Introduction
Ovarian cancer (OC) is one of the most fatal
malignancies in gynecologic cancer worldwide owing to its frequent
detection at an advanced stage (1,2). Based
on the statistics from The American Cancer Society in 2013, OC
accounts for 4% of all cancer types diagnosed in women, with an
estimated 22,000 new cases and 14,000 deaths per annum in the
United States alone (3,4). OC has a low 5-year survival rate, and
up to 70% of patients exhibit invasion and metastasis (5). The epithelial-mesenchymal transition
(EMT) and collective cell migration to neighboring tissues are key
steps in OC progression and metastasis, although the precise
underlying mechanisms are unclear (6).
MicroRNAs (miRNAs/miRs) are small, single-stranded
non-coding RNAs that regulate gene expression by binding to
specific target mRNA sequences, thereby degrading mRNAs or
inhibiting their translation (7). A
previous study reported an association between epithelial ovarian
cancer (EOC) and aberrant miRNA regulation (8). Various miRNAs are known to be
dysregulated in the advanced stages of OC, suggesting that they are
involved in malignancy and metastasis (9).
Additional studies have shown that miRNAs can be
secreted into the extracellular space, mostly in the form of
exosomes, and function in intercellular communication (10–12).
Exosomal miRNAs are also important biomarkers of diagnosis and
progression of OC (13). For
instance, the expression of members of the miR-373 and miR-200
families were significantly upregulated in patients with OC
compared with healthy women (14). A
previous study reported that exosomal miR-30a-5p may be a potential
biomarker for OC (15). miR-34b
belongs to the miR-34 family and is directly transactivated by the
p53 tumor suppressor (16). In
general, miR-34b can promote apoptosis and cell cycle arrest
resulting in p53 activation, thereby acting as a mediator of tumor
suppression by p53 (17). Several
studies have shown that miR-34b is downregulated in OC, but its
mechanism of action and precise role in intercellular communication
are poorly understood (18,19).
The present study examined the expression and
biological functions of exosomal miR-34b and investigated the
mechanisms underlying its role in OC.
Materials and methods
Cell culture
The human ovarian surface epithelium cell line,
IOSE-80 and the OC cell lines SKOV3, A2780 and OVCAR3 were
purchased from the Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences, and cultured in RPMI-1640 medium
(Invitrogen; Thermo Fisher Scientific, Inc.) containing 10% FBS,
100 units/ml penicillin and 100 µg/ml streptomycin in 5%
CO2 at 37°C.
Exosome purification
Exosomes were isolated from the culture medium by
differential centrifugation. Briefly, the conditioned medium was
centrifuged at 1,500 × g for 20 min at 4°C to separate the cells,
followed by centrifugation at 12,000 × g for 35 min at room
temperature and filtration through a 0.22-µm filter to remove cell
debris. Exosomes were pelleted by ultracentrifugation at 120,000 ×
g for 90 min at room temperature, and washed with PBS.
Subsequently, the exosomes were pelleted at 120,000 × g for 70 min
at room temperature and resuspended in 100 µl of PBS.
Nanoparticle tracking analysis
(NTA)
The Nanosight NTA NS300 (Malvern Instruments, Inc.)
was used to identify the concentration and size of isolated
exosomes by tracking the Brownian motion of particles. The samples
were captured for 60 sec at room temperature with manual
shutter.
Electron microscopy
For electron microscopy analysis, exosomes isolated
from the cell line, IOSE-80 dissolved in PBS were loaded to
carbon-coated nickel grids and negatively stained with 2%
methylamine tungstate (Sigma-Aldrich, Inc; Merck KGaA) at 37°C for
10 min. After drying at room temperature for 20 min, the samples
were viewed using a JEM-1230 transmission electron microscope
(Nikon Corporation) at 80 kV.
RNA preparation and reverse
transcription quantitative (RT-q)PCR
Total RNA was extracted from cells or using
TRIzol® reagent (Takara Biotechnology Co., Ltd).
Briefly, 1 µg of total RNA was reverse transcribed into cDNA using
the Mir-X™ miRNA FirstStrand Synthesis kit (Takara Biotechnology
Co., Ltd.) at 37°C for 60 min and then 85°C for 5 sec. qPCR
analyses were conducted using the SYBR Prime Script miRNA RT-PCR
kit (Takara Biotechnology Co., Ltd), following the manufacturers
procedure. All reagents used for RT-qPCR were obtained from Takara
Biotechnology Co., Ltd. The universal miRNA Reverse and U6 primers
were also obtained from Takara Biotechnology Co., Ltd. The miR-34b,
the universal miRNA Reverse, U6 and mRNA primer sequences are shown
in Table I.
 | Table I.Primer sequences used in reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used in reverse
transcription-quantitative PCR.
| Gene | Primer sequence (5′
→ 3′) |
|---|
| miR-34b |
|
|
Forward |
CAATCACTAACTCCACTGCCAT |
| U6 |
|
| Forward |
CGCAAGGATGACACGCAAATTCG |
| Universal
Reverse |
CCAGTGCAGGGTCCGAGGT |
| Notch2 |
|
|
Forward |
GGGACCCTGTCATACCCTCT |
|
Reverse |
GAGCCATGCTTACGCTTTCG |
| E-cadherin |
|
|
Forward |
AGGCTAGAGGGTCACCGCGTC |
|
Reverse |
GCTTTGCAGTTCCGACGCCAC |
| N-cadherin |
|
|
Forward |
AGTCAACTGCAACCGTGTGT |
|
Reverse |
AGCGTTCCTGTTCCACTCAT |
| Snail |
|
|
Forward | CCTCCC
TGTCAGATGAGGAC |
|
Reverse | CCAGG
CTGAGGTATTCCTTG |
| β-actin |
|
|
Forward |
AGCCATGTACGTAGCCATCC |
|
Reverse |
CTCTCAGCTGTGGTGGTGAA |
Western blot analysis
Proteins were extracted from cells using RIPA lysis
buffer (Beyotime Institute of Biotechnology) and the total protein
content was measured using the bicinchoninic acid assay (Beyotime
Biotechnology, Inc) by Fluoroskan (Thermo Fisher Scientific Inc.).
A total of 20 µg protein per lane was loaded onto a 12% SDS-PAGE
gel. Proteins were then transferred to polyvinylidene difluoride
membranes, and blocked using 5% BSA at room temperature for 1 h.
Then, immunoblotting was performed with primary antibodies against
TSG101 (1:2,000; cat. no. ab125011; Abcam), CD63 (1;2000, cat. no.
sc-5275; Santa Cruz Biotechnology Inc.), Notch2 (cat. no. 5732S),
E-cadherin (cat. no. 14472), N-cadherin (cat. no. 13116), Snail
(cat. no. 3879S) and β-actin (cat. no. 3700S) (1:2,000; all Cell
Signaling Technology, Inc.) at 4°C overnight. After washing, the
membranes were incubated with goat anti-rabbit or anti-mouse
antibody conjugated to horseradish peroxidase (1:2,000; Cell
Signaling Technology) for 1 h at room temperature. An enhanced
chemiluminescence system (Pierce; Thermo Fisher Scientific Inc.)
was used to detect the bands. Densitometry was performed using
ImageJ v1.8.0 software (National Institutes of Health).
Proliferation assay
Cell Counting Kit (CCK)-8 assays (Dojindo Molecular
Technologies, Inc.) were used to evaluate cell viability. Briefly,
exponentially growing cells were counted and seeded in 96-well
plates at 1×104 cells/ well. After 72 h, CCK-8 was added
to each well according to the manufacturers instructions and the
plates were incubated for at 37°C for 2 h. Then, the absorbance was
measured with Fluoroskan (Thermo Fisher Scientific Inc.) at 490
nm.
Wound healing assay
For the wound healing assay, SKOV3 cells were seeded
in 6-cm culture dishes. Following serum starvation for 24 h, a
straight scratch was created using a 200-µl pipette tip and the
wound was visualized under a light microscope (magnification,
×100). Images of cells were captured under the microscope and the
percentage of the scratch healing was calculated.
Transfection
SKOV3 cells were seeded on 6-well plates, and
transfected using TurboFect Transfection Reagent (Thermo Fisher
Scientific, Inc.) according to the manufacturers instructions.
anti-miR-34b (100 nM), miR-34b mimics (50 nM) and small interfering
(si)-Notch2 (100 nM) were used to regulate the expression of
miR-34b and Notch2. Scrambled miR-control and silencer negative
control 1 siRNA were used as negative controls. Cells were
collected 48 h post-transfection for further experimentation. The
sequences used were as follows: miR-34b mimics sense,
5-AGGCAGUGUAAUUAGCUGAUUGU-3 and antisense,
5-AAUCAGCUAAUUACACUGCCUUU-3; anti-miR-34b
5-ACAAUCAGCUAAUUACACUGCCU-3; and si-Notch2 sense,
5-GGAGGUCUCAGUGGAUAUATT-3 and antisense,
5-UAUAUCCACUGAGACCUCCTT-3.
Luciferase reporter assay
SKOV3 cells were evaluated using the recombinant
pMIR-reporter luciferase vector (Guangzhou RiboBio Co., Ltd.) with
a luciferase reporter assay (Promega Corporation). The same
transfection methodology was used as described in the previous
paragraph. Briefly, cells were transfected with the Notch2 3
untranslated region (UTR) wildtype (WT) and Notch2 3UTR mutant
(Mut) plasmids, together with the negative control RNA and miR-34b
mimics using TurboFect Transfection reagent (Thermo Fisher
Scientific, Inc.) in 24-well plates. pRL-CMV luciferase plasmids
served as the internal control. At 48 h after transfection, cells
were harvested and lysed. Luminescence was determined using the
dual-luciferase reporter assay system (Promega Corporation).
Firefly luciferase activity was normalized to Renilla luciferase
activity.
Prediction of targets of miR-34b
Three online prediction algorithms PicTar
(https://pictar.mdc-berlin.de/),
TargetScan (http://www.targetscan.org/vert_71/) and miRDB
(http://mirdb.org/) were used to identified targets
gene of miR-34b and then the common genes selected from the 3
algorithms were identified as the candidates.
Statistical analysis
All data are presented as mean ± standard error of
the mean from three individual experiments. GraphPad Prism version
7 (GraphPad Software) was used for data analyses. Paired Students
t-test and a one-way analysis of variance (ANOVA) with Tukeys post
hoc test were used to assess the differences. Pearsons correlation
analysis was used to analyze the correlation between the expression
of exosomal miR-34b and Notch2 mRNA. P<0.05 was considered to
indicate a statistically significant difference.
Results
Exosomal miR-34b expression is
downregulated in OC cells
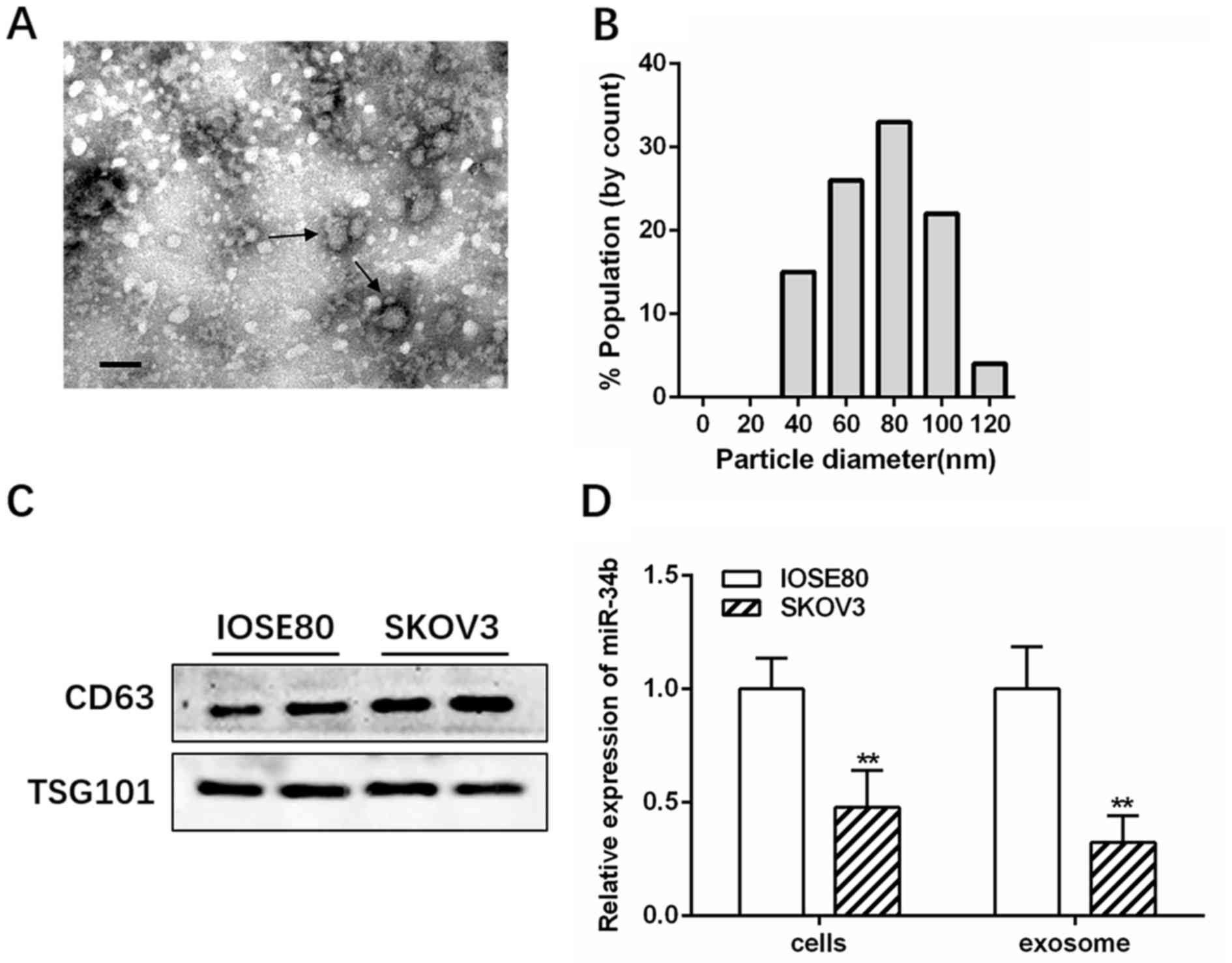
Transmission electron microscopy and NanoSight
analysis showed that IOSE-80 exosomes had a double-layer membrane
and were primarily 40–100 nm in diameter (Fig. 1A and B). Western blotting further
confirmed the presence of exosomes by identifying the
well-established markers, CD63 and TSG101 (Fig. 1C). RT-qPCR revealed that miR-34b was
highly expressed in the IOSE-80 cell line, and had decreased levels
in SKOV3 cells compared with several other OC cell lines (A2780 and
OVCAR3) (Fig. S1; *P<0.05;
**P<0.01). Therefore, IOSE-80 and SKOV3 cells were selected for
further study. The RT-qPCR results showed that miR-34b levels were
significantly lower in SKOV3-derived exosomes (SK-Exos) compared
with those in the IOSE-80-derived exosomes (IO-Exos), consistent
with the cellular expression (Fig.
1D; **P<0.01).
Exosomal miR-34b attenuates
proliferation and EMT in ovarian cancer
RT-qPCR revealed that miR-34b expression was
significantly upregulated in SKOV3 co-cultured with IOSE-80 cells
compared with that of SKOV3 cells alone (Fig. 2A; **P<0.01), suggesting that
miR-34b containing exosomes were delivered from IOSE-80 cells in
the upper Transwell chamber to the recipient SKOV3 cells in the
lower chamber. RT-qPCR also revealed anti-miR-34b transfection
significantly decreased miR-34b expression in exosomes compared
with IOSE-80 exosomes (NC-Exos) cells (Fig. S2). The CCK 8 and wound healing
assays indicated that IO-Exos markedly attenuated the proliferation
and invasion of SKOV3 cells, while exosomes derived from
miR-34b-antagonized IOSE-80 cells decreased IOSE-80
(IO)-Exos-induced target cell proliferation and invasion (Fig. 2B and C; #P<0.05;
##P<0.01; $$P<0.01 and
$$$P<0.001).
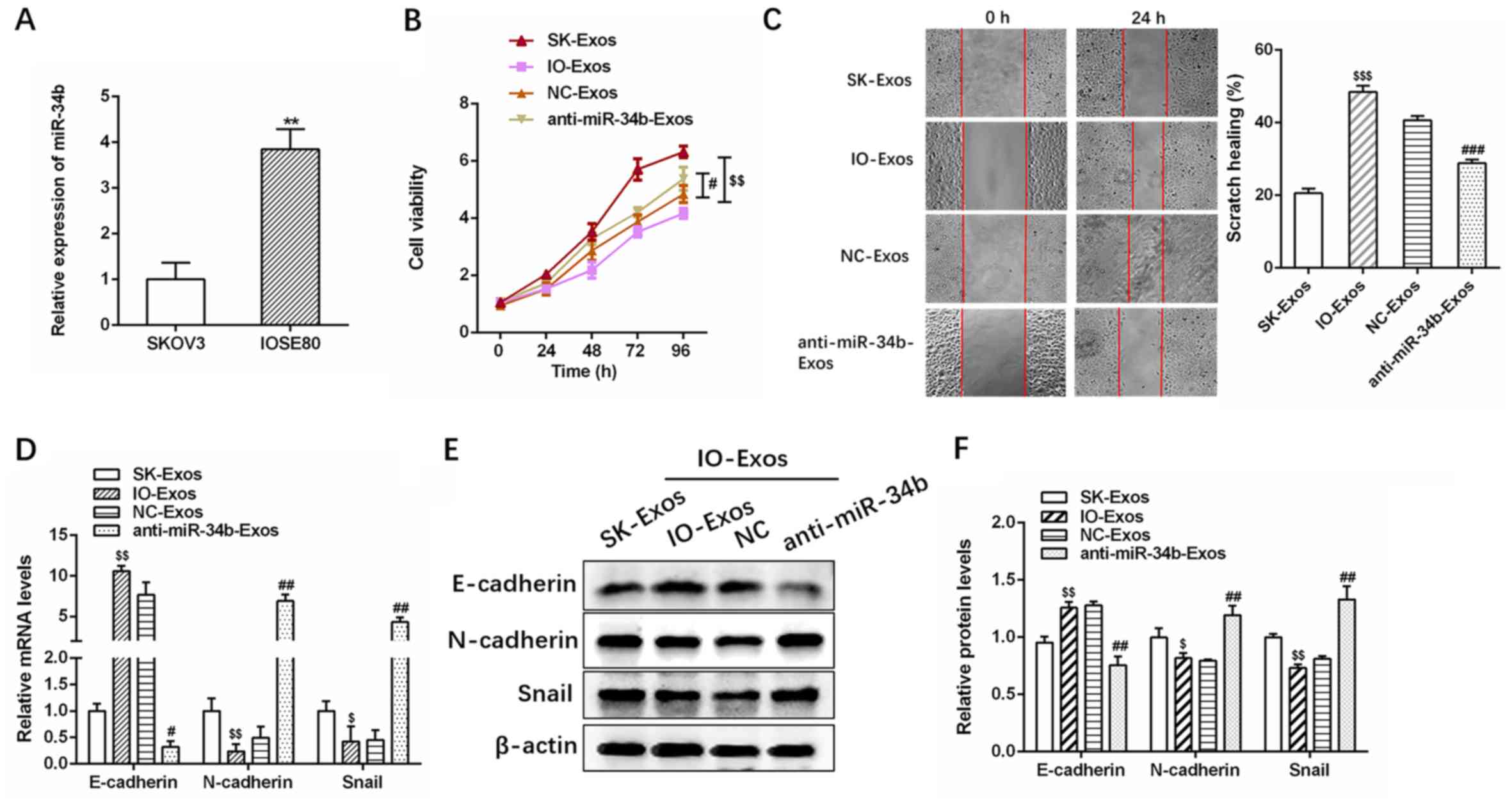 | Figure 2.Exosomal miR-34b suppresses cell
proliferation and EMT in ovarian cancer cells. (A) Relative
expression levels of miR-34b in SKOV3 cells co-cultured with SKOV3
or IOSE-80. (B) Cell viability assays and (C) cell invasion assays
in SKOV3 cells cultured with exosomes isolated from SKOV3, IOSE-80,
IOSE-80+NC or IOSE-80+anti-miR-34b. (D) Relative expression levels
of EMT markers (E-cadherin, N-cadherin and Snail) in SKOV3 cells
co-cultured with SKOV3, IOSE-80, IOSE-80+NC or
IOSE-80+anti-miR-34b. (E) and (F) Western blotting analysis of EMT
markers (E-cadherin, N-cadherin, and Snail) (20,21) in
SKOV3 cells co-cultured with SKOV3, IOSE-80, IOSE-80+NC or
IOSE-80+anti-miR-34b. **P<0.01 vs. SKOV3. $P<0.05,
$$P<0.01 and $$$P<0.001 vs. SK-Exos,
#P<0.05, ##P<0.01 vs. NC-Exos. EMT,
epithelial-mesenchymal transition; NC, negative control; miR,
microRNA; SK, SKOV3; IO, IOSE-80; Exos, exosomes. |
Furthermore, as demonstrated by RT-qPCR and western
blotting, IO-Exos was associated with elevated expression of
E-cadherin (epithelial marker) (20)
and reduced expression of N-cadherin and Snail (mesenchymal
markers) (21) at both the mRNA and
protein levels in SKOV3 cells (Fig.
2D-F). In contrast, anti-miR-34b-Exos was associated with
reduced E-cadherin and increased N-cadherin and Snail expression
(Fig. 2D-F; #P<0.05;
##P<0.01; $P<0.05 and
$$P<0.01). In summary, the aforementioned results
demonstrated that exosomal miR-34b attenuates the proliferation and
EMT in OC cells.
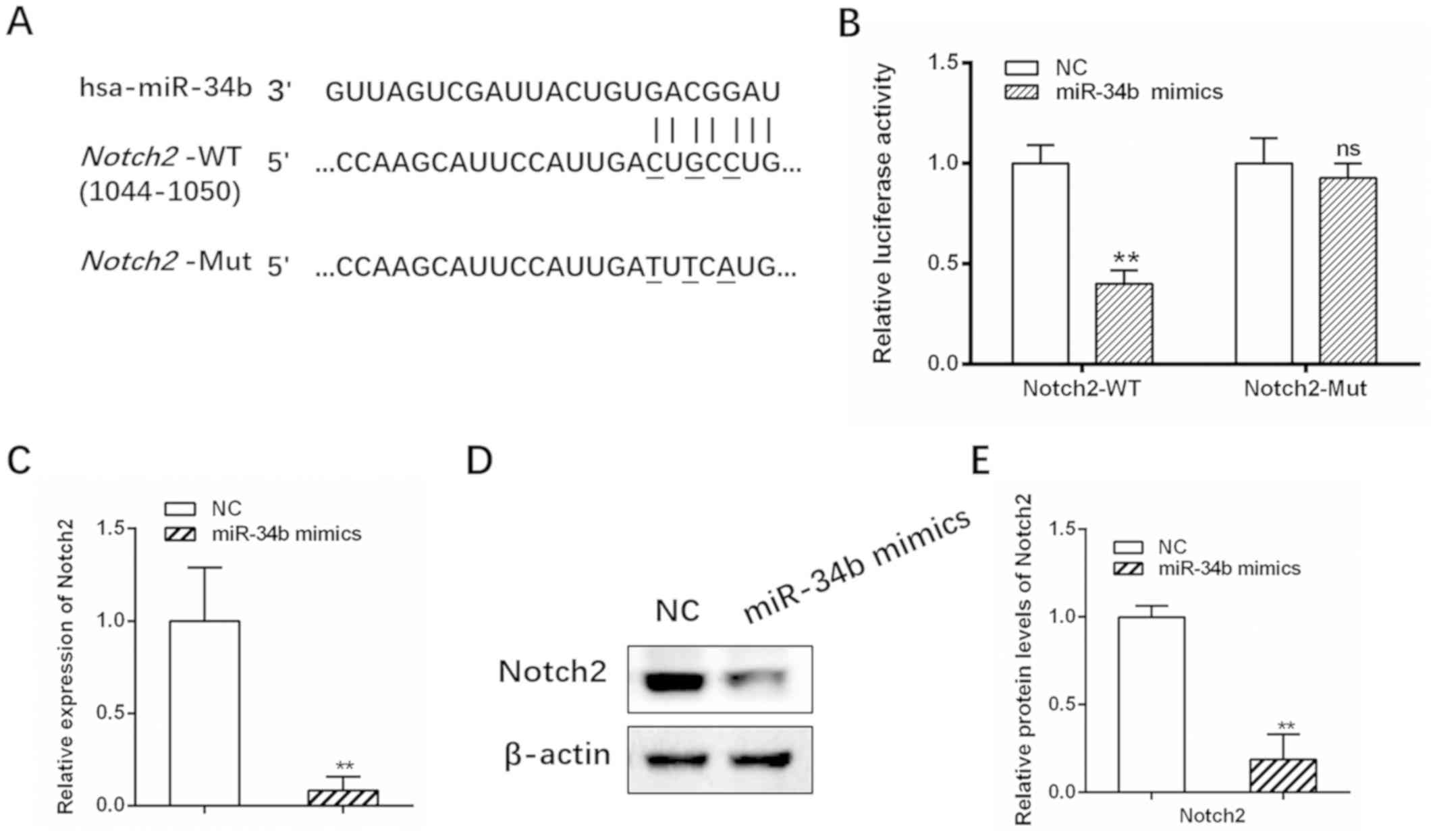
Notch2 is a direct target of
miR-34b
miRNAs are typically involved in the regulation of
target genes. Three online prediction algorithms (PicTar,
TargetScan and miRDB) identified Notch2 as a potential target gene
of miR-34b, with a putative binding site at base pairs 1044–1050
(Fig. 3A). Elevated Notch signaling
activity has been reported in previous studies investigating OC
(22,23). The dual-luciferase reporter assay
showed that luciferase activity for the WT Notch2 3-UTR was
significantly suppressed in cells transfected with miR-34b mimics
compared with the control (Fig. 3B;
**P<0.01), while no significant change in activity was observed
for Mut Notch2 (Fig. 3B;
**P<0.01). Furthermore, Notch2 mRNA and protein levels were
decreased following treatment with miR-34b mimics (Fig. 3C-E; **P<0.01). Collectively, these
results demonstrated that Notch2 is a direct target of miR-34b.
Notch2 is negatively correlated with
exosomal miR-34b
The mRNA and protein levels of Notch2 were lower in
cells co-cultured with IOSE-80 compared with those in SKOV3 cells
alone (Fig. 4A-C;
##P<0.01; ###P<0.001;
$P<0.05 and $$P<0.01). In contrast,
Notch2 mRNA and protein levels were increased in cells treated with
anti-miR-34b-Exos (Fig. 4A-C). These
results confirmed that Notch2 is negatively correlated with
exosomal miR-34b (r=−0.8493, Fig.
4D).
 | Figure 4.Notch2 is negatively associated with
exosomal miR-34b. (A) Relative expression levels of Notch2 in SKOV3
cells cultured with exosomes isolated from SKOV3, IOSE-80,
IOSE-80+NC or IOSE-80+anti-miR-34b. (B) and (C) Western blotting of
Notch2 in SKOV3 cells co-cultured with SKOV3, IOSE-80, IOSE-80+NC
or IOSE-80+anti-miR-34b. (D) Notch2 is negatively correlated with
exosomal miR-34b. $P<0.05, $$P<0.01 vs.
SK-Exos. ##P<0.01, ###P<0.001 vs.
NC-Exos. miR, microRNA; SK, SKOV3; IO, IOSE-80; Exos, exosomes. |
Notch2 knockdown decreases the effect
of exosomal anti-miR-34b on cell proliferation and EMT in OC
The efficiency of Notch2 siRNA transfection was
examined in SKOV3 cells (Fig. S3),
and then successfully transfected SKOV3 cells were treated with
IO-Exos-NC or anti-miR-34b IO-Exos (Fig.
5A). As demonstrated by the CCK-8 and wound healing assays,
Notch2 knockdown significantly inhibited the proliferation and
invasion induced by exosomal anti-miR-34b (Fig. 5B and C; **P<0.01; ***P<0.001
#P<0.05 and ##P<0.01). In addition,
exosomal anti-miR-34b-induced EMT inhibition was partly decreased
by Notch2-knockdown in SKOV3 cells (Fig.
5D-F; *P<0.05; **P<0.01; #P<0.05 and
##P<0.01). Together, these results suggested that
exosomal miR-34b mediates cell proliferation and EMT via
Notch2.
 | Figure 5.Notch2 knockdown abates the effect of
exosomal anti-miR-34b on cell proliferation and the EMT in ovarian
cancer cells. (A) Relative expression levels of Notch2 in SKOV3
cells co-cultured with IOSE-80, IOSE-80+NC or IOSE-80+anti-miR-34b.
(B) Cell viability and (C) cell invasion assays in SKOV3 cells
cultured with exosomes isolated from SKOV-3, IOSE-80, IOSE-80+NC or
IOSE-80+anti-miR-34b. (D) Relative expression levels of EMT markers
(E-cadherin, N-cadherin and Snail) in SKOV3 cells cultured with
exosomes isolated from SKOV3, IOSE-80, IOSE-80+NC or
IOSE-80+anti-miR-34b. (E) and (F) western blotting of E-cadherin,
N-cadherin and Snail in SKOV3 cells co-cultured with IOSE-80,
IOSE-80+NC, or IOSE-80+anti-miR-34b. **P<0.01, ***P<0.001 vs.
NC-Exos. #P<0.05, ##P<0.01 vs.
anti-miR-34b-Exos. EMT, epithelial-mesenchymal transition; NC,
negative control; Exos, exosomes; IO, IOSE-80; SK, SKOV3; si, small
interfering. |
Discussion
Despite improvements in surgical approaches, OC
still has a high mortality rate, which can be primarily attributed
to late-stage diagnosis and a high rate of metastasis (24). Previous studies have demonstrated the
critical roles of miRNAs in tumorigenesis, progression and
metastasis in OC (25,26). Approximately 400 dysregulated miRNAs
have been identified in OC, some of which are secreted in exosomes,
and are involved in diverse biological functions, including cell
proliferation, migration and resistance to paclitaxel and
Cisplatin® (27,28). In addition, exosomal miRNAs,
including miR-145, miR-940 and Let-7, have been identified as
potential diagnostic biomarkers for OC (29–31).
Therefore, understanding the roles of dysregulated exosomal miRNAs
in OC may help improve diagnosis and treatment. The present study
demonstrated that exosomal miR-34b expression is decreased in OC
and has an inhibitory effect on cell proliferation and EMT. These
results may improve our existing understanding of the fundamental
mechanisms underlying the pathogenesis of OC.
miR-34b can act as a tumor suppressor via several
mechanisms, including a positive feedback loop between miR-34b and
p53 (32). Decreased expression of
miR-34b has been detected in various types of cancer, such as
colorectal, pancreatic, gastric and ovarian cancer, due to the
inactivation of its promoter by CpG methylation (19,33–35).
Consequently, miR-34b has become the first miR target to reach the
phase 1 clinical trials for solid tumors, such as lung, colon,
prostate as well as other disorders, such as cardiac fibrosis,
cardiometabolic disease and chronic heart failure (36,37).
Moreover, miR-34b has been investigated in body fluids, such as
plasma and urine and tissues and has been identified as a biomarker
for clear cell renal cell carcinoma (38,39).
Several studies have shown that miR-34b expression is lower in OC
tissues compared with that in normal ovaries (16,30).
However, the functions and underlying mechanisms of action of
exosomal miR-34b in OC have not been explored. The present study
demonstrated that exosomal miR-34b is highly dysregulated in OC
cells. Exosomal miR-34b can suppress cell proliferation and
expression of the epithelial adhesion molecule, E-cadherin, and
increase the expression of the mesenchymal markers, N-cadherin and
Snail, suggesting that miR-34b inhibits EMT in OC (20,40).
Notch2, a member of the Notch family of proteins, is
a conserved cell surface receptor that mediates cellular
interactions, and is associated with the initiation and development
of various types of tumors, such as liver, brain and gastric tumors
(41,42). Indeed, Notch signaling has a wide
range of functions, and, depending on the cancer type, can function
as either a tumor promoter or suppressor, highlighting its complex
role in cancer (43). In ovaries,
Notch signaling regulates granulosa cell proliferation and
coordinates follicular growth; therefore, it is involved in ovarian
follicle development (44). In
addition, increased Notch2 expression is significantly correlated
with poor progression-free survival rate in OC, suggesting that it
has prognostic value (45). The
present study is the first to report that Notch2 is a direct target
gene of miR-34b in OC, to the best of our knowledge. Furthermore,
Notch2 was significantly negatively associated with exosomal
miR-34b levels, and knockdown of Notch2 abated the decreased cell
proliferation and EMT mediated by exosomal anti-miR-34b in OC.
EMT is generally associated with the invasion and
migration ability of cells (46).
Wound healing and Transwell assays are methods to analyze cell
migration. The present study, revealed that exosomal miR-34b
attenuates EMT in OC by downregulation of E-cadherin and
upregulation of N-cadherin and Snail. However, the Transwell assay
was not performed using Matrigel, therefore invasion could not be
analyzed, which is a limitation of the present study and worthy of
further investigation. In addition, the critical role of miR-34b
was only explored in OC cell lines, relevant OC animal models
should be studied to further confirm the regulatory function of
miR-34b in OC.
Overall, the regulatory network of exosomal miR34b
and the mechanisms underlying its suppressive effects on cell
proliferation and EMT in OC were identified. It was demonstrated
that miR-34b acts by directly binding to and regulating Notch2
expression. These findings highlight the important role of exosomal
miR-34b in OC, suggesting that it could act as a promising
diagnostic and therapeutic target in OC in the future.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was funded by the Changhai Hospital Youth
Startup Fund (grant no. 2018QNB004)
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors contributions
SL and WL designed the study and performed the
experiments. HS participated in the experimental design and
analysis of data, and drafted and critically revised the
manuscript. HZ participated in the experiment design and the
subject establishment of this article, in addition he wrote and
critically revised the manuscript and gave final approval for the
manuscript to be published. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Karimi-Zarchi M, Mortazavizadeh SMR,
Bashardust N, Zakerian N, Zaidabadi M, Yazdian-Anari P and Teimoori
S: The clinicopathologic characteristics and 5-year survival rate
of epithelial ovarian cancer in Yazd, Iran. Electron Physician.
7:1399–1406. 2015.PubMed/NCBI
|
|
2
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer Statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hannibal CG, Cortes R, Engholm G and Kjaer
SK: Survival of ovarian cancer patients in Denmark: Excess
mortality risk analysis of five-year relative survival in the
period 1978–2002. Acta Obstet Gynecol Scand. 87:1353–1360. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ozols RF, Bookman MA, Connolly DC, Daly
MB, Godwin AK, Schilder RJ, Xu X and Hamilton TC: Focus on
epithelial ovarian cancer. Cancer Cell. 5:19–24. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chebouti I, Kasimir-Bauer S, Buderath P,
Wimberger P, Hauch S, Kimmig R and Kuhlmann JD: EMT-like
circulating tumor cells in ovarian cancer patients are enriched by
platinum-based chemotherapy. Oncotarget. 8:48820–48831. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dragomir MP, Knutsen E and Calin GA:
SnapShot: unconventional miRNA functions. Cell. 174:1038–1038.e1.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reddy KB: MicroRNA (miRNA) in cancer.
Cancer Cell Int. 15:382015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
De Cecco L, Bagnoli M, Canevari S,
Califano D, Perrone F, Pignata S and Mezzanzanica D: miRNA-based
signature for predicting epithelial ovarian cancer recurrence.
Transl Cancer Res 6 (S1). S232–S234. 2017. View Article : Google Scholar
|
|
10
|
Lutgendorf SK, Thaker PH, Arevalo JM,
Goodheart MJ, Slavich GM, Sood AK and Cole SW: Biobehavioral
modulation of the exosome transcriptome in ovarian carcinoma.
Cancer. 124:580–586. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thomou T, Mori MA, Dreyfuss JM, Konishi M,
Sakaguchi M, Wolfrum C, Rao TN, Winnay JN, Garcia-Martin R,
Grinspoon SK, et al: Adipose-derived circulating miRNAs regulate
gene expression in other tissues. Nature. 542:450–455. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei Y, Lai X, Yu S, Chen S, Ma Y, Zhang Y,
Li H, Zhu X, Yao L and Zhang J: Exosomal miR-221/222 enhances
tamoxifen resistance in recipient ER-positive breast cancer cells.
Breast Cancer Res Treat. 147:423–431. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pan C, Stevic I, Müller V, Ni Q,
Oliveira-Ferrer L, Pantel K and Schwarzenbach H: Exosomal microRNAs
as tumor markers in epithelial ovarian cancer. Mol Oncol.
12:1935–1948. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meng X, Müller V, Milde-Langosch K,
Trillsch F, Pantel K and Schwarzenbach H: Diagnostic and prognostic
relevance of circulating exosomal miR-373, miR-200a, miR-200b and
miR-200c in patients with epithelial ovarian cancer. Oncotarget.
7:16923–16935. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou J, Gong G, Tan H, Dai F, Zhu X, Chen
Y, Wang J, Liu Y, Chen P, Wu X, et al: Urinary microRNA-30a-5p is a
potential biomarker for ovarian serous adenocarcinoma. Oncol Rep.
33:2915–2923. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Corney DC, Hwang CI, Matoso A, Vogt M,
Flesken-Nikitin A, Godwin AK, Kamat AA, Sood AK, Ellenson LH,
Hermeking H, et al: Frequent downregulation of miR-34 family in
human ovarian cancers. Clin Cancer Res. 16:1119–1128. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hosseinpour Z, Salehi Z, Talesh Sasani S
and Aminian K: p53 and miR-34b/c genetic variation and their impact
on ulcerative colitis susceptibility. Br J Biomed Sci. 75:46–49.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang S, Lu Z, Unruh AK, Ivan C, Baggerly
KA, Calin GA, Li Z, Bast RC Jr and Le XF: Clinically relevant
microRNAs in ovarian cancer. Mol Cancer Res. 13:393–401. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vogt M, Munding J, Grüner M, Liffers ST,
Verdoodt B, Hauk J, Steinstraesser L, Tannapfel A and Hermeking H:
Frequent concomitant inactivation of miR-34a and miR-34b/c by CpG
methylation in colorectal, pancreatic, mammary, ovarian,
urothelial, and renal cell carcinomas and soft tissue sarcomas.
Virchows Arch. 458:313–322. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chaw SY, Abdul Majeed A, Dalley AJ, Chan
A, Stein S and Farah CS: Epithelial to mesenchymal transition (EMT)
biomarkers--E-cadherin, beta-catenin, APC and Vimentin--in oral
squamous cell carcinogenesis and transformation. Oral Oncol.
48:997–1006. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Groeneweg JW, Foster R, Growdon WB,
Verheijen RH and Rueda BR: Notch signaling in serous ovarian
cancer. J Ovarian Res. 7:952014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang CP, Yang JL, Zhang J, Li L, Huang L,
Ji SY, Hu ZY, Gao F and Liu YX: Notch signaling is involved in
ovarian follicle development by regulating granulosa cell
proliferation. Endocrinology. 152:2437–2447. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yeung TL, Leung CS, Yip KP, Au Yeung CL,
Wong ST and Mok SC: Cellular and molecular processes in ovarian
cancer metastasis. A Review in the Theme: Cell and molecular
processes in cancer metastasis. Am J Physiol Cell Physiol.
309:C444–C456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Srivastava AK, Banerjee A, Cui T, Han C,
Cai S, Liu L, Wu D, Cui R, Li Z, Zhang X, et al: Inhibition of
miR-328-3p impairs cancer stem cell function and prevents
metastasis in ovarian cancer. Cancer Res. 79:2314–2326. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao W, Han T, Li B, Ma Q, Yang P and Li
H: miR-552 promotes ovarian cancer progression by regulating PTEN
pathway. J Ovarian Res. 12:1212019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deb B, Uddin A and Chakraborty S: miRNAs
and ovarian cancer: An overview. J Cell Physiol. 233:3846–3854.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Au Yeung CL, Co NN, Tsuruga T, Yeung TL,
Kwan SY, Leung CS, Li Y, Lu ES, Kwan K, Wong KK, et al: Exosomal
transfer of stroma-derived miR21 confers paclitaxel resistance in
ovarian cancer cells through targeting APAF1. Nat Commun.
7:111502016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Christenson LK: MicroRNA control of
ovarian function. Anim Reprod. 7:129–133. 2010.PubMed/NCBI
|
|
30
|
Xie YL, Yang YJ, Tang C, Sheng HJ, Jiang
Y, Han K and Ding LJ: Estrogen combined with progesterone decreases
cell proliferation and inhibits the expression of Bcl-2 via
microRNA let-7a and miR-34b in ovarian cancer cells. Clin Transl
Oncol. 16:898–905. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen X, Ying X and Wang X, Wu X, Zhu Q and
Wang X: Exosomes derived from hypoxic epithelial ovarian cancer
deliver microRNA-940 to induce macrophage M2 polarization. Oncol
Rep. 38:522–528. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yamakuchi M and Lowenstein CJ: MiR-34,
SIRT1 and p53: The feedback loop. Cell Cycle. 8:712–715. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hiyoshi Y, Schetter AJ, Okayama H, Inamura
K, Anami K, Nguyen GH, Horikawa I, Hawkes JE, Bowman ED, Leung SY,
et al: Increased microRNA-34b and −34c predominantly expressed in
stromal tissues is associated with poor prognosis in human colon
cancer. PLoS One. 10:e01248992015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tsai KW, Wu CW, Hu LY, Li SC, Liao YL, Lai
CH, Kao HW, Fang WL, Huang KH, Chan WC, et al: Epigenetic
regulation of miR-34b and miR-129 expression in gastric cancer. Int
J Cancer. 129:2600–2610. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu C, Cheng H, Shi S, Cui X, Yang J, Chen
L, Cen P, Cai X, Lu Y, Wu C, et al: MicroRNA-34b inhibits
pancreatic cancer metastasis through repressing Smad3. Curr Mol
Med. 13:467–478. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chakraborty C, Sharma AR, Sharma G, Doss
CGP and Lee SS: Therapeutic miRNA and siRNA: Moving from bench to
clinic as next generation medicine. Mol Ther Nucleic Acids.
8:132–143. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jung WJ, Koh Y, Kim S, Park H and Yoon SS:
Activity of microRNA replacement reagent, MRX34, in multiple
myeloma in vivo model. Cancer Res. 76:1081. 2016.
|
|
38
|
Butz H, Nofech-Mozes R, Ding Q, Khella
HWZ, Szabó PM, Jewett M, Finelli A, Lee J, Ordon M, Stewart R, et
al: Exosomal MicroRNAs Are Diagnostic Biomarkers and Can Mediate
Cell-Cell Communication in Renal Cell Carcinoma. Eur Urol Focus.
2:210–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ramezani A, Devaney JM, Cohen S, Wing MR,
Scott R, Knoblach S, Singhal R, Howard L, Kopp JB and Raj DS:
Circulating and urinary microRNA profile in focal segmental
glomerulosclerosis: A pilot study. Eur J Clin Invest. 45:394–404.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang Y, Shi J, Chai K, Ying X and Zhou BP:
The role of snail in EMT and tumorigenesis. Curr Cancer Drug
Targets. 13:963–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Siebel C and Lendahl U: Notch signaling in
development, tissue homeostasis, and disease. Physiol Rev.
97:1235–1294. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xiu MX and Liu YM: The role of oncogenic
Notch2 signaling in cancer: A novel therapeutic target. Am J Cancer
Res. 9:837–854. 2019.PubMed/NCBI
|
|
43
|
Koch U and Radtke F: Dual function of
notch signaling in cancer: oncogene and tumor suppressor. Targeting
Notch in Cancer. Miele L and Artavanis-Tsakonas S: Springer; New
York, NY: pp. 55–86. 2018, View Article : Google Scholar
|
|
44
|
Vanorny DA, Prasasya RD, Chalpe AJ, Kilen
SM and Mayo KE: Notch signaling regulates ovarian follicle
formation and coordinates follicular growth. Mol Endocrinol.
28:499–511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen C, Wang X, Huang S, Wang L, Han L and
Yu S: Prognostic roles of Notch receptor mRNA expression in human
ovarian cancer. Oncotarget. 8:32731–32740. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mittal V: Epithelial mesenchymal
transition in tumor metastasis. Annu Rev Pathol. 13:395–412. 2018.
View Article : Google Scholar : PubMed/NCBI
|