Introduction
Malignant obstructive jaundice (MOJ) is caused by
intrahepatic and extrahepatic bile duct obstruction due to
malignant obstructive tumor invasion or oppression, which is mainly
manifested by hyperbilirubinemia and scleral yellow staining
(1). Obstruction can cause a series
of pathophysiological disorders of the organism, including bile
duct dilation, increased capillary bile duct permeability, the
flowing of bile composition into blood and the reverse inflow of
bile into the blood and lymph, which is the leading cause of death
(2).
The main treatment for malignant biliary obstruction
is resection and drainage (3).
However, due to the malignancy of the tumor itself and its special
anatomical structure, most patients have lost the opportunity of
radical surgery at the time of diagnosis. Therefore, 90% of
patients diagnosed with malignant biliary obstruction can only
benefit from palliative resection (4). In addition, effective preoperative
drainage can significantly improve the prognosis of patients
undergoing radical surgery (4). The
harm of malignant biliary obstruction is not only the tumor itself
but also the organ damage caused by hyperbilirubinemia. Therefore,
effective biliary drainage is an important treatment for patients
in the advanced stage. Reducing jaundice can protect liver and
kidney function, improve quality of life and prolong survival
(5). Tibble et al (6), have revealed that due to the delayed
diagnosis, the resectability of high biliary tract tumors is only
15–20%. Therefore, removing obstruction, unobstructed drainage and
rapid and effective reduction of jaundice is the key to treatment.
According to bile discharge methods, preoperative biliary drainage
can be divided into internal drainage and external drainage, and
based on the drainage pathway, it can be divided into percutaneous
transhepatic biliary drainage (PTBD) and endoscopic biliary
drainage (EBD) (7). However, the
method of preoperative biliary drainage is still controversial.
Some scholars believe that PTBD is prone to complications such as
pancreatitis, cholangitis, biliary perforation, biliary bleeding
and stent displacement (8). However,
other studies have found that compared with EBD, PTBD causes less
surgical trauma, fewer complications and faster bilirubin decrease
(9).
At present, the specific selection of preoperative
biliary drainage methods for malignant obstructive jaundice has not
been determined. In this meta-analysis, therefore, the advantages
and disadvantages of these two drainage methods were analyzed from
the perspective of tumor implantation metastasis after drainage. In
addition, all included controlled clinical trials were designed to
compare the incidence rate of implantation metastasis between EBD
and PTBD in preoperative biliary drainage of MOJ, in order to
provide references for clinical application.
Materials and methods
Search strategy
PubMed (https://pubmed.ncbi.nlm.nih.gov/?term=), Embase
(https://www.embase.com/), Web of Science
(www.isiknowledge.com/) and Cochrane
Library (https://www.cochranelibrary.com/) were utilized.
Literature published from the database establishment to January
2019 as well as similar literature and references attached to the
search results were consulted. The retrieval strategy was
‘malignant obstruction jaundice’ (Title/Abstract) or ‘perihilar
cholangiocarcinoma’ (Title/Abstract) or ‘distal cholangiocarcinoma’
(Title/Abstract) or ‘pancreatic cancer’ (Title/Abstract) and
‘preoperative biliary drainage’ (Title/Abstract) or ‘percutaneous
transhepatic biliary drainage’ (Title/Abstract) or ‘endoscopic
biliary drainage’ (Title/Abstract) and ‘seeding metastasis’
(Title/Abstract) or ‘peritoneal metastasis’ (Title/Abstract).
Inclusion and exclusion criteria
The inclusion criteria were as follows: i) Clinical
study of preoperative biliary drainage in patients with MOJ; ii)
EBD and PTBD were preoperative biliary drainage methods; iii) The
incidence rate of implantation metastasis was the main objective of
the study; iv) The methodology used in the study was reliable; and
v) The data provided were complete and accurate. The exclusion
criteria were as follows: i) Irrelevant or in vitro
experiments; ii) Case report, review, letter or conference paper;
or iii) Repeated reports. If the data of a center were published
numerous times, the most recently published data would be selected.
If a study was reported more than once, the data with the longest
follow-up time would be used.
Data extraction and quality
assessment
Two reviewers independently read the literature
titles and abstracts according to the aforementioned inclusion and
exclusion criteria and after excluding the studies that evidently
did not meet the inclusion criteria, the full text of the remaining
studies was read to determine whether they met the inclusion
criteria and extract relevant data. In case of disagreement,
inclusion was determined by a third reviewer. The extracted
contents included: i) General information: Title, first author,
publication date and literature source; ii) Research
characteristics: Grouping methods and included cases of the
subjects; iii) Outcome index (incidence rate of implantation
metastasis); and iv) Information related to literature quality
assessment.
Two reviewers evaluated the quality of the included
studies according to the Newcastle Ottawa scale (NOS) (10) and the third reviewer evaluated the
study when the scores were inconsistent. The full score of the NOS
is 9 points, and the evaluation criteria include the selection,
comparability and outcome of the cohort studies. Selection
indicates the selection of cases and controls with a total score of
4, including the typicality of the exposed cohorts (1 score),
non-exposed cohorts from the same community (1 score), reliable
determination of exposure (1 score), and the unpresented outcomes
at the beginning of the study (1 score). Comparability indicates
the comparability of cases and control group with a total score of
2, including the control of the most important confounders (1
score) and the control of other confounders (1 score). Outcome
indicates methods of investigation and assessment of exposure with
a total score of 3, including independent blind evaluation (1
score), adequate follow-up time (1 score) and full follow-up of all
subjects (1 score). The study with a score equal to or greater than
6 points is divided into a high-quality study.
Intervention measures and observed
indicators
Intervention measures were as follows: PTBD,
percutaneous transhepatic biliary drainage, including internal
drainage and external drainage, EBD, endoscopic biliary drainage,
including internal drainage and external drainage.
Observed indicators were as follows: Implantation
metastasis, (a) The area through which the catheter passed,
including the skin poke and the abdominal chest wall layer; (b)
Right pleural implantation metastasis; (c) Peritoneal implantation
metastasis; (d) Intrahepatic metastasis; and (e) Surgical success
rate. Deaths in hospital and severe postoperative complications
(such as infection) were considered surgical failures.
Catheter-related implantation metastasis included (a) (b) (d).
Statistical analysis
Meta-analysis was performed using the RevMan5.3.0
software provided by the Cochrane Collaboration. Heterogeneity
between studies was analyzed. P>0.05 or I2<50%
indicated that there was a small possibility of heterogeneity among
studies, and the fixed effect models were used. Conversely,
P<0.05 or I2>50% indicated that there was
heterogeneity among the studies, and the random effect models were
used. Enumeration data were analyzed according to the odds ratio
(OR), and the 95% confidence interval (95% CI) was calculated. The
funnel plot was drawn by RevMan5.3.0 software to determine whether
there were any publication biases.
Results
Study selection and study
characteristics
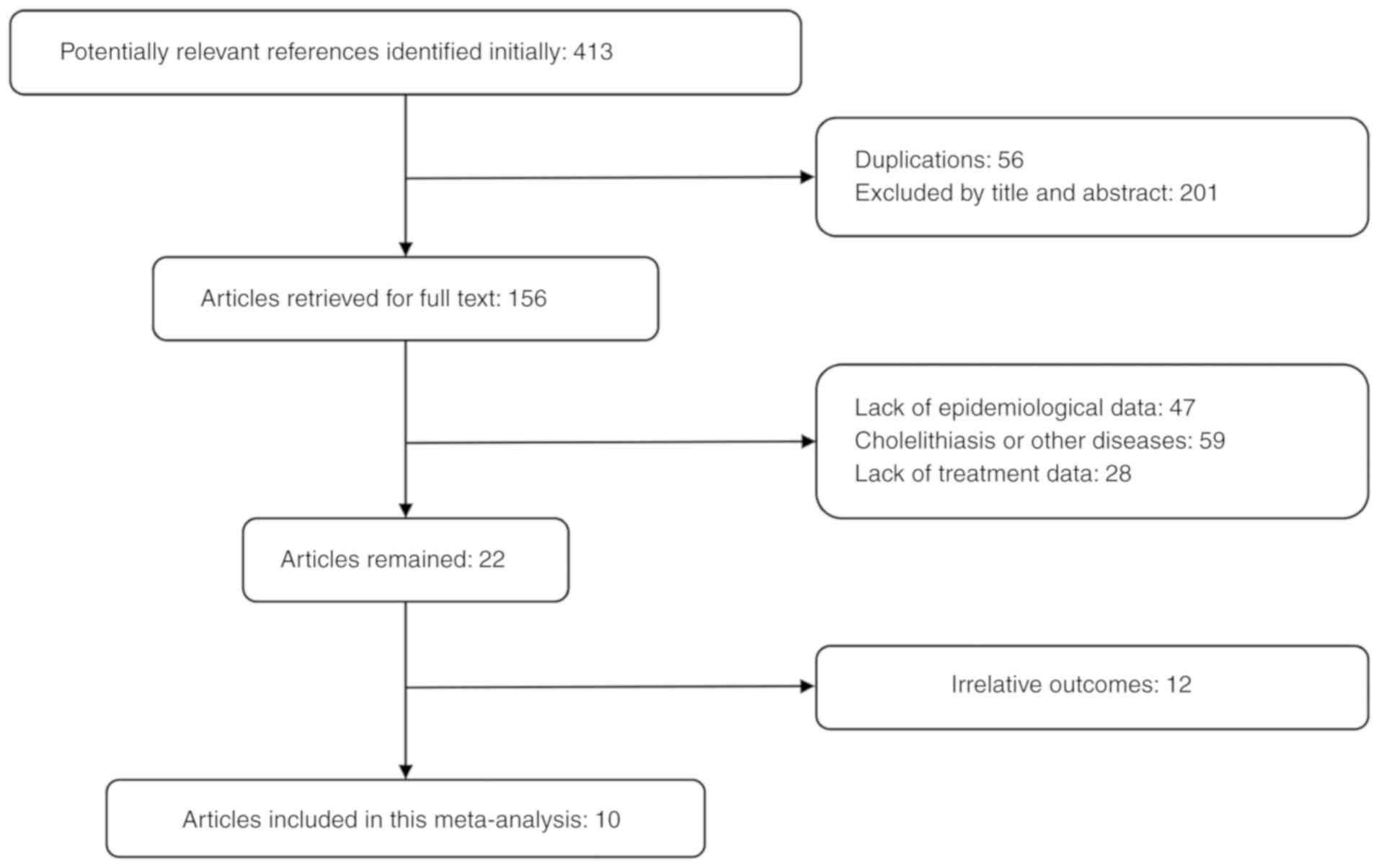
Ten studies were included (9,11–19),
including six for hilar cholangiocarcinoma, two for distal
cholangiocarcinoma, and two for pancreatic carcinoma. The retrieval
and screening processes are presented in Fig. 1. Baseline data of the included
literature are presented in Table I.
All the studies included PTBD and EBD schemes, all of which were
retrospective cohort studies with NOS scores ranging from 7 to 8
points, as presented in Table
II.
 | Table I.Basic characteristics of included
studies. |
Table I.
Basic characteristics of included
studies.
| Author (refs.) | Publication year | Country | Follow-up time
(years) | No. of patients
(PTBD/EBD) | Mean ages (PTBD/EBD,
years) | Primary
locations | Outcomes |
|---|
| Higuchi et al
(11) | 2017 | Japan | 5 | 87/76 | 67/70 | Hilar
cholangiocarcinoma | a, d, g |
| Hirano et al
(12) | 2014 | Japan | 12 | 67/74 | 68/68.5 | Hilar
cholangiocarcinoma | a, b, c, d, g |
| Hwang et al
(13) | 2012 | Korea | 10 | 171/62 | 59/59 | Hilar
cholangiocarcinoma | a, b, d |
| Kawakami et al
(14) | 2011 | Japan | 5 | 48/80 | 71/70 | Hilar
cholangiocarcinoma | a, b, d, g |
| Komaya et al
(15) | 2016 | Japan | 5 | 189/187 | 68/70 | Distal
cholangiocarcinoma | a, b, c, e |
| Komaya et al
(16) | 2017 | Japan | 5 | 168/152 | 66/67 | Hilar
cholangiocarcinoma | a, b, c, d |
| Miura et al
(17) | 2017 | Japan | 5 | 25/63 | 70.2/70.8 | Distal
cholangiocarcinoma | a, b, c, e, g |
| Murakami et
al (18) | 2015 | Japan | 5 | 20/73 | 69/69 | Pancreatic
carcinoma | a, c, f |
| Uemura et al
(19) | 2015 | Japan | 10 | 166/407 | 67/67 | Pancreatic
carcinoma | a, b, c, f, g |
| Wiggers et
al (9) | 2015 | Holland | 5 | 88/157 | 61/65 | Hilar
cholangiocarcinoma | a, b, c, d |
 | Table II.NOS scores of included studies. |
Table II.
NOS scores of included studies.
| Author (refs.) | Publication
year |
Selectiona |
Comparabilitya |
Outcomea | Totalb |
|---|
| Higuchi et
al (11) | 2017 | 4 | 1 | 2 | 7 |
| Hirano et al
(12) | 2014 | 3 | 2 | 2 | 7 |
| Hwang et al
(13) | 2012 | 4 | 2 | 2 | 8 |
| Kawakami et
al (14) | 2011 | 4 | 2 | 2 | 8 |
| Komaya et al
(15) | 2016 | 3 | 2 | 2 | 7 |
| Komaya et al
(16) | 2017 | 4 | 2 | 2 | 8 |
| Miura et al
(17) | 2017 | 4 | 1 | 2 | 7 |
| Murakami et
al (18) | 2015 | 4 | 2 | 2 | 8 |
| Uemura et al
(19) | 2015 | 4 | 2 | 2 | 8 |
| Wiggers et
al (9) | 2015 | 4 | 2 | 2 | 8 |
Comparison of the effects of EBD and
PTBD on the overall incidence rate of implantation metastasis in
resected MOJ
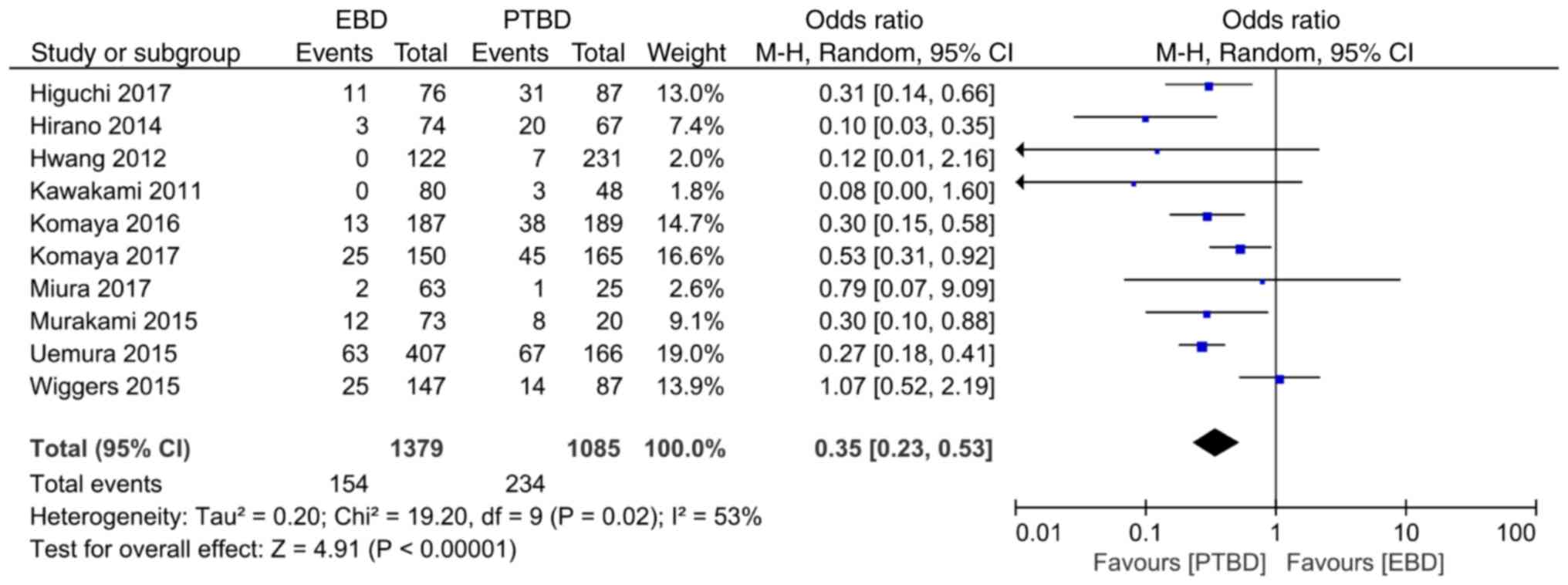
A total of ten studies reported the comparison of
the incidence rate of implantation metastasis. The results of the
heterogeneity analysis (I2=53%, P=0.02) demonstrated
that there was heterogeneity among the studies, and the random
effect model was used for analysis. Meta-analysis results revealed
that the overall incidence rate of implantation metastasis in the
EBD and PTBD groups was 11.2% (154/1379) and 21.2% (234/1085),
respectively, with statistically significant differences between
the two groups (OR=0.35, 95% CI: 0.23–0.53, P<0.00001; Fig. 2).
Comparison of the effects of EBD and
PTBD on the incidence rate of different types of implantation
metastases in resected MOJ
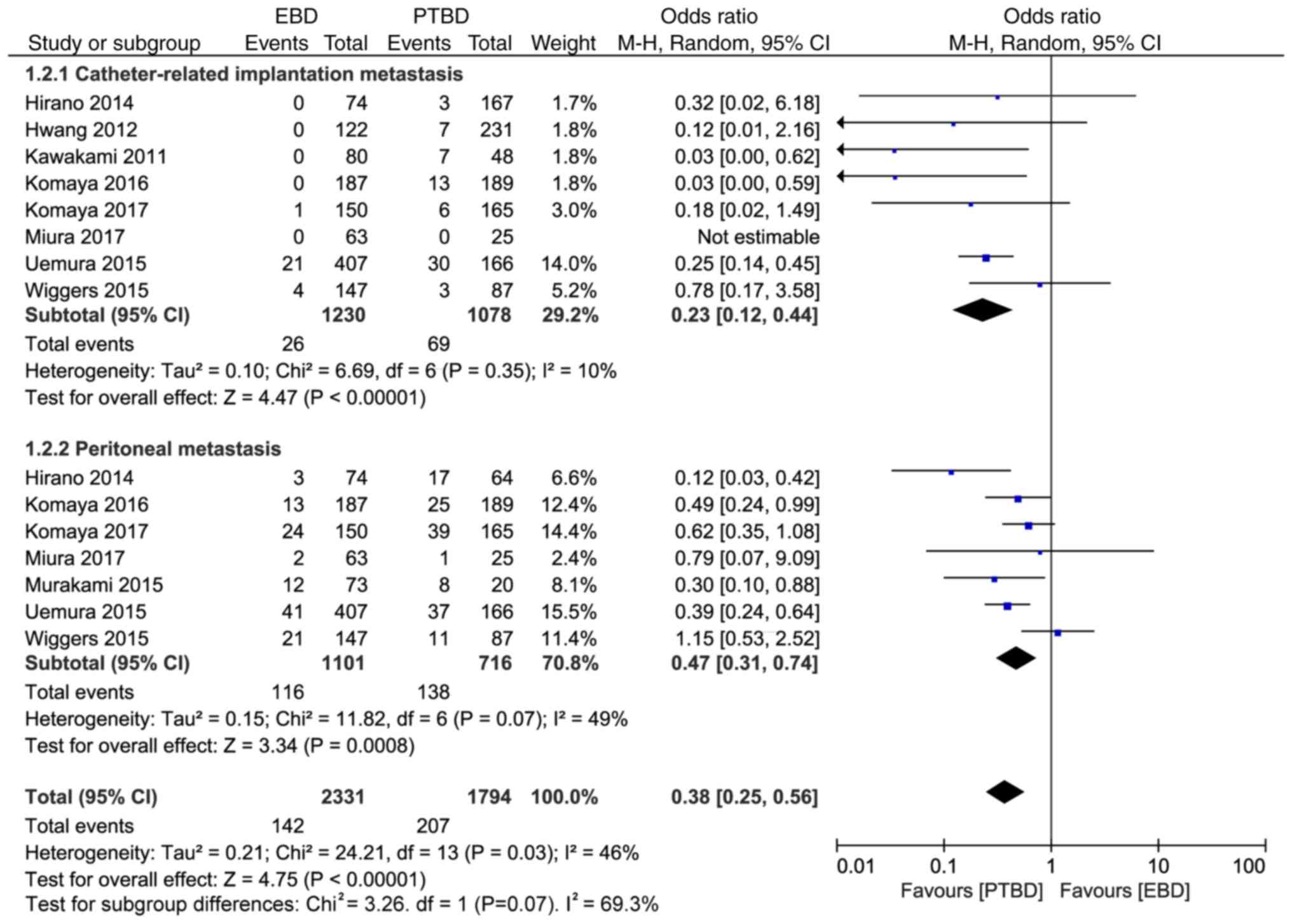
Implantation metastases can be divided into
peritoneal metastasis and catheter-related implantation metastasis.
The subgroup analysis results revealed that the incidence rates of
catheter-related implantation metastasis and peritoneal
implantation metastasis in the EBD and PTBD groups were 2.1%
(26/1230) vs. 6.4% (69/1078) and 10.5% (116/1101) vs. 19.3%
(138/716), respectively, and the differences were statistically
significant (OR=0.23, 95% CI: 0.12–0.44, P<0.00001; and OR=0.47,
95% CI: 0.31–0.74, P=0.0008; Fig.
3).
Comparison of the effects of EBD and
PTBD on the incidence rate of implantation metastasis in different
parts of resected MOJ
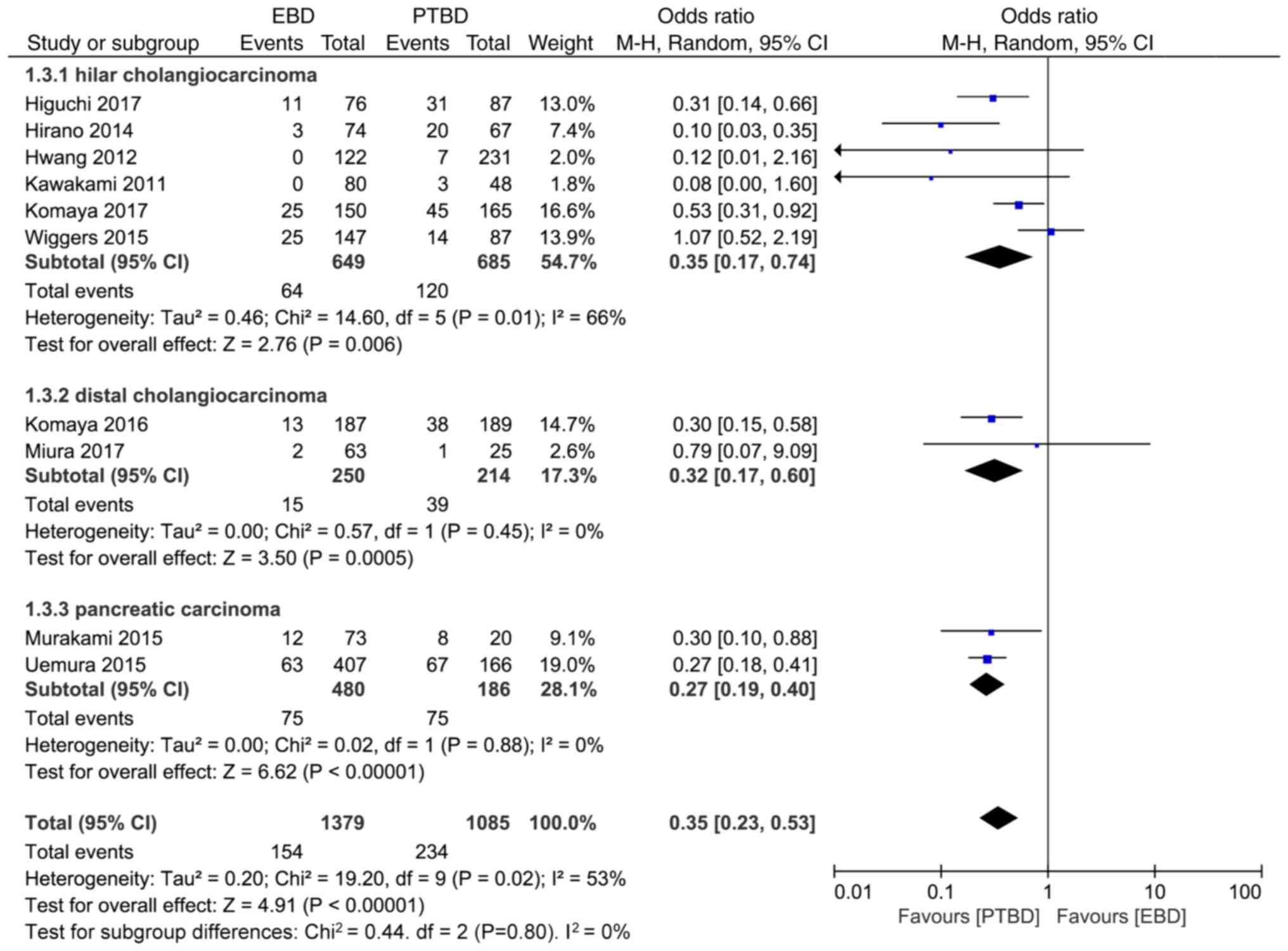
Obstructive jaundice can be caused by hilar
cholangiocarcinoma, distal cholangiocarcinoma and pancreatic
carcinoma. The results of subgroup analysis revealed that the
incidence rate of implantation metastasis in hilar
cholangiocarcinoma, distal cholangiocarcinoma and pancreatic
carcinoma of the EBD and PTBD groups were 9.7% (64/649) vs. 17.5%
(120/685), 6% (15/250) vs. 18.2% (39/214), 15.6% (75/480) vs. 40.3%
(75/186), respectively, and the differences were statistically
significant (OR=0.35, 95% CI: 0.17–0.74, P=0.006; OR=0.32, 95% CI:
0.17–0.60, P=0.0005; and OR=0.27, 95%CI: 0.19–0.40, P<0.00001;
Fig. 4).
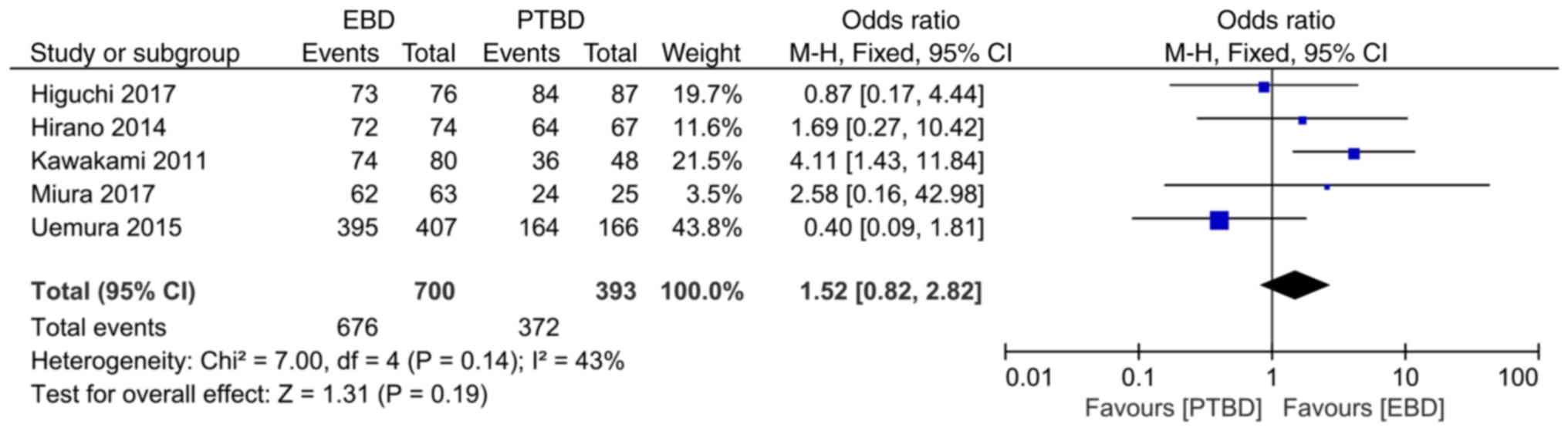
Comparison of the effects of EBD and
PTBD on the surgical success rate in resected MOJ
A total of 5 studies reported the comparison of the
surgical success rate. The heterogeneity analysis results
(I2=43%, P=0.14) demonstrated that there was no obvious
heterogeneity among the studies, and fixed effect model analysis
was used. Meta-analysis results revealed that the surgical success
rate of the EBD and the PTBD groups were 96.6% (676/700) and 94.6%
(372/393), respectively, and there was no statistically significant
difference between the two groups (OR=1.52, 95% CI: 0.82–2.82,
P=0.19; Fig. 5).
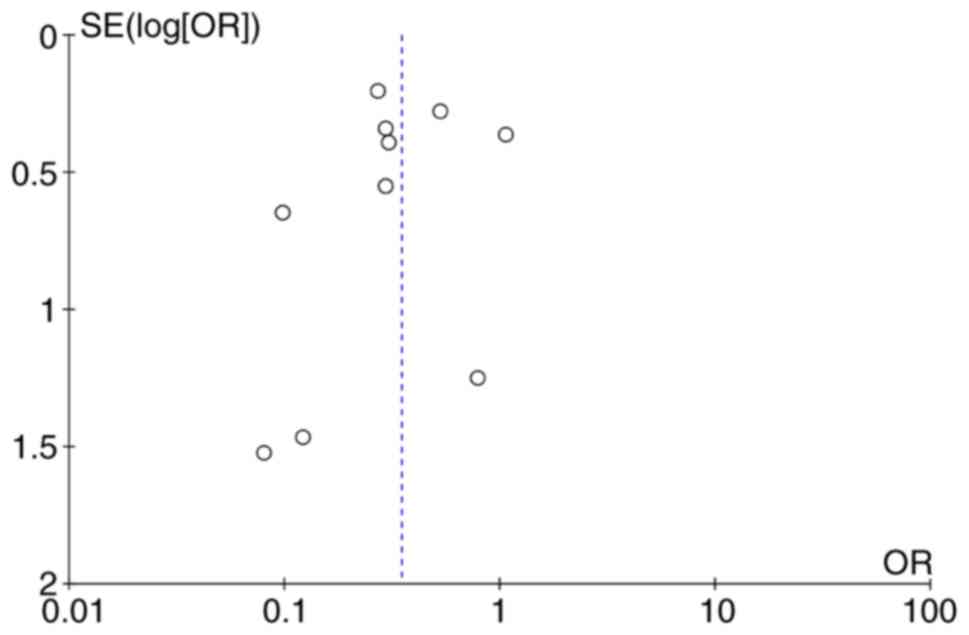
Publication bias analysis
A funnel plot was used to analyze the incidence rate
of implantation metastasis in the EBD and PTBD groups, and the
results revealed that funnel plot was basically symmetrical,
indicating that publication bias had little impact on meta-analysis
results (Fig. 6).
Discussion
Implantation metastasis is an important metastatic
route for abdominal malignancy and invasive procedures, while
preoperative biliary drainage may directly or indirectly increase
the risk of implantation metastasis (20). In this meta-analysis, the incidence
rate of implantation metastasis in the application of two common
preoperative biliary drainage methods was analyzed, and it was
found that the incidence rate of implantation metastasis in EBD was
lower than that in PTBD for resected MOJ.
Surgery remains the most effective treatment for
resected hepatobiliary and pancreatic malignancies (21). For malignant hepatobiliary and
pancreatic tumors with obstructive jaundice, how to effectively
remove biliary obstruction and reduce hyperbilirubinemia is of
great importance to improve the success rate of surgery. However, a
growing body of studies have found that preoperative biliary
drainage (PBD) cannot prolong the survival of MOJ patients, and it
extends the length of hospital stay and increases the postoperative
complications (22). Nevertheless,
the US (23), European (24) and Japanese (25) guidelines recommend appropriate PBD
for patients with MOJ. However, there remains controversy in the
international hepatobiliary and pancreatic community about which
drainage method to select: The European and American guidelines
recommend PTBD, while the Japanese guidelines strongly recommend
EBD. Compared with EBD, PTBD surgery is simple and easy to perform.
The latest meta-analysis confirmed that PTBD was more effective in
reducing postoperative complications than EBD (26.5% vs. 44.3%,
P=0.0009) (26). As supporters of
the EBD procedure, the Japanese consider implantation metastasis as
the most important factor (27).
Implantation metastasis is one of the distant
metastatic pathways of abdominal malignant tumors. It can be
divided into direct dissemination and hematogenous or lymphatic
dissemination according to the metastatic pathway, and into
thoracoperitoneal implantation and body wall implantation according
to implantable location. Hepatobiliary and pancreatic malignancies,
especially hilar cholangiocarcinoma, are more prone to implantation
metastasis due to their unique anatomical location. A study found
that implantation metastasis occurred in up to 15.9% of patients
with MOJ, even without preoperative biliary drainage (11). In this meta-analysis, the total
implantation metastasis rate of preoperative biliary drainage was
13.6% (336/2464), with 9.6% (132/1379) and 18.8% (204/1085) in the
EBD and PTBD groups, respectively, and the difference was
statistically significant (P<0.00001). In addition, PTBD
significantly increased the risk of catheter-related implantation
metastasis (4.3% vs. 0.6%, P<0.0001). In conclusion, the
prevalence and poor prognosis of implantation metastasis after PTBD
surgery indicates that attention must be paid to the problem of
implantation metastasis after PBD surgery.
Although this meta-analysis studies the effect of
drainage in MOJ, drainage is not necessary for MOJ in some cases.
Some scholars theorize that pancreaticoduodenectomy can be
performed without cholangitis in the presence of obstructive
jaundice (28). All the ten studies
included in this study were retrospective cohort studies that were
clearly grouped, and no selective results were reported. Although
literature retrieval and strict inclusion criteria were utilized in
the present study, there are still limitations. In spite of the
retrieval procedure adopted widely, some data such as supplements,
conference papers, and certain grey literature were unavailable.
Furthermore, as the original data of the included studies were not
sufficient, the meta-analysis could only comment on relevant
indicators, thus potential publication bias cannot be avoided. In
addition, a large number of retrospective cohort studies were
included in this study, lacking large sample, multi-center
randomized controlled studies. There was no clear definition of MOJ
implantation metastasis. Intrahepatic metastasis belongs to the
implantation metastasis related to PBD of pancreatic carcinoma, but
it is controversial for hilar cholangiocarcinoma and distal
cholangiocarcinoma (29). The
categories of implantable abdominal metastasis are different, and
the definitions of abdominal invasion, abdominal dissemination and
abdominal metastasis are confounding. The PBD schemes included in
the studies were different, and the PBD including PTBD and EBD
technologies used varied among hospitals, resulting in
heterogeneity of the studies, which may affect the evidence
strength and credibility of the results of this meta-analysis. In
addition, there was no significant difference in the surgical
success rate between the two groups. Due to the limitation of the
original research, there is a lack of relevant research in western
countries. Therefore, more studies in western countries are
expected to be carried out to draw more convincing conclusions.
In conclusion, this meta-analysis indicated that the
incidence rate of implantation metastasis in EBD was lower than
that in PTBD for resected MOJ. However, it should be noted that at
present, sufficient evidence-based medicine is still lacking. In
view of the lack of multi-center randomized controlled studies with
a large sample size and the limitations of the literature quality,
the aforementioned results should be confirmed by future
studies.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Project of the
Department of Education, Sichuan Province (grant no.
16TDD00025).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GY, YX, JL and JS collected the data. TT, WL and GW
analyzed the data. GY and JL prepared and wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chandrashekhara SH, Gamanagatti S, Singh A
and Bhatnagar S: Current status of percutaneous transhepatic
biliary drainage in palliation of malignant obstructive jaundice: A
review. Indian J Palliat Care. 22:378–387. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu X, Li J, Wu J, Zhu R and Ji W: A
systematic review and meta-analysis of intraluminal brachytherapy
versus stent alone in the treatment of malignant obstructive
jaundice. Cardiovasc Intervent Radiol. 41:206–217. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lorenz JM: Management of malignant biliary
obstruction. Semin Intervent Radiol. 33:259–267. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fukasawa M, Takano S, Shindo H, Takahashi
E, Sato T and Enomoto N: Lorenz JM. Management of malignant biliary
obstruction. Clin J Gastroenterol. 10:485–490. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hucl T: Malignant biliary obstruction. Cas
Lek Cesk. 155:30–37. 2016.(In Czech). PubMed/NCBI
|
|
6
|
Tibble JA and Cairns SR: Role of
endoscopic endoprostheses in proximal malignant biliary
obstruction. J Hepatobiliary Pancreat Surg. 8:118–123. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hameed A, Pang T, Chiou J, Pleass H, Lam
V, Hollands M, Johnston E, Richardson A and Yuen L: Percutaneous
vs. endoscopic pre-operative biliary drainage in hilar
cholangiocarcinoma-A systematic review and meta-analysis. HPB
(Oxford). 18:400–410. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsumoto K, Takeda Y, Onoyama T, Kawata
S, Kurumi H, Koda H, Yamashita T and Isomoto H: Endoscopic
treatment for distal malignant biliary obstruction. Ann Transl Med.
5:1902017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wiggers JK, Groot KB, Coelen RJ, Doussot
A, van Dieren S, Rauws EA, Schattner MA, van Lienden KP, Brown KT,
Besselink MG, et al: Percutaneous preoperative biliary drainage for
resectable perihilar cholangiocarcinoma: No association with
survival and no increase in seeding metastases. Ann Surg Oncol. 22
(Suppl 3):S1156–S1163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stang A: Critical evaluation of the
newcastle-ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Higuchi R, Yazawa T, Uemura S, Izumo W,
Chaudhary RJ, Furukawa T and Yamamoto M: ENBD is associated with
decreased tumor dissemination compared to PTBD in perihilar
cholangiocarcinoma. J Gastrointest Surg. 21:1506–1514. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hirano S, Tanaka E, Tsuchikawa T,
Matsumoto J, Kawakami H, Nakamura T, Kurashima Y, Ebihara Y and
Shichinohe T: Oncological benefit of preoperative endoscopic
biliary drainage in patients with hilar cholangiocarcinoma. J
Hepatobiliary Pancreat Sci. 21:533–540. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hwang S, Song GW, Ha TY, Lee YJ, Kim KH,
Ahn CS, Sung KB, Ko GY, Kim MH, Lee SK, et al: Oncological benefit
of preoperative endoscopic biliary drainage in patients with hilar
cholangiocarcinoma. World J Surg. 36:379–385. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawakami H, Kuwatani M, Onodera M, Haba S,
Eto K, Ehir N, Yamato H, Kudo T, Tanaka E, Hirano S, et al:
Endoscopic nasobiliary drainage is the most suitable preoperative
biliary drainage method in the management of patients with hilar
cholangiocarcinoma. J Gastroenterol. 46:242–248. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Komaya K, Ebata T, Fukami Y, Sakamoto E,
Miyake H, Takara D, Wakai K and Nagino M; Nagoya Surgical Oncology
Group, : Percutaneous biliary drainage is oncologically inferior to
endoscopic drainage: A propensity score matching analysis in
resectable distal cholangiocarcinoma. J Gastroenterol. 51:608–619.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Komaya K, Ebata T, Yokoyama Y, Igami T,
Sugawara G, Mizuno T, Yamaguchi J and Nagino M: Verification of the
oncologic inferiority of percutaneous biliary drainage to
endoscopic drainage: A propensity score matching analysis of
resectable perihilar cholangiocarcinoma. Surgery. 161:394–404.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miura F, Sano K, Wada K, Shibuya M, Ikeda
Y, Takahashi K, Kainuma M, Kawamura S, Hayano K and Takada T:
Prognostic impact of type of preoperative biliary drainage in
patients with distal cholangiocarcinoma. Am J Surg. 214:256–261.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Murakami Y, Uemura K, Hashimoto Y, Kondo
N, Nakagawa N, Sasaki H, Hatano N, Kohmo T and Sueda T: Does
preoperative biliary drainage compromise the long-term survival of
patients with pancreatic head carcinoma? J Surg Oncol. 111:270–276.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uemura K, Murakami Y, Satoi S, Sho M,
Motoi F, Kawai M, Matsumoto I, Honda G, Kurata M, Yanagimoto H, et
al: Impact of preoperative biliary drainage on long-term survival
in resected pancreatic ductal adenocarcinoma: A multicenter
observational study. Ann Surg Oncol. 22 (Suppl 3):S1238–S1246.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Manzotti C, Audisio RA and Pratesi G:
Importance of orthotopic implantation for human tumors as model
systems: Relevance to metastasis and invasion. Clin Exp Metastasis.
11:5–14. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tempero MA, Malafa MP, Al-Hawary M, Asbun
H, Bain A, Behrman SW, Benson AI III, Binder E, Cardin DB, Cha C,
et al: Pancreatic adenocarcinoma, version 2.2017, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
15:1028–1061. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Celotti A, Solaini L, Montori G, Coccolini
F, Tognali D and Baiocchi G: Preoperative biliary drainage in hilar
cholangiocarcinoma: Systematic review and meta-analysis. Eur J Surg
Oncol. 43:1628–1635. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mansour JC, Aloia TA, Crane CH, Heimbach
JK, Nagino M and Vauthey JN: Hilar cholangiocarcinoma: Expert
consensus statement. HPB (Oxford). 17:691–699. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Banales JM, Cardinale V, Carpino G,
Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes
SJ, Fouassier L, et al: Expert consensus document:
Cholangiocarcinoma: Current knowledge and future perspectives
consensus statement from the European network for the study of
cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol.
13:261–280. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rerknimitr R, Angsuwatcharakon P,
Ratanachu-ek T, Khor CJ, Ponnudurai R, Moon JH, Seo DW,
Pantongrag-Brown L, Sangchan A, Pisespongsa P, et al: Asia-Pacific
consensus recommendations for endoscopic and interventional
management of hilar cholangiocarcinoma. J Gastroenterol Hepatol.
28:593–607. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Al MA, Menahem B, Fohlen A, Dupont B,
Alves A, Launoy G and Lubrano J: Preoperative biliary drainage in
patients with resectable perihilar cholangiocarcinoma: Is
percutaneous transhepatic biliary drainage safer and more effective
than endoscopic biliary drainage? A meta-analysis. J Vasc Interv
Radiol. 28:576–582. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miyazaki M, Yoshitomi H, Miyakawa S,
Uesaka K, Unno M, Endo I, Ota T, Ohtsuka M, Kinoshita H, Shimada K,
et al: Clinical practice guidelines for the management of biliary
tract cancers 2015: The 2nd english edition. J Hepatobiliary
Pancreat Sci. 22:249–273. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Morano WF, Shaikh MF, Gleeson EM, Galvez
A, Khalili M, Lieb J, Renza-Stingone EP and Bowne WB:
Reconstruction options following pancreaticoduodenectomy after
Roux-en-Y gastric bypass: A systematic review. World J Surg Oncol.
16:1682018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Strom TJ, Klapman JB, Springett GM,
Meredith KL, Hoffe SE, Choi J, Hodul P, Malafa MP and Shridhar R:
Comparative long-term outcomes of upfront resected pancreatic
carcinoma after preoperative biliary drainage. Surg Endosc.
29:3273–3281. 2015. View Article : Google Scholar : PubMed/NCBI
|