistochemical
HER2 detection results in primary tumour sections prepared after
single or microarray blocking. |
Table II.
Comparison of immunohistochemical
HER2 detection results in primary tumour sections prepared after
single or microarray blocking.
|
| IHC HER2 single
block score |
|
|
|
|---|
|
|
|
|
|
|
|---|
|
| 0 | 1 | 2 | 3 |
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| IHC HER2 microarray
blocking score | n | % | n | % | n | % | n | % | P-value | κ | CR, % |
|---|
| 0 | 42 | 100 | 2 | 40 | 2 | 40 | 2 | 25 | <0.001 | 0.681 | 87 |
| 1 | 0 | 0 | 3 | 60 | 0 | 0 | 0 | 0 |
|
|
|
| 2 | 0 | 0 | 0 | 0 | 2 | 40 | 1 | 13 |
|
|
|
| 3 | 0 | 0 | 0 | 0 | 1 | 20 | 5 | 63 |
|
|
|
Comparison of HER2 results obtained by
immunohistochemical and SISH methods using microarray blocked
sections derived from primary tumours
Using the SISH method, HER2 amplification was
detected in 8% of 60 cases of gastric carcinoma (data not shown).
In the 9 HER2+ cases that had HER2 scores of 2–3
according to immunohistochemistry, the SISH method detected HER2
amplification in 56% (n=5) of cases, but not in 44% (n=4) of cases
(Table III). In the four cases
without HER2 amplification, 50% (n=2) had a HER2 score of 2 and 50%
(n=2) had a HER2 score of 3 according to immunohistochemistry (data
not shown). Using the SISH method, HER2 amplification was not
detected in 100% of the 51 no or low HER2 expression cases (HER2
score of 0–1 according to immunohistochemistry; Table III). In primary tumours, there was
a high degree of concordance (93% concordance rate, κ=0.681,
P<0.001) between the HER2 results derived from the
immunohistochemical and SISH methods in microarray blocked
sections. When comparing the results of the SISH method to those of
the immunohistochemical method for primary tumours, sensitivity,
specificity, positive predictive and negative predictive values,
and the false-negative rate of the SISH method were 56, 100, 100,
93 and 44%, respectively (data not shown).
 | Table III.Comparison of HER2 results obtained
by IHC and SISH methods using microarray blocked sections derived
from primary tumours. |
Table III.
Comparison of HER2 results obtained
by IHC and SISH methods using microarray blocked sections derived
from primary tumours.
|
| Primary tumours IHC
HER2 |
|
|
|
|---|
|
|
|
|
|
|
|---|
|
| Positive | No or low
expression |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Primary tumours
SISH HER2 | n | % | n | % | P-value | κ | CR, % |
|---|
| Amplification
(+) | 5 | 56 | 0 | 0 | <0.001 | 0.681 | 93 |
| Amplification
(−) | 4 | 44 | 51 | 100 |
|
|
|
Comparison of HER2 expression in
primary tumours and metastatic lymph nodes using
immunohistochemistry in microarray blocked sections
Of the 48 cases that had a HER2 score of 0 in the
primary tumour, 100% had a HER2 score of 0 in the metastatic lymph
nodes. Of the three cases that had a HER2 score of 1 in the primary
tumour, 67% (n=2) and 33% (n=1) had HER2 score of 0 and 1,
respectively, in the metastatic lymph nodes. Of the three cases
that had a HER2 score of 2 in the primary tumour, 67% (n=2) and 33%
(n=1) had HER2 scores of 2 and 3, respectively, in the metastatic
lymph nodes. Of the six cases with a HER2 score of 3 in their
primary tumour, 50% (n=3) had a HER2 score of 2 and 3 in the
metastatic lymph nodes (Table IV).
There was a high degree of concordance (90% concordance rate,
κ=0.689, P<0.001) between the HER2 scores for primary tumours
and those for metastatic lymph nodes when immunohistochemical
testing of microarray blocked sections was used. When HER2 scores
of 0 and 1 were grouped as no or low HER2 expression, and scores of
2 and 3 were grouped as HER2+, there was a 100%
concordance rate between HER2 results for the primary tumours and
those for the metastatic lymph nodes; this association was
statistically significant (ƙ=1, P<0.001; data not shown).
Therefore, when comparing the HER2 results from the metastatic
lymph nodes and the primary tumours, the sensitivity, specificity,
positive predictive and negative predictive value of metastatic
lymph node results were all 100% (data not shown).
 | Table IV.Comparison of HER2 expression in
primary tumours and metastatic lymph nodes using IHC in microarray
blocked sections. |
Table IV.
Comparison of HER2 expression in
primary tumours and metastatic lymph nodes using IHC in microarray
blocked sections.
|
| Primary tumours IHC
HER2 score |
|
|
|
|---|
|
|
|
|
|
|
|---|
|
| 0 | 1 | 2 | 3 |
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Metastatic lymph
nodes IHC HER2 score | n | % | n | % | n | % | n | % | P-value | κ | CR, % |
|---|
| 0 | 48 | 100 | 2 | 67 | 0 | 0 | 0 | 0 | <0.001 | 0.689 | 90 |
| 1 | 0 | 0 | 1 | 33 | 0 | 0 | 0 | 0 |
|
|
|
| 2 | 0 | 0 | 0 | 0 | 2 | 67 | 3 | 50 |
|
|
|
| 3 | 0 | 0 | 0 | 0 | 1 | 33 | 3 | 50 |
|
|
|
Comparison of HER2 amplification in
primary tumours and metastatic lymph nodes using SISH in microarray
blocked sections
In 100% of the five cases with HER2 amplification in
the primary tumour, HER2 amplification was also detected in the
lymph nodes. In contrast, HER2 amplification was detected in the
lymph nodes in only 2% (n=1) of the 55 cases where HER2
amplification was not detected in the primary tumours (Table V). There was a very strong
concordance (98% concordance rate, κ=0.900, P<0.001) between the
HER2 amplification in primary tumours and metastatic lymph nodes
when the SISH method was used to detect HER2 amplification. When
comparing the SISH-detected HER2 amplification in primary tumours
and metastatic lymph nodes, the sensitivity, specificity, positive
predictive and negative predictive values of the metastatic lymph
node were 100, 98, 83 and 100%, respectively (data not shown). A
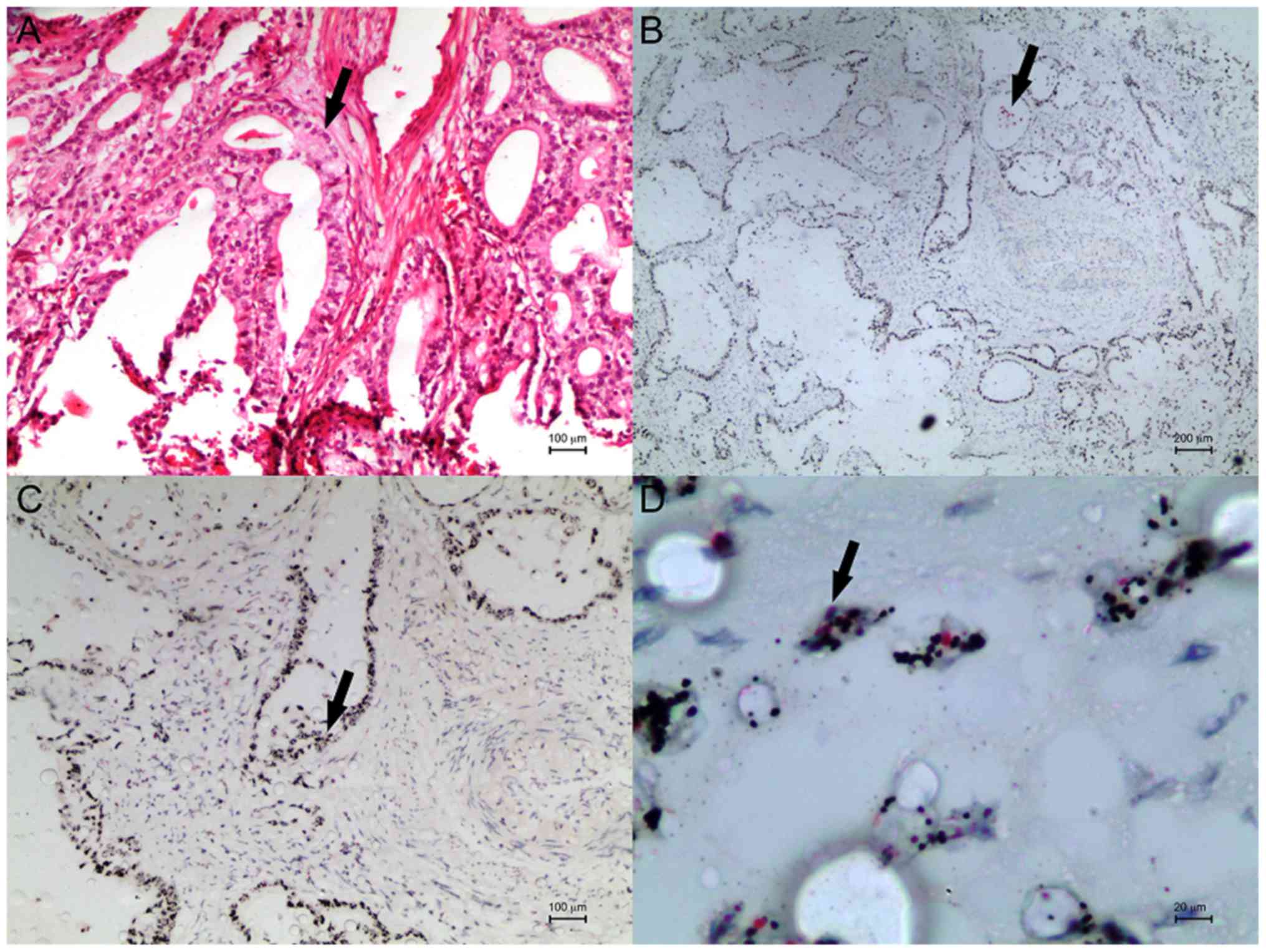
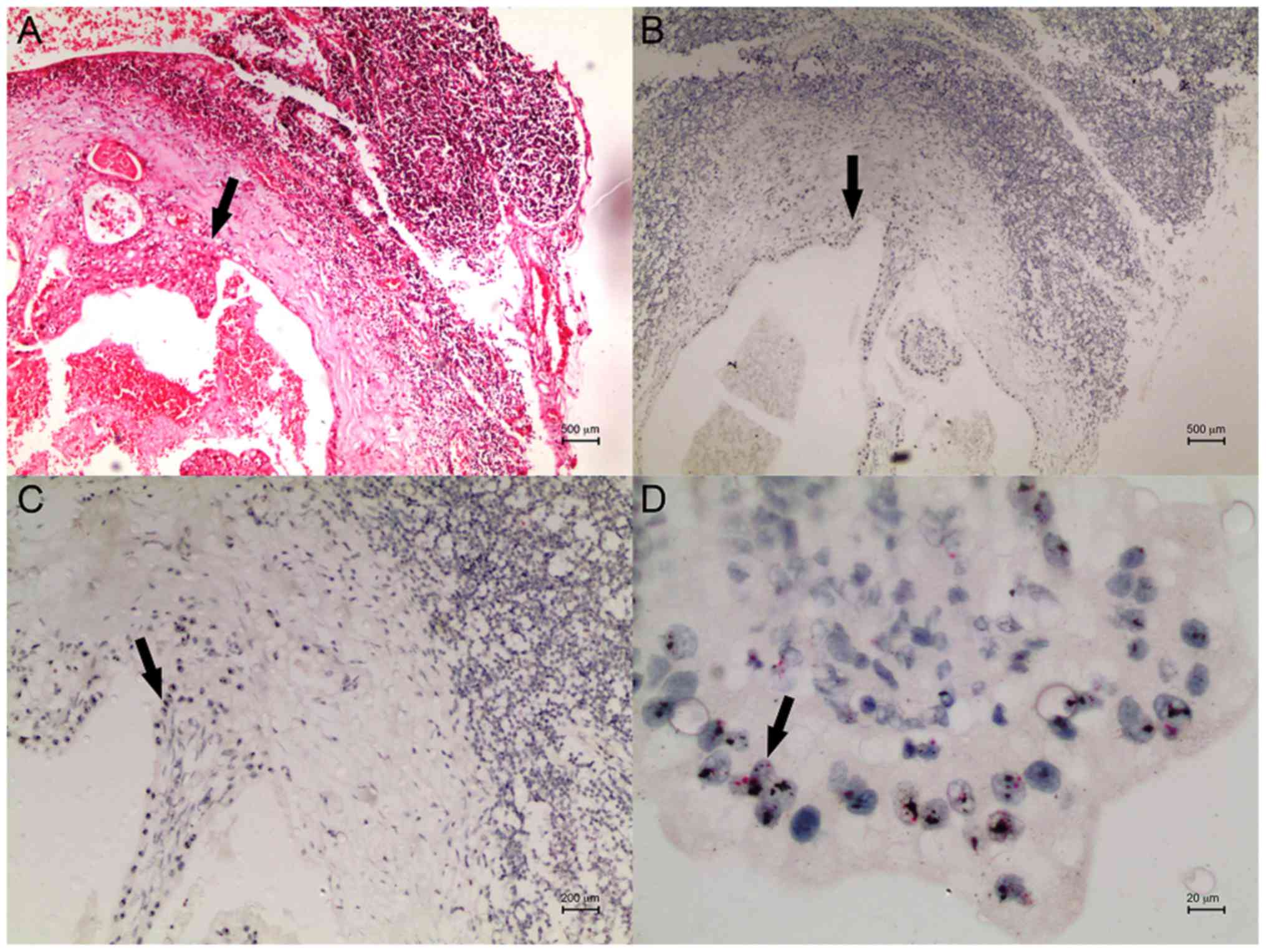
case with HER2 amplification in the primary tumour and lymph node
after microarray blocking is shown in Figs. 2 and 3.
 | Table V.Comparison of HER2 amplification in
primary tumours and metastatic lymph nodes using SISH in microarray
blocked sections. |
Table V.
Comparison of HER2 amplification in
primary tumours and metastatic lymph nodes using SISH in microarray
blocked sections.
|
| Primary tumours
SISH HER2 |
|
|
|
|---|
|
|
|
|
|
|
|---|
|
| Amplification
(+) | Amplification
(−) |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Metastatic lymph
nodes SISH HER2 | n | % | n | % | P-value | κ | CR, % |
|---|
| Amplification
(+) | 5 | 100 | 1 | 2 | <0.001 | 0.900 | 98 |
| Amplification
(−) | 0 | 0 | 54 | 98 |
|
|
|
Discussion
Gastric cancer was the fifth most common type of
cancer in the world in 2018 (1).
Overexpression and gene amplification of HER2 has been
detected in breast, stomach, colon, lung and ovarian cancer
(23). Furthermore, a previous study
showed that the evaluation of HER2 was important for trastuzumab
treatment in gastric carcinoma (5).
However, as heterogeneity is common in gastric carcinomas, >1
method may be required to detect HER2 amplification and expression
accurately. Differences in HER2 expression in the primary tumour
and lymph node metastasis were investigated in the present
study.
A wide range of prevalence has been reported for
HER2 gene amplification (7-42%) and protein expression
(7-34%) in gastric cancer (9). Such
a high degree of variation may be due to the increased tumour
heterogeneity in gastric carcinomas, as well as the different
approaches used to assess HER2 amplification and expression. For
example, Asioli et al (24),
evaluated HER2 in gastric cancer with single and multiple blocks
tests, and reported that HER2 positivity increased when the latter
approach was used. In another study, immunohistochemical HER2
levels in sections prepared from one and two paraffin blocks in
surgically resected gastric carcinomas were compared; HER2
positivity was 14.4% in sections prepared from one paraffin block
but 19.3% in sections prepared from two paraffin blocks (25).
In gastric carcinomas, adequate and accurate
histological evaluation of endoscopic biopsy materials requires 6–8
endoscopic biopsies (26). Tominaga
et al (27) reported that an
agreement between the resection material and biopsies in gastric
carcinomas could be achieved using ≥5 biopsies (27). The use of the microarray method for
HER2 evaluation in gastric carcinomas is controversial because of
the high frequency of intratumoral heterogeneity in gastric cancer.
For example, using gastrectomy material, Warneke et al
(28) compared HER2 overexpression
in a microarray prepared from five sections and in whole tissue
samples prepared from a single block; the group reported a
false-negative rate of 24% for HER2 overexpression when the
microarray method was used. In addition, Stahl et al
(29) demonstrated that HER2
amplification and overexpression results may be inaccurate when
small biopsies and the microarray method are used because of the
heterogeneity of gastric carcinomas. This group also suggested that
these inaccuracies could potentially limit treatment decisions.
In the present study, 13 of the 60 gastric carcinoma
cases had a HER2 score 2 or 3 in the primary tumours using
immunohistochemical analysis, and the HER2 positivity rate was 22%,
which was consistent with the previous literature (4,8,9). To re-evaluate HER2 expression
immunohistochemically for the 60 cases, the primary tumours were
used to obtain new sections by blocking 3–4 tissues from each case
using the microarray method, and 15% HER2 positivity was detected
in microarray blocked sections. Furthermore, in primary tumour
sections, there was a high degree of concordance between HER2
expression results from the single block method and the microarray
method (87% concordance rate). Compared with the single block
method, the sensitivity and specificity of the microarray method
were 69 and 100%, respectively. Additionally, the rate of false
negatives for the microarray method was 30%. When using the single
block method, two cases had a HER2 score of 3 and two had a HER2
score of 2, meanwhile these cases had a HER2 score of 0 when the
microarray method was used. This showed that the rate of false
negatives increased because a smaller tumour area was evaluated
using the microarray method. The heterogeneity of gastric
carcinomas may also be a contributing factor for these
discrepancies. Overall, these findings suggested that the greater
the amount of tissue taken during the microarray method, the lower
the rate of false negatives and the higher the number of positive
cases. In addition to potentially lowering the reliability of
results, the microarray method is more costly and time-consuming
compared with the single block method. Therefore, with respect to
time, cost and reliability, priority should be given to the single
block method over the microarray method when assessing HER2 in
gastric carcinoma.
Previous studies have reported a high degree of
agreement between the results of immunohistochemical methods and
fluorescence in situ hybridisation (FISH) for the evaluation
of HER2 in gastric carcinoma (7–9,13,17).
Werner et al (30)
investigated the agreement between HER2 results obtained using
immunohistochemical method and SISH in gastric carcinoma. The
agreement was 100, 98.96, 95.83 and 96.88% in cases with an
immunohistochemistry HER2 score of 0, 1, 2 and 3, respectively. Kim
et al (31) compared the HER2
RNA-ISH method to immunohistochemistry, FISH and SISH in gastric
carcinoma; the HER2 amplifications obtained using FISH or SISH
methods were found to have a good agreement. In another previous
study, there was an 87% agreement between HER2 results obtained
using FISH and immunohistochemistry (5). Additionally, patients who were
immunohistochemically HER2− and had HER2 amplification
according to the ISH method did not benefit from trastuzumab
treatment, concluding that the immunohistochemical method should be
the primary method for evaluation of HER2 (5).
In the present study, the SISH method was applied to
microarray blocked sections from primary tumours and HER2
amplification was detected in 8% of 60 gastric carcinoma cases,
which is consistent with previous literature (2,7,9). In these microarray blocked sections
from primary tumours, a high degree of concordance (93%) was
observed between immunohistochemical HER2 expression and HER2
amplification using the SISH method, which is consistent with
previous literature (30,31). When compared with the
immunohistochemical method, the sensitivity and specificity of the
SISH method were 56 and 100%, respectively, while the
false-negative rate was 44%. This suggested that HER2+
cases were detected less frequently by the SISH method and no or
low HER2 expression cases were detected 100% of the time using
SISH. Therefore, although there is a high degree of compatibility
between the immunohistochemical and SISH methods, it was concluded
that immunohistochemistry should be the first step during
evaluation of HER2 positivity in gastric carcinoma treatment plans
as SISH alone can produce false-negative results.
Several studies have compared HER2 positivity in the
primary tumours and metastatic lymph nodes of gastric carcinoma. In
studies by Ieni et al (19)
and Fusco et al (32) the
agreement between HER2 results for primary gastric tumours and
synchronised lymph node metastases were revealed to be high (95 and
90.74%, respectively). Furthermore, Kochi et al (18) Bozzetti et al (17) and Marx et al (7) found high degrees of agreement between
HER2 results obtained using immunohistochemistry and FISH in
primary tumours and metastatic lymph nodes. Kochi et al
(18) also suggested that analysing
the primary tumour alone for HER2 levels is not always sufficient
to inform treatment selection, concluding that metastatic lymph
nodes should also be evaluated for HER2. Kim et al (10) reported a divergence rate of 21.8%
between HER2 results obtained using immunohistochemistry in primary
and metastatic tumours. Furthermore, Pagni et al (20) revealed a significant divergence
between the HER2 results from primary tumours and lymph node
metastases and suggested that this diversity was due to
intratumoral heterogeneity.
In the present study, when using
immunohistochemistry to assess microarray blocked sections, a high
degree of concordance (90%) was observed between the HER2 scores
for primary tumours and metastatic lymph nodes. HER2 scores for
primary and metastatic lymph nodes were not associated in 10% of
the 60 cases; of the six discordant cases, 50% (n=3) had HER2 score
of 3 for primary tumour and HER2 score of 2 for metastatic lymph
nodes. This suggested that discrepancies in HER2 score may exist
between primary tumours and lymph node metastases in gastric
carcinoma due to intratumoral heterogeneity. However, when the HER2
scores were grouped as either positive or no or low expression, a
100% concordance between HER2 results from primary tumours and
those from metastatic lymph nodes was observed. Primary tumours and
metastatic lymph nodes were HER2+ in nine patients, and
primary tumours and metastatic lymph nodes were HER2 no or low
expression in 51 patients. Therefore, although differences in HER2
scores existed, complete agreement for HER2 positivity in primary
tumours and metastatic lymph nodes was observed when HER2 scores of
0 and 1 were grouped as no or low HER2 expression, and scores of 2
and 3 were grouped as HER2+. Moreover, the sensitivity
and specificity for detecting the metastatic lymph nodes were both
100% when compared with those of the primary tumours. Thus,
immunohistochemical HER2 evaluation of primary tumours may be
sufficient for planning trastuzumab treatment; however, evaluation
of HER2 in metastatic lymph nodes could be used when primary tumour
tissues cannot be obtained.
When using SISH in microarray blocked sections, a
98% concordance rate was observed between the HER2 amplification
results from the primary tumour and those from the metastatic lymph
node. In the five cases with HER2 amplification in the primary
tumour, there was also HER2 amplification in the metastatic lymph
nodes. Among 55 cases in which HER2 amplification was not observed
in the primary tumour, only one case also had amplification in the
metastatic lymph node. In this specific case, the
immunohistochemical method yielded a HER2 score of 3 in the primary
tumour sections prepared from a single block. In contrast, the
immunohistochemical method resulted in a HER2 score of 0 in
microarray blocked sections of the primary tumour and HER2
amplification was not detected by the SISH method. These
discrepancies may have been due to heterogeneity. Furthermore, in
the metastatic lymph nodes of this case, the immunohistochemical
method resulted in a HER2 score of 0 in the sections blocked by the
microarray method. The results of this anomalous case suggested
that HER2 positivity and amplification cannot always be detected in
the primary tumour, but HER2 positivity and/or amplification may
still be detected in the metastatic lymph nodes due to
heterogeneity. Moreover, there may be cases of gastric carcinoma in
which HER2 positivity cannot be detected in metastatic lymph nodes
using the immunohistochemical method but HER2 amplification can
found using SISH. Therefore, it was concluded that both the
immunohistochemical method and SISH method may be used for
analysing HER2 amplification to identify patients suitable for
trastuzumab treatment, and that this should be a focus of future
work.
In conclusion, priority should be given to the
single block method when evaluating HER2 by immunohistochemical
staining in gastric carcinomas. The limitation of the present study
was obtaining 3–4 tissue samples from the primary tumour and
metastatic lymph nodes in microarray block sections. This is
because microarray blocked sections are evaluated in smaller areas,
and therefore, false-negative results for HER2 amplification and
overexpression may be found due to intratumoral heterogeneity. To
ensure trastuzumab treatment planning is reliable and effective,
when using the microarray method, a large amount of tissue should
be blocked and evaluated in each case. The immunohistochemical
method should also be used as a first step in treatment planning as
it is more accurate compared with the SISH method, which when used
alone may lead to false-negative results. Finally, investigating
numerous tumour areas and tumour types (both primary and lymph node
metastases) using immunohistochemical and ISH methods may increase
the chances of successful trastuzumab treatment, and this should be
a focus of future work.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
GG, BY and NYD contributed to the design and
conception of the study, data collection, data analysis and
literature research. GG, BY and NYD performed histopathological
examination of gastric carcinomas. GG wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee for Non-Interventional Clinical Research at Pamukkale
University (dated 12.06.2018; approval no. 60116787-020/47233).
Patient consent for participation was waived by The Ethics
Committee for Non-Interventional Clinical Research at Pamukkale
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clın. 68:394–424. 2018. View Article : Google Scholar
|
|
2
|
Tanner M, Hollmén M, Junttila TT, Kapanen
AI, Tommola S, Soini Y, Helin H, Salo J, Joensuu H, Sihvo E, et al:
Amplification of HER-2 in gastric carcinoma: Association with
Topoisomerase IIalpha gene amplification, intestinal type, poor
prognosis and sensitivity to trastuzumab. Ann Oncol. 16:273–278.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jørgensen JT and Hersom M: HER2 as a
prognostic marker in gastric cancer-a systematic analysis of data
from the literature. J Cancer. 3:137–144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park DI, Yun JW, Park JH, Oh SJ, Kim HJ,
Cho YK, Sohn CI, Jeon WK, Kim BI, Yoo CH, et al: HER-2/neu
amplification is an independent prognostic factor in gastric
cancer. Dig Dis Sci. 51:1371–1379. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omura Y, Satoh T,
et al: ToGA Trial Investigators. Trastuzumab in combination with
chemotherapy versus chemotherapy alone for treatment of
HER2-positive advanced gastric or gastro-oesophageal junction
cancer (ToGA): A phase 3, open-label, randomised controlled trial.
Lancet. 376:687–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cortés-Funes H, Rivera F, Alés I, Márquez
A, Velasco A, Colomer R, García-Carbonero R, Sastre J, Guerra J and
Grávalos C: Phase II of trastuzumab and cisplatin in patients with
advanced gastric cancer with HER2/neu overexpression/amplification.
J Clin Oncol. 25 (Suppl 18):46132007. View Article : Google Scholar
|
|
7
|
Marx AH, Tharun L, Muth J, Dancau AM,
Simon R, Yekebas E, Kaifi JT, Mirlacher M, Brümmendorf TH,
Bokemeyer C, et al: HER-2 amplification is highly homogenous in
gastric cancer. Hum Pathol. 40:769–777. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takehana T, Kunitomo K, Kono K, Kitahara
F, Iizuka H, Matsumoto Y, Fujino MA and Ooi A: Status of c-erbB-2
in gastric adenocarcinoma: A comparative study of
immunohistochemistry, fluorescence in situ hybridization and
enzyme-linked immuno-sorbent assay. Int J Cancer. 98:833–837. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hofmann M, Stoss O, Shi D, Büttner R, van
de Vijver M, Kim W, Ochiai A, Rüschoff J and Henkel T: Assessment
of a HER2 scoring system for gastric cancer: Results from a
validation study. Histopathology. 52:797–805. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim MA, Lee HJ, Yang HK, Bang YJ and Kim
WH: Heterogeneous amplification of ERBB2 in primary lesions is
responsible for the discordant ERBB2 status of primary and
metastatic lesions in gastric carcinoma. Histopathology.
59:822–831. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Francis GD, Jones MA, Beadle GF and Stein
SR: Bright-field in situ hybridization for HER2 gene amplification
in breast cancer using tissue microarrays: Correlation between
chromogenic (CISH) and automated silver-enhanced (SISH) methods
with patient outcome. Diagn Mol Pathol. 18:88–95. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ross JS, Slodkowska EA, Symmans WF,
Pusztai L, Ravdin PM and Hortobagyi GN: The HER-2 receptor and
breast cancer: Ten years of targeted anti-HER-2 therapy and
personalized medicine. Oncologist. 14:320–368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yano T, Doi T, Ohtsu A, Boku N, Hashizume
K, Nakanishi M and Ochiai A: Comparison of HER2 gene amplification
assessed by fluorescence in situ hybridization and HER2 protein
expression assessed by immunohistochemistry in gastric cancer.
Oncol Rep. 15:65–71. 2006.PubMed/NCBI
|
|
14
|
Grabsch H, Sivakumar S, Gray S, Gabbert HE
and Müller W: HER2 expression in gastric cancer: Rare,
heterogeneous and of no prognostic value- conclusions from 924
cases of two independent series. Cell Oncol. 32:57–65.
2010.PubMed/NCBI
|
|
15
|
Drev P, Grazio SF and Bracko M: Tissue
microarrays for routine diagnostic assessment of HER2 status in
breast carcinoma. Appl Immunohistochem Mol Morphol. 16:179–184.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Simon R, Nocito A, Hübscher T, Bucher C,
Torhorst J, Schraml P, Bubendorf L, Mihatsch MM, Moch H, Wilber K,
et al: Patterns of Her-2/neu amplification and overexpression in
primary and metastatic breast cancer. J Natl Cancer Inst.
93:1141–1146. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bozzetti C, Negri FV, Lagrasta CA, Crafa
P, Bassano C, Tamagnini I, Gardini G, Nizzoli R, Leonardi F,
Gasparro D, et al: Comparison of HER2 status in primary and paired
metastatic sites of gastric carcinoma. Br J Cancer. 104:1372–1376.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kochi M, Fujii M, Masuda S, Kanamori N,
Mihara Y, Funada T, Tamegai H, Watanabe M, Suda H and Takayama T:
Differing deregulation of HER2 in primary gastric cancer and
synchronous related metastatic lymph nodes. Diagn Pathol.
8:1912013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ieni A, Barresi V, Caltabiano R, Caleo A,
Bonetti LR, Lanzafame S, Zeppa P, Caruso RA and Tuccari G:
Discordance rate of HER2 status in primary gastric carcinomas and
synchronous lymph node metastases: A multicenter retrospective
analysis. Int J Mol Sci. 15:22331–22341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pagni F, Zannella S, Ronchi S, Garanzini C
and Leone BE: HER2 status of gastric carcinoma and corresponding
lymph node metastasis. Pathol Oncol Res. 19:103–109. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: World Health Organisation classification of tumors of
the digestive system. IARC; Lyon: 2010
|
|
22
|
Simon R, Mirlacher M and Sauter G: Tissue
microarrays. Methods Mol Med. 114:257–268. 2005.PubMed/NCBI
|
|
23
|
David L, Seruca R, Nesland JM, Soares P,
Sansonetty F, Holm R, Børresen AL and Sobrinho-Simões M: C-erbB-2
expression in primary gastric carcinomas and their metastases. Mod
Pathol. 5:384–390. 1992.PubMed/NCBI
|
|
24
|
Asioli S, Maletta F, Verdun di Cantogno L,
Satolli MA, Schena M, Pecchioni C, Botta C, Chiusa L, Molinaro L,
Conti L, et al: Approaching heterogeneity of human epidermal growth
factor receptor 2 in surgical specimens of gastric cancer. Hum
Pathol. 43:2070–2079. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ge X, Wang H, Zeng H, Jin X, Sujie A, Xu
C, Liu Y, Huang J, Ji Y, Tan Y, et al: Clinical significance of
assessing Her2/neu expression in gastric cancer with dual tumor
tissue paraffin blocks. Hum Pathol. 46:850–857. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
National Comprehensive Cancer Network
clinical practice guidelines in oncology (version 2.2015), .
Gastric cancer.
|
|
27
|
Tominaga N, Gotoda T, Hara M, Hale MD,
Tsuchiya T, Matsubayashi J, Kono S, Kusano C, Itoi T, Fujimoto K,
et al: Five biopsy specimens from the proximal part of the tumor
reliably determine HER2 protein expression status in gastric
cancer. Gastric Cancer. 19:553–560. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Warneke VS, Behrens HM, Böger C, Becker T,
Lordick F, Ebert MPA and Röcken C: Her2/neu testing in gastric
cancer: Evaluating the risk of sampling errors. Ann Oncol.
24:725–733. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stahl P, Seeschaaf C, Lebok P, Kutup A,
Bockhorn M, Izbicki JR, Bokemeyer C, Simon R, Sauter G and Marx AH:
Heterogeneity of amplification of HER2, EGFR, CCND1 and MYC in
gastric cancer. BMC Gastroenterol. 15:72015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Werner D, Battmann A, Steinmetz K, Jones
T, Lamb T, Martinez M, Altmannsberger HM and Al-Batran SE: The
validation of a novel method combining both HER2
immunohistochemistry and HER2 dual-colour silver in situ
hybridization on one slide for gastric carcinoma testting. J Transl
Med. 12:1602014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim MA, Jung JE, Lee HE, Yang HK and Kim
WH: In situ analysis of HER2 mRNA in gastric carcinoma: Comparison
with fluorescence in situ hybridization, dual-color silver in situ
hybridization, and immunohistochemistry. Hum Pathol. 44:487–494.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fusco N, Rocco EG, Del Conte C, Pellegrini
C, Bulfamante G, Nuovo FD, Romagnoli S and Bosari S: HER2 in
gastric cancer: A digital image analysis in pre-neoplastic, primary
and metastatic lesions. Mod Pathol. 26:816–824. 2013. View Article : Google Scholar : PubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBI
|