Introduction
Globally, gastric cancer (GC) ranks as the fifth
most diagnosed cancer the third leading cause of mortality
associated with cancer (1). In 2018,
456,124 new cases of GC and 390,182 deaths were recorded in China,
which accounted for >50% of global GC deaths (2). Unfortunately, GC frequently remain
undetected until advanced stages because initial symptoms are
comparable to those of other diseases, such as chronic gastritis
(3). Despite development of novel
combined treatment strategies and increased understanding of the
underlying mechanisms, the mortality rate of patients with GC
remains relatively high (4).
Therefore, there is an urgent need to investigate novel molecular
pathways associated with the pathophysiology of GC so that
innovative therapeutic intervention strategies for GC can be
developed.
Lauren's classification, which divides GC into
‘intestinal’ and ‘diffuse’ types according to the morphological
characteristics of the tumor, is frequently applied worldwide
(5). Although reductions in the
incidence of gastric intestinal-type adenocarcinoma (GITA) has been
observed, it remains to be the most frequent type of GC found in
high-incidence populations (6). The
occurrence of GITA is a multi-step process that involves the
transformation of the normal gastric mucosa to non-atrophic
gastritis, multifocal atrophic gastritis, intestinal metaplasia,
low-grade intraepithelial neoplasia, high-grade intraepithelial
neoplasia and finally into carcinoma (7). Surmounting evidence has indicated that
tumor cells reactivate underlying developmental processes to
effectively perform this aforementioned multi-step process of
tumorigenesis (8). However, the
molecular mechanisms that regulate carcinogenesis and promote GITA
tumorigenesis remain to be elucidated. A number of studies have
previously indicated that transcription factors that are associated
with embryonic development may serve vital roles in this
pathological process (9–11).
Limb-bud and heart (LBH) is an important
transcription cofactor involved in embryonic development and
encodes a highly conserved nuclear protein that mediates
transcriptional activation in mouse embryo tissue culture cells
(12). Rieger et al (13) previously reported that LBH is a
direct target of the Wnt signaling pathway during epithelial
development, which was aberrantly overexpressed in highly invasive
ER-negative, basal subtype human breast cancer types. In another
study, Liu et al (14)
demonstrated that LBH overexpression induced nasopharyngeal
carcinoma cell cycle arrest during the G1/S transition and
inhibited the growth of transplanted nasopharyngeal carcinoma
tumors in vivo, by downregulating latent membrane
protein-1-mediated NF-κB transcriptional activity. Recently, Deng
et al (15) reported that LBH
expression was significantly downregulated in lung cancer tissue
samples, where it associated with the prognosis and clinical
characteristics of patients with lung cancer. Furthermore, knocking
out the LBH gene has been found to promote the proliferation,
migration and invasion of lung adenocarcinoma cells (15), where further bioinformatics analysis
revealed that LBH was significantly associated with signaling
pathways regulating cell adhesion (15). However, the role of LBH in GITA
remain poorly understood.
Therefore, in the present study, The Cancer Genome
Atlas (TCGA) and the Oncomine databases were used to compare the
expression levels of LBH mRNA in GITA and healthy gastric tissues.
In addition, the relationship between LBH gene expression and
survival in patients with GITA was analyzed. Functional enrichment
analysis was subsequently performed to examine the biological
pathways associated with the LBH regulatory network in GITA.
Materials and methods
RNA sequencing of patient data and
bioinformatics analysis
The Oncomine 4.5 database (https://www. oncomine.org/resource/login.html) is
a publicly accessible online cancer microarray database and
web-based data-mining platform containing 715 datasets and 86,733
samples. In the database, the gene was set as ‘LBH’, analysis type
as ‘cancer vs. normal analysis’, cancer type as ‘GITA’ and the data
type as ‘mRNA’. Under this search conditions, three datasets
including Chen Gastric, DErrico Gastric and Cho Gastric were used
to predict the expression levels of LBH mRNA in GITA and healthy
gastric tissues. Boxplots were then produced to compare the
differences in the expression of LBH between GITA and healthy
gastric mucosal tissues.
Expression profiles of GC samples and corresponding
clinical information were downloaded from the official GDC portal
of the TCGA database(https://portal.gdc.cancer.gov/, Data Release 16.0).
Search keywords were as follows: Primary Site: stomach, Project:
TCGA-STAD; Disease Type: adenocarcinomas, Data Category:
transcriptome profiling, Experimental Strategy: RNA-Seq, Workflow
Type: HTSeq-Counts. A total of 191 cases containing LBH gene
expression information were downloaded (Tables SI and SII). Run ‘R’ software to normalize the
data. At the same time, the clinical and pathological information
including survival time was screened from the downloaded data, and
diffuse gastric cancer cases were excluded. Combining data
containing LBH gene expression information and clinicopathological
characteristics data, a total of 163 GITA cases were obtained for
further analysis. Boxplots were used to visualize expression
differences of LBH according to discrete variables, including
pathological stage T, lymph node metastasis, distant metastasis,
histological grade (16) and tumor
stage (17).
Statistical analysis
All statistical analyses and figures were performed
using R software (https://www.r-project.org/, version 3.5.0). The
association between the baseline tumor characteristics of the
patients in each group were assessed using Pearson's χ2
test. The association between pathological stage, lymph node
metastasis, distant metastasis, histological grade and tumor stage
in patients with GITA and LBH expression was analyzed using the
Wilcoxon rank-sum test. Univariate survival analysis of patients
with TCGA in relation to LBH expression were tested using the
log-rank test. Kaplan-Meier survival analysis with log-rank test
was used to compare the overall survival of the two groups. The
Kaplan Meier-plotter database (18)
was used to verify differences in the survival rates further. Cox
regression was used for the multivariate analysis on the effects of
LBH expression and clinical characteristics, including sex, age,
histological grade and staging, on patient survival. P<0.05 was
considered to indicate a statistically significant difference.
Function enrichment analysis
Pearson's χ2 test was used to determine
LBH co-expressed genes. The co-expressed gene of LBH was defined as
the correlation coefficient r>0.6 and P<0.05. Subsequently,
in the R program, the ‘cluster profiler’ (18,19)
package was used to analyze and visualize gene ontology (GO) in the
cellular component (CC), molecular function (MF) and biological
process (BP) categories. Furthermore, Kyoto Encyclopedia of Genes
and Genomes (KEGG, Release 90.0) pathway enrichment analysis was
performed, where adjusted-P<0.05 was considered to indicate a
statistically significant difference (17).
Results
LBH mRNA overexpression in GITA
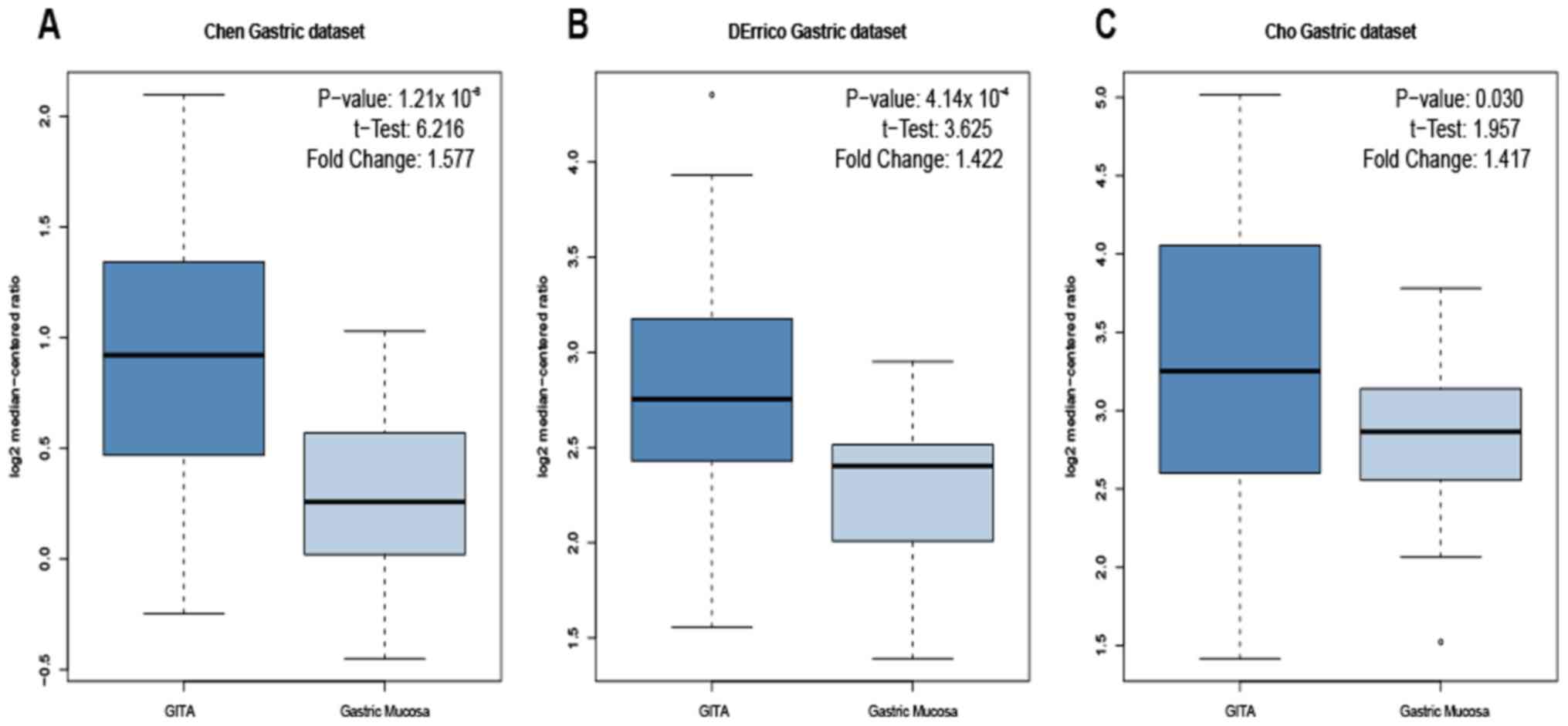
A total of 3 gastric datasets [Chen Gastric
(20), DErrico Gastric (21) and Cho Gastric (22)] in the Oncomine database were used to
examine the expression levels of LBH mRNA in GITA and healthy
gastric mucosa tissues. In the datasets, the P-values and t-test
results for the comparison of LBH mRNA levels in GITA and healthy
gastric mucosa were as follows: P=1.21×10−8,
t-test=6.216 (Chen Gastric dataset; Fig.
1A); P=4.14×10−4, t-test=3.625 (DErrico Gastric
dataset; Fig. 1B); and P=0.030,
t-test=1.957 (Cho Gastric dataset; Fig.
1C). The expression level of LBH mRNA in GITA tissue was
significantly higher compared with that in healthy gastric tissue
for all three datasets.
Association between LBH and
clinicopathological parameters in patients with GITA
Among the 163 patients, 57 were male and 106 female.
The age range was 30–90 years, in which 72 patients were aged
<67 years and 88 patients were aged >67 years with three
patients had unknown data for age. A total of 44 patients were in
pathological stages T1-2 according to the American Joint Committee
on Cancer Tumor-Node-Metastasis staging system (17), 117 in stages T3-4 and two exhibited
unknown stages. Furthermore, 117 patients exhibited lymph node
metastasis, 44 patients did not and 2 patients had unknown data for
lymph node metastasis. A total of 14 patients exhibited distant
metastasis, 147 patients did not and the data was unknown for 2
patients. Additionally, 90 patients were in histological grades 1–2
(17), 69 patients were in grade 3
and four patients had unknown grades. A total of 23 patients
exhibited tumor stages I–II (17),
137 patients in stages III–IV whereas stages were unknown for 3
patients (Table I). Median overall
survival (OS) was 17.18 months and median disease-free survival
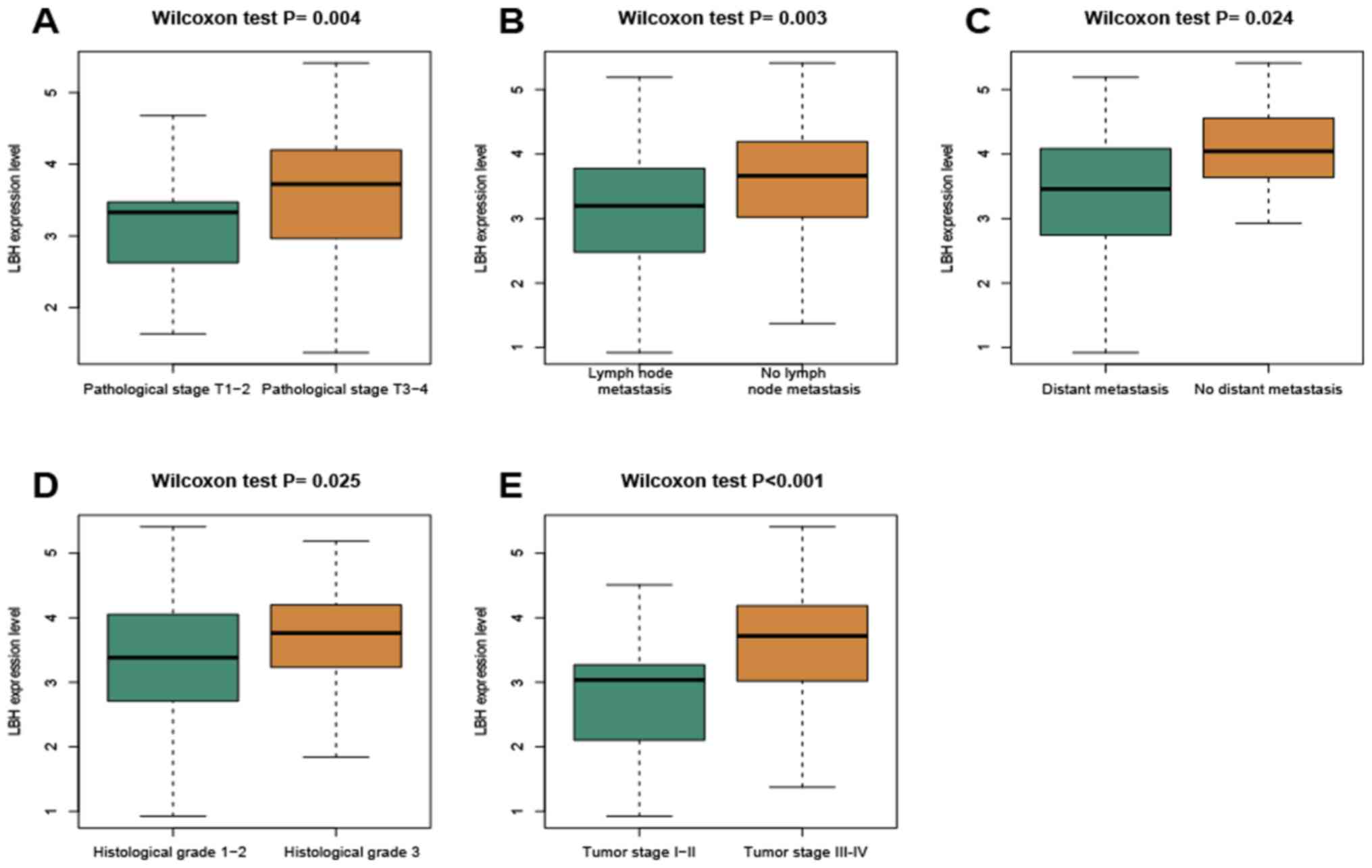
(DFS) was 8.71 months. Wilcoxon rank-sum test was used to
distinguish the LBH expression in patients stratified into the
different groups according to the clinicopathological parameters
(Fig. 2). The expression of LBH was
found to be increased in patients in pathological stage T3-4 (T3-4
vs. T1-2; P=0.004), no lymph node metastasis (no vs. yes; P=0.003),
no distant metastasis (no vs. yes; P=0.024), histological grade 3
(grade 3 vs. grades 1–2; P=0.025) and tumor stage III–IV (III–IV
vs. I–II; P<0.001). These results suggest that the elevated
expression of LBH is associated with advanced tumor staging and
poor differentiation.
 | Table I.Baseline patient and tumor
characteristics in each group. |
Table I.
Baseline patient and tumor
characteristics in each group.
|
|
| LBH expression, n
(%) |
|
|---|
|
|
|
|
|
|---|
| Characteristic | No. of patients, n
(%) | High | Low | P-value |
|---|
| Age, years |
|
<67 | 72 (44.2) | 37 (51.4) | 35 (48.6) | 0.975 |
|
>67 | 88 (54.0) | 45 (51.1) | 43 (48.9) |
|
| Sex |
|
Male | 57 (35.0) | 33 (58.0) | 24 (42.0) | 0.192 |
|
Female | 106 (65.0) | 50 (47.2) | 56 (52.8) |
|
| Histological
grade |
| Grade
1–2 | 90 (55.2) | 43 (47.8) | 47 (52.2) | 0.465 |
| Grade
3 | 69 (42.3) | 37 (53.6) | 32 (46.4) |
|
| Tumor stage |
| Stage
I–II | 23 (14.1) | 11 (47.8) | 12 (52.2) | 0.772 |
| Stage
III–IV | 137 (84.0) | 70 (51.1) | 67 (48.9) |
|
| Pathological stage
T |
|
T1-2 | 44 (27.0) | 20 (45.5) | 24 (54.5) | 0.394 |
|
T3-4 | 117 (71.8) | 62 (53.0) | 55 (47.0) |
|
| Lymph node
metastasis |
|
Yes | 117 (71.8) | 61 (52.1) | 56 (47.9) | 0.809 |
| No | 44 (27.0) | 22 (50.0) | 22 (50.0) |
|
| Distant
metastasis |
|
Yes | 14 (8.6) | 7 (50.0) | 7 (50.0) | 0.981 |
| No | 147 (90.2) | 74 (50.3) | 73 (49.7) |
|
Increased LBH expression is associated
with poor survival in GITA
The prognostic significance of LBH expression levels
in patients with GITA was next investigated. TCGA data of 163
patients was divided into high-expression (n=83) and the
low-expression (n=80) groups using the median expression of LBH
value as the cut-off value.
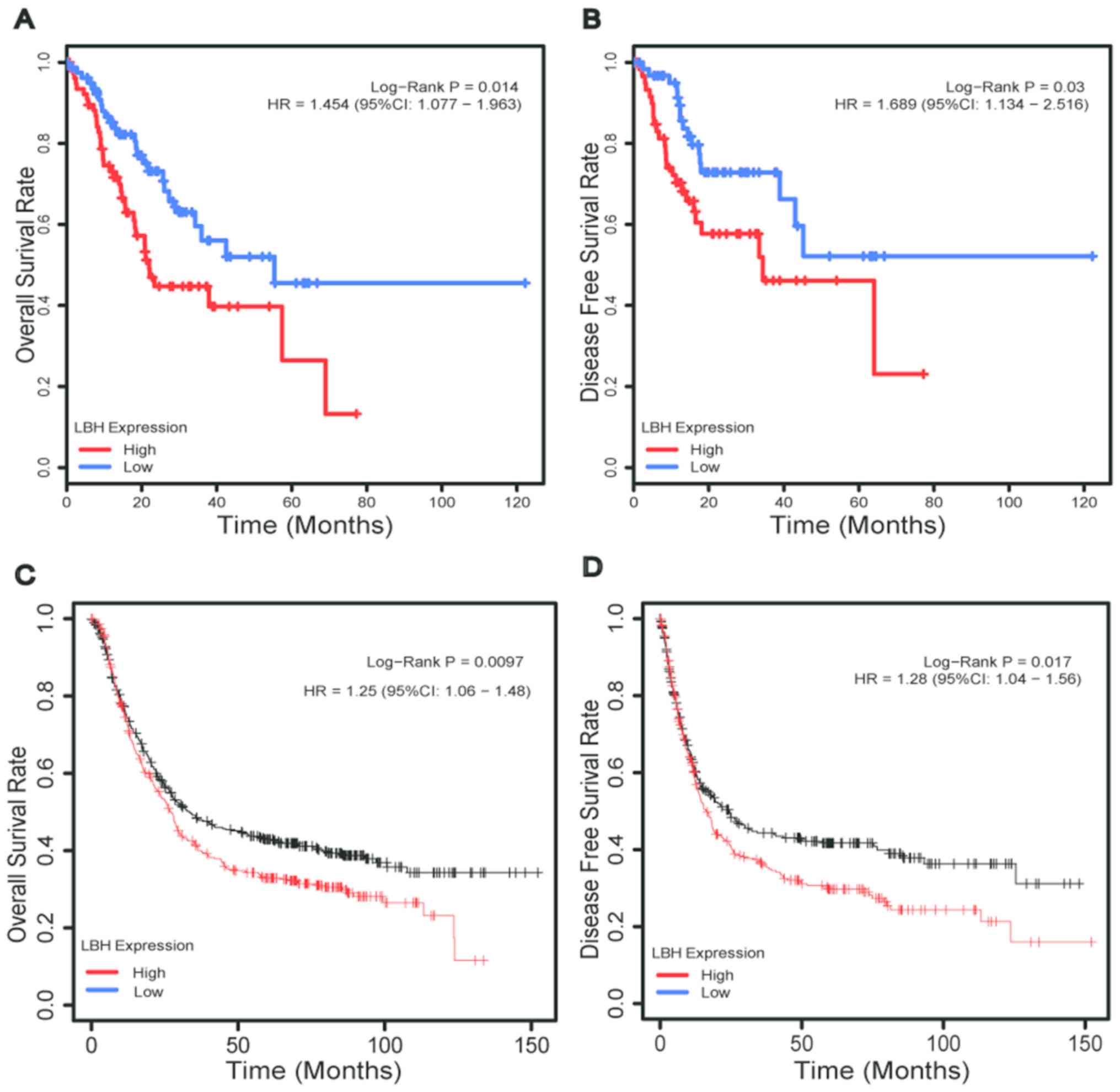
Kaplan-Meier survival analysis with log-rank test
was used to compare the overall survival of the two groups. The
hazard ratios (HRs) of the two groups were calculated using
univariate Cox regression analysis. The results demonstrated that
patients in the low expression group had significantly prolonged OS
and DFS compared with those in the high expression group (both
P<0.05; Fig. 3A and B). The HR in
the high and low LBH expression groups were 1.454 [95% confidence
interval (CI)=1.077–1.963] and 1.689 (95% CI=1.134–2.516),
respectively, as per the univariate Cox regression analysis.
Additionally, using the cut-off value established as
aforementioned, further verification in the Kaplan Meier-plotter
database demonstrated that patients in the low expression group
exhibited prolonged OS and DFS compared with those in the high
expression group (both, P<0.05; Fig.
3C and D). The HR in the high and low-expression groups were
1.25 (95% CI=1.06–1.48) and 1.28 (95% CI=1.04–1.56), respectively,
according to the univariate Cox regression analysis.
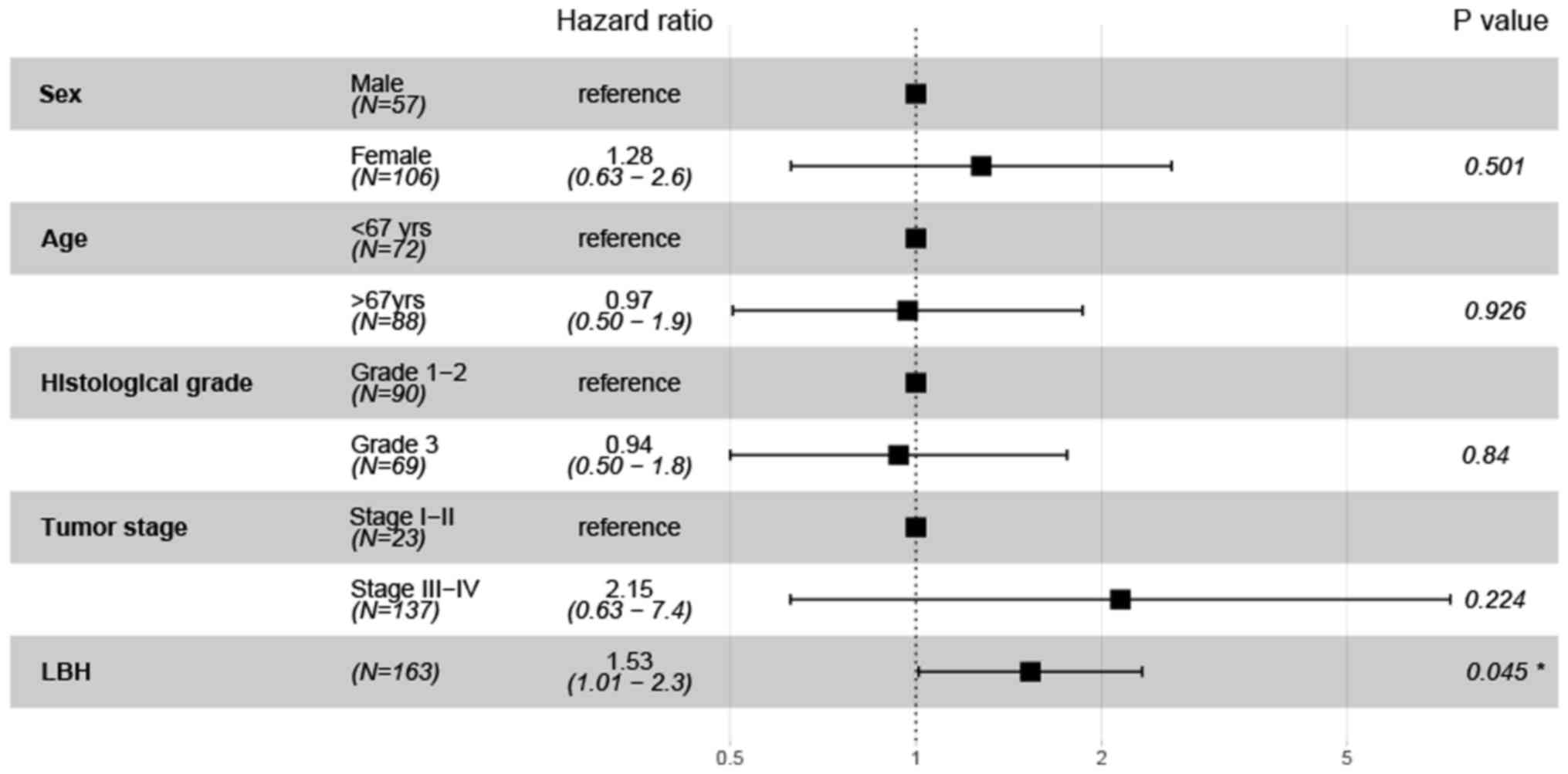
To evaluate the independent prognostic value of LBH
expression, multivariate Cox regression analysis was performed. The
results indicated that LBH expression was independently associated
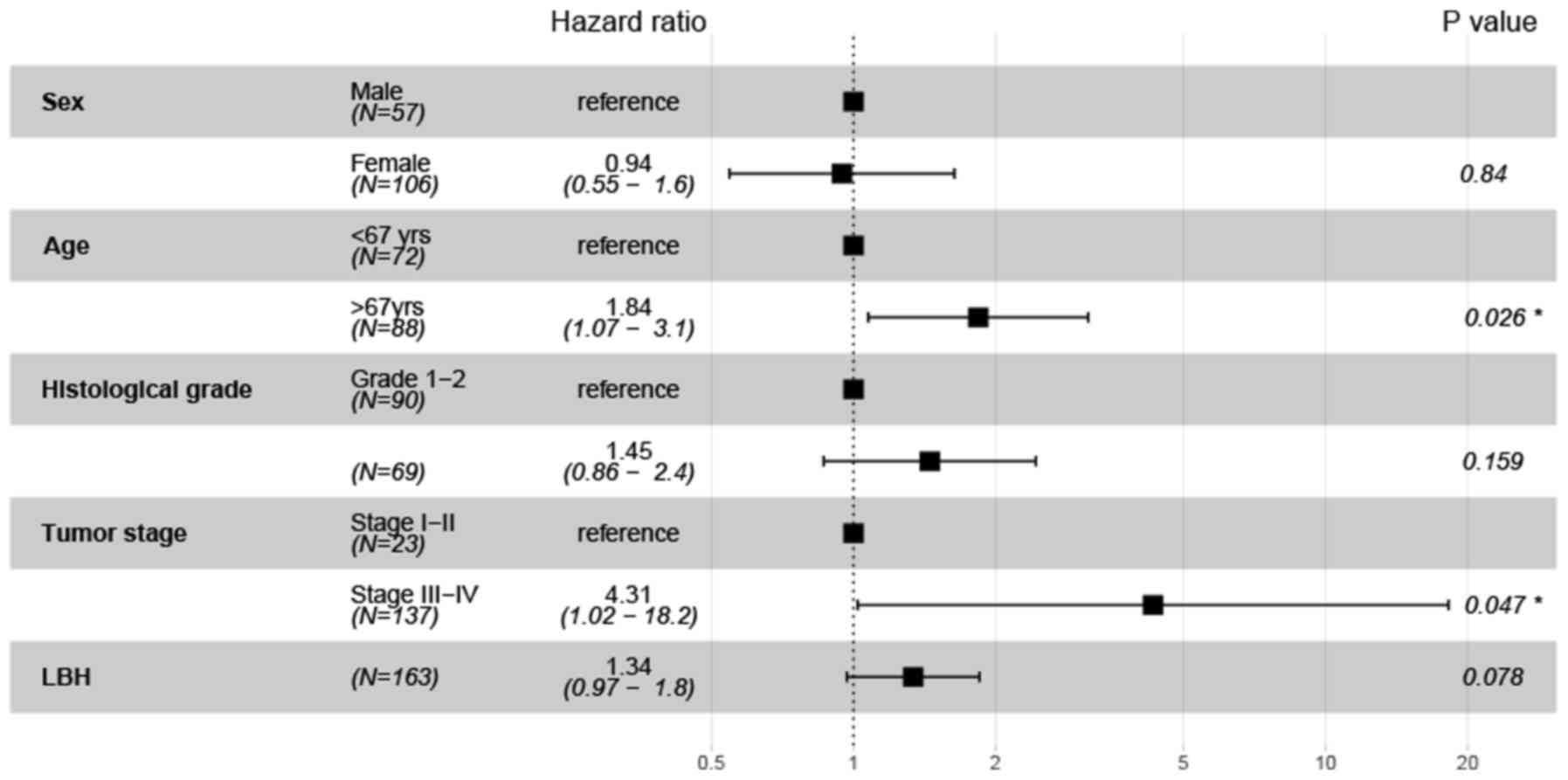
with DFS (P=0.045; HR=1.53; 95% CI=1.01–2.3; Fig. 4). However, LBH was not found to be
independently associated with OS (P=0.078; HR=1.34; 95%
CI=0.97–1.8; Fig. 5), but tumor
stage (P=0.047; HR=4.31; 95% CI=1.02–18.2) and age (P=0.026;
HR=1.84; 95% CI=1.07–3.1) were independent prognostic indicators of
OS (Fig. 5).
Functional enrichment analysis of the
LBH co-expression network
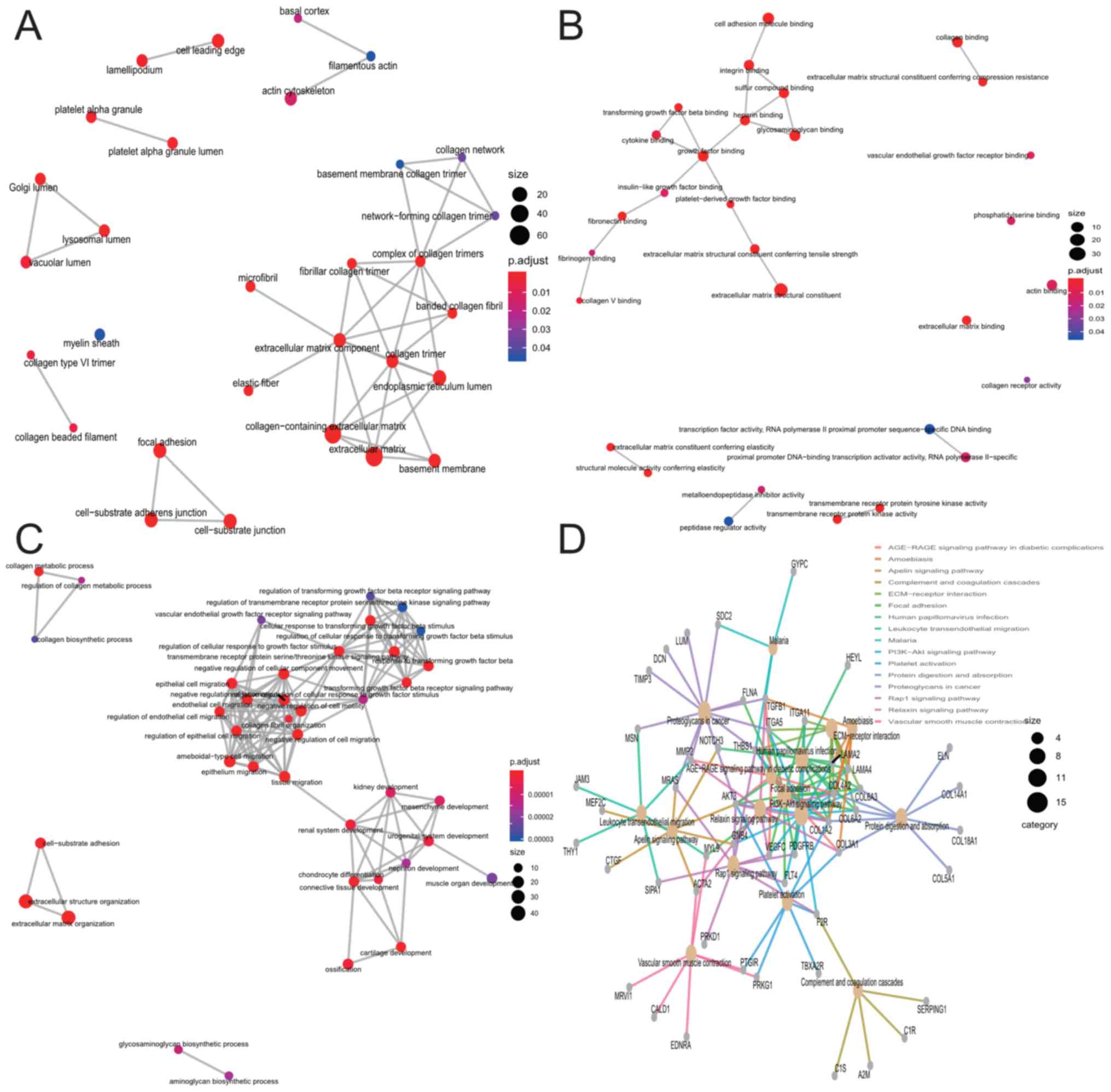
A total of 258 genes, which differentially expressed
as a result of CENPK alteration, were screened using the threshold
of absolute Pearson's r>0.6 (Table
SIII). The GO enrichment analysis of the co-expressed mRNA
indicated that ‘collagen-containing extracellular matrix (ECM)’,
‘ECM structural constituent’ and ‘ECM organization’ were the most
significant categories of enriched CC, MF and BP (Fig. 6A-C; Table
SIII). The KEGG pathway enrichment analysis indicated that
‘focal adhesion’ and ‘ECM-receptor interaction’ were the most
significant enrichment pathways (Fig.
6D; Table SIV). Additionally,
LBH is involved in a series of cancer-related biological processes
or signaling pathways, including proteoglycans in cancer and
phosphatidylinositol 3-kinase/protein kinase B (PI3K-AKT) signaling
pathways in tumors.
Discussion
LBH is a highly conserved and tissue-specific
transcription regulator that serves an essential role in the
embryonic development of vertebrates (12). Embryonic development and
tumorigenesis have been reported to exhibit similar molecular
mechanisms (23). LBH is a direct
target of the Wnt/β-catenin signaling pathway (13), which is fundamental to the genetic
network for stem cell control and oncogenesis in various epithelial
tissues, such as colorectal and breast tissue (24). Ashad-Bishop et al (25) previously revealed that LBH is
required for Wnt-induced mammary hyperplasia and tumor formation.
Reduced mammary hyperplasia in LBH-deficient mouse mammary tumor
virus-Wnt1 mice at pre-neoplastic stages was associated with
reduced cell proliferation and increased cell death, suggesting
that LBH promoted mammary epithelial cell hyperproliferation.
Lindley et al (26)
demonstrated that LBH is an essential regulatory factor for the
expansion and maintenance of basal multifunctional breast stem
cells (MaSC), acting upstream of the ΔNp63 oncogene to promote a
multipotent basal MaSC state and repress luminal differentiation.
In another previous study, Chen et al (27) demonstrated that high levels of LBH
expression could be detected in 20/226 (8.8%) of hepatocellular
carcinoma (HCC) samples. The mean survival time was prolonged in
patients with HCC who exhibited low LBH expression compared with
those with high expression. Therefore, LBH overexpression may
contribute to the development and progression of HCC.
These previous studies aforementioned have implied
that LBH may function as an oncogene, which appeared to be
consistent with the findings of the present study. Elevated
expression of LBH in GITA tissues compared with that in normal
gastric mucosa was found using Oncomine and TCGA public databases.
The differential expression levels of LBH were found in patients
with GITA who were stratified according to the clinicopathological
parameters, including pathological stage T, lymph node metastasis,
distant metastasis, histological grade and tumor stage.
Subsequently, the association between LBH expression and prognosis
in patients with GITA was assessed. Data of patients with GITA
obtained from the TCGA database were first divided into the low-
and high-expression groups. Kaplan-Meier survival analyses reported
that patients in the high expression group exhibited significantly
shorter OS and DFS compared with those in the low expression group.
This result was consistent with data obtained using the
Kaplan-Meier plotter database. Multivariate Cox analysis indicated
that LBH was an independent prognostic factor of DFS, but not of
OS. Age (>67 years) and stages T3-4 were found to be independent
predictors of unfavorable prognosis in patients with GITA.
The results also revealed that the genes that were
co-expressed with LBH in GITA were particularly enriched in the
‘collagen-containing ECM’, ‘ECM structural constituent’, ‘ECM
organization’ and ‘ECM-receptor interaction and focal adhesion’.
ECM organization has been demonstrated to be associated with cell
migration-related GO processes, which are linked with tumor
metastasis (28), whilst
ECM-receptor interaction and focal adhesion are pathways associated
with metastasis (28). Additionally,
LHB was reported to be involved in the PI3K-AKT signaling pathway,
which has been demonstrated to be connected with the cell
proliferation (29). The PI3K-AKT
pathway is the primary signaling pathway downstream of multiple
growth factor receptors and is one of the most active signaling
pathways in human tumors. Through the phosphorylation of the PI3K
and AKT proteins, tumor cell proliferation and malignant
transformation are promoted whereas tumor cell apoptosis is
inhibited (30,31). Consequently, PI3K-AKT inhibitors are
widely used in cancer treatment (32). Several previous studies have reported
the role of the PI3K-AKT pathway in promoting GC proliferation and
invasion (32,33). Therefore, the present study
hypothesized that LBH may promote the malignant proliferation of
GITA through the PI3K-AKT pathway. The results suggest that LBH
serves an oncogenic function in GITA and cn be applied as a
potential biomarker of disease prognosis.
However, the present study is not consistent with
previous studies conducted in human nasopharyngeal cancer models
and the prostate cancer cell line PC3M, which indicated that LBH is
a tumor suppressor (14,33). LBH expression was found to be
downregulated in prostate cancer tissues and cell lines compared
with that in healthy prostate epithelial cells (33). In addition, LBH overexpression was
revealed to inhibit PC3M cell proliferation and tumor growth by
inducing cell cycle arrest via downregulation of cyclin D1 and
cyclin E2 gene expression (33).
Therefore, it should be noted that LBH can function both as an
oncogene and a tumor suppressor gene.
However, the current study has limitations. Firstly,
the sample size analyzed was small. Secondly, the results of the
enrichment analysis require further research to determine the
potential molecular mechanisms by which LBH regulate GITA.
In summary, the present study demonstrated that LBH
is highly expressed in GITA, where LBH overexpression predicted
worse prognosis. LBH was an independent DFS predictor in GITA.
Furthermore, the co-expressed genes enriched by LBH are associated
with the migration, proliferation and metastasis of tumors.
Therefore, LBH may be a potential prognostic biomarker and a
therapeutic target for GITA.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the Oncomine (oncomine.org) and TCGA
(portal.gdc.cancer.gov).
Authors' contributions
SW and YH conceived the study and performed
bioinformatic analysis. JC collected the data and drafted the
manuscript or revising it critically for important intellectual
content. YY performed the experiments and analyzed data. HL and WC
prepared the figures and/or tables and interpreted the data. All
authors read and approved the final manuscript and agree to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
LBH
|
limb-bud and heart
|
|
GITA
|
gastric intestinal-type
adenocarcinoma
|
|
TCGA
|
The Cancer Genome Atlas
|
|
GO
|
Gene Ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genome
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2020. CA Cancer J Clin. 0:1–24. 2020.
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wadhwa R, Song S, Lee JS, Yao Y, Wei Q and
Ajani JA: Gastric cancer-molecular and clinical dimensions. Nat Rev
Clin Oncol. 10:643–655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ang TL and Fock KM: Clinical epidemiology
of gastric cancer. Singapore Med J. 55:621–628. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lauren P: The two histological main types
of gastric carcinoma: Diffuse and so called intestinal type
carcinoma. An attempt at a histo clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Figueiredo C, Costa S, Karameris A and
Machado JC: Pathogenesis of Gastric Cancer. Helicobacter. 20 (Suppl
1):30–35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park YH and Kim N: Review of atrophic
gastritis and intestinal metaplasia as a premalignant lesion of
gastric cancer. J Cancer Prev. 20:25–40. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Briegel KJ: Embryonic transcription
factors in human breast cancer. IUBMB Life. 58:123–132. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ben-Porath I, Thomson MW, Carey VJ, Ge R,
Bell GW, Regev A and Weinberg RA: An embryonic stem cell-like gene
expression signature in poorly differentiated aggressive human
tumors. Nat Genet. 40:499–507. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Francis R, Guo H, Streutker C, Ahmed M,
Yung T, Dirks PB, He HH and Kim TH: Gastrointestinal transcription
factors drive lineage specific developmental programs in organ
specification and cancer. Sci Adv. 5:eaax88982019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raghoebir L, Bakker ER, Mills JC,
Swagemakers S, Kempen MB, Munck AB, Driegen S, Meijer D, Grosveld
F, Tibboel D, et al: SOX2 redirects the developmental fate of the
intestinal epithelium toward a premature gastric phenotype. J Mol
Cell Biol. 4:377–385. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Briegel KJ and Joyner AL: Identification
and characterization of Lbh, a novel conserved nuclear protein
expressed during early limb and heart development. Dev Biol.
233:291–304. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rieger ME, Sims AH, Coats ER, Clarke RB
and Briegel KJ: The embryonic transcription cofactor LBH is a
direct target of the Wnt signaling pathway in epithelial
development and in aggressive basal subtype breast cancers. Mol
Cell Biol. 30:4267–4279. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Q, Guan X, Lv J, Li X, Wang Y and Li
L: Limb-bud and Heart (LBH) functions as a tumor suppressor of
nasopharyngeal carcinoma by inducing G1/S cell cycle arrest. Sci
Rep. 5:76262015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deng M, Yu R, Wang S, Zhang Y, Li Z, Song
H, Liu B, Xu L, Wang X, Zhang Z, et al: Limb-Bud and heart
attenuates growth and invasion of human lung adenocarcinoma cells
and predicts survival outcome. Cell Physiol Biochem. 47:223–234.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kruppa J and Jung K: Automated multigroup
outlier identification in molecular high-throughput data using
bagplots and gemplots. BMC Bioinformatics. 18:2322017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen J, Hu B, Wang W, Qian XJ, Shan BJ and
He YF: A six-microRNA signature to predict outcomes of patients
with gastric cancer. FEBS Open Bio. 9:538–547. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nagy Á, Lánczky A, Menyhárt O and Győrffy
B: Validation of miRNA prognostic power in hepatocellular carcinoma
using expression data of independent datasets. Sci Rep. 8:92272018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jing JJ, Wang ZY, Li H, Sun LP and Yuan Y:
Key elements involved in Epstein-Barr virus-associated gastric
cancer and their network regulation. Cancer Cell Int. 18:1462018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen X, Leung SY, Yuen ST, Chu KM, Ji J,
Li R, Chan AS, Law S, Troyanskaya OG, Wong J, et al: Variation in
gene expression patterns in human gastric cancers. Mol Biol Cell.
14:3208–3215. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
D'Errico M, de Rinaldis E, Blasi MF, Viti
V, Falchetti M, Calcagnile A, Sera F, Saieva C, Ottini L, Palli D,
et al: Genome-wide expression profile of sporadic gastric cancers
with microsatellite instability. Eur J Cancer. 45:461–469. 2009.
View Article : Google Scholar
|
|
22
|
Cho JY, Lim JY, Cheong JH, Park YY, Yoon
SL, Kim SM, Kim SB, Kim H, Hong SW, Park YN, et al: Gene expression
signature-based prognostic risk score in gastric cancer. Clin
Cancer Res. 17:1850–1857. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kahn M: Can we safely target the WNT
pathway? Nat Rev Drug Discov. 13:513–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ashad-Bishop K, Garikapati K, Lindley LE,
Jorda M and Briegel KJ: Loss of Limb-Bud-and-Heart (LBH) attenuates
mammary hyperplasia and tumor development in MMTV-Wnt1 transgenic
mice. Biochem Biophys Res Commun. 508:536–542. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lindley LE, Curtis KM, Sanchez-Mejias A,
Rieger ME, Robbins DJ and Briegel KJ: The WNT-controlled
transcriptional regulator LBH is required for mammary stem cell
expansion and maintenance of the basal lineage. Development.
142:893–904. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen J, Huang C, Chen K, Li S, Zhang X,
Cheng J, Cai M and Xiao Y: Overexpression of LBH is associated with
poor prognosis in human hepatocellular carcinoma. OncoTargets Ther.
11:441–448. 2018. View Article : Google Scholar
|
|
28
|
Liu H, Wu N, Zhang Z, Zhong X, Zhang H,
Guo H, Nie Y and Liu Y: Long Non-coding RNA LINC00941 as a
potential biomarker promotes the proliferation and metastasis of
gastric cancer. Front Genet. 10:52019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu Y, Li L, Wu G, Zhuo H, Liu G and Cai J:
Effect of PI3K/Akt signaling pathway on PRAS40Thr246
phosphorylation in gastric cancer cells. Iran J Public Health.
48:2196–2204. 2019.PubMed/NCBI
|
|
30
|
Hu M, Zhu S, Xiong S, Xue X and Zhou X:
MicroRNAs and the PTEN/PI3K/Akt pathway in gastric cancer (Review).
Oncol Rep. 41:1439–1454. 2019.PubMed/NCBI
|
|
31
|
Jia L, Zhu Z, Li H and Li Y: Shikonin
inhibits proliferation, migration, invasion and promotes apoptosis
in NCI-N87 cells via inhibition of PI3K/AKT signal pathway. Artif
Cells Nanomed Biotechnol. 47:2662–2669. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang C, Lin X, Zhao Q, Wang Y, Jiang F,
Ji C, Li Y, Gao J, Li J and Shen L: YARS as an oncogenic protein
that promotes gastric cancer progression through activating
PI3K-Akt signaling. J Cancer Res Clin Oncol. 146:329–342. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Q, Li E, Huang L, Cheng M and Li L:
Limb-bud and heart overexpression inhibits the proliferation and
migration of PC3M Cells. J Cancer. 9:424–432. 2018. View Article : Google Scholar : PubMed/NCBI
|