Introduction
Anaplastic thyroid cancer (ATC) is a highly
malignant undifferentiated subset of thyroid cancer (1). Although ATC represents 1–2% of thyroid
cancer cases, patients with ATC diagnosed at the Mary Babb Randolph
Cancer Center (Morgantown, USA) between 1987 and 2007 had a median
survival of 3–5 months after diagnosis, due to its aggressiveness
and the lack of effective treatment options (1). Despite informational and technological
improvement in medical therapies, the median survival period in
months for ATC has not improved. While some studies report that the
incidence rates of ATC have decreased worldwide (2,3), another
study reports an increased incidence rate of ATC between 1973–2014
in the United States (4). For the
treatment of ATC, it is preferable to resect the tumor and treat
with external beam radiation for loco-regional control, followed by
single or in-combination chemotherapy (3). Due to the challenging molecular
complexity of ATC, targeted therapies are considered to be the most
encouraging treatment modalities to improve prognosis in patients
with ATC. However, novel targeted therapies tested over the last 10
years have not yet been approved for ATC. However, clinical trials
are still ongoing and present promising results (2).
In addition to wound healing,
epithelial-to-mesenchymal transition (EMT) is a crucial step in
embryonic developmental processes, through which epithelial cells
are converted to mesenchymal cells; EMT also occurs in cancer
invasion and metastasis, so it is proposed as a promising target
for cancer therapy (5). During EMT,
a complex network of EMT transcription factors, including snail
family transcriptional repressor 1 and 2 (SNAI1 and SNAI2), zinc
finger E-box binding homeobox 1 and 2 (ZEB1 and ZEB2), and twist
family bHLH transcription factor 1 and 2 (TWIST1 and TWIST2), are
targeted to repress epithelial markers (such as E-cadherin) while
activating mesenchymal markers (such as vimentin) via different
levels of epigenetic, transcriptional and post-transcriptional
regulations (5). Additionally, low
E-cadherin expression is an important prognostic marker for ATC and
is associated with an aggressive phenotype of thyroid cancer
(6,7).
ZEB1 is a zinc finger E-box binding protein that
regulates the promoter of E-cadherin and serves a major role in EMT
(8). In various types of cancer,
such as oral cancer, and hepatocellular and renal carcinoma, ZEB1
upregulation has been identified as a prognostic factor (9–11). ZEB1
is regulated via numerous mechanisms, one of which acts via the
microRNA (miR)-200 family; miR-200 is a tumor suppressor family
with five members clustered as miR-200b/c/429 and miR-200a/141
based on their chromosomal region (12). These miRs are considered as
determining factors of the epithelial phenotype of cancer cells via
targeting the E-cadherin repressor ZEB1 (12).
The miR-200 family is involved in EMT, as well as in
the repression, self-renewal and differentiation of cancer stem
cells, modulation of cell division, apoptosis and the reversal of
chemo-resistance (13). Therefore,
numerous human cancer studies have focused on miR-200 (13). In a previous review, miR-200b was
defined as an important regulator of EMT via regulating ZEB1 and
ZEB2 in ATC (14). In the current
study, the role of miR-200b on mesenchymal-to-epithelial transition
was examined in ATC.
Materials and methods
Tissue collection
A total of 14 human ATC collected from 2003 to 2016
(from 5 male and 9 female patients; age range, 56–82 years; median
age, 69 years) and 15 non-cancerous human thyroid tissues collected
from 2014 to 2017 (from 2 male and 13 female individuals; age
range, 43–83 years; median age, 65 years), namely adenomatous
goiter (AG) tissues (used as controls), from different patients
were obtained from the Noguchi Thyroid Clinic and Hospital
Foundation (Beppu, Japan) in accordance with ethical approval from
the Noguchi Thyroid Clinic and Hospital Foundation (approval no.
020) and Wakayama Medical University School of Medicine (approval
no. 2449). All patients provided written informed consent for
participation in the present study.
Immunohistochemical staining
Immunohistochemical staining was performed on 10%
formalin-fixed paraffin-embedded tissue blocks using a Discovery
Auto-Stainer with automated protocols (Ventana Medical Systems,
Inc.). The primary antibodies used were anti-E-cadherin (1:400;
cat. no. 3195) and anti-vimentin (1:200; cat. no. 5741) from Cell
Signaling Technology, Inc., and anti-ZEB1 (1:100; cat. no.
ab203829) from Abcam, in addition to the Discovery Universal
secondary antibody (cat. no. 760-4205). Two clinical pathologists
(FS and YM) independently scored the staining intensity in ATC and
non-cancerous tissues using a light microscope (magnification, ×40;
Nikon Eclipse TS2; Nikon Corporation) for E-cadherin, vimentin and
ZEB1 expression. Staining scores were recorded as follows: 0,
negative staining in the cell membrane; 1, <10% of weak staining
in the cell membrane; 2, >10% of weak or moderate staining in
the cell membrane; and 3, >50% of strong staining in the cell
membrane (15).
Cell culture
The ATC 8505c, ASH-3 and KMH-2 cell lines were
purchased from the Japanese Collection of Research Bioresources
Cell Bank, and the normal human thyroid follicular epithelial
Nthy-ori 3-1 cell line was purchased from Sigma-Aldrich (Merck
KGaA). The 8505c cell line contained a C:G to G:C transversion at
codon 248 of the p53 gene, and there were chromosomal abnormalities
in the ASH-3 and KMH-2 cell lines at chromosome numbers 79–89 and
92–108, respectively.
The ATC and normal human thyroid follicular
epithelial cell lines were grown in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS (HyClone;
Cytiva), 1% penicillin/streptomycin and 1 µl/ml amphotericin B
(Gibco; Thermo Fisher Scientific, Inc.). All cell lines were
cultured in a humidified incubator at 37°C with 5%
CO2.
mRNA processing and reverse
transcription-quantitative PCR (RT-qPCR)
Preparation of cDNA from total RNA was performed
directly from cultured cell lysates using the TaqMan Gene
Expression Cells-to-CT kit (Ambion; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Briefly, cultured
cells (5×104 cells/well; 70% confluence) in 96-well
plates were washed with PBS and lysed with lysis buffer, and RNA
was released into the supernatant. RNA was reverse transcribed into
cDNA at 37°C for 60 min and 95°C for 5 min using the RT Enzyme mix
and appropriate RT buffer (Ambion; Thermo Fisher Scientific, Inc.).
Finally, cDNA was amplified by RT-qPCR using the TaqMan Gene
Expression Master mix and the specific TaqMan primer/probe assay
designed for the investigated genes: E-cadherin (accession no.
Hs01023894_m1), vimentin (accession no. Hs00185584_m1), ZEB1
(accession no. Hs00232783_m1) and GAPDH (accession no.
Hs02758991_m1) (Applied Biosystems; Thermo Fisher Scientific,
Inc.).
Gene expression levels were normalized to the
expression levels of the housekeeping gene, GAPDH, and expressed as
the relative fold change compared with the control sample. The
amplification profile was initiated by 10 min of incubation at
95°C, followed by a two-step amplification of 15 sec at 95°C and 60
sec at 60°C for 40 cycles, and a final extension step at 95°C for 5
min. To exclude reagent contamination, all experiments were
performed with non-template controls. Quantification was performed
using the 2−ΔΔCq calculation method (16). Each sample was analyzed in triplicate
on a 7500 Real-time PCR system in a 96-well plate (Applied
Biosystems; Thermo Fisher Scientific, Inc.).
miRNA processing and RT-qPCR
Preparation of cDNA from miRNA was performed
directly from cultured cell lysates using the TaqMan MicroRNA
Cells-to-CT kit (Ambion; Thermo Fisher Scientific, Inc.) according
to the manufacturer's protocol. Briefly, the cultured cells in
96-well plates were washed with PBS and lysed with lysis buffer,
releasing the RNA into the supernatant. RNA was reverse transcribed
into cDNA at 37°C for 60 min, followed by 95°C for 5 min and 4°C
until further use using the RT Enzyme mix, RT buffer and
appropriate RT primer (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Finally, the cDNA of each mature miRNA was
amplified with the same conditions as the mRNAs (10 min at 95°C,
followed by a two-step amplification of 15 sec at 95°C and 60 sec
at 60°C for 40 cycles, and a final extension step at 95°C for 5
min) using the TaqMan Universal Master mix and the specific primer
and probe mix included in pre-designed TaqMan MicroRNA assays for
hsa-miR-200b (cat. no. 4427975; Applied Biosystems; Thermo Fisher
Scientific, Inc.). Quantification of miRNA expression was performed
using the aforementioned quantification method, and RNU6B RNA (cat.
no. 001093; P/N 4373381; Applied Biosystems; Thermo Fisher
Scientific, Inc.) was used as the internal control. miRNA levels
were expressed as the fold change relative to the expression in
untreated cells. Each sample was analyzed in triplicate.
Cell transfection with miR-200b
mimic
The stability-enhanced miR-200b mimic and the
negative control, double-stranded RNA, were obtained from Applied
Biosystems (Thermo Fisher Scientific, Inc.). Transfection of miRNA
200b mimic was carried out using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) in accordance with the
manufacturer's protocol. Briefly, the ASH-3 and KMH-2 cell lines
(4×105 cells in 4 ml RPMI-1640 with 10% FBS per plate)
were seeded in 96-well culture plates and grown overnight in
antibiotic-free medium before transfection. For western blot
experiments, ASH-3 and KMH-2 cells (1×106 cells in 2 ml
of RPMI-1640 per dish) were seeded in 6-well culture dishes and
grown overnight in antibiotic-free medium before transfection. The
miR-200b-3p mimic (10 µM; 5′-UAAUACUGCCUGGUAAUGAUGA-3′) or the
negative control (cat. no. 4464061; Thermo Fisher Scientific, Inc.)
were transfected at the indicated concentrations into the cells
using 0.3 µl Lipofectamine® 2000 transfection reagent in
10 µl Opti-MEM (Gibco; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. The negative controls were scrambled
oligonucleotides that were validated to not produce identifiable
effects on known miRNA functions. miR-200b expression in the
transfected cells was confirmed by RT-qPCR (using TaqMan MicroRNA
assays) 24 h after transfection as aforementioned. Each sample was
analyzed in triplicate.
Western blot analysis
Preparation of whole-cell lysates from human ATC
cells has been previously described (15). Briefly, cultured cells were washed
with PBS and homogenized in a solution containing 50 mM Tris
buffer, 150 mM NaCl, 1 mM ethylene-diaminetetraacetic acid, 0.1%
Triton-X 100 (Sigma Aldrich; Merck KGaA), 1% NP-40 and
proteinase/phosphatase inhibitors. Following centrifugation at
16,000 rpm for 10 min at 4°C, cell lysates were used for subsequent
western blot analysis. Protein concentration was determined using
the Pierce Coomassie Plus Protein assay kit (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Subsequently, proteins (20 µg/lane) were separated via 10%
SDS-PAGE, transferred to polyvinylidene fluoride membranes using
Direct Blot (Gellex International), and immunostained using an
automated iBind Western Device (Thermo Fisher Scientific, Inc.) at
24°C, according to the manufacturer's protocol. The primary
antibodies used were anti-vimentin (1:1,000; cat. no. 5741) and
anti-ZEB1 (1:200; cat. no. 3396) from Cell Signaling Technology,
Inc., and anti-GAPDH (1:2,000; cat. no. ab9484) from Abcam. The
horseradish peroxidase-conjugated anti-mouse immunoglobulin G (IgG;
1:1,000; cat. no. 7076) or anti-rabbit IgG (1:1,000; cat. no. 7074)
from Cell Signaling Technology, Inc., were used as the secondary
antibodies, and subsequently detected using an enhanced
chemiluminescence (ECL) system (Amersham ECL Prime; Cytiva). For
control of protein loading, the blots were stripped using Re-Blot
Plus (EMD Millipore) and re-probed with GAPDH. ImageJ64 software
(v1.48; National Institutes of Health) was used for quantification
of band intensities.
Wound healing assay
Wound healing assay was performed using the ATC
ASH-3 and KMH-2 cell lines. The ATC cells were transfected
separately with the miR-200b mimic or the scrambled negative
control obtained from Ambion (Thermo Fisher Scientific, Inc.).
Transfection was performed as aforementioned using
Lipofectamine® 2000 and Opti-MEM without FBS. When the
cell confluence reached ~80%, wounds were created using a 200-µl
pipette tip and were then rinsed with RPMI-1640 medium to remove
any free-floating cells and debris using the CytoSelect 24-well
wound healing assay kit (Cell Biolabs, Inc). After 4 h, the medium
was replaced with fresh RPMI-1640 (with 10% FBS), and after 8 h
transfection was repeated, and after 4 h, replacement with
RPMI-1640 was repeated. Wound healing was observed at different
time points (0, 12, 36 and 72 h) within the scrape line, and
representative images were captured using a light microscope at
magnifications of ×4 and ×10 (Nikon Eclipse TS2; Nikon
Corporation). Nikon NIS-Element BR Analysis v5.11.00 software
(Nikon Corporation) was used for the quantification of wound
healing assays.
Statistical analysis
Data were analyzed using GraphPad Prism 5.0
(GraphPad Software, Inc.). To evaluate significant differences
between two groups, the non-parametric Mann-Whitney U test was
used. Data were presented as the mean ± SD (n=3). P<0.05 was
considered to indicate a statistically significant difference.
Results
Mesenchymal markers are upregulated in
human ATC tissues
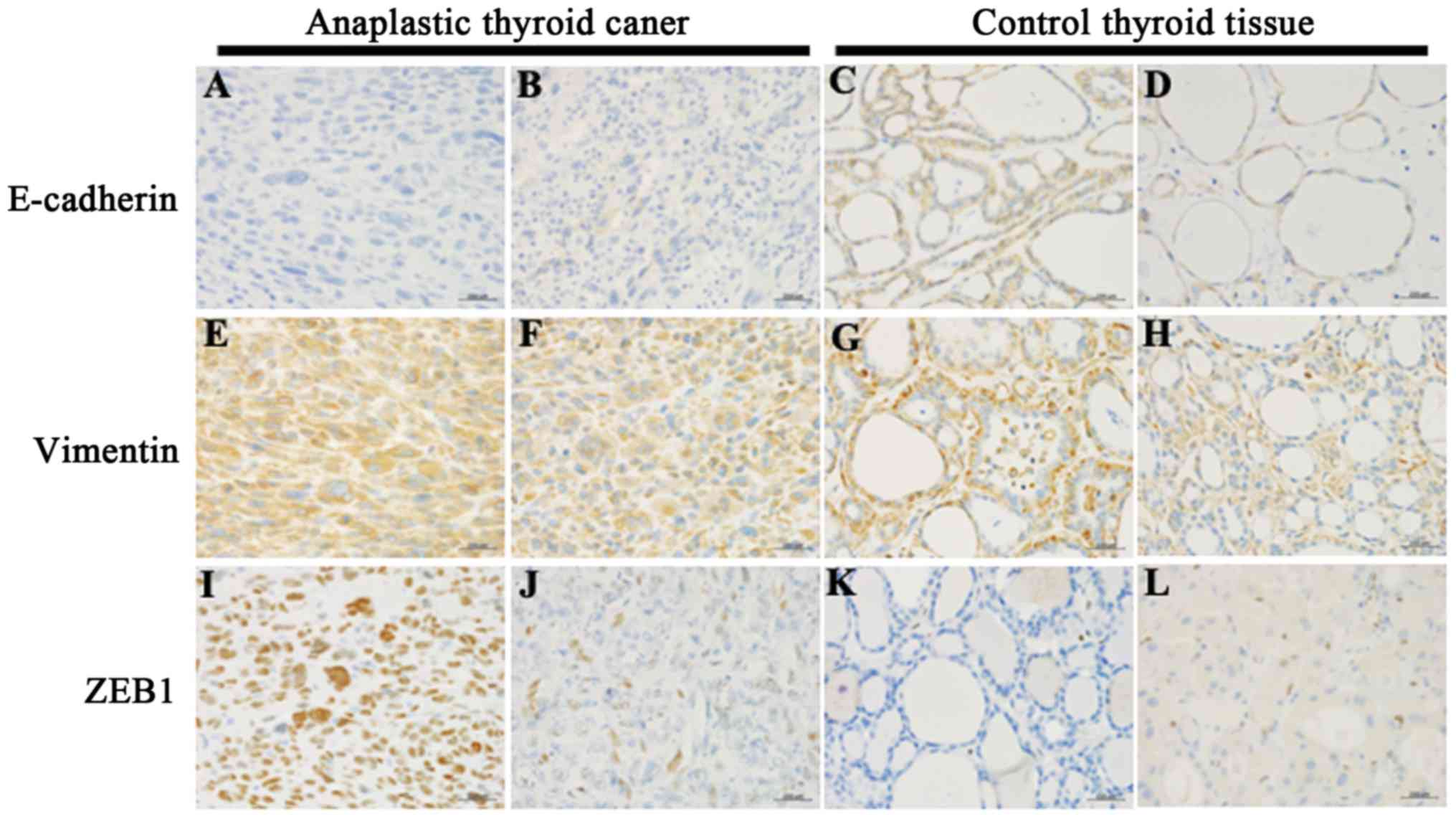
To investigate the EMT state in ATC tissues, the
expression levels of E-cadherin, vimentin and ZEB1 were examined by
immunohistochemical staining (Fig.
1). The patient clinicopathological characteristics and their
immunohistochemical scores are summarized in Table I. Marked E-cadherin immunoreactivity
in the cell membrane of cancer cells (score 2–3) was detected in
non-cancerous control tissues (100%; 15/15), but not detected
(score 0–1) in the ATC tissues (0%; 14/14). Vimentin
immunoreactivity score of ≥2 was detected in all ATC and normal or
AG tissues (100%; 14/14 and 15/15, respectively). Marked ZEB1
immunoreactivity scoring ≥2 was observed in all ATC tissues (100%;
14/14), but not in AG tissues (0%; 0/15).
 | Table I.Immunohistochemical expression of
E-cadherin, vimentin and ZEB1 proteins in human ATC and control AG
tissues. |
Table I.
Immunohistochemical expression of
E-cadherin, vimentin and ZEB1 proteins in human ATC and control AG
tissues.
|
|
|
|
| Scorea |
|---|
|
|
|
|
|
|
|---|
| Case no. | Age | Sex | Histology | E-cadherin | Vimentin | ZEB1 |
|---|
| 1 | 57 | F | ATC | 0 | 3 | 3 |
| 2 | 59 | F | ATC | 0 | 3 | 3 |
| 3 | 82 | M | ATC | 0 | 3 | 3 |
| 4 | 61 | M | ATC | 0 | 3 | 2 |
| 5 | 64 | F | ATC | 0 | 3 | 2 |
| 6 | 70 | F | ATC | 0 | 3 | 3 |
| 7 | 75 | M | ATC | 1 | 3 | 3 |
| 8 | 78 | F | ATC | 0 | 2 | 3 |
| 9 | 69 | M | ATC | 0 | 3 | 2 |
| 10 | 56 | F | ATC | 0 | 3 | 2 |
| 11 | 56 | M | ATC | 0 | 3 | 2 |
| 12 | 74 | F | ATC | 1 | 3 | 2 |
| 13 | 69 | F | ATC | 0 | 3 | 2 |
| 14 | 77 | F | ATC | 0 | 3 | 2 |
| 15 | 82 | F | AG | 3 | 3 | 0 |
| 16 | 53 | F | AG | 3 | 3 | 0 |
| 17 | 53 | M | AG | 3 | 3 | 0 |
| 18 | 66 | F | AG | 3 | 2 | 0 |
| 19 | 83 | F | AG | 3 | 3 | 0 |
| 20 | 65 | F | AG | 3 | 3 | 1 |
| 21 | 67 | F | AG | 3 | 3 | 0 |
| 22 | 46 | F | AG | 3 | 3 | 0 |
| 23 | 59 | F | AG | 3 | 3 | 0 |
| 24 | 73 | F | AG | 2 | 3 | 0 |
| 25 | 66 | F | AG | 2 | 3 | 0 |
| 26 | 64 | F | AG | 3 | 3 | 0 |
| 27 | 43 | F | AG | 2 | 3 | 0 |
| 28 | 81 | F | AG | 3 | 3 | 0 |
| 29 | 58 | M | AG | 3 | 3 | 0 |
Mesenchymal markers are upregulated in
ATC cell lines
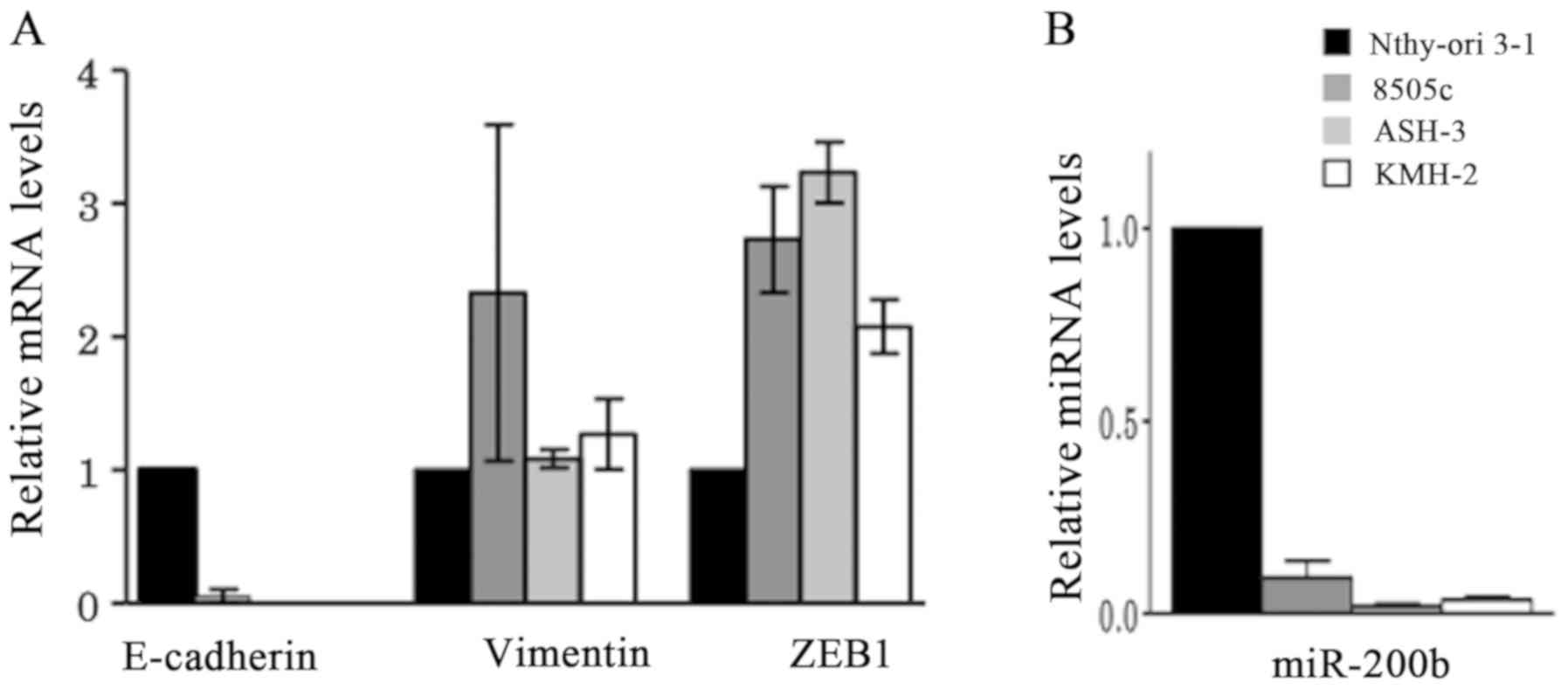
To compare the expression levels of epithelial and
mesenchymal markers at the RNA level, the normal thyroid follicular
epithelium Nthy-ori 3–1 cell line and the undifferentiated thyroid
carcinoma 8505c, ASH-3 and KMH-2 cell lines were used for RT-qPCR
(Fig. 2). Compared with Nthy-ori 3-1
cells, E-cadherin expression was either not detected or very low in
the ATC 8505c, ASH-3 and KMH-2 cell lines, while vimentin
expression was high in 8505c cells, but were similar to Nthy-ori
3-1 cells in ASH-3 and KMH-2 cell lines. The expression levels of
the mesenchymal marker ZEB1 were high in all three ATC cell lines
compared with Nthy-ori 3-1 cells (Fig.
2A). Furthermore, the ATC cell lines exhibited markedly
decreased miR-200b expression compared with Nthy-ori 3-1 cells
(Fig. 2B).
miR-200b regulates EMT markers in ATC
cell lines
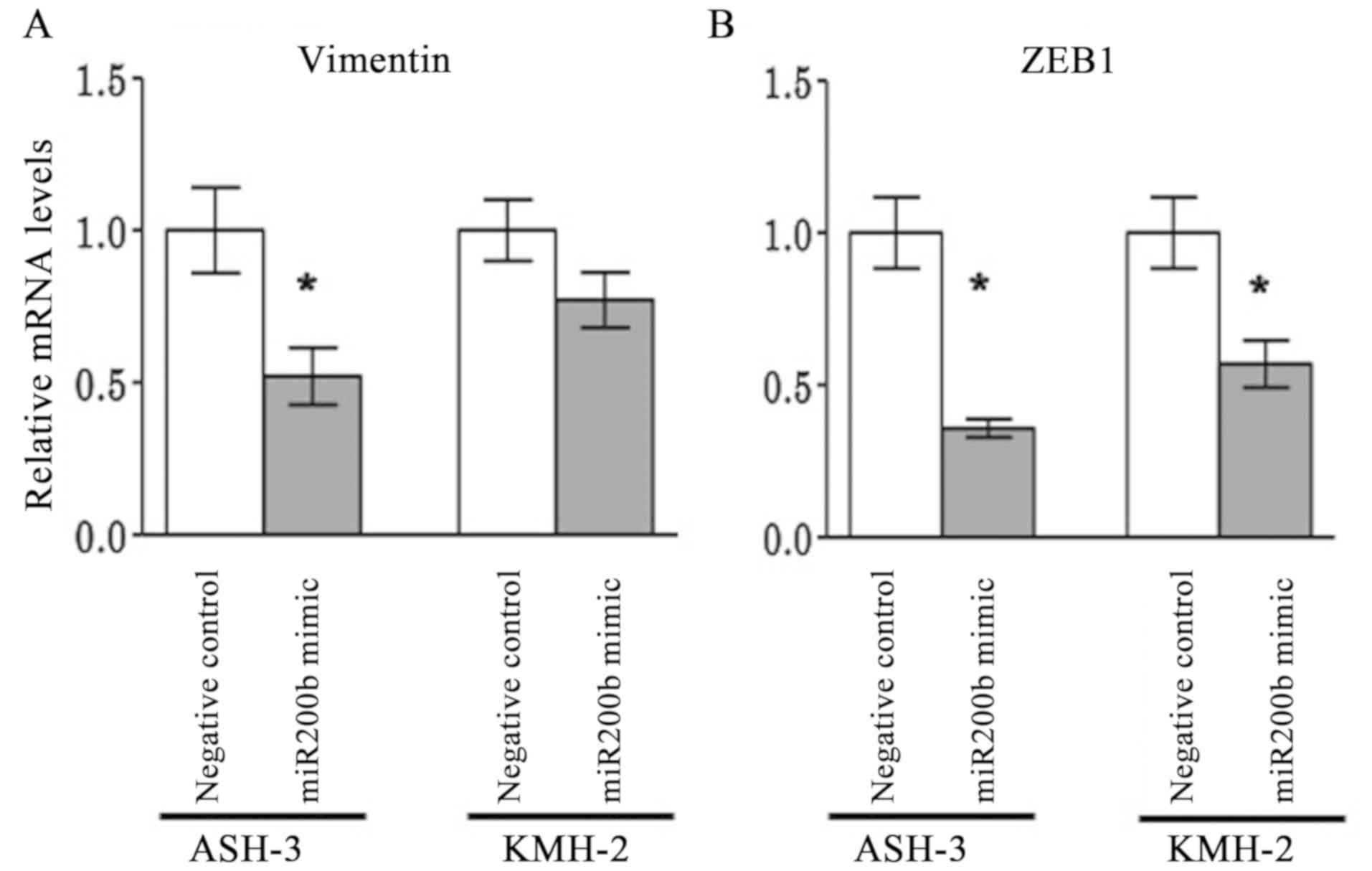
To further investigate the association between
miR-200b and EMT markers (E-cadherin, vimentin and ZEB1), the
effects miR-200b overexpression were analyzed on E-cadherin,
vimentin and ZEB1 expression by transfecting the ASH-3 and KMH-2
cell lines with mimic miRNAs. Relative quantification was used to
evaluate the expression levels of miR-200b following transfection
with a negative control and with mimic miR-200b. Although no
miR-200b expression was detected following transfection with the
negative control, overexpression of miR-200b following transfection
with the miRNA mimic was confirmed by RT-qPCR (data not shown).
miR-200b overexpression resulted in a significant
downregulation of vimentin expression in ASH-3 cells (P=0.028) and
a marked downregulation in KMH-2 cells (P=0.200) compared with that
in negative control-transfected cells (Fig. 3A). Similarly, ZEB1 expression was
significantly downregulated in ASH-3 (P=0.028) and KMH-2 cells
(P=0.028) compared with negative control-transfected cells
(Fig. 3B). E-cadherin expression
remained below the measurement sensitivity, suggesting no direct
association with miR-200b (data not shown). The present findings
suggested that miR-200b promoted epithelial transition through
suppression of ZEB1, which in turn repressed the mesenchymal marker
vimentin in ATC cell lines.
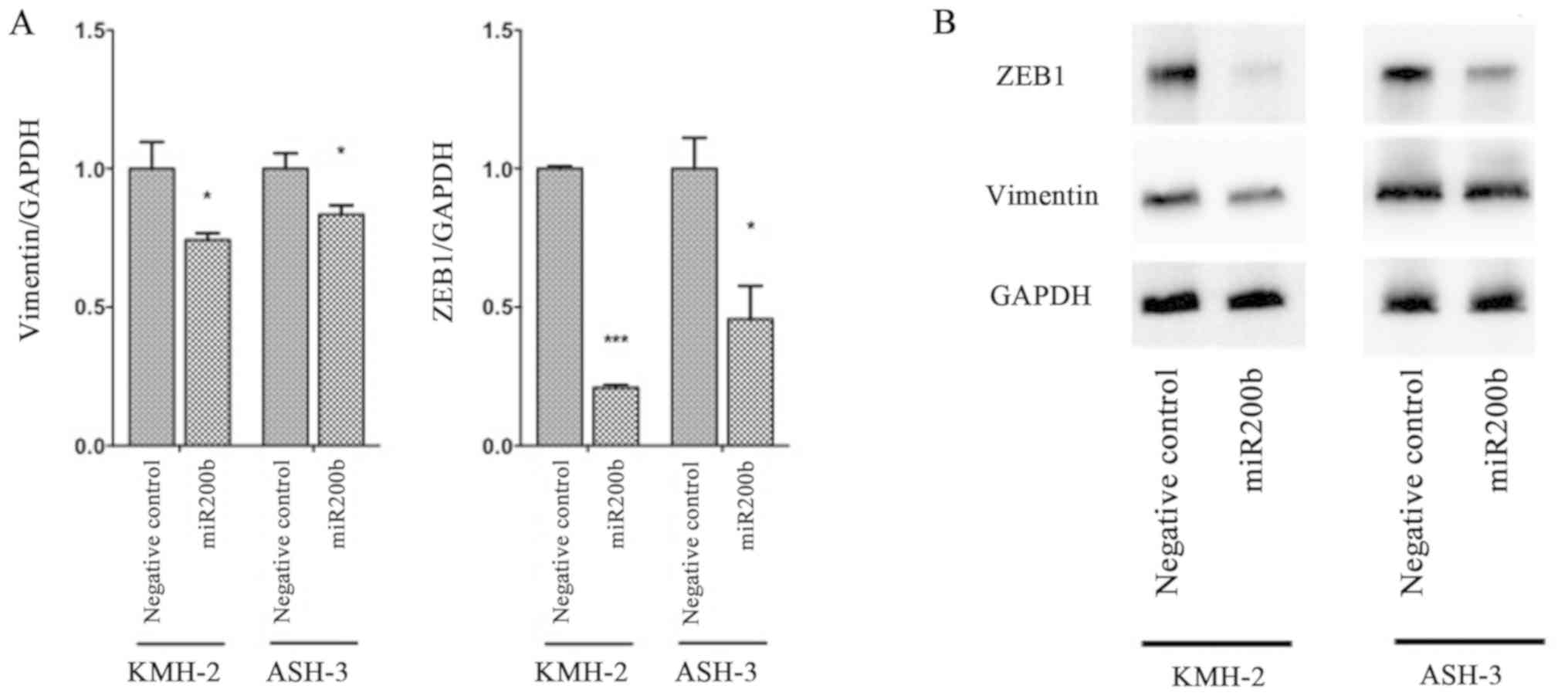
Subsequently, changes in protein levels of EMT
markers were evaluated via western blotting in ASH-3 and KMH-2
cells following transfection with miR-200b mimic (Fig. 4). Similar to RT-qPCR results,
compared with negative control-transfected cells, the protein
levels of vimentin were significantly decreased in both KMH-2 and
ASH-3 cells following transfection with the miR-200b mimic (P=0.030
and P=0.031, respectively). Furthermore, ZEB1 protein expression
was significantly suppressed in both KMH-2 and ASH-3 cells
(P<0.001 and P=0.015, respectively) compared with negative
control-transfected cells. The present results suggested that
miR-200b promoted mesenchymal-to-epithelial transition in human ATC
cell lines.
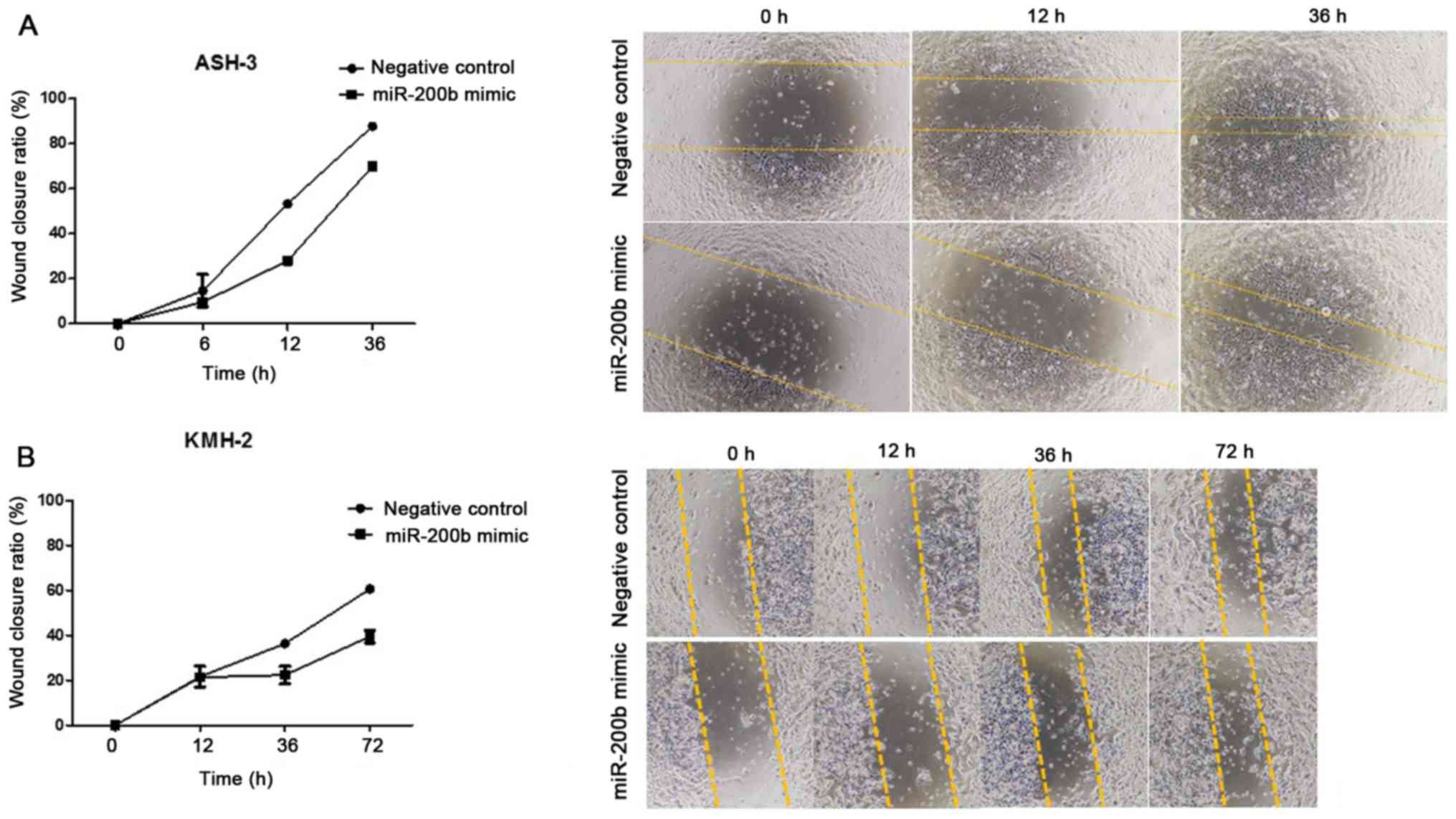
miR-200b suppresses cell
migration
Subsequently, the present study evaluated whether
cell migration was associated with miR-200b. ASH-3 and KMH-2 cells
were transfected with scrambled negative control and miR-200b
mimic. Following transfection, miR-200b mimic-transfected cells
migrated slower than the control cells, suggesting that wound
healing was slower for cells with enforced miR-200b expression
(Fig. 5). Although 10% FBS was used
in the wound healing assays and a few cells were left in the middle
of the wound during wound creation, which may have increased the
proliferative activity and stimulated wound closure, the wound
closure ratio was 90% in negative control cells and 70% in ASH-3
cells with miR-200b overexpression after 36 h (Fig. 5A). In KMH-2 cells after 72 h, wound
closure was 60% for negative control cells and 40% for miR-200b
mimic-transfected cells (Fig. 5B).
The present findings suggested that miR-200b may reduce cell
migration.
Discussion
EMT is considered to be an important phenomenon in
invasion and metastasis. Whether genetically or epigenetically
regulated, during EMT cancer cells first lose their epithelial
properties and then gain mesenchymal characteristics and start
migrating (17). Downregulated
E-cadherin expression is a distinctive feature of EMT, resulting in
loss of the epithelial phenotype (17). In the current study, extremely low
expression levels of E-cadherin were detected in ATC tissues
compared with non-cancerous human thyroid tissues. Similarly,
E-cadherin expression was not detected or was very low in the ATC
8505c, ASH-3 and KMH-2 cell lines, supporting the distinctive
feature of EMT.
ZEB1 protein is thought to trigger the repression of
epithelial genes while upregulating mesenchymal factors, leading to
a dedifferentiation program (18).
The findings of the present study revealed upregulated ZEB1
expression in ATC tissues and 8505c, ASH-3 and KMH-2 cell lines
compared with non-cancerous tissues and cells, respectively.
Additionally, the expression levels of a representative mesenchymal
marker, vimentin, did not differ between ATC and non-cancerous
human thyroid tissues. Both ATC and AG tissues displayed high
vimentin expression. Conversely, the results of vimentin expression
in ATC cell lines exhibited varied expression levels: While
expression in the ATC ASH-3 and KMH-2 cell lines did not differ
from that in control cells, 8505c cells displayed higher vimentin
expression compared with Nthy-ori 3-1 cells. In a previous study,
vimentin expression was analyzed in ASH-3 and KMH-2 cell lines, and
although vimentin expression was detected, this was lower compared
with the ATC IHH-4 cell line, whereas the ATC TMH-1 cell line did
not exhibit vimentin expression (19). This is in accordance with the present
data revealing that while vimentin expression was high in 8505c
cells, other cell lines, such as ASH-3 and KMH-2, displayed weaker
vimentin expression. A potential explanation may be that vimentin
is subject to epigenetic modifications in cancer (20). Additionally, vimentin is a
biochemically diverse protein, and its diversity may be promoted by
its size, assembly state, post-transcriptional modifications,
co-assembly with other intermediate filaments and interactions with
non-intermediate filaments (21).
ZEB and SNAI transcription factors, and members of
the miR-200 and miR-34 families are involved in the EMT regulatory
network (22). Additionally, in
cells with an epithelial phenotype, there are high expression
levels of miR-200 and miR-34, and low expression levels of ZEB and
SNAI, while in cells with a mesenchymal phenotype there are high
expression levels of ZEB and SNAI, and low expression levels of
miR-200 and miR-34 (22). Loss of
the miR-200 family is associated with aggressive cancer cell
phenotypes, and downregulation of miR-200 leads to EMT, invasion
and metastasis in cancer (23).
Gregory et al reported that ZEB1 has five putative binding
sites for miR-200b at the 3′-untranslated region, confirming
previous data of ZEB1 being targeted by miR-200 family members
(24). The present study revealed
markedly decreased expression levels of miR-200b in ATC cell lines,
and transfection with miR-200b mimic downregulated the mRNA
expression levels of ZEB1 and vimentin in ASH-3 and KMH-2 cell
lines. Additionally, the western blot results confirmed that the
protein levels of ZEB1 and vimentin were also downregulated in
ASH-3 and KMH-2 cell lines via enforced miR-200b expression,
suggesting a potential role of miR-200b in EMT marker
regulation.
miR-200 expression is decreased in ATC (25,26).
Zhang et al (26)
demonstrated that epidermal growth factor (EGF)/EGF
receptor-induced EMT was regulated by the miR-200b family. As
miR-200b restoration downregulated vimentin expression, the present
results of the mesenchymal marker vimentin were similar to those
reported by Zhang et al (26), although miR-200b restoration did not
upregulate E-cadherin expression in the present study (data not
shown). This may be due to various other mechanisms, such as
methylation, that regulate E-cadherin expression (27). Although E-cadherin expression was not
upregulated via miR-200b overexpression, the present results
revealed that miR-200b overexpression decreased cell migration. The
current data suggested an independent role of miR-200b from
E-cadherin in cell migration.
The present study indicated that enforced miR-200b
expression downregulated ZEB1 and vimentin expression, and
suppressed cell migration in ATC cell lines. miR-200b may therefore
promote mesenchymal-to-epithelial transition in ATC, and future
studies may help to identify improved treatment modalities through
the prevention of metastasis and cell invasion.
Acknowledgements
The authors would like to acknowledge proofreading
and editing by Mr Benjamin Phillis at the Clinical Study Support
Center of Wakayama Medical University (Wakayama, Japan).
Funding
The present study was partially supported by a
Grant-in-Aid for Scientific Research (KAKENHI) from the Ministry of
Education, Culture, Sports, Science and Technology of Japan (grant
no. 18K16852).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ST, MG and MH designed the study. ST and KE acquired
the data. ST, KE, FS, EG, MG, SU and YM analyzed the data. ST and
EG prepared the manuscript. KE, FS, MG and SU edited the
manuscript. MH controlled the quality of the data. YM and MH
reviewed the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study received ethical approval from the
Noguchi Thyroid Clinic and Hospital Foundation (grant no. 020) and
Wakayama Medical University School of Medicine (grant no. 2449).
All patients provided written informed consent to participate in
the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nagaiah G, Hossain A, Mooney CJ,
Parmentier J and Remick SC: Anaplastic thyroid cancer: A review of
epidemiology, pathogenesis, and treatment. J Oncol.
2011:5423582011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Molinaro E, Romei C, Biagini A, Sabini E,
Agate L, Mazzeo S, Materazzi G, Sellari-Franceschini S, Ribechini
A, et al: Anaplastic thyroid carcinoma: From clinicopathology to
genetics and advanced therapies. Nat Rev Endocrinol. 13:644–660.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fagin JA and Wells SA Jr: Biologic and
Clinical Perspectives on Thyroid Cancer. N Engl J Med.
375:1054–1067. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Janz TA, Neskey DM, Nguyen SA and Lentsch
EJ: Is the incidence of anaplastic thyroid cancer increasing: A
population based epidemiology study. World J Otorhinolaryngol Head
Neck Surg. 5:34–40. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Simeone P, Trerotola M, Franck J, Cardon
T, Marchisio M, Fournier I, Salzet M, Maffia M and Vergara D: The
multiverse nature of epithelial to mesenchymal transition. Semin
Cancer Biol. 58:1–10. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsiambas E, Ragos V, Georgakopoulos G,
Rigopoulos DN, Fotiades PP, Chatziioannidis A, Stamatelopoulos A,
Vilaras G and Karameris A: E-cadherin/α-catenin deregulated
co-expression in thyroid carcinoma based on tissue microarray
digital image analysis. J Buon. 21:450–5. 2016.PubMed/NCBI
|
|
7
|
Fourati A, El Amine O, Ben Ayoub W, Cherni
I, Goucha A, El May MV, Gamoudi A and El May A: Expression profile
of biomarkers altered in papillary and anaplastic thyroid
carcinoma: Contribution of Tunisian patients. Bull Cancer.
104:433–441. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Xu L, Li A and Han X: The roles
of ZEB1 in tumorigenic progression and epigenetic modifications.
Biomed Pharmacother. 110:400–408. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yao X, Sun S, Zhou X, Zhang Q, Guo W and
Zhang L: Clinicopathological significance of ZEB-1 and E-cadherin
proteins in patients with oral cavity squamous cell carcinoma. Onco
Targets Ther. 10:781–790. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wan T, Zhang T, Si X and Zhou Y:
Overexpression of EMT-inducing transcription factors as a potential
poor prognostic factor for hepatocellular carcinoma in Asian
populations: A meta-analysis. Oncotarget. 8:59500–59508. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Harb OA, Elfeky MA, El Shafaay BS, Taha
HF, Osman G, Harera IS, Gertallah LM, Abdelmonem DM and Embaby A:
SPOP, ZEB-1 and E-cadherin expression in clear cell renal cell
carcinoma (cc-RCC): Clinicopathological and prognostic
significance. Pathophysiology. 25:335–345. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park SM, Gaur AB, Lengyel E and Peter ME:
The miR-200 family determines the epithelial phenotype of cancer
cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes
Dev. 22:894–907. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng X, Wang Z, Fillmore R and Xi Y:
miR-200, a new star miRNA in human cancer. Cancer Lett. 344:166–73.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fuziwara CS and Kimura ET: MicroRNA
deregulation in anaplastic thyroid cancer biology. Int J
Endocrinol. 2014:7434502014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Enomoto K, Sato F, Tamagawa S, Gunduz M,
Onoda N, Uchino S, Muragaki Y and Hotomi M: A novel therapeutic
approach for anaplastic thyroid cancer through inhibition of LAT1.
Sci Rep. 9:146162019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta C(T)) method. Methods. 25:402–8. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Das V, Bhattacharya S, Chikkaputtaiah C,
Hazra S and Pal M: The basics of epithelial-mesenchymal transition
(EMT): A study from a structure, dynamics, and functional
perspective. J Cell Physiol. 2019.(Epub ahead of print). View Article : Google Scholar
|
|
18
|
Caramel J, Ligier M and Puisieux A:
Pleiotropic Roles for ZEB1 in Cancer. Cancer Res. 78:30–35. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sekiguchi M, Shiroko Y, Arai T, Kishino T,
Sugawara I, Kusakabe T, Suzuki T, Yamashita T, Obara T, Ito K and
Hasumi K: Biological characteristics and chemosensitivity profile
of four human anaplastic thyroid carcinoma cell lines. Biomed
Pharmacother. 55:466–74. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Satelli A and Li S: Vimentin in cancer and
its potential as a molecular target for cancer therapy. Cell Mol
Life Sci. 68:3033–3046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Danielsson F, Peterson MK, Caldeira Araújo
H, Lautenschläger F and Gad AKB: Vimentin diversity in health and
disease. Cells. 7:1472018. View Article : Google Scholar
|
|
22
|
Garg M: Epithelial, mesenchymal and hybrid
epithelial/mesenchymal phenotypes and their clinical relevance in
cancer metastasis. Expert Rev Mol Med. 19(e3)2017.PubMed/NCBI
|
|
23
|
Mongroo PS and Rustgi AK: The role of the
miR-200 family in epithelial-mesenchymal transition. Cancer Biol
Ther. 10:219–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Braun J, Hoang-Vu C, Dralle H and
Hüttelmaier S: Downregulation of microRNAs directs the EMT and
invasive potential of anaplastic thyroid carcinomas. Oncogene.
29:4237–44. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Z, Liu ZB, Ren WM, Ye XG and Zhang
YY: The miR-200 family regulates the epithelial-mesenchymal
transition induced by EGF/EGFR in anaplastic thyroid cancer cells.
Int J Mol Med. 30:856–62. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jensen K, Patel A, Hoperia V, Larin A,
Bauer A and Vasko V: Dynamic changes in E-cadherin gene promoter
methylation during metastatic progression in papillary thyroid
cancer. Ther Med. 1:457–462. 2010. View Article : Google Scholar
|