Introduction
Precancerous cervical lesions are defined as
localized, identifiable cervical lesions that carry an increased
risk of developing into cancer, that are treatable and that can be
eradicated to prevent the occurrence of cervical cancer. Large
population-based screening programs for precursor lesions have been
shown to be highly effective in the prevention of cervical cancer
(1,2).
Over the past 100 years, however, the definition of
‘precancerous cervical lesions’ has been vague and variable, from
carcinoma in situ (CIS) dysplasia to cervical
intraepithelial neoplasia (CIN) (3).
Furthermore, according to its potential to develop into cervical
cancer, CIN can be divided into grades corresponding to mild,
moderate or severe dysplasia and CIS (4,5).
In order to better understand the biology and
epidemiology of cervical cancer and to improve the consistency of
cervical biopsies, a two-tiered system of nomenclature for squamous
intraepithelial lesion (SIL) was developed to replace the
three-grade CIN classification (6–10). In
this system, low-grade SILs (LSILs) equate to CIN1, and high-grade
SILs (HSILs) equate to CIN3 and to most CIN2 lesions.
p16-immunohistochemistry (IHC) was used to further classify CIN2
lesions into LSILs (p16-negative) and HSILs (p16-positive)
(11–13). Since most LSILs are expected to
regress naturally within ~2 years and are conservatively treated by
observation, whereas HSILs usually require excision by
electrosurgery, it is important to distinguish LSILs from HSILs
(6).
Currently the diagnosis of HSILs and LSILs is based
on histopathology. In total, 60% of LSILs will regress naturally
without treatment, and the lesions are characterized by features
that include hyperplasia of squamous epithelial basement and
subbasal cells, mild nuclear disorder and mild atypia (6). LSILs are typically limited to the first
one-third of the subepithelial layer and show an absence of p16
staining or positive scattered dots in the epithelium. HSILs, which
may develop into infiltrating carcinoma, present with nuclear
polarity disorder, an increased proportion of nucleoplasm (higher
nuclear-to-cytoplasmic ratio) and increased cellular mitosis
(6). Atypical cells extend to the
subepithelial two-thirds of the epithelium or even throughout the
whole epithelial layer with continuously positive p16 staining
(6,14). SIL classification requires tissue
biopsy, which is uncomfortable and invasive, and causes patients to
be less compliant. Furthermore, it is difficult to distinguish
human papilloma virus (HPV) infection from unequivocal LSIL, and
HPV infection alone is increasingly being included in the category
of LSIL by cytopathologists (15).
Although HPV testing and the thinprep cytologic test (TCT) has
improved the diagnosis of SIL (16),
the final diagnosis still relies mainly on the histopathology. A
non-invasive and simplified strategy to discriminate between HSILs
and LSILs would greatly facilitate the diagnosis and treatment of
these lesions.
A blood-based method to diagnose cancer by detecting
circulating tumor cells or tumor DNA in peripheral blood has been
proposed as a simpler, non-invasive strategy for cancer detection
(17). This method, however, is not
suitable for detecting precancerous lesions or cancer at early
stages, as tumor-derived molecules released from a tiny cancer
focus would rarely be detectable or would be undetectable. By
contrast, we previously reported a novel ‘liquid biopsy’ (18) for cancer, using peripheral blood
transcriptomic biomarkers in the diagnosis of various
non-hematological disorders. As a blood-mRNA based rather than
tumor dependent diagnostic, this technique is especially useful for
detection in the early and pre-cancerous disease stages (19–22).
The present study uses peripheral blood
transcriptome profiling to identify candidate genes for
distinguishing HSILs from LSILs. The genes identified in this study
may be clinically useful as the basis of a new blood test for the
diagnosis of SILs, and may further promote our understanding of the
pathophysiology of SILs and cervical cancer.
Materials and methods
Ethics
The present study was approved by the Ethics
Committee of the Qingdao Women and Children's Hospital (Qingdao,
China; approval no. QFELL-KY-2019-46). Sample acquisition for HSILs
and LSILs was conducted between July 2019 and October 2019 at the
Qingdao Women and Children's Hospital. All 102 participants,
including 66 patients with HSILs and 36 with LSILs, were enrolled
and provided written informed consent. The inclusion criteria were
as follows: i) Ages from 20–65 years; and ii) HPV infection lasting
longer than 6 months. The exclusion criteria were: i) Having
autoimmune disease or immunodeficiency disease; ii) having cervical
cancer or other malignancy; iii) pregnancy; and iv) having taken
drugs affecting immune function within the previous 6 months. The
age distribution of the patients is listed in Table SI. In order to verify the
effectiveness of this method both for identifying transcriptomic
biomarkers for LSIL and HSIL and for differentiating SIL from
healthy populations, blood samples were also taken from 65 healthy
female volunteers without cervical disease collected between July
2019 and October 2019 at the Qingdao Women and Children's Hospital.
All healthy volunteers were recruited from patients who underwent a
health examination in the hospital and who provided written
informed consent.
Study population
A total of 102 blood samples were collected from the
women before undergoing cervical tissue biopsy and before they had
undergone any form of treatment, including hormone therapy,
radio/chemo-therapy or surgery. SIL was categorized based on
surgical pathological examination. LSIL was characterized as the
proliferation of basal-like cells extending no more than one-third
of the epithelial thickness and with normal mitoses. HSIL was
characterized as the proliferative cell compartment extending into
the middle one-third or the superficial one-third of the epithelium
and with abnormal mitoses. All patients underwent HPV testing and
TCT before cervical tissue biopsy.
Blood collection, RNA isolation and
RNA quality control
Peripheral whole blood (2.5 ml) was collected in
PaxGene Blood RNA tubes (PreAnalytix GmbH) and total RNA was
isolated using an accessory PaxGene Blood RNA kit (PreAnalytix
GmbH) according to the manufacturer's instructions. The isolated
RNA quality was accessed using Agilent 2100 Bioanalyzer RNA 6000
Nano Chips (Agilent Technologies, Inc.). All the samples for
microarray analysis met the following quality criteria: RNA
integrity number ≥7.0 and 28S:18S rRNA ≥1.0. RNA quantity was
determined by a NanoDrop 1000 UV–Vis spectrophotometer (Thermo
Fisher Scientific, Inc.).
HPV test
A single cervical specimen was collected from each
participant using a Rovers Cervex-Brush device (Rovers Medical
Devices B.V.), and cells were suspended into BD SurePath collection
vials containing preservative solution (Becton, Dickinson and
Company), according to the manufacturer's instructions. HPV types,
including 7 high-risk types 16/44/45/52/53/58/61, were detected
with an Aptima HPV assay targeting E6/E7 mRNA (Aptima; Hologic,
Inc.) according to the protocol previously described (23,24). The
Aptima Auto Detect kit (Aptima; Hologic Inc.) was used to test
these specimens with the Panther Fusion system (Hologic, Inc.),
according to the manufacturer's instructions.
TCT test
Exfoliated cervical cells were collected from the
ectocervix and endocervix with Rovers Cervex-Brush device (Rovers
Medical Devices B.V.) and were analyzed using TCT tests according
to the Bethesda classification system 2009 (25).
Microarray hybridization
Whole blood RNA from the 102 samples (66 HSILs and
36 LSILs) was analyzed using Gene Profiling Array cGMP U133 P2
microarray (Affymetrix; Thermo Fisher Scientific, Inc.) following
the manufacturer's instructions. Gene expression signal intensity
was processed using Affymetrix Expression Console software (version
1.4.1; Affymetrix; Thermo Fisher Scientific, Inc.) and normalized
by MAS5 normalization, in which the global signal intensity value
was adjusted to 500 for each microarray to make it possible to
compare profiling variations between microarrays.
Microarray data mining
To identify candidate genes that distinguish HSILs
from LSILs, the entire 54,675 microarray probe sets were treated
according to the following criteria: i) Only the probe sets
reliably detected as ‘present’ in all of the samples were retained;
and ii) the probe sets were included within the MicroArray Quality
Control (MAQC) list from MAQC Consortium (26). The intensity values of gene
expression signals were then log transformed to conform to a
Gaussian distribution.
To select gene expression signatures, the ensemble
learning strategy AdaBoost method was employed (27). This data mining method does not make
restrictive assumptions on the training set, unlike traditional
methods. Instead, AdaBoost creates a series of weak classifiers and
then combines them into a single strong classifier by assigning
each weak classifier its proper weight. In this way, AdaBoost
outperforms existing methods in accuracy and training time
(27). In the present study, the
final 10 genes were selected using the AdaBoost method and then
used to construct the predictive model via a logistic regression
algorithm. To evaluate the performance of the predictive model for
classifying the HSIL and LSIL groups, the model was characterized
by the area under the receiver operating characteristic curve (ROC
AUC), the sensitivity, the specificity and the accuracy.
As the sample size in this study was small, it was
not practical to evaluate the performance of the predictive model
by partitioning the entire group of samples into traditional
training and test sets. Instead, as in our previously reported
study, a 2-fold cross validation process was conducted to avoid
data overfitting (28). Half of the
LSIL and HSIL samples were randomly selected as a training fold to
generate the predictive model; the remainder of the samples were
included into a test fold, for prediction over 1,000
iterations.
An additional problem is that the final 10 genes
identified may, due to clinical bias and limited sample size,
derive from random chance. To avoid this problem, a null set
analysis was performed to verify that the results were not due to
chance and to reduce the bias owing to the limited number of HSIL
and LSIL samples. The cross validation process was tested once
again; the model was used to predict the sample cohorts with
disease status randomly reassigned (null set). The distributions of
diagnostic parameters of sensitivity, specificity, accuracy and ROC
AUC were then compared between the two cross validation
processes.
Bioinformatics analysis
Gene Ontology (GO) annotations of the candidate
biomarkers were queried from the COXPRESdb v7 database (http://coxpresdb.jp) (29). The proteins interacting with the
candidate biomarkers were downloaded from the STRING database
(https://string-db.org/) with total confidence
≥0.7. Reactome (https://reactome.org/) pathway
enrichment analysis using the clusterProfiler R package (version
3.16.0) (30) was performed on
signature genes and their correlative proteins. Reactome pathways
were identified with a strict cut-off of adjusted P<0.05,
corrected with the Benjamini-Hochberg method and with a
false-discovery rate (FDR) of <0.05. The visualization of the
protein-protein interaction network was performed with Cytoscape
software (https://cytoscape.org/; version
3.8.0).
Results
Basic and clinicopathological
characteristics of patients with HSILs and LSILs
In the present study, a total of 102 samples were
collected, including 66 HSIL and 36 LSIL samples. The mean ages of
the two groups were not statistically significantly different
(two-tailed Student's t-test; P=0.0642). Most patients were
distributed between the ages of 31 and 40 years (Table SI).
HPV test information for all patients was
summarized, as shown in Table SII.
In the HSIL group, HPV16 was the most prevalent HPV type and
accounted for more than half of all HSIL patients (37/66, 56.1%).
HPV16 also predominated in the LSIL group, but its HPV16-positive
rate was only one-fifth that found in the LSIL group (8/36, 22.2%).
Except for HPV16, other single HPV subtypes comprised <8% of the
total.
Peripheral blood gene expression
profiling
Gene expression profiling was performed for
peripheral blood samples taken from the 66 patients with HSILs and
36 patients with LSILs. Genome-wide expression profiles generated
with Affymetrix Gene Profiling Array cGMP U133 P2 microarray were
analyzed and correlated as between HSIL and LSIL. Finally, 10
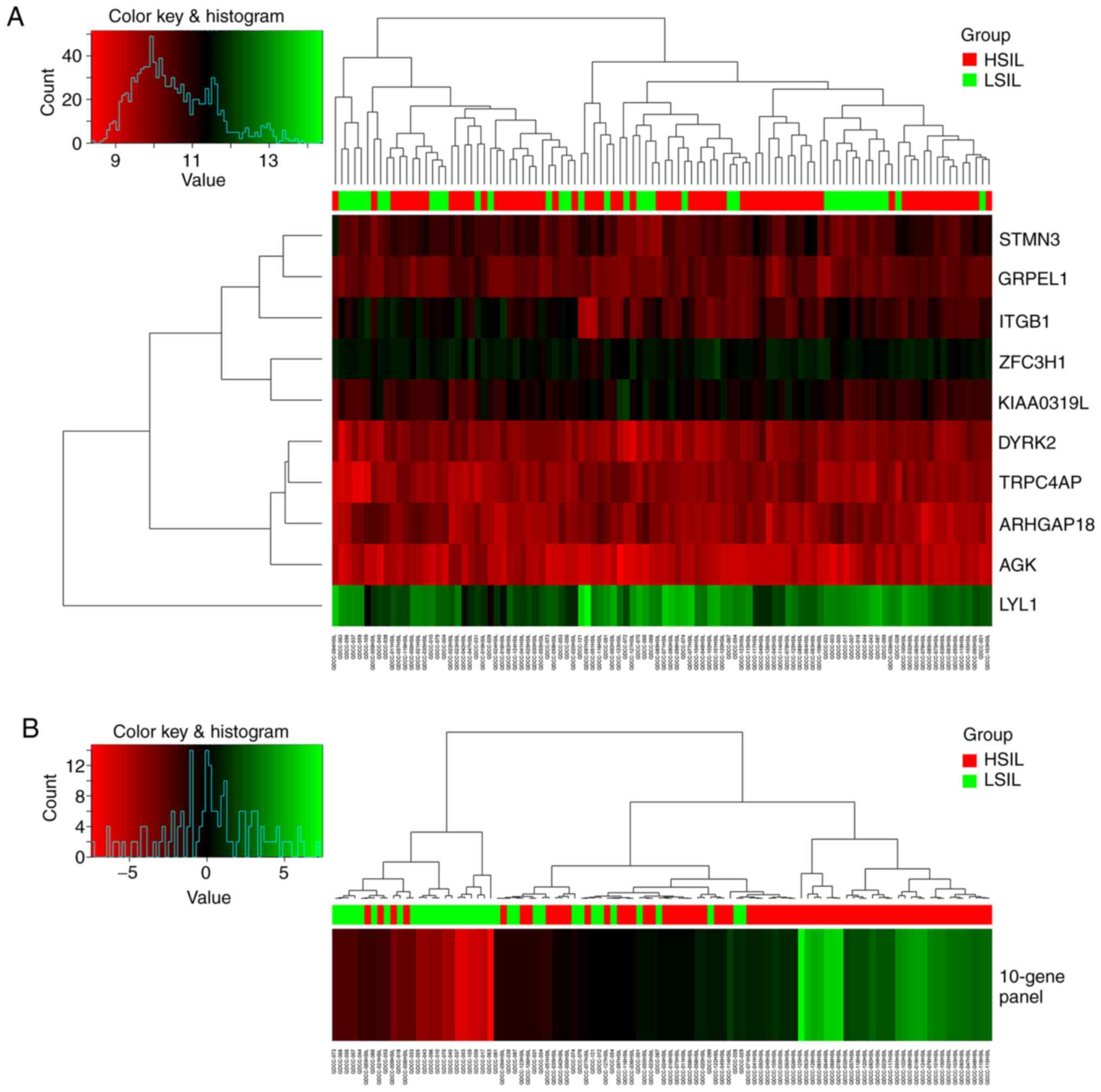
candidate genes were identified as distinguishing HSILs from LSILs:
STMN3, TRPC4AP, DYRK2, AGK, KIAA0319L, GRPEL1, ZFC3H1, LYL1,
ITGB1 and ARHGAP18. The corresponding gene symbols and
names of the final 10 probe sets are listed in Table I, as well as the fold-change between
the two cohorts.
 | Table I.Final 10 candidate genes for
distinguishing HSILs from LSILs. |
Table I.
Final 10 candidate genes for
distinguishing HSILs from LSILs.
| Probe set | Gene symbol | Gene name | Fold-change |
|---|
| 222557_at | STMN3 | Stathmin-like
3 | 1.23 |
| 212059_s_at | TRPC4AP | Transient receptor
potential cation channel, subfamily C, member 4 associated
protein | 1.19 |
| 202968_s_at | DYRK2 | Dual specificity
tyrosine-(Y)-phosphorylation regulated kinase 2 | 1.09 |
| 222132_s_at | AGK | Acylglycerol
kinase | 1.09 |
| 222468_at | KIAA0319L | KIAA0319-like | 1.08 |
| 212434_at | GRPEL1 | GrpE-like 1,
mitochondrial | 1.08 |
| 213065_at | ZFC3H1 | Zinc finger,
C3H1-type containing | 1.04 |
| 210044_s_at | LYL1 | Lymphoblastic
leukemia-associated hematopoiesis regulator 1 | −1.09 |
| 1553530_a_at | ITGB1 | Integrin β1 | −1.23 |
| 225173_at | ARHGAP18 | Rho GTPase
activating protein 18 | −1.23 |
Model construction and performance
estimation
Based on the 10-gene panel identified, a predictive
model was constructed for discriminating HSILs from LSILs using
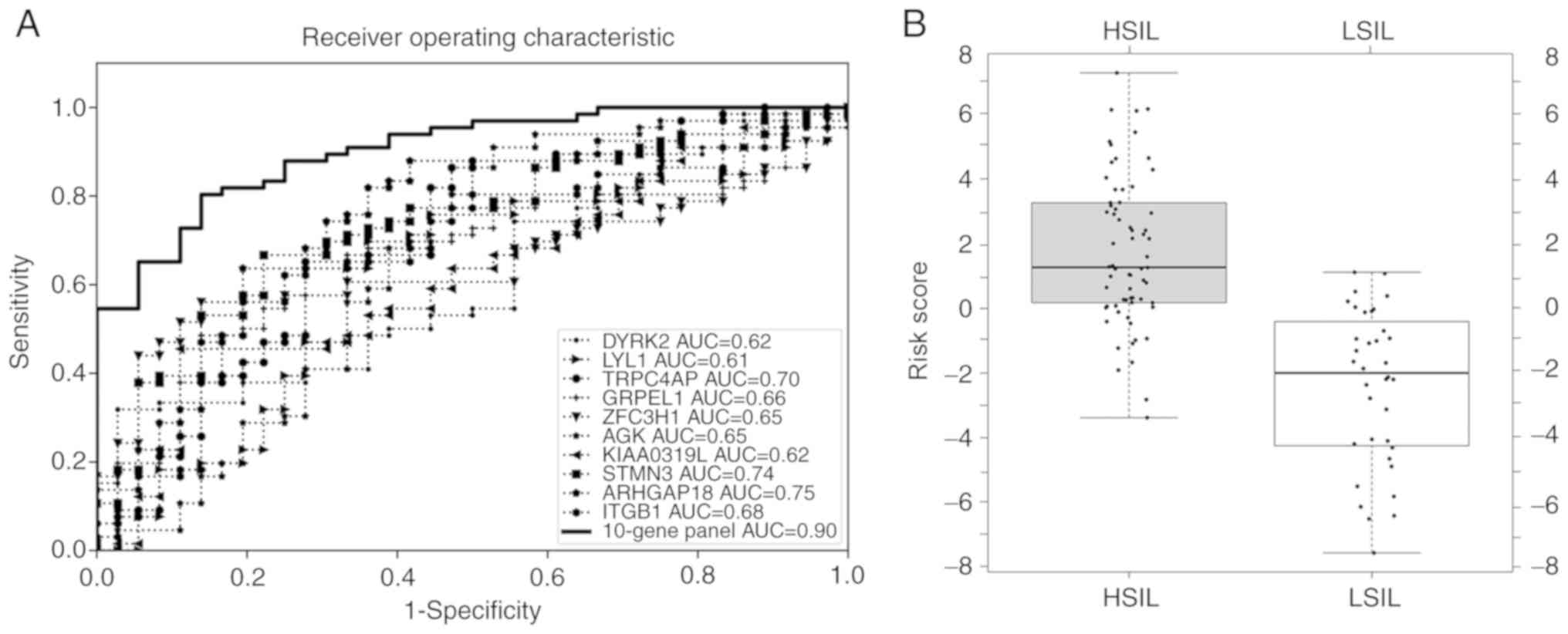
logistic regression. Fig. 1 shows
the performance of the 10 candidate genes separately and the
10-gene panel as a whole for discriminating HSILs from LSILs for
the total 102 samples. Use of the 10-gene panel showed better
clustering of the HSIL samples.
As the sample size in the present study was too
small to be divided into a training set and a test set, the entire
data set was used to construct the model. It was found that a
10-gene panel could discriminate HSILs from LSILs (sensitivity,
81.8%; specificity, 83.3%; accuracy, 82.4%). The 10-gene panel
exhibited the highest ROC AUC of 0.90 as compared with that of each
of 10 candidate genes (0.61–0.75) separately, as shown in Fig. 2A for the total 102 samples. The
box-whisker plot (Fig. 2B) also
illustrated a well-separated distribution of prediction scores of
HSIL and the LSIL, based on the 10-gene panel and logistic
regression algorithm.
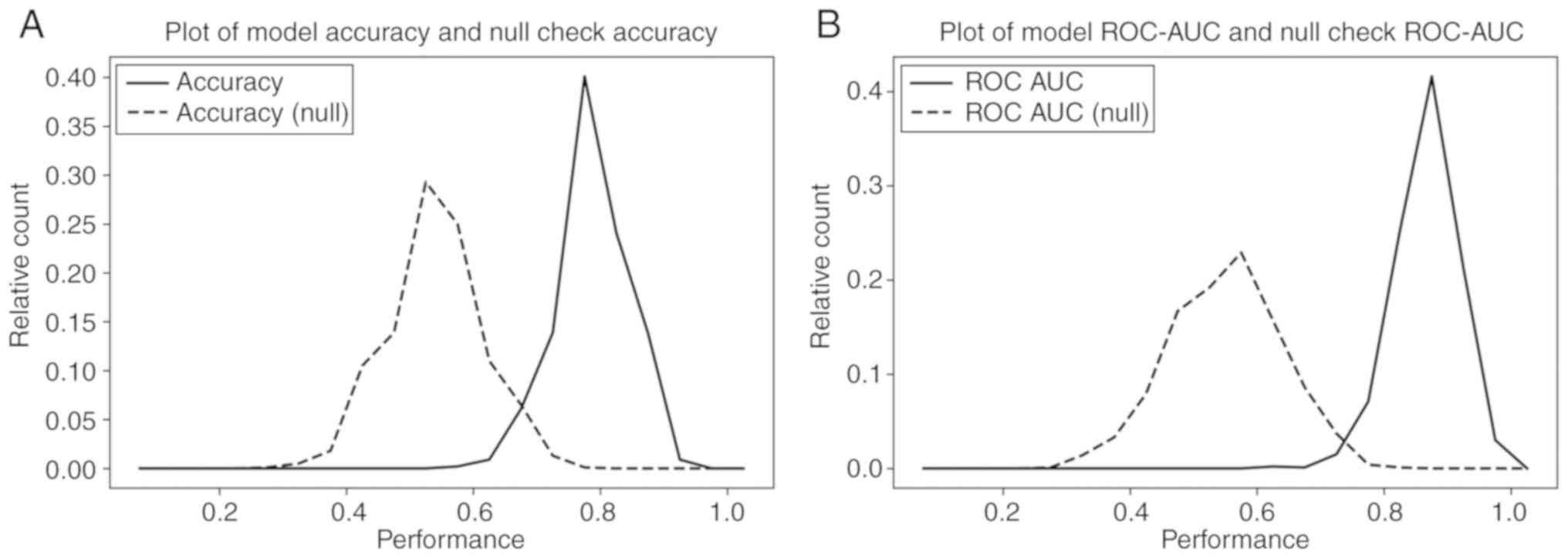
In order to validate the stability of the model, a
2-fold cross-validation method was performed, iterated 1,000 times.
For each iteration, the samples were randomly and equally divided
into training and test folds. The predictive model was constructed
based on the samples in the training fold and then its performance
was evaluated on the sample in the test fold. The distributions of
accuracy and AUC ROC values over the 1,000 iterations exhibited
good prediction performance, with a model mean accuracy of 82%
(Fig. 3A) and ROC AUC of 0.9
(Fig. 3B) (solid line). To rule out
the possibility that the final 10-gene panel was merely derived
from chance and clinical bias, a null set analysis process was
performed for 1,000 iterations, similar to the aforementioned
2-fold cross-validation process, but with HSIL/LSIL status randomly
re-assigned. Comparing the prediction results with the known
HSIL/LSIL status of the samples, it was found that the null set
analysis results were significantly lower, with a model accuracy of
52% (Fig. 3A) and ROC AUC of 0.58
(Fig. 3B) (dashed line). The
distribution of each analysis resulted in 2 well-separated curves
with <5% overlap, from which it was concluded that the
performance of the 10-gene panel for distinguishing HSILs from
LSILs was unlikely to be merely the result of random chance.
To further validate efficiency, this data mining
method was performed in an analysis of blood-based gene expression
profiles of women with cervical SILs and healthy volunteers.
Transcriptomic biomarkers were identified and were found to exhibit
good performance for differentiating cervical SIL patients from
healthy volunteers. Detailed information is presented in Fig. S1 and Table SIII.
Protein-protein interaction and
functional enrichment categorization
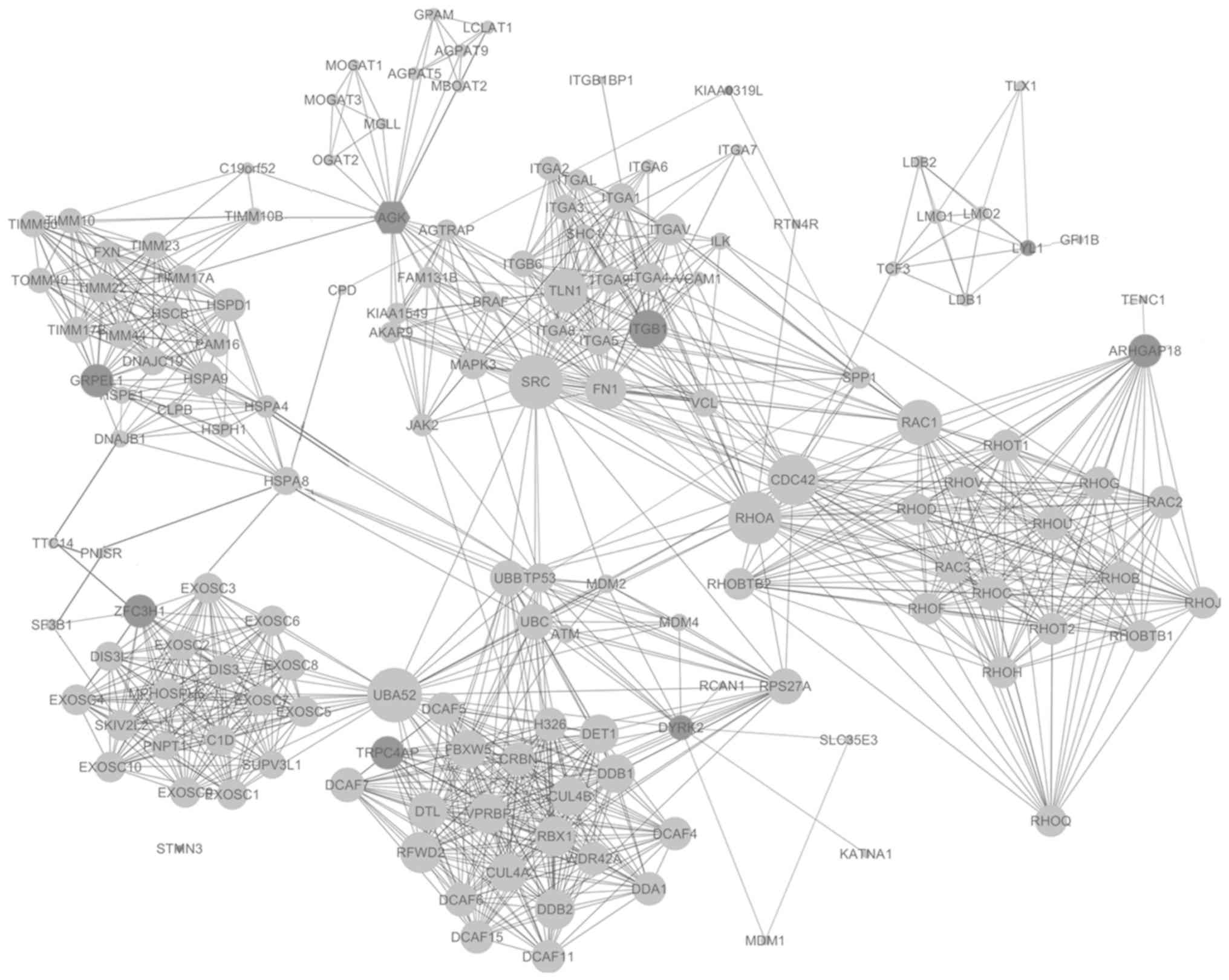
Proteins interacting among the 10 candidate genes
were downloaded from the STRING database individually, with a total
confidence ≥0.7, followed by the protein-protein interactions of
the total 149 proteins thus identified. The protein-protein
interaction network is shown in Fig.
4.
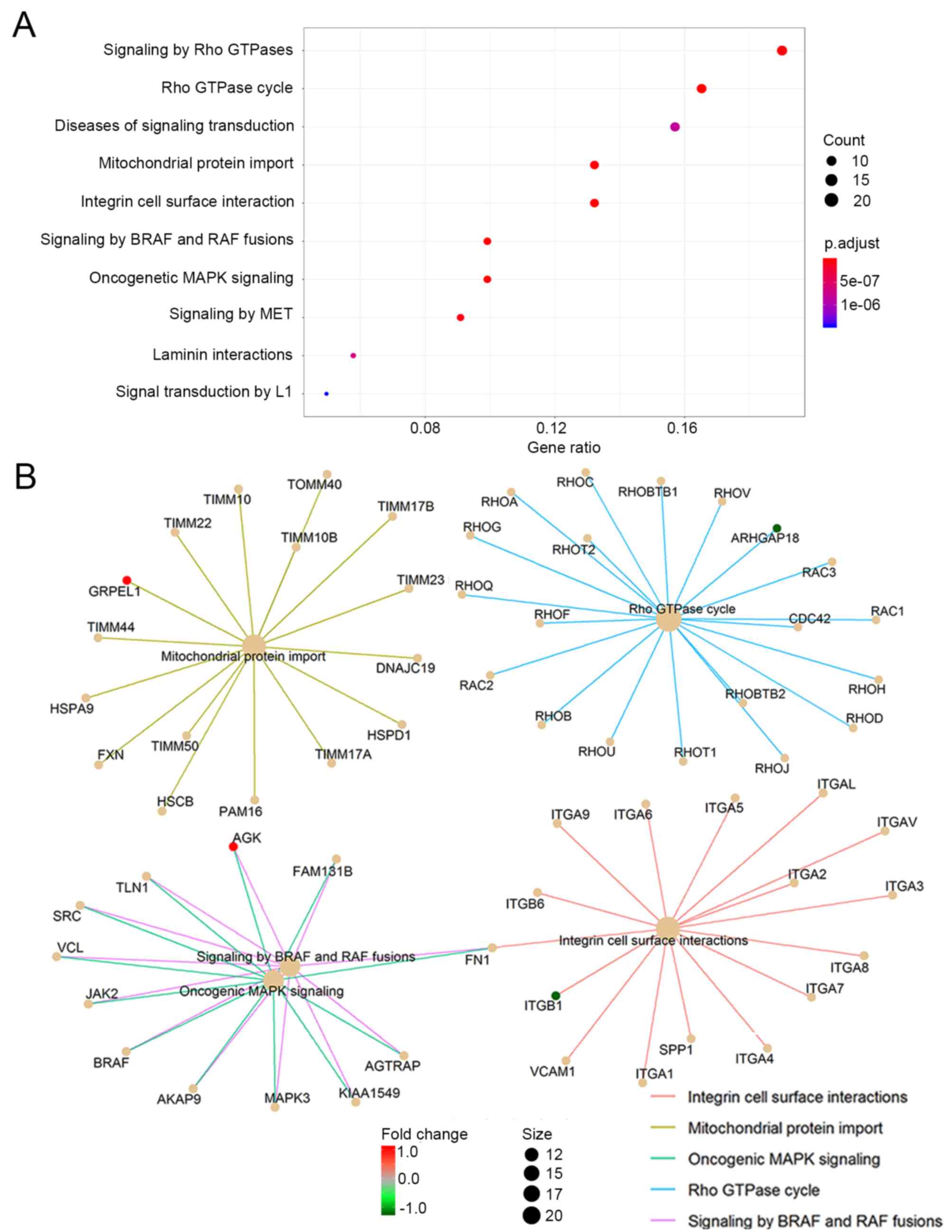
The genes corresponding to the selected 149 proteins
were functionally categorized based on Reactome pathways. Reactome
pathways were identified with a strict cut-off of P<0.05 and FDR
of <0.05. When the top 10 significantly enriched pathways were
analyzed, it was found that the 10 candidate genes and their
interactive proteins were mainly engaged in the ‘Rho GTPase cycle’,
‘mitochondrial protein import’, ‘oncogenic MAPK signaling’,
‘integrin cell surface interaction’ and ‘signaling by BRAF and RAF
fusions’, as shown in Fig. 5A. Since
the Rho GTPase proteins act as binary switches by cycling between
active GTP-bound and inactive GDP-bound conformations (Rho GTPase
cycle) (31), the ‘signaling by Rho
GTPase’ pathway mainly reflects the ‘Rho GTPase cycle’ and thus
could be aligning to the pathway of ‘Rho GTPase cycle’ in the
present study. The interactions of the engaged processes and the
related candidate genes of each process are also indicated in
Fig. 5B.
Discussion
The present study was undertaken to explore a blood
transcriptomic profiling method for distinguishing cervical HSILs
from LSILs. Peripheral blood samples of patients with cervical
HSILs and LSILs were collected, and blood transcriptomic features
were identified using the AdaBoost algorithm. A panel of 10
candidate genes that successfully discriminated cervical HSILs from
LSILs, with an accuracy of 82.4%, was identified. Functional
enrichment analysis indicated that the candidate genes were mainly
involved in the ‘Rho GTPase cycle’, ‘mitochondrial protein import’,
‘oncogenic MAPK signaling’, ‘integrin cell surface interaction’ and
‘signaling by BRAF and RAF fusions’. These preliminary results are
promising; however, further research is needed to validate the
findings in larger cohorts and in a multi-site validation clinical
study.
Most types of cervical cancer are caused by HPV
infections, and HPV is detected in ~99.7% of cervical cancer cases
(32). Not all HPV infections,
however, lead to cervical cancer. Most of these infections are
temporary, resulting only in LSILs; it is only persistent HPV
infection that may result in an HSIL (33). The comparative risks of progression
to cervical cancer differ sharply between HSILs and LSILs (34). From a clinical perspective, the
management of the two types of lesion is also different, with HSILs
regarded as true cervical cancer precursor lesions, requiring
colposcopy and treatment (35).
Currently, the diagnosis of HSIL is mainly dependent on colposcopy
and biopsy, but neither test is completely satisfactory. A
non-invasive test would be welcome to distinguish HSILs from LSILs
during the premalignant stage and to prevent cervical cancer.
In current clinical practice, the tools used for
cervical cancer screening and diagnosis include HPV testing,
cytology testing, colposcopy and cervical biopsy. Although
combinations of different techniques have improved the screening
and diagnosis of cervical cancer, the sensitivity, specificity,
reliability and repeatability are still unsatisfactory due to
sampling errors and/or screening errors, and the final diagnosis is
still dependent on cervical biopsy (36). Thus, the development of a sensitive,
non-invasive approach for the screening and diagnosis of HSILs and
LSILs is essential to complement existing techniques.
The present study was based on our previous blood
transcriptome profiling study (37)
and on our previous reports in cancer research (19,21,22,37–39). In
the present study, a 10-gene panel (Table I) was identified that can
successfully distinguish HSILs from LSILs. A predictive model
performed well in the training set (Figs. 1 and 2), with a sensitivity of 81.8%, specificity
of 83.3% and accuracy of 82.4% (Table
II). The 10 candidate genes (10-gene panel) used to construct
the predictive model were: STMN3, TRPC4AP, DYRK2, AGK,
KIAA0319L, GRPEL1, ZFC3H1, LYL1, ITGB1 and ARHGAP18. The
last 3 genes (LYL1, ITGB1 and ARHGAP18) were
downregulated in HSILs as compared with LSILs, while the other 7
genes were upregulated.
 | Table II.Predictive performance of the
training set. |
Table II.
Predictive performance of the
training set.
| Performance | HSILs, n | LSILs, n | Sensitivity, % | Specificity, % | Accuracy, % | ROC AUC |
|---|
| Positive
prediction | 54 | 6 |
|
|
|
|
| Negative
prediction | 12 | 30 |
|
|
|
|
| Total | 66 | 36 | 81.8 | 83.3 | 82.4 | 0.9 |
To verify that this method is also efficient for
identifying blood-based transcriptomic biomarkers suitable for
discriminating HSILs/LSILs from healthy populations, the blood gene
expression profiles of samples from healthy women and samples from
women with cervical SIL were analyzed. It was found that the
biomarkers identified by the present data mining method were able
to differentiate SILs from healthy samples with relatively high
accuracy, similar to the HSIL and LSIL classification results.
Detailed information is provided in Fig. S1 and Table SIII. This result also confirmed the
feasibility of applying blood-based transcriptomic biomarkers for
SIL and cervical cancer screening.
To further explore the function of the 10-gene
panel, the protein-protein interaction network was mapped and a
total of 149 proteins were identified interacting with the
candidate genes. The genes corresponding to the 149 interactive
proteins were then functionally categorized into several biological
processes, including the ‘Rho GTPase cycle’, ‘mitochondrial protein
import’, ‘oncogenic MAPK signaling’, ‘integrin cell surface
interaction’ and ‘signaling by BRAF and RAF fusions’, as shown in
Fig. 5A. Of these, GRPEL1 is
involved in ‘mitochondrial protein import’, ARHGAP18 in ‘Rho GTPase
cycling’, ITGB1 in ‘integrin cell surface interaction’, and AGK in
‘oncogenic MAPK signaling’ and ‘BRAF and RAF fusions’, as indicated
in Fig. 5B. These findings suggest
that these processes may be associated with the progression of
LSILs to HSILs.
Mitochondrial protein import is essential for
maintaining cellular homeostasis. During this process, the
mitochondria and nucleus co-ordinately communicate with each other,
and the adaptive responses to stress depend on modulation of
mitochondrial import (40).
Dysfunction of this process plays a role in human disorders such as
mitochondrial neuropathies, myopathies and cancer (41–43). It
has been reported that cervical cancer HeLa cells can revert from
an apoptotic state even after the induction of widespread
mitochondrial-membrane permeabilization, and that mitochondrial
protein import plays a role in the anastatic process of HeLa cells
(44). Since most mitochondrial
proteins are encoded in the nucleus and are imported into the
matrix compartment where they are properly folded, the process is
facilitated by mitochondrial heat shock protein 70 (mtHsp70)
(45) GrpE-like 1 (GrpEL1), as a
putative nucleotide exchange factors ortholog, acts as a
hetero-oligomeric subcomplex to associate with mtHsp70 and regulate
mtHsp70 function (46). Therefore,
the GrpEL1-related mitochondrial protein import process may be a
potential therapeutic target for cervical cancer and precancerous
lesions.
The best characterized members of the Rho GTPase
family, RhoA, Rac1 and Cdc42 (47),
are associated with abnormalities in Rho GTPase function that have
major consequences for tumorigenesis (48), cancer progression (49) and cancer immune suppression (50). The Rho GTPase member, Rac1, is
expressed in the nucleus of epithelial cells in SIL and cervical
cancer cell lines, and inhibition of Rac1 can decrease cellular
proliferation, suggesting that Rho-GTPases may have a role in
cervical cancer progression (51).
One study demonstrated that stimulation of G12 proteins is capable
of promoting cervical cancer invasion through RhoA/ROCK-JNK
activation (52). RhoA has also
shown a positive correlation with the progression and metastatic
potential of cervical cancer, both in vivo and in
vitro (53). ARHGAP18 is
one of the crucial factors for the regulation of RhoA, whose
knockdown and overexpression in HeLa cells in a previous study
resulted in the inhibition and promotion of cell migration,
respectively. Furthermore, it was also required for the
polarization of cells for migration (54). Since ARHGAP18 was the most
downregulated candidate gene in the present study, we speculate
that this gene may be a biomarker associated with a good prognosis
in cervical cancer, as has been reported in breast cancer (55).
Integrins belong to the family of heterodimeric cell
surface receptors and trigger various cellular responses by forming
physical crosstalk between the inside and outside of cells, and
these receptors can bi-directionally control the signals for cell
adhesion, migration, proliferation, survival and differentiation
(56). Due to their crucial role in
physiological functions, integrins also play a pivotal role in
tumorigenesis (57). Integrins are
heterodimers that include 8β and 18α subunits. ITGB1 protein,
namely the integrin β1, which is a member of the β sub-family,
forms dimers with different α subunits (α1–7 and αV) 55(t.). ITGB1
protein together with another integrin, ITGA6 protein, was found to
be downregulated in Pap-cell smears of high-risk human
papillomavirus-positive squamous cervical carcinoma in a previous
study (58). The present results
were consistent with this, as the gene expression of ITGB1
was also decreased in HSILs compared with that in LSILs. There was
also a contradictory study, which suggested that ITGB1 was
negatively mediated by miR-183-5p in cervical cancer cells; since
miR-183-5p serves as a latent anti-oncogene, the investigators
regarded ITGB1 as a metastasis-promoter gene (59). The precise role of ITGB1 in
SILs and cervical cancer thus requires further research
clarification.
Oncogenic MAPK signalling, as well as BRAF and RAF
fusions with AGK, were the other biological processes identified in
the present study. AGK protein, namely acylglycerol kinase, is a
novel lipid kinase, which produces lysophosphatidic acid from
monoacylglycerol (60). AGK
is a cancer-related gene that is overexpressed in various human
cancer types (61–64). Sun et al found that AGK
protein and mRNA expression was significantly upregulated in
cervical cancer cell lines and cancer tissues and demonstrated that
the AGK protein expression level was an independent prognostic
factor for the survival of patients with early-stage cervical
squamous cell cancer (65). In the
present study, it was also found that AGK was upregulated in
HSILs, which suggested that AGK plays a role in the
progression of LSILs to HSILs, and can be used as a prognostic
marker for SIL and cervical cancer evolution.
On the journey of cancer as it progresses from
precursor lesions towards full-scale malignancy, gene expression
undergoes changes. Those genetic events in turn establish an
environment permissive for neoplastic progression (66). We propose that the downregulation of
ITGB1 and ARHGAP18 in women with HSILs leads to the
dysfunction of cell adhesion and the promotion of cell migration.
Dysfunction of cell adhesion then allows cells to migrate and to be
more invasive, which occurs during the transformation of LSILs into
HSILs. We also suggest that the upregulation of AGK and
GRPEL1 in women with HSILs may disrupt the process of
protein localization and transport (67,68),
processes that establish and maintain proteins during oncogenesis.
The present findings thus indicate that protein transport and cell
migration may play important roles in the progression of LSILs to
HSILs.
Conceptual progress for the hallmarks of cancer has
been made over the past decade. In the present study, the
downregulated genes, ITGB1 and ARHGAP18, were found
to be involved in the integrin cell surface interaction and the Rho
GTPase cycle pathways. Integrin, as one of the family of
heterodimeric cell surface receptors, can mediate cell adhesion to
the extracellular matrix, which is a network of macro-molecules.
Integrins can bi-directionally control the signals for cell
adhesion, migration, proliferation, survival and differentiation
(56). The activation of Rho GTPases
can trigger changes in the organization of the cytoskeleton,
thereby regulating cell polarity and cell-cell junctions (47). RhoA has also shown a positive
correlation with the progression and metastatic potential of
cervical cancer (53). In brief,
these two enriched pathways identified in the present study are
associated with cell invasion and metastasis, two important
hallmarks of cancer (69).
The upregulated genes, AGK and GRPEL1,
are involved in the oncogenic MAPK signaling and mitochondrial
protein import pathways. Accumulating evidence shows that the
RAS/RAF/MAPK cascade is a critical pathway for cancer cell
proliferation, differentiation and survival (70). Mitochondrial protein import is
essential for maintaining cellular homeostasis (71). GRPEL1 acts as a
hetero-oligomeric subcomplex to interact with mtHsp70, facilitating
the process in which mitochondrial proteins are encoded and
imported into the matrix (45), and
regulating mtHsp70 function (46).
In brief, these two enriched pathways identified in the present
study are associated with selective growth and proliferative
advantage, which are hallmarks of cancer (69,72).
A question arises as to any possible correlations
that may be identified between blood gene expression and specific
HPV types. In the present study, HPV16 was the most prevalent HPV
type and accounted for more than half of all HSIL patients (37/66,
56.1%). A limitation of this study, however, is that only 20
samples in the HSIL cohort presented with other types of HPV, a
number inadequate for statistically significant correlation
analysis between blood gene expression and specific HPV type. This
issue will be studied in future in larger cohorts.
Another notable future project would be to study
variations in blood-based gene expression profiling between women
with HSIL and women with cervical cancer. Such a study would be of
clinical benefit for diagnosing cervical cancer.
In conclusion, the present study identified a panel
of 10 blood transcriptomic biomarkers that discriminate cervical
HSILs from LSILs, and which could form the basis of a novel blood
test for SIL classification. Gene function investigation has
indicated that these genes are mainly engaged in the ‘Rho GTPase
cycle’, ‘mitochondrial protein import’, ‘oncogenic MAPK signaling’,
‘integrin cell surface interaction’ and ‘signaling by BRAF and RAF
fusions’. We propose that these biological processes play important
roles in the progression of LSILs to HSILs. We also suggest that
these processes may be associated with cell invasion and
metastasis, selective growth and proliferative advantage, which are
important hallmarks of cancer. Moreover, the 10 candidate genes
identified in this study (STMN3, TRPC4AP, DYRK2, AGK, KIAA0319L,
GRPEL1, ZFC3H1, LYL1, ITGB1 and ARHGAP18) may
participate in events leading to precancerous cervical lesions and
require additional investigation. The genes identified here may
also be used as diagnostic biomarkers and targeted therapy for
cervical SILs.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Ms. Qian Shi,
Shanghai Homeostasis Bio-Technology Inc., who conducted the
Affymetrix microarray experiments and Ms. Isolde Prince, Shanghai
Homeostasis Bio-Technology Inc., who helped with the editing of the
original manuscript.
Funding
This study was sponsored by Shanghai Homeostasis
Bio-Technology Inc. (Shanghai, China).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CZ was responsible for conception, study design and
acquisition of data. YL was responsible for conceptualization,
study design and writing the original draft of the manuscript. YC,
CS, HL and JJ were involved in acquisition and analysis of data. MW
was responsible for analysis and interpretation of data. RZ was
responsible for analysis and interpretation of data. CC was
involved in conception and design of the study, analysis and
interpretation of data, drafting of the initial manuscript,
revising the manuscript for important intellectual content and
providing approval for the final version of the manuscript to be
published. CCL was responsible for conceptualization and designing
of the study, resources and revising the manuscript for important
intellectual content. SZ was responsible for conception and design
of the study, acquisition and analysis of data and revising the
manuscript for important intellectual content. All authors have
read and approved the manuscript.
Ethics approval
This study was approved by the Ethics Committee of
the Qingdao Women and Children's Hospital (institutional review
board no. QFELL-KY-2019-46). Sample acquisition for HSILs and LSILs
was conducted between July 2019 and October 2019 at the Qingdao
Women and Children's Hospital. All 102 participants, including 66
HSIL and 36 LSIL patients, were enrolled and provided written
informed consent. All 65 healthy volunteers also provided informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors wish to declare the following competing
interests: CC, YL, ME and RZ are employees of Shanghai Homeostasis
Bio-Technology Inc., who sponsored this research. CCL was the
scientific consultant at Shanghai Homeostasis Bio-Technology Inc.,
and the chair and founder of Golden Health Diagnostics. The authors
declare that they have no competing interests.
References
|
1
|
Godoy-Vitorino F, Romaguera J, Zhao C,
Vargas-Robles D, Ortiz-Morales G, Vázquez-Sánchez F,
Sanchez-Vázquez M, de la Garza-Casillas M, Martinez-Ferrer M, White
JR, et al: Cervicovaginal fungi and bacteria associated with
cervical intraepithelial neoplasia and high-risk human
papillomavirus infections in a hispanic population. Front
Microbiol. 9:25332018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
World Health Organization: WHO guidelines
for screening and treatment of precancerous lesions for cervical
cancer prevention. 2013.
|
|
3
|
Reagan JW, Seidemann IL and Saracusa Y:
The cellular morphology of carcinoma in situ and dysplasia or
atypical hyperplasia of the uterine cervix. Cancer. 6:224–234.
1953. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Richart RM: A theory of cervical
carcinogenesis. Obstet Gynecol Surv. 24:874–879. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hoffman SR, Le T, Lockhart A, Sanusi A,
Dal Santo L, Davis M, McKinney DA, Brown M, Poole C, Willame C and
Smith JS: Patterns of persistent HPV infection after treatment for
cervical intraepithelial neoplasia (CIN): A systematic review. Int
J Cancer. 141:8–23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Voltaggio L, Cimino-Mathews A, Bishop JA,
Argani P, Cuda JD, Epstein JI, Hruban RH, Netto GJ, Stoler MH,
Taube JM, et al: Current concepts in the diagnosis and pathobiology
of intraepithelial neoplasia: A review by organ system. CA Cancer J
Clin. 66:408–436. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nguyen HN and Nordqvist SR: The Bethesda
system and evaluation of abnormal pap smears. Semin Surg Oncol.
16:217–221. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Solomon D and Ritu N: The bethesda system
for reporting cervical cytology: Definitions, criteria, and
explanatory notes. Comprehensive cytopathology. 10. pp. 77–90.
2008
|
|
9
|
Darragh TM, Colgan TJ, Thomas Cox J,
Heller DS, Henry MR, Luff RD, McCalmont T, Nayar R, Palefsky JM,
Stoler MH, et al: The lower anogenital squamous terminology
standardization project for HPV-associated lesions: Background and
consensus recommendations from the College of American pathologists
and the American society for colposcopy and cervical pathology. Int
J Gynecol Pathol. 32:76–115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stoler MH, Ronnett BM, Joste NE, Hunt WC,
Cuzick J and Wheeler CM; New Mexico HPV Pap Registry Steering
Committee, : The interpretive variability of cervical biopsies and
its relationship to HPV status. Am J Surg Pathol. 39:729–36. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Charoonwatana T, Boonlikit S and Yanaranop
M: Progression of precancerous cervical lesion predicted by p16
protein immunohistochemistry in Rajavithi Hospital. Asian Pac J
Cancer Prev. 20:1809–1815. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu Z and Chen J: Introduction of WHO
classification of tumours of female reproductive organs fourth
edition. Zhonghua Bing Li Xue Za Zhi. 43:649–650. 2014.(In
Chinese). PubMed/NCBI
|
|
13
|
Liu Y, Alqatari M, Sultan K, Ye F, Gao D,
Sigel K, Zhang D and Kalir T: Using p16 immunohistochemistry to
classify morphologic cervical intraepithelial neoplasia 2:
Correlation of ambiguous staining patterns with HPV subtypes and
clinical outcome. Hum Pathol. 66:144–151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wentzensen N, Bergeron C, Cas F,
Eschenbach D, Vinokurova S and von Knebel Doeberitz M: Evaluation
of a nuclear score for p16INK4a-stained cervical squamous cells in
liquid-based cytology samples. Cancer. 105:461–467. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Sigel K and Gaisa MM: Human
papillomavirus genotypes predict progression of anal low-grade
squamous intraepithelial lesions. J Infect Dis. 218:1746–1752.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Johnson CA, James D, Marzan A and Armaos
M: Cervical cancer: An overview of pathophysiology and management.
Semin Oncol Nurs. 35:166–174. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schwarzenbach H, Hoon DS and Pantel K:
Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev
Cancer. 11:426–437. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liew CC: Method for detection of gene
transcripts in blood and uses thereof. 2009.
|
|
19
|
Shi J, Cheng C, Ma J, Liew CC and Geng X:
Gene expression signature for detection of gastric cancer in
peripheral blood. Oncol Lett. 15:9802–9810. 2018.PubMed/NCBI
|
|
20
|
Omar H, Lim CR, Chao S, Lee MM, Bong CW,
Ooi EJ, Yu CG, Tan SS, Abu Hassan MR, Menon J, et al: Blood gene
signature for early hepatocellular carcinoma detection in patients
with chronic hepatitis B. J Clin Gastroenterol. 49:150–157. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mok CS, Kim JH, Skates SJ, Schorge JO,
Cramer DW, Lu KH and Liew CC: Use of blood-based mRNA profiling to
identify biomarkers for ovarian cancer screening. Gynecol Obstet
(Sunnyvale). 7:4432017. View Article : Google Scholar
|
|
22
|
Marshall KW, Mohr S, Khettabi FE, Nossova
N, Chao S, Bao W, Ma J, Li XJ and Liew CC: A blood-based biomarker
panel for stratifying current risk for colorectal cancer. Int J
Cancer. 126:1177–1186. 2010.PubMed/NCBI
|
|
23
|
Monsonego J, Hudgens MG, Zerat L, Zerat
JC, Syrjӓnen K, Halfon P, Ruiz F and Smith JS: Evaluation of
oncogenic human papillomavirus RNA and DNA tests with liquid-based
cytology in primary cervical cancer screening: The FASE study. Int
J Cancer. 129:691–701. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ratnam S, Coutlee F, Fontaine D, Bentley
J, Escott N, Ghatage P, Gadag V, Holloway G, Bartellas E, Kum N, et
al: Aptima HPV E6/E7 mRNA test is as sensitive as hybrid capture 2
assay but more specific at detecting cervical precancer and cancer.
J Clin Microbiol. 49:557–564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nayar R and Wilbur DC: The Bethesda system
for reporting cervical cytology. Definitions, criteria, and
explanatory Notes. 3rd. Springer International Publishing; Cham,
Switzerland: 2015
|
|
26
|
MAQC Consortium, . Shi L, Reid LH, Jones
WD, Shippy R, Warrington JA, Baker SC, Collins PJ, de Longueville
F, Kawasaki ES, et al: The microarray quality control (MAQC)
project shows inter- and intraplatform reproducibility of gene
expression measurements. Nat Biotechnol. 24:1151–1161. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qi Z, Meng F, Tian Y, Niu L, Shi Y and
Zhang P: Adaboost-LLP: A boosting method for learning with label
proportions. IEEE Trans Neural Netw Learn Syst. 29:3548–3559. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chao S, Cheng C and Liew CC: Mining the
dynamic genome: A method for identifying multiple disease
signatures using quantitative RNA expression analysis of a single
blood sample. Microarrays (Basel). 4:671–689. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Obayashi T, Kagaya Y, Aoki Y, Tadaka S and
Kinoshita K: COXPRESdb v7: A gene coexpression database for 11
animal species supported by 23 coexpression platforms for technical
evaluation and evolutionary inference. Nucleic Acids Res. 47(D1):
D55–D62. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu G, Wang LG, Han Y and He QY:
ClusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tcherkezian J and Lamarche-Vane N: Current
knowledge of the large RhoGAP family of proteins. Biol Cell.
99:67–86. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
McCormack PL: Quadrivalent human
papillomavirus (types 6, 11, 16, 18) recombinant vaccine
(gardasil(®)): A review of its use in the prevention of
premalignant anogenital lesions, cervical and anal cancers, and
genital warts. Drugs. 74:1253–1283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stanley MA and Sterling JC: Host responses
to infection with human papillomavirus. Curr Probl Dermatol.
45:58–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Steenbergen RD, Snijders PJ, Heideman DA
and Meijer CJ: Clinical implications of (epi)genetic changes in
HPV-induced cervical precancerous lesions. Nat Rev Cancer.
14:395–405. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bierkens M, Wilting SM, Wieringen WN, van
de Wiel MA, Ylstra B, Meijer CJ, Snijders PJ and Steenbergen RD:
HPV type-related chromosomal profiles in high-grade cervical
intraepithelial neoplasia. BMC Cancer. 12:362012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Karia N, Van Loon A, Simoens C, Benoy I
and Bogers J: The positive predictive value of high-grade squamous
intraepithelial lesion on cytology for the histological diagnosis
of cervical intraepithelial neoplasia 2 or higher: A systematic
review. Acta Cytol. 63:206–214. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mohr S and Liew CC: The peripheral-blood
transcriptome: New insights into disease and risk assessment.
Trends Mol Med. 13:422–432. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Osman I, Bajorin DF, Sun TT, Zhong H,
Douglas D, Scattergood J, Zheng R, Han M, Marshall KW and Liew CC:
Novel blood biomarkers of human urinary bladder cancer. Clin Cancer
Res. 12:3374–3380. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chao S, Ying J, Liew G, Marshall W, Liew
CC and Burakoff R: Blood RNA biomarker panel detects both left- and
right-sided colorectal neoplasms: A case-control study. J Exp Clin
Cancer Res. 32:442013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nicolas E, Tricarico R, Savage M, Golemis
EA and Hall MJ: Disease-associated genetic variation in human
mitochondrial protein import. Am J Hum Genet. 104:784–801. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guerra F, Guaragnella N, Arbini AA, Bucci
C, Giannattasio S and Moro L: Mitochondrial dysfunction: A novel
potential driver of epithelial-to-mesenchymal transition in cancer.
Front Oncol. 7:2952017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Raimundo N: Mitochondrial pathology:
Stress signals from the energy factory. Trends Mol Med. 20:282–292.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Madamba SM, Damri KN, Dejean LM and
Peixoto PM: Mitochondrial ion channels in cancer transformation.
Front Oncol. 5:1202015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Seervi M, Sumi S, Chandrasekharan A,
Sharma AK and Santhosh Kumar TR: Molecular profiling of anastatic
cancer cells: Potential role of the nuclear export pathway. Cell
Oncol (Dordr). 42:645–661. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Neupert W and Brunner M: The protein
import motor of mitochondria. Nat Rev Mol Cell Biol. 3:555–565.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
46
|
Srivastava S, Savanur MA, Sinha D, Birje
A, R V, Saha PP and D'Silva P: Regulation of mitochondrial protein
import by the nucleotide exchange factors GrpEL1 and GrpEL2 in
human cells. J Biol Chem. 292:18075–18090. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cherfils J and Zeghouf M: Regulation of
small GTPases by GEFs, GAPs, and GDIs. Physiol Rev. 93:269–309.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Karlsson R, Pedersen ED, Wang Z and
Brakebusch C: Rho GTPase function in tumorigenesis. Biochim Biophys
Acta. 1796:91–98. 2009.PubMed/NCBI
|
|
49
|
Jansen S, Gosens R, Wieland T and Schmidt
M: Paving the Rho in cancer metastasis: Rho GTPases and beyond.
Pharmacol Ther. 183:1–21. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chaker M, Minden A, Chen S, Weiss RH,
Chini EN, Mahipal A and Azmi AS: Rho GTPase effectors and NAD
metabolism in cancer immune suppression. Expert Opin Ther Targets.
22:9–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mendoza-Catalán MA, Cristóbal-Mondragón
GR, Adame-Gómez J, del Valle-Flores HN, Coppe JF, Sierra-López L,
Romero-Hernández MA, del Carmen Alarcón-Romero L, Illades-Aguiar B
and Castañeda-Saucedo E: Nuclear expression of Rac1 in cervical
premalignant lesions and cervical cancer cells. BMC Cancer.
12:1162012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yuan B, Cui J, Wang W and Deng K: Gα12/13
signaling promotes cervical cancer invasion through the
RhoA/ROCK-JNK signaling axis. Biochem Biophys Res Commun.
473:1240–1246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu X, Chen D and Liu G: Overexpression of
RhoA promotes the proliferation and migration of cervical cancer
cells. Biosci Biotechnol Biochem. 78:1895–1901. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Maeda M, Hasegawa H, Hyodo T, Ito S, Asano
E, Yuang H, Funasaka K, Shimokata K, Hasegawa Y, Hamaguchi M and
Senga T: ARHGAP18, a GTPase-activating protein for RhoA, controls
cell shape, spreading, and motility. Mol Biol Cell. 22:3840–3852.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Aleskandarany MA, Sonbul S, Surridge R,
Mukherjee A, Caldas C, Diez-Rodriguez M, Ashankyty I, Albrahim KI,
Elmouna AM, Aneja R, et al: Rho-GTPase activating-protein 18: A
biomarker associated with good prognosis in invasive breast cancer.
Br J Cancer. 117:1176–1184. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Desgrosellier JS and Cheresh DA: Integrins
in cancer: Biological implications and therapeutic opportunities.
Nat Rev Cancer. 10:9–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Pan B, Guo J, Liao Q and Zhao Y: β1 and β3
integrins in breast, prostate and pancreatic cancer: A novel
implication. Oncol Lett. 15:5412–5416. 2018.PubMed/NCBI
|
|
58
|
Manavi M, Hudelist G, Fink-Retter A,
Gschwandtler-Kaulich D, Pischinger K and Czerwenka K: Gene
profiling in Pap-cell smears of high-risk human
papillomavirus-positive squamous cervical carcinoma. Gynecol Oncol.
105:418–426. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang W, Zhang M, Liu L, Jin D, Wang P and
Hu J: MicroRNA-183-5p inhibits aggressiveness of cervical cancer
cells by targeting integrin subunit beta 1 (ITGB1). Med Sci Monit.
24:7137–7145. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bektas M, Payne SG, Liu H, Goparaju S,
Milstien S and Spiegel S: A novel acylglycerol kinase that produces
lysophosphatidic acid modulates cross talk with EGFR in prostate
cancer cells. J Cell Biol. 169:801–811. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Nouh MA, Wu XX, Okazoe H, Tsunemori H,
Haba R, Abou-Zeid AM, Saleem MD, Inui M, Sugimoto M, Aoki J and
Kakehi Y: Expression of autotaxin and acylglycerol kinase in
prostate cancer: Association with cancer development and
progression. Cancer Sci. 100:1631–1638. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang X, Lin C, Zhao X, Liu A, Zhu J, Li X
and Song L: Acylglycerol kinase promotes cell proliferation and
tumorigenicity in breast cancer via suppression of the FOXO1
transcription factor. Mol Cancer. 13:1062014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chen X, Ying Z, Lin X, Lin H, Wu J, Li M
and Song L: Acylglycerol kinase augments JAK2/STAT3 signaling in
esophageal squamous cells. J Clin Invest. 123:2576–2589. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Cui Y, Lin C, Wu Z, Liu A, Zhang X, Zhu J,
Wu G, Wu J, Li M, Li J and Song L: AGK enhances angiogenesis and
inhibits apoptosis via activation of the NF-κB signaling pathway in
hepatocellular carcinoma. Oncotarget. 5:12057–12069. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sun F, Xiong Y, Zhou XH, Li Q, Xiao L,
Long P, Li LJ, Cai MY, Wei YX, Ma YL and Yu YH: Acylglycerol kinase
is over-expressed in early-stage cervical squamous cell cancer and
predicts poor prognosis. Tumour Biol. 37:6729–6736. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gius D, Funk MC, Chuang EY, Feng S,
Huettner PC, Nguyen L, Bradbury CM, Mishra M, Gao S, Buttin BM, et
al: Profiling microdissected epithelium and stroma to model genomic
signatures for cervical carcinogenesis accommodating for
covariates. Cancer Res. 67:7113–7123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Bauer NC, Detsch PW and Corbett AH:
Mechanisms regulating protein localization. Traffic. 16:1039–1061.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kang Y, Fielden LF and Stojanovski D:
Mitochondrial protein transport in health and disease. Semin Cell
Dev Biol. 76:142–153. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Molina JR and Adjei AA: The Ras/Raf/MAPK
pathway. J Thorac Oncol. 1:7–9. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kotiadis VN, Duchen MR and Osellame LD:
Mitochondrial quality control and communications with the nucleus
are important in maintaining mitochondrial function and cell
health. Biochim Biophys Acta. 1840:1254–1265. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Fouad YA and Aanei C: Revisiting the
hallmarks of cancer. Am J Cancer Res. 7:1016–1036. 2017.PubMed/NCBI
|