Introduction
Gastric cancer (GC) is caused by a combination of
genetic and epigenetic alterations in gastric mucosal cells, such
as TP53, PTEN gene mutations or DNA methylation or noncoding
RNA regulation (1). GC is the fourth
most common cancer worldwide (2);
951,600 new GC cases were reported, and 723,100 deaths occurred in
2012 (2). In general, the highest
incidence rates of GC are observed in East Asian countries
(3). As most cases are diagnosed at
an advanced stage, the long-term survival rate is low (10–20%)
(4). However, when GC is diagnosed
at an early stage, the long-term survival rate is high (>90%)
(5,6). The underlying mechanisms of GC
tumorigenesis and recurrence are complex. Zinc is one of the
indispensable trace elements in the human body, and abnormal
expression of metalloenzymes leads to abnormal absorption of
elemental zinc, which causes tumorigenesis and tumor development
(7). A review summarized evidence
indicating that zinc metalloenzymes may be utilized for diagnostic
and prognostic purposes in child brain tumors (8). Similar to aminopeptidase N (APN, also
termed CD13), other zinc metalloenzymes are upregulated in multiple
types of cancer and on the surface of vasculature undergoing
angiogenesis (9).
The human carbonic anhydrase IV (CA4) gene, located
on chromosome 17q22, was the first identified membrane-bound
isozyme in the 16-member carbonic anhydrase (CA) gene family and
contains 1,170 base pairs (10). The
CA4 protein is a zinc metalloenzyme that catalyzes the reversible
hydration and dehydration of CO2 and
HCO3− (10).
The CA4 enzyme is involved in the formation of gastric acid and
participates in acid-base homeostasis (11). CA4 is expressed in normal human
stomach tissues (11). CA2 is
expressed at low levels in GC tissues (12). Similarly, CA9, another member of the
carbonic anhydrase family, also exhibits loss of expression in GC
(13). In contrast to CA2 and CA9,
CA12 is highly expressed in GC (14). However, whether CA4 is expressed in
GC has not yet been determined. The present study aimed to
determine the expression of CA4 in GC tissues and assess its impact
on cell proliferation.
Materials and methods
Study subjects
Tissue samples (age range, 25–88; mean age ± SD:
62.95±13.12; males, 37; females, 34), including GC and adjacent
normal mucosal tissues, were collected from surgically resected
specimens obtained between April 2013 and December 2015. The
distance between the tumor tissues and normal adjacent tissues was
5 cm. Normal biopsy tissues (age range, 29–80; mean age ± SD:
60.37±10.23; male, 17; female, 15) were collected from patients
undergoing endoscopy between May 2015 and July 2015 at Ningbo First
Hospital (Ningbo, China). As soon as the specimens were resected
from the patients, tissue samples were obtained, preserved in RNA
fixer (Bioteke Corporation) and stored at −80°C until use.
Paraffin-embedded specimens were collected from the Ningbo
Diagnostic Pathology Center in August 2017. Approval was obtained
from the Human Research Ethics Committee of Ningbo First Hospital.
Each tumor was staged in accordance with the primary
Tumor-Node-Metastasis (TNM) staging system (NCCN V.1.2011)
(15). The included patients did not
receive any neoadjuvant therapy. Inclusion criteria for the
patients were as follows: i) Age ≥18; ii) Informed consent
obtained; and iii) Primary gastric cancer without chemotherapy or
radiotherapy. Exclusion criteria for the patients were: i) Age
<18; ii) Pregnancy or lactation; and iii) No full informed
consent from the patient or his next of kin.
Cell lines and culture
A normal human gastric epithelial cell line (GES-1)
and GC cell lines (AGS and HGC-27) were obtained from the Shanghai
Institute of Biochemistry and Cell Biology, Chinese Academy of
Sciences (Shanghai, China). Cells were cultured in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) in a humidified atmosphere
containing 5% CO2 at 37°C (16).
Plasmids, DNA and transfection
Human pcDNA3.1-CA4 and pcDNA3.1 clones were
purchased from Thermo Fisher Scientific, Inc. Cells at 80%
confluence were transfected with 1.5 µg pcDNA3.1 negative control
(trans-NC) or pcDNA3.1-CA4 (trans-CA4) vectors using
Lipofectamine® 2000 reagent (Thermo Fisher Scientific,
Inc.) in 6-well plates according to the manufacturer's
instructions. The transfected cells were cultured in Opti-MEM I
Reduced Serum Medium (Thermo Fisher Scientific, Inc.) for 6 h and
then in routine growth medium (RPMI-1640) for an additional 48 h
before functional assays or protein expression analyses were
performed.
Real-time analysis of cell
proliferation
A Roche DP real-time cell analyzer (RTCA), an
impedance-based xCELLigence System with E-Plates 96 (Roche Applied
Science), was used to conduct proliferation assays as previously
described (17). Impedance was
measured by determining the cell index (CI).
Clonogenic assay
Cells were seeded into 6-well plates (500
cells/well) and cultured for 2 weeks. Colonies were then fixed with
ethanol for 15 min and stained with 0.05% Giemsa for 1 h at room
temperature. Each cell line was cultured in biological triplicate,
and the surviving colonies (>50 cells/colony) were counted.
Flow cytometric analysis of the cell
cycle
The cell cycle distribution was analyzed using a BD
FACSCalibur flow cytometer (BD Biosciences). A total of
1×106 GES-1 and AGS cells/well were fixed with 70%
ethanol and then propidium iodide (PI)/RNase staining buffer
(QIAGEN GmbH) was added to stain the cells. Analysis was performed
according to the manufacturer's instructions. Data were analyzed
using Cell Quest Pro v5.1 software (BD Biosciences).
Total RNA extraction and reverse
transcription-quantitative (RT-q) PCR
Total RNA was obtained from freshly cultured cells
and human tissues using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. A NanoDrop spectrophotometer (Thermo Fisher
Scientific, Inc.) was used to determine the concentrations and
purity of RNA samples using the A260/A280 ratio (13). A GoTaq 2-Step RT-qPCR system (Promega
Corporation) and an Mx3005P QPCR system (Stratagene; Agilent
Technologies, Inc.) were used to assess the levels of CA4 and
β-actin mRNA according to the manufacturer's instructions. The
primer sequences are provided in Table
I. All experiments were repeated in triplicate. The -ΔCt method
was used to analyze the tissue samples, and the 2−ΔΔCt
method was used for the relative quantification of gene expression
in cell lines (18).
 | Table I.The specific primer sequences. |
Table I.
The specific primer sequences.
| Gene | Primer sequence
(5′→3′) |
|---|
| CA4 | F:
TTGGTGGTGACGATGTTGAT |
|
| R:
CACTGGTGCTACGAGGTTCA |
| β-actin | F:
GCTGTCACCTTCACCGTTCC |
|
| R:
CTCCATCCTGGCCTCGCTGT |
Western blotting
Radioimmunoprecipitation (RIPA) lysis buffer (Cell
Signaling Technology, Inc.) was used to isolate total protein from
GES-1/AGS cells transfected with pcDNA3.1-CA4 and pcDNA3.1. The
protein concentration was quantified using Bradford assay reagents
(Beyotime Institute of Biotechnology) according to the
manufacturer's instructions; proteins (20 µg) were separated by 12%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred to polyvinylidene fluoride membranes (EMD Millipore).
The PVDF membranes were blocked in blocking solution (Beyotime
Institute of Biotechnology) for 1 h in a shaker, and then washed 4
times with TBST for 5 min each time. The membranes were then
incubated with primary antibodies at 4°C on a shaker overnight.
Primary antibodies against CA4 (1:500; cat. no. sc-74527),
cyclin-dependent kinase 2 (1:500; Cdk2; cat. no. sc-6248), Cyclin
B1 (1:400; cat. no. sc-245), p21 (1:600; cat. no. sc-6246) and
β-actin (dilution 1:1,000; cat. no. sc-47778) were obtained from
Santa Cruz Biotechnology, Inc.. Horseradish peroxidase
(HRP)-conjugated goat anti-mouse IgG (H + L) (1:1,000; cat. no.
A0216; Beyotime Institute of Biotechnology) and Western Bright ECL
kits (Advansta, Inc.) were utilized to detect the desired proteins.
Pre-stained protein molecular weight markers (Thermo Fisher
Scientific, Inc.) were included in each gel. The results were
analyzed using Image J software version 1.8.0 (National Institute
of Health).
Immunohistochemistry (IHC)
Formalin-fixed paraffin-embedded specimens were
sliced into 2-µm sections. After deparaffinization and dehydration,
the sections were incubated with 0.3% hydrogen peroxide for 10 min
for blocking. The sections were placed in EDTA buffer (pH 8.0) and
autoclaved at 100°C for 20 min for antigen retrieval and were then
incubated with a primary anti-CA4 mouse monoclonal antibody (1:50;
cat. no. sc-74527; Santa Cruz Biotechnology, Inc.) overnight at
4°C, and an HRP-conjugated goat anti-mouse-IgG antibody (1:2,000;
cat. no. PV-9000; Beijing Zhongshan Jinqiao Biotechnology Co.;
OriGene Technologies) was used as the secondary antibody and added
for 30 min at 37°C. A sample in which diluted PBS replaced the
primary antibody during incubation served as a negative control.
All sections were stained at the same time under the same
conditions. All sections were observed by a light microscope
(Olympus BX43; magnifications; ×20 and ×40).
IHC scoring
The histology of the samples was examined
independently by two histopathologists blinded to the
clinicopathological information. The sections were scored as
previously described (19). The
intensity of immunostaining was scored as negative (−, 0 points),
weak (+, 1 point), moderate (++, 2 points), or strong (+++, 3
points). The percentage of positive tumor cells was assigned to
five categories: i) 0–5%, 0 points; ii) 6–25%, 1 point; iii)
26–50%, 2 points; iv) 51–75%, 3 points; and v) 76–100%, 4 points.
The staining intensity and percentage of positive tumor cell scores
were multiplied to determine the final score for each tumor
specimen. The scores were grouped as low (which included scores
between 0 and +4) and high (which included scores between +6 and
+12).
Statistical analysis
Data are presented as the mean ± standard deviation.
The data were analyzed using SPSS Statistics 18.0 software (SPSS,
Inc.) and GraphPad Prism 6.0 (GraphPad Software, Inc.). One-way
analysis of variance, Pearson's χ2 and two-tailed
Student's t-tests were used as appropriate. P<0.05 was
considered to indicate a statistically significant difference.
Results
CA4 expression is downregulated in GC
tissues
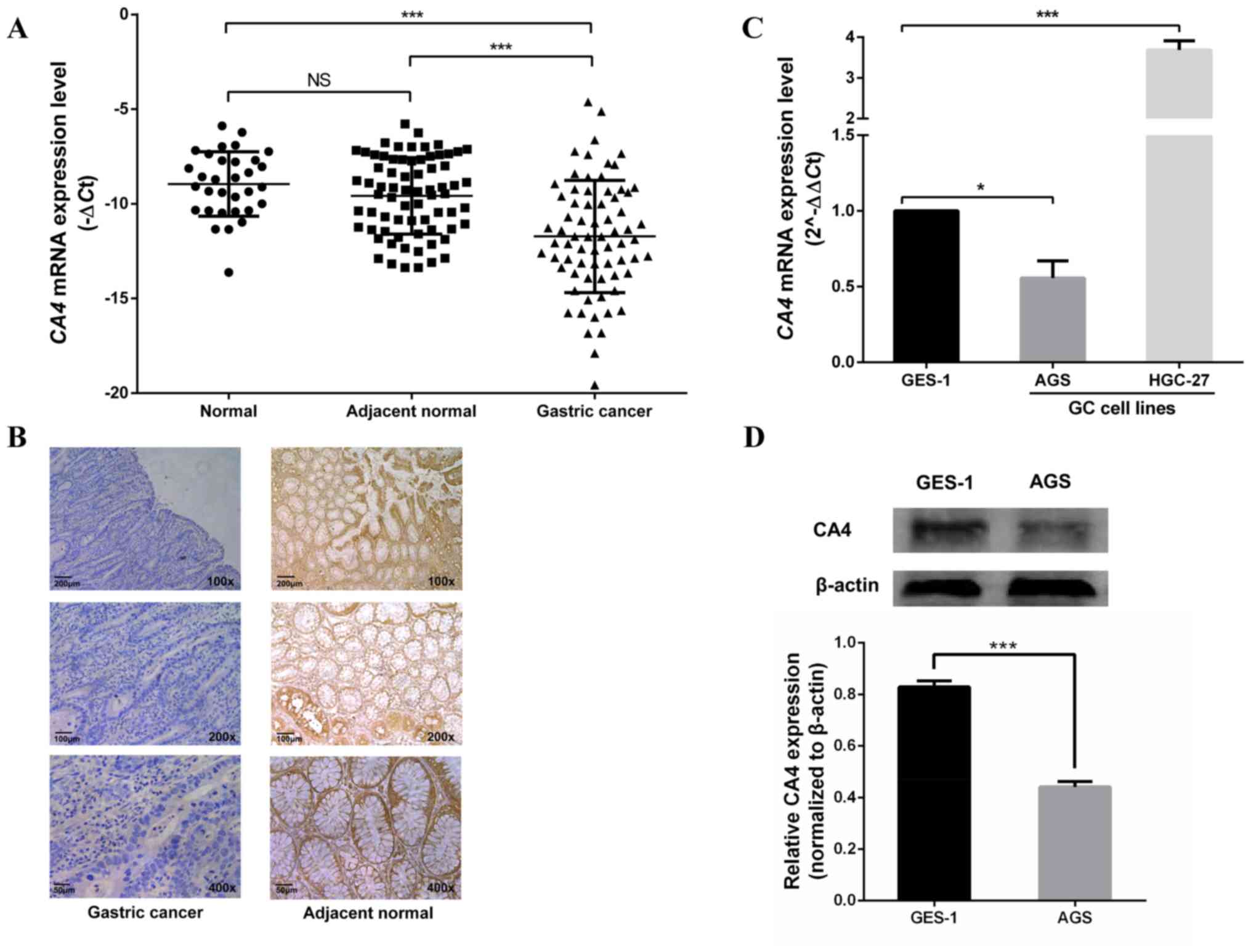
GC tissues expressed lower levels of CA4 compared
with the adjacent gastric mucosa and normal biopsy tissues
(Fig. 1A), but no significant
difference was observed in CA4 expression between adjacent gastric
mucosa and normal biopsy tissues (Fig.
1A). The expression of the CA4 protein in GC tissues was also
investigated by immunohistochemistry (Fig. 1B). The results demonstrated that
among the 39 cases, 84.6% (33/39) patients exhibited low CA4
expression (score <8) and were used for analysis and presented
in the table; however, the other 15.6% (6/39) of cases displayed
high CA4 expression (all 6 cases had ≥76% stained cells and a
staining intensity score of 3) were excluded and their results were
not presented (Table II).
 | Table II.Clinicopathological features of CA4 in
gastric cancer. |
Table II.
Clinicopathological features of CA4 in
gastric cancer.
|
| CA4 |
|
|---|
|
|
|
|
|---|
| Feature | Low (%) | High (%) | P-value |
|---|
| Age, years |
|
| 0.580 |
| ≥60 | 13 (61.9) | 8 (38.1) |
|
|
<60 | 9 (75.0) | 3 (25.0) |
|
| Sex |
|
| 0.696 |
| Male | 16 (69.6) | 7 (30.4) |
|
|
Female | 6 (60.0) | 4 (40.0) |
|
| Diameter, cm |
|
| 0.547 |
| ≥5 | 13 (68.4) | 6 (31.6) |
|
|
<5 | 9 (64.3) | 5 (35.7) |
|
|
Differentiation |
|
| 0.045 |
|
High | 8 (80.0) | 2 (20.0) |
|
|
Moderate | 8 (61.5) | 5 (38.5) |
|
|
Low | 6 (60.0) | 4 (40.0) |
|
| Lauren's
classification |
|
| 0.163 |
|
Intestinal | 14 (77.8) | 4 (22.2) |
|
|
Diffuse | 8 (53.3) | 7 (46.7) |
|
| Depth of
invasion |
|
| 0.721 |
|
T1-T2 | 10 (62.5) | 6 (37.5) |
|
|
T3-T4 | 12 (70.6) | 5 (29.4) |
|
| Lymphatic
metastasis |
|
| 0.282 |
|
Negative | 13 (76.5) | 4 (23.5) |
|
|
Positive | 9 (56.2) | 7 (43.8) |
|
| TNM stage |
|
| 0.465 |
| I and
II | 12 (75.0) | 4 (25.0) |
|
| III and
IV | 10 (58.8) | 7 (41.2) |
|
The expression of CA4 in GC cell
lines
The mRNA and protein levels of CA4 in two GC cell
lines were investigated. CA4 mRNA expression was low in the AGS
cell line and high in the HGC-27 cell line compared with that in
the normal gastric mucosal cell line GES-1 (Fig. 1C). In addition, western blot analysis
demonstrated that the protein expression levels of CA4 in AGS cells
were also lower compared with those in GES-1 cells (Fig. 1D). Thus, the AGS and GES-1 cell lines
were used in the subsequent functional study.
Associations between CA4 expression,
pathological findings and tumor markers
The level of CA4 mRNA was associated with tumor
size, depth of invasion and differentiation (Table III). With increasing pathological
severity, the CA4 mRNA expression level decreased, however, this
trend was not statistically significant. Simultaneously, no
statistical difference was observed with any other pathological
factors. Among the included patients, the rates of carcinoembryonic
antigen (CEA) and carbohydrate antigen 19–9 (CA19-9) positivity
were 56.3 and 45.1%, respectively. In addition, the
clinicopathological features of CA4 in GC tissues were also
examined. According to the median score (IHC score=2) of CA4, the
low expression patients (n=33) presented in Table II were divided into two groups: 17
were considered weakly positive (IHC score 0–2), and 16 were
selected as strongly positive (IHC score 3–6). In addition, the
expression levels of CA4 were higher in female patients with GC
compared with those in male patients. The CA4 levels increased from
well-differentiated GC tissues to moderately and poorly
differentiated GC tissues (Table
II).
 | Table III.The association between CA4 mRNA
expression levels in cancer tissues and clinicopathological factors
of patients with gastric cancer. |
Table III.
The association between CA4 mRNA
expression levels in cancer tissues and clinicopathological factors
of patients with gastric cancer.
|
Characteristics | No. of
patients | CA4 level, -ΔCq
mean ± SD | P-value |
|---|
| Age |
|
| 0.360 |
|
≥60 | 44 | −11.53±2.89 |
|
|
<60 | 27 | −12.01±3.51 |
|
| Sex |
|
| 0.764 |
|
Male | 37 | −11.81±3.17 |
|
|
Female | 34 | −11.57±3.07 |
|
| Diameter, cm |
|
| 0.038 |
| ≥5 | 33 | −12.58±3.34 |
|
|
<5 | 38 | −10.97±2.73 |
|
| CEA |
|
| 0.296 |
|
Positive | 40 | −12.00±3.09 |
|
|
Negative | 31 | −11.14±3.14 |
|
| CA19-9 |
|
| 0.391 |
|
Positive | 32 | −11.34±3.40 |
|
|
Negative | 39 | −12.02±2.83 |
|
| Depth of
invasion |
|
| 0.039 |
| T1 and
T2 | 26 | −10.65±2.26 |
|
| T3 and
T4 | 45 | −12.25±3.36 |
|
|
Differentiation |
|
| 0.018 |
|
High | 7 | −9.54±1.50 |
|
|
Moderate | 23 | −11.67±2.23 |
|
|
Low | 41 | −12.66±3.56 |
|
| Lymphatic
metastasis |
|
| 0.702 |
|
Negative | 24 | −11.49±2.14 |
|
|
Positive | 47 | −11.81±3.50 |
|
| TNM stage |
|
| 0.321 |
| I and
II | 32 | −11.35±1.99 |
|
| III and
IV | 39 | −12.02±3.58 |
|
Role of CA4 in cell proliferation
IHC and western blotting analysis demonstrated lower
CA4 levels in AGS GC cells compared with those in GES-1 gastric
epithelial cells (Fig. 1C and D);
these cells were transfected with a CA4 expression vector to
determine the biological functions of CA4 in human GC cells.
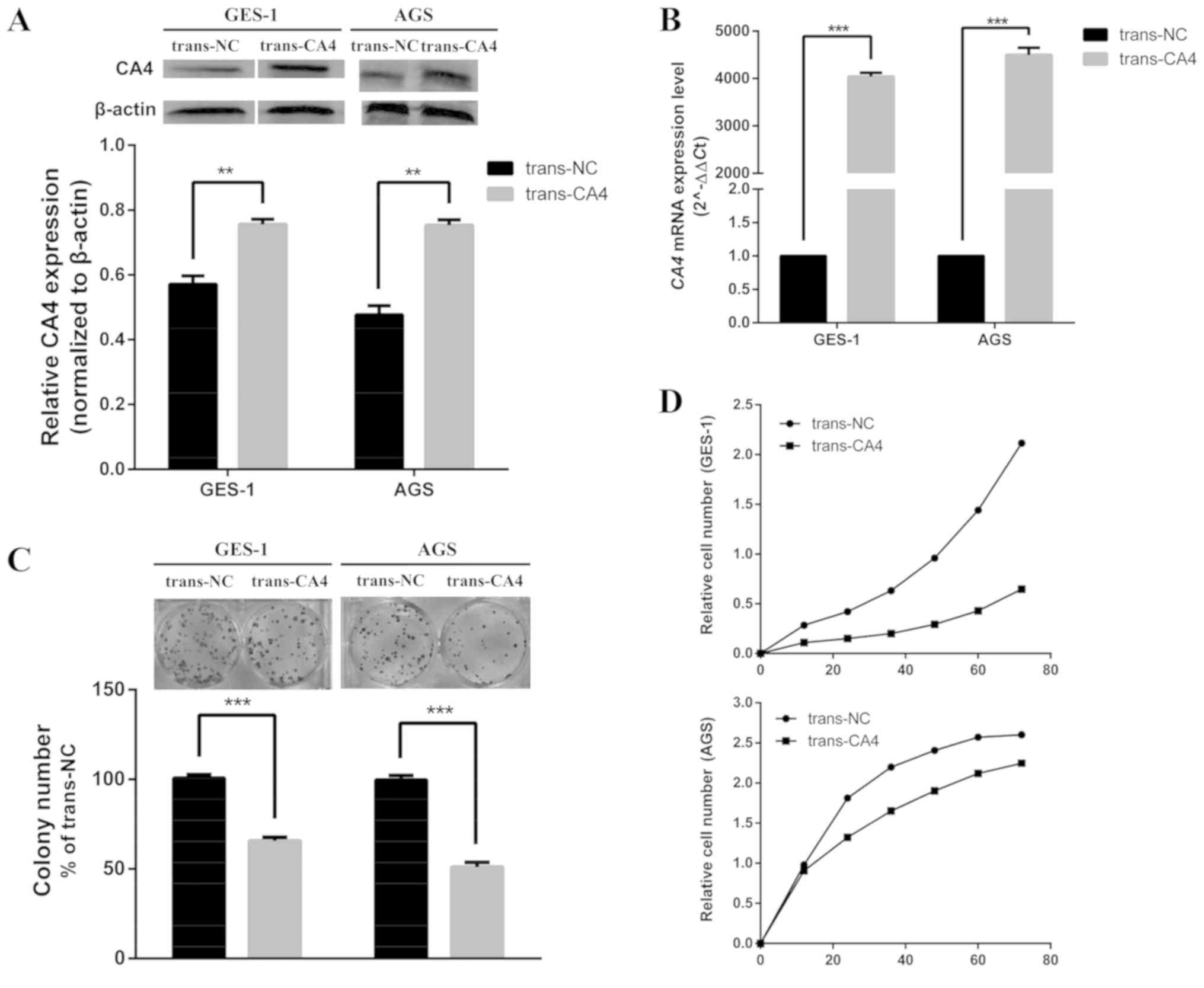
Overexpression of CA4 protein and mRNA was confirmed by western
blotting (Fig. 2A) and RT-qPCR
(Fig. 2B), respectively. Based on
the results of the clonogenic assay, fewer clones of a smaller size
were observed in trans-CA4 cells compared with trans-NC cells
(Fig. 2C).
Subsequently, the tumor-suppressive activity of CA4
was examined using a real-time cell analyzer. Trans-CA4 cells
exhibited a significantly lower growth rate compared with that of
trans-NC cells (Fig. 2D).
Overexpression of CA4 effectively inhibited cell proliferation
beginning at 24 h after transfection in both AGS and GES-1
cells.
Overexpression of CA4 in GES-1 and AGS
cells arrests the cell cycle at the G2/M phase
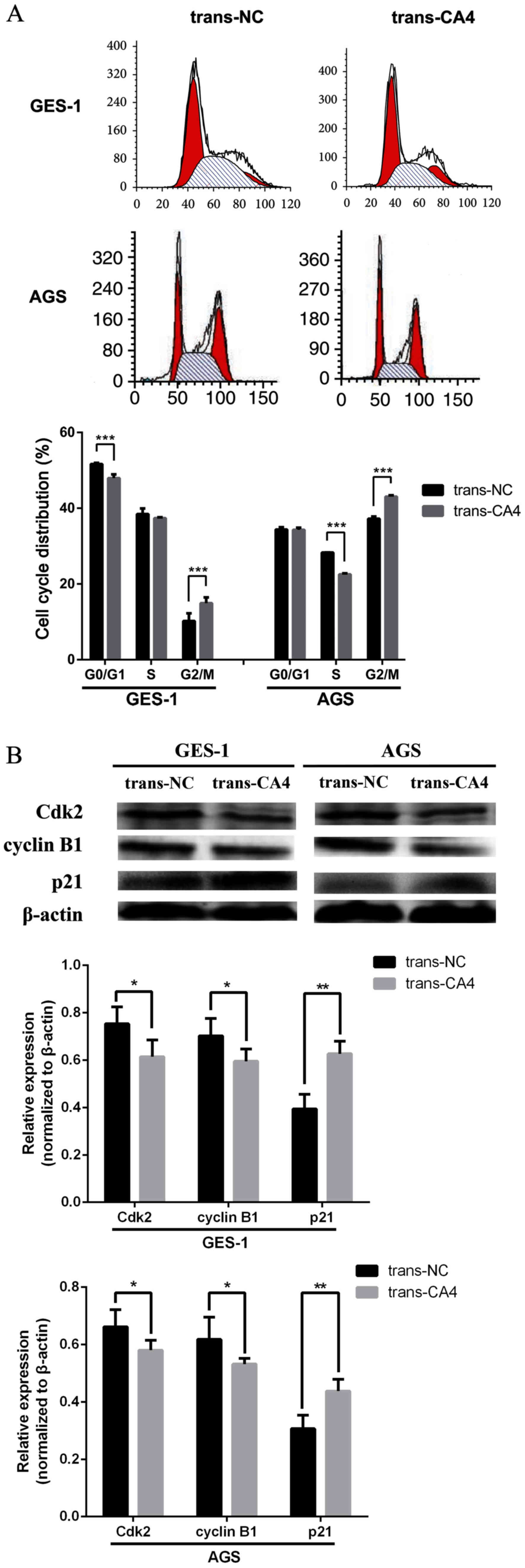
CA4 overexpression was associated with an increased
population of trans-CA4 AGS cells in the G2/M phase (43.07%)
compared with that in trans-NC AGS cells (37.22%; Fig. 3A). Similar findings were observed in
trans-CA4-GES-1 cells. According to the western blot analysis, CA4
overexpression modulated the expression of G2/M phase-related
proteins; the levels of Cdk2 and cyclin B1 were decreased, and the
levels of p21 were increased compare with those in trans-NC cells
(Fig. 3B).
Discussion
The results of the present study demonstrated that
CA4 was expressed at lower levels in GC tissues compared with those
in adjacent normal tissues and normal biopsy tissues, and its level
was associated with sex, tumor size, depth of invasion and
differentiation. The results of the present study also indicated
that CA4 overexpression was associated with the inhibition of cell
proliferation and the cell cycle of AGS and GES-1 cells, possibly
by modulating the expression of cell cycle-associated proteins. To
the best of our knowledge, the present study was the first to
identify the clinical and biological functions of CA4 in GC. CA4
was previously studied in colon cancer by Zhang et al
(20), who reported that CA4
exhibited tumor-suppressing properties. In addition, CA4
methylation levels serve as a prognostic factor for colon cancer
recurrence (20). Overexpression of
CA4 in transfected cells suppresses the proliferative and migratory
abilities of colon cancer cells (20).
In addition to CA4, other members of the carbonic
anhydrase family have been reported to possess cancer-associated
functions; however, only CA2, CA9 and CA12 have been reported to be
associated with GC. According to Hu et al (12), downregulation of CA2 expression is
associated with tumor aggressiveness and may predict the overall
survival of patients with GC. Fidan et al (21), demonstrated that CA9 levels were
increased in patients with GC compared with healthy controls,
suggesting that CA9 may be used as a biomarker to predict the
occurrence of GC. Leppilampi et al (14), confirmed that CA12 expression was
slightly increased in gastric adenocarcinoma samples compared with
non-neoplastic gastric mucosa samples. In the present study, the
expression levels of CA4 were examined in two GC cell lines (AGS
and HGC-27): The AGS cell line was ultimately selected as the CA4
mRNA and protein expression levels were lower in AGS cells compared
with those in GES-1 cells; the expression of CA4 in HGC-27 GC cells
was higher compared with that in GES-1 cells, which may be related
to tumor heterogeneity. Similarly, CA4 was not downregulated in all
GC tissues, but was upregulated in a small number of GC tissues. A
series of functional experiments in the present study confirmed
that CA4 overexpression inhibited the proliferation of AGS cells,
suggesting a tumor-suppressive role of CA4 in this GC cell line.
However, the inhibitory effect of CA4 on cell proliferation is not
only reflected in GC cell lines, its overexpression also inhibits
the growth of GES-1. Further research indicated that the inhibition
of cell proliferation may be related to cell cycle arrest. The cell
cycle distribution analysis results indicated that CA4
overexpression arrested AGS and GES-1 in the G2/M phase. The
overexpression of CA4 caused a significant reduction in the S phase
of AGS cells, while GES-1 cells were significantly reduced in the
G0/G1 phase. This result revealed that CA4 impacts on GC cells as
well as normal cells. If used in the clinical treatment of GC, CA4
needs a more specific therapeutic target. In subsequent analysis in
the present study, CA4 overexpression was associated with reduced
expression of cyclin B1 and Cdk2, and increased expression of p21,
the key Cdk inhibitor, compared with the NC group. In the G2/M
phase of the GC cell cycle, cyclin B1 and Cdk2 are upregulated, and
p21 is expressed at low levels (22–25).
Based on the results from the present study, CA4 suppression may be
responsible for the alterations in the expression of these
regulatory proteins in GC.
Commonly used biomarkers, such as CEA or CA19-9,
were detected at low rates in GC, as demonstrated in the present
study. Consistent with these findings, the positive detection rates
of CEA and CA19-9 in another study were 64.0 and 53.3%,
respectively (26). Therefore, CA4
may serve as a more sensitive biomarker compared with CEA or CA19-9
for the detection of GC.
Limitations of the present study should be
acknowledged. Only 71 GC samples were used in this study.
Additional analyses with a larger sample size may further define
the clinical value of CA4 in GC. In addition, the role of CA4 in
tumorigenesis requires further research.
In conclusion, CA4 may act as a tumor suppressor
gene in GC by arresting cell division in G2/M phase. Future studies
should investigate whether CA4 is useful for the diagnosis and
treatment of GC to improve the survival of patients with GC.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Natural
Science Foundation of Zhejiang (grant no. LQ18H160015), The Natural
Science Foundation of Ningbo (grant nos. 2014A610226 and
2016A610158) and The Scientific Benefit for People Project of
Ningbo (grant no. 2014C51001).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
BW, XD and HS conceived and designed the
experiments, and wrote the article. BW, HJ, XW, YW and PL performed
the experiments. HJ, XZ and JG analyzed the data. All authors have
read and approved the manuscript.
Ethics approval and consent to
participate
Approval was obtained from the Human Research Ethics
Committee of Ningbo First Hospital (approval no. 2018-R045).
Written informed consent was provided by all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shi J, Qu YP and Hou P: Pathogenetic
mechanisms in gastric cancer. World J Gastroenterol.
20:13804–13819. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ko KP, Shin A, Cho S, Park SK and Yoo KY:
Environmental contributions to gastrointestinal and liver cancer in
the asia-pacific region. J Gastroenterol Hepatol. 33:111–120. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Suzuki H, Oda I, Abe S, Sekiguchi M, Mori
G, Nonaka S, Yoshinaga S and Saito Y: High rate of 5-year survival
among patients with early gastric cancer undergoing curative
endoscopic submucosal dissection. Gastric Cancer. 19:198–205. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanabe S, Ishido K, Higuchi K, Sasaki T,
Katada C, Azuma M, Naruke A, Kim M and Koizumi W: Long-term
outcomes of endoscopic submucosal dissection for early gastric
cancer: A retrospective comparison with conventional endoscopic
resection in a single center. Gastric Cancer. 17:130–136. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ohnita K, Isomoto H, Shikuwa S, Yajima H,
Minami H, Matsushima K, Akazawa Y, Yamaguchi N, Fukuda E, Nishiyama
H, et al: Early and long-term outcomes of endoscopic submucosal
dissection for early gastric cancer in a large patient series. Exp
Ther Med. 7:594–598. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Skrajnowska D and Bobrowska-Korczak B:
Role of zinc in immune system and anti-cancer defense mechanisms.
Nutrients. 11:22732019. View Article : Google Scholar
|
|
8
|
Hrabeta J, Eckschlager T, Stiborova M,
Heger Z, Krizkova S and Adam V: Zinc and zinc-containing
biomolecules in childhood brain tumors. J Mol Med (Berl).
94:1199–1215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schreiber CL and Smith BD: Molecular
imaging of aminopeptidase N in cancer and angiogenesis. Contrast
Media Mol Imaging. 2018:53151722018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Waheed A and Sly WS: Membrane associated
carbonic anhydrase IV (CA IV): A personal and historical
perspective. Subcell Biochem. 75:157–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fleming RE, Parkkila S, Parkkila AK,
Rajaniemi H, Waheed A and Sly WS: Carbonic anhydrase IV expression
in rat and human gastrointestinal tract regional, cellular, and
subcellular localization. J Clin Invest. 96:2907–2913. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu X, Huang Z, Liao Z, He C and Fang X:
Low CA II expression is associated with tumor aggressiveness and
poor prognosis in gastric cancer patients. Int J Clin Exp Pathol.
7:6716–6724. 2014.PubMed/NCBI
|
|
13
|
Chen J, Röcken C, Hoffmann J, Krüger S,
Lendeckel U, Rocco A, Pastorekova S, Malfertheiner P and Ebert MP:
Expression of carbonic anhydrase 9 at the invasion front of gastric
cancers. Gut. 54:920–927. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leppilampi M, Saarnio J, Karttunen TJ,
Kivelä J, Pastoreková S, Pastorek J, Waheed A, Sly WS and Parkkila
S: Carbonic anhydrase isozymes IX and XII in gastric tumors. World
J Gastroenterol. 9:1398–1403. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ajani JA, Bentrem DJ, Besh S, D'Amico TA,
Das P, Denlinger C, Fakih MG, Fuchs CS, Gerdes H, Glasgow RE, et
al: Gastric cancer, version 2.2013: Featured updates to the NCCN
guidelines. J Natl Compr Canc Netw. 11:531–546. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang Z, Guo J, Xiao B, Miao Y, Huang R,
Li D and Zhang Y: Increased expression of miR-421 in human gastric
carcinoma and its clinical association. J Gastroenterol. 45:17–23.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deng H, Guo Y, Song H, Xiao B, Sun W, Liu
Z, Yu X, Xia T, Cui L and Guo J: MicroRNA-195 and microRNA-378
mediate tumor growth suppression by epigenetical regulation in
gastric cancer. Gene. 518:351–359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou H, Guo JM, Lou YR, Zhang XJ, Zhong
FD, Jiang Z, Cheng J and Xiao BX: Detection of circulating tumor
cells in peripheral blood from patients with gastric cancer using
microRNA as a marker. J Mol Med (Berl). 88:709–717. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi R, Liu W, Liu B, Xu Z, Chen L and
Zhang Z: Slit2 expression and its correlation with subcellular
localization of beta-catenin in gastric cancer. Oncol Rep.
30:1883–1889. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang J, Tsoi H, Li X, Wang H, Gao J, Wang
K, Go MY, Ng SC, Chan FK, Sung JJ and Yu J: Carbonic anhydrase IV
inhibits colon cancer development by inhibiting the wnt signalling
pathway through targeting the WTAP-WT1-TBL1 axis. Gut.
65:1482–1493. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fidan E, Mentese A, Ozdemir F, Deger O,
Kavgaci H, Karahan SC and Aydin F: Diagnostic and prognostic
significance of CA IX and suPAR in gastric cancer. Med Oncol.
30:5402013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Begnami MD, Fregnani JH, Nonogaki S and
Soares FA: Evaluation of cell cycle protein expression in gastric
cancer: Cyclin B1 expression and its prognostic implication. Hum
Pathol. 41:1120–1127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee SM, Kwon JI, Choi YH, Eom HS and Chi
GY: Induction of G2/M arrest and apoptosis by water extract of
strychni semen in human gastric carcinoma AGS cells. Phytother Res.
22:752–758. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang R, Li YX, Wang LS, Song Y, Huang QJ
and Zhang DG: Aqueous extracts of fructus ligustri lucide induce
gastric carcinoma cell apoptosis and G2/M cycle arrest. Int J Clin
Exp Med. 8:12307–12316. 2015.PubMed/NCBI
|
|
25
|
Zhang C, Chen Z, Zhou X, Xu W, Wang G,
Tang X, Luo L, Tu J, Zhu Y, Hu W, et al: Cantharidin induces G2/M
phase arrest and apoptosis in human gastric cancer SGC-7901 and
BGC-823 cells. Oncol Lett. 8:2721–2726. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song H, Sun W, Ye G, Ding X, Liu Z, Zhang
S, Xia T, Xiao B, Xi Y and Guo J: Long non-coding RNA expression
profile in human gastric cancer and its clinical significances. J
Transl Med. 11:2252013. View Article : Google Scholar : PubMed/NCBI
|