Introduction
Acute myeloid leukemia (AML) is characterized by the
dysfunction of differentiation and maturation of bone marrow
precursor cells, leading to damage or even loss of normal
hematopoietic function and poor prognosis (1). With an aging population worldwide, the
morbidity of AML is increasing, and the recurrence rate of AML is
high. In the United States, 21,000 new AML patients are diagnosed
each year, of which approximately 10,000 succumb to the disease;
moreover, the mortality rate of patients over 65 years of age is
higher than 90% (2,3). Thus, the identification of biological
indicators closely related to AML is quite significant for the
early diagnosis and prognosis of AML patients.
Bone marrow aspiration cytology is still the gold
standard for AML, but this method is more traumatic, especially in
young AML patients. MicroRNAs (miRNAs) are small non-coding RNAs
found in eukaryotes, which can form a complex biological process
control network and participate in life activities, including the
occurrence of cancer (4,5). In recent years, many studies have
reported that miRNAs are closely related to AML, which are not only
possible novel markers for the diagnosis and prognosis of AML, but
also potential therapeutic targets (6). Studies have found that miRNAs play a
vital role in almost all aspects of AML disease progression,
including cell proliferation and differentiation (7). Zhao et al (8) discovered that the plasma miR-372 level
in AML patients was significantly upregulated, and tumor-suppressor
gene PTEN was targeted to inhibit the migration and cloning of
tumor cells. In addition, miR-495 was found to be down-regulated in
AML and ectopic expression of miR-495 greatly inhibited the
activity of tumor cells and increased apoptosis (9). Nevertheless, the significance of
miR-372 and miR-495 in the diagnosis and prognosis of AML has not
been reported.
The clinical significance of miR-372 and miR-495 in
AML may provide guidance for clinical AML diagnosis and patient
prognosis.
Patients and methods
Research subjects
From March 2012 to January 2014, 81 AML patients
(research group) and 60 healthy subjects (control group) aged 20–65
years were enrolled at The First Hospital of Lanzhou University.
Inclusion criteria precluded that all patients were diagnosed with
AML by bone marrow puncture needle aspiration cytology and
presented with primary AML; the medical records and follow-up data
were complete; the survival time of prognosis was more than 2
months, and there were no pregnant or lactating women included.
Those subjects in the control group had no clinicopathological
symptoms or history of cancer. Exclusion criteria were as follows:
Patient survival was less than 2 months; patients receiving
previous bone marrow transplantation; patients presenting with
other tumors and blood diseases, severe liver and kidney
insufficiency, systemic infectious diseases, immune diseases and
mental and communication disorders. This study conformed to the
Helsinki Declaration and was approved by the Ethics Committee of
The First Hospital of Lanzhou University (Lanzhou, Gansu, China).
All subjects were consulted by telephones or mails, and an informed
consent was signed by all subjects.
Acquisition of information
Sex, age, FAB typing, white blood cell count,
hemoglobin, platelets, the proportion of peripheral blood immature
cells, the proportion of bone marrow immature cells, chromosome
typing, karyotype, CD34, CD56 and gene mutation of patients were
extracted from the patient medical records.
Collection of peripheral blood
The nurses in our hospital collected the peripheral
blood of the subjects through vacuum venous blood collection. The
blood was sent for examination within 1 h after the completion of
blood collection.
Observation indicators
The expression levels of miR-372 and miR-495 in the
peripheral blood of the included subjects were detected using
reverse transcriptase quantitative PCR (RT-qPCR), and their
diagnostic and prognostic value in regards to AML were analyzed.
Risk factors affecting the prognosis and survival of AML patients
were assessed via Cox regression analysis. Peripheral blood was
collected in the early morning when subjects had an empty
stomach.
RT-qPCR
Peripheral blood of all subjects was collected, and
total RNA was extracted from the cells using TRIzol lysate
(Guangzhou Lanji Biotechnology Co., Ltd.). The extraction procedure
was carried out according to the instructions of the kit. The
concentration and purity of the extracted RNA were analyzed by
micro-ultraviolet spectrophotometer DanoProp 1000 (Thmorgan
Biotechnology Co., Ltd.). The A260/A280 value was between 1.8 and
2.1, which was considered to meet the experimental requirements.
The integrity of RNA was analyzed by 3% agarose gel electrophoresis
(Gel Electrophoresis kit; Shanghai Jingke Chemical Technology Co.,
Ltd.). RT-qPCR reaction was conducted after RNA extraction. The
reverse transcription reaction system was as follows: 5X
TransScript® All-in-One Superfix for PCR (4 µl; TransGen
Biotech, China), total RNA (2 µg), ribonuclease-free distilled
water added to 20 µl; 25°C for 10 min, 42°C for 30 min,
deactivation of reverse transcriptase at 85°C for 5 sec. The
reaction was terminated. The PCR amplification system was as
follows: cDNA template (2 µl), 2X TransTaq® High
Fidelity (HiFi) PCR SuperMix II (25 µl; TransGen Biotech, China),
upstream primer and downstream primer 1 µl each, double-distilled
water added to 50 µl. Afterward pre-denaturation was carried out at
95°C for 3 min, 94°C for 2 min, 94°C for 30 sec, 55°C for 30 sec,
72°C for 1–2 kb/min, for a total of 42 cycles, and extension was
carried out at 72°C for 5 min after the completion of cycles. U6
was used as the reaction internal reference and the results were
analyzed by 2−ΔCq (10).
The primer sequences were designed and synthesized by Hepeng
(Shanghai) Biotechnology Co., Ltd. More details are shown in
Table I.
 | Table I.Primer sequences for RT-qPCR. |
Table I.
Primer sequences for RT-qPCR.
| Variable | Forward primer | Reverse primer |
|---|
| miR-372 |
5′-ACACTCCAGCTGGGAAAAGCTGGGTTGAGA-3′ |
5′-TGGTGTCGTGGAGT-3′ |
| miR-495 |
5′-TCCGATTCTTCACGTGGTAC-3′ |
5′-GTGCAGGGTCCGAGGT-3′ |
| U6 |
5′-GCGCGTCGTGAAGCGTTC-3′ |
5′-GTGCAGGGTCCGAGGT-3′ |
Statistical analysis
SPSS 19.0 (Asian Analytics formerly SPSS, China) was
applied. All measurement data are expressed as mean ± standard
deviation (SD). The comparison between two groups was conducted by
independent-samples t-test. Receiver operating characteristic (ROC)
analysis was used to analyze the diagnosis of miR-372 and miR-495
in AML. Kaplan-Meier (K-M) curve was used to analyze the
relationship between miR-372, miR-495 and the 5-year survival rate
of AML patients, and the log rank test was used for variance
analysis. COX regression was used to analyze the risk factors
affecting the prognosis and survival of AML patients. A P-value
less than 0.05 was considered to indicate statistical
significance.
Results
Demographical data
Eighty-one subjects were included in the research
group, including 43 males and 38 females with a mean age of
42.37±10.13 years. Sixty subjects were included in the control
group, including 40 males and 20 females with a mean age of
40.72±8.63 years. There was no statistical difference in the sex
ratio (P=0.105) and age (P=0.359) between the two groups. Other
basic data of patients in the two groups are shown in Table II.
 | Table II.Demographical data and biochemical
parameters. |
Table II.
Demographical data and biochemical
parameters.
| Data/parameters | Research group
(n=81) | Control group
(n=60) | χ2/t | P-value |
|---|
| Sex, n (%) |
|
| 2.625 | 0.105 |
| Male | 43 (53.09) | 40 (66.67) |
|
|
|
Female | 38 (46.91) | 20 (33.33) |
|
|
| Age (years) | 42.37±10.13 | 40.72±8.63 | 0.921 | 0.359 |
| FAB typing, n
(%) |
|
|
|
|
| M1 | 5 (6.17) |
|
|
|
| M2 | 28 (34.58) |
|
|
|
| M3 | 13 (16.05) |
|
|
|
| M4 | 14 (17.28) |
|
|
|
| M5 | 19 (23.46) |
|
|
|
| M6 | 1 (1.23) |
|
|
|
| M7 | 1 (1.23) |
|
|
|
| White blood cell
count (×109/l) | 57.76±9.08 | 4.62±1.03 | 45.077 | <0.001 |
| Hemoglobin (g/l) | 54.37±10.28 | 101.75±8.45 | 29.139 | <0.001 |
| Platelets
(×109/l) | 48.73±9.82 | 135.63±12.64 | 45.943 | <0.001 |
| Proportion of
immature cells in peripheral blood | 44.84±9.24 | Negative |
|
|
| Proportion of
immature cells in bone marrow | 68.98±15.20 |
|
|
|
| Chromosome typing, n
(%) |
|
|
|
|
|
Low-risk group | 16 (19.75) |
|
|
|
|
Non-low-risk group | 65 (80.25) |
|
|
|
| Karyotype, n
(%) |
|
|
|
|
|
Normal | 19 (23.46) |
|
|
|
| t
(8:21) | 23 (28.40) |
|
|
|
| t
(15:17) | 11 (13.58) |
|
|
|
|
11q23 | 8 (9.88) |
|
|
|
| inv
(16) | 9 (11.11) |
|
|
|
|
Rests | 11 (13.58) |
|
|
|
| CD34 (+/-) | 37/44 |
|
|
|
| CD56 (+/-) | 39/42 |
|
|
|
| Gene mutation |
|
|
|
|
| c-KIT
(+/-) | 12/69 |
|
|
|
| CEBPA
(+/-) | 13/68 |
|
|
|
|
FLT3-ITD (+/-) | 7/74 |
|
|
|
|
FLT3-TKD (+/-) | 3/78 |
|
|
|
|
Negative (+/-) | 46/35 |
|
|
|
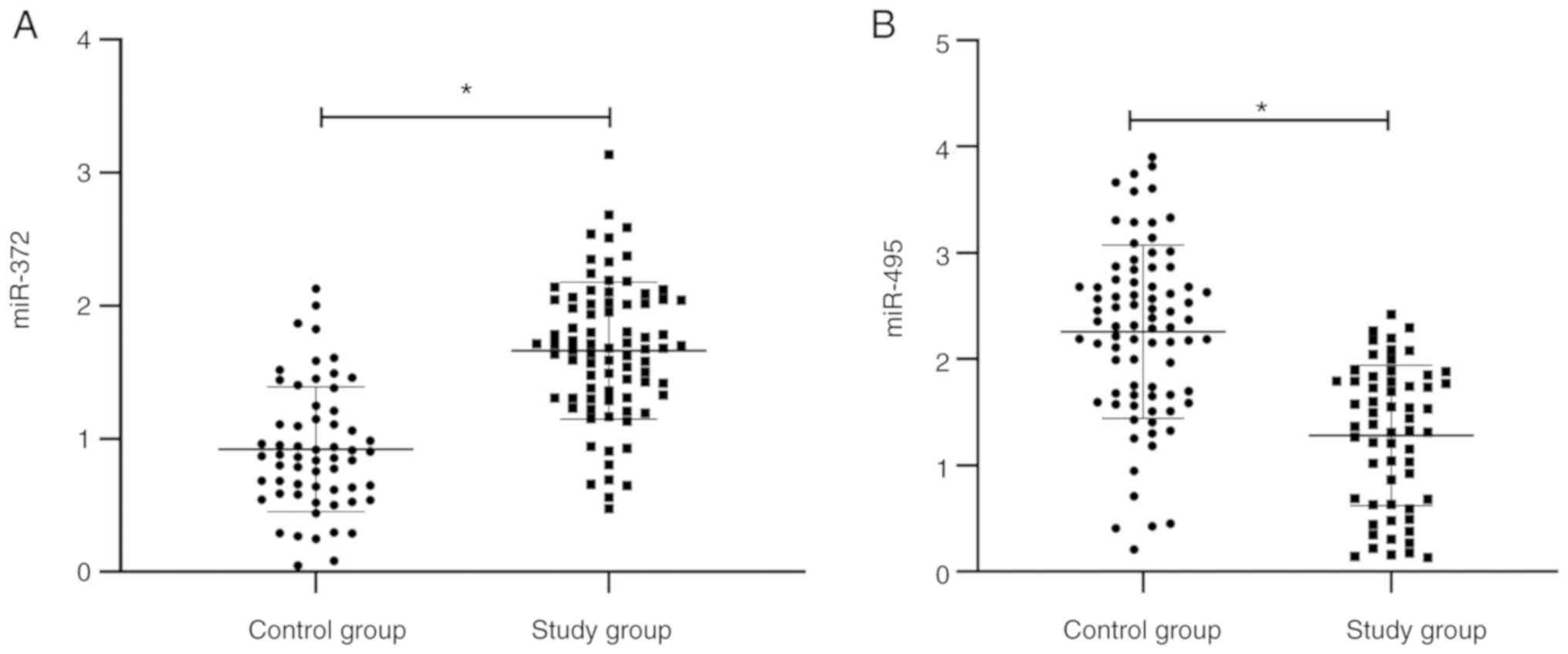
Expression levels of miR-372 and
miR-495 in AML
The miRNA level was tested by RT-qPCR. The miR-372
level in peripheral blood of patients in the research group was
significantly higher than that in the control group, and the
miR-495 level was significantly lower than that in the control
group (P<0.05) (Fig. 1).
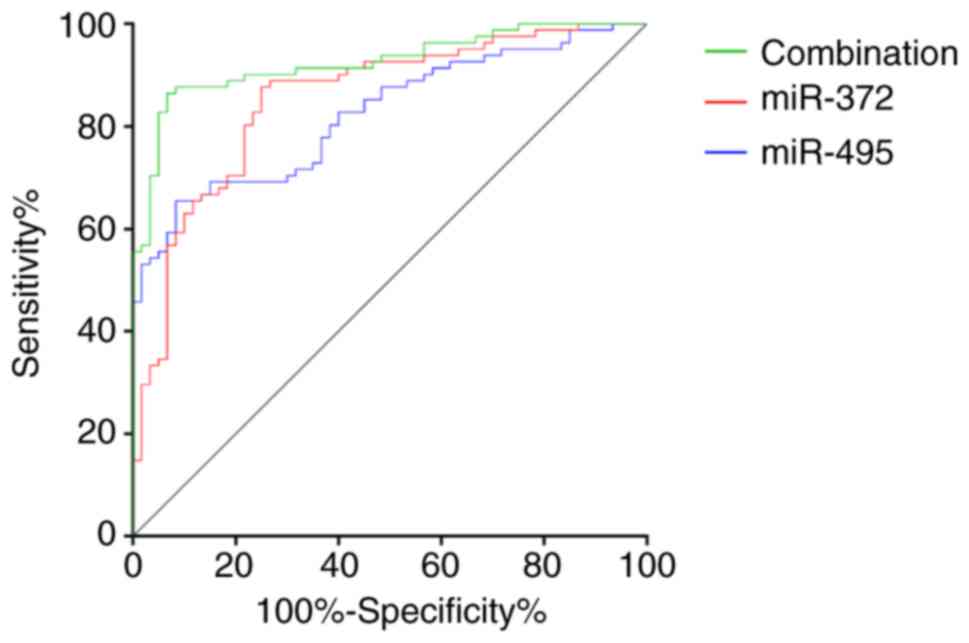
Diagnostic value of miR-372 and
miR-495 in AML
The area under the curve (AUC), cut-off value,
sensitivity, and specificity of miR-372 in the diagnosis of AML
were 0.853, 1.150, 87.65, and 75.00%, respectively. The AUC,
cut-off value, sensitivity, and specificity of miR-495 in the
diagnosis of AML were 0.824, 2.097, 65.43, and 91.67%,
respectively. The AUC, sensitivity and specificity of miR-372
combined with miR-495 in the diagnosis of AML were 0.925, 86.43,
and 93.33%, respectively (Fig.
2).
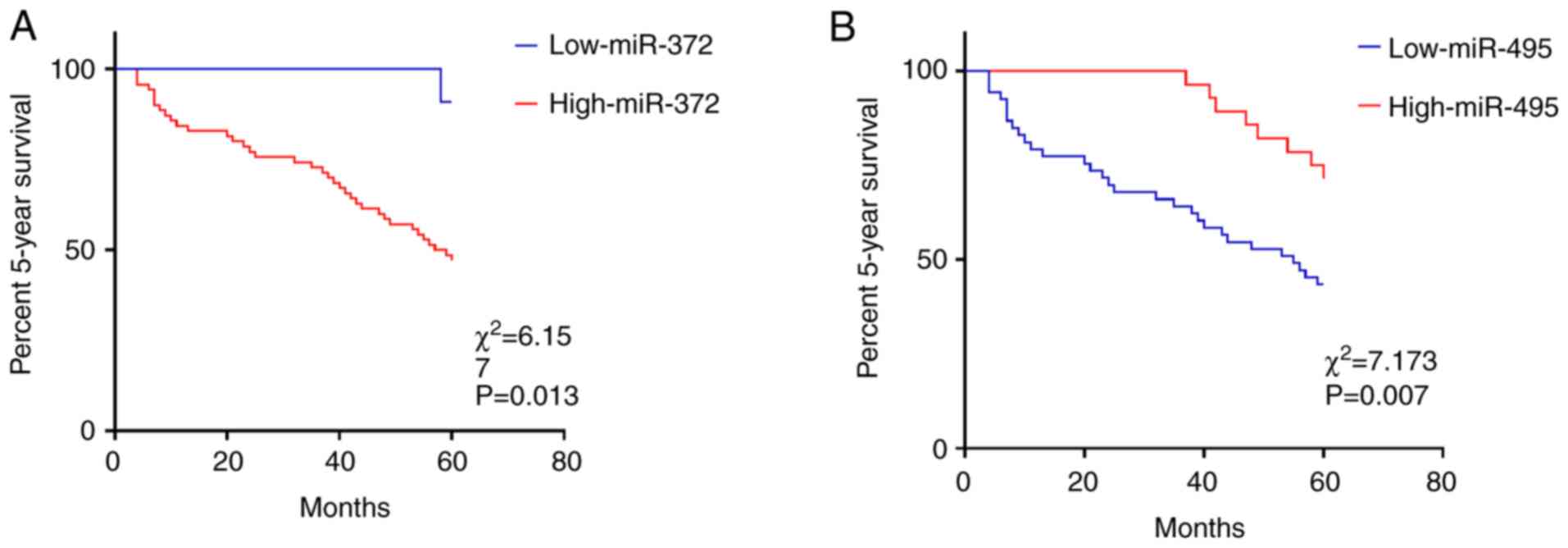
Association between miR-372, miR-495,
and the 5-year survival rate of AML patients
Using the cut-off value as the critical value,
values equal or greater than the cut-off value was used to
designate patients in a high expression group and values less than
the cut-off value were used to designate patients to a low
expression group. K-M curve analysis results revealed that the
5-year survival rate of patients with high expression of miR-372
was lower than that of patients with low expression of miR-372
(P=0.013) (Fig. 3A), and the 5-year
survival rate of patients with high expression of miR-495 was
higher than that of patients with low expression of miR-495
(P=0.007) (Fig. 3B).
Risk factors for survival and
prognosis of AML patients
COX regression analysis showed that white blood cell
count (P=0.009), proportion of immature cells in peripheral blood
(P=0.022), chromosome typing (P=0.024), gene mutation (P=0.028),
miR-372 (P=0.016), and miR-495 (P=0.017) were independent risk
factors for the prognosis and death of AML patients (Tables III and IV).
 | Table III.Results of the COX univariate
analysis. |
Table III.
Results of the COX univariate
analysis.
|
|
|
|
|
|
|
| 95% CI Exp (B) |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Variables | B | SE | Wald | df | Sig. | Exp (B) | Lower part | Upper part |
|---|
| Sex | −0.158 | 0.326 | 0.234 | 1 | 0.628 | 0.854 | 0.450 | 1.619 |
| Age (years) | 0.075 | 0.017 | 20.687 | 1 | <0.001 | 1.078 | 1.044 | 1.114 |
| FAB typing | −0.021 | 0.115 | 0.034 | 1 | 0.854 | 0.979 | 0.782 | 1.226 |
| White blood cell
count | 0.051 | 0.018 | 8.445 | 1 | 0.004 | 1.052 | 1.017 | 1.089 |
| Hemoglobin | −0.036 | 0.015 | 5.841 | 1 | 0.016 | 0.964 | 0.936 | 0.993 |
| Platelet | −0.007 | 0.017 | 0.188 | 1 | 0.665 | 0.993 | 0.960 | 1.027 |
| Proportion of
immature cells in peripheral blood | 0.066 | 0.017 | 15.77 | 1 | <0.001 | 1.068 | 1.034 | 1.104 |
| Proportion of
immature cells in bone marrow | 0.009 | 0.01 | 0.719 | 1 | 0.396 | 1.009 | 0.988 | 1.030 |
| Chromosome
typing | −2.132 | 0.359 | 35.191 | 1 | <0.001 | 0.119 | 0.059 | 0.240 |
| Karyotype | −0.141 | 0.096 | 2.127 | 1 | 0.145 | 0.869 | 0.719 | 1.050 |
| CD34 | −0.203 | 0.325 | 0.391 | 1 | 0.532 | 0.816 | 0.432 | 1.542 |
| CD56 | −0.401 | 0.327 | 1.511 | 1 | 0.219 | 0.669 | 0.353 | 1.269 |
| Gene mutation | −0.363 | 0.096 | 14.179 | 1 | <0.001 | 0.696 | 0.576 | 0.840 |
| miR-372 | 0.953 | 0.229 | 17.339 | 1 | <0.001 | 2.594 | 1.656 | 4.064 |
| miR-495 | −0.615 | 0.172 | 12.731 | 1 | <0.001 | 0.541 | 0.386 | 0.758 |
 | Table IV.Results of the COX multivariate
analysis. |
Table IV.
Results of the COX multivariate
analysis.
|
|
|
|
|
|
|
| 95% CI Exp (B) |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Variables | B | SE | Wald | df | Sig. | Exp (B) | Lower part | Upper part |
|---|
| Age (years) | 0.029 | 0.022 | 1.776 | 1 | 0.183 | 1.029 | 0.986 | 1.074 |
| White blood cell
count | −0.015 | 0.024 | 0.359 | 1 | 0.009 | 2.986 | 0.940 | 4.034 |
| Hemoglobin | 0.003 | 0.027 | 0.016 | 1 | 0.901 | 1.003 | 0.952 | 1.057 |
| Proportion of
immature cells in peripheral blood | 0.063 | 0.028 | 5.232 | 1 | 0.022 | 1.065 | 1.009 | 1.124 |
| Chromosome
typing | −1.728 | 0.41 | 17.773 | 1 | 0.024 | 0.178 | 0.080 | 0.397 |
| Gene mutation | −0.251 | 0.114 | 4.831 | 1 | 0.028 | 0.778 | 0.622 | 0.973 |
| miR-372 | 0.484 | 0.358 | 1.828 | 1 | 0.016 | 1.623 | 0.804 | 4.273 |
| miR-495 | −0.168 | 0.297 | 0.322 | 1 | 0.017 | 2.845 | 0.472 | 4.511 |
Discussion
At present, the pathogenesis of AML has not been
fully studied. Since gene mutations often occur in AML patients and
their prognosis is also variable, the identification of accurate
indicators for prognosis and early diagnosis of AML is quite
significant to guide the clinical treatment of AML and improve the
prognosis of patients.
The present study analyzed the clinical significance
of the miR-372 and miR-495 expression levels in AML. The research
results showed that, consistent with the results reported in
previous studies, the miR-372 expression in peripheral blood of
patients with AML increased while the miR-495 expression decreased
(8,9). Then, the diagnostic value of miR-372
and miR-495 in AML was analyzed. At present, there are few reports
on the diagnosis of miRNAs in AML. Wang et al (11) reported the diagnostic value of
miR-29a and miR-142-3p in 52 AML patients and 100 healthy adults.
Their research results showed that the AUC of miR-29a combined with
miR-142-3p in the diagnosis of AML could reach 0.97, and the
sensitivity and specificity were 90 and 100%, respectively. This
study signified that the AUC of miR-372 combined with miR-495 in 81
AML patients and 60 healthy subjects was 0.925, and the sensitivity
and specificity were 86.43 and 93.33% respectively, which were
slightly lower than those of Wang et al. Ohyashiki et
al (12) reported that the AUC
of miR-92a in 91 AML patients and 25 healthy subjects was 0.9959,
while Yan et al (13)
indicated that the AUC of miR-217 in 89 AML patients and 60 healthy
adults was 0.836, with sensitivity and specificity of 74.16 and
83.33% respectively. Differences between the results of these
research studies were not only caused by different miRNAs, but also
caused by different sample sources and sample sizes. We will
further carry out multi-center clinical research verification.
The research results on the prognostic relationship
between miR-372, miR-495, and AML patients showed that the 5-year
survival rate of patients with high miR-372 expression was lower
than that of patients with low miR-372 expression, while the 5-year
survival rate of patients with high miR-495 expression was higher
than that of patients with low miR-495 expression. In many studies,
it has been reported that miR-372 and miR-495 can affect a variety
of biological behaviors of tumor cells, such as modulating miR-372
to inhibit proliferation and invasion of renal cancer cells
(14). Up-regulating miR-372 was
also found to promote the migration of squamous cell carcinoma
cells in the head and neck by downregulating p62, and the
expression of miR-372 was also found to be up-regulated in a
hypoxic environment, promoting the progression of squamous cell
carcinoma in the head and neck (15). miR-495 was reported to have similar
results. Mao et al (16)
reported that miR-495 could inhibit proliferation, migration, and
invasion of esophageal cancer cells. Chen et al (17) reported that miR-495 demethylation
could inhibit the invasion of breast cancer cells and promote their
apoptosis. These functions of miR-372 and miR-495 are key factors
affecting the prognosis of cancer patients. Yu et al
(18) confirmed that miR-372 was a
biomarker for the diagnosis and prognosis of patients with early
colorectal cancer. Wang et al (19) verified that miR-495 was a prognostic
factor for medulloblastoma. The results of this study also showed
that, similar to the previously reported results, age, white blood
cell count, proportion of immature cells in peripheral blood,
chromosome typing, and gene mutation were independent risk factors
for prognosis and survival of AML patients (20–23). It
was found in this study that miR-372 and miR-495 were also
independent risk factors for the prognosis and survival of AML
patients, which were rarely reported in AML. Gu et al
(24) confirmed that high expression
of miR-372 was an independent predictor for poor prognosis of
patients with liver cancer, and there had been few such reports on
miR-495. However, this also improved the credibility of our
research results to a certain extent.
There may be some limitations in this study.
Invasive fungal infection and bone marrow failure are also factors
affecting the prognosis of AML (25,26).
Because of genetic abnormality, some AML patients with t (8; 21)
(q22; q22.1) mutations have a good prognosis; therefore, the
relationship between miR-372, miR-495 and AML should be further
analyzed on the basis of the prognosis of patients; in addition,
different AML subtypes have different treatment plans, thus we need
to further analyze the clinical significance of miR-372 and miR-495
expression in AML subtypes, However, the data collected in this
study were insufficient. We will continue to deepen our research
and supplement more comprehensive results. What's more, we also
need to further analyze the mechanisms of miR-372 and miR-495 in
AML, which require more research verification.
In conclusion, miR-372 was expressed higher in AML
patient peripheral blood samples, while miR-495 was expressed
lower. miR-372 and miR-495 may be effective indicators for the
early diagnosis and prognosis of AML.
Acknowledgements
Not applicable.
Funding
This study was funded by the Gansu Natural Science
Foundation “Proteomics Research and Bioinformatics Analysis of Bone
Marrow Serum in Patients with Acute Myeloid Leukemia”. Grant
number: 18JR3RA356
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WZ conceived the study and wrote the manuscript. BW
performed the PCR analysis. BL analyzed and interpreted the patient
data. SW and LZ conducted the statistical analysis. All authors
read and approved the manuscript and agree to be accountable for
all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The First Hospital of Lanzhou University (Lanzhou, Gansu, China).
Patients who participated in this research, signed the informed
consent and had complete clinical data. Signed written informed
consents were obtained from all the patients and/or their
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ramsey HE, Fischer MA, Lee T, Gorska AE,
Arrate MP, Fuller L, Boyd KL, Strickland SA, Sensintaffar J, Hogdal
LJ, et al: A novel MCL1 inhibitor combined with venetoclax rescues
venetoclax-resistant acute myelogenous leukemia. Cancer Discov.
8:1566–1581. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin B, Londoño-Joshi A, Kang Q, Tewari
M, Rhim AD and Malek SN: Ultrasensitive mutation detection
identifies rare residual cells causing acute myelogenous leukemia
relapse. J Clin Invest. 127:3484–3495. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abelson S, Collord G, Ng SWK, Weissbrod O,
Mendelson Cohen N, Niemeyer E, Barda N, Zuzarte PC, Heisler L,
Sundaravadanam Y, et al: Prediction of acute myeloid leukaemia risk
in healthy individuals. Nature. 559:400–404. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartel DP: Metazoan MicroRNAs. Cell.
173:20–51. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Berindan-Neagoe I, Monroig PC, Pasculli B
and Calin GA: MicroRNAome genome: A treasure for cancer diagnosis
and therapy. CA Cancer J Clin. 64:311–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Trino S, Lamorte D, Caivano A, Laurenzana
I, Tagliaferri D, Falco G, Del Vecchio L, Musto P and De Luca L:
MicroRNAs as new biomarkers for diagnosis and prognosis, and as
potential therapeutic targets in acute myeloid leukemia. Int J Mol
Sci. 19(pii): E4602018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wallace JA and O'Connell RM: MicroRNAs and
acute myeloid leukemia: Therapeutic implications and emerging
concepts. Blood. 130:1290–1301. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao Q, Feng J, Li W, Shen K, Chen S, Xie
Y, Zhao C and Hou Y: Expression and mechanism of plasma miR-372 in
acute myeloid leukemia patients. J Pract Med. 34:2030–2034.
2018.
|
|
9
|
Jiang X, Huang H, Li Z, He C, Li Y, Chen
P, Gurbuxani S, Arnovitz S, Hong GM, Price C, et al: miR-495 is a
tumor-suppressor microRNA down-regulated in MLL-rearranged
leukemia. Proc Natl Acad Sci USA. 109:19397–19402. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang F, Wang XS, Yang GH, Zhai PF, Xiao Z,
Xia LY, Chen LR, Wang Y, Wang XZ, Bi LX, et al: miR-29a and
miR-142-3p downregulation and diagnostic implication in human acute
myeloid leukemia. Mol Biol Rep. 39:2713–2722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohyashiki JH, Umezu T, Kobayashi C,
Hamamura RS, Tanaka M, Kuroda M and Ohyashiki K: Impact on cell to
plasma ratio of miR-92a in patients with acute leukemia: In vivo
assessment of cell to plasma ratio of miR-92a. BMC Res Notes.
3:3472010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yan J, Wu G, Chen J, Xiong L, Chen G and
Li P: Downregulated miR-217 expression predicts a poor outcome in
acute myeloid leukemia. Cancer Biomark. 22:73–78. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang X, Huang M, Kong L and Li Y: miR-372
suppresses tumour proliferation and invasion by targeting IGF2BP1
in renal cell carcinoma. Cell Prolif. 48:593–599. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yeh LY, Liu CJ, Wong YK, Chang C, Lin SC
and Chang KW: miR-372 inhibits p62 in head and neck squamous cell
carcinoma in vitro and in vivo. Oncotarget. 6:6062–6075. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mao Y, Li L, Liu J, Wang L and Zhou Y:
miR-495 inhibits esophageal squamous cell carcinoma progression by
targeting Akt1. Oncotarget. 7:51223–51236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Y, Luo D, Tian W, Li Z and Zhang X:
Demethylation of miR-495 inhibits cell proliferation, migration and
promotes apoptosis by targeting STAT-3 in breast cancer. Oncol Rep.
37:3581–3589. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu J, Jin L, Jiang L, Gao L, Zhou J, Hu Y,
Li W, Zhi Q and Zhu X: Serum miR-372 is a diagnostic and prognostic
biomarker in patients with early colorectal cancer. Anticancer
Agents Med Chem. 16:424–431. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang C, Yun Z, Zhao T, Liu X and Ma X:
miR-495 is a predictive biomarker that downregulates GFI1
expression in medulloblastoma. Cell Physiol Biochem. 36:1430–1439.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chou WC, Chou SC, Liu CY, Chen CY, Hou HA,
Kuo YY, Lee MC, Ko BS, Tang JL, Yao M, et al: TET2 mutation is an
unfavorable prognostic factor in acute myeloid leukemia patients
with intermediate-risk cytogenetics. Blood. 118:3803–3810. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Green ML, Leisenring WM, Xie H, Walter RB,
Mielcarek M, Sandmaier BM, Riddell SR and Boeckh M: CMV
reactivation after allogeneic HCT and relapse risk: Evidence for
early protection in acute myeloid leukemia. Blood. 122:1316–1324.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Björkholm M, Derolf AR, Hultcrantz M,
Kristinsson SY, Ekstrand C, Goldin LR, Andreasson B, Birgegård G,
Linder O, Malm C, et al: Treatment-related risk factors for
transformation to acute myeloid leukemia and myelodysplastic
syndromes in myeloproliferative neoplasms. J Clin Oncol.
29:2410–2415. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thol F, Damm F, Lüdeking A, Winschel C,
Wagner K, Morgan M, Yun H, Göhring G, Schlegelberger B, Hoelzer D,
et al: Incidence and prognostic influence of DNMT3A mutations in
acute myeloid leukemia. J Clin Oncol. 29:2889–2896. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gu H, Guo X, Zou L, Zhu H and Zhang J:
Upregulation of microRNA-372 associates with tumor progression and
prognosis in hepatocellular carcinoma. Mol Cell Biochem. 375:23–30.
2013.PubMed/NCBI
|
|
25
|
Michallet M, Bénet T, Sobh M, Kraghel S,
El Hamri M, Cannas G, Nicolini FE, Labussière H, Ducastelle S,
Barraco F, et al: Invasive aspergillosis: An important risk factor
on the short- and long-term survival of acute myeloid leukemia
(AML) patients. Eur J Clin Microbiol Infect Dis. 31:991–997. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deschler B and Lübbert M: Acute myeloid
leukemia: Epidemiology and etiology. Cancer. 107:2099–2107. 2006.
View Article : Google Scholar : PubMed/NCBI
|