Introduction
Human bladder cancer is a heterogeneous disease that
is one of the most common types of cancer worldwide, with ~550,000
new diagnosed cases annually (1). In
the Western world, bladder cancer is the 4th most common malignancy
in men; the incidence in women is approximately one-third of that
in men (2). Bladder cancer is
classified into non-muscle-invasive bladder cancer (NMIBC) and
muscle-invasive bladder cancer (MIBC): ~75% of patients with
bladder cancer exhibit NMIBC, and 15–20% of NMIBCs progress to
MIBCs; the remaining 25% of patients exhibit MIBC at first
diagnosis (3,4). However, MIBC is highly aggressive, and
patients with invasive bladder cancer exhibit a poor prognosis with
a 5-year survival rate of <50% (5). The treatment options for bladder cancer
include radiation alone, chemotherapy alone, combination treatment
with radiation and chemotherapy or surgery, and the selected
treatment is dependent on the age of the patient, the
aggressiveness of the disease and drug effectiveness (6). Despite the various treatment options,
metastasis is fatal for patients; therefore, uncovering the
underlying mechanism of metastasis and screening for antimetastatic
drugs is urgently needed.
Chronic inflammation increases the risk of cancer
and contributes to tumor development through the induction of
oncogenic mutations, enhanced angiogenesis and early tumor
promotion (7). Tumor-associated
inflammation is associated with tumor progression; evidence has
indicated that inflammation impacts every step of tumorigenesis,
from initiation through promotion to invasion and metastasis
(8). Traditional Chinese medicine
provides the material basis and molecular structure framework for
the screening of antitumor drugs. Heat-clearing and detoxifying
herbs have been reported to serve important roles in
anti-inflammation, tumor cells death and suppression of tumor
proliferation as well as metastasis (9). Paris polyphylla (chonglou) is a
heat-clearing and detoxifying herb mainly used in traditional
Chinese medicine to treat headaches, fevers and wounds, as well as
for neutralizing snake poison (10).
Extracts from Paris polyphylla have also been demonstrated
to exert anticancer effects in breast (11), lung (12) and ovarian (13) cancer. In the Chinese Pharmacopoeia,
Polyphyllin (PP) I, PPII, PPVI and PPVII are the key components for
identifying Paris polyphylla (14). Therefore, these four extracts have
gained increasing attention and have been chosen to be studied in
cancer treatment.
The epithelial-mesenchymal transition (EMT) serves
vital roles in angiogenesis, cell invasion and metastasis (15). The key changes during EMT include a
decrease in the levels of the epithelial marker E-cadherin (CDH1)
and increases in those of the mesenchymal marker N-cadherin (CDH2),
as well as snail family transcriptional repressor 2 (SNAI2) and
twist family bHLH transcription factor 1 (TWIST1), which are two
transcriptional inhibitors of CDH1 (16). PPs, especially PPI, have been
demonstrated to suppress tumor cell invasion and migration in
vitro. PPI, which is an active component of Paris
polyphylla, suppresses metastasis in hepatocellular carcinoma
by downregulating the formation of vasculogenic mimicry via the
TWIST1/VE-cadherin pathway (17).
PPI has also been demonstrated to inhibit gastric cancer cell
invasion by suppressing the EMT-associated CIP2A/PP2A/Akt signaling
pathway (18). However, to the best
of our knowledge the role and the underlying mechanism of PPs,
especially PPII, in the inhibition of bladder cancer metastasis
have not been studied.
The aim of the present study was to screen sensitive
heat-clearing and detoxifying herbs and their effective components
in bladder cancer. In addition, the present study aimed to identify
the inhibitory effect and mechanism of PPII, a vital component of
ethanol extracts of Paris polyphylla (PPE), on the invasion
and migration of human bladder cancer cells in vitro.
Materials and methods
Cell culture
Human bladder cancer cell lines T24 and 5637 (kindly
provided by Professor Shengtian Zhao, Shandong Provincial Hospital,
Jinan, China) were cultured in RPMI-1640 medium (cat. no. CM10041;
Macgene Technology Co., Ltd.) supplemented with 10% (v/v) fetal
bovine serum (FBS, cat. no. 04-001-1ACS; Biological Industries).
Cells were incubated in a humidified atmosphere with 5%
CO2 at 37°C.
Reagents
PPI (cat. no. A0386), PPII (cat. no. A0387), PPVI
(cat. no. A0389) and PPVII (cat. no. A0390) of ≥98% purity were
purchased from Chengdu Must Bio-Technology Co., Ltd. and dissolved
in DMSO (cat. no. D8370; Sigma-Aldrich; Merck KGaA). Antibodies
against CDH1 (cat. no. 20874-1-AP) and ACTB (cat. no. 66009-1-Ig)
were purchased from Proteintech Group, Inc. Antibodies against CDH2
(cat. no. ab98952), TWIST1 (cat. no. ab50581) and SNAI2 (cat. no.
ab27568) were obtained from Abcam, and antibodies against matrix
metallopeptidase (MMP) 2 (cat. no. 87809) and MMP9 (cat. no. 13667)
were obtained from Cell Signaling Technology, Inc. The secondary
antibodies HRP-labeled Goat Anti-Rabbit IgG (H+L) (cat. no. A0208)
and HRP-labeled Goat Anti-Mouse IgG (H+L) (cat. no. A0216) were
purchased from Beyotime Institute of Biotechnology.
Compound extraction
PPE were prepared using EtOH/H2O (60/40,
v/v) for 2 h twice under reflux conditions to generate a crude
extract, followed by filtration with 150 µm filter cloth. The
filtrates were combined, concentrated and separated from the liquid
reaction mixture by water precipitation. Following 12-h incubation
at 4°C, the extracted solution was filtered, precipitated and
dried. Finally, dry powders were obtained and dissolved in DMSO to
be used for bladder cell treatment. A total of five compounds
including Scutellaria barbata, Pulsatillae decoction,
Dahuang Huanglian Xiexin decoction, Bazhengsan and Hedyotis
diffusa combined with S. barbata were immersed in 10X
pure water for 1 h. Subsequently, the compounds were boiled twice
over a light flame, and the filtrates were merged and condensed
into extracts using a rotary evaporator. The condensed extracts
were freeze-dried, and each powder was weighed and dissolved in
water prior to experimental use.
Cell viability assay
The sensitivity to the six extracts and cisplatin of
bladder cancer cells was detected using the Cell Counting Kit-8
(CCK-8; cat. no. CK04; Dojindo Molecular Technologies, Inc.)
according to the manufacturer's instructions. Briefly, T24
(6.0×103 cells/well) and 5637 (8.0×103
cells/well) cells were cultured in quintuplicate in 96-well plates
at 37°C overnight and treated with the drugs at different
concentrations: [Cisplatin, T24 (0, 0.5, 1, 2, 4, 8 µg/ml) and 5637
(0, 1, 3, 9, 27, 81 µg/ml)]; [PPE, T24 (0, 1, 2, 4, 8, 16 µg/ml)
and 5637 (0, 1, 3, 9, 27, 81 µg/ml)]; [Scutellaria barbata,
T24 (0, 0.1, 0.2, 0.4, 0.8, 1.6 mg/ml) and 5637 (0, 0.25, 0.5, 1,
2, 4 mg/ml)]; Hedyotis diffusa combined with S.
barbata, T24 and 5637 (0, 0.25, 0.5, 1, 2, 4 mg/ml);
[Pulsatillae decoction, T24 (0, 0.25, 0.5, 1, 2, 4 mg/ml)
and 5637 (0, 0.05, 0.1, 0.2, 0.4, 0.8 mg/ml)]; [Dahuang Huanglian
Xiexin decoction; T24 (0, 0.25, 0.5, 1, 2, 4 mg/ml) and 5637 (0,
0.05, 0.1, 0.2, 0.4, 0.8 mg/ml)]; and Bazhengsan, T24 and 5637 (0,
0.25, 0.5, 1,2, 4 mg/ml) for 48 h. Subsequently, the medium was
removed, and the cells were incubated with 90 µl new culture medium
supplemented with 10 µl CCK-8 reagent for 2–4 h. Absorption at 450
nm was detected using a spectrophotometer (Type, 1510; Thermo
Fisher Scientific Inc.).
Wound healing assay
The bladder cancer cells T24 (6.5×105
cells/well) and 5637 (8.0×105 cells/well) were seeded in
6-well plates. At 24 h, when the cells had reached 90% confluence,
linear scratch wounds were created on the cell monolayers in
triplicate using a sterile 200-µl pipette tip. The cells were
maintained in RPMI-1640 medium containing 1% FBS. Images were
captured at 0, 24 and 48 h using an inverted fluorescence
microscope (Vert. A1; Carl Zeiss AG) to measure mean distance
between edges of wound area. Representative images of wound treated
with PPE were observed at ×50 magnification; images of wound for
T24 cells treated with PPII were observed at ×50 and 5637 cells at
×100 magnification.
Transwell assay
A 24-well plate was used to determine the effect of
drugs on bladder cancer invasion. Bladder cancer cells were
harvested and resuspended in serum-free medium supplemented with
drugs at different concentrations (PPE for T24 cells at 0, 0.6 and
1.2 µg/ml; and for 5637 at 0, 0.95 and 1.9 µg/ml; PPII for cells at
0, 0.1 and 0.2 µg/ml). An 8-µm pore size filter was pre-coated with
Matrigel (cat. no. 354234; BD Biosciences) at 37°C for 30 min, and
the lower chamber was filled with medium containing 10% FBS.
Suspended bladder cancer cells (2.5×104 cells/well) were
seeded in the upper chamber and incubated for 48 h at 37°C.
Subsequently, the filter was fixed with 4% paraformaldehyde fix
solution (cat. no. P0099; Beyotime Institute of Biotechnology) for
15 min and stained with 0.1% crystal violet for 30 min at room
temperature. Finally, the cells and Matrigel on the upper chamber
were scraped with a cotton swab, and the invasive cells on the
lower surface were counted under an inverted fluorescence
microscope (Vert. A1; Carl Zeiss AG). Three fields for each sample
were captured at ×100 magnification.
Western blotting
The total proteins were obtained from bladder cancer
cells treated with PPII for 48 h at different concentrations (0,
0.1, 0.2 and 0.4 µg/ml). Cell lysates were acquired using RIPA cell
lysis buffer and centrifuged at 16,363 × g at 4°C for 15 min. A
bicinchoninic acid assay kit (cat. no. P0012S; Beyotime Institute
of Biotechnology) was used to determine the protein concentration,
and 10% SDS-PAGE was applied to separate equal amounts of proteins
(40 µg protein loaded per lane). After the proteins were
transferred to PVDF membranes, the membranes were blocked with 5%
nonfat milk for 1 h at room temperature and incubated with primary
antibodies at a 1:1,000 dilution at 4°C overnight. Appropriate
secondary antibodies conjugated with horseradish peroxidase at
1:2,000 dilution were used to incubate membrane-bound primary
antibodies at room temperature for 1 h, followed by development of
immunoblots using an enhanced chemiluminescence kit (cat. no.
WBKLS0050; Merck KGaA). The densitometry analysis was performed
using Image-Pro Plus version.6.0 (Media Cybernetics Inc.).
Statistical analysis
All experiments were conducted at least three times.
Data are presented as the mean ± SEM. Statistical analysis was
performed using GraphPad Prism 5.0 software (GraphPad Software,
Inc.) by one-way ANOVA with Dunnett's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Bladder cancer cells are sensitive to
PPE
To identify heat-clearing and detoxifying herbs that
affect bladder cancer cells, 5637 and T24 cells were challenged
with PPE (Fig. S1B), S.
barbata (Fig. S1C), Hedyotis
diffusa combined with S. barbata (Fig. S1D), Pulsatillae decoction
(Fig. S1E), Dahuang Huanglian
Xiexin decoction (Fig. S1F) and
Bazhengsan (Fig. S1G) for 48 h at
different concentrations. Cisplatin was used as a positive control
(Fig. S1A). Bladder cancer cells
T24 and 5637 were more sensitive to PPE compared with the other
herbs. The IC50 of each compound in different cells was
calculated and analyzed; the results demonstrated that PPE
treatment exerted a stronger inhibitory effect on the viability of
T24 and 5637 cells compared with that of other compounds, with
IC50 values of 4.43±0.08 and 7.87±0.39 µg/ml,
respectively (Table I), at 48 h. The
inhibitory effect of PPE on bladder cancer cells was similar to
that of the positive control cisplatin.
 | Table I.IC50 values of six classic
heat-clearing and detoxicating herbs and cisplatin |
Table I.
IC50 values of six classic
heat-clearing and detoxicating herbs and cisplatin
|
|
IC50 |
|---|
|
|
|
|---|
| Compound | T24 cells | 5637 cells |
|---|
| Cisplatin,
µg/ml | 1.30±0.17 | 2.62±0.30 |
| PPE, µg/ml | 4.43±0.08 | 7.87±0.39 |
| Scutellaria
barbata, mg/ml | 0.75±0.06 | 0.67±0.04 |
| Pulsatillae
decoction, mg/ml | 0.45±0.02 | 0.16±0.01 |
| Dahuang Huanglian
Xiexin decoction, mg/ml | 0.82±0.02 | 0.14±0.03 |
| Bazhengsan,
mg/ml | 1.84±0.09 | 0.85±0.05 |
| Hedyotis
diffusa combined with S. barbata, mg/ml | 0.67±0.04 | 0.45±0.03 |
PPE suppresses the migration and
invasion of bladder cancer cells
In order to determine the appropriate drug
concentration, CCK-8 was used to evaluate the viability of bladder
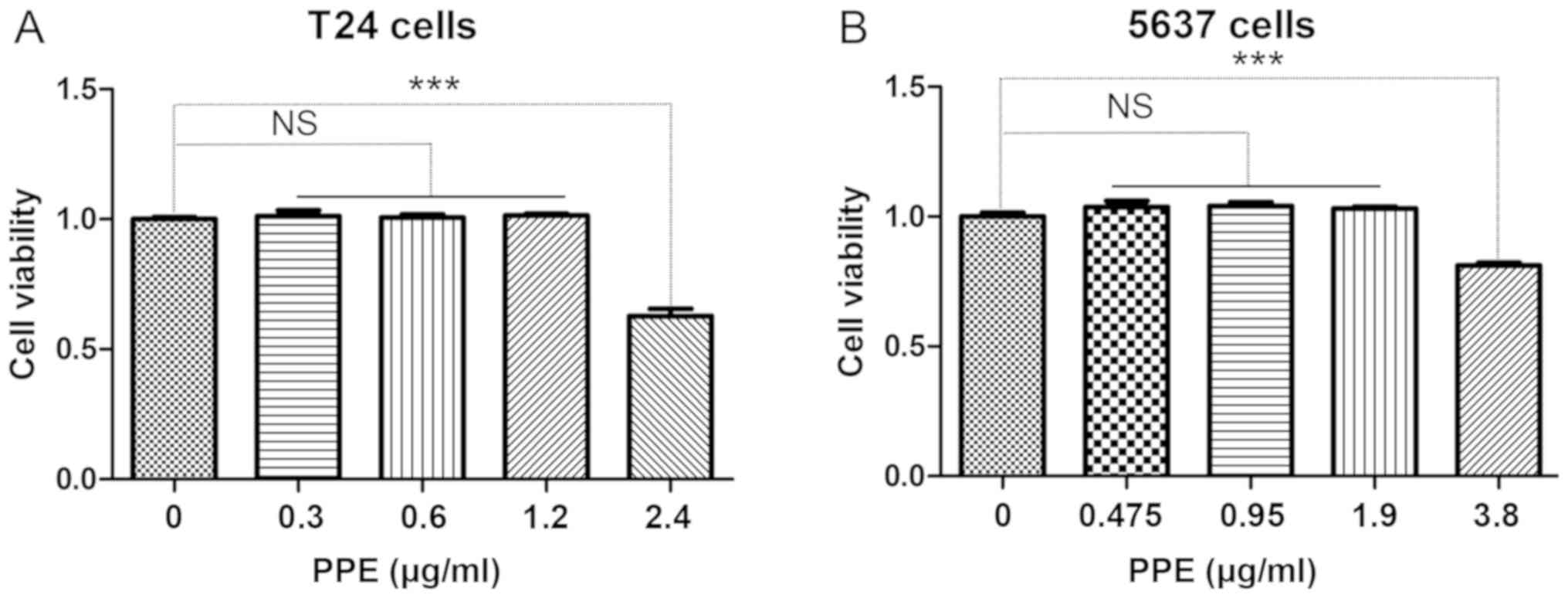
cancer cells following PPE treatment. The results demonstrated that
PPE inhibited the viability of T24 (Fig.
1A) and 5637 (Fig. 1B) cells at
>1.2 and 1.9 µg/ml, respectively (P<0.001). Therefore,
different concentrations of PPE were used in subsequent experiments
to treat T24 (0, 0.6 and 1.2 µg/ml) and 5637 (0, 0.95 and 1.9
µg/ml) cells.
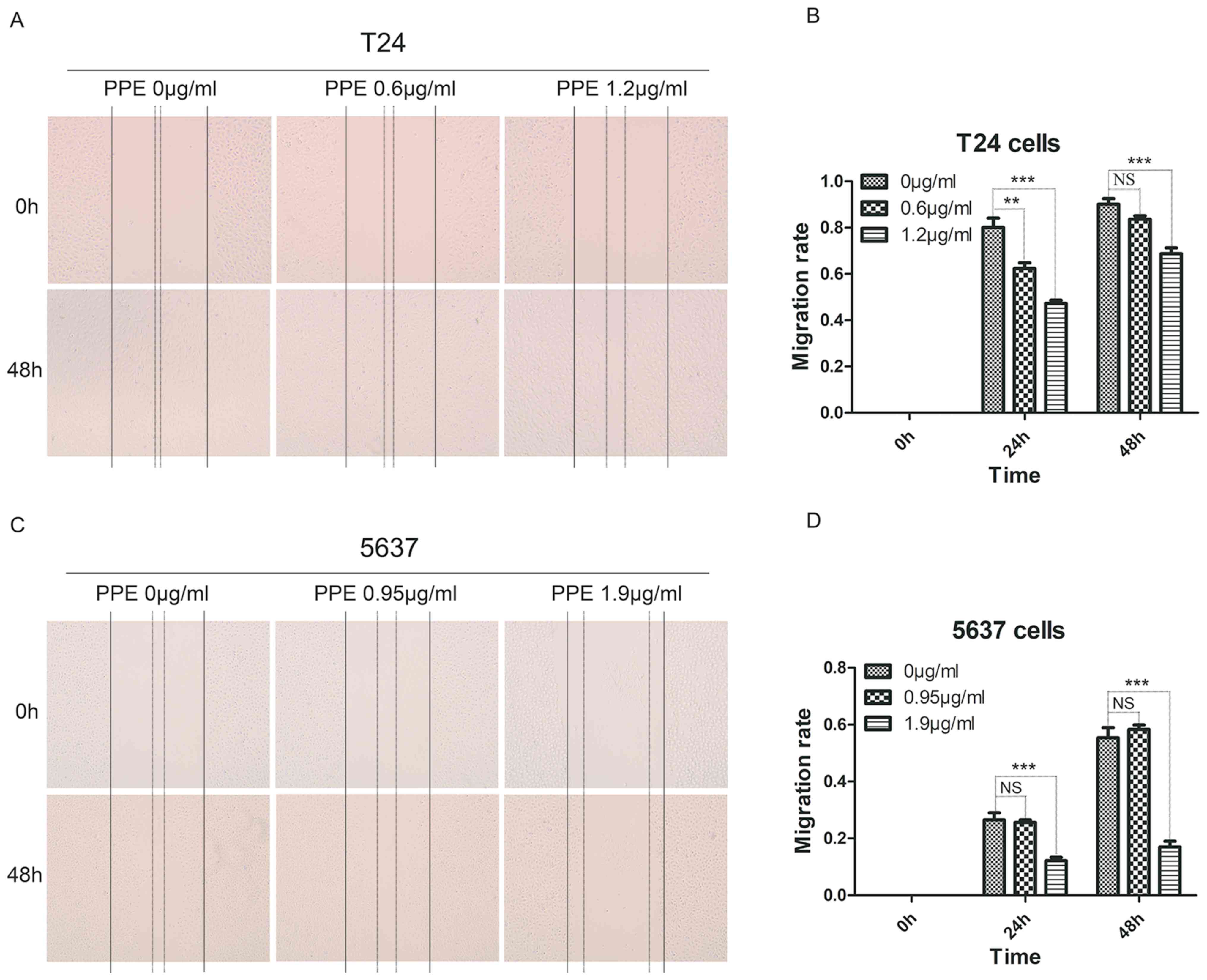
To investigate the effects of PPE on bladder cancer
invasion and migration, wound healing and Transwell assays were
performed in T24 and 5637 cells treated with PPE. The results
revealed that PPE significantly suppressed the wound closure of T24
cells at 1.2 µg/ml (Fig. 2A and B)
and 5637 cells (Fig. 2C and D) at
1.9 µg/ml (P<0.001). The effect of PPE on the invasiveness of
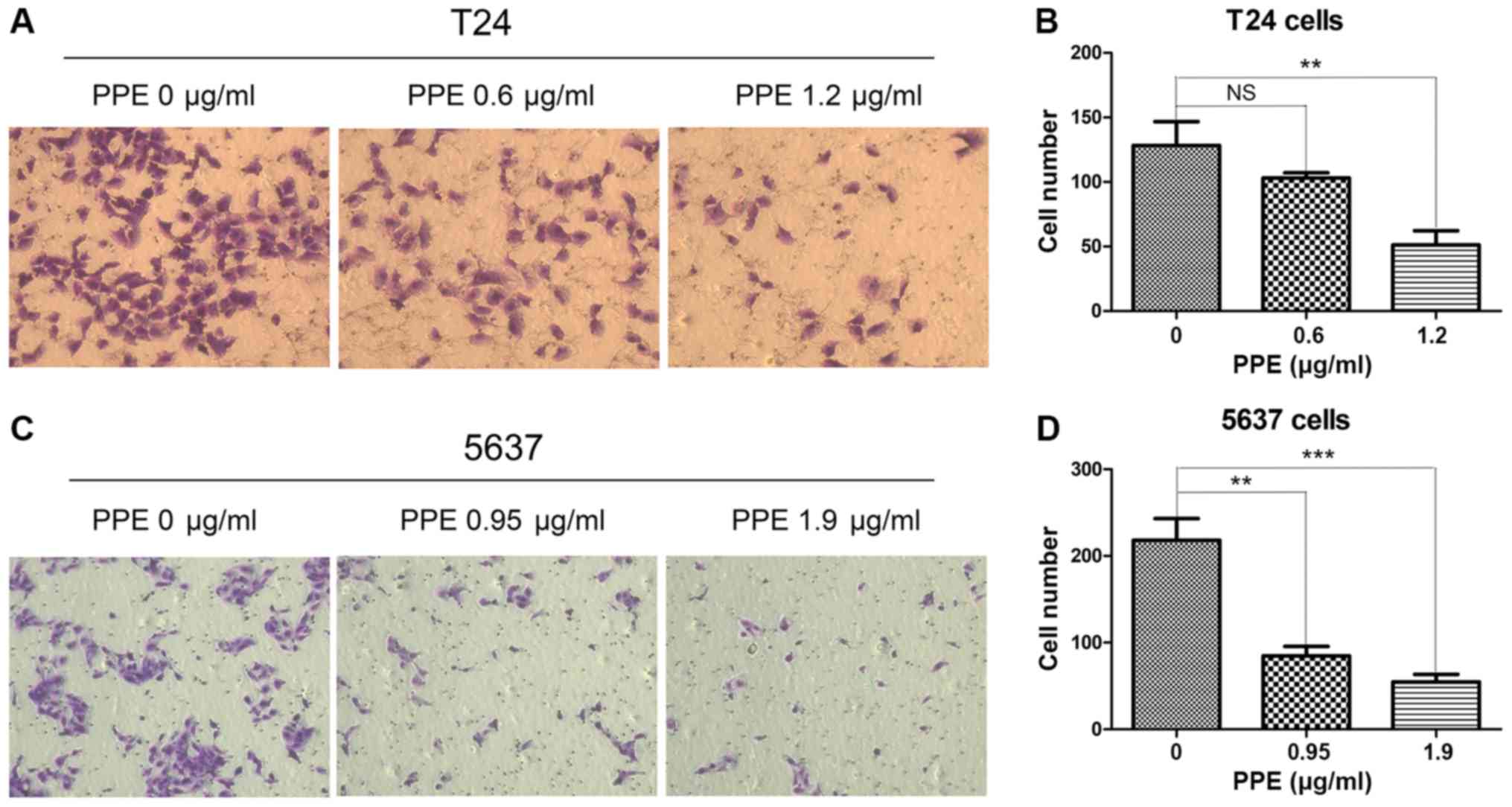
bladder cancer cells was further examined, and the results
demonstrated that PPE suppressed the invasive ability of T24
(Fig. 3A and B, P<0.01 at 1.2
µg/ml) and 5637 (Fig. 3C and D,
P<0.01 at 0.95 µg/ml and P<0.001 at 1.9 µg/ml) cells compared
with that of the control group. Therefore, PPE inhibited the
invasive and migratory abilities of T24 and 5637 bladder cancer
cells.
Bladder cancer cells are sensitive to
PPII
To determine which component from PPE served a role
in the anticancer activity of PPE in bladder cancer cells, the
essential components of PPE, including PPI (Fig. S2B), PPII (Fig. S2C), PPVI (Fig. S2D) and PPVII (Fig. S2E) were used to treat T24 and 5637
cells at different concentrations. Cisplatin was also used as a
positive control (Fig. S2A).
According to the IC50 values, T24 and 5637 cells were
more sensitive to PPII compared with PPI, PPVI and PPVII (Table II).
 | Table II.IC50 values of four
components from Paris polyphylla and cisplatin. |
Table II.
IC50 values of four
components from Paris polyphylla and cisplatin.
|
| IC50,
µg/ml |
|---|
|
|
|
|---|
| Compound | T24 cells | 5637 cells |
|---|
| Cisplatin | 1.15±0.11 | 3.25±0.33 |
| Polyphyllin I | 0.42±0.04 | 2.48±0.13 |
| Polyphyllin II | 0.64±0.03 | 0.71±0.23 |
| Polyphyllin VI | 0.84±0.02 | 3.79±0.40 |
| Polyphyllin
VII | 0.53±0.02 | 3.51±0.27 |
PPII suppresses bladder cancer cell
migration and invasion
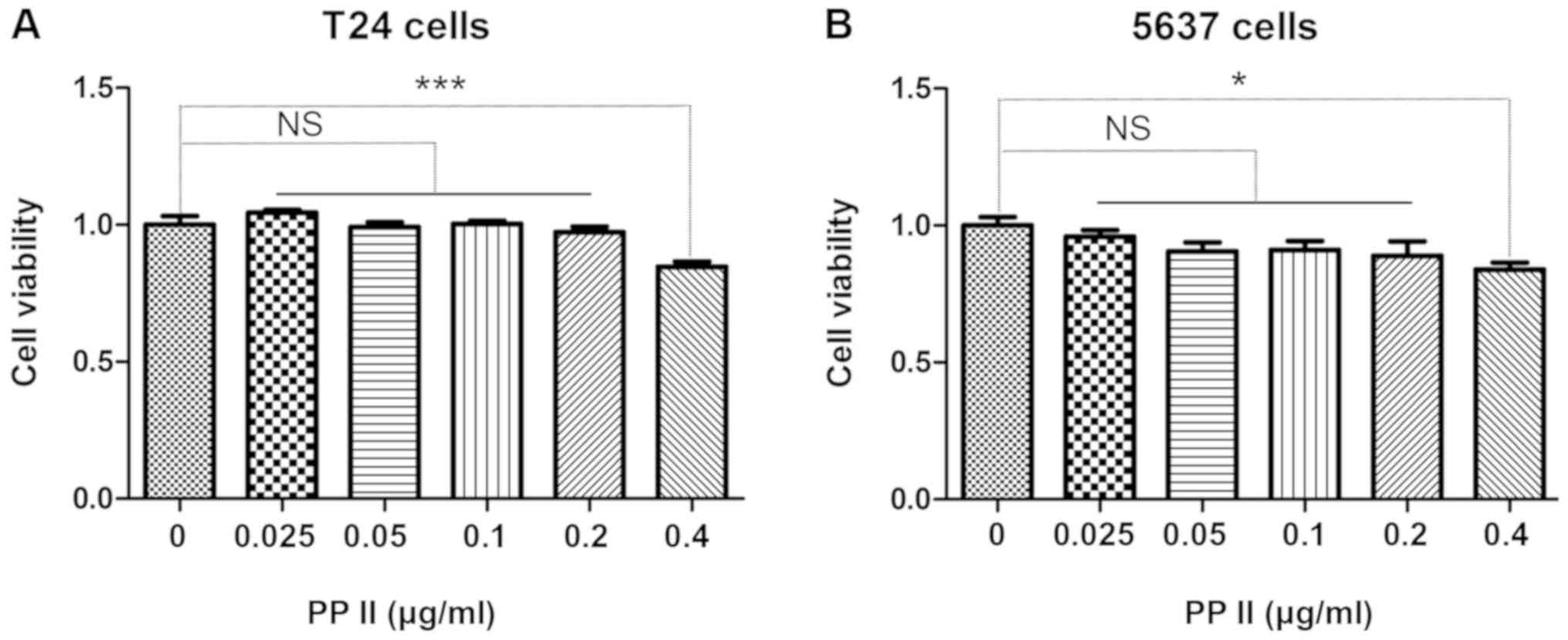
The bladder cancer cells T24 (Fig. 4A) and 5637 (Fig. 4B) were treated with 0, 0.025, 0.05,
0.1, 0.2 or 0.4 µg/ml PPII for 48 h. The viability of bladder
cancer cells was inhibited when the concentration of PPII was 0.4
µg/ml (P<0.05). Therefore, the antimetastatic activity of PPII
was further analyzed using 0, 0.1 and 0.2 µg/ml PPII.
To further investigate the effects of PPII on
bladder cancer cell migration and invasion, T24 and 5637 cells were
treated with 0, 0.1 and 0.2 µg/ml PPII for 48 h. Cell migration was
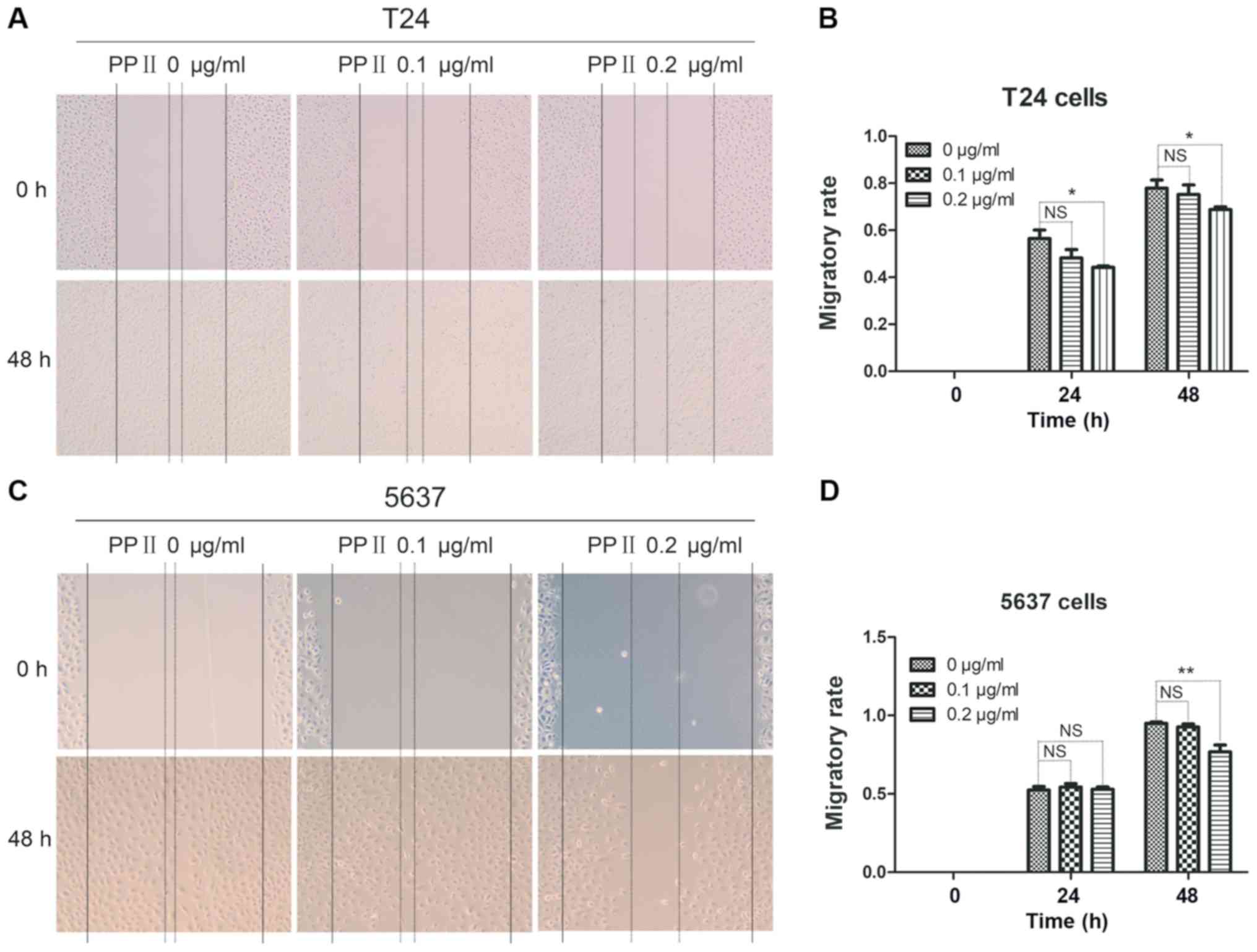
analyzed using a wound healing assay. PPII significantly inhibited
the migratory rates of T24 cells at 0.2 µg/ml (Fig. 5A and B; P<0.05) and 5637 cells at
0.2 µg/ml treated for 48 h (Fig. 5C and
D; P<0.01) compared with those in the control group. The
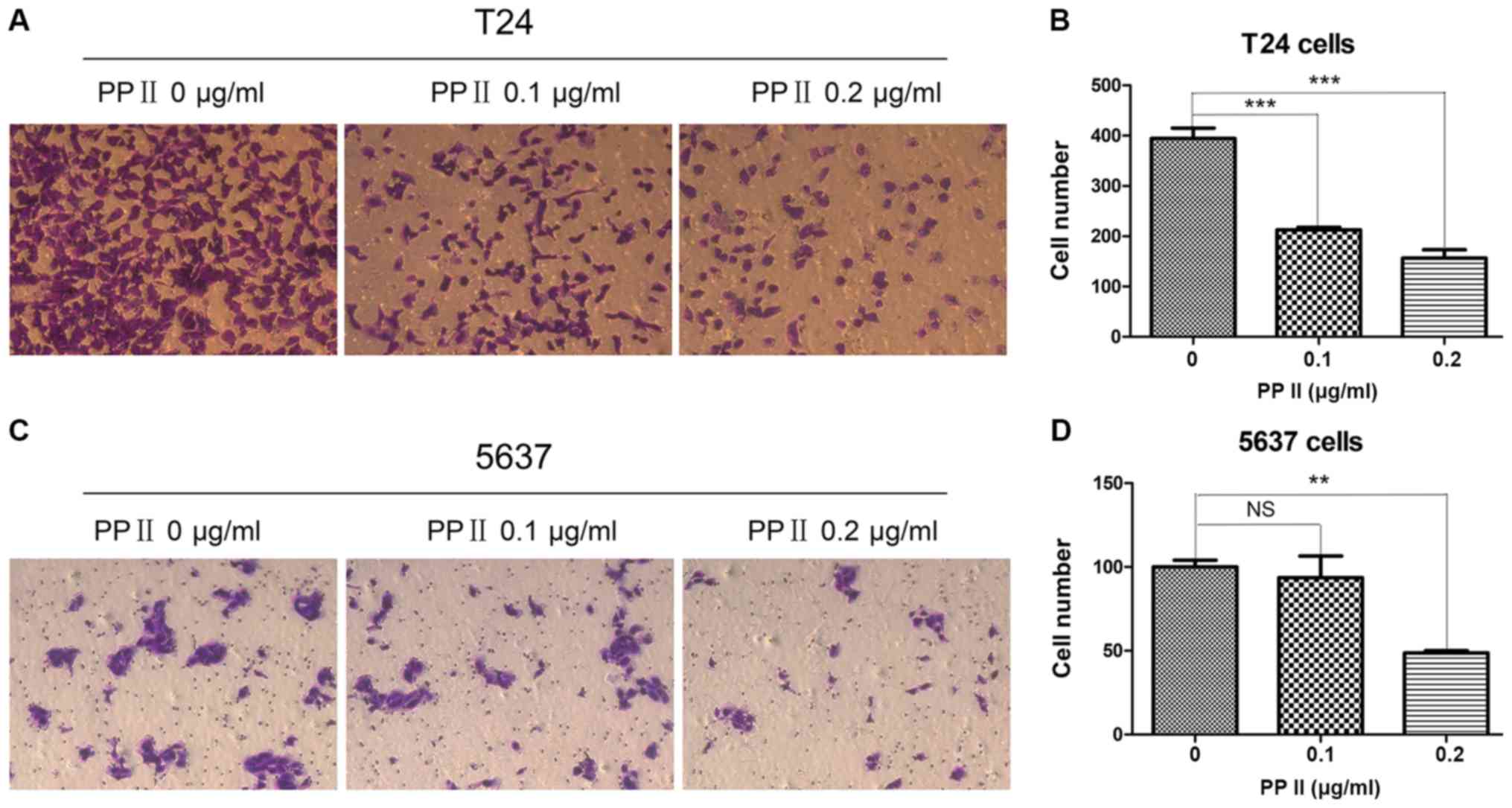
invasive ability of bladder cancer cells was examined by a
Transwell assay. The results indicated that PPII also significantly
decreased the ability of T24 cells (Fig.
6A and B; P<0.001) and 5637 cells at 0.2 µg/ml (Fig. 6C and D; P<0.01) to traverse the
membrane. These results suggested that PPII may inhibit the
invasive and migratory abilities of bladder cancer cells.
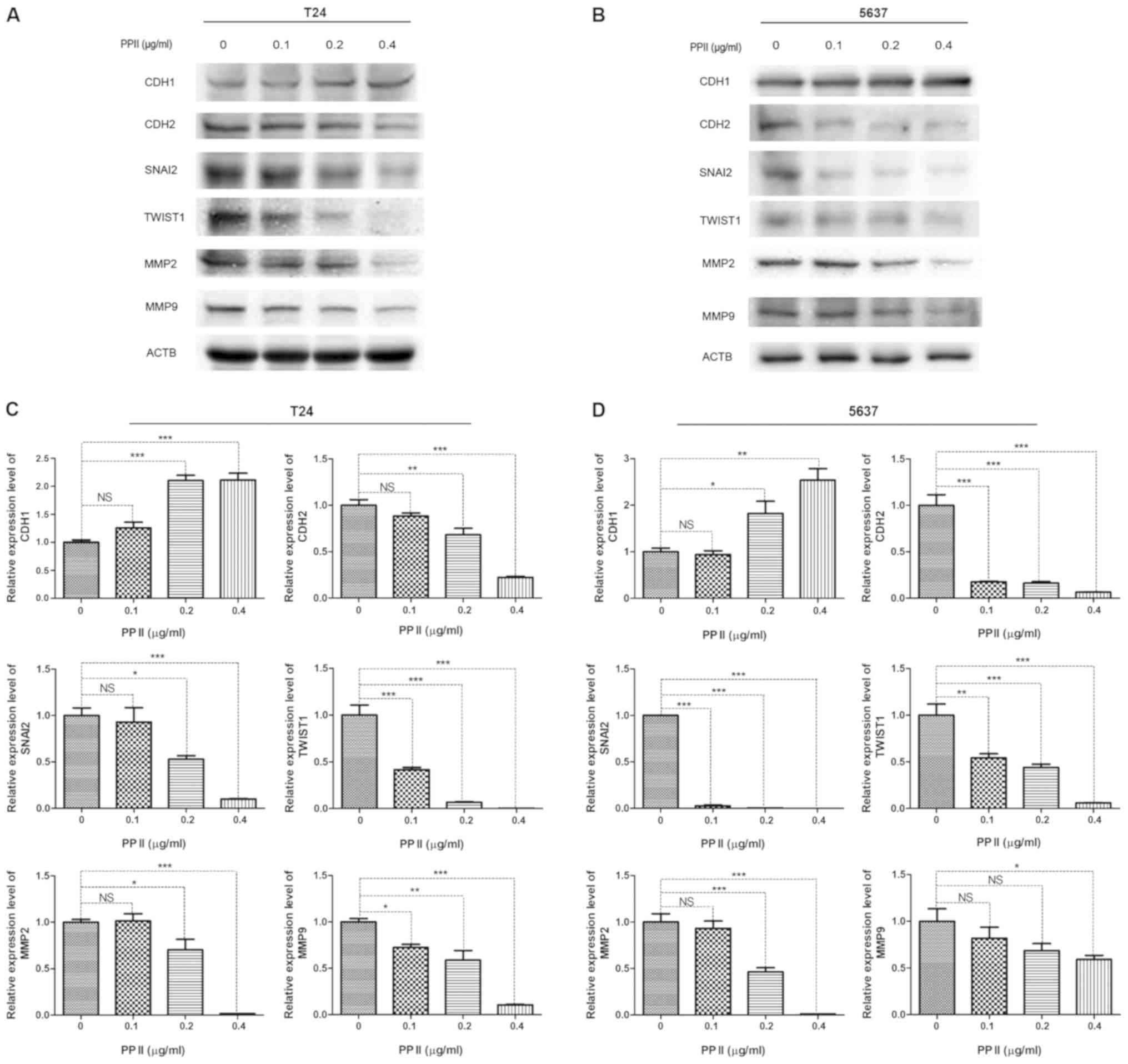
PPII affects the expression of
EMT-associated proteins and MMPs
Since EMT-inducing transcription factors promote
cancer metastasis, the present study examined whether PPII
inhibited bladder cancer migration and invasion by regulating the
expression of EMT-associated proteins. As markers of EMT, the
expression levels of CDH1 and CDH2 were detected by western blot
analysis. The results demonstrated that the expression of CDH1 in
T24 (Fig. 7A) and 5637 (Fig. 7B) cells was significantly increased
following PPII treatment, whereas that of CDH2 was decreased
compared with the control group. In addition, the expression levels
of the transcriptional repressors of CDH1, including SNAI2 and
TWIST1, were decreased in PPII-treated cells compared with those in
the untreated control group (Fig. 7C and
D). The expression levels of gelatinases MMP2 and MMP9, which
serve crucial roles in maintaining extracellular matrix homeostasis
and tumor invasion, were also significantly inhibited by PPII
compared with those in the control group (Fig. 7C and D). These results suggested that
PPII-induced migration and invasion inhibition may occur through
the suppression of EMT and MMPs expression.
Discussion
Although neoadjuvant chemotherapy, surgery and
combinational chemotherapy have been widely used in bladder cancer
treatment, it is still one of the most common tumors with a poor
prognosis and a low 5-year survival rate (1). Drug resistance is a major obstacle to
bladder cancer chemotherapy, and >50% of NMIBC patients will
recur in the future (19). Of note,
bladder cancer has become the most expensive disease among all
types of cancer due to the need for life-long recurrence monitoring
(20), and bladder cancer metastasis
is still a fatal disease. Thus, there is an urgent need to identify
effective drugs to inhibit the progression of bladder cancer,
especially metastasis, and to elucidate the detailed underlying
molecular mechanisms. Increasing evidence has indicated that
heat-clearing and detoxifying Chinese medicines exert anticancer
effects without significant toxic effects (21–24).
Chinese medicine provides a library with which to develop potential
drugs for the prevention of tumor formation and metastasis.
Tumor metastasis is considered the primary lethal
factor for patients with cancer (25). Therefore, the prevention of cancer
metastasis is the key means to improving the survival rate of
patients with cancer (26). In the
present study, with the aim of identifying natural antimetastatic
drugs against bladder cancer and investigating the associated
molecular mechanisms, the effects of six heat-clearing and
detoxifying compounds on the viability of bladder cancer cells were
evaluated. The results demonstrated that bladder cancer cells were
sensitive to PPE, which had an inhibitory effect on cell migration
and invasion. To further investigate the antimetastatic mechanisms
of PPE, the major components PPI, PPII, PPVI and PPVII were applied
to treat bladder cancer cells; the results demonstrated that cancer
cells were more sensitive to PPII compared with the other
polyphyllins. When PPE, PPII and cisplatin were used to treat T24
and 5637 cells, respectively, the IC50 value of PPII was
lower compared with that of PPE and cisplatin. In addition,
exposure to PPII significantly inhibited bladder cancer cell
invasion and migration by regulating the expression of
EMT-associated proteins and MMPs.
EMT is a key step in cancer metastasis characterized
by high expression of mesenchymal markers and low expression of
epithelial markers (27,28). SNAI2 and TWIST1 are the major
transcription factors regulating EMT that contribute to cancer
metastasis via enhanced cell invasion (29). SNAI2 is an essential mediator of
TWIST1-induced EMT (30); thus, the
expression of these two EMT inducers was estimated in the present
study and their levels obviously decreased after PPII treatment. In
addition, CDH1 and CDH2, which are EMT markers, were dysregulated
in bladder cancer cells, consistent with a previous study (31). Of note, the role and involvement of
MMPs in cancer metastasis have been extensively investigated
(32,33). MMP2 and MMP9 are widely studied MMPs
in cancer progression and serve important roles in regulating tumor
angiogenesis, invasion and metastasis mainly through degrading the
extracellular matrix (34). In the
present study, PPII treatment significantly increased the
expression of CDH1 and decreased that of CDH2, SNAI2, TWIST1, MMP2
and MMP9 in bladder cancer cells compared with the untreated
control. These results indicated that PPII may inhibit bladder
cancer cell invasion and migration by regulating the expression of
EMT-related proteins and MMPs.
Anticancer activities of extracts of Paris
polyphylla have been previously reported. PPE suppressed the
proliferation and promoted apoptosis of human osteosarcoma cells
(35). Furthermore, an aqueous
extract of Paris polyphylla was previously demonstrated to
suppress ovarian cancer proliferation and migration in vitro
via downregulation of peroxisome proliferator-activated receptor γ
coactivator 1α (36). However, the
effect of PPE on bladder cancer metastasis has not been
investigated. In the present study, using six heat-clearing and
detoxifying Chinese medicines, it was demonstrated that PPE
significantly suppressed bladder cancer migration and invasion. To
identify the effective component of bladder cancer, PPI, PPII, PPVI
and PPVII were used to treat bladder cancer cells. The results
demonstrated that bladder cancer cells exhibited the highest
sensitivity to PPII; the antitumor effects of PPII have been
previously demonstrated in other cancer cell, such as HepaRG
(37). However, the role of PPII in
inhibiting the metastasis of bladder cancer has not been studied.
The present study broadened the antimetastatic scope of PPII and
verified that PPII exerted an inhibitory effect on the migration
and invasion of bladder cancer cells. However, the antimetastatic
roles of the other polyphyllins should not be ignored; PPI has been
extensively studied and proposed to serve antimetastatic functions,
and its roles in ovarian (38) and
lung (39) cancer have been
identified. Bladder cancer cells were more sensitive to PPII
compared with other polyphyllins in the present study, so PPII was
selected for the experiments, but the antimetastatic functions of
PPI, PPVI and PPVII in bladder cancer will be verified in future
studies.
In conclusion, the results of the present study
demonstrated that PPE inhibited bladder cancer migration and
invasion and that the PPII component served an important role in
the inhibitory effects. These results suggested that PPII may be a
potential candidate for antimetastatic drug design. In addition,
the results of the present study demonstrated that PPII, at least
in part, targeted the expression of EMT-associated proteins and
MMPs to suppress bladder cancer cell migration and invasion. Thus,
these results lay the foundation for further studies of the
antimetastatic mechanisms of PPE and PPII.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Professor Shengtian
Zhao (Shandong Provincial Hospital, Jinan, China) for providing the
T24 and 5637 cell lines.
Funding
The present study was supported by the National
Nature Science Foundation (grant nos. 81573989, 81673807, 81674014
and 81873330), the SDU-KI Cooperative Research Project of Qilu
Hospital of Shandong University (grant no. SDU-KI-2019-13) and the
Shandong Province: Taishan Scholars Project (grant no.
tsqn201909185).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZYL conceived and designed the study. WPN, LX, ZYL,
YXG, WS and YHJ conducted the experiments. JWL, YZ, HQL, CCM and
ZHS performed the statistical analysis. WPN, LX, ZHS, HQL and YXG
drafted the manuscript. JWL, WS, YHJ, CCM, YZ and ZYL revised the
manuscript for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Richters A, Aben KKH and Kiemeney LALM:
The global burden of urinary bladder cancer: An update. World J
Urol. Nov 1–2019.(Epub ahead of print). PubMed/NCBI
|
|
2
|
Antoni S, Ferlay J, Soerjomataram I, Znaor
A, Jemal A and Bray F: Bladder cancer incidence and mortality: A
global overview and recent trends. Eur Urol. 71:96–108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharma S, Ksheersagar P and Sharma P:
Diagnosis and treatment of bladder cancer. Am Fam Physician.
80:717–723. 2009.PubMed/NCBI
|
|
4
|
Allory Y, Beukers W, Sagrera A, Flández M,
Marqués M, Márquez M, van der Keur KA, Dyrskjot L, Lurkin I,
Vermeij M, et al: Telomerase reverse transcriptase promoter
mutations in bladder cancer: High frequency across stages,
detection in urine, and lack of association with outcome. Eur Urol.
65:360–366. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim WJ, Kim EJ, Kim SK, Kim YJ, Ha YS,
Jeong P, Kim MJ, Yun SJ, Lee KM, Moon SK, et al: Predictive value
of progression-related gene classifier in primary non-muscle
invasive bladder cancer. Mol Cancer. 9:32010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Milowsky MI, Rumble RB, Booth CM, Gilligan
T, Eapen LJ, Hauke RJ, Boumansour P and Lee CT: Guideline on
Muscle-Invasive and Metastatic Bladder Cancer (European Association
of Urology Guideline): American Society of Clinical Oncology
Clinical Practice Guideline Endorsement. J Clin Oncol.
34:1945–1952. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quirk JT and Kupinski JM: Chronic
infection, inflammation, and epithelial ovarian cancer. Med
Hypotheses. 57:426–428. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Y, Liang Y and He C: Anticancer
activities and mechanisms of heat-clearing and detoxicating
traditional Chinese herbal medicine. Chin Med. 12:202017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Man S, Gao W, Wei C and Liu C: Anticancer
drugs from traditional toxic Chinese medicines. Phytother Res.
26:1449–1465. 2012.PubMed/NCBI
|
|
11
|
Lee MS, Yuet-Wa JC, Kong SK, Yu B,
Eng-Choon VO, Nai-Ching HW, Chung-Wai TM and Fung KP: Effects of
polyphyllin D, a steroidal saponin in Paris polyphylla, in
growth inhibition of human breast cancer cells and in xenograft.
Cancer Biol Ther. 4:1248–1254. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He H, Zheng L, Sun YP, Zhang GW and Yue
ZG: Steroidal saponins from Paris polyphylla suppress
adhesion, migration and invasion of human lung cancer A549 cells
via down-regulating MMP-2 and MMP-9. Asian Pac J Cancer Prev.
15:10911–10916. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiao X, Zou J, Bui-Nguyen TM, Bai P, Gao
L, Liu J, Liu S, Xiao J, Chen X, Zhang X, et al: Paris saponin II
of Rhizoma Paridis - a novel inducer of apoptosis in human ovarian
cancer cells. Biosci Trends. 6:201–211. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Commission CP: Pharmacopoeia of The
People's Republic of China. 1:(1st). People's Medical Publishing
House. (Beijing). 2015.
|
|
15
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao T, Zhong W, Zhao J, Qian B, Liu H,
Chen S, Qiao K, Lei Y, Zong S, Wang H, et al: Polyphyllin I
suppresses the formation of vasculogenic mimicry via
Twist1/VE-cadherin pathway. Cell Death Dis. 9:9062018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Huang P, Liu X, Xiang Y, Zhang T,
Wu Y, Xu J, Sun Z, Zhen W, Zhang L, et al: Polyphyllin I inhibits
growth and invasion of cisplatin-resistant gastric cancer cells by
partially inhibiting CIP2A/PP2A/Akt signaling axis. J Pharmacol
Sci. 137:305–312. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu H, Meng Q, Lei T and Zhang M:
Nucleophosmin1 associated with drug resistance and recurrence of
bladder cancer. Clin Exp Med. 15:361–369. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fankhauser CD and Mostafid H: Prevention
of bladder cancer incidence and recurrence: Nutrition and
lifestyle. Curr Opin Urol. 28:88–92. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hsiao WL and Liu L: The role of
traditional Chinese herbal medicines in cancer therapy - from TCM
theory to mechanistic insights. Planta Med. 76:1118–1131. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chow SE, Chang YL, Chuang SF and Wang JS:
Wogonin induced apoptosis in human nasopharyngeal carcinoma cells
by targeting GSK-3β and ΔNp63. Cancer Chemother Pharmacol.
68:835–845. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Z, Lv J, Lei X, Li S, Zhang Y, Meng
L, Xue R and Li Z: Baicalein reduces the invasion of glioma cells
via reducing the activity of p38 signaling pathway. PLoS One.
9:e903182014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weifeng T, Feng S, Xiangji L, Changqing S,
Zhiquan Q, Huazhong Z, Peining Y, Yong Y, Mengchao W, Xiaoqing J,
et al: Artemisinin inhibits in vitro and in vivo
invasion and metastasis of human hepatocellular carcinoma cells.
Phytomedicine. 18:158–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fidler IJ and Kripke ML: The challenge of
targeting metastasis. Cancer Metastasis Rev. 34:635–641. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Radisky DC: Epithelial-mesenchymal
transition. J Cell Sci. 118:4325–4326. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kudo-Saito C, Shirako H, Takeuchi T and
Kawakami Y: Cancer metastasis is accelerated through
immunosuppression during Snail-induced EMT of cancer cells. Cancer
Cell. 15:195–206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Casas E, Kim J, Bendesky A, Ohno-Machado
L, Wolfe CJ and Yang J: Snail2 is an essential mediator of
Twist1-induced epithelial mesenchymal transition and metastasis.
Cancer Res. 71:245–254. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F
and Liu Y: TGF beta induced upregulation of malat1 promotes bladder
cancer metastasis by associating with suz12. Clin Cancer Res.
20:1531–1541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Halbersztadt A, Haloń A, Pajak J,
Robaczyński J, Rabczynski J and St Gabryś M: The role of matrix
metalloproteinases in tumor invasion and metastasis. Ginekol Pol.
77:63–71. 2006.(In Polish). PubMed/NCBI
|
|
34
|
Klein G, Vellenga E, Fraaije MW, Kamps WA
and de Bont ES: The possible role of matrix metalloproteinase
(MMP)-2 and MMP-9 in cancer, e.g., acute leukemia. Crit Rev Oncol
Hematol. 50:87–100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yao N, Ren K, Wang Y, Jin Q, Lu X, Lu Y,
Jiang C, Zhang D, Lu J, Wang C, et al: Paris polyphylla
suppresses proliferation and vasculogenic mimicry of human
osteosarcoma cells and inhibits tumor growth in vivo. Am J Chin
Med. 45:575–598. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang CW and Tai CJ, Choong CY, Lin YC, Lee
BH, Shi YC and Tai CJ: Aqueous extract of Paris polyphylla
(AEPP) inhibits ovarian cancer via suppression of peroxisome
proliferator activated receptor gamma coactivator (PGC) 1alpha.
Molecules. 21:7272016. View Article : Google Scholar
|
|
37
|
Wang W, Dong X, You L, Sai N, Leng X, Yang
C, Yin X and Ni J: Apoptosis in HepaRG and HL-7702 cells inducted
by polyphyllin II through caspases activation and cell-cycle
arrest. J Cell Physiol. 234:7078–7089. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gu L, Feng J, Xu H, Luo M and Su D:
Polyphyllin I inhibits proliferation and metastasis of ovarian
cancer cell line HO 8910PM in vitro. J Tradit Chin Med.
33:325–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lou W, Chen Y, Zhu KY, Deng H, Wu T and
Wang J: Polyphyllin I overcomes EMT-associated resistance to
erlotinib in lung cancer cells via IL-6/STAT3 pathway inhibition.
Biol Pharm Bull. 40:1306–1313. 2017. View Article : Google Scholar : PubMed/NCBI
|