Introduction
Breast cancer is one of the most common malignancies
affecting the life of women worldwide. Although the widespread use
of adjuvant chemotherapy and hormonal drugs has reduced breast
cancer mortality, breast cancer remains the leading cause of
mortality among women <50 years of age (1). Breast cancer is a complex polygenic
disease and an abnormal metabolic disease. It is well known that
the viability of tumour cells is associated with their specific
metabolism. Cancer cells are usually hyperproliferative and exhibit
a higher rate of glycolysis compared with normal cells. Glycolysis
is part of the energy metabolism of cancer cells and is an
important way to generate ATP. Cancer cells reprogram their
metabolism to shift from using pyruvate for oxidative
phosphorylation to using lactate, which means tumour cells use a
large amount of glucose for glycolysis and produce a large amount
of lactate; this shift is known as the Warburg effect (2). The high rate of glycolysis in tumour
cells maintains the acidic characteristics of the tumour
microenvironment, which is associated with tumour invasion
features, such as growth advantages and increased survival,
migration, invasion and angiogenes (3,4).
Monocarboxylate transporters (MCTs) mediate the
transport of various monocarboxylates, including lactate, pyruvate
and ketone, across cell membranes (5). Tumour cells rely on MCTs to transport
large amounts of lactate out of the cell, thereby avoiding
intracellular acidification and cell death. The MCT family consists
of 14 members. Among these, MCT1-4 are preferentially involved in
the transport of lactic acid (6).
MCT-1 is relatively ubiquitously expressed in a number of tissues
and serves a role in lactate shuttles in the heart, slow-twitch
muscles, red blood cells and liver (7). Other MCT proteins exhibit stronger
tissue-specific expression, such as MCT-2 being present in neurons,
and MCT-3 being restricted to retinal pigment and choroid plexus
epithelium (8). MCT-4 is limited to
the glycolysis pathway. MCT-4 is located in the cell membrane and
transports lactic acid through a pH gradient. MCT-4 has been
demonstrated to be overexpressed in multiple types of cancer,
including melanoma, and cervical, colorectal, kidney and lung
cancer (9–19). It has been demonstrated that MCT-4,
as a marker of glycolysis and lactic acid production, can prevent
pH lowering and inhibit sustained glycolysis by exporting lactic
acid in tumour cells (20).
However, the clinical relevance of MCT-4 expression
in breast cancer has not been fully elucidated. Therefore, the
present study aimed to investigate the predictive effect of MCT-4
expression on overall survival (OS) in patients with breast cancer,
and to evaluate its clinicopathological significance, thus
potentially identifying a novel potential therapeutic target for
the treatment of breast cancer.
Materials and methods
Patients and tissues
A total of 145 samples were collected from 145
female patients with confirmed breast cancer at The First People's
Hospital of Yibin (Yibin, China) affiliated with Southwest Medical
University. The average age of the patients was 60 years (range,
33–88 years). The follow-up began on the day of surgery and ended
in July 2014, ranging between 2 and 119 months. All patients
underwent surgery between August 2004 and December 2008, including
modified radical mastectomy or lumpectomy with axillary
lymphonodectomy. According to the World Health Organization
Classification of Tumors of the Breast (21), the basic clinicopathological data are
shown in Table I. The haematoxylin
and eosin-stained tissue specimens were reviewed from 145 paraffin
samples. Cylindrical core tissue samples (0.6-mm diameter) were
taken from the most representative area of each paraffin block and
aligned into a new acceptor paraffin block (20×35 mm) using
precision instruments. If the histopathological diagnosis lacked a
clear date or the sample did not contain enough cancer cells at the
tissue chip point, the sample was excluded from the present study.
OS time was defined as the date from initial diagnosis to the last
follow-up or death. The exclusion criteria were as follows: i)
Patients lost to follow-up; ii) died of other diseases, iii) died
of accidents, iv) lacked a clear date of death and v) the sample
did not contain enough cancer cells at the tissue chip point. The
clinicopathological parameters of patients in the present study and
the relevant dates of long-term follow-up were obtained from
hospitals. In addition, 30 fresh breast cancer tissues and paired
adjacent normal tissues (5 cm away from primary tumour site) were
collected from The First People's Hospital of Yibin affiliated with
Southwest Medical University and stored at −80°C prior to protein
extraction. The mean age of the patients was 52 years (range, 35–67
years). The date of surgery was between January 5, 2018 and
December 22, 2018. The present study was conducted in accordance
with the Declaration of Helsinki and the Guiding Principles of The
First People's Hospital of Yibin Ethics Review Committee and
Southwest Medical University.
 | Table I.Patient clinicopathological
characteristics (n=145). |
Table I.
Patient clinicopathological
characteristics (n=145).
| Characteristics | Samples, n | % |
|---|
| Age, years |
| ≤60 | 83 | 57.2 |
|
>60 | 62 | 42.8 |
| pT stage |
|
|
|
pT1 | 36 | 24.8 |
|
pT2 | 90 | 62.1 |
|
pT3 | 17 | 11.7 |
|
pT4 | 2 | 1.4 |
| N stage |
| N0 | 74 | 51.0 |
| N1 | 38 | 26.2 |
| N2 | 18 | 12.4 |
| N3 | 15 | 10.4 |
| M stage |
|
|
| M0 | 145 | 100 |
| M1 | 0 | 0 |
| Histologic
grade |
| G1 | 4 | 2.8 |
| G2 | 121 | 83.4 |
| G3 | 20 | 13.8 |
| TNM stage |
| I | 23 | 15.9 |
| II | 83 | 57.2 |
|
III | 39 | 26.9 |
| Oestrogen
receptor |
|
Negative | 57 | 41.6 |
|
Positive | 80 | 58.4 |
| Progesterone
receptor |
|
Negative | 88 | 64.2 |
|
Positive | 49 | 35.8 |
| Human epidermal
growth factor receptor |
|
Negative | 97 | 70.3 |
|
Positive | 41 | 29.7 |
| Ki67, % |
| Low
(<14) | 85 | 62.5 |
| High
(≥14) | 51 | 37.5 |
| Androgen
receptor |
|
Negative | 43 | 30.9 |
|
Positive | 96 | 69.1 |
Cell culture and transfection
The MCF-7 cell line, obtained from the American Type
Culture Collection, was maintained in Minimum Essential Medium
supplemented (MEM; cat. no. SH30024) with 10% FBS (cat. no.
SH30396; both from Hyclone; Cytiva), 2 mM/l glutamine and 100 U/ml
penicillin/streptomycin. Cells were maintained at 37°C in a 5%
(v/v) CO2 atmosphere and sub-cultured every 3 days.
Transfection with small interfering (si)RNAs (50 nM; Guangzhou
RiboBio Co., Ltd.) against the MCT-4 gene (siMCT-4;
5′-TCCCATGGCCAGGAGGGTTG-3′) was performed using
Lipofectamine® 3000 (Thermo Fisher Scientific, Inc.).
Scrambled siRNA (5′-CCAUGAGGAGUACUGCCAATT-3′) was used as a
negative control. A total of 2×105 cells/well were
seeded in a 6-well plate in complete medium (2 ml/well). According
to the manufacturer's protocol, when the cells reached 80%
confluence, Solution A (P3000™ Reagent with siRNAs) and Solution B
(Lipofectamine™ 3000 reagent; both from the
Lipofectamine® 3000 kit; Thermo Fisher Scientific, Inc.)
were diluted in serum-free medium, respectively, mixed with each
other and incubated at room temperature for 10–15 min. Cells were
washed with 2 ml serum-free medium, the complex solution was added
to each well and cells were cultured at 37°C in a 5% CO2
humidified incubator for 24 h. Subsequently, the culture medium
with transfection regents was replaced with fresh complete medium
to remove the influence of transfection regents on cell biological
behaviours. After 72 h incubation at 37°C, western blotting was
performed to detect whether MCT-4 expression was successfully
silenced.
Cell Counting Kit-8 (CCK-8) and
5-ethynyl-2′-deoxyuridine (EdU) incorporation assay
To assess cell viability, a CCK-8 assay
(Sigma-Aldrich; Merck KGaA) was performed according to the
manufacturer's protocol. Subsequently, EdU integration (Guangzhou
RiboBio Co., Ltd.) was performed. After transfection, cells were
seeded into 96-well plates with ~5×103 cells/well and
incubated at 37°C until cells reached 30% confluence. According to
the manufacturer's protocol, the EdU assay was performed using a
Cell-Light EdU Apollo 567 in vitro kit (cat. no. 100T;
Changzhou Ruibo Biotechnology Co., Ltd.).
Immunohistochemical staining
Samples were fixed in 4% paraformaldehyde (cat. no.
P6148; Merck KGaA) for 48 h at room temperature.
Immunohistochemical staining was used to assess the expression
levels of MCT-4. Tissue microarrays (TMAs, 4 µm) were dewaxed twice
in xylene for 15 min each at room temperature and rehydrated three
times in a descending alcohol series (100, 100, 95 and 80%).
Antigen retrieval was performed with citric acid buffer (10 mM; pH
6.0) in a microwave (800 W) for 15 min, and samples were cooled at
room temperature for 30 min. Subsequently, sections were blocked
with 0.5% goat serum (cat. no. SL038; Beijing Solarbio Science
& Technology Co., Ltd.) and incubated with 3%
H2O2 (cat. no. 323381; Merck KGaA) to inhibit
endogenous peroxidase activity, both at room temperature for 30
min. The samples were incubated with anti-MCT-4 primary antibody
(dilution, 1:100 in TBS; cat. no. sc-376140; Santa Cruz
Biotechnology, Inc.) at 4°C overnight. The TMAs were subsequently
washed with PBS for 5 min and incubated with a goat anti-mouse
secondary antibody (dilution, 1:2,000 for immunohistochemistry;
cat. no. HA1001; Hangzhou HuaAn Biotechnology Co., Ltd) for 1 h at
37°C. The colour was developed with 3–3′-diaminobenzidine.
Subsequently, sections were counterstained with hematoxylin
solution (cat. no. 51275; Merck KGaA) for 5 min, rinsed with tap
water repeatedly until the water became clear, and subsequently
differentiated using 1–2% hydrochloric acid (cat. no. H1758; Merck
KGaA) for 1–2 sec, all of which were performed at room temperature.
The nuclei and cytoplasm of breast cancer cells were observed using
a light microscope (magnification, ×200).
Western blotting
Fresh tissue samples (~100 mg) were collected from
30 patients with breast cancer and cut into pieces with surgical
scissors in lysis buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris
pH 7.4, 1 mM EDTA, 1 mM EGTA pH 8.0, 0.2 mM sodium orthovanadate
and protease inhibitors), and the protein concentration was
quantified using a bicinchoninic acid protein assay kit. Protein
lysate (50 µg/lane) were separated via SDS-PAGE on a 10% gel and
transferred to a PVDF membrane, which were blocked with 5% skimmed
milk for 1 h at room temperature. Membranes were incubated with
anti-MCT-4 primary antibody (dilution, 1:1,000 in BSA; cat. no.
sc-376140; Santa Cruz Biotechnology, Inc.) and non-conjugated
internal reference antibodies, anti-GAPDH (dilution, 1:5,000; cat.
no. EM1101) and anti-β-actin (dilution, 1:5,000; cat. no. EM21002)
at 4°C overnight (both from Hangzhou HuaAn Biotechnology Co.,
Ltd.). The membrane was washed with TBS-Tween (0.1%) and incubated
with goat anti-mouse secondary antibody for 60 min at 37°C. Pierce
enhanced chemiluminescent reagent (cat. no. NCI4106; Pierce; Thermo
Fisher Scientific, Inc.) was added, and the membrane was exposed to
X-ray film in the dark. Protein bands were semi-quantitatively
analysed using Quantity One software (version 4.6.6, Bio-Rad
Laboratories, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from breast cancer tissues
and paired adjacent tissues using TRIzol reagent (Takara Bio,
Inc.). The PrimeScript RT kit (Takara Bio, Inc.) was used for
reverse transcription to cDNA with the following temperature
protocol: 35°C for 5 min, 42°C for 40 min and 75°C for 5 min.
Subsequently, qPCR was performed using SYBR Premix Ex Taq II
(Takara Bio, Inc.) and the LightCycler system (Roche Diagnostics
GmbH). The results were analysed using the 2−ΔΔCq method
(22). GAPDH was used as an internal
control. The primer sequences were as follows: MCT-4 forward,
5′-CCATGCTCTACGGGACAGG-3′ and reverse, 5′-GCTTGCTGAAGTAGCGGTT-3′;
and GAPDH forward, 5′-GGAGCGACATCCGTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCAATCTTCTCATGG-3′. The thermocycling conditions for
qPCR were as follows: Initial activation step at 95°C for 15 min,
followed by 40 cycles of denaturation at 94°C for 15 sec, annealing
at 55°C for 30 sec, extension at 72°C for 30 sec and a final
extension at 72°C for 5 min, and the amplification product was kept
at 4°C.
Scoring of the staining results
MCT-4 expression was observed and analysed based on
the intensity of staining (IS; 0, negative; 1, weak; 2, moderate;
3, strong) and the area of positive staining (AP; 0, <5%; 1,
5–25%; 2, 26–50%; 3, 51–75%; 4, >75%). The final
immunoreactivity score of MCT-4 expression was determined using the
following formula: Intensity distribution (ID)=AP × IS. To
determine the cut-off value of the expression level (high or low)
of MCT-4, receiver operating characteristic (ROC) curve analysis of
the OS rate was performed. The TMA was independently analysed by
two experienced pathologists who were blinded to the clinical
characteristics of patients.
Statistical analysis
Statistical analysis was performed using SPSS
software 19.0 (IBM Corp.) and GraphPad Prism software 6.0 (GraphPad
Software, Inc.). Data are presented as the mean ± standard
deviation. The paired t-test was used for comparisons between two
groups. ROC curve analysis was performed to determine the cut-off
point for high or low MCT-4 expression. The association between
MCT-4 immunofluorescence staining and clinicopathological
parameters of patients with breast cancer was analysed by
χ2 test and Fisher analysis. The Kaplan-Meier method was
used to assess the importance of prognosis and the log-rank test
was used to assess the survival curves. Univariate and multivariate
analyses of survival data were performed using the Cox proportional
hazard model approach to analyse independent prognostic values.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
At the end of the follow-up period, 102 patients
(70.3%) survived, while 43 (29.7%) died of breast cancer. The
follow-up period ranged between 2 and 119 months (mean, 76 months;
Table SI). With regard to the
tumour diameter (pT), 36 samples (24.8%) were pT1, 90 samples
(62.1%) were pT2, 17 samples (11.7%) were pT3 and 2 samples (1.4%)
were pT4. With regard to the degree of lymph node involvement (N),
74 (51.0%) patients were N0, 38 (26.2%) were N1, 18 (12.4%) were N2
and 15 (10.4%) were N3. There was no distant metastasis (M)
observed. Considering these three aspects, 23 samples (15.9%) were
classified as TNM stage I, 83 samples (57.2%) were stage II and 39
samples (26.9%) were stage III. In terms of histologic grade, 4
patients (2.8%) were G1, 121 (83.4%) were G2 and 20 (13.8%) were
G3. Other clinicopathological factors are listed in Table I.
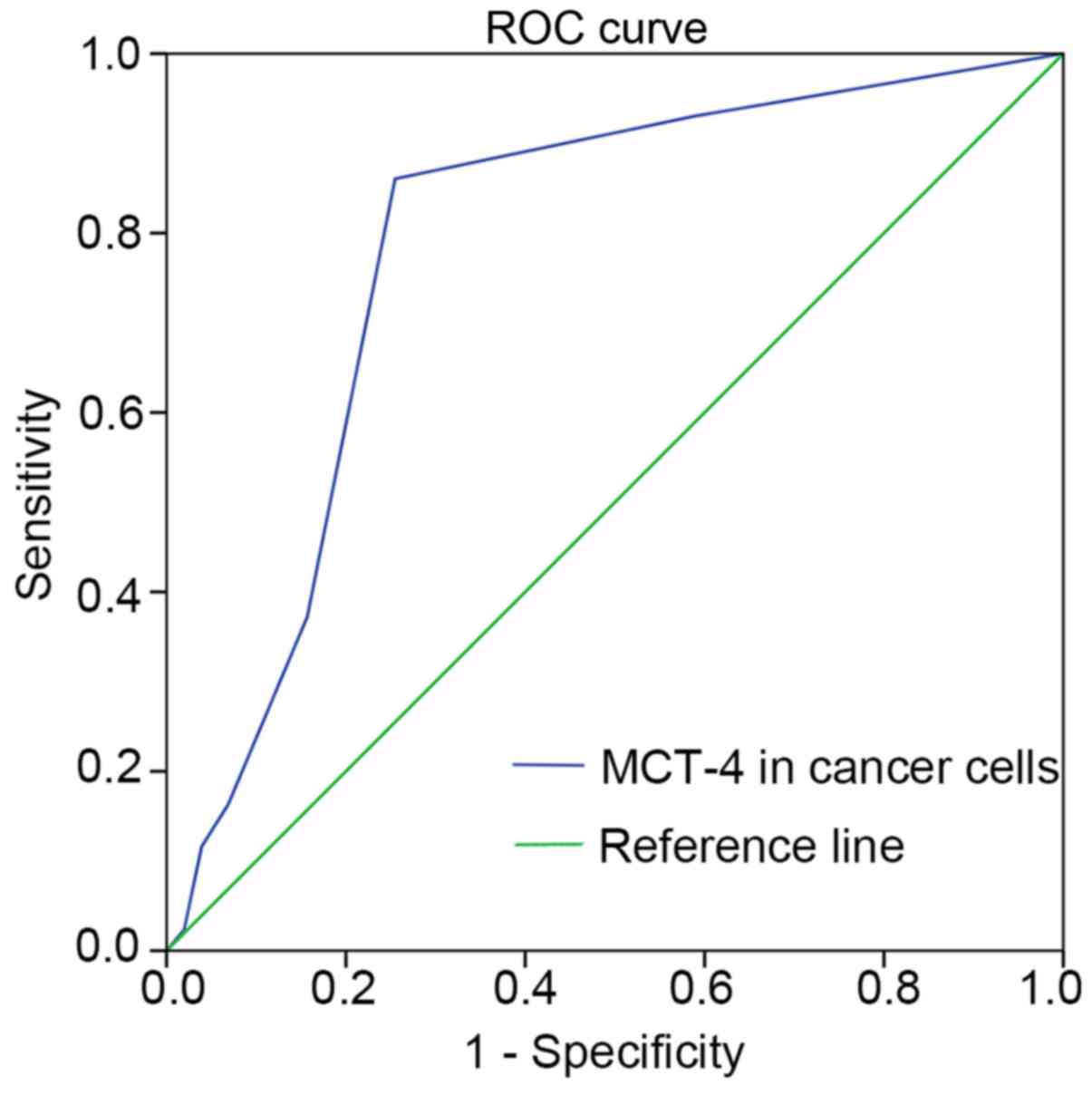
Cut-off value of MCT-4 expression
To accurately obtain cut-off values for high and low
expression levels, ROC curve analysis was performed for OS
(Fig. 1). Based on the optimal
sensitivity and specificity, an ID score of 1.5 was determined as
the cut-off score for MCT-4 expression in breast cancer (ID score
≥1.5 indicated high expression and <1.5 indicated low
expression).
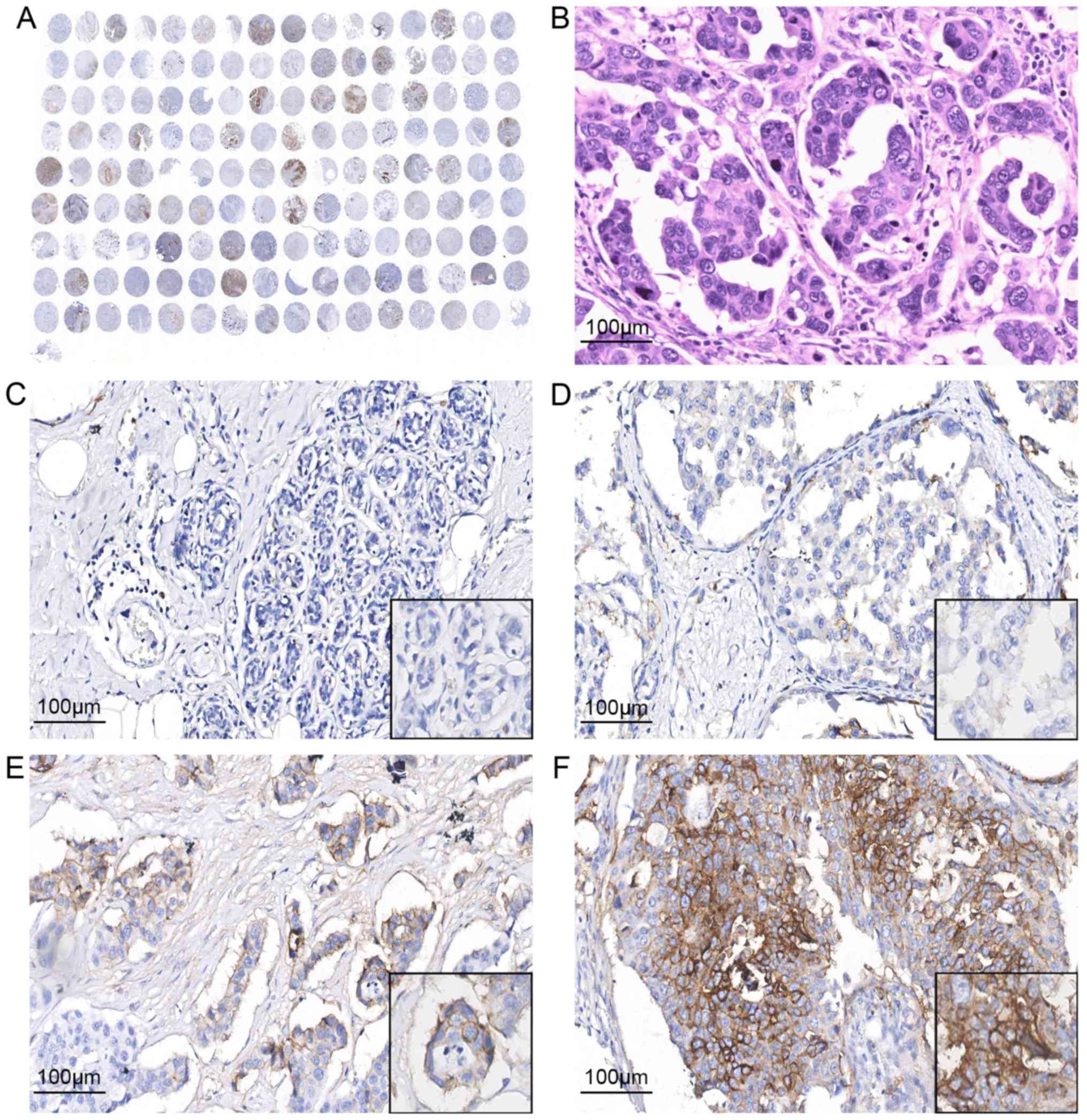
MCT-4 expression in normal breast and
breast cancer tissues
A positive MCT-4 signal was mainly observed on the
cell membrane, as well as in the cytoplasm of tumour cells
(Fig. 2). A total of 63 (43.4%)
samples exhibited high MCT-4 expression, while 82 (56.6%) exhibited
low MCT-4 expression. No high MCT-4 expression was detected in the
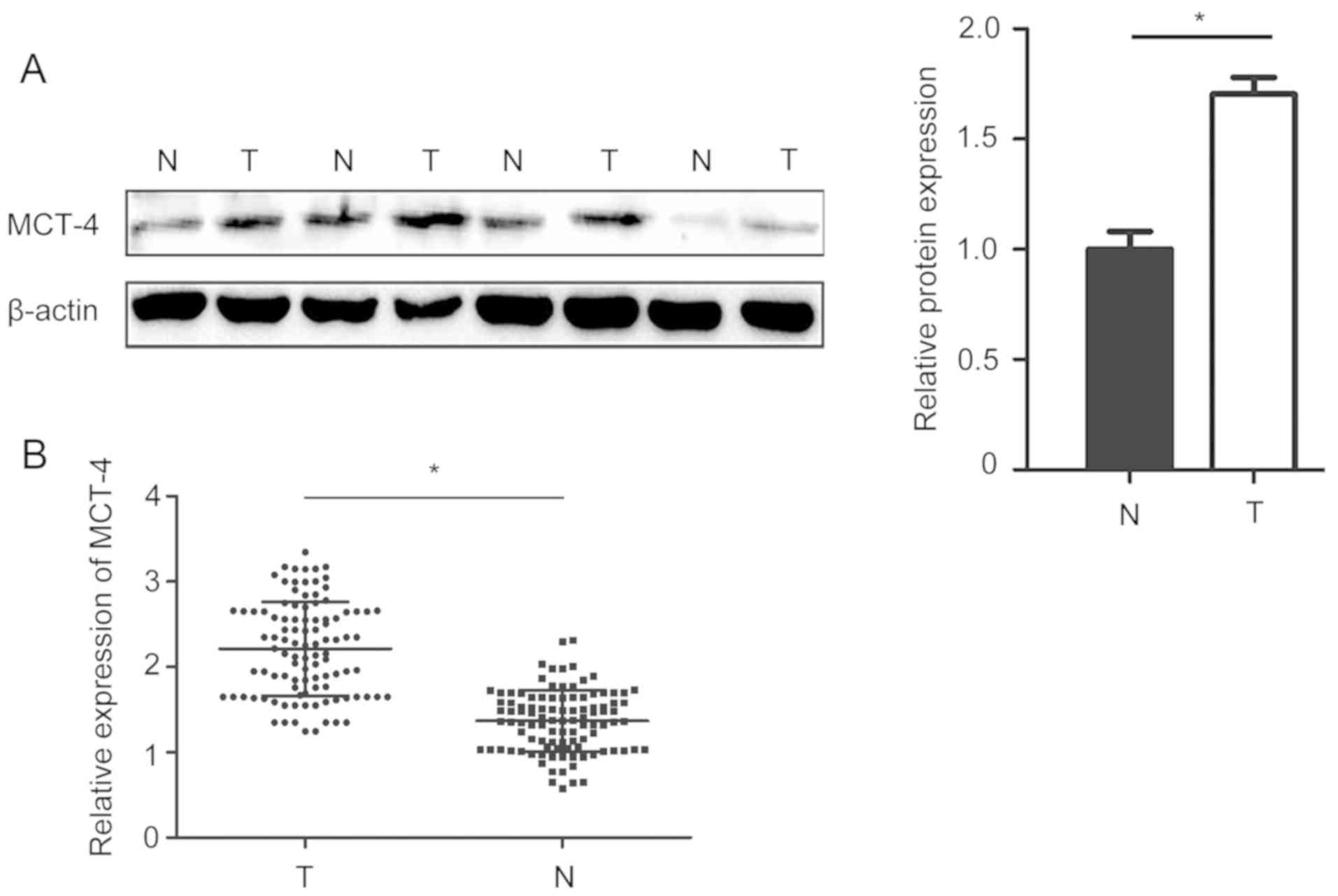
30 corresponding normal breast cancer tissues. Additionally,
RT-qPCR and western blotting demonstrated that MCT-4 expression in
breast cancer tissues was significantly higher than in adjacent
normal tissues (Fig. 3).
Clinical significance and prognostic
value of MCT-4 expression
To analyse the effect of MCT-4 expression on tumour
aggressiveness, the association between MCT-4 expression and
clinicopathological features was assessed using the χ2
test (Table II). A significant
association was detected between high MCT-4 expression and pT stage
(P=0.018). High MCT-4 expression was associated with oestrogen
receptor (ER) status (P=0.001), progesterone receptor (PR) status
(P=0.004), Ki67 index (P=0.043) and androgen receptor (AR) status
(P=0.033). In addition, a close association was observed between
MCT-4 expression and histological grade (P=0.030). No significant
association was identified between MCT-4 expression and any other
clinicopathological variable (Table
II).
 | Table II.Association between MCT-4 expression
and clinicopathological characteristics of patients with breast
cancer (n=145). |
Table II.
Association between MCT-4 expression
and clinicopathological characteristics of patients with breast
cancer (n=145).
|
|
| MCT-4
expression |
|
|---|
|
|
|
|
|
|---|
| Features | Total no. | Low, n (%) | High, n (%) | P-value |
|---|
| Age, years | 145 |
|
| 0.719 |
|
≤60 |
| 48 (57.8) | 35 (42.2) |
|
|
>60 |
| 34 (54.8) | 28 (45.2) |
|
| pT stage | 145 |
|
| 0.018 |
|
pT1/pT2 |
| 76 (60.3) | 50 (39.7) |
|
|
pT3/pT4 |
| 6 (31.6) | 13 (68.4) |
|
| N stage | 145 |
|
| 0.471 |
| N0 |
| 44 (59.5) | 30 (40.5) |
|
|
N1/N2/N3 |
| 38 (53.5) | 33 (46.5) |
|
| Histologic
grade | 145 |
|
| 0.030 |
| G1 |
| 3 (75) | 1 (25) |
|
| G2 |
| 73 (60.3) | 48 (39.7) |
|
| G3 |
| 6 (30) | 14 (70) |
|
| Clinical stage | 145 |
|
| 0.507 |
| I |
| 14 (60.92) | 9 (39.1) |
|
| II |
| 49 (59.0) | 34 (41.0) |
|
|
III |
| 19 (48.7) | 20 (51.3) |
|
| Oestrogen
receptor | 137 |
|
| 0.001 |
|
Negative |
| 22 (38.6) | 35 (61.4) |
|
|
Positive |
| 57 (71.3) | 23 (28.7) |
|
| Progesterone
receptor | 137 |
|
| 0.004 |
|
Negative |
| 42 (47.7) | 46 (52.3) |
|
|
Positive |
| 36 (73.5) | 13 (26.5) |
|
| Human epidermal
growth factor receptor 2 | 138 |
|
| 0.980 |
|
Negative |
| 57 (58.8) | 40 (41.2) |
|
|
Positive |
| 24 (58.5) | 17 (41.5) |
|
| Ki67 | 136 |
|
| 0.043 |
|
Low |
| 55 (64.7) | 30 (35.3) |
|
|
High |
| 24 (47.1) | 27 (52.9) |
|
| Androgen
receptor | 139 |
|
| 0.033 |
|
Negative |
| 19 (44.2) | 24 (55.8) |
|
|
Positive |
| 61 (63.5) | 35 (36.5) |
|
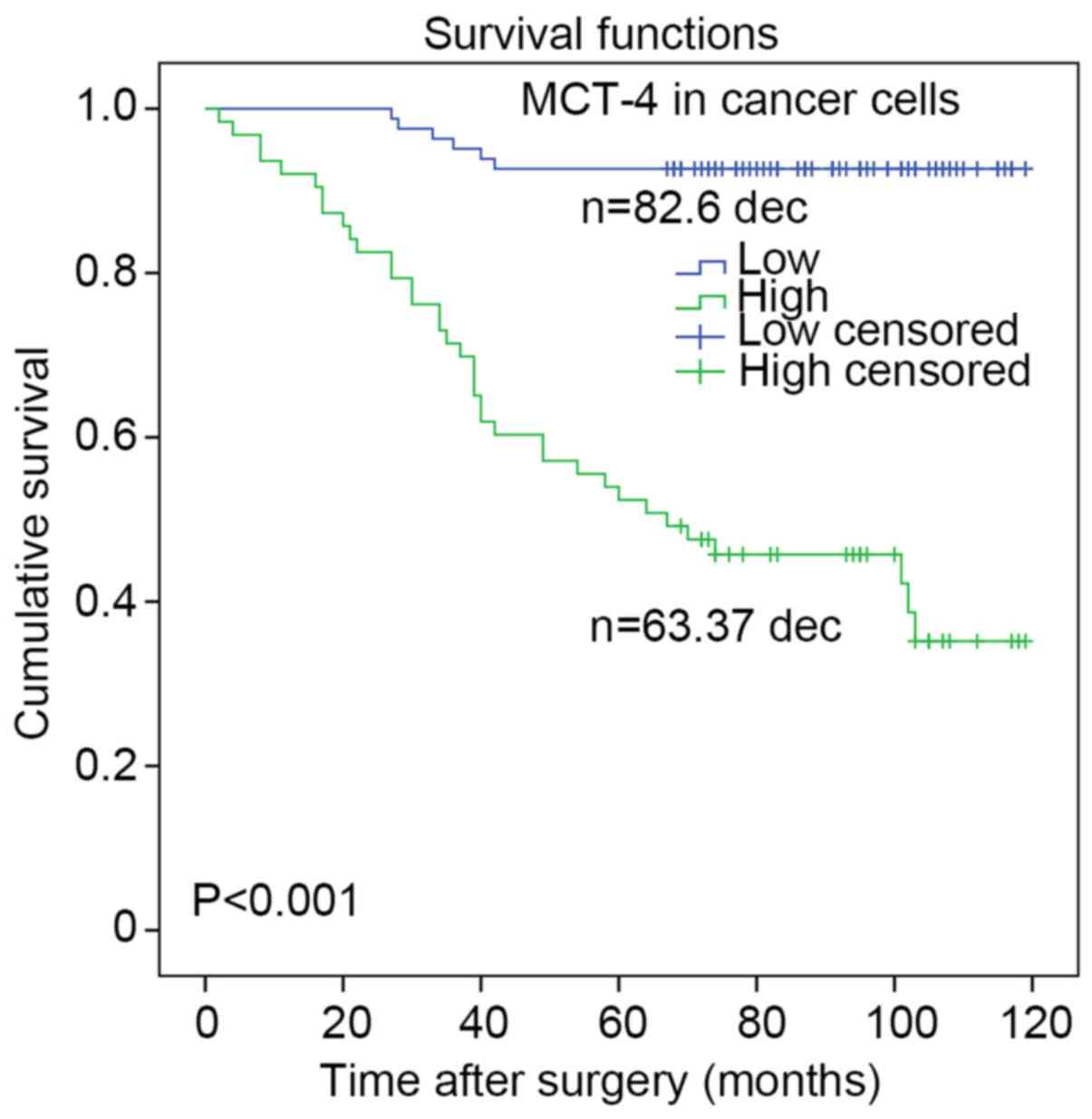
In addition, the Kaplan-Meier method with a log-rank
test was used to estimate survival curves for OS rates and to
assess differences in survival between patients with high and low
MCT-4 expression. The mean OS time for the high MCT-4 expression
group was 62.1 months, whereas that for the low MCT-4 expression
group was 87.9 months. Survival curves indicated that high MCT-4
expression in breast cancer predicted a significantly reduced
likelihood of survival (P<0.001; Fig.
4). In addition, the Cox proportional hazards regression model
was used to identify independent prognostic factors in patients
with breast cancer. Multivariate analysis revealed that MCT-4
expression and lymph node involvement were significantly associated
with OS in patients with breast cancer (P=0.027). However, other
clinicopathological features failed to independently predict breast
cancer prognosis (Table III). The
results of the present study indicated that low MCT-4 expression
was significantly associated with a reduced risk of mortality
compared with high MCT-4 expression in patients with breast cancer.
Multivariate analysis was performed as shown in Table III. Similar to univariate analysis,
MCT-4 expression and lymph node involvement were significant
independent predictors of breast cancer prognosis.
 | Table III.Cox proportional hazard model of the
overall survival rate of patients with breast cancer. |
Table III.
Cox proportional hazard model of the
overall survival rate of patients with breast cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | P-value | HR (95% CI) | P-value | HR (95% CI) |
|---|
| Age (≤60 vs. >60
years) | 0.983 | 1.007
(0.549–1.846) | 0.447 | 0.743
(0.345–1.599) |
| T (≤4 vs. >4
cm) | 0.079 | 0.547
(0.279–1.073) | 0.806 | 0.907
(0.416–1.979) |
| HER2 (negative vs.
positive) | 0.591 | 0.835
(0.432–1.613) | 0.877 | 0.933
(0.390–2.236) |
| ER (negative vs.
positive) | 0.115 | 1.627
(0.888–2.979) | 0.788 | 0.886
(0.365–2.147) |
| PR (negative vs.
positive) | 0.204 | 1.544
(0.790–3.018) | 0.897 | 0.937
(0.351–2.502) |
| Ki67 (negative vs.
positive) | 0.386 | 0.759
(0.407–1.416) | 0.747 | 0.892
(0.446–1.784) |
| AR (negative vs.
positive) | 0.430 | 1.295
(0.682–2.460) | 0.553 | 1.271
(0.575–2.814) |
| Lymph node
metastasis (N0 vs. N1/2/3) | 0.036 | 0.513
(0.275–0.958) | 0.027 | 0.419
(0.194–0.904) |
| MCT-4 expression
(low vs. high) | 0.001 | 0.092
(0.039–0.217) | 0.001 | 0.096
(0.039–0.240) |
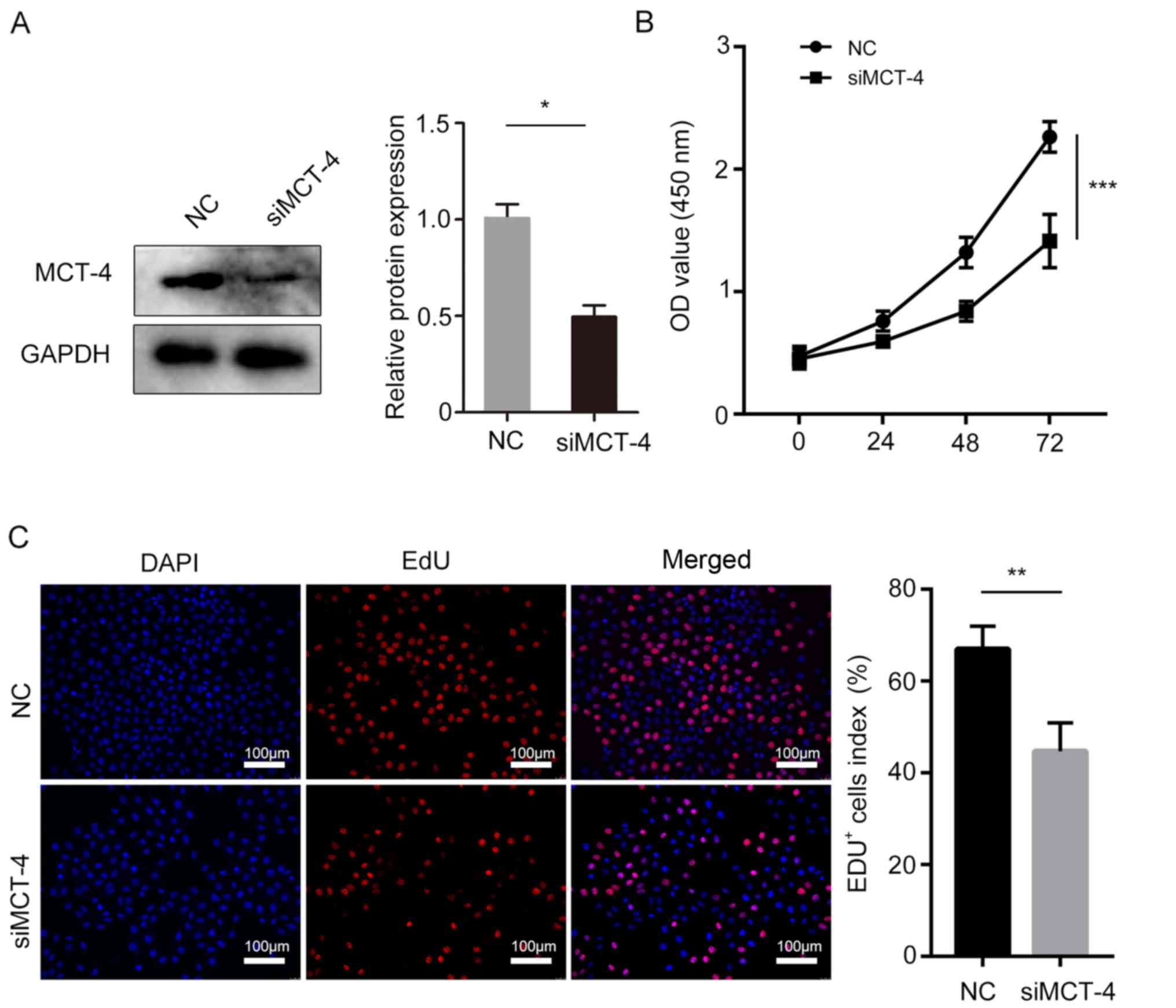
MCT-4 promotes cell viability in
vitro
To investigate the biological effects of MCT-4 on
breast cancer cell viability, MCT-4 expression was silenced in the
MCF-7 cell line (Fig. 5A), and the
CCK-8 analysis revealed that MCT-4 knockdown significantly
decreased MCF-7 cell viability (Fig.
5B). Additionally, reduced MCT-4 expression significantly
inhibited MCF-7 cell viability as assayed by EdU incorporation
(Fig. 5C).
Discussion
The role of the MCT family in abnormal tumour
metabolism has received increasing attention from researchers in
recent years. The present study revealed that staining of MCT-4
appeared mainly in the cell membrane and cytoplasm of tumour cells,
and that it was expressed at low levels in the stromal cells of
breast cancer tissues. Additionally, the association between MCT-4
expression and clinical parameters associated with prognosis was
explored. High MCT-4 expression was associated with advanced stage
(pT3+pT4) and histological grade of breast cancer. MCT-4 knockdown
significantly reduced MCF-7 cell viability in vitro.
Therefore, high MCT-4 expression may predict poor prognosis in
breast cancer, and MCT-4 may be a candidate therapeutic target in
patients with breast cancer. However, the present study contains a
limitation, in that only one cell line was used to explore MCT-4
expression in breast cancer cells. Multiple breast cancer cell
lines should be included in future experiments.
The findings of the present study are consistent
with previous studies by Pinheiro et al (5) demonstrating an increase in MCT-4
expression in breast cancer and by Maria et al (23) reporting that MCT-4 is highly
expressed in a patient with breast cancer with a high histological
grade. However, both of these studies lacked detailed information
on the association between MCT-4 expression and clinical features
of breast cancer, including ER, PR, Ki67, AR and human epidermal
growth factor receptor 2 (HER2) expression. In addition, they did
not report the association between MCT-4 expression and
pathological features or long-term survival rate. In the present
study, silencing the MCT-4 gene significantly attenuated breast
cancer cell viability. The high expression levels of MCT-4 in the
cytoplasm may indicate that it is involved in other cellular
functions (24).
Interestingly, in the present study, high MCT-4
expression was closely associated with triple-negative breast
cancer (TNBC; P=0.024). As the breast cancer type with the worst
prognosis, TNBC is characterized by a lack of ER, PR and HER2
expression. Due to a lack of known specific therapeutic targets,
patients with TNBC do not benefit from conventional
endocrine-targeted therapy, anti-HER2 drugs or other types of
chemotherapy, resulting in high mortality (25,26). The
highly-specific MCT-4 expression in TNBC may become a potential
novel target for prediction and treatment. Unlike most other
studies, the present study thoroughly investigated the association
between MCT-4 expression and AR, which is the most widely expressed
steroid receptor protein in normal breast tissues and is detectable
in ~90% of primary breast cancers and 75% of metastatic lesions. It
has been reported that high AR expression is linked to adverse
reactions to endocrine therapy (27). In the present study, MCT-4 expression
was significantly associated with AR status and may provide a novel
perspective for the treatment of breast cancer.
In previous years, the clinical and prognostic value
of MCT-4 has been identified in colorectal cancer (12,19),
oral squamous cell carcinoma (13),
prostate cancer (13) and lung
adenocarcinoma (28). Although
experimental evidence suggests that MCT-4 may be a potential target
for cancer therapy (29), the
function of this membrane protein in breast cancer remains unclear.
In liver cancer, Luo et al (30) demonstrated that hypoxia-inducible
factor 1-α-mediated MCT-4 expression enhanced glycolysis in liver
cancer cells, resulting in the release of products, such as lactic
acid, into the extracellular environment. The acidic
microenvironment promotes the production of pro-inflammatory
cytokines, contributing to arsenite-induced liver cancer (30). Compared with the normal breast
tissues in the present study, the positive rate of MCT-4 expression
in breast cancer samples was significantly increased, indicating
that there may be different metabolic mechanisms between normal
breast and breast cancer cells. Additionally, high MCT-4 expression
was significantly associated with short OS, acting as an
independent predictor of poor prognosis in patients with breast
cancer, which is consistent with the findings of Curry et al
(31) in head and neck squamous cell
carcinoma. In addition, high MCT4 expression in the stroma is
associated with the progression of gastric cancer and predicts a
poor prognosis (32). At present,
the understanding of the molecular basis of MCT-4 mainly focuses on
its role in transporting lactic acid from tumour cells to the
extracellular matrix (5,33), which increases the acidity of the
extracellular environment and maintains an acidic tumour
microenvironment (5). This shift
results in the activation of a number of cytokines by matrix
metalloproteases or other proteases to facilitate tumour
neovascularization, cell survival and epithelial-mesenchymal
transition to aid cancer cell survival (33).
In conclusion, MCT-4 expression was significantly
higher in breast cancer tissues compared with adjacent normal
tissues. Furthermore, MCT-4 high expression was demonstrated to be
associated with pT status, ER status, PR status, Ki67 and AR
status, and was closely associated with pathological grade.
Silencing MCT-4 decreased cell viability of breast cancer cells
(MCF-7). Taken together, these results suggest that MCT-4 may be
used as a novel potential predictor and treatment target for
patients with breast cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key Research
Projects on Application Foundation of Sichuan Science and
Technology Department (grant no. 2017JY0030).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SX, YS and MX designed and conducted the
experiments, and wrote the manuscript. HZ, ZW and HW provided the
research materials and analyzed the data. All authors agree to be
accountable for all aspects of the research. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki and approved by the Ethics Review
Committee of the Yibin First People's Hospital (Yibin, China;
approval no. Y2018010601) and the Ethics Review Committee of
Southwest Medical University (Luzhou, China; approval no.
Z2018-178-01). Written informed consent was obtained from all
patients for the use of their tissues in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xi J, Feng J, Li Q, Li X and Zeng S: The
long non-coding RNA lncFOXO1 suppresses growth of human breast
cancer cells through association with BAP1. Int J Oncol.
50:1663–1670. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lu J, Tan M and Cai Q: The Warburg effect
in tumor progression: Mitochondrial oxidative metabolism as an
anti-metastasis mechanism. Cancer Lett. 356:156–164. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gatenby RA and Gillies RJ: Why do cancers
have high aerobic glycolysis? Nat Rev Cancer. 4:891–899. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fang JS, Gillies RD and Gatenby RA:
Adaptation to hypoxia and acidosis in carcinogenesis and tumor
progression. Semin Cancer Biol. 18:330–337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pinheiro C, Longatto-Filho A,
Azevedo-Silva J, Casal M, Schmitt FC and Baltazar F: Role of
monocarboxylate transporters in human cancers: State of the art. J
Bioenerg Biomembr. 44:127–139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Halestrap AP: The monocarboxylate
transporter family-Structure and functional characterization. IUBMB
Life. 64:1–9. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bonen A, Miskovic D, Tonouchi M, Lemieux
K, Wilson MC, Marette A and Halestrap AP: Abundance and subcellular
distribution of MCT1 and MCT4 in heart and fast-twitch skeletal
muscles. Am J Physiol Endocrinol Metab. 278:E1067–E1077. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Philp NJ, Yoon H and Lombardi L: Mouse
MCT3 gene is expressed preferentially in retinal pigment and
choroid plexus epithelia. Am J Physiol Cell Physiol.
280:C1319–C1326. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Doyen J, Trastour C, Ettore F, Peyrottes
I, Toussant N, Gal J, Ilc K, Roux D, Parks SK, Ferrero JM and
Pouysségur J: Expression of the hypoxia-inducible monocarboxylate
transporter MCT4 is increased in triple negative breast cancer and
correlates independently with clinical outcome. Biochem Biophys Res
Commun. 451:54–61. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fisel P, Kruck S, Winter S, Bedke J,
Hennenlotter J, Nies AT, Scharpf M, Fend F, Stenzl A, Schwab M and
Schaeffeler E: DNA methylation of the SLC16A3 promoter regulates
expression of the human lactate transporter MCT4 in renal cancer
with consequences for clinical outcome. Clin Cancer Res.
19:5170–5181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fisel P, Schaeffeler E and Schwab M:
Clinical and functional relevance of the monocarboxylate
transporter family in disease pathophysiology and drug therapy.
Clin Transl Sci. 11:352–364. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gotanda Y, Akagi Y, Kawahara A, Kinugasa
T, Yoshida T, Ryu Y, Shiratsuchi I, Kage M and Shirouzu K:
Expression of monocarboxylate transporter (MCT)-4 in colorectal
cancer and its role: MCT4 contributes to the growth of colorectal
cancer with vascular endothelial growth factor. Anticancer Res.
33:2941–2947. 2013.PubMed/NCBI
|
|
13
|
Hao J, Chen H, Madigan MC, Cozzi PJ,
Beretov J, Xiao W, Delprado WJ, Russell PJ and Li Y: Co-expression
of CD147 (EMMPRIN), CD44v3-10, MDR1 and monocarboxylate
transporters is associated with prostate cancer drug resistance and
progression. Br J Cancer. 103:1008–1018. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hemdan T, Malmstrom PU, Jahnson S and
Segersten U: Emmprin expression predicts response and survival
following cisplatin containing chemotherapy for bladder cancer: A
validation study. J Urol. 194:1575–1581. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim Y, Choi JW, Lee JH and Kim YS:
Expression of lactate/H+ symporters MCT1 and MCT4 and
their chaperone CD147 predicts tumor progression in clear cell
renal cell carcinoma: Immunohistochemical and The Cancer Genome
Atlas data analyses. Hum Pathol. 46:104–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pinheiro C, Sousa B, Albergaria A, Paredes
J, Dufloth R, Vieira D, Schmitt F and Baltazar F: GLUT1 and CAIX
expression profiles in breast cancer correlate with adverse
prognostic factors and MCT1 overexpression. Histol Histopathol.
26:1279–1286. 2011.PubMed/NCBI
|
|
17
|
Szubert S, Szpurek D, Moszynski R, Nowicki
M, Frankowski A, Sajdak S and Michalak S: Extracellular matrix
metalloproteinase inducer (EMMPRIN) expression correlates
positively with active angiogenesis and negatively with basic
fibroblast growth factor expression in epithelial ovarian cancer. J
Cancer Res Clin Oncol. 140:361–369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng D, Zhu X, Ding X, Zhu X, Yin Y and
Li G: Sensitive detection of CD147/EMMPRIN and its expression on
cancer cells with electrochemical technique. Talanta. 105:187–191.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu S, Chu D, Zhang Y, Wang X, Gong L, Han
X, Yao L, Lan M, Li Y and Zhang W: EMMPRIN/CD147 expression is
associated with disease-free survival of patients with colorectal
cancer. Med Oncol. 30:3692013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McClelland GB and Brooks GA: Changes in
MCT 1, MCT 4, and LDH expression are tissue specific in rats after
long-term hypobaric hypoxia. J Appl Physiol (1985). 92:1573–1584.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lokuhetty D, White VA, Watanabe R, Cree
IA, et al: WHO Classification of Tumours, 5th Edition, Volume 2:
Breast Tumours. (Lyon, France). IARC Press. 2019.PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maria RM, Altei WF, Selistre-de-Araujo HS
and Colnago LA: Impact of chemotherapy on metabolic reprogramming:
Characterization of the metabolic profile of breast cancer
MDA-MB-231 cells using 1H HR-MAS NMR spectroscopy. J
Pharm Biomed Anal. 146:324–328. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Long Y, Gao Z, Hu X, Xiang F, Wu Z, Zhang
J, Han X, Yin L, Qin J, Lan L, et al: Downregulation of MCT4 for
lactate exchange promotes the cytotoxicity of NK cells in breast
carcinoma. Cancer Med. 7:4690–4700. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Perou CM, Sorlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kreike B, van Kouwenhove M, Horlings H,
Weigelt B, Peterse H, Bartelink H and van de Vijver MJ: Gene
expression profiling and histopathological characterization of
triple-negative/basal-like breast carcinomas. Breast Cancer Res.
9:R652007. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bleach R and McIlroy M: The divergent
function of androgen receptor in breast cancer; analysis of steroid
mediators and tumor intracrinology. Front Endocrinol (Lausanne).
9:5942018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ruan Y, Zeng F, Cheng Z, Zhao X, Fu P and
Chen H: High expression of monocarboxylate transporter 4 predicts
poor prognosis in patients with lung adenocarcinoma. Oncol Lett.
14:5727–5734. 2017.PubMed/NCBI
|
|
29
|
Mathupala SP, Parajuli P and Sloan AE:
Silencing of monocarboxylate transporters via small interfering
ribonucleic acid inhibits glycolysis and induces cell death in
malignant glioma: An in vitro study. Neurosurgery. 55:1410–1419.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luo F, Zou Z, Liu X, Ling M, Wang Q, Wang
Q, Lu L, Shi L, Liu Y, Liu Q and Zhang A: Enhanced glycolysis,
regulated by HIF-1α via MCT-4, promotes inflammation in
arsenite-induced carcinogenesis. Carcinogenesis. 38:615–626. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Curry J, Tassone P, Gill K, Tuluc M, BarAd
V, Mollaee M, Whitaker-Menezes D, Rodeck U, Luginbuhl A, Cognetti
D, et al: Tumor metabolism in the microenvironment of nodal
metastasis in oral squamous cell carcinoma. Otolaryngol Head Neck
Surg. 157:798–807. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yan P, Li YH, Tang ZJ, Shu X and Liu X:
High monocarboxylate transporter 4 protein expression in stromal
cells predicts adverse survival in gastric cancer. Asian Pac J
Cancer Prev. 15:8923–8929. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sanita P, Capulli M, Teti A, Galatioto GP,
Vicentini C, Chiarugi P, Bologna M and Angelucci A: Tumor-stroma
metabolic relationship based on lactate shuttle can sustain
prostate cancer progression. BMC cancer. 14:1542014. View Article : Google Scholar : PubMed/NCBI
|