Introduction
Lung cancer is one of the most common cancer types
with a high mortality rate globally (1). Non-small cell lung cancer (NSCLC)
represents a large proportion of lung cancer cases (2). The 5-year survival rate of patients
with lung cancer is only 15%, which is due to metastasis (3). Early stage diagnosis of NSCLC might
lead to higher survival rates and patients could be effectively
treated by surgery, radiotherapy or chemotherapy sooner (4). Therefore, it is important to gain an
improved understanding of NSCLC progression.
Long non-coding RNA (lncRNA) is a form of non-coding
RNA, ~200 nucleotides in length, that cannot be translated into
protein (5). A number of lncRNAs
regulate cancer metastasis and progression. In several studies,
Opa-interacting protein 5 antisense RNA 1 (OIP5-AS1; transcribed
into the antisense strand of OIP5) functions as an oncogenic lncRNA
in several cancer types, including oral squamous cell carcinoma
(6), bladder cancer (7) and hemangioma (8). OIP5-AS1 was reported to promote lung
cancer progression (9,10). Nonetheless, the underlying molecular
mechanisms of OIP5-AS1 function in this phenomenon have not been
fully elucidated.
MicroRNAs (miRs/miRNAs) are non-coding RNAs ~22
nucleotides in length (11). miRs
regulate gene expression levels via occupying 3′-untranslated
(3′-UTR) regions of target genes (12). miRs are involved in a number of
cancer types, including NSCLC (13,14).
miR-140-5p was demonstrated to inhibit proliferation, invasion and
drug resistance of NSCLC via the vascular endothelial growth factor
A (VEGFA) and Wnt signaling pathways (15,16).
Histone deacetylase 7 (HDAC7) is a class 2 member of
the HDAC family (17). Previous
studies have shown that HDACs affect gene transcription through
complexing with classical transcription factors, such as STAT3,
hypoxia-inducible factor-1α, forkhead box protein (FOX) P3 and
FOXA1 (18–20). Caslini et al (21) suggested that HDAC7 was necessary for
stem cell-like properties of breast cancer cells. Additionally,
HDAC7 deletion in endothelial progenitor cells repressed the
neovascularization of NSCLC tumors via regulating the transcription
of angiogenic genes, such as VEGF (22). However, whether HDAC7 exerts a role
in NSCLC cells remains to be elucidated.
The present study aimed to investigate and resolve
the novel molecular mechanism underlying OIP5-AS1-induced
metastasis in NSCLC. These findings may help advance the
understanding of NSCLC progression and aid the development of novel
therapeutic targets for treating NSCLC.
Materials and methods
Cell lines
Human lung cancer cells (A549 and H1299) were
obtained from American Type Culture Collection. Lung cancer cells
were cultured in DMEM (HyClone; Cytiva) supplemented with 10% FBS
(Hyclone; GE Healthcare Life Sciences) and 1%
penicillin/streptomycin at 5% CO2 and 37°C. In addition,
human dermal lymphatic endothelial cells (HDLECs) were obtained
from PromoCell GmbH. HDLECs were cultured in endothelial cell
growth medium (PromoCell GmbH) at 5% CO2 and 37°C. After
72 h, the conditioned medium (CM) from A549 and H1299 cells were
harvested and filtered. HDLECs (2×105 per well) were
plated in 6-well cell culture plates.
Cell transfection
HDAC7 and VEGFA cDNA sequences were cloned and
ligated into a pcDNA6 vector (Youbio Inc., http://www.youbio.cn/). In brief, RNAs were extracted
from A549 cells using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). The concentration and purity of
RNA were determined using NanoDrop™ 2000 (Thermo Fisher Scientific,
Inc.). cDNA was synthesized from 2 µg RNA using the PrimeScript RT
kit (Takara Biotechnology Co., Ltd.) at 42°C for 30 min and 85°C
for 5 sec. cDNA samples were diluted with 50 µl RNase-free water.
HDAC7 and VEGFA cDNA were cloned and amplified by PCR. Q5
High-Fidelity DNA Polymerase (M0491, New England Biolabs Inc.) was
used to amplify DNA. HDAC7, forward:
5′-GGTACCATGCACAGCCCCGGCGCTGATGG-3′ and reverse:
5′-CTCGAGTTAGAGATTCATAGGTTC-3′; VEGFA, forward:
5′-GGTACCATGACGGACAGACAGACAGACAC-3′ and reverse:
5′-CTCGAGTCACCGCCTCGGCTTGTCA-3′. The following thermocycling
conditions were used: Initial denaturation at 98°C for 30 sec,
followed by 30 cycles of 98°C for 10 sec, 60°C for 20 sec and 72°C
for 30 sec with final extension at 72°C for 2 min. The constructed
vectors (2 µg), NC mimic (50 µM), NC inhibitor (50 µM), miR-140-5p
mimic (50 µM) and miR-140-5p inhibitor (50 µM) were transiently
transfected into A549 cells using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc). NC mimic:
5′-GGUUCCAUCGUACACUGUUCA-3′; miR-140-5p 5′-mimic:
CUCACAGAGAAGGGCAGUG-3′; NC inhibitor: 5′-CCAUCAGUCCCCAUCGCCA-3′;
miR-140-5p inhibitor: 5′-CAGUAGUAGAACGGCG-3′. After 48 h of
transfection, the cells were used for subsequent experimentation.
Transfection efficiency reached 50–70% through visualizing EGFP
positive cells under microscopy. Small interfering (si)-negative
control (NC) (20 µM), si-OIP5-AS1 (20 µM), miR-140-5p mimic and
inhibitor were all synthesized by Shanghai GenePharma Co., Ltd. The
sequences were as follows: si-NC:UACCGACUGGCAAUUCAUG;
si-OIP5-AS1:GGCAGUAGAAUCACUUAAA and
si-HDAC7:GGGCUGACAAAGAAGAAGU.
Gene cloning bacterial
transformation
The cDNAs and pcDNA6 vector were double-digested
with restriction enzymes (KpnI and XhoI) at 37°C for
12 h. The digested linear sequences were ligated using T4 ligase at
16°C for 12 h. The ligated vectors were then transformed into DH5α
competent bacteria (Sangon Biotech Co., Ltd). A tube containing
ligated vectors and competent bacteria were incubated at 42°C for
90 sec and then placed on ice.
Bioinformatic prediction
Starbase version 2.0 online program (developed by
Sun Yat-sen University) was to perform prediction of interaction
between OIP5-AS1 and miR-140-5p. TargetScan (developed by the
Massachusetts Institute of Technology) was also used to predict
interaction between miR-140-5p and HDAC7.
Dual-luciferase reporter assay
Wild-type and mutant sequences of OIP5-AS1 and HDAC7
were subcloned into a pGL3 vector (Promega Corporation). HDAC7 and
VEGFA cDNA were cloned and amplified by PCR. Following 12 h of A549
cell inoculation at density of 3×104 cells/well (24-well
plate), the vectors were transfected into cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After 48 h, cells were collected. Luciferase
activities were determined using a Dual-Luciferase Reporter Assay
system (Promega Corporation). Firefly luciferase activity was
normalized to Renilla luciferase activity.
Transwell invasion assay
H1299 and A549 cells (~1×105 cells per
well) were resuspended in 250 µl DMEM. Cells were plated in the
upper chamber containing Matrigel-coated membranes (precoating for
12 h at 4°C). Approximately 300 µl DMEM supplemented with 50% FBS
was plated in the bottom chambers. Following incubation for 48 h,
invaded cells were stained with 0.005% crystal violet for 2 h at
room temperature and visualized under light microscope
(magnification, ×400).
Reverse transcription-quantitative PCR
(RT-qPCR)
RNA extraction was performed as aforementioned.
Subsequently, 0.5 µl cDNA was used for qPCR in a total volume of 10
µl using the SYBR Green I Master Mix kit (Thermo Fisher Scientific,
Inc.) and primers. qPCR was performed on an ABI 7500 instrument
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The following
thermocycling conditions were used for the two-step qPCR program:
Initial denaturation at 94°C for 15 min; followed by 40 cycles of
94°C for 30 sec, 60°C for 30 sec and 70°C for 30 sec. GAPDH served
as the internal control for OIP5-AS1, VEGFA and HDAC7, whereas U6
was the internal control for miR-140-5p. Relative gene expression
was calculated using 2−ΔΔCq method (23). The following primer pairs were used
for the qPCR: OIP5-AS1 forward, 5′-TGCGAAGATGGCGGAGTAAG-3′ and
reverse, 5′-TAGTTCCTCTCCTCTGGCCG-3′; miR-140-5p forward,
5′-CAGTGGTTTTACCCTATGGTAG-3′ and reverse,
5′-ACCATAGGGTAAAACCACTGTT-3′; HDAC7 forward,
5′-TGCTCTTGTCCTTGTGGAGA-3′ and reverse, 5′-CCACCACCTCTTCCTAGCAG-3′;
VEGFA forward, 5′-GGCCAGCACATAGGAGAGAT-3′ and reverse,
5′-ACGCTCCAGGACTTATACCG-3′; GAPDH forward,
5′-CTGACTTCAACAGCGACACC-3′ and reverse, 5′TGCTGTAGCCAAATTCGTTG-3′;
U6 forward, 5′CGCTTCGGCAGCACATATACTA-3′ and reverse,
5′GAATTTGCGTGTCATCCTTGCG-3′.
Lymphatic vessel formation assay
HDLECs were subjected to CM treatment on Growth
Factor Reduced Matrigel. Matrigel was diluted with PBS buffer at a
1:5 ratio. The resultant solution was used to coat 24-well plates
(0.2 ml/well) and incubated at 37°C for 5 h. HDLECs were cultured
in DMEM in wells at a density of ~5×104 cells/well. When
experiment was finished (6 h after incubation), an inverted
microscope (magnification, ×400) was used to observe and capture
images. Mean tube lengths were quantified (normalized to the
control) using the Image-Pro® Plus software (Media
Cybernetics, Inc.).
Western blotting
Total protein was extracted from A549 cells using 1×
RIPA buffer (Beyotime Institute of Biotechnology). The protein
concentration of supernatant was determined using the bicinchoninic
acid method. Approximately 50 µg protein/lane were separated by 10%
SDS-PAGE. The separated proteins were then transferred to a
nitrocellulose membrane (EMD Millipore). Blocking was performed
using 5% non-fat milk for 1 h at room temperature. Subsequently,
the immunoblotted membrane was incubated with the following primary
antibodies: Anti-HDAC7 (cat. no. ab50212, 1:1,000, Abcam),
anti-VEGFA (cat. no. ab51745, 1:1,000, Abcam) and anti-GAPDH (cat.
no. GTX124502, 1:1,000, GeneTex Inc.) overnight at 4°C. The
following day, the membrane was washed with PBS-Tween-20 (0.05%)
(PBS-T) and incubated with secondary antibodies for 1 h at room
temperature. anti-mouse IgG-HRP (cat. no. SE131, 1:2,000, Beijing
Solarbio Science & Technology Co., Ltd.), anti-rabbit IgG-HRP
(cat. no. SE134, 1:2,000. Beijing Solarbio Science & Technology
Co., Ltd.). After washing with PBS-T, protein signal intensity was
measured using an ECL exposure system.
The Cancer Genome Atlas (TCGA)
analysis
The gene expression datasets of lung tumors were
downloaded from the Genomic Data Commons (GDC) lung adenocarcinoma
TCGA datasets (160 cancer and 369 normal tissues). The datasets
used were originally from api.gdc.cancer.gov/data/. For stage plot analysis, we
grouped the patients according to stage I, II, III and IV lung
cancer. Then, we input genes (OIP5-AS1, miR-140-5p, HDAC7, VEGFA)
expression data into GraphPad Prism (GraphPad Software) to draw the
stage plot. For Kaplan-Meier plot analysis, the clinical
information of survival was used to draw survival curve using
GraphPad Prism software.
Statistical analysis
GraphPad Prism 8.0 (GraphPad Software, Inc.) was
used to perform statistical analysis. The data of three replicates
were calculated as the mean ± SD. Comparisons between two or more
experimental groups were analyzed using unpaired Student's t-test
and one-way ANOVA with Tukey's post hoc test. Lung tumor tissues
and paired adjacent normal tissues of TCGA were evaluated using
paired Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
OIP5-AS1 promotes NSCLC
metastasis
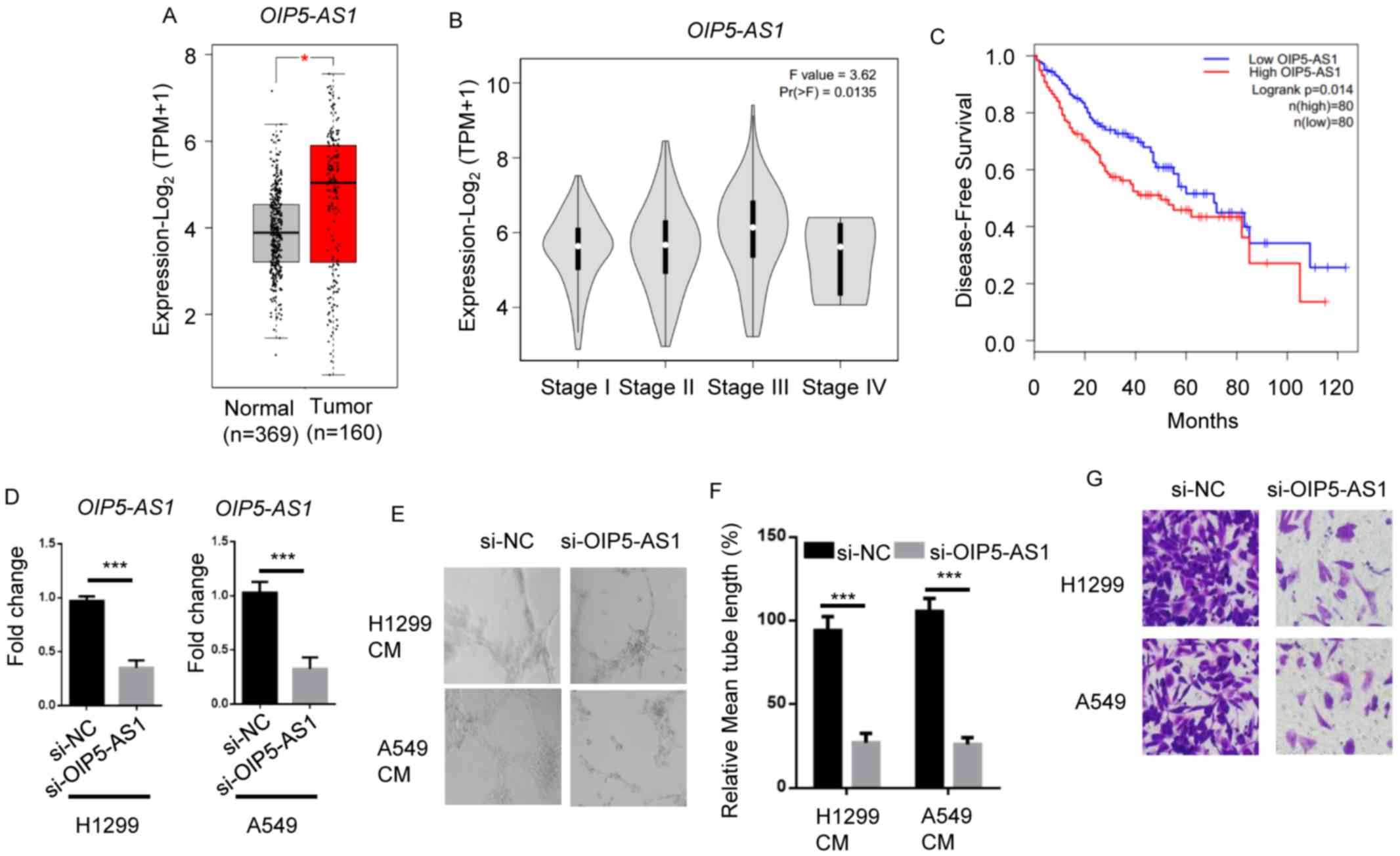
To determine the role of OIP5-AS1 in NSCLC
metastasis, TCGA datasets of NSCLC were analyzed. OIP5-AS1 was
significantly upregulated in lung tumor tissues compared with
normal tissues (Fig. 1A). Moreover,
higher levels of OIP5-AS1 were observed in advanced stages of NSCLC
tumors (stage III and IV) compared with lower stages of tumors
(stage I and II) (Fig. 1B). Low
levels of OIP5-AS1 were associated with higher overall survival
rate of patients with NSCLC (Fig.
1C).
Next, OIP5-AS1 knockdown in H1299 and A549 cells was
performed, and RT-qPCR analysis showed that OIP5-AS1 levels
significantly decreased in si-OIP-AS1 groups compared with si-NC
groups (Fig. 1D). Compared with
si-NC groups, OIP5-AS1 knockdown led to a significant reduction in
lymphatic vessel formation ability in HDLECs incubated with H1299
or A549 CM (Fig. 1E and F).
Likewise, OIP5-AS1 knockdown attenuated the invasion of H1299 or
A549 cells compared with the si-NC group (Fig. 1G). These data showed that OIP5-AS1
plays a role in NSCLC progression.
miR-140-5p rescues OIP5-AS1-induced
NSCLC metastasis
miRNAs were reported to function in several cancer
types by being sponged by lncRNAs (24–26).
Based on these findings, the present study aimed to identify
potential miRNAs responsible for OIP5-AS1-enhanced NSCLC
progression. miR-140-5p was identified as the candidate gene using
bioinformatics analysis (Fig. 2A).
To validate the findings, a dual-luciferase reporter assay was
performed using A549 cells, as this is the most used cell line in
previous stduies investigating lung cancer (27,28). The
results demonstrated that the miR-140-5p mimic significantly
reduced the luciferase activity of A549 cells compared with NC
mimic, whereas miR-140-5p inhibitor significantly increased
luciferase activity compared with the NC inhibitor group. There is
no significant alteration in mutant OIP5-AS1-expressing A549 cells
(Fig. 2B). Furthermore, miR-140-5p
mimic significantly reduced OIP5-AS1 expression levels compared
with the NC mimic group, while miR-140-5p inhibitor significantly
increased OIP5-AS1 expression levels compared with the NC inhibitor
group. The miR-140-5p mimic greatly increased miR-140-5p levels and
the miR-140-5p inhibitor downregulated miR-140-5p expression
(Fig. 2C). Clinically, it was found
that miR-140-5p levels were significantly lower in NSCLC tumors
compared with normal tissues (Fig.
2D).
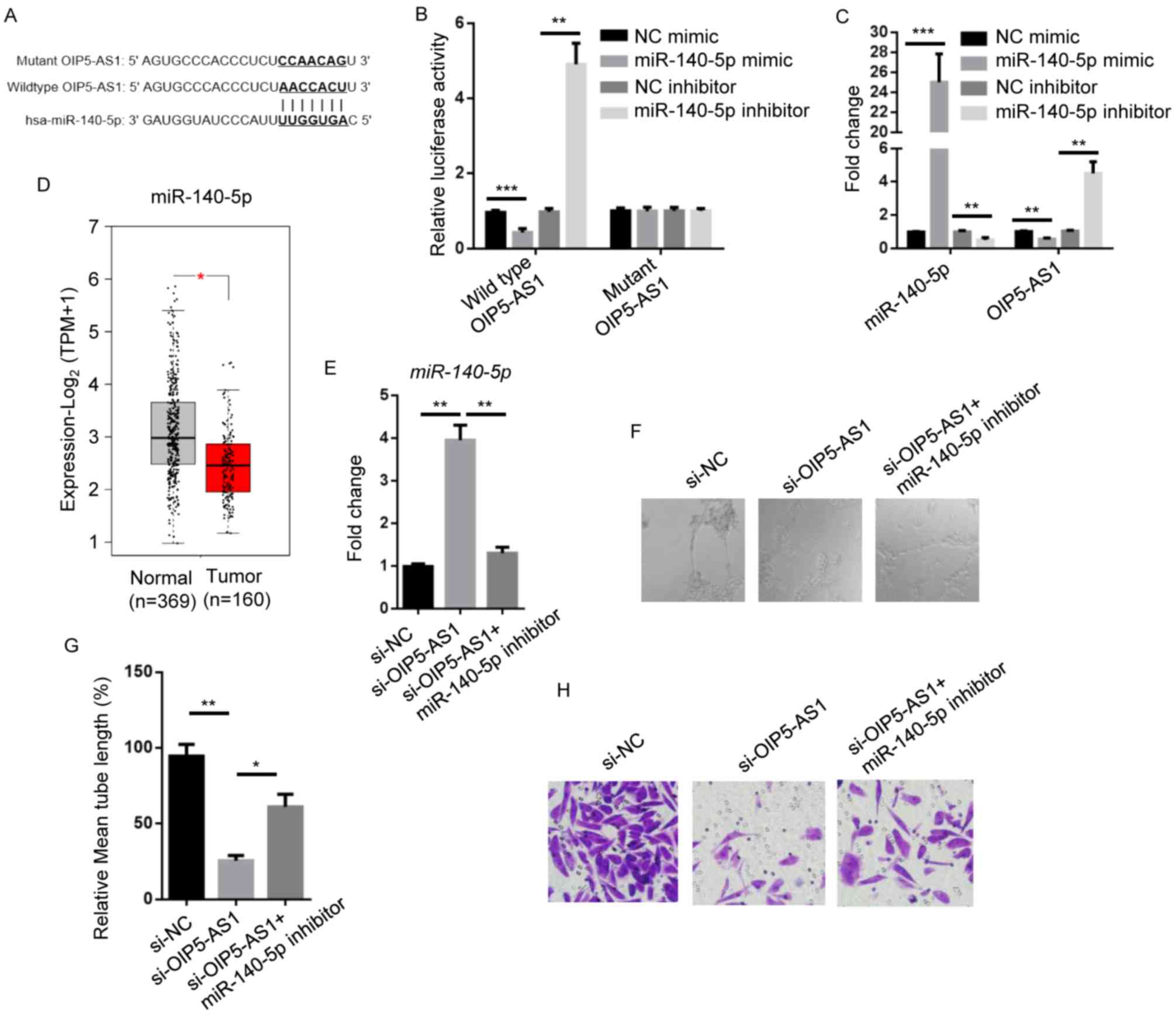 | Figure 2.miR-140-5p rescues OIP5-AS1-regulated
NSCLC metastasis. (A) Bioinformatics analysis showing the binding
site of OIP5-AS1 by miR-140-5p. Complementary sequences are shown
in bold. (B) Luciferase activity in A549 cells transfected with NC
mimic, miR-140-5p mimic, NC inhibitor and miR-140-5p inhibitor. (C)
RT-qPCR showing miR-140-5p and OIP5-AS1 expression levels in A549
cells transfected with NC mimic, miR-140-5p mimic, NC inhibitor and
miR-140-5p inhibitor. (D) Bioinformatic analysis of The Cancer
Genome Atlas datasets showing miR-140-5p levels in human normal
lung tissues (n=369) or lung adenocarcinoma tumors (n=160). (E)
RT-qPCR showing miR-140-5p levels in A549 cells transfected with
si-NC, si-OIP5-AS1 or si-OIP5-AS1 + miR-140-5p inhibitor. (F)
Representative microscopy images (400× magnification) and (G)
quantification (normalized to the control) of lymphatic vessel
formation in human dermal lymphatic endothelial cells cultured with
conditioned medium of A549 cells transfected with si-NC,
si-OIP5-AS1 or si-OIP5-AS1 + miR-140-5p inhibitor. (H) Transwell
assay showing invasion of A549 cells transfected with si-NC,
si-OIP5-AS1 or si-OIP5-AS1 + miR-140-5p inhibitor (400×
magnification). Data are presented as the mean ± standard
deviation. *P<0.05; **P<0.01 and ***P<0.001. miR,
microRNA; OIP5-AS1, Opa-interacting protein 5 antisense RNA 1;
NSCLC, non-small cell lung cancer; NC, negative control; RT-qPCR,
reverse transcription-quantitative PCR; si, small interfering; TPM,
Transcripts Per Million. |
To investigate the role of miR-140-5p in
OIP5-AS1-regulated metastasis, miR-140-5p levels were examined in
A549 cells treated with si-OIP5-AS1, si-OIP5-AS1 plus miR-140-5p
inhibitor. si-OIP5-AS1 increased miR-140-5p levels by 4-fold and
miR-140-5p inhibitor restored miR-140-5p levels compared with the
si-NC (Fig. 2E). Transfection with
si-OIP5-AS1 attenuated the lymphatic vessel formation ability in
HDLECs cultured with A549 medium, which was partly rescued by
miR-140-5p inhibitor (Fig. 2F and
G). OIP5-AS1 knockdown reduced cell invasion compared with the
si-NC group. Cell invasion was rescued by miR-140-5p inhibitor
(Fig. 2H). Taken together, these
data demonstrated that miR-140-5p is a mediator of
OIP5-AS1-regulated NSCLC metastasis.
HDAC7 is involved in
miR-140-5p-modulated metastatic phenotypes of NSCLC
The aforementioned findings led to the investigation
of the downstream targets of miR-140-5p in NSCLC. TargetScan was
used and predicted the epigenetic regulator HDAC7 as a potential
candidate (Fig. 3A). The
dual-luciferase reporter assay results showed that compared with
their respective NC groups, miR-140-5p mimic significantly reduced
luciferase activity of A549 cells containing the wild-type 3′-UTR
of HDAC7, whereas miR-140-5p inhibitor significantly increased
luciferase activity. There is no significant alteration in mutant
3′-UTR of HDAC7-expressing A549 cells (Fig. 3B). Compared with their respective NC
groups, western blotting results demonstrated miR-140-5p mimic
attenuated HDAC7 protein levels, while miR-140-5p inhibitor
increased HDAC7 protein levels (Fig.
3C). In addition, HDAC7 levels were significantly higher in
NSCLC tumors compared with normal adjacent tissues (Fig. 3D).
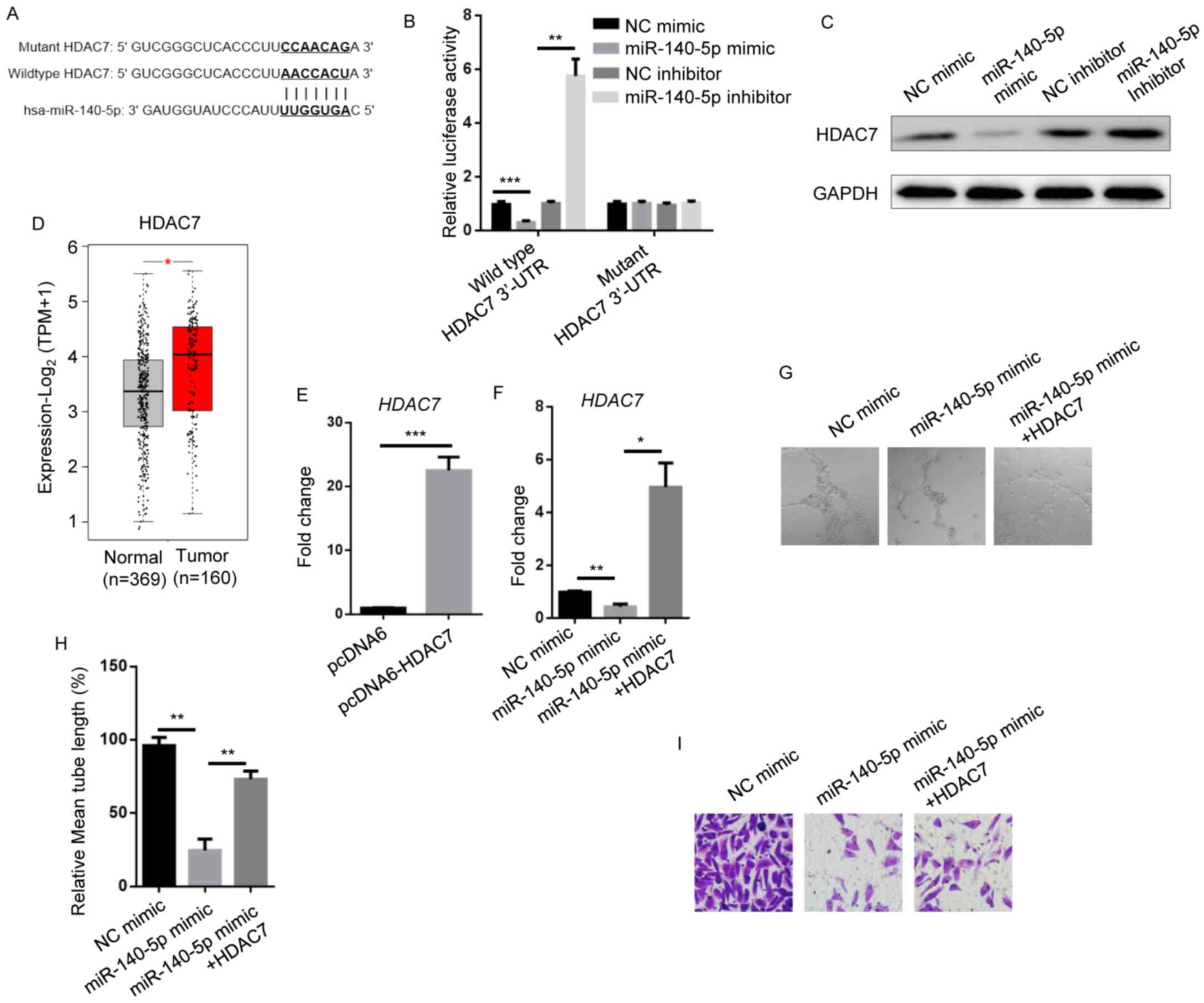 | Figure 3.HDAC7 is involved in
miR-140-5p-modulated metastatic phenotypes of non-small cell lung
cancer. (A) Bioinformatics of the putative binding site at the
3′-UTR of HDAC7 by miR-140-5p. Complementary sequences are shown in
bold. (B) Luciferase activity in A549 cells transfected with NC
mimic, miR-140-5p mimic, NC inhibitor and miR-140-5p inhibitor. (C)
Western blotting showing HDAC7 expression levels in A549 cells
transfected with NC mimic, miR-140-5p mimic, NC inhibitor and
miR-140-5p inhibitor. GAPDH was the internal control. (D)
Bioinformatic analysis of The Cancer Genome Atlas datasets showing
HDAC7 levels in human normal lung tissues (n=369) or lung
adenocarcinoma tumors (n=160). Reverse transcription-quantitative
PCR showing HDAC7 levels in A549 cells transfected with (E) pcDNA6
and pcDNA6-HDAC7 and (F) NC mimic, miR-140-5p mimic and miR-140-5p
mimic + HDAC7. (G) Representative microscopy images (400×
magnification) and (H) quantification (normalized to the control)
of lymphatic vessel formation in human dermal lymphatic endothelial
cells cultured with conditioned medium of A549 cells transfected
with NC mimic, miR-140-5p mimic and miR-140-5p mimic + HDAC7. (I)
Transwell invasion assay showing invasion of A549 cells transfected
with NC mimic, miR-140-5p mimic and miR-140-5p mimic + HDAC7 (400×
magnification). Data are presented as the mean ± standard
deviation. *P<0.05; **P<0.01 and ***P<0.001. HDAC7, human
dermal lymphatic endothelial cells; miR, microRNA; NC, negative
control; 3′UTR, 3′-untranslated region; TPM, Transcripts Per
Million. |
To evaluate whether HDAC7 plays a role in
miR-140-5p-regulated metastasis, HDAC7 was elevated by ~24-fold in
pcDNA6-HDAC7-transfected A549 cells compared with
pcDNA6-transfected A549 cells. Meanwhile, miR-140-5p mimic
downregulated HDAC7 expression and HDAC7 overexpression in
miR-140-5p mimic-transfected A549 cells resulted in 4–5-fold
upregulation of HDAC7 compared with the NC mimic (Fig. 3E and F). Compared with the NC mimic
group, miR-140-5p mimic significantly decreased the lymphatic
vessel formation ability of HDLECs incubated with A549 medium,
which was rescued by HDAC7 overexpression (Fig. 3G and H). miR-140-5p mimic reduced the
number of invaded cells, and this trend was enhanced by HDAC7
(Fig. 3I). The results suggested
that HDAC7 plays a role miR-140-5p-inhibited NSCLC metastasis.
VEGFA acts as an effector for
HDAC7-regulated NSCLC metastasis
It was recently reported that HDAC7 contributed to
cancer progression via regulating VEGFA expression levels (21). VEGFA has also been shown to be
implicated in cancer metastasis (29,30).
Thus, it was hypothesized that VEGFA acted as a downstream effector
for the OIP5-AS1/miR-140-5p/HDAC7 axis in NSCLC cells. RT-qPCR and
western blotting were performed to analyze VEGFA expression levels
in HDAC7-knockdown A549 cells (Fig. 4A
and B). HDAC7 expression was markedly reduced in A549 cells
transfected with si-HDAC7 compared with si-NC. Consistent with
previous results, HDAC7 knockdown resulted in downregulation of
VEGFA levels compared with the si-NC group. VEGFA expression levels
were significantly upregulated in NSCLC tumors compared with normal
adjacent tissues (Fig. 4C).
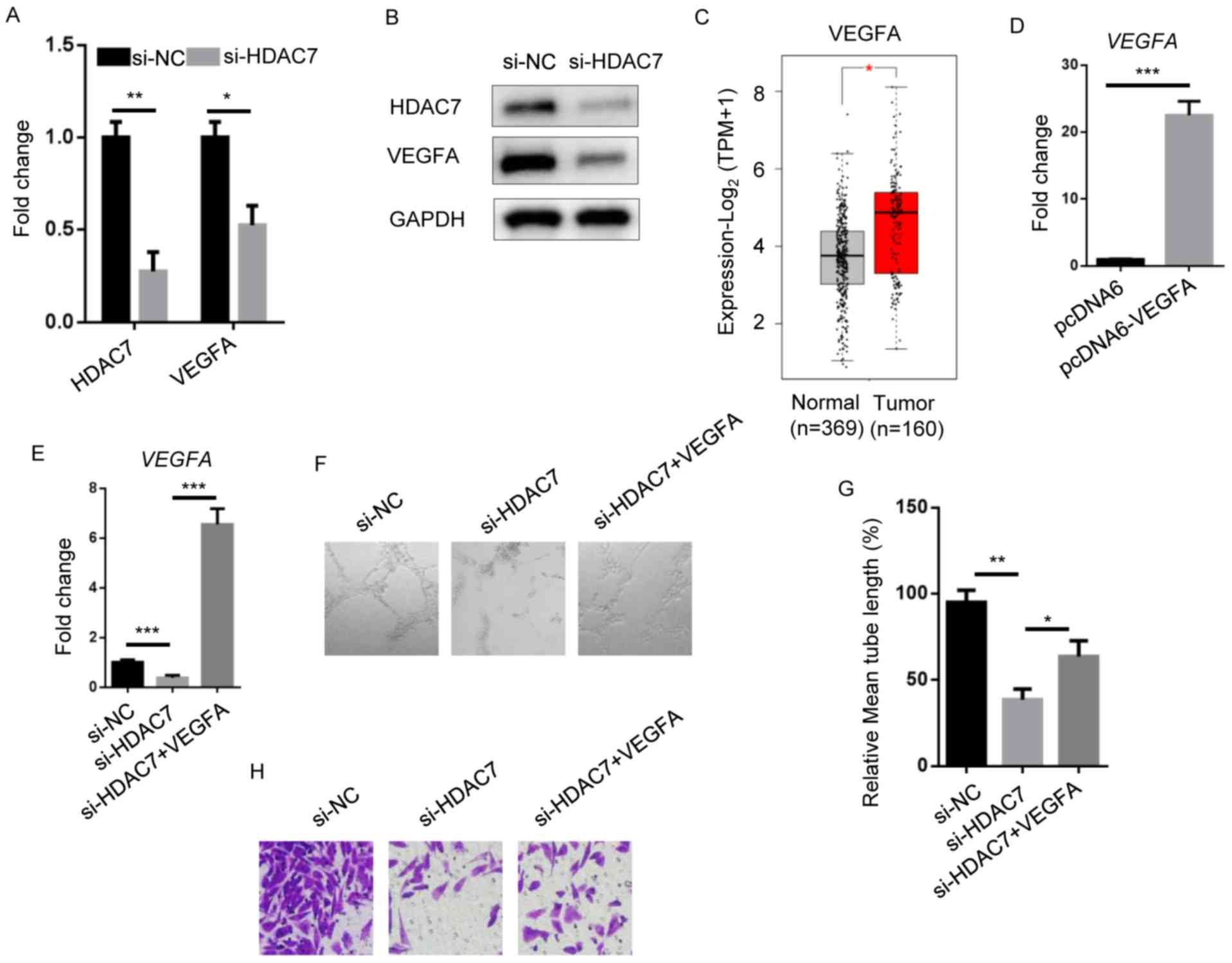 | Figure 4.VEGFA functions as an effector for
HDAC7-regulated non-small cell lung cancer metastasis. (A) RT-qPCR
showing HDAC7 and VEGFA levels in A549 cells transfected with si-NC
and si-HDAC7. (B) Western blotting of HDAC7 and VEGFA levels in
A549 cells transfected with si-NC and si-HDAC7. GAPDH was the
internal control. (C) Bioinformatic analysis of The Cancer Genome
Atlas datasets showing VEGFA levels in human normal lung tissues
(n=369) or lung adenocarcinoma tumors (n=160). RT-qPCR showing
VEGFA levels in A549 cells transfected with (D) pcDNA6 and
pcDNA-VEGFA and (E) si-NC, si-HDAC7 and si-HDAC7 + VEGFA. (F)
Representative microscopy images (400× magnification) and (G)
quantification (normalized to the control) of lymphatic vessel
formation in human dermal lymphatic endothelial cells cultured with
conditioned medium of A549 cells transfected with si-NC, si-HDAC7
and si-HDAC7 + VEGFA. (H) Transwell invasion assay showing invasion
of A549 cells transfected with si-NC, si-HDAC7 and si-HDAC7 + VEGFA
(400× magnification). Data are presented as the mean ± standard
deviation. *P<0.05; **P<0.01 and ***P<0.001. HDAC7, human
dermal lymphatic endothelial cells; RT-qPCR, reverse
transcription-quantitative PCR; si, small interfering; NC, negative
control; HDAC7, human dermal lymphatic endothelial cells; VEGFA,
vascular endothelial growth factor A; TPM, Transcripts Per
Million. |
To assess the function of VEGFA in NSCLC, VEGFA was
overexpressed in HDAC7-depleted A549 cells (Fig. 4D and E). HDAC7 knockdown impaired
lymphatic vessel formation of HDLEC cells incubated with A549
medium; however, VEGFA overexpression significantly restored this
phenotype (Fig. 4F and G). In
addition, si-HDAC7 cells reduced invasion ability compared with the
si-NC group, which was reverted by VEGFA overexpression (Fig. 4H). The results suggested that VEGFA
plays a role in OIP5-AS1/miR-140-5p/HDAC7 axis-induced tumor
metastasis of NSCLC.
Discussion
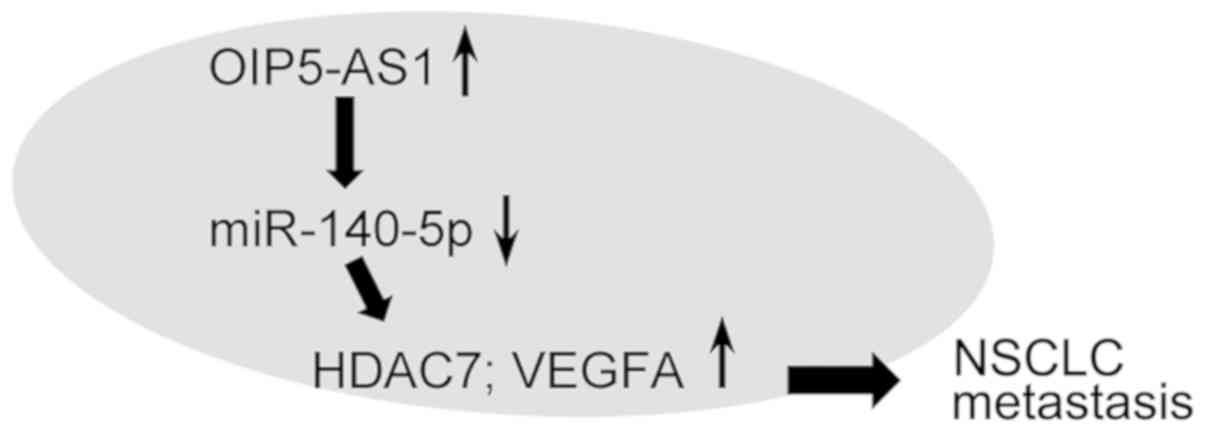
The present study demonstrated that OIP5-AS1
negatively interacted with miR-140-5p, thereby regulating HDAC7 and
VEGFA expression levels (Fig. 5).
miR-140-5p functioned as an effector for the biological role of
OIP5-AS1 in lung cancer cell metastasis. HDAC7 and VEGFA rescued
OIP5-AS1/miR-140-5p-related tumor phenotypes, and it was observed
that the OIP5-AS1/miR-140-5p/HDAC7 axis was dysregulated in TCGA
lung tumors.
Generally, patients with NSCLC are treated with
chemotherapy agents, such as cisplatin, for most cases (31). Although the overall survival rate of
patients with NSCLC has improved recently, the therapeutic effect
of chemotherapy reaches a plateau with a 20% response rate and
median survival of 8–10 months (32). More notably, molecular targeted
therapy is one of the most novel strategies for NSCLC treatment,
such as epidermal growth factor receptor (EGFR) mutation and
anaplastic lymphoma kinase (ALK) rearrangement (33). Gefitinib and crizotinib are the two
targeted drugs for EGFR and ALK tyrosine kinase inhibitors,
respectively (33). In the present
study, it was confirmed that OIP5-AS1 exerted a role in NSCLC
progression, suggesting that OIP5-AS1 may have value as a
diagnostic biomarker and therapeutic target for NSCLC
treatment.
LncRNAs function as endogenous miRNA decoys in
cancer to modulate the expression of downstream target genes
(34). It was shown that OIP5-AS1
acts as a competitive endogenous RNA for sponging miRNAs, such as
miR-338-3p (6), miR-448 (9) and miR-378a-3p (10). In the present study, we demonstrated
that OIP5-AS1 negatively regulated miR-140-5p by complementary
binding.
A study indicated that miR-140-5p suppressed the
migration, proliferation and invasion of NSCLC cells (14). The lncRNA HANR negatively regulated
miR-140-5p to aggravate the proliferation, migration and invasion
of NSCLC (35). Consistently, the
present study found that miR-140-5p inhibited the invasion and
lymphatic vessel formation ability of A549 cells. In addition,
miR-140-5p levels were relatively lower in human clinical lung
tumors tissues compared with normal tissues. Additionally,
miR-140-5p was shown to partly restore OIP5-AS1-induced
phenotypes.
HDAC7 is a member of the HDAC family. Commonly,
HDAC7 upregulates the acetylation of histones (especially H3) in an
enzymatic activity-dependent manner, thereby activating the
expression of target genes (36).
However, HDAC7 also exerts non-enzymatic dependent activity,
instead functioning via interaction with other transcriptional
factors (18,37). The present data showed that HDAC7
positively regulated VEGFA, which is in agreement with previously
published study (21). Caslini et
al (21), indicated that HDAC7
reduced H3K27ac, a super enhancer-related gene, levels in
human breast cancer cells. Caslini et al reported that
HDAC7-knockdown influenced chromatin configuration and blocked the
expression of oncogenes. Moreover, the present results confirmed
the regulatory relationship between HDAC7 and VEGFA using RT-qPCR
and western blot analysis.
VEGF was shown to induce angiogenesis in a
VEGFR2-dependent manner, which is important for tumor metastasis
(38). Recently, homeoprotein Six1,
a member of Six family of homeodomain transcription factors,
enhanced colorectal cancer metastasis and angiogenesis via
upregulating VEGF (39). miR-195
inhibited VEGF to regulate squamous cell lung cancer metastasis and
angiogenesis (40). Sinha et
al (41) suggested that VEGF was
involved in breast cancer metastasis and angiogenesis via the focal
adhesion kinase/matrix metalloproteinase-9 signaling pathway. In
agreement with the present data, it was also observed that VEGFA
promoted lung cancer invasion and lymphatic vessel formation.
In summary, the present study suggested the
oncogenic role of lncRNA OIP5-AS1 in NSCLC metastasis via
miR-140-5p at the cellular and clinical levels. HDAC7 and VEGFA are
two downstream effectors for mediating
OIP5-AS1/miR-140-5p-regulated NSCLC metastasis. The present
findings may provide an improved understanding of NSCLC progression
and accelerate the development of diagnostic and therapeutic
strategies for the treatment of NSCLC.
Acknowledgements
Not applicable.
Funding
The study was supported by Project jointly built by
HENAN Medical Science and Technology Public Relations Plan (grant
no. 2018020837).
Availability of data and materials
The datasets analyzed in this study are available in
The Cancer Genome Atlas, (https://cancergenome.nih.gov/).
Authors' contributions
JM conceived and designed the experiments, and wrote
the manuscript. GL, WW, PX and DY performed the experiments. HB and
RL analyzed the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhuang Y, Jiang H, Li H, Dai J, Liu Y, Li
Y, Miao L, Cai H, Xiao Y, Xia H, et al: Down-regulation of long
non-coding RNA AFAP1-AS1 inhibits tumor cell growth and invasion in
lung adenocarcinoma. Am J Transl Res. 9:2997–3005. 2017.PubMed/NCBI
|
|
2
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morgensztern D, Ng SH, Gao F and Govindan
R: Trends in stage distribution for patients with non-small cell
lung cancer: A national cancer database survey. J Thorac Oncol.
5:29–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu G, Chen J, Pan Q, Huang K, Pan J, Zhang
W, Chen J, Yu F, Zhou T and Wang Y: Long noncoding RNA expression
profiles of lung adenocarcinoma ascertained by microarray analysis.
PLoS One. 9:e1040442014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li M, Ning J, Li Z, Fei Q, Zhao C, Ge Y
and Wang L: Long noncoding RNA OIP5-AS1 promotes the progression of
oral squamous cell carcinoma via regulating miR-338-3p/NRP1 axis.
Biomed Pharmacother. 118:1092592019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y, Shi F, Xia Y and Zhao H: LncRNA
OIP5-AS1 predicts poor prognosis and regulates cell proliferation
and apoptosis in bladder cancer. J Cell Biochem. 2018.(Online ahead
of print).
|
|
8
|
Zhang J, Zhao T, Tian L and Li Y: LncRNA
OIP5-AS1 promotes the proliferation of hemangioma vascular
endothelial cells via regulating miR-195-5p/NOB1 axis. Front
Pharmacol. 10:4492019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deng J, Deng H, Liu C, Liang Y and Wang S:
Long non-coding RNA OIP5-AS1 functions as an oncogene in lung
adenocarcinoma through targeting miR-448/Bcl-2. Biomed
Pharmacother. 98:102–110. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang M, Sun X, Yang Y and Jiao W: Long
non-coding RNA OIP5-AS1 promotes proliferation of lung cancer cells
and leads to poor prognosis by targeting miR-378a-3p. Thorac
Cancer. 9:939–949. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Acunzo M, Romano G, Wernicke D and Croce
CM: MicroRNA and cancer-a brief overview. Adv Biol Regul. 57:1–9.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao Y, Ma J, Xue Y, Wang P, Li Z, Liu J,
Chen L, Xi Z, Teng H, Wang Z, et al: Knockdown of long non-coding
RNA XIST exerts tumor-suppressive functions in human glioblastoma
stem cells by up-regulating miR-152. Cancer Lett. 359:75–86. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun Y and Qin B: Long noncoding RNA MALAT1
regulates HDAC4-mediated proliferation and apoptosis via decoying
of miR-140-5p in osteosarcoma cells. Cancer Med. 7:4584–4597. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou W, Wang X, Yin D, Xue L, Ma Z, Wang
Z, Zhang Q, Zhao Z, Wang H, Sun Y and Yang Y: Effect of miR-140-5p
on the regulation of proliferation and apoptosis in NSCLC and its
underlying mechanism. Exp Ther Med. 18:1350–1356. 2019.PubMed/NCBI
|
|
15
|
Shi SL and Zhang ZH: Long non-coding RNA
SNHG1 contributes to cisplatin resistance in non-small cell lung
cancer by regulating miR-140-5p/Wnt/β-catenin pathway. Neoplasma.
66:756–765. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang P, Xiong J, Zuo L, Liu K and Zhang H:
miR1405p regulates cell migration and invasion of nonsmall cell
lung cancer cells through targeting VEGFA. Mol Med Rep.
18:2866–2872. 2018.PubMed/NCBI
|
|
17
|
Witt O, Deubzer HE, Milde T and Oehme I:
HDAC family: What are the cancer relevant targets? Cancer Lett.
277:8–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jensen ED, Schroeder TM, Bailey J,
Gopalakrishnan R and Westendorf JJ: Histone deacetylase 7
associates with Runx2 and represses its activity during osteoblast
maturation in a deacetylation-independent manner. J Bone Miner Res.
23:361–372. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kato H, Tamamizu-Kato S and Shibasaki F:
Histone deacetylase 7 associates with hypoxia-inducible factor
1alpha and increases transcriptional activity. J Biol Chem.
279:41966–41974. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li B, Samanta A, Song X, Iacono KT, Bembas
K, Tao R, Basu S, Riley JL, Hancock WW, Shen Y, et al: FOXP3
interactions with histone acetyltransferase and class II histone
deacetylases are required for repression. Proc Natl Acad Sci USA.
104:4571–4576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Caslini C, Hong S, Ban YJ, Chen XS and
Ince TA: HDAC7 regulates histone 3 lysine 27 acetylation and
transcriptional activity at super-enhancer-associated genes in
breast cancer stem cells. Oncogene. 38:6599–6614. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wei Y, Zhou F, Zhou H, Huang J, Yu D and
Wu G: Endothelial progenitor cells contribute to neovascularization
of non-small cell lung cancer via histone deacetylase 7-mediated
cytoskeleton regulation and angiogenic genes transcription. Int J
Cancer. 143:657–667. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lei H, Gao Y and Xu X: LncRNA TUG1
influences papillary thyroid cancer cell proliferation, migration
and EMT formation through targeting miR-145. Acta Biochim Biophys
Sin (Shanghai). 49:588–597. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu Y, Shen HM, Fang DM, Meng QJ and Xin
YH: LncRNA HCP5 promotes the development of cervical cancer by
regulating MACC1 via suppression of microRNA-15a. Eur Rev Med
Pharmacol Sci. 22:4812–4819. 2018.PubMed/NCBI
|
|
26
|
Yao N, Yu L, Zhu B, Gan HY and Guo BQ:
LncRNA GIHCG promotes development of ovarian cancer by regulating
microRNA-429. Eur Rev Med Pharmacol Sci. 22:8127–8134.
2018.PubMed/NCBI
|
|
27
|
Gobillot TA, Humes D, Sharma A, Kikawa C
and Overbaugh J: The robust restriction of zika virus by type-I
interferon in A549 cells varies by viral lineage and is not
determined by IFITM3. Viruses. 12:5032020. View Article : Google Scholar
|
|
28
|
Massa D, Baran M, Bengoechea JA and Bowie
AG: PYHIN1 regulates pro-inflammatory cytokine induction rather
than innate immune DNA sensing in airway epithelial cells. J Biol
Chem. 295:4438–4450. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Q, Lu S, Li T, Yu L, Zhang Y, Zeng
H, Qian X, Bi J and Lin Y: ACE2 inhibits breast cancer angiogenesis
via suppressing the VEGFa/VEGFR2/ERK pathway. J Exp Clin Cancer
Res. 38:1732019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo J, Chen M, Ai G, Mao W, Li H and Zhou
J: Hsa_circ_0023404 enhances cervical cancer metastasis and
chemoresistance through VEGFA and autophagy signaling by sponging
miR-5047. Biomed Pharmacother. 115:1089572019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Duma N, Santana-Davila R and Molina JR:
Non-small cell lung cancer: Epidemiology, screening, diagnosis, and
treatment. Mayo Clin Proc. 94:1623–1640. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH; Eastern
Cooperative Oncology, : Comparison of four chemotherapy regimens
for advanced non-small-cell lung cancer. N Engl J Med. 346:92–98.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kumarakulasinghe NB, van Zanwijk N and Soo
RA: Molecular targeted therapy in the treatment of advanced stage
non-small cell lung cancer (NSCLC). Respirology. 20:370–378. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cao MX, Jiang YP, Tang YL and Liang XH:
The crosstalk between lncRNA and microRNA in cancer metastasis:
Orchestrating the epithelial-mesenchymal plasticity. Oncotarget.
8:12472–12483. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li SJ, Wu YX, Liang YH, Gao Y, Wu AB,
Zheng HY and Yang ZX: LncRNA HANR aggravates the progression of
non-small cell lung cancer via mediating miRNA-140-5p. Eur Rev Med
Pharmacol Sci. 24:704–711. 2020.PubMed/NCBI
|
|
36
|
Dressel U, Bailey PJ, Wang SC, Downes M,
Evans RM and Muscat GE: A dynamic role for HDAC7 in MEF2-mediated
muscle differentiation. J Biol Chem. 276:17007–17013. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma C and D'Mello SR: Neuroprotection by
histone deacetylase-7 (HDAC7) occurs by inhibition of c-jun
expression through a deacetylase-independent mechanism. J Biol
Chem. 286:4819–4828. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Saharinen P, Eklund L, Pulkki K, Bono P
and Alitalo K: VEGF and angiopoietin signaling in tumor
angiogenesis and metastasis. Trends Mol Med. 17:347–362. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu H, Zhang Y, Pena MM, Pirisi L and Creek
KE: Six1 promotes colorectal cancer growth and metastasis by
stimulating angiogenesis and recruiting tumor-associated
macrophages. Carcinogenesis. 38:281–292. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu H, Chen Y, Li Y, Li C, Qin T, Bai M,
Zhang Z, Jia R, Su Y and Wang C: miR195 suppresses metastasis and
angiogenesis of squamous cell lung cancer by inhibiting the
expression of VEGF. Mol Med Rep. 20:2625–2632. 2019.PubMed/NCBI
|
|
41
|
Sinha S, Khan S, Shukla S, Lakra AD, Kumar
S, Das G, Maurya R and Meeran SM: Cucurbitacin B inhibits breast
cancer metastasis and angiogenesis through VEGF-mediated
suppression of FAK/MMP-9 signaling axis. Int J Biochem Cell Biol.
77:41–56. 2016. View Article : Google Scholar : PubMed/NCBI
|