Introduction
Cancer, one of the top ten malignant diseases, is a
severe threat to all human beings (1). World Health Organization (WHO) and
International Agency for Research on Cancer (IARC) concluded that
there were 14.09 million new cases of malignant tumors such as
breast, lung and colorectal cancer, and 8.2 million deaths in 2012
(2). Approximately 1.2 million women
are diagnosed with breast cancer every year in the world, and about
500,000 women die of breast cancer every year (3). In 2012, in China there were about
190,000 women diagnosed with breast cancer, ranking first in the
incidence of female diseases, and 50,000 deaths, ranking sixth. It
is the most common with the highest incidence malignant tumors
among women in the world (4), and
the second cause of death of female cancer in the world (5). Moreover, in recent years, the incidence
of breast cancer is still rising (6,7) and the
proportion of the incidence and death of all malignant tumors in
women worldwide is also increasing (8,9).
Although China has a low incidence of breast cancer, the burden of
breast cancer in China is still increasing (10). At present, the sensitivity and
specificity of clinical markers, such as CEA and CA19-9, are not
high. In recent years, many studies have shown the value of serum
miRNA in tumor diagnosis (11).
miRNA is involved in the regulation of various
biological behaviors and has been found to be a target for
diagnosis and treatment of various diseases (12). A previous study pointed out that
miRNA can be used for disease diagnosis, treatment monitoring and
predicting prognosis (13).
MicroRNAs (miRNAs) are found in many organisms such as animals,
plants and viruses. They are a class of endogenous non-coding small
RNAs (14) with a length of about 22
nt. It has been shown in literature that miRNAs can be involved as
proto-oncogenes or tumor suppressor genes in the development and
progression of tumors (15,16). Studies have also found that miRNAs
can participate in the regulation of their downstream target genes
in the case of multidrug resistance (MDR) (17,18). As
tumor-associated miRNAs in many cancers such as breast, gastric,
bladder, ovarian, liver cancer and oral squamous cell carcinoma,
miR-129-5p (19–21) and miR-433 (22,23) can
affect proliferation, invasion, migration and apoptosis of many
cancer cells, and can also regulate chemotherapeutic resistance.
Studies have shown the mechanism of action of miR-433 and
miR-129-5p in breast cancer (24,25), but
miR-433 and miR-129-5p have rarely been studied in the diagnosis of
breast cancer.
In the present study, the expression of miR-129-5p
and miR-433 in breast cancer was detected to study the relationship
between the expression levels of miR-129-5p, miR-433 and
clinicopathological characteristics, and to explore the
relationship between the expression levels of miR-129-5p and
miR-433 in the blood of breast cancer patients with different
clinical stages and differentiation. The values of single and
combined diagnosis of miR-129-5p and miR-433 in breast cancer were
compared to provide a theoretical basis for early diagnosis and
treatment of breast cancer.
Patients and methods
General information
Seventy-eight patients with breast cancer diagnosed
in Zhengzhou Central Hospital Affiliated to Zhengzhou University
(Zhengzhou, China) from February 2016 to September 2017 were
enrolled in the research group, aged 58.52±7.96 years. There were
16 cases highly differentiated, 45 cases moderately differentiated
and 17 cases poorly differentiated. Clinical stages, 16 cases in
stage I, 25 cases in stage II, 21 cases in stage III and 16 cases
in stage IV. Seventy-two healthy people who received physical
examination in the same period were included in the control group,
aged 57.98±8.57 years.
Inclusion criteria: Patients with complete clinical
and pathological data; patients without neoadjuvant chemotherapy,
radiotherapy and immunotherapy; patients who received three routine
examinations, liver and kidney function, electrocardiogram and
other routine examinations; patients who were diagnosed with breast
cancer by postoperative pathological reports; patients who were
accompanied by family members at the time of admission.
Exclusion criteria: Patients with history of mental
illness and family history of mental illness; patients with
autoimmune deficiency; patients with history of severe organ
disease, craniocerebral trauma, drug dependence; patients with
communication disorder due to aphasia, irritability,
unconsciousness and other factors and cannot cooperate with the
examiner.
This study was approved by the Ethics Committee of
the Zhengzhou Central Hospital Affiliated to Zhengzhou University.
The patients and their families were given a detailed description
of the experimental procedures prior to the study, and a complete
informed consent was signed by the patients and/or guardians.
Blood collection
A total of 4 ml peripheral blood on an empty stomach
was collected in the morning from patients in the research group
and put into an anticoagulant tube. Fasting peripheral blood (4 ml)
was also collected from the controls in the morning on the day of
physical examination and was put into an anticoagulant tube. After
coagulation for 60 min at 20–25°C, it was centrifuged at 1,006.2 ×
g for 10 min, with a radius of 10 cm at 4°C, the upper serum was
separated for storage, avoiding repeated freezing and thawing.
Laboratory instruments and agents
TRIzol kit (Shanghai Mingjing Biology Co., Ltd.;
5003050), DNase 1 (Shanghai Hengfeng Biotechnology Co., Ltd.;
K003399P), cDNA reverse transcription kit (Shanghai Jianqiao
Technology Co., Ltd.; 4368814), ultraviolet spectrophotometer
(Shanghai Hengfei Biotechnology Co., Ltd.; UV-1100), fluorescence
quantitative PCR kit (Beijing Baioleibo Technology Co., Ltd.;
ALH190-UBN), and real-time PCR detector (Agilent Technologies Co.
Ltd.; MX3000P).
Fluorescence quantitative polymerase
chain reaction (RT-PCR) experimental procedures
Total RNA in serum was extracted in accordance with
the instructions of TRIzol kit. Template RNA was digested by DNase
I (RNA free) to eliminate genomic DNA contamination. The purity and
concentration were determined by ultraviolet spectrophotometer, and
the integrity of RNA was detected by 1.5% agarose gel
electrophoresis. The concentration of RNA was adjusted to 500
ng/μl, and RNA samples were reverse transcribed into cDNAs
by using reverse transcriptase in strict accordance with the
instructions. RT-PCR system was 20 μl, 2× Ultra SYBR-Green
one step Qrt-PCR buffer 10 μl, RNA template 2 μl,
nuclease-free water 5.5 μl, 1 μl of upstream and
downstream primers, and super enzyme mix 0.5 μl. RT-PCR
reaction conditions were: Pre-denaturation at 95°C for 10 min,
denaturation at 95°C for 15 sec, annealing, and extension at 60°C
for 1 min, for a total of 40 cycles. The primers in this experiment
were designed by Primer Premier 5.0 (Premier Biosoft International)
primer design software, and generated by Tianjin Sell Biotechnology
Co., Ltd. U6 was used as an internal reference. The specific primer
sequences are shown in Table I. The
above system configuration was strictly in accordance with the
instructions. In the results, the number of cycles of the
fluorescent signal cycle Ct is the number of cycles corresponding
to the inflection point at which the background begins to enter the
exponential growth phase during the amplification process. The
relative expression levels of the target gene miR-129-5p and
miR-433 were calculated by 2−ΔCt.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Genes | Upstream
primers | Downstream
primers |
|---|
| miR-129-5p |
5′-CTTTTTGCGGTCTGGGCTTG-3′ |
5′-AACGCTTCACGAATTTGCGT-3′ |
| miR-433 |
5′-GGATCATGATGGGCTCCT-3′ |
5′-CAGTGCGTGTCGTGGAGT-3′ |
| U6 |
5′-CTCGCTTCGGCAGCACA-3′ |
5′-AACGCTTCACGAATTTGC-3′ |
Observation indicators
The basic clinical data of the two groups were
compared. The expression levels of miR-129-5p and miR-433 in
peripheral blood of the research group and the control group were
observed. The relationship between the expression of miR-129-5p,
miR-433 and clinicopathological features was analyzed. The
association between the expression levels of miR-129-5p, miR-433,
the clinical stage, and differentiation of breast cancer was
analyzed.
Statistical analysis
The experimental data were analyzed by SPSS 19.0
software system (IBM Corp.). The counting data were expressed as n
(%), and Chi-square test was used for comparison between groups.
The measured data were expressed as (mean ± SD). Paired t-test was
used to compare the two groups. One-way ANOVA was used to compare
the mean of multiple groups. LSD t-test was used for post-test.
Spearman's correlation coefficient was used to evaluate the
correlation between the expression levels of miR-129-5p, miR-433,
clinical stages and degree of differentiation. The sensitivity and
specificity of single and combined detection were evaluated by the
receiver operating curve (ROC). The diagnostic value of combined
detection of miR-129-5p and miR-433 in breast cancer was analyzed
by binary logistic regression. P<0.05 was considered
statistically significant.
Results
Comparison of general data
General clinical data of age, body mass index,
smoking and drinking, diastolic blood pressure, systolic blood
pressure, white blood cell (WBC), hemoglobin (HB), red blood cell
(RBC) count and platelet (PLT) count were compared between the
research and the control group. There was no difference in general
clinical data between the two groups (P>0.05) (Table II).
 | Table II.Comparison of general clinical data
between the two groups [mean ± SD, n (%)]. |
Table II.
Comparison of general clinical data
between the two groups [mean ± SD, n (%)].
| Variables | Research group
(n=78) | Control group
(n=72) |
t/χ2 | P-value |
|---|
| Age (years) | 58.52±7.96 | 57.98±8.57 | 0.40 | 0.69 |
| BMI
(kg/m2) | 20.22±2.08 | 20.12±2.11 | 0.29 | 0.77 |
| Smoking |
|
| 0.05 | 0.83 |
|
Yes | 24 (30.77) | 21 (29.16) |
|
|
| No | 54 (69.23) | 51 (70.84) |
|
|
| Drinking |
|
| 0.01 | 0.94 |
|
Yes | 21 (26.92) | 19 (26.39) |
|
|
| No | 57 (73.08) | 53 (73.61) |
|
|
| Diastolic pressure
(mmHg) | 77.23±11.13 | 77.56±11.45 | 0.18 | 0.86 |
| Systolic pressure
(mmHg) | 112.34±15.24 | 111.19±16.15 | 0.45 | 0.65 |
| WBC
(×109/l) | 5.59±4.35 | 5.71±3.91 | 0.18 | 0.86 |
| HB (gm/dl) | 12.17±2.11 | 11.69±1.96 | 1.44 | 0.15 |
| PLT
(×109/l) | 153.97±20.91 | 154.27±21.61 | 0.09 | 0.93 |
| RBC (1,012/l) | 4.56±0.41 | 4.64±0.38 | 1.24 | 0.22 |
Comparison of the expression levels of
miR-129-5p and miR-433 in two groups
The expression levels of miR-129-5p and miR-433 in
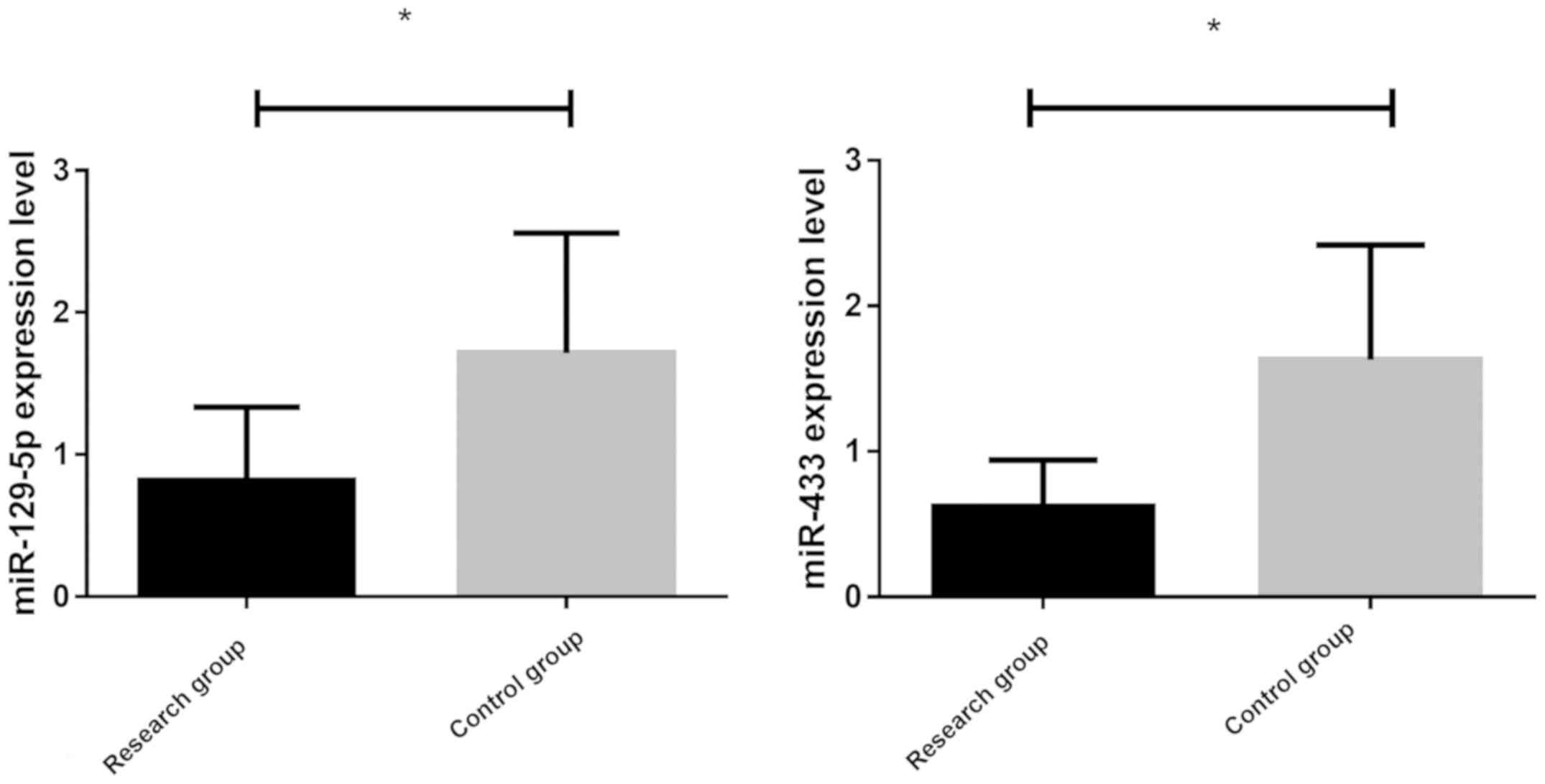
the two groups were detected by RT-PCR (Fig. 1). The expression level of miR-129-5p
in the research group (0.84±0.45) was significantly lower than that
in the control group (1.58±0.89) (P<0.05). The expression level
of miR-433 in the research group (0.59±0.32) was significantly
lower than that in the control group (1.68±0.87) (P<0.05).
Relationship between the expression
levels of miR-129-5p, miR-433 and clinicopathological features in
breast cancer patients
Clinical information of the research group (Table III), indicated that the expression
level of miR-129-5p in the blood of breast cancer patients was not
significantly correlated with age (P>0.05), but it was
correlated with tumor size, differentiation degree, lymph node
metastasis, invasion depth and clinical stage (P<0.05). The
expression level of miR-433 was not significantly correlated with
age or tumor size (P>0.05), but was correlated with
differentiation, lymph node metastasis, depth of invasion and
clinical stage P<0.05).
 | Table III.Relationship between the expression
levels of miR-129-5p, miR-433 and clinicopathological features
(mean ± SD). |
Table III.
Relationship between the expression
levels of miR-129-5p, miR-433 and clinicopathological features
(mean ± SD).
| Variables | Cases | miR-129-5p | F/t | P-value | miR-433 | F/t | P-value |
|---|
| Age, years |
|
| 0.27 | 0.78 |
| 0.40 | 0.69 |
|
<50 | 42 | 0.88±0.49 |
|
| 0.57±0.21 |
|
|
|
≥50 | 36 | 0.91±0.47 |
|
| 0.59±0.23 |
|
|
| Tumor size, cm |
|
| 2.78 | 0.01 |
| 1.01 | 0.31 |
|
<5 | 56 | 1.12±0.44 |
|
| 0.54±0.20 |
|
|
| ≥5 | 22 | 0.82±0.40 |
|
| 0.49±0.19 |
|
|
|
Differentiation |
|
| 53.47 | <0.01 |
| 41.28 | <0.01 |
| High
differentiation | 16 | 1.23±0.36 |
|
| 0.91±0.24 |
|
|
| Medium
differentiation | 45 | 0.93±0.11 |
|
| 0.52±0.21 |
|
|
| Low
differentiation | 17 | 0.55±0.12 |
|
| 0.29±0.10 |
|
|
| Lymph node
metastasis |
|
| 5.45 | <0.01 |
| 5.38 | <0.01 |
|
Yes | 48 | 0.65±0.34 |
|
| 0.38±0.24 |
|
|
| No | 30 | 1.18±0.52 |
|
| 0.69±0.26 |
|
|
| Infiltration
depth |
|
| 6.28 | <0.01 |
| 3.82 | <0.01 |
|
T1+T2 | 28 | 1.08±0.33 |
|
| 0.72±0.27 |
|
|
|
T3+T4 | 50 | 0.61±0.31 |
|
| 0.51±0.21 |
|
|
| Clinical
stages |
|
| 33.42 | <0.01 |
| 79.50 | <0.01 |
| I | 16 | 1.28±0.43 |
|
| 0.91±0.11 |
|
|
| II | 25 | 0.82±0.31 |
|
| 0.71±0.16 |
|
|
|
III | 21 | 0.54±0.11 |
|
| 0.42±0.13 |
|
|
| IV | 16 | 0.40±0.12 |
|
| 0.27±0.11 |
|
|
Correlation between the expression
level of miR-129-5p, miR-433, clinical stage and the degree of
differentiation
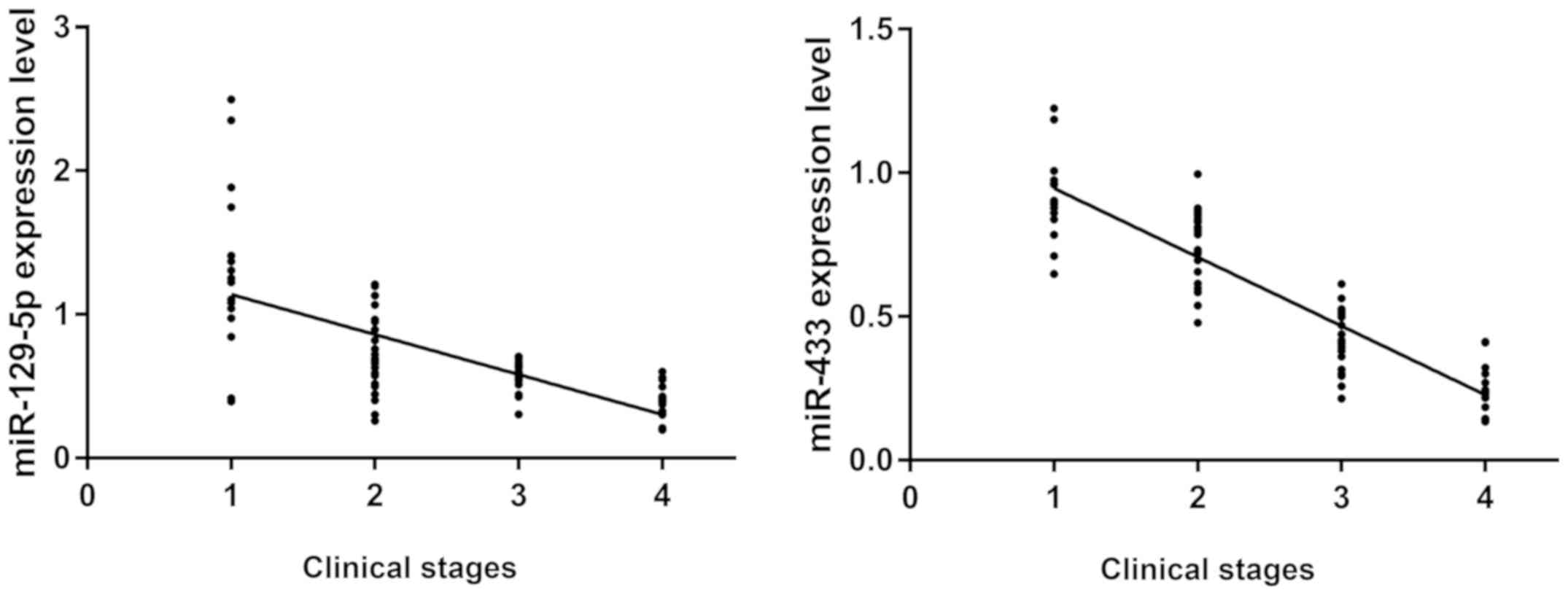
Expression levels of miR-129-5p and miR-433 in the
two groups were detected by RT-PCR, and the correlation between the
expression levels of miR-129-5p, miR-433 and the clinical stage of
breast cancer was analyzed (Fig. 2).
The expression of miR-129-5p and miR-433 was negatively correlated
with clinical stages (r=−0.6595, −0.8947; P<0.05). The
expression levels of miR-129-5p and miR-433 decreased gradually
with the aggravation of patient's condition.
Correlation between the expression
level of miR-129-5p, miR-433 and the degree of differentiation
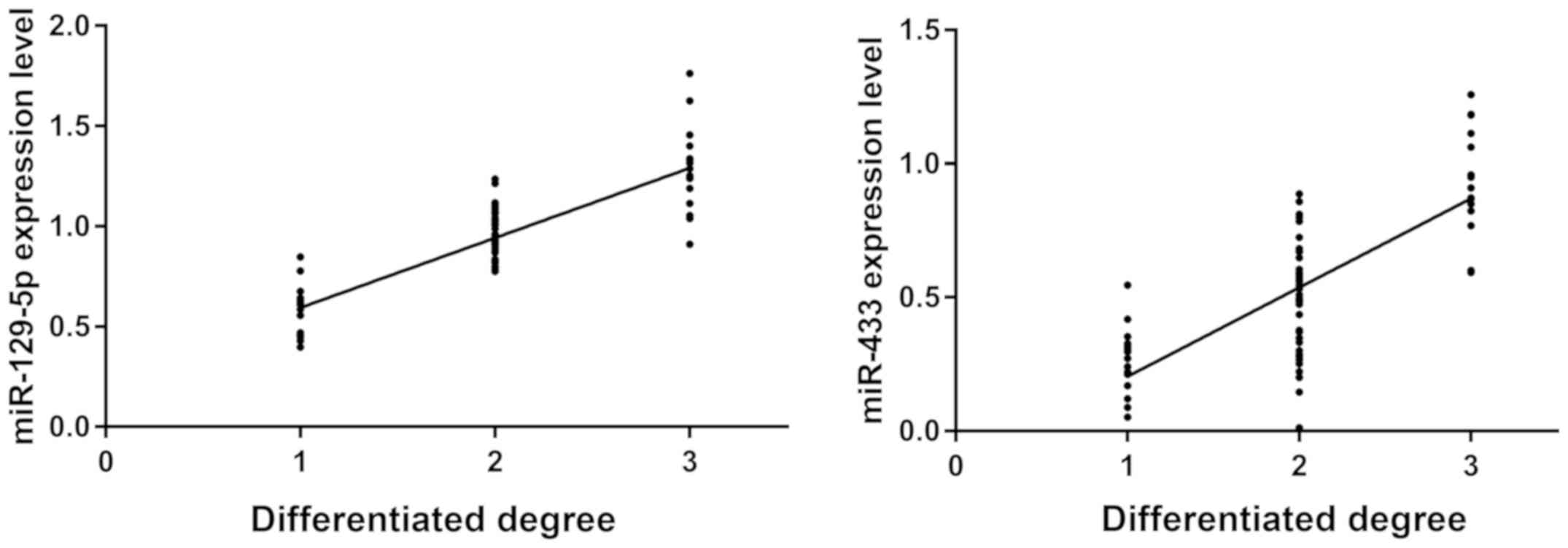
The expression levels of miR-129-5p and miR-433 were
detected by RT-PCR. Correlation between the expression levels of
miR-129-5p, miR-433 and the differentiation of breast cancer cells
was analyzed (Fig. 3). There was a
significant positive correlation between the expression of
miR-129-5p, miR-433 and the differentiation of cancer cells
(r=0.8507, r=0.7522; P<0.05). The higher the differentiation of
cancer cells, the higher the expression levels of miR-129-5p and
miR-433.
Comparison of the value of miR-129-5p
and miR-433 in the diagnosis of breast cancer by single and
combined detection
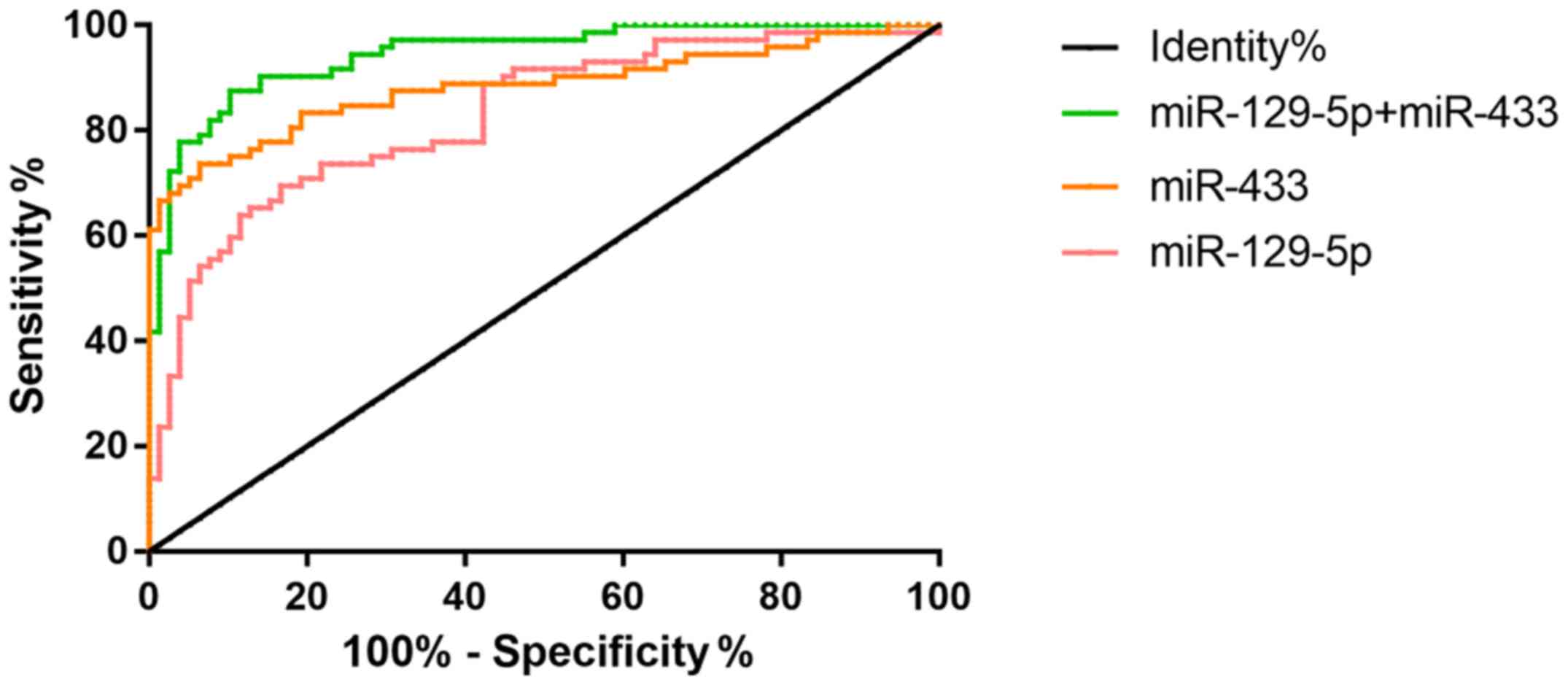
Sensitivity, specificity and AUC of single and
combined detection of miR-129-5p and miR-433 were compared between
the research group and the control group (Table IV). High to low sensitivity was
combined, detection rate (87.5%), miR-433 (73.61%) and miR-129-5p
(69.44%). The specificity from high to low was miR-433 (93.59%),
combined detection (89.74%) and miR-129-5p (83.33%). The highest
level of AUC combined detection was 0.95 (Fig. 4).
 | Table IV.Comparison of the diagnostic value of
single and combined detection of miR-129-5p and miR-433 in breast
cancer. |
Table IV.
Comparison of the diagnostic value of
single and combined detection of miR-129-5p and miR-433 in breast
cancer.
|
|
|
|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Detection | Sensitivity
(%) | Specificity
(%) | Youden index | Optimum critical
value | AUC | P-value | Upper limit | Lower limit |
|---|
| miR-129-5p | 69.44 | 83.33 | 0.03 | >1.30 | 0.83 | <0.01 | 0.89 | 0.76 |
| miR-433 | 73.61 | 93.59 | 0.03 | >1.14 | 0.88 | <0.01 | 0.94 | 0.82 |
|
miR-129-5p+miR-433 | 87.5 | 89.74 | 0.02 | >0.46 | 0.95 | <0.01 | 0.99 | 0.91 |
Association between miR-129-5p,
miR-433 and prognosis of breast cancer patients
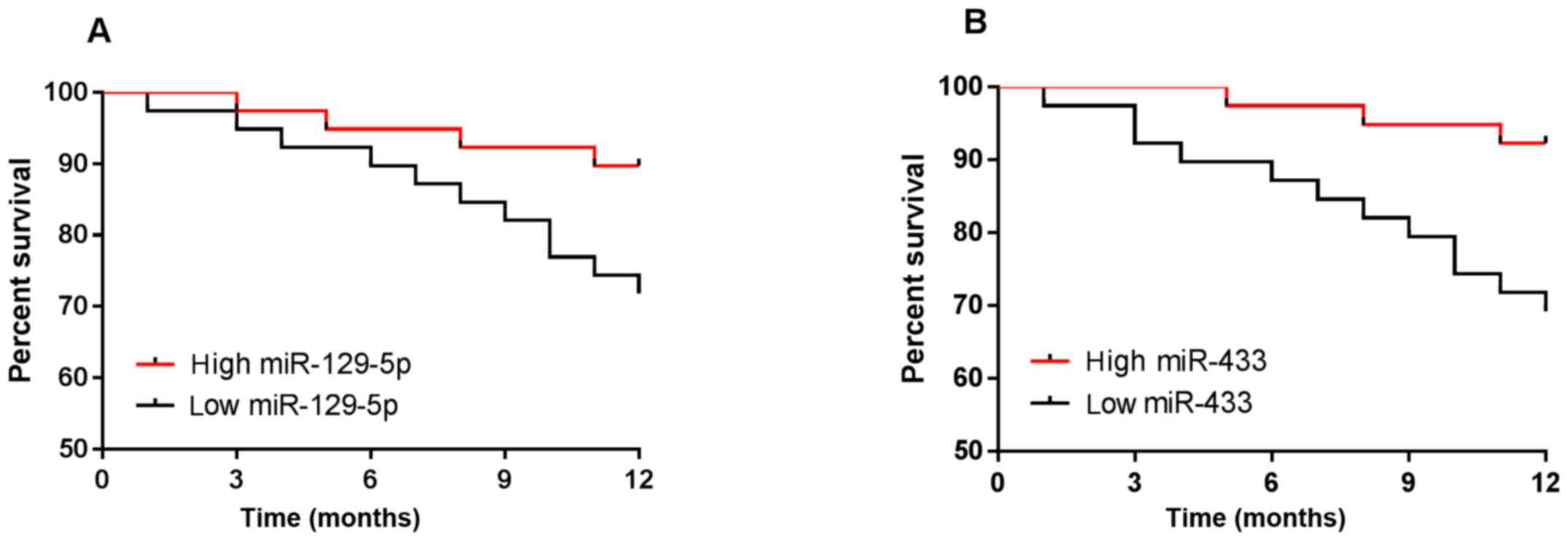
Patients were followed up for 1 year, and no
patients were lost. The results showed that the survival rate of
breast cancer was 80.77%. According to the median values of the
expression levels of miR-129-5p and miR-433, patients were divided
into high-expression groups and low expression groups, and the
survival curve was plotted. miR-129-5p low expression group (n=39)
had lower survival than the high expression group (n=39), and the
miR-433 low expression group (n=39) had lower survival than the
high expression group (n=39) (P<0.05) (Fig. 5).
Discussion
Breast cancer patients often have poor prognosis due
to early noninvasive diagnosis, drug resistance and metastasis in
the treatment of breast cancer (26). Therefore, it is worth exploring the
ideal molecular targets for early diagnosis and targeted therapy of
breast cancer (27,28). MicroRNAs play a very important
regulatory role in the early diagnosis, clinical stages and
prognosis, and in reversing drug resistance of tumors (29).
miR-129-5p was discovered as a tumor-suppressing
microRNA. In the study of Luan et al (30), miR-129-5p expression was low in
breast cancer and regulated multidrug resistance (MDR) in breast
cancer cells. In the present study, the expression level of
miR-129-5p in the research group was significantly lower than that
in the control group (P<0.05). The expression level of
miR-129-5p in the blood of breast cancer patients was positively
correlated with tumor size, degree of differentiation, lymph node
metastasis, invasion depth and clinical stage (P<0.05). It is
positively correlated with the degree of differentiation. The
higher the degree of differentiation, the higher the expression
level, indicating that miR-129-5p plays an important role in the
occurrence and progression of breast cancer. Moreover, it is
negatively correlated with clinical stage. The lower the expression
level, the higher the severity of the disease, indicating that
miR-129-5p can be used to judge the severity of the disease. In the
study by Luo et al (31),
HMGB1 is shown as direct functional target of miR-129-5p in breast
cancer cells. miR-129-5p may inhibit autophagy of breast cancer
cells by targeting HMGB-1, and may also reduce the radiation
resistance of breast cancer cells. It has also been shown that
targeting inhibition of HMGB1 expression by miR-129-5p inhibits the
proliferation and migration of osteosarcoma cells (32) and enhance the sensitivity of breast
cancer MCF-7 and MDA-MB-23l cells to radiotherapy (33). The authors speculate that miR-129-5p
can promote breast cancer cell apoptosis by targeting inhibition of
HMGB1 or similar nuclear proteins, and may also be an important way
to regulate the radiosensitivity of breast cancer cells. The
expression of miR-433 in the research group was significantly lower
than that in the control group (P<0.05). The expression of
miR-433 was correlated with the degree of tumor differentiation,
lymph node metastasis, depth of invasion and clinical stage
(P<0.05). There is a positive correlation with the degree of
differentiation. The higher the degree of differentiation, the
higher the expression level indicating that miR-433 also plays an
important role in the occurrence and progression of breast cancer.
There was a significant negative correlation with clinical stage.
As the condition becomes more serious, the expression level
declines, indicating that miR-433 can be used to judge the severity
of patient's condition. Gotanda et al (34) and Wang et al (35) studied apoptosis of retinoblastoma and
human dental pulp cells induced by miR-433. Zhang et al
(36) revealed the effect of miR-433
on apoptosis, migration and proliferation of breast cancer cells.
The results of the above studies confirmed that Rap1a, as a direct
target gene of miR-433, participates in the function of miR-433.
Rap1a activated the MAPK signaling pathway, promoting cell
migration, proliferation and inhibiting apoptosis. These findings
highlight that miR-433 is an anti-oncogene that regulates the
progression and metastasis potential of breast cancer and may
contribute to the future development of treatment for breast
cancer. Referring to the relevant literature, there are few studies
on the diagnosis of breast cancer by miR-129-5p and miR-433, and no
studies on the combined diagnosis of the two miRs. In this study,
the diagnostic significance of miR-129-5p and miR-433 with single
and combined detection in breast cancer is compared, and it was
found that the sensitivity and specificity of miR-129-5p with
single detection were 73.61 and 83.33%, respectively. The
sensitivity and specificity with single detection of miR-433 were
73.61 and 93.59%, respectively. The sensitivity and specificity in
the combined detection of miR-129-5p and miR-433 were 87.5 and
89.74%, respectively. The optimal thresholds of miR-129-5p and
miR-433 were 1.30 and 1.14, respectively, and the diagnostic
efficiency was the highest at this point. The results suggest that
the combined detection of miR-129-5p and miR-433 is the most
sensitive. The value of the combined detection of miR-129-5p and
miR-433 in the diagnosis of breast cancer was evaluated. ROC curves
were drawn based on the sensitivity and specificity with the single
and combined detection. The larger the AUC, the greater the
diagnostic value. The combined diagnosis AUC (0.95) is larger than
the single diagnosis AUC (0.83, 0.88) (Fig. 4). The above indicates that the value
of the combined detection of miR-129-5p and miR-433 is higher than
the value of its single detection. It has been reported that low
level of miR-129-3p is associated with short-term disease-free
survival and overall survival in patients with renal cell carcinoma
(37). Moreover, Zheng et al
(38) proposed that down-regulation
of miR-433 level may be related to poor prognosis of patients with
gastrointestinal cancer. Therefore, the one-year survival of breast
cancer patients was analyzed in this study. The results showed that
the low expression groups of miR-129-5p and miR-433 had lower
survival than the high expression groups, suggesting that the poor
prognosis of breast cancer patients may be related to the low
expression of miR-129-5p and miR-433.
In the present study, the expression levels of
miR-129-5p and miR-433 in the blood of patients were studied in
many aspects, which provided a reference for clinical research.
There were some limitations in this study. In recent years, there
have been many studies on serum diagnostic indicators since they
are convenient and easy to obtain. Therefore, this study mainly
focused on serology to explore its value. Due to the short
follow-up time, coupled with the differences in the treatment, the
relationship between miR-129-5p and miR-433 and the long-term
survival prognosis of breast cancer patients needs to be further
studied.
In conclusion, the expression levels of miR-129-5p
and miR-433 in peripheral blood of patients with breast cancer are
lower than those of healthy people, and are closely related to the
clinical stage of breast cancer and the degree of differentiation
of cancer cells. It is expected to provide reference for judging
the condition of breast cancer patients and to guide the treatment
of breast cancer. The combined detection of miR-129-5p and miR-433
is of great significance for the diagnosis and treatment of breast
cancer, and will provide a new entry point for targeted treatment
of breast cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JX conceived the study and wrote the manuscript. XZ
and PH conceived and designed the study. YH and YX were responsible
for the collection and analysis of the experimental data. RL and MZ
interpreted the data and drafted the manuscript. JX and PH revised
the manuscript critically for important intellectual content. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Zhengzhou Central Hospital Affiliated to Zhengzhou University
(Zhengzhou, China). Signed informed consents were obtained from the
patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cronin KA, Lake AJ, Scott S, Sherman RL,
Noone AM, Howlader N, Henley SJ, Anderson RN, Firth AU, Ma J, et
al: Annual report to the nation on the status of cancer, part I:
National cancer statistics. Cancer. 124:2785–2800. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yardley DA: Pharmacologic management of
bone-related complications and bone metastases in postmenopausal
women with hormone receptor-positive breast cancer. Breast Cancer
(Dove Med Press). 8:73–82. 2016.PubMed/NCBI
|
|
4
|
Futakuchi M and Singh RK: Animal model for
mammary tumor growth in the bone microenvironment. Breast Cancer.
20:195–203. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shioi Y, Kashiwaba M, Inaba T, Komatsu H,
Sugai T and Wakabayashi G: Long-term complete remission of
metastatic breast cancer, induced by a steroidal aromatase
inhibitor after failure of a non-steroidal aromatase inhibitor. Am
J Case Rep. 15:85–89. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fitzmaurice C, Dicker D, Pain A, Hamavid
H, Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R,
Wolfe C, et al Global burden of disease cancer collaboration, : The
global burden of cancer 2013. JAMA Oncol. 1:505–527. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang ML, Huang ZZ and Zheng Y: Estimates
and prediction on incidence, mortality and prevalence of breast
cancer in China, 2008. Zhonghua Liu Xing Bing Xue Za Zhi.
33:1049–1051. 2012.(In Chinese). PubMed/NCBI
|
|
10
|
Chen WQ and Zheng RS: Incidence, morality
and survival analysis of breast cancer in China. Chin J Clin Oncol.
42:668–674. 2015.(In Chinese).
|
|
11
|
He K, Li WX, Guan D, Gong M, Ye S, Fang Z,
Huang JF and Lu A: Regulatory network reconstruction of five
essential microRNAs for survival analysis in breast cancer by
integrating miRNA and mRNA expression datasets. Funct Integr
Genomics. 19:645–658. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
elife. 2015.4:e05005doi: 10.7554/eLife.05005. View Article : Google Scholar
|
|
14
|
Calin GA, Sevignani C, Dumitru CD, Hyslop
T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M,
et al: Human microRNA genes are frequently located at fragile sites
and genomic regions involved in cancers. Proc Natl Acad Sci USA.
101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peng X, Cao P, He D, Han S, Zhou J, Tan G,
Li W, Yu F, Yu J, Li Z and Cao K: MiR-634 sensitizes nasopharyngeal
carcinoma cells to paclitaxel and inhibits cell growth both in
vitro and in vivo. Int J Clin Exp Pathol. 7:6784–6791.
2014.PubMed/NCBI
|
|
18
|
Ye J, Zhang Z, Sun L, Fang Y, Xu X and
Zhou G: MiR-186 regulates chemo-sensitivity to paclitaxel via
targeting MAPT in non-small cell lung cancer (NSCLC). Mol Biosyst.
12:3417–3424. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Duan L, Hao X, Liu Z, Zhang Y and Zhang G:
MiR-129-5p is down-regulated and involved in the growth, apoptosis
and migration of medullary thyroid carcinoma cells through
targeting RET. FEBS Lett. 588:1644–1651. 2016. View Article : Google Scholar
|
|
20
|
Jiang Z, Wang H, Li Y, Hou Z, Ma N, Chen
W, Zong Z and Chen S: MiR-129-5p is down-regulated and involved in
migration and invasion of gastric cancer cells by targeting
interleukin-8. Neoplasma. 63:673–680. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luan QX, Zhang BG, Li XJ and Guo MY:
MiR-129-5p is downregulated in breast cancer cells partly due to
promoter H3K27m3 modification and regulates epithelial-mesenchymal
transition and multi-drug resistance. Eur Rev Med Pharmacol Sci.
20:4257–4265. 2016.PubMed/NCBI
|
|
22
|
Luo H, Zhang H, Zhang Z, Zhang X, Ning B,
Guo J, Nie N, Liu B and Wu X: Down-regulated miR-9 and miR-433 in
human gastric carcinoma. J Exp Clin Cancer Res. 28:822009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tan Y, Ge G, Pan T, Wen D, Chen L, Yu X,
Zhou X and Gan J: A serum microRNA panel as potential biomarkers
for hepatocellular carcinoma related with hepatitis B virus. PLoS
One. 9:e1079862014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang T, Jiang K, Zhu X, Zhao G, Wu H,
Deng G and Qiu C: miR-433 inhibits breast cancer cell growth via
the MAPK signaling pathway by targeting Rap1a. Int J Biol Sci.
14:622–632. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meng R, Fang J, Yu Y, Hou LK, Chi JR, Chen
AX, Zhao Y and Cao XC: miR-129-5p suppresses breast cancer
proliferation by targeting CBX4. Neoplasma. 65:572–578. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zdenkowski N, Butow P, Tesson S and Boyle
F: A systematic review of decision aids for patients making a
decision about treatment for early breast cancer. Breast. 26:31–45.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu X, Zeng R, Wu S, Zhong J, Yang L and Xu
J: Comprehensive expression analysis of miRNA in breast cancer at
the miRNA and isomiR levels. Gene. 557:195–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
D'Angelo B, Benedetti E, Cimini A and
Giordano A: MicroRNAs: A puzzling tool in cancer diagnostics and
therapy. Anticancer Res. 36:5571–5575. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yan JW, Lin JS and He XX: The emerging
role of miR-375 in cancer. Int J Cancer. 135:1011–1018. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luan QX, Zhang BG, Li XJ and Guo MY:
MiR-129-5p is downregulated in breast cancer cells partly due to
promoter H3K27m3 modification and regulates epithelial-mesenchymal
transition and multi-drug resistance. Eur Rev Med Pharmacol Sci.
20:4,257–4,265. 2016.
|
|
31
|
Luo J, Chen J and He L: miR-129-5p
attenuates irradiation-induced autophagy and secreases
radioresistance of breast cancer cells by targeting HMGB1. Med Sci
Monit. 21:4122–4129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu K, Huang J, Ni J, Song D, Ding M, Wang
J, Huang X and Li W: MALAT1 promotes osteosarcoma development by
regulation of HMGB1 via miR-142-3p and miR-129-5p. Cell Cycle.
16:578–587. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Luo J, Chen J and He L: Mir-129-5p
attenuates irradiation-induced autophagy and decreases
radioresistance of breast cancer cells by targeting HMGB1. Med Sci
Monit. 21:4122–4129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gotanda K, Hirota T, Matsumoto N and Ieiri
I: MicroRNA-433 negatively regulates the expression of thymidylate
synthase (TYMS) responsible for 5-fluorouracil sensitivity in HeLa
cells. BMC Cancer. 13:3692013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang K, Li L, Wu J, Qiu Q, Zhou F and Wu
H: The different expression profiles of microRNAs in elderly and
young human dental pulp and the role of miR-433 in human dental
pulp cells. Mech Ageing Dev. 146-148:1–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang T, Jiang K, Zhu X, Zhao G, Wu H,
Deng G and Qiu C: miR-433 inhibits breast cancer cell growth via
the MAPK signaling pathway by targeting Rap1a. Int J Biol Sci.
14:622–632. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen X, Ruan A, Wang X, Han W, Wang R, Lou
N, Ruan H, Qiu B, Yang H and Zhang X: miR-129-3p, as a diagnostic
and prognostic biomarker for renal cell carcinoma, attenuates cell
migration and invasion via downregulating multiple
metastasis-related genes. J Cancer Res Clin Oncol. 140:1295–1304.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zheng Q, Chen C, Guan H, Kang W and Yu C:
Prognostic role of microRNAs in human gastrointestinal cancer: A
systematic review and meta-analysis. Oncotarget. 8:46611–46623.
2017. View Article : Google Scholar : PubMed/NCBI
|