Introduction
Extranodal natural killer/T cell lymphoma-nasal type
(EN-NK/T-NT) is a type of lymphoma which primarily occurs in the
nasal cavity and nasal pharynx (1–5).
Furthermore, the incidence rate is higher in Asian countries
compared with that in Western countries (1–4). In a
study published in 2017, the incidence rate in Asia and Latin
America (10% of all non-Hodgkin's lymphoma) was reported to be
higher than that in Europe and North America (<1%) (5). As shown in our previous study,
EN-NK/T-NT is the most common subtype of peripheral T cell lymphoma
in China (1). EN-NK/T-NT occurs
primarily in adult males, and often presents as a localized disease
involving the nasal cavity or its surrounding structures;
furthermore, it is characterized by vascular destruction, necrosis
and a cytotoxic immunophenotype (1–4).
EN-NK/T-NT is named ‘NK/T’ as opposed to ‘NK’ primarily as the
majority cases appear to be true NK-cell tumors, a few cases can
manifest as cytotoxic T-cell immunophenotype (2,3).
Epstein-Barr virus (EBV) infection can be detected in the tumor
cells in the form of clonal epistasis, which suggests that this
virus might have an important role in the pathogenesis of lymphoma,
independent of race and geographical distribution (2,3).
The association between EN-NK/T-NT and EBV infection
was first described in 1988 and assists in the diagnostic and
pathological understanding of the disease (6). Harabuchi et al (7) and Ho et al (8) identified an association between
EN-NK/T-NT and EBV using Southern blot hybridization for EBV DNA.
Moreover, a seminar co-sponsored by the University of Hong Kong and
the Society for Hematopathology held on October 9, 1994, discussed
the definition, diagnosis, differential diagnosis and epidemiology
of angiocentric lymphomas occurring in the nose and other
extra-nodal sites, including the skin, subcutis and
gastrointestinal tract (9). The term
‘nasal T/natural killer (NK) cell lymphoma’ identifies its
association with EBV, which assists in the clinical diagnosis of
the disease; however, the term lacks a definition of lineage. Thus,
the term EN-NK/T-NT has been classified as a clinicopathological
disease, which is associated with EBV by the World Health
Organization (WHO) since 2001 (2,3,10,11). In
the WHO 2008 classification, the presence of EBV was included in
the definition of the disease by evaluating the EBV-encoded small
RNA (EBER), and EBV may be associated with the pathogenesis of the
disease (2). Positive identification
of EBER is considered to be a requisite for the diagnosis of this
disease, and the detection of EBER using in situ
hybridization (ISH) in paraffin-embedded samples remains the gold
standard for EBV detection, as expression of latent
membraneprotein1 (LMP1) is inconsistently detected in EBV-positive
tumors (2,3,11).
Furthermore, the combination of immunostaining of CD3, CD20, CD56,
TIA1 and granzyme B, and T-cell receptor (TCR) gene rearrangement
analysis is required for the accurate diagnosis of T cell and NK
cell lymphomas (1–3,10).
EN-NK/T-NT is associated with EBV infection, which
is different from other types of mature T/NK cell lymphoma,
including peripheral T-cell lymphoma, not otherwise specified,
anaplastic large cell lymphoma, adult T-cell leukemia/lymphoma and
hepatosplenic T-cell lymphoma (1–3,10). Previous studies have revealed that
EBER can be detected in nearly all EN-NK/T-NT cases (1–3,10). Moreover, there is a higher incidence
rate of EBV infection in EN-NK/T-NT in Asian compared with Western
countries; however, cases without detectable EBV may still be
suspected of diagnosis (2,3,12).
Classic features of EN-NK/T-NT have been widely accepted and
include patients being from Asian and Central and South American
countries, the tumor being located in the upper aerodigestive
tract, being morphologically characterized by vascular destruction
and necrosis, expressing NK or T cell markers, and ≥1 cytotoxic
molecules, consistent association with EBV and germline TCR gene;
however, controversy remains regarding atypical or discordant
cases, such as the tumor occurring in other extranodal sites
(including the skin, subcutis, testes and gastrointestinal tract),
the tumor cells being small and mixed with inflammatory
infiltratory cells without angiodestruction characteristic, and
demonstrating an atypical phenotype (such as being CD3-, CD56- or
TIA-1-negative and CD30-positive, and having an aberrant CD20
expression), and particularly the absence of EBV (9,13).
A previous seminar by the Society for
Hematopathology/European Association for Hematopathology on the
NK/T cell malignant tumors (13)
discussed three cases with typical location or immunotype of
EN-NK/T-NT, but with EBV-negative expression. Moreover, the seminar
assessed challenges faced with the classical definition of the
disease; however, the lymphomas classification involving NK
features remains controversial. Data on patients with EBV-negative
EN-NK/T-NT are limited. The present study identified seven
EBV-negative cases from a total of 99 EN-NK/T-NT, and
retrospectively analyzed the clinicopathological and molecular
characteristics of the lymphoma in China. Furthermore, the results
were also compared with that in EBV-positive cases.
Materials and methods
Patients and tissue samples
The pathology archives at the Department of
Pathology, First Hospital of Peking University, as well as the data
files, were searched between January 2001 and December 2016, and 99
EN-NK/T-NT cases were identified. In addition, seven patients with
EBV-negative EN-NK/T-NT were further analyzed, retrospectively, for
clinical information, including age, sex, location (including the
upper aerodigestive tract and others), Ann Arbor stage (14), treatment (chemotherapy and/or
radiotherapy) and survival status. The follow-up data were
available for 62 patients, including 5 EBV-negative EN-NK/T-NT
patients. Histologic sections were stained with hematoxylin and
eosin, and all the sections in the database were reviewed by three
pathologists blinded to the study. The pathologic diagnosis
criteria were based on the 2001, 2008 and 2016 WHO classification:
i) Patients presenting with extranodal/upper aerodigestive tract
lesion; ii) tumor cells were evaluated for the presence of
cytological features, angiocentricity or angioinvasion, necrosis
and inflammatory cells; iii) immunophenotyping, including the
expression of CD3, CD56 and cytotoxic molecules (TIA1 and granzyme
B) in the absence of B-cell markers (CD20); and iv) EBER-positive
expression using ISH (2,3,10). An
angiocentric pattern could only be assessed in 84 patients and
necrosis in 91 patients due to sampling, as many nasal biopsies
were small, and the tumor cells may have been deformed or
degenerated.
Immunophenotypical analysis
Immunohistochemical staining was observed under a
light microscope with ×400 magnification. Due to the extremely
limited samples, it was not possible to analyze all the markers on
the same sample simultaneously. Immunohistochemistry (IHC) was
performed on a total of 99 samples using 4-µm thick sections from
representative formalin-fixed (using 10% formalin at room
temperature for 24 h) and paraffin-embedded tissue blocks, using
the Dako EnVision detection kit (Dako; Agilent Technologies, Inc.).
Briefly, before dewaxing, the tissue section was heated to 65°C for
ten min to remove the wax. The slides were subsequently washed
twice with xylene for dewaxing for 10 min, then dehydrated in an
ethanol descending gradient series (100, 100, 95,80 and 70%, for 2
min each time), and washed with distilled deionized water. Next,
the slides were washed with PBS (5 times, 10 min each time), and
the tissue sections underwent heat-induced antigen retrieval in
EDTA-Tris (pH 9.0) at 97°C for 20 min (PT Link; Dako; Agilent
Technologies, Inc.). After the samples were washed with PBS for an
additional 10min, 3% hydrogen peroxide was used to treat the
samples for 10 min, followed by an additional wash with PBS for 5
min. Tissues were subsequently probed with primary antibodies for 1
h at room temperature. The following primary antibodies were used:
Anti-CD3 (cat. no. LN10; 1: 50–100 depending on the tissue samples;
OriGene Technologies, Inc.), anti-CD56 (cat. no. UMAB83; 1:100;
OriGene Technologies, Inc.), anti-CD20 (cat. no. L26; 1:100; Dako;
Agilent Technologies, Inc.), anti-TIA-1 (cat. no. 2G9A10F5; 1:100;
OriGene Technologies, Inc.), anti-granzyme B (cat. no. EP230; 1:
50; OriGene Technologies, Inc.), anti-T-bet (cat. no. H-210; 1:100;
Santa Cruz Biotechnology, Inc.) and ETS1 (cat. no. C-4; 1:100;
Santa Cruz Biotechnology, Inc.). The tissue sections were
additionally washed with PBS (3 times, 5 min each time) and then
probed for 20 min with a secondary antibody conjugated to
horseradish peroxidase (cat. no. PV-6000-D; 1: 500; OriGene
Technologies, Inc.) at 37°C for 30 min. An additional PBS wash (3
times, 5 min each time) was subsequently performed, and the
chromogenic 3,3′-diaminobenzidine mixture (OriGene Technologies,
Inc.) was used to stain the samples at room temperature for 5 min.
Then, the samples were dehydrated with an ascending ethanol series
(75, 95, 100 and 100%) and washed with xylene, and natural gum was
used to seal the samples.
The staining results were semi-quantitatively
assessed using the following criteria: i) -, Positive cell ≤5%; ii)
1 +, positive cell 6–20%; iii) 2 +, positive cell 21–50%; and iv) 3
+, positive cell >50% (15).
Positive and negative controls were used. From the aforementioned
pathology archives, normal lymph nodes were used as the positive
control for CD3 and CD20 expression, and EN-NK/T-NT samples with
definite diagnosis were used as the positive control for CD56,
TIA1, granzyme B, T-bet and ETS1 expression. PBS was used as the
negative control.
In addition, immunohistochemical analysis was
performed for anti-PR/SET domain 1 (PRDM1) (cat. no. C14A4; 1:100;
Cell Signaling Technology, Inc.). Nuclear staining of PRDM1 in
>10% of tumor cells was interpreted as positive. PRDM1 staining
was semi-quantitatively assessed according to the follow criteria:
i) -, No positive cell or positive cell <10%; ii) 1+, positive
cell 10-≤50%; iii) 2+, positive cell >50-100%). For the negative
control reactions, PBS was used (16). Analysis of the IHC results from the
database was performed by three pathologists blinded to the study.
Due to some samples being of poor quality, the results of some
markers were lost.
ISH analysis
All cases were tested for EBER using ISH according
to manufacturer's instructions. The probe for EBER-1 was supplied
by OriGene Technologies, Inc. To assess staining, an EBV-positive
nasopharyngeal carcinoma sample from our tissue bank was used as a
positive control. Tumor nuclei stained with brown granules were
interpreted as positive. The percentage of positive tumor cells was
semi-quantitatively estimated as the standard in IHC. The seven
EBV-negative cases were assessed two times for the 2 consulted
cases and three times for the other 5 cases by repeat assays.
Molecular analysis for TCR gene
rearrangement
TCR gene rearrangement was investigated in 7/99
cases, including 2 EBV-positive cases and 5 EBV-negative cases. TCR
gene rearrangement was not performed for the remaining two
EBV-negative consulted cases due to limited paraffin blocks. DNA
was extracted from paraffin-embedded tissue samples using a Qiagen
DNeasy blood and tissue kit (Qiagen GmbH), according to the
manufacturer's instructions. TCR-γ (TCRG) and TCR-β (TCRB) chain
clonality analysis was performed using PCR with the Identi Clone
T-cell clonality assays (Invivoscribe, Inc.). The TCRG/B gene
primers and the PCR protocols were designed according to a previous
study (17). The PCR products were
analyzed via 10% polyacrylamide gel electrophoresis, stained with 1
µg/ml ethidium bromide and observed under an ultraviolet
illuminator. The results were interpreted following the
manufacturer's instructions.
Fluorescence in situ hybridization
(FISH) analysis
A total of 2 EBV-negative EN-NK/T-NT cases, from
limited specimens of paraffin-embedded samples, were analyzed using
FISH to detect gene aberration following the manufacturer's
instructions. The DNA probes 6q21 (length, 409 kb) and PRDM1
(length, not available) FISH were used to detect the deletion of
these two genes (Empire Genomics LLC). Slides were prepared and the
results were analyzed as previously described (16).
Statistical analysis
The association between EBV expression and the
clinicopathological characteristics of the patients, including age,
sex, primary sites, Ann Arbor stage, angiocentricity and/or
angioinvasion, necrosis and the expression levels of IHC markers
(CD56, TIA1, granzyme B, T-bet and ETS1) were analyzed using either
Fisher's exact or χ2 tests. The clinicopathological
features of EBV-positive and -negative cases, which occurred in the
upper aerodigestive tract were also compared using either Fisher's
exact or χ2 tests. Overall survival (OS), which was
defined as the day of initial diagnosis to the day of mortality due
to any cause or the last follow-up, was determined using the
Kaplan-Meier method and the comparison of differences between the
OS of the EBV-positive and -negative groups was evaluated using the
log-rank test. All the data are presented as number of cases, and
all statistical analyses were performed three times using SPSS
software (v23.0; IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical data
Of the 99 patients with EN-NK/T-NT, there were 57
men and 42 women (male:female ratio, 1.6:1). The median age was 43
years (range, 8–88 years). For one patient, the biopsy location and
follow-up information were not available, leaving a total of 98
biopsies. The most common biopsy sites for initial diagnosis
included the upper aerodigestive tract, which accounted for 75.5%
(74/98) of cases, followed by 24.5% (24/98) of non-upper
aerodigestive tract involvement, including the skin, lymph node,
gastrointestinal tract and uterine cervix. There were clinical
stage data for 64 patients, in which 23 patients (35.9%) presented
at stage I–II, whilst 41 patients (64.1%) were stage III–IV, due to
bone marrow and/or liver involvement. Furthermore, 30/58 patients
(51.7%) showed B symptoms (fever, malaise and weight loss) on
disease presentation.
The clinical characteristics of the seven patients
with EBV-negative EN-NK/T-NT (median age, 32 years; age range, 8–59
years) are summarized in Table I.
The results indicated a high female predominance (male:female
ratio, 1:6). In addition, there was a significant difference in the
sex of the patients between EBV-positive and -negative expression
(P=0.045; Table II). The initial
involvement sites of the seven patients were all in the upper
aerodigestive tract, and 5/7 patients were diagnosed at Ann Arbor
stage I while three patients had accompanying B symptom (Table I). There were no significant
differences in age, involvement sites and stage between patients
with EBV-positive and -negative expression (P>0.05; Table II).
 | Table I.Summary of Clinical findings of EBV
negative EN-NK/T-NT. |
Table I.
Summary of Clinical findings of EBV
negative EN-NK/T-NT.
| Case no. | Age, years | Sex | Primary site | Ann Arbor
stage | Therapy | Time, months | Survival |
|---|
| 1 | 36 | Female | Nasopharynx | IA | NA | NA | NA |
| 2 | 31 | Male | Nasal septum
posterior extremity | IVB | ND | 1 | Died of
disease |
| 3 | 24 | Female | Right tonsil | IA | R | 113 | CR |
| 4 | 59 | Female | Hard Palate | IA | NA | NA | NA |
| 5 | 8 | Female | back of the
tongue | IVB | C | 9 | Died of MOF |
| 6 | 32 | Female | Skin of the left
ala nasi | IB | C+R | 40 | CR |
| 7 | 54 | Female | Nasal cavity | IA | C+R | 37 | CR |
 | Table II.Differences in the clinical and
pathological features between EBV-positive (n=92) and -negative
cases (n=7). |
Table II.
Differences in the clinical and
pathological features between EBV-positive (n=92) and -negative
cases (n=7).
| Characteristic | EBV-positive,
n | EBV-negative,
n | P-value |
|---|
| Age, years |
|
| 0.345 |
|
>60 | 21 | 0 |
|
|
≤60 | 71 | 7 |
|
| Sex |
|
| 0.045a |
|
Male | 56 | 1 |
|
|
Female | 36 | 6 |
|
| Primary sites |
|
| 0.845 |
|
Nasal | 69 | 6 |
|
|
Extranasal | 23 | 1 |
|
| Ann Arbor
stage |
|
| 0.495 |
|
I/II | 20 | 5 |
|
|
III/IV | 39 | 2 |
|
| NA | 33 | 0 |
|
| B symptom |
|
| 0.999 |
|
Yes | 28 | 3 |
|
| No | 27 | 4 |
|
| NA | 37 | 0 |
|
| Angioinvasive |
|
| 0.183 |
|
Yes | 42 | 1 |
|
| No | 36 | 6 |
|
| NA | 14 | 0 |
|
| Necrosis |
|
| 0.121 |
|
Yes | 73 | 4 |
|
| No | 11 | 3 |
|
| NA | 8 | 0 |
|
| CD56 |
|
| 0.581 |
| 0 | 11 | 0 |
|
| 1+ | 12 | 2 |
|
| 2+ | 23 | 1 |
|
| 3+ | 39 | 4 |
|
| NA | 7 | 0 |
|
| TIA1 |
|
| 0.508 |
| 0 | 3 | 0 |
|
| 1+ | 11 | 2 |
|
| 2+ | 23 | 1 |
|
| 3+ | 53 | 4 |
|
| NA | 2 | 0 |
|
| Granzyme B |
|
| 0.315 |
| 0 | 5 | 1 |
|
| 1+ | 18 | 3 |
|
| 2+ | 25 | 1 |
|
| 3+ | 40 | 2 |
|
| NA | 4 | 0 |
|
| T-bet |
|
| 0.015a |
| 0 | 2 | 2 |
|
| 1+ | 13 | 2 |
|
| 2+ | 35 | 1 |
|
| 3+ | 40 | 2 |
|
| NA | 2 | 0 |
|
| ETS-1 |
|
| 0.097 |
| 0 | 21 | 1 |
|
| 1+ | 28 | 3 |
|
| 2+ | 24 | 0 |
|
| 3+ | 12 | 3 |
|
| NA | 7 | 0 |
|
| Survival |
|
| 0.762 |
|
Alive | 24 | 3 |
|
|
Dead | 33 | 2 |
|
| NA | 35 | 2 |
|
Histology results
The histological examination of all the 99
extranodal/non-nasal lesion biopsy specimens revealed similar
histological characteristics (data not shown). For example, the
morphological lineage of tumor cells was extensive, characterized
by mixed cell types of small, medium to large size. Furthermore,
there was irregular nuclear morphology, chromatin granules,
inconspicuous or small nucleoli and minimal-to-medium cytoplasm. In
addition, there was a variable magnitude of inflammatory cells,
including small lymphocytes, histiocytes, plasma cells, eosinophils
and neutrophils. An angiocentric and/or angioinvasion pattern was
also observed in 43/84 patients (51.2%) and necrosis of tumor
tissue in 77/91 (84.6%) cases (data not shown).
Among the seven EBV-negative EN-NK/T-NT cases,
epidermotropism, which was characterized by the invasion of tumor
cells into the glandular epithelium or the surface mucosa, was
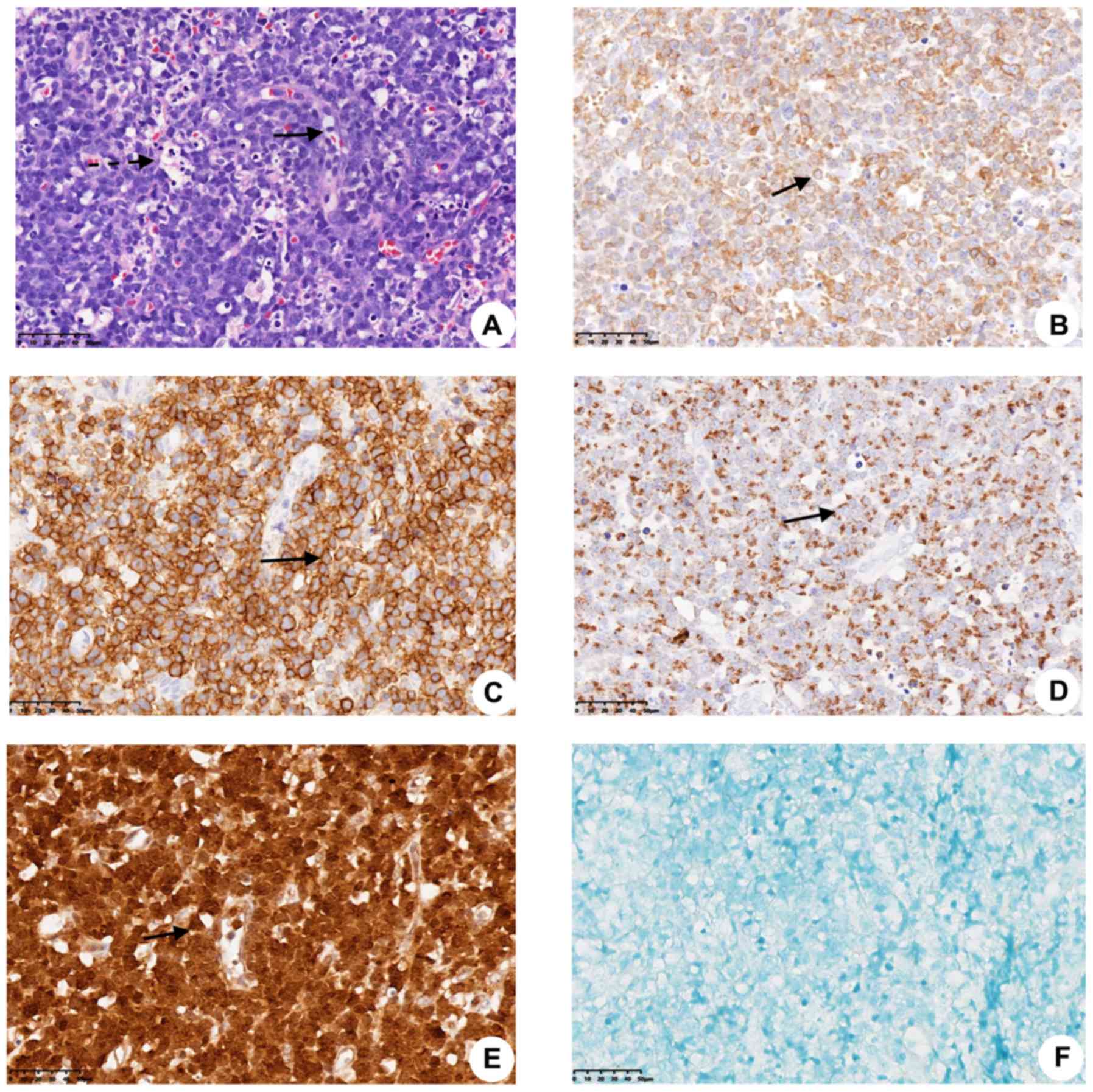
observed in 5/7 cases (Fig. 1A). In
addition, an angiocentric and/or angiodestructive infiltration was
found in 1/7 (14.3%) cases, and necrosis was observed in 4/7
(57.1%) cases (Fig. 1A; Table III). However, no significant
differences were demonstrated in the morphological features between
EBV-positive and -negative cases (Table
II).
 | Table III.Summary of the pathology results in
EBV-negative extranodal NK/T-cell lymphoma, nasal type cases. |
Table III.
Summary of the pathology results in
EBV-negative extranodal NK/T-cell lymphoma, nasal type cases.
|
|
|
| Protein
expression |
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Case no. | Angioinvasive | Necrosis | CD3 | CD56 | TIA-1 | GB | T-bet | ETS1 | PRDM1 | EBER | TCR | 6q21 deletion | PRDM1 gene
deletion |
|---|
| 1 | No | No | 1+ | 1+ | 1+ | – | – | – | – | – | No | ND | ND |
| 2 | No | Yes | 3+ | 1+ | 2+ | 2+ | 1+ | 1+ | – | – | No | ND | ND |
| 3 | No | No | 2+ | 3+ | 1+ | 1+ | 3+ | 3+ | + | – | No | No | No |
| 4 | No | Yes | 3+ | 3+ | 3+ | 1+ | 1+ | 1+ | + | – | No | ND | ND |
| 5 | No | No | 3+ | 3+ | 3+ | 1+ | – | 3+ | – | – | No | Yes | Yes |
| 6 | No | Yes | 3+ | 2+ | 3+ | 3+ | 3+ | 3+ | ND | – | ND | ND | ND |
| 7 | Yes | Yes | 3+ | 3+ | 3+ | 3+ | 2+ | 1+ | – | – | ND | ND | ND |
Immunophenotypical results
All 99 cases of EN-NK/T-NTs had negative and
positive staining for CD20 and CD3 expression, respectively. There
was also positive expression for CD56 in 81/92 cases (88%), TIA-1
in 94/97 cases (96.9%) and granzyme B in 89/95 cases (93.7%).
Furthermore, there were more patients who were positive for T-bet
than for ETS-1, 93/97 (95.9%) and 70/92 (76.1%), respectively. In
addition, PRDM1 expression was observed in 6/23 cases (26.1%).
All the seven cases of EBV-negative EN-NK/T-NTs were
CD3-positive (7/7; 100%; Fig. 1B)
and CD20-negative (7/7; 100%). In addition, more than half the
patients had positive expression for CD56 (7/7; 100%; Fig. 1C), TIA1 (7/7; 100%; Fig. 1D), granzyme B (6/7; 85.7%; Fig. 1E), T-bet (5/7; 71.4%; data not shown)
and ETS (16/7; 85.7%; data not shown). The levels of expression for
the afore mentioned markers are shown in Table III. There was weak positive
expression for PRDM1in only two cases (2/6; 33.3%). Furthermore,
all the immunostaining results were similar with those found in the
EBV-positive cases (Table III).
Among the aforementioned markers, a significant difference in the
expression of T-bet between EBV positive and negative expression
was found (P=0.015; Table
III).
EBV in situ hybridization
The results indicated that the tumor cells in 92/99
EN-NK/T-NT cases (92.9%) were positive for EBER mRNA using ISH, and
the seven cases were EBER-negative (Table II; Fig.
1F). Additionally, the present study reviewed the literature
and identified that there were indeed some EBV-negative EN-NK/T-NT
cases, most of which were published in the form of case reports or
small series (Table IV).
 | Table IV.Distribution of cases reported as
EN-NK/T-NT without EBV infection in different studies or case
reports. |
Table IV.
Distribution of cases reported as
EN-NK/T-NT without EBV infection in different studies or case
reports.
| Author, year | Country of
residence | Number of cases
(%) | (Refs.) |
|---|
| Harabuchi et
al, 1996 | Japan | 2/18 (11) | (44) |
| Nakamura et
al, 1997 | Japan | 5/32 (16) | (12) |
| Cuadra-Garcia et
al, 1999 | USA | 1/14 (7) | (45) |
|
Quintanilla-Martinez et al,
1999 | Peru | 1/28 (4) | (46) |
| Ko et al,
2000 | Korea | 6/46 (13) | (4) |
| Jung et al,
2001 | Korea | 4/13 (31) | (47) |
| Ohshima et
al, 2002 | Japan | 3/9 (33) | (48) |
| Kim et al,
2003 | Korea | 10/35 (29) | (49) |
| Ko et al,
2004 | Korea | 11/49 (22) | (26) |
| Ng et al,
2004 | Singapore | 1/42 (2) | (50) |
| Miyazato et
al, 2004 | Japan | 11/34 (32) | (51) |
| Tai et al,
2004 | Malaysia | 1/20 (5) | (52) |
| Cabrera et
al, 2007 | Chile | 2/9 (22) | (53) |
| Matsuda et
al, 2009 | Japan | 1/1 (100) | (54) |
| Teo et al,
2011 | China | 1/1 (100) | (22) |
| Bouchekioua et
al, 2013 | France | 1/23 (4) | (24) |
| Kim et al,
2015 | Korea | 1/1 (100) | (55) |
| Tian et al,
2015 | China | 1/1 (100) | (56) |
| Nicolae et
al, 2017 | USA | 7/7 (100) | (27) |
| Tsuyama et
al, 2018 | Japan | 1/1 (100) | (23) |
| Zeng et al,
2017 | China | 22/56 (39) | (57) |
| Asif et al,
2019 | USA | 1/1 (100) | (43) |
Genotype results
TCR gene rearrangement was detected in only 7/99
EN-NK/T-NT cases at diagnosis, of which 6/99 (6.1%) had germline
TCR gene rearrangements. However, 5/7 (71.4%) EBV-negative cases
all had germline TCR gene rearrangement, thus suggesting an origin
in the NK-lineage (Table II). FISH
detection of 6q21 and PRDM1 was performed in only two EBV-negative
EN-NK/T-NTs specimens. In one case, both 6q21 and PRDM1 genes were
deleted, while in the other case neither gene was abnormal
(Table II).
Therapy, outcome and statistical
analysis
In total, 62/99 patients had available follow-up
data and the median OS was 22 months (range, 1–147 months).
Overall, 43.5% (27/62) of patients were alive with or without
lymphoma, while mortality occurred in 56.5% (35/62) of patients at
the end of the follow-up period, due to tumor progression or
related complications, as a result of drug toxicity, infection,
systemic failure or other unknown reasons.
There was follow-up data for 5/7 patients with
EBV-negative EN-NK/T-NT. The median OS was 37 months (range, 1–133
months) and the median follow-up time was 40 months (range, 37–113
months). Furthermore, 3/5(60%) patients were in remission following
local radiotherapy or combined radiotherapy and chemotherapy. In
total, mortality occurred in 2/5 patients (40%) due to rapid
disease progression, both of whom died at stage IVB. In particular,
1 patient stopped treatment due to economic reasons and mortality
occurred rapidly within 1 month following diagnosis. The remaining
four patients were treated, including one patient receiving only
chemotherapy, one receiving radiotherapy alone and two patients
receiving both radiotherapy and chemotherapy. Progressive
dissemination and chemo-resistance developed in 1 patient, and
mortality occurred due to multi-organ failure within 9 months.
Treatment and follow-up data are shown in Table I. There was no significant difference
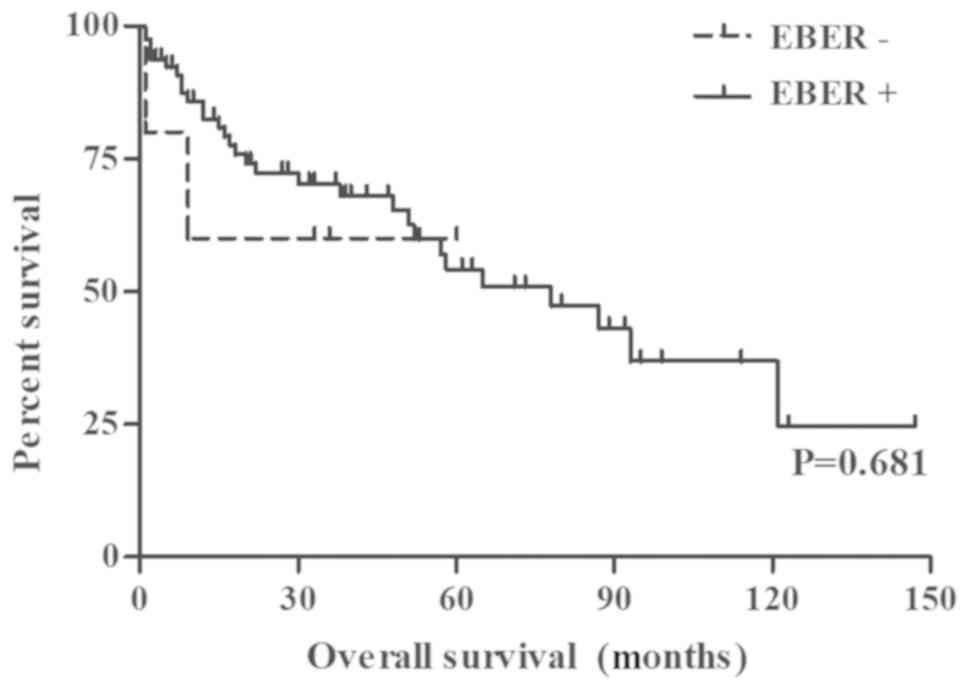
in OS between the EBV-positive and EBV-negative cases (P=0.762;
Fig. 2; Table III). Furthermore, EN-NK/T-NTs
occurred in the upper aerodigestive tract, irrespective of
EBV-positive or EBV-negative status, and were hypothesized to have
similar clinicopathological features, except for gender (P=0.037;
Table SI) and T-bet expression
(P<0.001; Table SI).
Discussion
EN-NK/T-NT is an extranodal aggressive mature T or
NK-cell lymphoma, which has been associated with EBV infection and
cytotoxic tissue-destructive, and its incidence rate is higher in
Asian countries compared with that in Western countries (1–4,10,12). A
study published in 2017 reported that the incidence rate in Asia
and Latin America (10% of all non-Hodgkin's lymphoma) was higher
than that in Europe and North America (<1%) (5). However, EBV-negative EN-NK/T-NT is
rare, even in Asia, where there is a high infection rate of EBV
(3,18). The findings of the present study are
similar with those of EBV-negative EN-NK/T-NT cases obtained
through a literature review (Table
IV). EBV-negative EN-NK/T-NT is rarely seen, most of which are
case report or a small series of studies and the geographical
distribution is mainly in Asian countries and other countries such
as Central America and South America (Table IV). Whether these atypical cases
should be defined as EBV-negative EN-NK/T-NT of the same disease or
as an independent EN-NK/T-NT remains controversial, and the
detailed clinicopathological features are limited.
In the etiology study of T-cell and NK-cell tumors,
malignant transformation caused by EBV infection is a well-known
factor in tumorigenesis, although the exact carcinogenic function
of EBV remains unknown (2,3,11).
Immunosuppression is an important risk factor for individuals who
are susceptible to EBV infection-mediated malignant transformation
(19). However, EBV has been
associated with NK/T cell lymphoma in individuals who are not
immunocompromised, suggesting that EBV infection may be
opportunistic (20). In addition,
most patients with persistent EBV infection will never develop
EN-NK/T-NT, indicating that EBV does not function alone and that it
is likely that host genetic and environmental cofactors or
lifestyle differences are also implicated (11,21).
Moreover, it is suspected that NK or T cells may be infected when
the organism's immunity is reduced or immunosuppressed, and undergo
malignant transformation, which may be caused by other pathogenic
factors unrelated to EBV infection (2,11,19),
such as the possibility of ethnic, geographic heterogeneity,
genetic background and environmental cofactors, or lifestyle
differences (11,21); however, this requires further
investigation.
The present study described the clinicopathological
features of seven patients with lymphoma of the upper aerodigestive
tract, with the presence of neoplasms morphology and angioinvasion.
Furthermore, these patients presented with necrosis and expressed
the NK/T-cell phenotype (positive expression for CD3, CD56, TIA1
and granzyme B). TCR gene rearrangement analysis was not routinely
performed due to the poor quality of specimens, including small
sample size and/or wide necrosis areas. It was found that two cases
had monoclonal TCR gene rearrangements and were therefore
classified as T cell lineage, while the remaining cases were
categorized into NK cell lineage. The diagnosis of EN-NK/T-NT was
supported by the comprehensive analysis of clinicopathological
features, immunophenotypic data and molecular results, but had a
negative expression of EBV, which is inconsistent with the
diagnostic criteria defined by the WHO (2,3,8). However, the results of the present
study and the research shown in Table
IV indicates that EBV-negative EN-NK/T-NT does exist, and
EBV-negative results do not exclude the possibility of an
EN-NK/T-NT diagnosis.
The existence of the EBV-negative cases requires
further investigation to determine whether: i) EBV is required or
solely acts as a ‘passenger’ in the oncogenic functions of
EN-NK/T-NT (22); ii) there is
another latent pattern of EBV expression that does not express
EBER, or alternative oncogenic mechanisms other than infection of
EBV, such as recurrent mutations in MLL2, BCOR, STAT3, JAK3, TP53
and KDM6A genes (23); iii) some
patients with EBV-negative cytotoxic lymphoma with NK cell
characteristics may lose EBV expression during clone amplification
(24,25).
In the present study, all the patients with EBV
negative EN-NK/T-NT were of Chinese ethnicity, which is consistent
with previous studies in which EN-NK/T-NT occurs at a higher rate
in Asian countries (1–4,10). The
median age of the patients was 32 years, which is slightly younger
compared with that in previous studies (2-4,10,12,23). Moreover,
there was a higher number of females, which is in contrast with
that in previous studies on EN-NK/T-NT, which found that males are
more frequently affected (2–4,10,12). The
present study compared the sex difference between EBV-positive and
-negative cases, and EBV infection was more likely in male patients
compared with that in female patients, which was significant.
However, the specific mechanism of this phenomenon has not been
fully elucidated and requires further investigation, although it
was hypothesized that the hormone levels of androgen and estrogen
may have an effect on EBV infection. The results from the present
study suggests that the upper aerodigestive tract was the most
common site of involvement (75.5%), which is consistent with
previous findings (1–4,10,12).
Moreover, patients present with nasal obstruction, rhinorrhea and
epistaxis due to mass or ulceration in the involvement sites
(2,3,10,12).
A total of two patients presented with stage IV
EN-NK/T-NT, and dissemination of the tumor cells to bilateral
eyelids and to the bone marrow or liver. Furthermore, mortality
occurred in one patient due to the disease at 1 month following
diagnosis, while the other patient died due to multiple organ
failure at 9 months following diagnosis; the mean OS of these two
patients was 5 months. Moreover, patients with stage I EN-NK/T-NT
had an improved survival rate. Previous studies have shown that the
prognosis of patients in stage I was improved compared with
patients at stage II and IV (2–4,12). However, the present study found no
significant difference between clinical stage and survival rate. It
has been reported that patients with EBV-negative EN-NK/T-NT have
an improved response to chemotherapy and less aggressive phenotype
compared with patients who are EBV-positive (12,26,27). Ko
et al (28) reported that in
EN-NK/T-NT cases, patients who were EBV-negative had a longer
survival time compared with those who were EBV-positive. However,
the follow-up data obtained in the present study could not be used
to evaluate the prognostic influence of EBV infection, and the
difference was not statistically significant, which may be due to
the small sample size, consistent with the study by Nakamura et
al (12). Thus, it was
hypothesized that the prognosis of patients may be associated with
other factors, such as advanced-stage disease (stage III or IV) and
invasion of bone or skin (3,12), and not EBV infection alone.
The histological features found in the EBV-negative
cases were similar with those in the EBV-positive cases. The
cytological spectrum of EN-NK/T-NT is broad; neoplastic cells in
the present study were predominantly medium size and inflammation
was present. The histological characteristics are associated with
angioinvasiveness and necrosis of tumor tissue (2,3,10). Furthermore, in the present study
angioinvasion and necrosis in EBV-negative cases were 3.9 and 1.9
times lower compared with that in EBV positive cases, respectively.
Kanno et al (29) found that
EBV-infected lymphoid cells adhered to the vascular wall via
cytokines, such as tumor necrosis factor (TNF)-α, interferon
(IFN)-γ and interleukin (IL)-1β, and interacted with adhesion
molecules, such as intercellular adhesion molecule-1 (ICAM-1) and
vascular cell adhesion molecule-1 (VCAM-1), on endothelial cells
irrespective of neoplastic transformation, which subsequently
initiated the destruction of vascular lesions in EBV-positive
NK/T-cell lymphomas; the terms ‘angiocentric’ or ‘angiodestructive
pattern’ are often used to describe these lesions (2,3,8). Moreover, CD56 can increase the ability
of tumor cells to strongly adhere to and destroy blood vessel
walls, which may also rely on cytokines (TNF-α and IFN-γ),
resulting in angioinvasive and angiodestruction (29,30).
Another previous study revealed that the upregulation of cytokines,
including murine IFN-γ-inducible protein and monokine
IFN-γ-inducible, was associated with the degree of necrosis
(26). The cytotoxic granule
proteins (TIA1 and granzyme B) may also affect angiodestruction and
necrosis (29,30). Takeshita et al (30) found that EBV infection had a reduced
effect on the histology of angiodestruction and necrosis. The
present study identified no significant difference between
angioinvasion or necrosis and EBV status, which is consistent with
these previous studies, this may be related to the limited sample
size, and more cases are required for further investigation.
It was demonstrated that all seven cases of
EN-NK/T-NT had positive staining for CD3, CD56 and ≥1 of the
cytotoxic molecules (7/7 for TIA-1 and 6/7 for granzyme B), and
negative expression of CD20. Moreover, it was found that there was
positive expression of CD3 in all of the seven cases; these
neoplasms are hypothesized to arise from NK-like cytotoxic T-cells
(2,8). T-bet and ETS-1 (5/7 for T-bet and 6/7
for ETS1) were also detected using immunohistochemistry in the
tumor cells. Our previous studies have shown that T-bet and ETS-1,
as transcription factors, serve an important biological role in
lymphomagenesis (1,15). Furthermore, these transcription
factors are upregulated in EN-NK/T-NT and are important markers in
the diagnosis of EN-NK/T-NT (15).
Lin et al (31) revealed
that, in NK cells infected with EBV, microRNA-BART20-5p, which is
encoded by EBV, inhibited the translation of T-bet, induced T-bet
to upregulate p53 and inhibited p53 in invasive EN-NK/T-NT.
Moreover, the results from the present study found a significant
association between T-bet expression and EBV-positive and -negative
cases, which indicates the interaction between T-bet and EBV to
contribute to lymphomagenesis. As important synergistic factors,
ETS-1 and T-bet regulate the terminal differentiation of NK and
cytotoxic T cells, activate cytotoxic expression and stimulate the
production of IFN-γ, which promotes lymphoma progression (15). The present study also found that
these two transcription factors were highly expressed in seven
EBV-negative EN-NK/T-NT cases (6/7, 85.7%) and both were markedly
expressed in two cases. Thus, these transcription factors may serve
a pathogenic role via non-EBV infection pathways (such as the
JAK/STAT1 and JAK/STAT4 pathways) and may be sensitive markers for
EN-NK/T-NT (32). In addition,
several cytokines and chemokines, including IFN-γ, IL-4, IL-5,
IL-9, IL-10 and IL-13, produced by NK/T tumor cells can also form a
network microenvironment, which promotes the expression of these
two transcription factors (32,33).
Moreover, these transcription factors can positively regulate the
development of NK cells to serve a cytotoxic role, thus producing
cytotoxic effectors that are associated with clinically aggressive
features, including extensive destructive midfacial lesions,
dissemination to various sites and potential complication by
hemophagocytic syndrome (3,15). However, the exact role of these two
transcription factors in EN-NK/T-NT cases with aggressive features
remains controversial. On the other hand, Lin et al
(31) found that the expression of
T-bet in EN-NK/T-NT cases was associated with reduced aggressive
clinical features. Therefore, further investigation is required to
determine the functions of these two transcription factors and
their involvement in the presence and absence of EBV. Moreover,
additional information regarding EBV stages (which was not
performed in the present study), which can be obtained from
peripheral blood EBV DNA, is required to further compare the
difference between EBV stage 0 and negative EBV samples, with
respect to the protein levels of T-bet. There was no significant
difference between the expression levels of ETS-1 and T-bet and
survival rate, which was consistent with our previous study
(15).
In the present study, EBV was not detected using ISH
in EN-NK-NT cases, which suggests that EBV may be a transient
infection or there was no EBV infection present. The ISH method is
the gold standard for EBV detection in clinical studies; however,
another method, rarely used in daily diagnosis, proposed by Mundo
et al (25) includes EBV
microRNA detection, which is a sensitive method to identify the
existence of EBV. It was found that EBV serves a causative role in
the pathogenesis of Burkitt lymphoma and that the EBV genome may be
lost following genetic changes, including DNA methylation and
histone modifications involving E-cadherin and PYCARD gene loci,
known as the ‘hit and run’ mechanism, which were not detectable
using conventional ISH methods (20,34,35).
This mechanism may also be used to understand the effects in
EBV-negative NK/T-cell lymphoma. However, a case of intestinal
aggressive NK-cell lymphoma, described by Martin et al
(36), found that the EBV genome was
not detectable. Tsuyama et al (23) also confirmed the lack of the EBV
genome in EN-NK/T-NT using second-generation DNA sequencing
analysis. Therefore, it was hypothesized that EBV infection was not
present in some EN-NK/T-NT cases. However, due to sample quantity
and quality, analysis of the EBV genome was not performed, and
additional studies are required to increase the understanding of
these atypical lymphomas and to further identify the
characteristics of the disease spectrum.
It has been reported that there are numerous
cytogenetic abnormalities in EN-NK/T-NT, including deletions of 1p,
6q, 11q, 13q and 17p, and gains of 1q, 2q, 7q, 17q and 20q
(16,37), and a complex karyotype in a patient
with EBV-negative EN-NK/T-NT has been reported by Gao et al
(38), but no characteristic genetic
abnormalities have been previously identified. The most common
abnormality is the deletion of 6q21, and it has been hypothesized
that this genetic alteration may serve a role in the occurrence and
development of EN-NK/T-NT (16).
However, whether this alteration plays an important role or is
associated with disease progression has not been fully understood.
Moreover, the downregulation of PRDM1 protein expression (via gene
deletion, DNA methylation and/or microRNA aberrant expression), a
tumor suppressor gene located on 6q21, has been considered as a
potential candidate gene associated with the development of
EN-NK/T-NT (16,39). The results from the present study
suggests that there was negative PRDM1 expression (four cases) or
weak expression (two cases) in the seven EBV-negative cases. Thus,
it was hypothesized that PRDM1 may be a pathogenic gene, which is
independent of EBV infection in EN-NK/T-NT, and is consistent with
a previous study (16). The present
study also identified differences in gene expression and PRDM1
protein expression, which may be due to the loss of heterozygotes
of 6q21 and PRDM1 genes, in which there was still an undeleted
allele in cells (16). Therefore,
PRDM1 and 6q21 may play a pathogenic role, which is independent of
EBV infection. However, further studies are required to analyze the
changes in 6q21 and the PRDM1 gene, which may involve decreased or
the loss of expression of PRDM1 via other pathogenic mechanisms,
such as promoter methylation and microRNA inhibition (16,39). Our
previous study found that the protein levels of PRDM1 were
negatively correlated with T-bet or ETS-1 expression, and that it
could interact with these two transcription factors to form a
transcriptional regulatory network, which together regulates the
growth and development of tumor cells (39); a similar association was also
identified in the present study.
With the rapid development of high-throughput
genomic and transcriptional analysis, progress has been made in
identifying key cellular pathways underlying the dysregulation in
EN-NK/T-NT, such as upregulation of JAK/STAT, RUNX3, PDGFRA,
NOTCH1, Aurora kinase A and NF-κB-associated genes, and
dysregulation of the c-Myc oncogene (11,40).
Tsuyama et al (23) analyzed
the gene expression profile of an EBV-negative EN-NK/T-NT case
using the second generation sequencing method, and found mutations
in KDM6A (V967G) and TP53 (G266R), which are commonly mutated in
EBV-positive EN-NK/T-NT (41,42),
suggesting that the epigenetic pathway of EBV-negative cases was
similar to that of EBV-positive cases (36). Moreover, Gao et al (38) found that the PRC2 pathway may
contribute to the development of EN-NK/T-NT. In EBV-negative cases,
the activation of the PRC2 pathway may be associated with the
upregulation of c-Myc, which then induces histone modification of
H3K27me3 via the interaction with EZH2 and other molecules
associated with PRC2, such as SUZ12 and EED, which is similar to
other EBV-positive EN-NK/T-NT cases (38). In addition, other key genes,
including genes encoding RNA helicase DDX3X, the JAK/STAT signaling
pathway (JAK3, STAT3 and STAT5B) and tumor suppressors, such as
TP53, MGA, PRDM1, protein tyrosine phosphatase κ, FOXO3, ATG, AIM
and HACE1, have been identified (37). Furthermore, genes encoding RAS gene
family and proto-oncogene (such as Myc), epigenetic modifiers
(including KMT2D, MLL2, EP300, ASXL3 and ARID1A) and cell cycle and
apoptotic regulators (including CDKN1A, CDKN2A, CDKN2B and FAS)
have been identified (11,20,37,38). The
pathogenicity of these genes may be independent of EBV infection;
however, this requires further investigation using large number of
EBV-negative EN-NK/T-NT cases. The primary limitation of the
present study was the inability to perform gene expression analysis
on all tissues due to the small sample size.
In conclusion, the results from the present study
may provide further evidence for the existence of EBV-negative
EN-NK/T-NT cases; however, these cases remain rare. The
clinicopathological characteristics of EBV-negative EN-NK/T-NT were
found to be similar with those of EBV-positive cases. However, it
was identified that more patients with EBV-negative EN-NK/T-NT were
female, compared with patients with EBV-positive EN-NK/T-NT. In
addition, two transcription factors, T-bet and ETS-1, were highly
expressed in EBV-negative EN-NK/T-NT, while there was negative or
weak expression of PRDM1. However, the present study did not
demonstrate whether the prognosis of patients with EBV-negative
expression was improved or worse compared with that in patients
with EBV-positive expression. Therefore, understanding the
EN-NK/T-NT EBV-negative variant is important for early diagnosis of
this aggressive neoplasm. At present, previous studies have
reported the mechanism of pathogenic genes, such as overexpression
of EZH2 and trimethylated H3K27, overexpression and amplification
of c-Myc, missense mutations of the STAT3 gene, strong expression
of PD-L1 and CD30, in EBV-negative EN-NK/T-NT, and these genes
could serve an important role in future targeted therapy (23,27,38,43).
Moreover, future progress for the disease depends upon more robust
diagnostic criteria with replicable molecular markers.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Ms. Ying Zhang, Mr.
Dong Li and Mr. Xin Li, from the Department of Pathology, Peking
University First Hospital (Beijing, China), for their technical
assistance.
Funding
The present study was financially supported by a
grant from the Natural Science Foundation of China, Beijing (grant
no. 81470359).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TL designed the study. WW, LL, YZ and LN collected
and analyzed the patient data. WW, LN and TL evaluated and
interpreted the pathological and immunohistochemical results. YZ
performed statistical analysis and interpreted the results. DL
performed the immunohistochemical staining. XL was responsible for
the technical operation of the ISH and FISH. WW, LN, LL and TL
wrote the manuscript. LN, LL and TL revised the manuscript. All
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Peking University First Hospital [approval no. 2013(571)].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EN-NK/T-NT
|
extranodal natural killer/T-cell
lymphoma-nasal type
|
|
EBV
|
Epstein-Barr virus
|
|
EBER
|
EBV-encoded RNA
|
|
ISH
|
In situ hybridization
|
|
FISH
|
fluorescence in situ
hybridization
|
|
TCR
|
T cell receptor
|
|
OS
|
overall survival
|
References
|
1
|
Ren YL, Nong L, Zhang S, Zhao J, Zhang XM
and Li T: Analysis of 142 Northern Chinese patients with peripheral
T/NK-Cell lymphomas: Subtype distribution, clinicopathologic
features, and prognosis. Am J Clin Pathol. 138:435–447. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chan JK, Quintanilla-Martinez L, Ferry JA,
et al: Extrannodal NK/T-cell lymphoma, nasal type. Swerdlow SH,
Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J and
Vardiman JW: WHO classification of tumours of haematopoietic and
lymphoid tissues Lyon, France: IARC Press; pp. 285–288. 2008
|
|
3
|
Chan JKC, Quintanilla-Martinez L and Ferry
JA: Extrannodal NK/T-cell lymphoma, nasal type. Swerdlow SH, Campo
E, Harris NL, Jaffe ES, Pileri SA, Stein H and Thiele J: WHO
classification of tumours of haematopoietic and lymphoid tissues,
revised. 4th. IARC Press; Lyon: pp. 368–371. 2017
|
|
4
|
Ko YH, Ree HJ, Kim WS, Choi WH, Moon WS
and Kim SW: Clinicopathologic and genotypic study of extranodal
nasal-type natural killer/t-cell lymphoma and natural killer
precursor lymphoma among Koreans. Cancer. 89:2106–2116. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bakos A, Szomor Á, Schneider T, Miltényi
Z, Marton I, Borbényi Z, Pammer J, Krenács L, Bagdi E and Piukovics
K: Incidence and treatment of extranodal natural killer/T-cell
lymphoma nasal type. Hungarian experiences. Orv Hetil.
158:1635–1641. 2017.(In Hungarian). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jones JF, Shurin S, Abramowsky C, Tubbs
RR, Sciotto CG, Wahl R, Sands J, Gottman D, Katz BZ and Sklar J:
T-cell lymphomas containing Epstein-Barr viral DNA in patients with
chronic Epstein-Barr virus infections. N Engl J Med. 318:733–741.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harabuchi Y, Yamanaka N, Kataura A, Imai
S, Kinoshita T, Mizuno F and Osato T: Epstein-Barr virus in nasal
T-cell lymphomas in patients with lethal midline granuloma. Lancet.
335:128–130. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ho FC, Srivastava G, Loke SL, Fu KH, Leung
BP, Liang R and Choy D: Presence of Epstein-Barr virus DNA in nasal
lymphomas of B and ‘T’ cell type. Hemafol Oncol. 8:271–281. 1990.
View Article : Google Scholar
|
|
9
|
Jaffe ES, Chan JK, Su IJ, Frizzera G, Mori
S, Feller AC and Ho FC: Report of the workshop on nasal and related
extranodal angiocentric T/natural killer cell lymphomas.
Definitions, differential diagnosis, and epidemiology. Am J Surg
Pathol. 20:103–111. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chan JK, Jafe ES and Ralfkiaer E:
Extranodal NK/T cell lymphoma, nasal type. Jafe ES, Harris NL,
Stein H, et al: World Health Organization classification of
tumours: Pathology and genetics of tumours of haematopoietic and
lymphoid tissues. IARC Press; Lyon, France: pp. 204–207. 2001
|
|
11
|
George LC, Rowe M and Fox CP: Epstein-Barr
virus and the pathogenesis of T and NK lymphoma: A mystery
unsolved. Curr Hematol Malig Rep. 7:276–284. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakamura S, Katoh E, Koshikawa T, Yatabe
Y, Nagasaka T, Ishida H, Tokoro Y, Koike K, Kagami Y, Ogura M, et
al: Clinicopathologic study of nasal T/NK-cell lymphoma among the
Japanese. Pathology Int. 47:38–53. 1997. View Article : Google Scholar
|
|
13
|
Hasserjian RP and Harris NL: NK-cell
lymphomas and leukemias: A spectrum of tumors with variable
manifestations and immunophenotype. Am J Clin Pathol. 127:860–868.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lister TA, Crowther D, Sutcliffe SB,
Glatstein E, Canellos GP, Young RC, Rosenberg SA, Coltman CA and
Tubiana M: Report of a committee convened to discuss the evaluation
and staging of patients with Hodgkin's disease: Cotswolds meeting.
J Clin Oncol. 7:1630–1636. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang S, Li T, Zhang B, Nong L and Aozasa
K: Transcription factors engaged in development of NK cells are
commonly expressed in nasal NK/T-cell lymphomas. Hum Pathol.
42:1319–1328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liang L, Zhang Z, Wang Y, Nong L, Zheng Y,
Qu L, Zhang B and Li T: The genetic deletion of 6q21 and PRDM1 and
clinical implications in extranodal NK/T cell lymphoma, nasal type.
Biomed Res Int. 2015:4354232015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Van Dongen JJ, Langerak AW, Brüggemann M,
Evans PA, Hummel M, Lavender FL, Delabesse E, Davi F, Schuuring E,
García-Sanz R, et al: Design and standardization of PCR primers and
protocols for detection of clonal immunoglobulin and T-cell
receptor gene recombinations in suspect lymphoproliferations:
Report of the BIOMED-2 concerted action BMH4-CT98-3936. Leukemia.
17:2257–2317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fujiwara S, Kimura H, Imadome K, Arai A,
Kodama E, Morio T, Shimizu N and Wakiguchi H: Current research on
chronic active Epstein-Barr virus infection in Japan. Pediatr Int.
56:159–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Delecluse HJ, Feederle R, O'Sullivan B and
Taniere P: Epstein-Barr virus-associated tumours: An update for the
attention of the working pathologist. J Clin Pathol. 60:1358–1364.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gru AA, Haverkos BH, Freud AG, Hastings J,
Nowacki NB, Barrionuevo C, Vigil CE, Rochford R, Natkunam Y,
Baiocchi RA and Porcu P: The Epstein-Barr virus (EBV) in T cell and
NK cell lymphomas: Time for a reassessment. Curr Hematol Malig Rep.
10:456–467. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li T, Hongyo T, Syaifudin M, Nomura T,
Dong Z, Shingu N, Kojya S, Nakatsuka S and Aozasa K: Mutations of
the p53 gene in nasal NK/T-cell lymphoma. Lab Invest. 80:493–499.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Teo WL and Tan SY: Loss of Epstein-Barr
virus-encoded RNA expression in cutaneous dissemination of natural
killer/T-cell lymphoma. J Clin Oncol. 29:e342–e343. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsuyama N, Asaka R, Dobashi A, Baba S,
Mishima Y, Ueda K, Oguchi M, Tsuji H, Hatake K and Takeuchi K:
Epstein-Barr virus-negative extranodal ‘true’ natural killer-cell
lymphoma harbouring a KDM6A mutation. Hematol Oncol. 36:328–335.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bouchekioua A, Scourzic L, de Wever O,
Zhang Y, Cervera P, Aline-Fardin A, Mercher T, Gaulard P, Nyga R,
Jeziorowska D, et al: JAK3 deregulation by activating mutations
confers invasive growth advantage in extranodal nasal-type natural
killer cell lymphoma. Leukemia. 28:338–348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mundo L, Ambrosio MR, Picciolini M, Lo
Bello G, Gazaneo S, Del Porro L, Lazzi S, Navari M, Onyango N,
Granai M, et al: Unveiling another missing piece in EBV-driven
lymphomagenesis: EBV-encoded MicroRNAs expression in EBER-negative
burkitt lymphoma cases. Front Microbiol. 8:2292017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ko YH, Cho EY, Kim JE, Lee SS, Huh JR,
Chang HK, Yang WI, Kim CW, Kim SW and Ree HJ: NK and NK-like T-cell
lymphoma in extranasal sites: A comparative clinicopathological
study according to site and EBV status. Histopathology. 44:480–489.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nicolae A, Ganapathi KA, Pham TH, Xi L,
Torres-Cabala CA, Nanaji NM, Zha HD, Fan Z, Irwin S, Pittaluga S,
et al: EBV-negative aggressive NK-cell leukemia/lymphoma: Clinical,
pathologic, and genetic features. Am J Surg Pathol. 41:67–74. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ko YH, Park S, Kim K, Kim SJ and Kim WS:
Aggressive natural killer cell leukemia: Is Epstein-Barr virus
negativity an indicator of a favorable prognosis? Acta Haematol.
120:199–206. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kanno H, Watabe D, Shimizu N and Sawai T:
Adhesion of Epstein-Barr virus-positive natural killer cell lines
to cultured endothelial cells stimulated with inflammatory
cytokines. Clin Exp Immunol. 151:519–527. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takeshita M, Yamamoto M, Kikuchi M, Kimura
N, Nakayama J, Uike N, Daimaru H, Sawada H and Okamura T:
Angiodestruction and tissue necrosis of skin-involving CD56+
NK/T-cell lymphoma are influenced by expression of cell adhesion
molecules and cytotoxic granule and apoptosis-related proteins. Am
J Clin Pathol. 113:201–211. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin TC, Liu TY, Hsu SM and Lin CW:
Epstein-Barr virus-encoded miR-BART20-5p inhibits T-bet translation
with secondary suppression of p53 in invasive nasal NK/T-cell
lymphoma. Am J Pathol. 182:1865–1875. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Strengell M, Matikainen S, Sirén J,
Lehtonen A, Foster D, Julkunen I and Sareneva T: IL-21 in synergy
with IL-15 or IL-18 enhances IFN-gamma production in human NK and T
cells. Immunol. 170:5464–5469. 2003. View Article : Google Scholar
|
|
33
|
Agnello D, Lankford CS, Bream J, Morinobu
A, Gadina M, O'Shea JJ and Frucht DM: Cytokines and transcription
factors that regulate T helper cell differentiation: New players
and new insights. J Clin Immunol. 23:147–161. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Queen KJ, Shi M, Zhang F, Cvek U and Scott
RS: Epstein-Barr virus-induced epigenetic alterations following
transient infection. Int J Cancer. 132:2076–2086. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Birdwell CE, Queen KJ, Kilgore PC,
Rollyson P, Trutschl M, Cvek U and Scott RS: Genome-wide DNA
methylation as an epigenetic consequence of Epstein-Barr virus
infection of immortalized keratinocytes. J Virol. 88:11442–11458.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Martin AR, Chan WC, Perry DA, Greiner TC
and Weisenburger DD: Aggressive natural killer cell lymphoma of the
small intestine. Mod Pathol. 8:467–472. 1995.PubMed/NCBI
|
|
37
|
Iqbal J, Kucuk C, Deleeuw RJ, Srivastava
G, Tam W, Geng H, Klinkebiel D, Christman JK, Patel K, Cao K, et
al: Genomic analyses reveal global functional alterations that
promote tumor growth and novel tumor suppressor genes in natural
killer-cell malignancies. Leukemia. 23:1139–1151. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gao J, Behdad A, Ji P, Wolniak KL,
Frankfurt O and Chen YH: EBV-negative aggressive NK-cell
leukemia/lymphoma: A clinical and pathological study from a single
institution. Mod Pathol. 30:1100–1115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang Z, Liang L, Li D, Nong L, Liu J, Qu
L, Zheng Y, Zhang B and Li T: Hypermethylation of PRDM1/Blimp-1
promoter in extranodal NK/T-cell lymphoma, nasal type: An evidence
of predominant role in its downregulation. Hematol Oncol.
35:645–654. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
de Mel S, Hue SS, Jeyasekharan AD, Chng WJ
and Ng SB: Molecular pathogenic pathways in extranodal NK/T cell
lymphoma. J Hematol Oncol. 12:332019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Quintanilla-Martinez L, Kremer M, Keller
G, Nathrath M, Gamboa-Dominguez A, Meneses A, Luna-Contreral L,
Cabras A, Hoefler H, Mohar A and Fend F: p53 Mutations in nasal
natural killer/T-cell lymphoma from Mexico: Association with large
cell morphology and advanced disease. Am J Pathol. 159:2095–2105.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Northrup D, Yagi R, Cui K, Proctor WR,
Wang C, Placek K, Pohl LR, Wang R, Ge K, Zhu J and Zhao K: Histone
demethylases UTX and JMJD3 are required for NKT cell development in
mice. Cell Biosci. 7:252017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Asif S, Begemann M, Bennett J, Fatima R,
Masood A and Raza S: Pembrolizumab in newly diagnosed EBV-negative
extranodal natural killer/T-cell lymphoma: A case report. Mol Clin
Oncol. 10:397–400. 2019.PubMed/NCBI
|
|
44
|
Harabuchi Y, Kataura A and Imai K:
Circulating intercellular adhesion molecule-1 and its cellular
expression in head and neck non-Hodgkin's lymphomas, including
lethal midline granuloma. Ann Otol Rhinol Laryngol. 105:634–642.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cuadra-Garcia I, Proulx GM, Wu CL, Wang
CC, Pilch BZ, Harris NL and Ferry JA: Sinonasal lymphoma: A
clinicopathologic analysis of 58 cases from the massachusetts
general hospital. Am J Surg Pathol. 23:1356–1369. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Quintanilla-Martinez L, Franklin JL,
Guerrero I, Krenacs L, Naresh KN, Rama-Rao C, Bhatia K, Raffeld M
and Magrath IT: Histological and immunophenotypic profile of nasal
NK/T cell lymphomas from Peru: High prevalence of p53
overexpression. Hum Pathol. 30:849–855. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jung CK, Lee KY, Kim Y, Han K, Shim SI,
Kim BK and Kang CS: Epstein-Barr virus infection, drug resistance
and prognosis in Korean T- and NK-cell lymphomas. Pathol Int.
51:355–363. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ohshima K, Liu Q, Koga T, Suzumiya J and
Kikuchi M: Classification of cell lineage and anatomical site, and
prognosis of extranodal T-cell lymphoma-natural killer cell,
cytotoxic T lymphocyte, and non-NK/CTL types. Virchows Arch.
440:425–435. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim JE, Kim YA, Jeon YK, Park SS, Heo DS
and Kim CW: Comparative analysis of NK/T-cell lymphoma and
peripheral T-cell lymphoma in Korea: Clinicopathological
correlations and analysis of EBV strain type and 30-bp deletion
variant LMP1. Pathol Int. 53:735–743. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ng SB, Lai KW, Murugaya S, Lee KM, Loong
SL, Fook-Chong S, Tao M and Sng I: Nasal-type extranodal natural
killer/T-cell lymphomas: A clinicopathologic and genotypic study of
42 cases in Singapore. Mod Pathol. 17:1097–1107. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Miyazato H, Nakatsuka S, Dong Z, Takakuwa
T, Oka K, Hanamoto H, Tatsumi Y, Kanamaru A and Aozasa K; Osaka
Lymphoma Study Group, : NK-cell related neoplasms in Osaka, Japan.
Am J Hematol. 76:230–235. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tai YC, Kim LH and Peh SC: High frequency
of EBV association and 30-bp deletion in the LMP-1 gene in CD56
lymphomas of the upper aerodigestive tract. Pathol Int. 54:158–166.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cabrera ME, Eizuru Y, Itoh T, Koriyama C,
Tashiro Y, Ding S, Rey S, Akiba S and Corvalan A: Nasal natural
killer/T-cell lymphoma and its association with type ‘i’/Xhol loss
strain Epstein-Barr virus in Chile. J Clin Pathol. 60:656–660.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Matsuda M, Iwanaga T, Hashimoto S, Uesugi
T and Itagaki N: Primary Epstein-Barr virus-negative nasal-type
natural killer/T cell lymphoma of the testis. Leuk Res.
33:e119–e120. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kim HS, Lee HW, Kim WS and Ko YH: Systemic
Epstein-Barr virus-negative mature natural killer-cell lymphoma
with cutaneous and visceral involvement. APMIS. 123:990–992. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tian C, Wang Y, Zhu L, Yu Y and Zhang Y:
Primary bone natural killer/T cell lymphoma, nasal type without EBV
infection: A case report. Int J Clin Exp Pathol. 8:14836–14839.
2015.PubMed/NCBI
|
|
57
|
Zeng LS, Huang WT, Qiu T, Shan L, Guo L,
Ying JM, Lyu N and Feng XL: Correlation between the
clinicopathological features and prognosis in patients with
extranodal natural killer/T cell lymphoma. Chronic Dis Transl Med.
3:252–259. 2017.PubMed/NCBI
|