A systematic review and meta-analysis has
demonstrated that the epidermal growth factor receptor (EGFR)
mutation global prevalence in patients with non-small cell lung
cancer (NSCLC) is 32.3%, which is different to the prevalence in
the European population (14.1%), but similar to that in China
(38.4%) (1). Exon 19 deletions and
exon 21 L858R mutations are the most common mutations (2). The T790M point mutation occurs in
50-60% of patients and confers resistance to first-generation
tyrosine kinase inhibitors (TKIs) (2–4).
For patients with wild-type EGFR and no resistance
mutations, especially the T790M mutation, osimertinib is an
effective third-generation irreversible inhibitor (5,6). FLAURA
Clinical Trials (Funded by AstraZeneca) have demonstrated that
previously untreated EGFR-mutant patients treated with osimertinib
had a significantly longer median progression-free survival (PFS)
time than those treated with standard EGFR-TKIs (18.9 vs. 10.2
months) (7). Similar results were
obtained in an Asian study (8).
However, the inclusion criteria for participation in
clinical trials are often so stringent that most patients in
clinical practice are unable to participate (9). In the real world, some patients may
progress rapidly and have a short PFS time, and this may be
associated with resistance mutations. Mechanisms of acquired
resistance to first-generation EGFR-TKIs include the T790M point
mutation and other gene alterations in PIK3CA, human epidermal
growth factor receptor 2 (HER2) and KRAS (10). However, to the best of our knowledge,
there has not been a clinical study to confirm the resistance
mechanism to osimertinib. The present study revealed, from a
clinical perspective, that patients with an EGFR mutation in
addition to complex mutations experienced poor survival, and
examined the resistance mechanism of osimertinib. Therefore, more
comprehensive genetic tests are required for patients treated with
osimertinib.
Data from 128 patients treated with osimertinib
diagnosed with NSCLC between March 2015 and November 2018 at the
Chinese People's Liberation Army General Hospital (Beijing, China)
were collected for the present study. Tissue for diagnosis was
obtained by biopsy. Inclusion criteria were as follows: i) NSCLC
patients diagnosed by histological examination; ii) osimertinib
used during treatment; iii) measurable lesion by CT or MRI scan;
and iv) age >18 years. Exclusion criteria were: i) Patients that
receive other treatments at the same time, such as biological
immunotherapy, radiotherapy, etc.; ii) other malignant tumors that
are not cured; iii) severe dysfunction of important organs such as
heart, liver and kidney and iv) severe history of drug allergy. The
diagnosis and staging of patients was based on National
Comprehensive Cancer Network (NCCN) clinical practice guidelines
(11). Among the 128 patients with
NSCLC, 124 (96.9%) were diagnosed with adenocarcinoma, 3 (2.3%)
with adenosquamous carcinoma and 1 (0.8%) with large cell
carcinoma. A total of 125 patients were diagnosed with stage IV
disease, while 3 were diagnosed with stage IIIB disease. In
addition, 111 patients had EGFR mutations according to
next-generation sequencing results and 82 patients underwent a
further biopsy after progression on first-generation EGFR-TKIs. The
details of the patient characteristics and EGFR mutation types are
presented in Tables I and II.
The molecular diagnostic method used in the present
study was next-generation sequencing. First, a clinical pathologist
evaluated the tumour content (≥30%) to confirm the adequacy of the
sample for sequencing. A DNA FFPE Tissue kit (Qiagen GmbH) was used
according to the manufacturer's instructions to extract DNA from
5-µm paraffin sections, and then standard sequencing was used to
analyse the tissues after library preparation, hybrid capture and
library quality assurance (12).
Oral osimertinib (Tagrisso™; AZD9291; AstraZeneca
PLC) was administered at a dose of 80 mg/day until disease
progression (from 0.20–38.6 months, one patient died 0.2 months
after trying osimertinib at the end of the systemic metastatic
disease without T790M detection). Clinical follow-up assessments
were performed, including radiological evaluations at an average of
every 4–6 weeks, physical examinations were performed if patients
were coughing or had another discomfort. Molecular pathological
analyses by next-generation sequencing were performed when disease
progression had been confirmed by CT or MRI scan in every 4–6 weeks
of examination. Experienced investigators evaluated the treatment
response according to the Response Evaluation Criteria In Solid
Tumours 1.1 guidelines (13). The
PFS time of the patients with osimertinib treatment was the main
study end point, and PFS time was defined as the time from the
beginning of osimertinib treatment to disease progression or to the
last follow-up date (October 31, 2018). The efficacy of osimertinib
treatment was represented by the objective response rate (ORR) and
the disease control rate (DCR). The ORR included patients with a
partial response (PR) or complete response (CR), and the DCR
included patients with stable disease (SD), PR or CR.
The Kaplan-Meier method was used to analyse PFS and
overall survival (OS) times, and the median PFS values between
different groups were compared using log-rank tests. Paired
Student's t-test was used to analyse the measurement data in
Table SI, and the χ2
test/Fisher's exact test was used to analyse categorical data.
P<0.05 (two-sided) was considered to indicate a statistically
significant difference. SPSS v22.0 (IBM Corp.) was used to perform
statistical analyses, and survival curves were generated with
GraphPad Prism v7.0 (GraphPad Software, Inc.).
A total of 128 patients with sufficient case report
data were available for analysis. A total of 120 (93.8%) patients
had at least one genetic test, which identified 111 (86.7%)
patients with EGFR mutations. In the present study, 17 patients
still required the use of osimertinib, although those patients did
not have EGFR mutations or the EGFR mutations were not detected.
The presence of an EGFR mutation did not affect the PFS time in the
present study (patients with EGFR mutation vs. patients without
EGFR mutation; median PFS time, 12.2 months and 95% CI, 9.7–14.7,
vs. median PFS time, 11.6 months and 95% CI, 7.7–15.5,
respectively; P=0.369) (Fig. S1).
The DCR was 81.3% (104/128), with 78 patients (60.9%) achieving PR.
The median PFS of all the patients was 12.17 months (95% CI,
10.6–13.8). A total of 45 (35.2%) patients died, and the median OS
was 29.33 months (95% CI, 27.1–31.6) (Fig. S2). The osimertinib treatment summary
is shown in Table III.
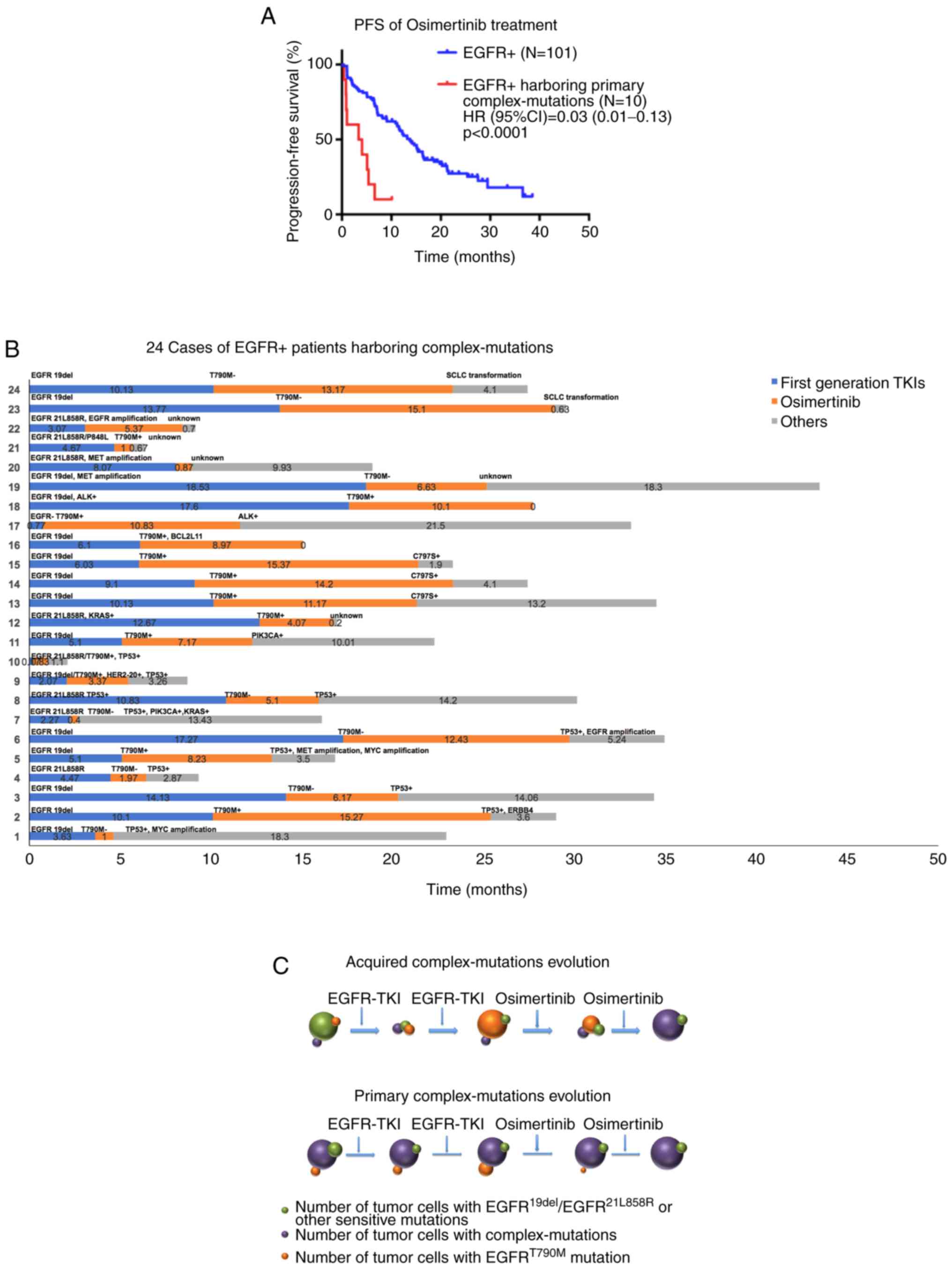
Among the 111 patients with EGFR mutations,
additional complex mutations were identified in 24 patients. Ten of
them had primary complex mutations (before the use of osimertinib)
and 14 of them had acquired complex mutations (after the use of
osimertinib). These additional complex mutations included TP53
mutations (usually in association with other mutations), the C797S
mutation, MYC amplification, EGFR amplification, KRAS or PIK3CA
mutations, SCLC transformation-associated mutations, B-cell
lymphoma 2-like 11 (BIM) deletion polymorphisms, HER2 exon 20
insertions and ALK rearrangements (Fig.
1B). Patients with these primary complex mutations had a
significantly shorter median PFS time compared with those without
the same mutations before the use of osimertinib (3.4 months and
95% CI, 0.0–8.1, vs. 13.7 months and 95% CI, 10.8–16.6,
respectively; P<0.0001; Fig. 1A).
Patients with the acquired complex mutations had a significantly
shorter median PFS time compared with those without the same
mutations after the use of osimertinib (11.2 vs. 14.6 months; 95%
CI, 8.5–13.9 and 95% CI, 10.6–18.6, respectively; P=0.0206;
Fig. S3). These results
demonstrated that these mutations may be responsible for
osimertinib resistance. Our schematic diagram showing different
patterns of the primary/acquired complex-mutations evolution.
(Fig. 1C)
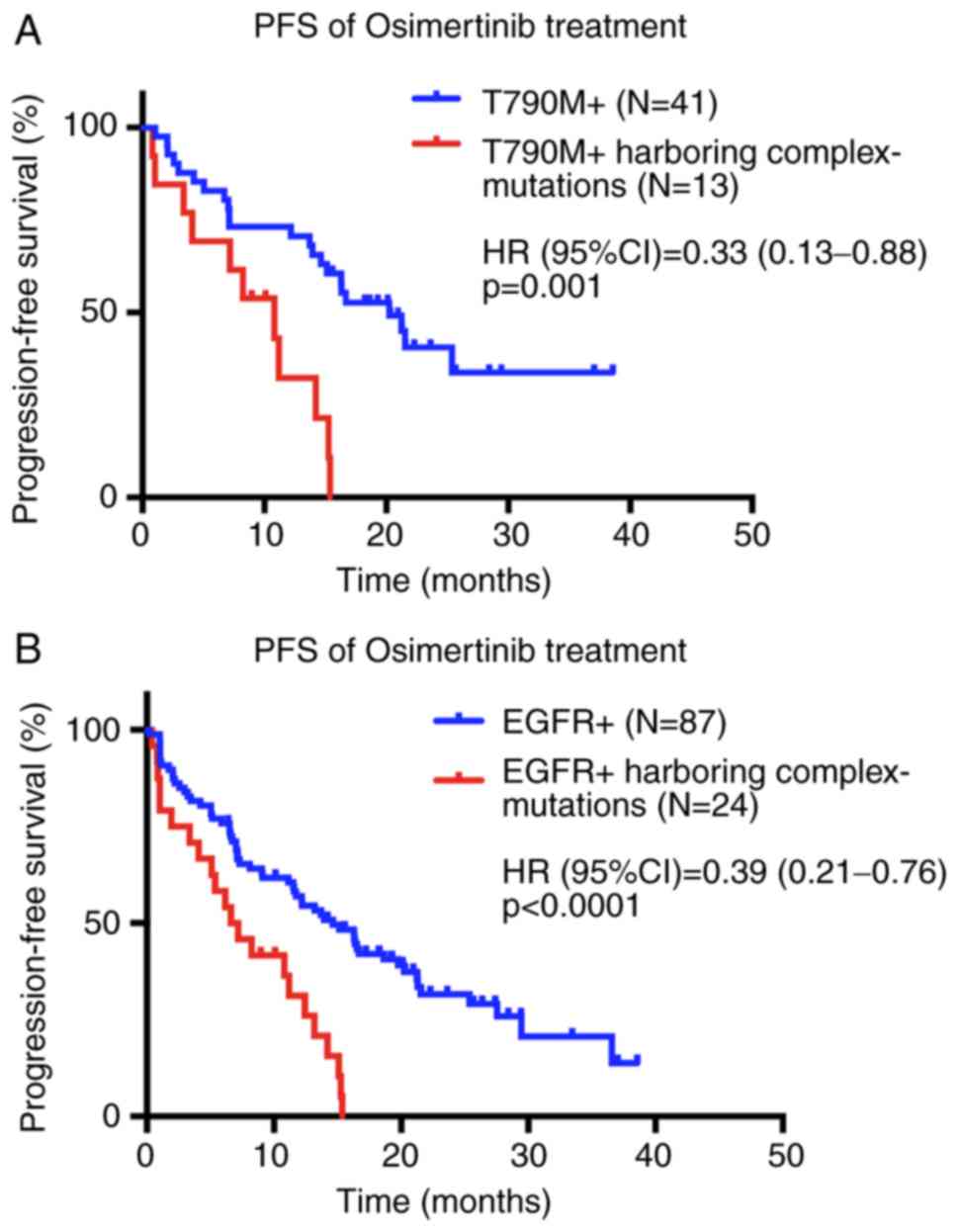
The median PFS time of 41 patients with the EGFR
T790M mutation, in which threonine at position 790 of exon 20 is
replaced by methionine (14), was
significantly longer than that of the 13 patients with the T790M
mutation and the aforementioned complex mutations (20.2 months and
95% CI, 10.5–25.9, vs. 10.8 months and 95% CI, 5.1–16.5,
respectively; P=0.001; Fig. 2A). The
basic characteristics of these 54 patients are shown in Table SI.
Additionally, the median PFS time of the 87 patients
with EGFR mutations was significantly longer than that of the 24
patients with EGFR and complex mutations (14.6 months and 95% CI,
10.62–18.65, vs. 6.6 months and 95% CI, 3.19–10.07, respectively;
P<0.0001; Fig. 2B). The basic
characteristics of these patients are shown in Table SII.
Osimertinib has been recommended by the National
Comprehensive Cancer Network guidelines for untreated patients with
advanced NSCLC with EGFR mutations. However, not all patients with
EGFR mutations benefit from this drug (7,11).
Therefore, it is important to distinguish patients that will
benefit from osimertinib from those that will be resistant.
There are numerous other EGFR-related mutations
associated with resistance mechanisms, such as the EGFR P848L
mutation (18–22), EGFR amplification (23,24), MET
amplification (25,26), HER2 mutations (27,28),
PIK3CA mutations (29–31) and mutations activating the RAS-MAPK
signalling pathway (32–35). The present study revealed that a
patient with the EGFR21L858R, P848L/T790M mutations experienced
rapid progression after TKI treatment, including osimertinib.
Therefore, complete genetic testing should be performed when
selecting TKI treatments, and it is important to distinguish
sensitivity mutations from resistance mutations. The present study
suggested that one of the potential resistance mechanisms to
osimertinib was MET amplification, even in the presence of an EGFR
mutation. Patients with NSCLC with EGFR mutations harbouring MET
amplifications may require a MET inhibitor after osimertinib
progression. A T790M-mutation patient harbouring the HER2 exon 20
insertion displayed a 3.37-month PFS time after osimertinib
treatment in the present study. The HER2 exon 20 insertion is a
factor that may influence the efficacy of EGFR-TKIs.
The aforementioned studies suggest that these
mutations may be important factors limiting the efficacy of
osimertinib. Future studies should investigate novel therapeutic
targets for NSCLC. Dual-targeting antibodies and combination
therapies may provide benefits for patients with EGFR and
additional complex mutations.
In conclusion, the present study investigated the
effects of osimertinib in patients with advanced NSCLC with EGFR
mutations, particularly T790M mutations. The present results
indicate that the efficacy of osimertinib is weakened in patients
with complex mutations, suggesting that complex mutations may be
responsible for the resistance to osimertinib.
Not applicable.
No funding was received.
All data generated or analyzed during this study are
included in this published article.
NC conceived and designed the study, drafted the
manuscript and revised it for important intellectual content. ZL
made substantial contributions to conception and design and given
final approval of the version to be published. JD, LW and ZD
collected and analysed the data. All authors have read and approved
the manuscript.
The present study was approved by the director of
the Department of Oncology at Chinese People's Liberation Army
General Hospital (Beijing, China). The authors were able to review
the patients' case records and obtain relevant information
(Data S1). The patients gave oral
consent to receive the targeted therapy while receiving treatment
and agreed to the researchers reviewing their case records.
The patients referred to in this study provided
consent for the publication of their information.
The authors declare that they have no competing
interests.
|
1
|
Zhang YL, Yuan JQ, Wang KF, Fu XH, Han XR,
Threapleton D, Yang ZY, Mao C and Tang JL: The prevalence of EGFR
mutation in patients with non-small cell lung cancer: A systematic
review and meta-analysis. Oncotarget. 7:78985–78993. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Massarelli E, Johnson FM, Erickson HS,
Wistuba II and Papadimitrakopoulou V: Uncommon epidermal growth
factor receptor mutations in non-small cell lung cancer and their
mechanisms of EGFR tyrosine kinase inhibitors sensitivity and
resistance. Lung Cancer. 80:235–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sequist LV, Waltman BA, Dias-Santagata D,
Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger
S, Cosper AK, et al: Genotypic and histological evolution of lung
cancers acquiring resistance to EGFR inhibitors. Sci Transl Med.
3:75ra262011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu HA, Arcila ME, Rekhtman N, Sima CS,
Zakowski MF, Pao W, Kris MG, Miller VA, Ladanyi M and Riely GJ:
Analysis of tumor specimens at the time of acquired resistance to
EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers.
Clin Cancer Res. 19:2240–2247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pao W, Miller V, Zakowski M, Doherty J,
Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, et al:
EGF receptor gene mutations are common in lung cancers from ‘never
smokers’ and are associated with sensitivity of tumors to gefitinib
and erlotinib. Proc Natl Acad Sci USA. 101:13306–13311. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Soria JC, Ohe Y, Vansteenkiste J,
Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura
F, Nogami N, Kurata T, et al FLAURA Investigators, : Osimertinib in
Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl
J Med. 378:113–125. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cho BC, Chewaskulyong B, Lee KH,
Dechaphunkul A, Sriuranpong V, Imamura F, Nogami N, Kurata T,
Okamoto I, Zhou C, et al: Osimertinib versus Standard of Care EGFR
TKI as First-Line Treatment in Patients with EGFRm Advanced NSCLC:
FLAURA Asian Subset. J Thorac Oncol. 14:99–106. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okamoto I, Morita S, Tashiro N, Imamura F,
Inoue A, Seto T, Yamamoto N, Ohe Y, Nakagawa K and Fukuoka M: Real
world treatment and outcomes in EGFR mutation-positive non-small
cell lung cancer: Long-term follow-up of a large patient cohort.
Lung Cancer. 117:14–19. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thress KS, Paweletz CP, Felip E, Cho BC,
Stetson D, Dougherty B, Lai Z, Markovets A, Vivancos A, Kuang Y, et
al: Acquired EGFR C797S mutation mediates resistance to AZD9291 in
non-small cell lung cancer harboring EGFR T790M. Nature Medicine.
21:560–562. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
National Comprehensive Cancer Network
(NCCN), . NCCN Guidelines® Insights - Non-Small Cell
Lung Cancer, Version 5. 2018.
|
|
12
|
Wang S, Yan B, Zhang Y, Xu J, Qiao R, Dong
Y, Zhang B, Zhao Y, Zhang L, Qian J, et al: Different
characteristics and survival in non-small cell lung cancer patients
with primary and acquired EGFR T790M mutation. Int J Cancer.
144:2880–2886. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao J, Li HR, Jin C, Jiang JH and Ding JY:
Strategies to overcome acquired resistance to EGFR TKI in the
treatment of non-small cell lung cancer. Clin Transl Oncol.
21:1287–1301. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Niederst MJ, Hu H, Mulvey HE, Lockerman
EL, Garcia AR, Piotrowska Z, Sequist LV and Engelman JA: The
Allelic Context of the C797S Mutation Acquired upon Treatment with
Third-Generation EGFR Inhibitors Impacts Sensitivity to Subsequent
Treatment Strategies Cancer Therapy. Clin Cancer Res. doi:
10.1158/1078-0432.CCR-15-0560.
|
|
16
|
Jia Y, Yun CH, Park E, Ercan D, Manuia M,
Juarez J, Xu C, Rhee K, Chen T, Zhang H, et al: Overcoming
EGFR(T790M) and EGFR(C797S) resistance with mutant-selective
allosteric inhibitors. Nature. 534:129–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Uchibori K, Inase N, Araki M, Kamada M,
Sato S, Okuno Y, Fujita N and Katayama R: Brigatinib combined with
anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated
non-small-cell lung cancer. Nat Commun. 8:147682017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamamoto H, Yatabe Y and Toyooka S:
Inherited lung cancer syndromes targeting never smokers. Transl
Lung Cancer Res. 7:498–504. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han B, Zhou X, Zhang RX, Zang WF, Chen ZY,
Song HD, Wan HY and Zheng CX: Mutations of the epidermal growth
factor receptor gene in NSCLC patients. Oncol Lett. 2:1233–1237.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng D, Hu M, Bai Y, Zhu X, Lu X, Wu C,
Wang J, Liu L, Wang Z, Ni J, et al: EGFR G796D mutation mediates
resistance to osimertinib. Oncotarget. 8:49671–49679. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Menon R, Müller J, Schneider P, Lakis S,
Thress K, Wolf J, Heukamp L, Heuckmann JM and Griesinger F: A novel
EGFR (C797) variant detected in a pleural biopsy specimen from an
osimertinib-treated patient using a comprehensive hy- brid
capture-based next-generation sequencing assay(J). J Thorac Oncol.
11:e105–e107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bersanelli M, Minari R, Bordi P, Gnetti L,
Bozzetti C, Squadrilli A, Lagrasta CA, Bottarelli L, Osipova G,
Capelletto E, et al: L718Q mutation as new mechanism of acquired
resistance to AZD9291 in EG- FR- mutated NSCLC(J). J Thorac Oncol.
11:e121–e123. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Knebel FH, Bettoni F, Shimada AK, Cruz M,
Alessi JV, Negrão MV, Reis LFL, Katz A and Camargo AA: Sequential
liquid biopsies reveal dynamic alterations of EGFR driver mutations
and indicate EGFR amplification as a new mechanism of resistance to
osimertinib in NSCLC. Lung Cancer. 108:238–241. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ercan D, Zejnullahu K, Yonesaka K, Xiao Y,
Capelletti M, Rogers A, Lifshits E, Brown A, Lee C, Christensen JG,
et al: Amplification of EGFR T790M causes resistance to an
irreversible EGFR inhibitor. Oncogene. 29:2346–2356. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Li L, Han R, Jiao L, Zheng J and
He Y: Clinical analysis by next-generation sequencing for NSCLC
patients with MET amplification resistant to osimertinib. Lung
Cancer. 118:105–110. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ou SI, Agarwal N and Ali SM: High MET
amplification level as a resistance mechanism to osimertinib
(AZD9291) in a patient that symptomatically responded to crizotinib
treatment post-osimertinib progression. Lung Cancer. 98:59–61.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Planchard D, Loriot Y, André F, Gobert A,
Auger N, Lacroix L and Soria JC: EGFR-independent mechanisms of
acquired resistance to AZD9291 in EGFR T790M-positive NSCLC
patients. Ann Oncol. 26:2073–2078. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu S, Li S, Hai J, Wang X, Chen T, Quinn
MM, Gao P, Zhang Y, Ji H, Cross DAE, et al: Targeting HER2
Aberrations in Non-Small Cell Lung Cancer with Osimertinib. Clin
Cancer Res. 24:2594–2604. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oxnard GR, Thress K, Paweletz C, Stetson
D, Dougherty Lai BZ, Markovets A, Felip AE, Vivancos A, Kuang Y, et
al: ORAL17.07-Mechanisms of Acquired Resistance to AZD9291 in EGFR
T790M Positive Lung Cancer. Proceedings of the WCLC 2015 - 16th
World Conference on Lung Cancer (Denver, CO). 2015.
|
|
30
|
Chaft JE, Arcila ME, Paik PK, Lau C, Riely
GJ, Pietanza MC, Zakowski MF, Rusch V, Sima CS, Ladanyi M, et al:
Coexistence of PIK3CA and other oncogene mutations in lung
adenocarcinoma-rationale for comprehensive mutation profiling. Mol
Cancer Ther. 11:485–491. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ludovini V, Bianconi F, Pistola L, Chiari
R, Minotti V, Colella R, Giuffrida D, Tofanetti FR, Siggillino A,
Flacco A, et al: Phosphoinositide-3-kinase catalytic alpha and KRAS
mutations are important predictors of resistance to therapy with
epidermal growth factor receptor tyrosine kinase inhibitors in
patients with advanced non-small cell lung cancer. J Thorac Oncol.
6:707–715. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ho CC, Liao WY, Lin CA, Shih JY, Yu CJ and
Yang JC: Acquired BRAF V600E Mutation as Resistant Mechanism after
Treatment with Osimertinib. J Thorac Oncol. 12:567–572. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Del Re M, Tiseo M, Bordi P, D'Incecco A,
Camerini A, Petrini I, Lucchesi M, Inno A, Spada D, Vasile E, et
al: Contribution of KRAS mutations and c.2369C > T (p.T790M)
EGFR to acquired resistance to EGFR-TKIs in EGFR mutant NSCLC: A
study on circulating tumor DNA. Oncotarget. 8:13611–13619. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eberlein CA, Stetson D, Markovets AA,
Al-Kadhimi KJ, Lai Z, Fisher PR, Meador CB, Spitzler P, Ichihara E,
Ross SJ, et al: Acquired Resistance to the Mutant-Selective EGFR
Inhibitor AZD9291 Is Associated with Increased Dependence on RAS
Signaling in Preclinical Models. Cancer Res. 75:2489–2500. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim JY, Welsh EA, Fang B, Bai Y, Kinose F,
Eschrich SA, Koomen JM and Haura EB: Phosphoproteomics Reveals MAPK
Inhibitors Enhance MET- and EGFR-Driven AKT Signaling in
KRAS-Mutant Lung Cancer. Mol Cancer Res. 14:1019–1029. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Piotrowska Z, Niederst MJ, Karlovich CA,
Wakelee HA, Neal JW, Mino-Kenudson M, Fulton L, Hata AN, Lockerman
EL, Kalsy A, et al: Heterogeneity Underlies the Emergence of
EGFRT790 Wild-Type Clones Following Treatment of T790M-Positive
Cancers with a Third-Generation EGFR Inhibitor. Cancer Discov.
5:713–722. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hirakawa H, Komiya K, Nakashima C, Ogusu
S, Nakamura T, Tanaka M, Takahashi K, Egashira Y, Kai K, Kimura S,
et al: A case of osimertinib-resistant lung adenocarcinoma
responded effectively to alternating therapy. Ann Transl Med.
6:4642018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Minari R, Bordi P, Del Re M, Facchinetti
F, Mazzoni F, Barbieri F, Camerini A, Comin CE, Gnetti L, Azzoni C,
et al: Primary resistance to osimertinib due to SCLC
transformation: Issue of T790M determination on liquid re-biopsy.
Lung Cancer. 115:21–27. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li X, Wang S, Li B, Wang Z, Shang S, Shao
Y, Sun X and Wang L: BIM Deletion Polymorphism Confers Resistance
to Osimertinib in EGFR T790M Lung Cancer: A Case Report and
Literature Review. Target Oncol. 13:517–523. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tanimoto A, Takeuchi S, Arai S, Fukuda K,
Yamada T, Roca X, Ong ST and Yano S: Histone Deacetylase 3
Inhibition Overcomes BIM Deletion Polymorphism-Mediated Osimertinib
Resistance in EGFR-Mutant Lung Cancer. Clin Cancer Res.
23:3139–3149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Iwama E, Sakai K, Azuma K, Harada D,
Nosaki K, Hotta K, Nishio M, Kurata T, Fukuhara T, Akamatsu H, et
al: Exploration of resistance mechanisms for epidermal growth
factor receptor-tyrosine kinase inhibitors based on plasma analysis
by digital polymerase chain reaction and next-generation
sequencing. Cancer Sci. 109:3921–3933. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Labbé C, Cabanero M, Korpanty GJ, Tomasini
P, Doherty MK, Mascaux C, Jao K, Pitcher B, Wang R, Pintilie M, et
al: Prognostic and predictive effects of TP53 co-mutation in
patients with EGFR-mutated non-small cell lung cancer (NSCLC). Lung
Cancer. 111:23–29. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ou SI, Cui J, Schrock AB, Goldberg ME, Zhu
VW, Albacker L, Stephens PJ, Miller VA and Ali SM: Emergence of
novel and dominant acquired EGFR solvent-front mutations at Gly796
(G796S/R) together with C797S/R and L792F/H mutations in one EGFR
(L858R/T790M) NSCLC patient who progressed on osimertinib. Lung
Cancer. 108:228–231. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jang J, Son JB, To C, Bahcall M, Kim SY,
Kang SY, Mushajiang M, Lee Y, Jänne PA, Choi HG, et al: Discovery
of a potent dual ALK and EGFR T790M inhibitor. Eur J Med Chem.
136:497–510. 2017. View Article : Google Scholar : PubMed/NCBI
|